Abstract
Non–thrombotic pulmonary embolism can occur from rare but diverse etiology and is not well understood. Increasing prevalence of osteoporosis in the aging population has contributed to increased utilization of percutaneous vertebral augmentation procedures of vertebroplasty and its recent modification, kyphoplasty. Though these procedures are relatively well tolerated, there is risk of potentially fatal complication of bone cement embolization to distant vasculature. We report a case of symptomatic pulmonary cement emboli developed 2 day's post kyphoplasty and its successful treatment with novel anticoagulant for 6 months. We also summarize evidence to assist clinicians and radiologists for early identification, treatment, and prevention of cement pulmonary emboli.
Key words: Vertebroplasty, Kyphoplasty, Polymethyl methacrylate, Cement pulmonary embolism, Anticoagulation
Introduction
Considering the aging American population, osteoporotic vertebral fractures have become a major public health concern with its significant impact on quality of life [1]. When conventional measures of treatment cannot be implemented, percutaneous minimally invasive vertebral augmentation methods (vertebroplasty [VP] and kyphoplasty [KP]) involving the injection of bone cement (polymethyl methacrylate cement [PMMA]) into the vertebral body are useful tools for the management of symptomatic fractures without neurologic impairment [2]. Since its introduction in 1987 by Galibert et al. [3], both vertebroplasty and a recent modification of balloon kyphoplasty have gained wider acceptance for the treatment of a variety of benign and malignant vertebral pathologies [2,4]. Though procedures are well tolerated with low rate of clinical complications, potentially serious, and fatal complication of distant organ cement embolization have been reported [5,6]. VP has been associated with higher risk of cement leakage and symptomatic embolization as compared to KP [5]. We report an interesting case of symptomatic cement pulmonary embolism 2 day's post KP and its successful treatment with novel anticoagulation agent for 6-months.
Case presentation
Forty-nine-year-old non–smoker female with past medical history of hypertension, hyperlipidemia, supraventricular tachycardia, asthma, fibromyalgia, and thyroid cancer status post thyroidectomy presented with sharp low back pain from T-10 compression fracture status post fall. After conservative medical management, pain persisted, and she was referred for percutaneous KP. During pre-operative assessment, the patient was asymptomatic except mid back pain. She underwent kyphoplasty of T-10 vertebral body (Fig. 1) which was complicated by extravasation of the cement material along the lateral wall of vertebral body (Fig. 2). Subsequently, infusion of cement was paused, and the introducer was withdrawn into the middle of vertebral body. After a delay of 20 seconds, remaining cement was infused (total amount = 2.5 mL) without further extravasation. Patient tolerated procedure well and was later discharged home with no symptoms during the discharge.
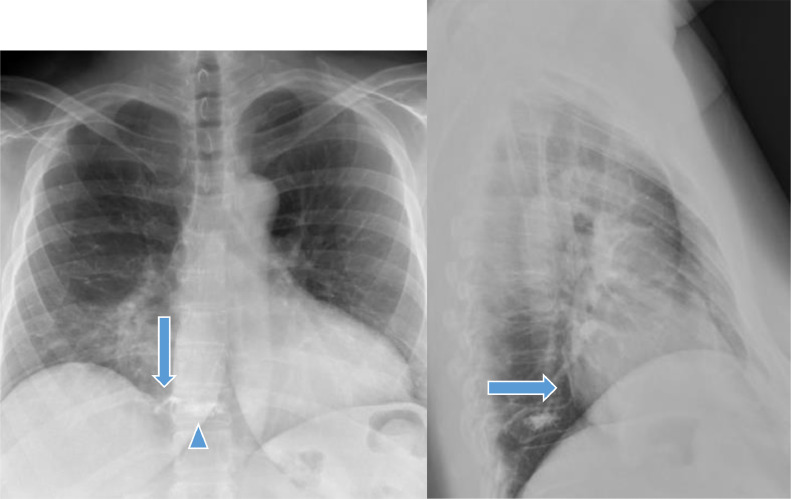
Fig. 1.
X ray chest AP and lateral views show kyphoplasty of T10 vertebral body (blue arrow head) for anterior wedging compression fracture. Paravertebral extravasation of cement (blue arrow) is seen on right lateral aspect on AP view and is seen tracking upward, likely in the paravertebral venous plexus (Color version of the figure is available online.)
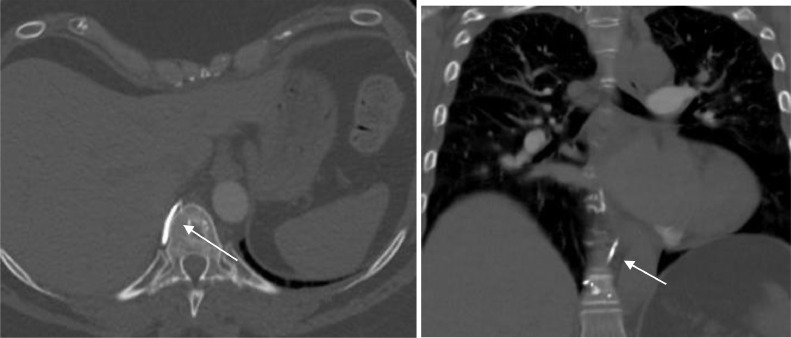
Fig. 2.
On axial and coronal images of CT thorax with IV contrast show paravertebral extravasation of cement (white arrow).
Two day's post procedure patient presented to the emergency department with complaint of pleuritic chest pain, new productive cough with gray colored sputum, and shortness of breath. Patient described pain as intermittent sharp stabbing sensation, aggravated by activity, and alleviated with rest. She denied any associated fevers, hemoptysis or GI symptoms. Patient was hemodynamically stable, normotensive with regular heart rate, and oxygen saturation of 97% on room air. Laboratory parameters were within normal limit except elevated d-dimer. CT PE was notable for distal sub-segmental pulmonary arterial embolization (bilateral upper and right lower lobe). Non–contrast hyperattenuation of bone cement within the pulmonary artery lumen was suggestive of non–thrombotic pulmonary embolism (Fig. 3). We could not determine the exact timings of occurrence of cement emboli; however, it is high likelihood that embolism of cement material occurred during the procedure at the time of extravasation. Considering her active symptoms, patient was hospitalized, and initiated on systemic anticoagulation. Novel oral anticoagulation agent (apixaban) was continued for 6 months’ after detailed discussion of risks of bleeding vs. benefits of preventing superimposed thrombus formation.
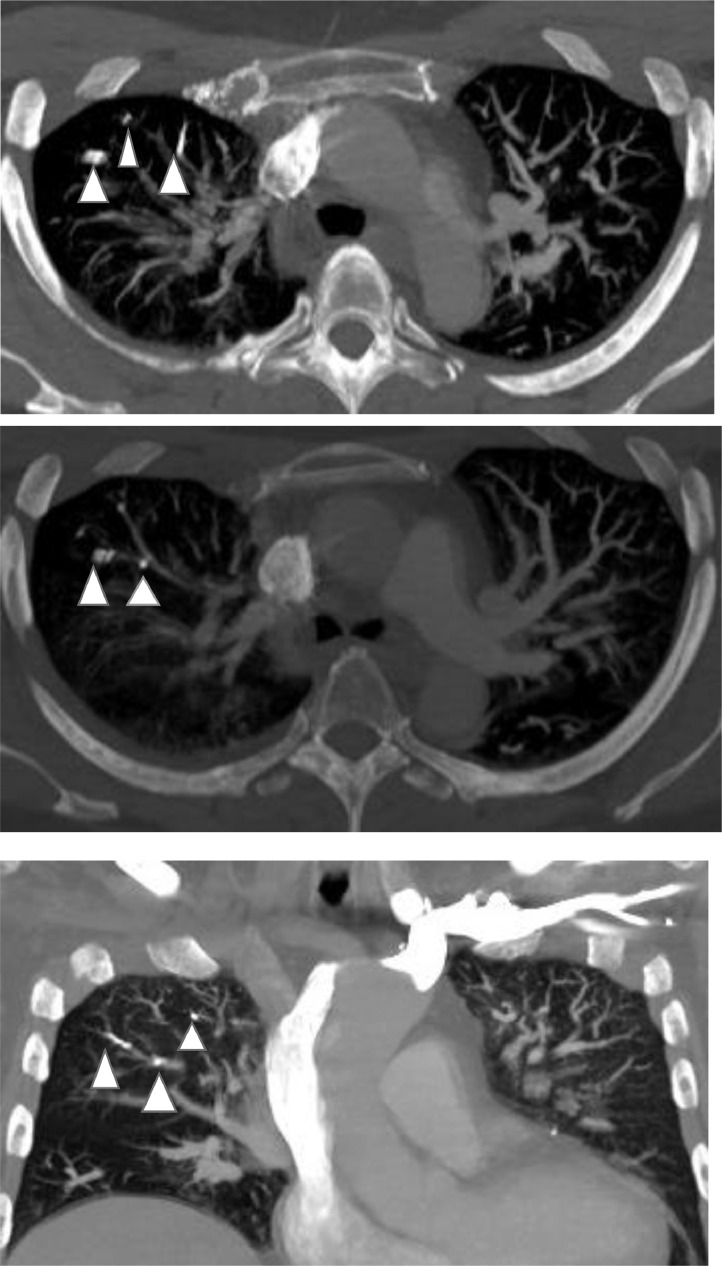
Fig. 3.
Axial and coronal images of CT chest with IV contrast in bone window show cement emboli (white arrow head) in sub-segmental right upper lobe pulmonary arteries.
Five months’ after the initial presentation, patient again presented to ED with increasing shortness of breath. On examination, she was found to be tachycardic (147 beats per minute), and had blood pressure of 130/100 mm Hg. Respiratory rate and oxygen saturation were within normal limits. Blood workup revealed elevated troponin (40-77 ng/L) and BNP (443 pg/mL). EKG showed supraventricular tachycardia with stable incomplete right bundle branch block. CT PE noted stable cement emboli with normal caliber of main pulmonary trunk and branches. Transthoracic echocardiography was unremarkable with normal biventricular size and systolic functions. Patient underwent diagnostic coronary angiogram for suspected non–ST elevation myocardial infarction and possible dyspnea as angina equivalent. Cardiac catheterization revealed normal coronary arteries, normal filling pressures, and cardiac output. There was no evidence of pulmonary hypertension on right heart cath. VQ scan did not identify unmatched perfusion defects, ruling out concerns for chronic thromboembolic pulmonary hypertension (CTEPH). Spirometry revealed restrictive process. She endorsed significant anxiety related to her cement emboli diagnosis and its contribution to her recurrent dyspnea spells. She was discharged home in stable condition with no need for supplemental oxygen.
Discussion
Non–thrombotic pulmonary embolism (NTPE) is defined as the embolization of non–thrombotic material into the pulmonary vasculature. The potential sources could be biologic (various cell types [adipocytes, hematopoietic, amniotic, trophoblastic, tumor], bacteria or fungi) or nonbiologic from foreign material or gas [7]. Bone cement (PMMA) pulmonary embolism is well-known but rare complication following percutaneous VP and KP vertebral augmentation procedures with reported prevalence of 0.9% and 0.4%, respectively [5]. Due to the lack of routine screening after these procedures, the frequency of asymptomatic pulmonary embolus is not well-documented. However, Choe Du et al. verified a frequency of 4.6% for asymptomatic pulmonary embolus [8], while Duran et al. reported a 6.8% rate of PMMA embolus following vertebroplasty [4]. Moreover, a significant correlation of this complication found with paravertebral venous cement leak but not with the number of vertebral bodies treated or with the type of procedure performed [8]. A higher risk of cement leakage has been noted in vertebroplasty (30%-75%) when compared to kyphoplasty (8%-33%) [9]. Biomechanical studies of KP have suggested that the risk of cement extravasation is reduced because balloon inflation creates a void within the vertebral body into which cement can be injected under relatively low pressure [6,10]. Our patient underwent percutaneous KP with intraoperative complication of paravertebral extravasation of cement and subsequent development of cement PE.
The vertebral venous system is an important collateral venous network serving as the main vehicle for embolic complications following VP and KP procedures. Post cement leak, a pathway for the migration of cement to the lungs involves its flow through the basivertebral vein and anterior external vertebral venous plexus, to the pulmonary arteries via the segmental spinal veins, vena radicularis magna, azygos vein, and accessory hemiazygos vein [11]. The lack of valves in the venous system often facilitates this migration. Several risk factors have been identified for the development of cement emboli. The lower viscosity of cement material may have a higher risk of embolism [12]. Leakage is expected to occur more frequently in neoplastic disease when compared with osteoporosis, possibly secondary to neo-angiogenesis. Reflecting this phenomenon, all patients in Choe et al. study had multiple myeloma [8]. However, Duran et al. had higher proportion of osteoporotic lesions undergoing procedure, arguing the potential role of reasons other than malignancy [4]. In addition, insufficient polymerization of PMMA at the time of injection, needle position with respect to the basivertebral vein, and overfill of vertebral body could aid the cement migration [13].
Following the classic Virchow's triad for development of venous thromboembolism, methyl methacrylate (MMA) polymers were shown to promote endothelial injury with local cytotoxicity, and release of procoagulant substances whereas the polymerized PMMA in the occluded vessel promotes local venous stasis [14]. Resultant superimposed thrombus formation may contribute to delayed clinical symptoms as reported previously in the literature [15]. Similarly, there were description of cement removed from the pulmonary artery in symptomatic cases was noted to be covered by thrombi [16]. Use of long-term anticoagulation in our patient may have played a role in preventing progression of cement PE.
With approximately 38,000 VP and 16,000 KP performed annually, radiologists are more likely to encounter complications of these procedures on routine imaging [17]. Considering the high density of PMMA, conventional X-rays may identify solitary or multiple tubular or branching radio-dense lines in the lung fields [8], raising the suspicion of cement PE. CT is the most effective modality in the diagnosis of PE. While the contrast media for the detection of thrombotic PE is mandatory on CT examinations, the unenhanced chest CT can readily detect the hyperdense (>500 Hounsfield units), often branching cement deposits within the pulmonary vessels. Bone window settings should be utilized for contrast-enhanced images to assist the recognition of cement emboli [8,18]. Additional concern of fat marrow emboli may represent a diagnostic challenge where the mediastinal window might aid in identification [4]. Bliemel et al. found low incidence rate of asymptomatic cement PE detection on screening plain radiographs post-procedure and recommended proceeding with confirmatory CT imaging for suspicious cases [19].
There is no established protocol for the management of cement pulmonary emboli. Treatment is recommended to reduce the risk of pulmonary embolism, pulmonary infarction, and respiratory failure. In addition to analgesics, bed rest and supplemental oxygen, anticoagulation is utilized to reduce the risk of secondary thrombus formation and complication of CTEPH. Systemic anticoagulation for 3-6 months is largely reserved for symptomatic cement PE patients [4,20]. In large central emboli with risk of cardio-respiratory decline, aggressive treatment with pulmonary arterial embolectomy has been suggested [13]. Interestingly, endovascular placement of IVC filter to capture cement emboli, and its successful removal via operative venotomy has been performed as primary preventive measure [9,21]. Considering dyspnea on presentation and bilateral distribution of subsegmental cement PE, our patient was treated with long term anticoagulation. Six-months’ post anticoagulation repeat imaging showed stable findings without propagation of clot and its long -erm hemodynamic complication of CTEPH.
In order to prevent potentially fatal complication of distant organ cement emboli, several measures have been suggested in the literature. Elevating intraabdominal pressure (prone-positioning) and intrathoracic pressures (manual insufflation under general anesthesia) and thereby increasing venous pressures might reduce the risk of extrusion of fat, bone marrow, air, and bone cement into the intra and extra-vertebral venous plexuses [22]. The risk of PMMA extravasation is increased in acute fractures (within 3-4 weeks) as fracture lines are not yet sealed by hematoma and callus. Careful patient and procedure selection (KP with balloon tamp producing intra-vertebral cavity) might limit PMMA extravasation [23]. Numerous technique related precautions have been discussed to alleviate the risk of cement emboli including use of ≤4-6 mL of PMMA to augment 1 vertebral body, use of large-caliber needles, correct handling of VP and/or KP mixture (strictly adhering the manufacturer directions, avoiding too early injection, injection during the transitional phase from liquid to solid), and staged and/or fractional injection of cement with an immediate pause for about 20 seconds following extravasation prior to proceeding again [16,22,24,25]. The role of additional imaging has been evaluated. Antecedent intravertebral venography may be beneficial to determine the optimal condition for VP and/or KP, however, recent investigations did not find significant impact of such approach, particularly when percutaneous VP and/or KP procedures are performed by qualified, experienced operators[25,26] Moreover, pooling of venography contrast may disturb the evaluation of intraoperative PMMA extravasation, particularly for osteoporotic fractures with compromised blood flow. Choe Du et al. proposed increasing the amount of barium in the PMMA mixture, in order to detect even a minimum of extravasated material [8]. Transesophageal echography may be considered because spinal instrumentation appeared to correlate significantly with intraoperative pulmonary fat and bone marrow embolism [27].
Conclusion
With the growing utilization of percutaneous vertebral augmentation techniques of VP and KP, there's increased recognition of cement embolization into the distant vasculature. Careful patient and procedure selection, appropriate handling of bone cement mixture, modified techniques, intra and peri-operative precautions, and early detection and management of patients might contribute to reduce the risk of potentially fatal complication of pulmonary cement emboli.
Footnotes
Competing Interests: Authors report no relevant conflict of interests in relation to the submitted manuscript.
References
- 1.Lenchik L, Rogers LF, Delmas PD, Genant HK. Diagnosis of osteoporotic vertebral fractures: importance of recognition and description by radiologists. AJR Am J Roentgenol. 2004;183(4):949–958. doi: 10.2214/ajr.183.4.1830949. [DOI] [PubMed] [Google Scholar]
- 2.Denaro V, Longo UG, Maffulli N, Denaro L. Vertebroplasty and kyphoplasty. Clin Cases Miner Bone Metab. 2009;6(2):125–130. http://www.ncbi.nlm.nih.gov/pubmed/22461161 Accessed on: 6/22/2021. [PMC free article] [PubMed] [Google Scholar]
- 3.Galibert P, Deramond H, Rosat P, Le Gars D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33(2):166–168. http://www.ncbi.nlm.nih.gov/pubmed/3600949 Accessed on: 6/22/2021. [PubMed] [Google Scholar]
- 4.Duran C, Sirvanci M, Aydoğan M, Ozturk E, Ozturk C, Akman C. Pulmonary cement embolism: a complication of percutaneous vertebroplasty. Acta Radiol. 2007;48(8):854–859. doi: 10.1080/02841850701422153. [DOI] [PubMed] [Google Scholar]
- 5.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2021;8(3):488–497. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Radcliff KE, Reitman CA, Delasotta LA. Pulmonary cement embolization after kyphoplasty: a case report and review of the literature. Spine J. 2010;10(10):e1–e5. doi: 10.1016/j.spinee.2010.07.394. [DOI] [PubMed] [Google Scholar]
- 7.McCabe BE, Veselis CA, Goykhman I, Hochhold J, Eisenberg D, Son H. Beyond pulmonary embolism; nonthrombotic pulmonary embolism as diagnostic challenges. Curr Probl Diagn Radiol. 2021;48(4):387–392. doi: 10.1067/j.cpradiol.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Choe DH, Marom EM, Ahrar K, Truong MT, Madewell JE. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol. 2004;183(4):1097–1102. doi: 10.2214/ajr.183.4.1831097. [DOI] [PubMed] [Google Scholar]
- 9.Agko M, Nazzal M, Jamil T, Castillo-Sang M, Clark P, Kasper G. Prevention of cardiopulmonary embolization of polymethylmethacrylate cement fragment after kyphoplasty with insertion of inferior vena cava filter. J Vasc Surg. 2010;51(1):210–213. doi: 10.1016/j.jvs.2009.07.110. [DOI] [PubMed] [Google Scholar]
- 10.Fourney DR, Schomer DF, Nader R. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1):21–30. doi: 10.3171/spi.2003.98.1.0021. Suppl. [DOI] [PubMed] [Google Scholar]
- 11.Khan M, Terk M. Cement pulmonary embolus complicating percutaneous vertebroplasty. Radiol case reports. 2009;4(2):282. doi: 10.2484/rcr.v4i2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine (Phila Pa 1976). 2002;27(19):E416-8. doi:10.1097/00007632-200210010-00021 [DOI] [PubMed]
- 13.Tozzi P, Abdelmoumene Y, Corno AF, Gersbach PA, Hoogewoud H-M, von Segesser LK. Management of pulmonary embolism during acrylic vertebroplasty. Ann Thorac Surg. 2002;74(5):1706–1708. doi: 10.1016/s0003-4975(02)03962-0. [DOI] [PubMed] [Google Scholar]
- 14.Dahl OE. Cardiorespiratory and vascular dysfunction related to major reconstructive orthopedic surgery. Acta Orthop Scand. 1997;68(6):607–614. doi: 10.3109/17453679708999038. [DOI] [PubMed] [Google Scholar]
- 15.Ross J, Bhatia R, Hyde T, Dixon G. Pulmonary embolism with coexistent incidental pulmonary cement embolism post vertebroplasty. BMJ Case Rep. 2021;14(3) doi: 10.1136/bcr-2020-237449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdul-Jalil Y, Bartels J, Alberti O, Becker R. Delayed presentation of pulmonary polymethylmethacrylate emboli after percutaneous vertebroplasty. Spine (Phila Pa 1976). 2007;32(20):E589-93. doi:10.1097/BRS.0b013e31814b84ba [DOI] [PubMed]
- 17.Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the food and drug administration medical device related web site. J Vasc Interv Radiol. 2004;15(11):1185–1192. doi: 10.1097/01.RVI.0000144757.14780.E0. [DOI] [PubMed] [Google Scholar]
- 18.Habib N, Maniatis T, Ahmed S. Cement pulmonary embolism after percutaneous vertebroplasty and kyphoplasty: an overview. Heart Lung. 2021;41(5):509–511. doi: 10.1016/j.hrtlng.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Bliemel C, Buecking B, Struewer J, Piechowiak EI, Ruchholtz S, Krueger A. Detection of pulmonary cement embolism after balloon kyphoplasty : should conventional radiographs become routine? Acta Orthop Belg. 2013;79(4):444–450. http://www.ncbi.nlm.nih.gov/pubmed/24205776 Accessed on: 6/22/2021. [PubMed] [Google Scholar]
- 20.Bopparaju S, Varon J, Surani S. Pulmonary embolism with vertebral augmentation procedures. Case Rep Pulmonol 2013;2013:785307. doi:10.1155/2013/785307 [DOI] [PMC free article] [PubMed]
- 21.Baumann A, Tauss J, Baumann G, Tomka M, Hessinger M, Tiesenhausen K. Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdisciplinary management. Eur J Vasc Endovasc Surg. 2006;31(5):558–561. doi: 10.1016/j.ejvs.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Groen RJM, du Toit DF, Phillips FM, et al. Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: a reappraisal of the vertebral venous system. Spine (Phila Pa 1976) 2004;29(13):1465-1471. doi:10.1097/01.brs.0000128758.64381.75 [DOI] [PubMed]
- 23.Ruiz Santiago F, Santiago Chinchilla A, Guzmán Álvarez L, Pérez Abela AL, Castellano García Mdel M, Pajares López M. Comparative review of vertebroplasty and kyphoplasty. World J Radiol. 2014;6(6):329–343. doi: 10.4329/wjr.v6.i6.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belkoff SM, Sanders JC, Jasper LE. The effect of the monomer-to-powder ratio on the material properties of acrylic bone cement. J Biomed Mater Res. 2002;63(4):396–399. doi: 10.1002/jbm.10258. [DOI] [PubMed] [Google Scholar]
- 25.Vasconcelos C, Gailloud P, Beauchamp NJ, Heck DV, Murphy KJ. Is percutaneous vertebroplasty without pretreatment venography safe? Evaluation of 205 consecutives procedures. AJNR Am J Neuroradiol. 2021;23(6):913–917. http://www.ncbi.nlm.nih.gov/pubmed/12063215 Accessed on 6/28/2021. [PMC free article] [PubMed] [Google Scholar]
- 26.Gaughen JR, Jensen ME, Schweickert PA, Kaufmann TJ, Marx WF, Kallmes DF. Relevance of antecedent venography in percutaneous vertebroplasty for the treatment of osteoporotic compression fractures. AJNR Am J Neuroradiol. 2002;23(4):594–600. http://www.ncbi.nlm.nih.gov/pubmed/11950650 Accessed on 6/28/2021. [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi S, Kitagawa H, Ishii T. Intraoperative pulmonary embolism during spinal instrumentation surgery. A prospective study using transoesophageal echocardiography. J Bone Joint Surg Br. 2003;85(1):90–94. doi: 10.1302/0301-620x.85b1.13172. [DOI] [PubMed] [Google Scholar]