Abstract
Background
Pain is an unpleasant sensory experience that usually plays a protective role. Inflammatory pain is often severe and stubborn, which has a great impact on the quality of life of patients. However, there has been no breakthrough in the treatment strategy and mechanism of inflammatory pain.
Methods
This study investigated the analgesic effect of tetrahydropalmatine (THP) in rats injected with complete Freund's adjuvant (CFA)-induced inflammatory pain. Allodynia and gait analysis of rats were used to evaluate the analgesic effect at different time points before and after operation. THP (2.5, 5, and 10 mg/kg) was administered intraperitoneally once daily for 7 days post Day 3. The expression levels of TNF-α and IL-1β in the spinal cord were measured by enzyme-linked immunosorbent assay. The activation of astrocytes and microglial cells in the spinal cord was tested by western blot before and after THP treatment. The apoptosis of glial cells was tested by flow cytometry after treatment with THP in the primary cultured glial cell model.
Results
CFA treatment induced significant allodynia and caused abnormal gait in rats. Administration of THP at 10 mg/kg significantly alleviated CFA-induced inflammatory pain behaviors. Moreover, CFA-induced activation of glial cells and the increased levels of TNF-α and IL-1β were inhibited by THP administration. In addition, THP promotes apoptosis in primary cultured glial cells. This study suggests the possible clinical utility of THP in the treatment of inflammatory pain.
Conclusion
THP plays an analgesic role by inhibiting the activation of glial cells and promoting apoptosis.
Keywords: Tetrahydropalmatine, glial cell, apoptosis, inflammatory pain
Introduction
Pain is an unpleasant sensory experience that usually plays a protective role. It warns us of possible harm to the body, enables us to quickly remove body parts from harmful stimuli, and allows us to learn to avoid such stimuli for a long time.1 Inflammatory pain is often severe and stubborn, which has a great impact on the quality of life of patients.2 However, there has been no breakthrough in the treatment strategy and mechanism of inflammatory pain. Therefore, new and more effective treatment methods are essential to improve the quality of life of patients.
Glial cells in the spinal cord, including astrocytes and microglia, play crucial roles in the occurrence and maintenance of inflammatory pain.3,4 The activation of these glial cells is usually concentrated in the intracellular signal cascade involving the phosphorylation of p38 MAP kinase, which leads to the increased expression and release of TNF-α, IL-1β, and IL-18.5,6 TNF-α and IL-1β can induce central sensitization directly by enhancing excitatory synaptic transmission of the spinal cord, which plays an important role in the maintenance of chronic inflammatory pain.7 After sciatic nerve transection or L5 spinal nerve injury, apoptosis of neurons and glial cells appears in the dorsal root ganglion of rats.8,9 The role of glial apoptosis in pain, especially in inflammatory pain, is still unclear.
Tetrahydropalmatine (THP), an active component of traditional Chinese herbs derived from the corydalis Yanhusuo, has been reported to possess anti‐inflammatory and antinociceptive effects.10,11 Although THP is a traditional analgesic medicine in the clinic, the mechanism of its antinociceptive effect is still unclear. Whether this traditional analgesic can attenuate the regulation of glial cell activation and apoptosis is still unknown.
In this study, we aimed to assess the effects of THP on CFA-induced inflammatory pain and its potential mechanisms.
Materials and methods
Materials
THP (purity ≥99.0%) was purchased from Shanghai Selleck Chemicals (Shanghai, China). CFA and lipopolysaccharides were purchased from Sigma (St. Louis, United States). Primary antibodies against GFAP, Iba-1, NF-κB, p-NF-κB and GAPDH were purchased from Abcam (Cambridge, UK). All secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from Beyotime (Shanghai, China). Fetal bovine serum was purchased from Gibco (St. Louis, United States). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Corning (Manassas, USA). All of the chemicals and reagents used were of standard biochemical quality.12
Animals
All animal experiments were conducted in accordance with the International Association for Study of Pain Guidelines and approved by the Animal Care and Use Committee of Jiaxing University (Jiaxing, China). Adult male Sprague-Dawley rats (180–200 g) were obtained from the Experimental Animal Center of Jiaxing University. Rats were randomly assigned and placed in plastic cages to drink and eat freely in a 12:12 h light/dark cycle. The rats adapted to the new environment for a week. All efforts were made to minimize animal suffering and the number of animals used.
Experimental designs and THP treatment
Rats were separated into 6 groups: the Con + Veh, Con + THP 10 mg/kg, CFA + Veh, CFA + THP 2.5 mg/kg, CFA + THP 5 mg/kg and CFA + THP 10 mg/kg groups (each group was 8 rats). The last 4 groups of rats, after disinfection of the skin of the left hind foot with iodophor, inhaled isoflurane (Yuyan, Shanghai, China) for short-term anesthesia. Then, 100 µL of CFA was injected subcutaneously in the center of the left hindpaw followed by gentle pressure to avoid leakage to induce inflammatory pain, while the control animals were injected with an equal volume of 0.9% saline in the same position.13 THP was suspended in 5% DMSO. Different concentrations of THP (2.5, 5, and 10 mg/kg) were injected (i.p.) on Day 3 once daily for 7 successive days and were freshly prepared prior to conducting the experiments.14 All behaviors of animals were tested at 2 h after administration during testing days. Animals were accommodated for 30 min in the testing room before behavioral tests and were sacrificed to quickly collect the L4-5 spinal segmental tissues after behavior tests on Day 9 to carry out the next experiment. At the end of the experiment, all animals were euthanized with a narcotic overdose (isoflurane).
Mechanical allodynia test
Mechanical allodynia was tested by using a set of von Frey monofilaments (Institute of Biological Medicine, Academy of Medical Science, China) according to a previous study.15 Before each test, rats were placed in a transparent plexiglass compartment (50 × 20 × 20 cm) for 30 min to adapt to the environment. After five consecutive tests with an interval of 10 s, the average of these values was taken to generate the paw withdrawal threshold (PWT). All behavioral testing was conducted by investigators blinded to the experimental treatment conditions.
Heat hyperalgesia test
Heat hyperalgesia was determined by a significantly shortened latency of foot withdrawal in response to heat stimulation. In brief, the heat plate was focused on a portion of the hind paw, and a radiant thermal stimulus was delivered to that site. The stimulus was shut off automatically when the hind paw moved (or after 20 s to prevent tissue damage). Thermal stimuli were delivered 3 times to each hind paw at 10-min intervals.
CatWalk gait analysis
On the same day as the last von Frey test, gait analysis was conducted using the CatWalk XT 10.0 system (Noldus Information Technology, Netherlands).16 CatWalk XT is a system for quantitative assessment of footfalls and gait in rats. It consists of a walkway, which the animal can move directly across. A closed corridor is fixed on a glass panel, with red background lights above the corridor, and a green light reflects on the glass panel. A camera was installed below the setting to record the paw prints illuminated by green light when the rat paw touched the glass plate as it walked along the corridor. Captured footprint data were analyzed by special software to calculate related variable values, such as the footprint length and maximum contact area of the paw.17–19
Primary cell culture and treatment
Primary glial cell cultures were extracted from cerebral cortices of postnatal day male Sprague-Dawley rats. In short, rat cerebral cortices were extracted in sterile cold PBS, dissociated into single cells with 0.125% trypsin at 37 °C and filtered through a 70-µm nylon mesh. All cells were plated onto 75 cm2 flasks for 2 weeks in high-glucose DMEM containing 10% FBS and 1% penicillin-streptomycin solution. To obtain primary astrocytes, 75 cm2 flasks containing mixed glial cells were sealed with foil and shaken at 250 rpm on a rotary shaker at room temperature (RT) overnight.20 The conditioned culture medium was then discarded, and the cells were dissociated using 0.25% trypsin and centrifuged at 2000 rpm for 30 min. After centrifugation, the pellet (primary astrocytes) was collected and used for experiments. To obtain primary microglia, mixed glial cultures were incubated with 0.25% trypsin diluted 1:4 in serum-free DMEM for 20–30 min in a 5% CO2 incubator at 37 °C.20 The upper layer of astrocytes was discarded, and the remaining microglia were used for the next experiment. To determine whether THP inhibits primary glial activation, rat primary cells were treated with lipopolysaccharide (LPS, 1 µg/ml) or PBS (1% DMSO) for 30 min followed by THP (100 µM) or PBS for 5.5 h. Then, we collected cell samples after trypsin digestion to detect the protein expression of proteins by western blot.
Immunofluorescence staining
To verify the identity and assess the purity of isolated glial cells, immunofluorescence microscopy was used. Briefly, cells plated on poly-D-lysine-coated coverslips were fixed in 4% paraformaldehyde at room temperature for 20 min and incubated with 2% bovine serum albumin at RT for 60 min. Then, the cells were incubated with primary antibodies against GFAP (1:200, mouse, Abcam) and IBA1 (1:100, rabbit, Abcam) overnight at 4 °C. The secondary antibodies used were DyLight 488-conjugated goat anti-rabbit IgG (1:200, EarthOx) and Alexa Fluor 594-conjugated goat anti-mouse IgG (1:200, Invitrogen) and incubated at RT for 60 min. Finally, Antifade Mounting Medium with DAPI (Vector Lab, USA) was added for nuclear staining. Images of glial cells were captured using a fluorescence microscope (Zeiss, Germany).
Western blot analysis
L4-5 spinal segmental tissue or cultured cells were homogenized for 30 min on ice in RIPA lysis buffer (Beyotime, China) containing a mixture of phosphatase and proteinase inhibitors. The sample was centrifuged at 4 °C at 16,000 rpm for 20 min, and then the protein supernatant was collected. The concentration of total protein was measured by BCA protein assay (Beyotime, China). Equal amounts of total proteins (30 µg/lane) were separated on a 10% SDS-PAGE gel and then transferred onto NC membranes. The membrane was blocked with 5% nonfat milk in PBS at RT for 1 h and incubated with antibodies against GFAP (1:1000, rabbit, Abcam), IBA1 (1:500, rabbit, Abcam), NF‐κB (1:500, rabbit, Abcam) and GAPDH (rabbit, 1:5000; CST) overnight at 4 °C. This membrane was washed and further incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000, Beyotime) at RT for 1 h. The signals were developed using Pierce ECL western blotting Substrate. The intensity of blots was quantified with densitometry using Alpha EaseFC software (Bio-Rad, USA). All protein bands were normalized to GAPDH.
Cytokine measurement
The L4-5 spinal segmental tissue was homogenized using PBS buffer containing protease inhibitors. The sample was centrifuged at 4 °C at 16,000 rpm for 15 min, and then the supernatant was collected. TNF‐α and IL‐1β levels were determined by ELISA using Abcam kits (Abcam, USA) according to the manufacturer's instructions. Reading was performed at absorbance (450 nm) using an enzyme-labeled instrument (Bio Tek, USA).
Cell apoptosis analysis
Cell apoptosis analysis was conducted with the Annexin V-FITC Apoptosis Detection Kit (Beyotime, China). The test was carried out following the manufacturer’s recommendations. Rat primary cells were grown for 12 h in 6-well plates and then treated with 1 µg/ml LPS or 200 µmol/L THP (with 1 µg/ml LPS) for 6 h; control wells were left untreated. Cells were collected by trypsinization and resuspended in binding buffer. Annexin V-FITC and propidium iodide (PI) were added to the binding buffer and incubated for 30 min at RT in the dark. Analyses were performed on a BD LSR Fortessa flow cytometer (BD, USA) as soon as possible.21
Statistical analysis
All results were given as means ± S.E.M. One-way or two-way analysis of variance followed by Tukey’s post hoc test was performed (GraphPad 5.0, USA). A p value of <0.05 was considered statistically significant. The analysis was carried out using SPSS software (SPSS Inc., USA).
Results
THP alleviates CFA-induced mechanical allodynia and heat hyperalgesia
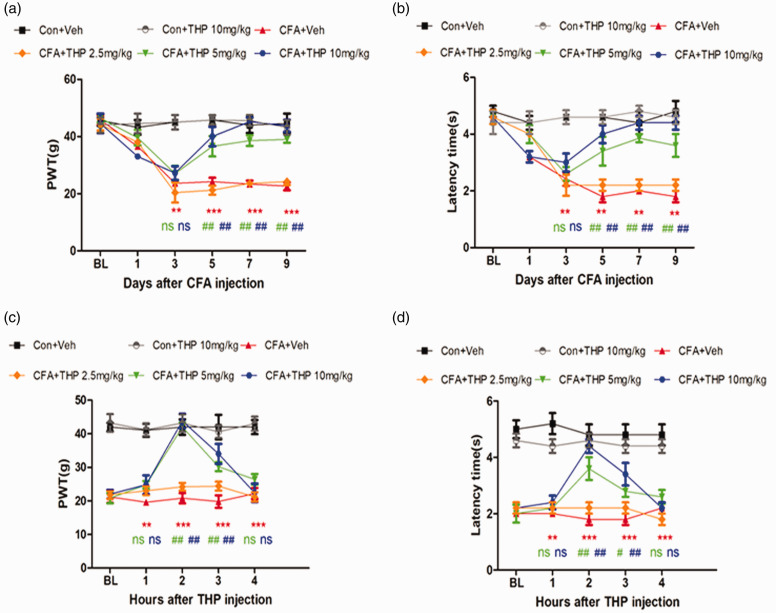
THP was reported to possess anti‐inflammatory and antinociceptive effects.22 We evaluated the antinociceptive effect of THP in CFA-induced inflammatory pain. After administration (i.p.) of CFA, the mechanical withdrawal threshold and latency time showed a significant decrease on Day 3 as a mark of successful modeling. The effect of THP at 5 mg/kg and 10 mg/kg (once daily for 7 days post Day 3) was initiated on Day 5 after the surgery (Figure 1(a) and (b)), and the dose of 2.5 mg/kg did not significantly relieve mechanical allodynia or heat hyperalgesia after CFA surgery. Last treatment with 5/10 mg/kg THP alleviated mechanical allodynia and heat hyperalgesia in CFA-induced inflammatory pain rats in a time-dependent manner (Figure 1(c) and (d)), and this effect was observed on Day 9. These data show that THP can relieve CFA-induced inflammatory pain.
Figure 1.
THP reduces CFA induced mechanical allodynia and heat hyperalgesia. Rats were treated with THP or vehicle (i. p.) once daily for 7 days post Day 3. (a) and(b) Intraperitoneal injection of THP dose dependently alleviated the mechanical allodynia and heat hyperalgesia in CFA induced inflammatory pain rats and this effect was observed at 5/10 mg/kg doses. (c) and (d) Intraperitoneal injection of THP time-dependently alleviated the mechanical allodynia and heat hyperalgesia in CFA induced inflammatory pain rats and this effect was observed at 2 h after THP injection. The results were exhibited as mean ± SD. (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Con + Veh group. #P < 0.05, ## P < 0.01 vs. CFA + Veh group.
Effect of THP on functional gait changes in CFA-induced inflammatory pain rats
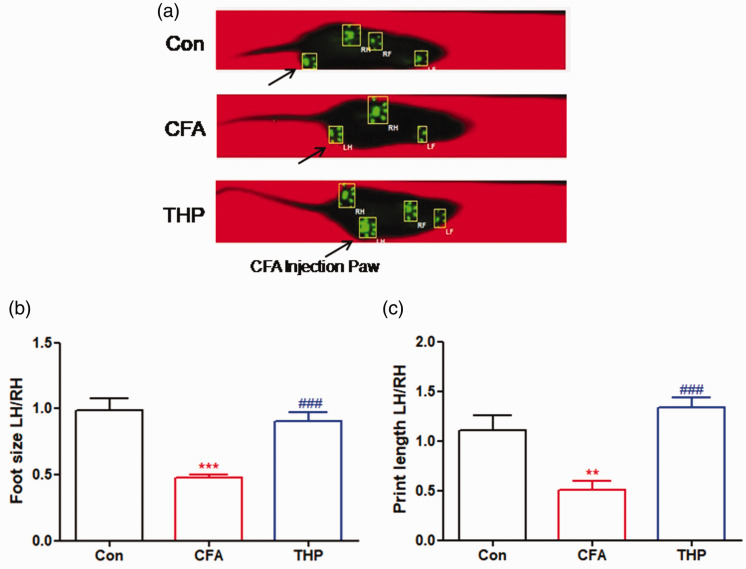
The evaluation of gait parameters using CatWalk gait analysis is considered to be an objective method to evaluate inflammatory pain caused by CFA.23 In this study, we measured the maximum foot size contact area and hind paw print length gait parameters to display pain-related behaviors in rats. As shown in Figure 2(a), Catwalk gait analysis was conducted after 2 h of administration of THP (10 mg/kg, i.p. once daily) on Day 7 after CFA injection surgery. In comparison with the Con group, the CFA group had obviously reduced the maximum contact area and print length gait parameters on Day 7 postsurgery (Figure 2(b) and (c)), which indicated CFA-induced pain hypersensitivity in rats. Meanwhile, these reductions were significantly reversed by THP treatment. These results suggest that THP treatment alleviates pain-related behaviors in rats with CFA-induced inflammatory pain, as indicated by the improvement of CatWalk gait parameters.
Figure 2.
Effect of THP on functional gait-changes in CFA induced inflammatory pain rats. Rats were treated with THP or vehicle (i. p.) once daily for 7 days post CFA injected surgery. (a) Images of rats walking on the CatWalk were showed as above. Comparing these demonstrates increased duration of contact with the surface for the uninjured limb, compared with the injured, while the print representations above (RH, RF, LH, LF) shows the print-area in contact with the plate. Notice that the injured hind paw (LH: Left Hind, arrowhead) has a much smaller print-area, primarily with the heel of the paw. (b) Maximum contact area (cm2); the maximum surface area of a paw that comes into contact with the glass plate. Presented as a ratio between surgery and unsurgery hind paws. (c) Print length (cm); the print length of a paw. Presented as a ratio between surgery and unsurgery hind paws. The results were exhibited as mean ± SD. (n = 5). **P < 0.01, ***P < 0.001 vs. Con group. ###P < 0.001 vs. CFA group.
THP promotes cell apoptosis in primary glial cells
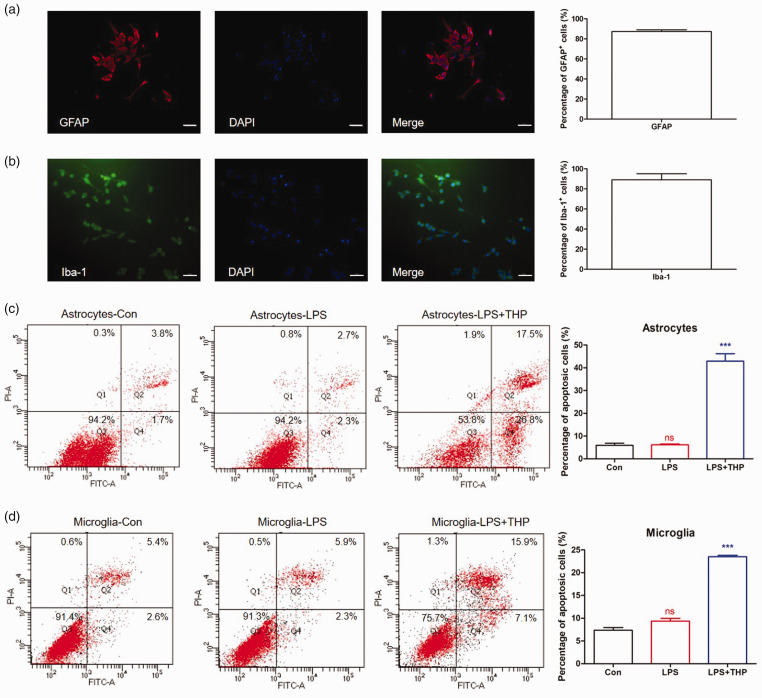
Glial cell activation was reported to play an important role in CFA-induced inflammatory pain.24,25 We studied the effect of THP on the apoptosis of astrocytes and microglia by flow cytometry in a primary cultured glial cell model. The purity of primary cultured astrocytes and microglial cells prepared from neonatal rat glia by shaking was approximately 90% by GFAP/Iba-1 and DAPI immunofluorescence staining identification (Figure 3(a) and (b)). As shown in Figure 3(c) and (d), THP significantly increased the percentage of apoptotic glial cells induced by LPS treatment. THP significantly promoted the apoptosis of astrocytes and microglia, as shown by flow cytometry.
Figure 3.
THP promotes primary glial cells apoptosis by LPS treatment. Following 6 h treatment with LPS (1 µg/ml)) or THP (100 µM), primary astrocytes and microglia were determined by flow cytometry. (a) and (b) The purity of primary cultured astrocytes and microglia cells prepared from neonatal rat glia by the shaking was approximate 90% (n = 3) by GFAP/Iba-1 and DAPI immunofluorescence staining identification. Scale bar 50 µm. (c) and (d) The percentage of astrocytes and microglia apoptosis significantly increased after THP treatment by flow cytometry. The results were exhibited as mean ± SD. (n = 4). ***P < 0.001 vs. LPS group.
THP attenuated the activation of astrocytes and microglia in the spinal cord of rats with inflammatory pain
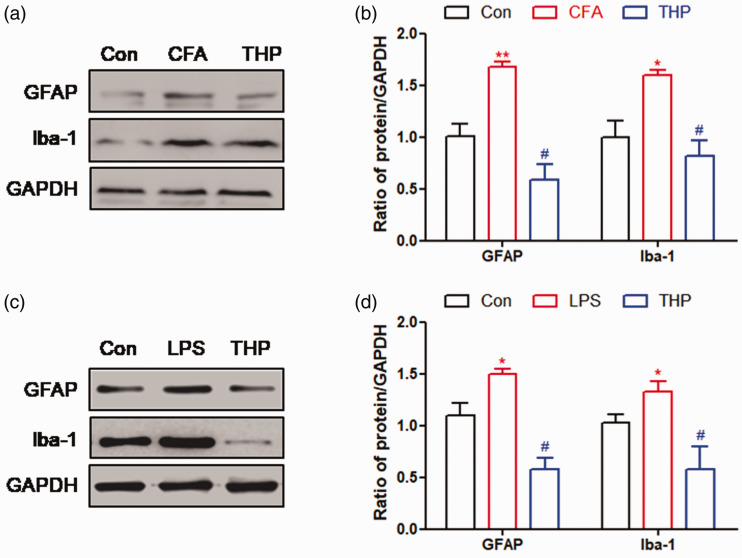
We explored whether astrocyte and microglial activation in the spinal cord of CFA-induced rats could be modulated by THP. Treatment with THP reduced CFA-induced expression of glial fibrillary acidic protein (GFAP, astrocyte activation marker) and induction of brown adipocyte 1 (Iba‐1, microglial activation marker), as observed by the reduction in intensity of western blotting (Figure 4(a) and (b)). We also explored the effect of THP on the activity of astrocytes and microglia in primary culture (Figure 4(c) and (d)), which was consistent with the results in the rat spinal cord. These results suggested that THP treatment inhibited the activation of astrocytes and microglia in the spinal cord of CFA-induced inflammatory pain and a primary glial cell model.
Figure 4.
THP inhibits astrocytes and microglia activation. (a) and (b) Repetitive administration of THP at 10 mg/kg (once daily for 7 days post Day 3) significantly suppressed astrocytes and microglial cells activation in spinal cord tissue of rats. (c) and (d) Treatment of THP at 100 µM significantly suppressed astrocytes and microglial cells activation in primary glial cells. The results were exhibited as mean ± SD. (n = 4). *P < 0.05, **P < 0.01 vs. Con group. #P < 0.05 vs. CFA group.
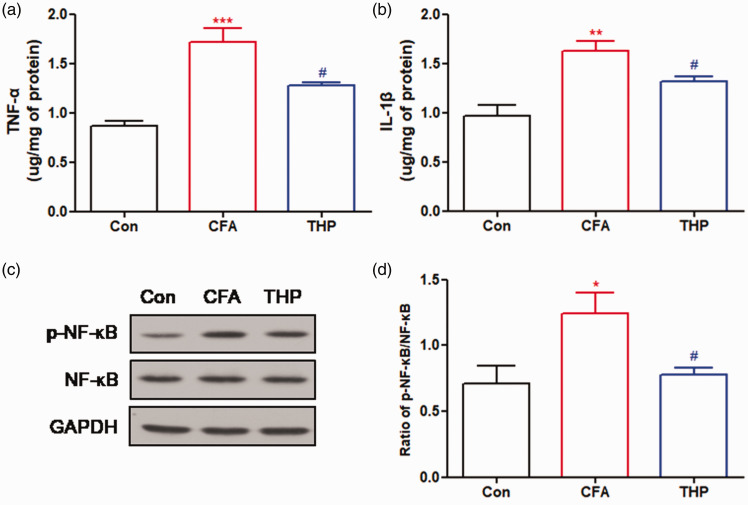
THP suppresses CFA-induced increased levels of cytokines and NF‐κB activation
Upregulation of inflammatory cytokines is usually associated with glial activation and NF‐κB activation, which are involved in the process of CFA-induced inflammatory pain.26–28 Therefore, we evaluated the efficacy of THP in CFA-induced spinal cord cytokine production and NF‐κB activation. CFA significantly increased the expression of TNF‐α (Figure 5(a)) and IL‐1β (Figure 5(b)), which were reduced after treatment with THP. In addition, CFA significantly increased the phosphorylation level of NF‐κB (Figure 5(c) and (d)), which was reduced after treatment with THP, as observed by the reduction in the p-NF‐κB/NF‐κB ratio. Importantly, treatment with 10 mg/kg THP reduced spinal cord inflammatory cytokine expression and NF‐κB activation.
Figure 5.
THP suppresses CFA induced spinal cord increased levels of cytokine and NF‐κB activation. Seven days after intraplantar injection of CFA (100 μl per paw), the spinal cord was dissected for determination of TNF‐α (a), IL‐1β (b) and NF‐κB activation (c) and (d) by ELISA. NF‐κB activation was observed as a reduction of phosphorylated NF‐κB/NF‐κB ratio. The results were exhibited as mean ± SD. (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 vs. Con group. #P < 0.05 vs. CFA group.
Discussion
Previous clinical and experimental studies have confirmed the analgesic effect of THP.22,29,30 Recent studies have shown that THP can alleviate hypochondriac pain, chest pain, headache and abdominal pain in humans 29,30 and inflammatory and bone cancer pain in experimental animals.14,31 However, the mechanism underlying the analgesic effect of THP remains unclear. Previous studies have shown that THP mediates analgesia by blocking D1/2 dopamine receptors in the striatum and hypothalamic arcuate nucleus, thus activating the descending antinociceptive system from the periaqueductal gray to the spinal dorsal horn and inhibiting nociceptive signal transduction.10,32 Few studies have shown the role of THP in the spinal cord, but the spinal cord is the main central system of pain. Therefore, this study addresses the analgesic effect of THP at the level of spinal cord tissue.
In this study, we explored the analgesic role of THP in a CFA-induced inflammatory pain model. Our results indicated that high-dose THP can delay and reverse allodynia and gait abnormalities induced by CFA in a dose-dependent manner. The analgesic effect of THP may be applicable by suppressing the activation of glial cells, promoting apoptosis, and inhibiting the increase in inflammatory factors. To the best of our knowledge, this study demonstrated that THP can significantly reduce the allodynia induced by CFA, which provides new experimental evidence for THP in the treatment of inflammatory pain and the expansion of clinical application.
In this study, by behavioral testing, we found that intraperitoneal injection of THP significantly inhibited and reversed CFA-induced pain-related behaviors in a dose-dependent manner. The analgesic effect of THP is related to inhibiting the activation of astrocytes and microglia induced by inflammation and reducing the activation of NF-κB and the production of TNF-α and IL-1β. In the bone cancer pain model, however, THP showed no effect on astrocyte activation and only significantly suppressed microglial cell activation. Our results showed that THP significantly inhibited the activation of astrocytes in a primary culture astrocyte model and a rat CFA-induced inflammatory pain model. THP has a strong inhibitory effect on primary cultured glial cells, leading to apoptosis of glial cells, especially microglia, which may be the reason that the expression of Iba-1 after THP application was much lower than that in the control group. Cytokines have been shown to play important roles in central and peripheral sensitization in chronic pain and are known to be upregulated after nerve injury.33 In addition, it has been reported that THP can inhibit the ERK/NF-κB pathway in a rat liver ischemia-reperfusion injury model.34 In this study, we also found that THP inhibited the activation of NF-κB in CFA-induced inflammatory pain.
THP alters the expression of molecules involved in apoptosis in primary glial cells. We studied the effect of THP on the apoptosis of astrocytes and microglia by flow cytometry in a primary cultured glial cell model. The results showed that THP could promote the apoptosis of two kinds of glial cells, which may be the mechanism by which THP inhibits the activation of glial cells. However, it was reported that THP inhibited neuronal apoptosis in the cerebral cortex.35 Immunohistochemistry staining (the apoptosis marker) using tissues from rats in control, CFA and CFA + THP groups could provide better evidence. In the future study, we will adding this experiment to more comprehensively confirm the role of THP in promoting glial cell apoptosis. In this study, we did not detect the effect of THP on apoptosis in primary cultured neurons, which requires further study.
In summary, THP at high doses can effectively relieve CFA-induced pain-related behavior. Mechanisms underlying THP-induced attenuation of inflammatory pain may be partially mediated by promoting glial apoptosis and inhibiting glial cell activation, as well as inhibiting NF-κB activation and release of the inflammatory cytokines TNF-α and IL-1β, in the spinal cord. However, current studies cannot exclude other possible mechanisms of THP in the treatment of inflammatory pain, such as sedation. This study provides experimental evidence to confirm the possible analgesic mechanism of THP in inflammatory pain and suggests the clinical application of THP in the treatment of inflammatory pain.
Footnotes
Ethics approval: The animal study was approved by the Animal Care and Use Committee of Jiaxing University. [JUMC2020-018]
Author contributions: Conceived and designed the experiments: MY LSX BBL. Performed the experiments: BBL ML DYM. Analyzed the data: HP HS JFS. Wrote the paper: MY LSX BBL. All authors have read and agreed with the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Science and Technology Project of Jiaxing City (CN) [grant number 2021AD30137], Key Discipline Established by Zhejiang Province and Jiaxing City Jointly–Pain Medicine (CN) [grant number 2019-ss-ttyx], and Key Discipline of Anesthesiology of Jiaxing City (CN) [grant number 2019-zc-06]. The grants supported the necessary funds for acquiring the materials and animals used in the research, the salaries for laboratory and veterinary personnel, and support staff for data collection and analysis.
ORCID iDs
Ming Yao https://orcid.org/0000-0002-4226-8473
Longsheng Xu https://orcid.org/0000-0002-6091-6323
References
- 1.Gao YJ, Ji RR.Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010; 7: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu A, Dong W, Liu S, Cheung JPY, Kwan KYH, Zeng X, Zhang K, Sun Z, Wang X, Cheung KMC, Zhou M, Zhao J.The prevalence and years lived with disability caused by low back pain in China, 1990 to 2016: findings from the global burden of disease study 2016. Pain 2019; 160: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis S.Pain: microglia take control in chronic pain. Nat Rev Neurosci 2013; 14: 154. [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Chamessian A, Zhang YQ.Pain regulation by non-neuronal cells and inflammation. Science 2016; 354: 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang HC, Lin LX, Hu XF, Zhu H, Li HP, Zhang RY, Hu L, Liu WT, Zhao YL, Shu Y, Pan HL, Li M.AMPK activation attenuates inflammatory pain through inhibiting NF-kappaB activation and IL-1beta expression. J Neuroinflammation 2019; 16: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu ML, Wei RD, Zhang T, Wang JM, Cheng Y, Qin FF, Fu SP, Lu ZG, Lu SF.Electroacupuncture relieves pain and attenuates inflammation progression through inducing IL-10 production in CFA-induced mice. Inflammation 2020; 43: 1233–1245. [DOI] [PubMed] [Google Scholar]
- 7.Gajtkó A, Bakk E, Hegedűs K, Ducza L, Holló K.IL-1beta induced cytokine expression by spinal astrocytes can play a role in the maintenance of chronic inflammatory pain. Front Physiol 2020; 11: 543331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaeffer V, Meyer L, Patte-Mensah C, Eckert A, Mensah-Nyagan AG.Sciatic nerve injury induces apoptosis of dorsal root ganglion satellite glial cells and selectively modifies neurosteroidogenesis in sensory neurons. Glia 2010; 58: 169–180. [DOI] [PubMed] [Google Scholar]
- 9.Zhou D, Zhang S, Hu L, Gu YF, Cai Y, Wu D, Liu WT, Jiang CY, Kong X, Zhang GQ.Inhibition of apoptosis signal-regulating kinase by paeoniflorin attenuates neuroinflammation and ameliorates neuropathic pain. J Neuroinflamm 2019; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YY, Wang TX, Zhou JC, Qu WM, Huang ZL.Dopamine D(1) and D(2) receptors mediate analgesic and hypnotic effects of l-tetrahydropalmatine in a mouse neuropathic pain model. Psychopharmacology (Berl) 2019; 236: 3169–3182. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Man Y, Wang X, Jin H, Sun X, Su X, Hao J, Mi W.Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci Rep 2014; 4: 3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su DJ, Li LF, Wang SY, Yang Q, Wu YJ, Zhao MG, Yang L.Pra-C exerts analgesic effect through inhibiting microglial activation in anterior cingulate cortex in complete Freund's adjuvant-induced mouse model. Mol Pain 2021; 17: 1744806921990934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, Verri WA., Jr.The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol 2019; 176: 1728–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang MY, Liu YP, Zhang LY, Yue DM, Qi DY, Liu GJ, Liu S.Levo-tetrahydropalmatine attenuates bone cancer pain by inhibiting microglial cells activation. Mediators Inflamm 2015; 2015: 752512–752016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ni H, Wang Y, An K, Liu Q, Xu L, Zhu C, Deng H, He Q, Wang T, Xu M, Zheng Y, Huang B, Fang J, Yao M.Crosstalk between NFkappaB-dependent astrocytic CXCL1 and neuron CXCR2 plays a role in descending pain facilitation. J Neuroinflammation 2019; 16: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deumens R, Jaken RJ, Marcus MA, Joosten EA.The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J Neurosci Methods 2007; 164: 120–130. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Tian NX, Bai QY, Chen Q, Sun XH, Wang Y.Gait assessment of pain and analgesics: comparison of the DigiGait and CatWalk gait imaging systems. Neurosci Bull 2019; 35: 401–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feehan AK, Zadina JE.Morphine immunomodulation prolongs inflammatory and postoperative pain while the novel analgesic ZH853 accelerates recovery and protects against latent sensitization. J Neuroinflamm 2019; 16: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X-y, Ma H-j, Xue M, Sun Y, L, Ren A, Li M-q, Huang Z-h, Huang C.Anti-nociceptive effects of Sedum Lineare Thunb on spared nerve injury-induced neuropathic pain by inhibiting TLR4/NF-κB signaling in the spinal cord in rats. Biomed Pharmacother 2021; 135: 111215. [DOI] [PubMed] [Google Scholar]
- 20.Ryu KY, Lee HJ, Woo H, Kang RJ, Han KM, Park H, Lee SM, Lee JY, Jeong YJ, Nam HW, Nam Y, Hoe HS.Dasatinib regulates LPS-induced microglial and astrocytic neuroinflammatory responses by inhibiting AKT/STAT3 signaling. J Neuroinflamm 2019; 16: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Shen J, Pan H, Xu L, Sheng H, Liu B, Yao M.Curcumin analog B14 has high bioavailability and enhances the effect of anti-breast cancer cells in vitro and in vivo. Cancer Sci 2021; 112: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou HH, Wu DL, Gao LY, Fang Y, Ge WH.L-Tetrahydropalmatine alleviates mechanical hyperalgesia in models of chronic inflammatory and neuropathic pain in mice. Neuroreport 2016; 27: 476–480. [DOI] [PubMed] [Google Scholar]
- 23.Hestehave S, Abelson KSP, Bronnum Pedersen T, Finn DP, Andersson DR, Munro G.The influence of rat strain on the development of neuropathic pain and comorbid anxio-depressive behaviour after nerve injury. Sci Rep 2020; 10: 20981–20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ.Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J Neurosci 2014; 39: 1391–1402. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z, Wang H, Fang S, Li L, Li X, Shi J, Zhu M, Tan Z, Lu Z.Annexin-1 mimetic peptide Ac2-26 suppresses inflammatory mediators in LPS-induced astrocytes and ameliorates pain hypersensitivity in a rat model of inflammatory pain. Cell Mol Neurobiol 2020; 40: 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Zhang N, Zhang R, Zhao W, Chen Y, Wang Z, Xu B, Zhang M, Shi X, Zhang Q, Guo Y, Xiao J, Chen D, Fang Q.Preemptive intrathecal administration of endomorphins relieves inflammatory pain in male mice via inhibition of p38 MAPK signaling and regulation of inflammatory cytokines. J Neuroinflamm 2018; 15: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu C, Yang LD, Yu W, Tian DD, Gao MR, Wang WJ, Li XB, Wu YM, Wang M.Paeonol ameliorates CFA-induced inflammatory pain by inhibiting HMGB1/TLR4/NF-κB p65 pathway. Metab Brain Dis 2021; 36: 273–283. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y, Jiang Y, Wang L, Huang J, Wen J, Lv H, Wu X, Wan C, Yu C, Zhang W, Zhao J, Zhou Y, Chen Y.Thymosin alpha-1 inhibits complete Freund's adjuvant-induced pain and production of microglia-mediated pro-inflammatory cytokines in spinal cord. Neurosci Bull 2019; 35: 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Zhou J, Wang S, Ye M, Jiang C, Fan G, Zou H.Combined comparative and chemical proteomics on the mechanisms of levo-tetrahydropalmatine-induced antinociception in the formalin test. J Proteome Res 2010; 9: 3225–3234. [DOI] [PubMed] [Google Scholar]
- 30.Liao ZG, Liang XL, Zhu JY, Zhao GW, Yang M, Wang GF, Jiang QY, Chen XL.Correlation between synergistic action of Radix Angelica dahurica extracts on analgesic effects of Corydalis alkaloid and plasma concentration of dl-THP. J Ethnopharmacol 2010; 129: 115–120. [DOI] [PubMed] [Google Scholar]
- 31.Liu TT, Qu ZW, Qiu CY, Qiu F, Ren C, Gan X, Peng F, Hu WP.Inhibition of acid-sensing ion channels by levo-tetrahydropalmatine in rat dorsal root ganglion neurons. J Neurosci Res 2015; 93: 333–339. [DOI] [PubMed] [Google Scholar]
- 32.Chu H, Jin G, Friedman E, Zhen X.Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol 2008; 28: 491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki Y, Zhang L, Cheng JK, Ji RR.Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 2008; 28: 5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Q, Wu L, Liu T, Li S, Feng J, Mao Y, Fan X, Guo C, Wu J.Protective effects of levo-tetrahydropalmatine on hepatic ischemia/reperfusion injury are mediated by inhibition of the ERK/NF-kappaB pathway. Int Immunopharmacol 2019; 70: 435–445. [DOI] [PubMed] [Google Scholar]
- 35.Sun R, Song Y, Li S, Ma Z, Deng X, Fu Q, Qu R, Ma S.Levo-tetrahydropalmatine attenuates neuron apoptosis induced by cerebral ischemia-reperfusion injury: involvement of c-Abl activation. J Mol Neurosci 2018; 65: 391–399. [DOI] [PubMed] [Google Scholar]