Abstract
There is a gap in the manuals for scoring sleep-related movements because of the absence of rules for scoring large movements. A taskforce of the International Restless Legs Syndrome Study Group (IRLSSG) elaborated rules that define the detection and quantification of movements involving large muscle groups. Consensus on each of the criteria in this article was reached by testing the presence of consensus on a first proposal; if no consensus was achieved, the concerns were considered and used to modify the proposal. This process was iterated until consensus was reached. A preliminary analysis of the duration of movements involving large muscle groups was also carried out on data from two previous studies, which, however, used a visual analysis of video-polysomnographic (PSG) recordings obtained from children or adults. Technical specifications and scoring rules were designed for the detection and quantification of large muscle group movements during sleep with a duration between 3 and 45 seconds in adults or 3 and 30 seconds in children, characterized by an increase in electromyographic activity and/or the occurrence of movement artifact in any combination of at least two recommended channels and not meeting the criteria for any other type of movement. Large muscle group movements are often accompanied by sleep stage changes, arousals, awakenings, and heart rate rises. The absence of clear and detailed rules defining them has likely impeded the development of studies that might disclose their clinical relevance; these new rules fill this gap.
Keywords: large muscle group movements during sleep, sleep-related movements, restless sleep disorder, sleep scoring, quantification, rules, criteria
Introduction
While the most studied motor phenomenon during sleep is represented by periodic limb movements during sleep [1, 2], in recent years it has become evident that other motor activities, such as nonperiodic leg movements [3–5] or movements involving different muscle groups [6–8] are often observed in polysomnographic (PSG) studies and can have clinical significance.
The study of large body movements during sleep is not new [9], but the recent identification of a new sleep-related movement disorder in children called restless sleep disorder (RSD) [10–12], has highlighted a gap in the current literature about how to score these large body movements. RSD is characterized above all by the frequent occurrence of movements involving large muscle groups while the child is asleep. Scoring of these movements has only been possible by the detailed analysis of video-PSG recordings, in the absence of a clear and standardized definition for these movements using PSG signals alone.
It is noteworthy that these types of motor activity, such as “major body movements,” have only been defined in the past (without particular detail) in order to overcome the problems posed by their occurrence obscuring sleep staging [1, 13]. It is possible that the lack of a definition has also delayed the recognition of their importance and significance in the diagnosis of conditions such as RSD. In fact, for the identification of RSD, but also for the analysis of movements in other conditions such as narcolepsy and rapid eye movement (REM) sleep behavior disorder, the synchronized video is analyzed instead of standard PSG signals, due to the absence of rules for the counting of movements in PSG recordings [11, 14, 15], with the exception of the different criteria to quantify REM sleep without atonia which, again, do not identify and count single movements and are limited to the analysis of EMG signals [16].
The evidence is overwhelming for the importance of a comprehensive analysis of sleep beyond sleep stages and respiratory events to accurately assess sleep quality and the presence of movement disorders. The full potential for gathering information currently collected in a nocturnal PSG is not completely utilized. The analysis of movements involving large muscle groups has proven effective and helpful in other conditions beyond RSD, such as central respiratory pauses [17] and Gilles de la Tourette syndrome [18], as examples, and experts in the field have delineated their own methods to assess body movements during sleep [19–22].
Rationale for Arranging Scoring Rules for Movements Involving Large Muscle Groups
There is a gap in the technical manuals for scoring sleep-related movement events because of the absence of rules for scoring large body movements during sleep. Currently, the AASM Manual for the Scoring of Sleep and Associated Events includes a mention of major body movement only in the framework of scoring sleep stages [1].
Experts in movement disorders have published their work on video analysis of large movements during sleep but different groups used different methods and there is lack of consensus [11, 20, 23]. Visual video-PSG analysis is a time-consuming task and, while it allows the detection of visible movements, EMG activities not resulting in movements can be missed, as well as brief motor events if blankets and sheets limit their visibility or parts of the body are out of the optic field of the camera. In addition, video-PSG is not always available due to technical limitations or to economic restrictions imposed by reimbursement rules.
Nevertheless, PSG recordings without video recording contain by default a number of channels able to pick up EMG potentials and movement artifacts that, in this case, can be treated as true signals and not as noise to discard. In fact, besides EMG channels, all leads attached to the skin and recording electric signals, such as EEG, ECG, and EOG, can pick up EMG potentials, also depending on the filters used. These channels also show movement artifacts, usually as very slow waves of very high amplitude, mixed with EMG activity. It is clear that this information is very useful when large muscle group movements are the focus of attention, and can be used for their detection and quantification.
Within this background and framework, the members of a specifically dedicated taskforce of the International Restless Legs Syndrome Study Group (IRLSSG), elaborated detailed rules that define the detection and quantification of movements involving large muscle groups. This was done using the signals routinely recorded for PSG and following standard criteria [1], but also taking into account additional data from channels available in standard PSG recordings.
The IRLSSG taskforce was formed by six members with known expertise in the assessment of sleep-related movement disorders, as also supported by their scientific publications on this specific topic. The taskforce began correspondence on June 2020 and held teleconferences during which the experts presented their methodology [11, 23, 24] to assess movements during sleep.
Ten consensus questions were then agreed upon to guide the development of scoring criteria. These were as follows and responses have been included, implicitly or explicitly, in different paragraphs of this article:
Is there a need to study movements involving large muscle groups?
Is “large muscle group movement” the most appropriate name and “LMM” the most suitable acronym for these motor activities during sleep?
Are signals recorded in a standard PSG study sufficient to detect and score LMM during sleep, without the analysis of synchronized video recordings?
Which are the signals that can pick up best LMM during sleep?
Which is the minimum set of signals that should be used for scoring LMM during sleep?
What minimal amplitude should be used for LMM during sleep scoring in the different signals?
What are the minimum and maximum duration of LMM during sleep?
What other movements during sleep need to be excluded when scoring LMM during sleep?
Are there differences between pediatric and adult LMM during sleep?
When are LMM during sleep associated with arousals/awakenings?
Based on the discussion raised by the need to answer the above questions, the consensus on each of the criteria exposed in this article was reached by testing the presence of consensus on a first proposal; if no consensus was achieved, the concerns were considered and used to modify the proposal. This process was iterated until consensus was reached.
In addition, in order to gather information useful for the decision on the duration of LMM, an analysis of the duration of movements involving large muscle groups was carried out on data available from two previous studies which, however, used a visual analysis of video recordings synchronized with PSG obtained from children [11] or adults [24] and used similar but not identical criteria for their scoring. The results of this analysis are reported in the Appendix, at the end of this article.
After approval by the members of the taskforce, the position statement was submitted to the IRLSSG Executive Committee for final approval.
Position
For scoring LMM it is the IRLSSG position that:
Technical specifications
-
The following signals that directly record electric fields should be obtained, following the latest version of the American Academy of Sleep Medicine (AASM) manual [1] specifications, as noted in the sleep staging rules chapters for adults and children (recommended):
a. Both tibialis anterior muscle EMG activity should be recorded, as specified for the detection of leg movements
b. Chin EMG
c. At least three EEG signals (F4-M1, C4-M1 and O2-M1 or F3-M2, C3-M2 and O1-M2).
d. Two EOG
e. ECG
Body position (optional)
-
Other EMG channels (optional)
a. Flexor digitorum superficialis or flexor digitorum communis sampled per AASM
b. Paraspinal muscles sampled per AASM
c. Masseter electrodes per AASM
Notes: (1) If optional EMG and/or EEG signals are included, they can all be used for the detection and measurement of large (muscle group) movements; (2) For research purposes, the set of signals used for the detection of LMM should be kept constant within the whole experimental protocol.
Scoring LMM
The following define an LMM:
Temporally overlapping increase in EMG activity and/or the occurrence of movement artifact in any combination of at least two recommended channels.
The increase in EMG activity or movement artifact signal must be at least twice the amplitude of the background signal amplitude (as defined by the AASM scoring manual [1]).
The onset of the increase in EMG activity and/or movement artifact starts during sleep, i.e. starts after at least 10 seconds of sleep without movement.
If the movement causes awakening during the same epoch of occurrence and the epoch is scored as W, the movement is counted and assigned to the sleep stage preceding it (see below).
LMM onset is defined as the beginning of the first detectable increase in EMG activity and/or movement artifact in any of the channels involved.
LMM end is defined as the return of its amplitude to below twice the baseline signal amplitude for more than 1 second of the last of the channels involved.
Duration is defined from LMM onset to LMM end, as defined above.
LLM not separated by more than 1 second from each other are considered as a single LMM.
The minimum duration of a LMM is 3 seconds.
The maximum duration of a LMM is 30 seconds for children and 45 seconds for adults.
In contrast to movement artifacts, technical artifacts should be excluded and not used for the scoring of an LMM.
The movement does not meet criteria for any other scored movement, following the AASM Manual [1]—e.g. periodic leg movements during sleep (PLMS), alternating leg movement activity, hypnagogic foot tremor, excessive fragmentary myoclonus, bruxism, etc.—or the World Association of Sleep Medicine (WASM) criteria for PLMS [2], and does not correspond to behaviors characterizing parasomnias or seizures, or movements triggered by environmental stimuli (as monitored by the sleep technician).
If scoring LMM, the following should be measured:
Time of occurrence.
Duration.
Sleep stage of occurrence (if the movement causes a stage change or awakening during the same epoch when it starts, assign it to the sleep stage of the preceding epoch).
An LMM and an awakening should be considered associated with each other when they occur simultaneously or when the awakening occurs while the movement lasts or within 0.5 second from its end.
An LMM and an arousal or a respiratory event should be considered associated with each other when they occur simultaneously, overlap, or when there is less than 0.5 second between the end of one event and the onset of the other event, regardless of which is first.
If scoring LMM, the following parameters should be reported:
-
LMM index (LMM number/hour of total sleep time):
a. total (recommended)
b. during NREM and REM sleep (optional)
-
Mean duration:
a. total (recommended)
b. during NREM and REM sleep (optional)
-
Index of LMM associated with arousals or awakening:
a. total (recommended)
b. during NREM and REM sleep (optional)
-
Index of LMM associated with arousals:
a. total (optional)
b. during NREM and REM sleep (optional)
-
Index of LMM associated with awakening:
a. total (optional)
b. during NREM and REM sleep (optional)
-
Index of LMM associated with respiratory events:
a. total (optional)
b. during NREM and REM sleep (optional)
Notes: The rules for counting LMM do not require any adaptation or changes of the AASM sleep stage scoring rules [1] and sleep staging is required prior to scoring LMM:
1) The AASM manual states that in an epoch where movement obscures the EEG to the point that the sleep stage cannot be determined then the epoch is scored as W if either the epoch BEFORE or the epoch AFTER is scored as W. In the case the epoch before is assigned the stage W, the movement is not counted because it does not occur while the subject is asleep; if only the epoch after is scored as W, the movement is assigned to the sleep stage of the epoch preceding the epoch in which it starts, as indicated above (see Figure 1 for some examples).
2) The AASM manual also states that in an epoch where movement obscures the EEG for more than 15 seconds to the point that the sleep stage cannot be determined and neither the epoch before or after is scored as W then the sleep stage of the epoch is scored the same as the epoch that comes AFTER it. In contrast, with the current rules the movement (which can last only 3 seconds or more) is assigned to the sleep stage of the epoch preceding the epoch in which it starts, while the AASM sleep stage scoring of the epoch in which the movement starts remains unchanged (see Figure 1 for some examples).
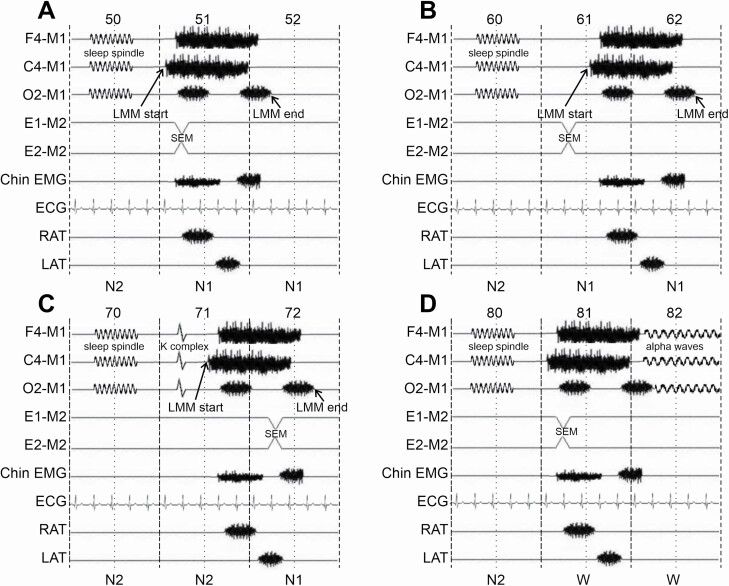
Figure 1.
In all panels, sleep stages are scored according to the AASM criteria. (A) An LMM is depicted starting in the first half of epoch 51 with obscured EEG activity and following epoch 50 scored as N2. Epoch 51 in which the LMM starts is scored as N1 and the LMM is assigned to the sleep stage of the same epoch 51 (N1), according to the scoring rules described in this article. (B) An LMM is depicted starting in the second half of epoch 61 and it is assigned to the sleep stage of the same epoch 61 (N1), according to the scoring rules described in this article. (C) An LMM is depicted starting in the second half of epoch 71 and the LMM is assigned to the sleep stage of the same epoch 71 (N2), according to the scoring rules described in this article. (D) An LMM is depicted starting in the first half of epoch 81 with obscured EEG activity, causing an awakening and following epoch 80 scored as N2. Epoch 81 in which the LMM starts is scored as W and the LMM is assigned to the sleep stage of the preceding epoch 80 (N2), according to the scoring rules described in this article.
Examples
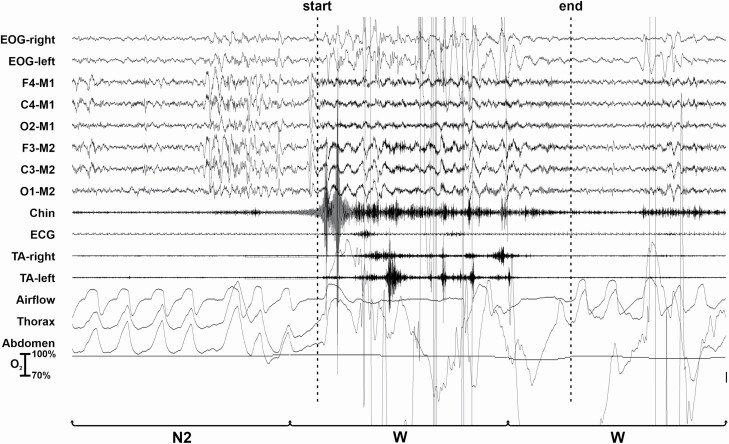
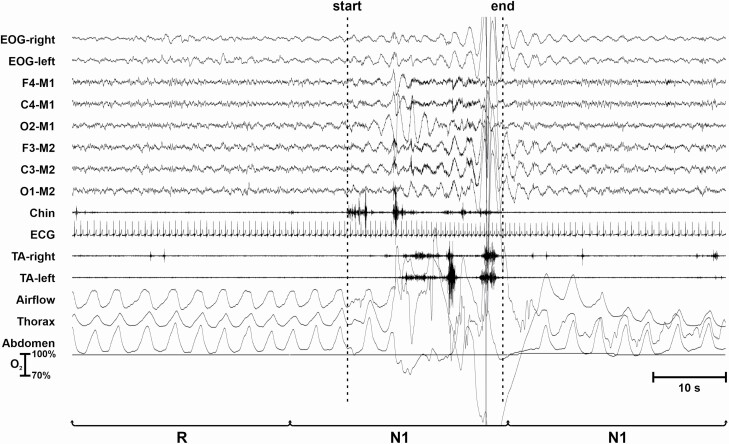
Figure 2 shows a LMM occurring after an epoch of NREM sleep stage N2 followed by an awakening. The LMM is characterized by the presence of high-amplitude EMG potentials in the EMG and EEG channels that obscure the underlying EEG activity and do not allow to score the sleep stage. As per AASM scoring manual criteria, this epoch is scored as W because it is followed by another epoch scored as W; however, the LMM is counted and assigned to the sleep stage assigned to the preceding epoch.
Figure 2.
LMM occurring during an epoch scored as W and preceded by sleep stage N2 (as schematically shown in Figure 1D).
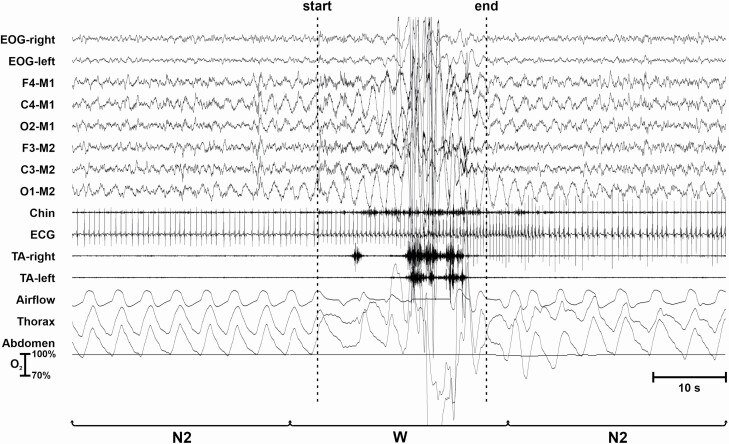
Figure 3 shows an LMM occurring during an epoch scored as W and preceded by sleep stage N2. The LMM is characterized by the presence of high-amplitude EMG potentials in the EMG and EEG channels, as well as by slow and high-amplitude movement artifacts in the EEG and EOG channels. This movement is counted and assigned to the preceding sleep stage (N2).
Figure 3.
LMM occurring during an epoch scored as W and preceded and followed by sleep.
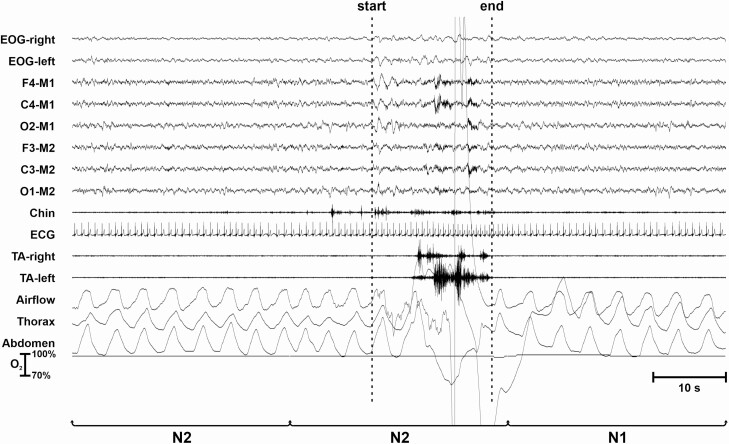
In Figure 4, a LMM is scored due to the occurrence of EMG potentials in several channels (EMG and EEG channels). Note the movement artifacts picked up by the respiratory channels (not mentioned by the LMM scoring rules). Sleep is maintained after the end of the LMM; the LMM is assigned to sleep stage N2, according to the rules described in this article.
Figure 4.
LMM occurring during NREM sleep.
In Figure 5, the LMM starts in an epoch scored as stage N1 and is assigned to this stage, according to the rules described in this article. The LMM is characterized by the presence of EMG potentials in the EMG and EEG channels. Slow and high-amplitude movement artifacts are evident in the EEG signals.
Figure 5.
LMM occurring during REM sleep.
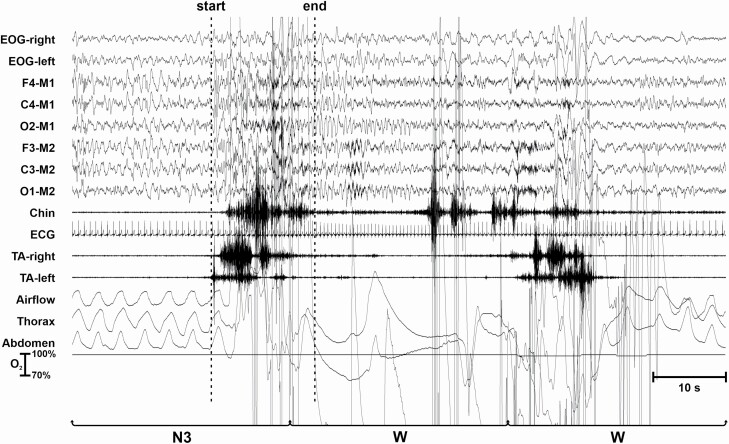
Figure 6 shows an LMM occurring in NREM sleep stage N3 followed by an awakening; the LMM is assigned to sleep stage N3, according to the rules described in this article. The LMM is characterized by the presence of high-amplitude EMG potentials in the EMG and EEG channels. Note the subsequent appearance of new EMG bursts that are not scored as LMM because they start during wakefulness.
Figure 6.
LMM occurring during NREM sleep stage N3.
Note: None of the movements recorded by the tibialis anterior channels in these examples qualified for PLMS.
Discussion
These criteria for LMM represent a consensus of experts to clearly and completely assess this type of motor activity during sleep. Previous literature did not provide detailed rules and was mostly based on video identification alone. Furthermore, the AASM scoring manual inclusion of large body movements, some of which, at least, might qualify as LMM, is limited to their effect on sleep staging. As further evidence arises in the future, adjustments may be needed; however, setting clearly defined criteria such as these is a necessary step, which was missing until now.
Most decisions by the taskforce were based on practical feasibility and complied with existing standard processes and rules. A major advantage of this pragmatic approach is to make possible the retrospective analysis of previously recorded studies, thus opening a potentially huge field of application. In addition, with the rules proposed, it is not necessary to include additional channels to standard PSG recordings, thus avoiding an increase in costs.
Probably, the most important parameter to be considered for LMM, beside the total number and index, is their duration. The taskforce felt, with unanimous consensus, that for reliable identification of LMM a minimum duration of 3 seconds was needed, taking into consideration other movements that could be considered “large.” This minimum duration, automatically excludes several different movements typically characterized by a shorter duration and already defined by international standards or the previous literature, such as sleep-related head jerks [7] or neck myoclonus [25], and hypnic jerks or sleep starts [26]. In addition, the criteria clearly state that LMM should not qualify for any other type of scored movement following the AASM Manual [1] or the WASM criteria for PLMS [2] and do not correspond to behaviors characterizing parasomnias or seizures, or triggered by environmental stimuli. Indeed, if the LMM includes any of these other types of movements/behaviors, it should not be classified as LMM and discarded from the analysis. It is recommended to score LMM after all other types of movements are scored.
LMM can also be scored in unattended PSG. However, in the case that LMM are scored in an attended video-PSG and environmental stimuli are noted that triggered an LMM, these should not be scored.
For the maximum duration of LMM, in the absence of clear data in the previous literature, an attempt was made to obtain some information from data collected for different purposes in two separate studies in children [11] and young adults [24], as detailed in the Appendix on the analysis of LMM duration by video-PSG. However, because these studies were primarily based on the analysis of video-PSG and not by PSG alone (therefore applying criteria different from those proposed here), the results of this analysis were taken into account cautiously. The taskforce felt that taking into consideration EMG and movement artifacts recorded by PSG, the movements might be characterized by a longer duration, due to the possibility of picking up EMG activity before and after the actual start and end of the visual movement seen in the video. For this reason, the maximum duration for LMM was set well above the 95th percentile of the statistical distribution of movement duration in both children and adults.
Another important point is the consideration of the association of movements not only with arousals but also with awakenings. In the second case, as the epoch in which the movement starts is likely to be classified as W, it has been necessary to specify that such a movement must be counted and assigned to the sleep stage preceding the awakening. Without this rule, several movements might be missed only because the epoch in which they occur is scored as W. However, even if these rules seem to be reasonable and based on practical points, they were decided by expert consensus and will need further evidence to be confirmed or changed; this holds true also for the proposed association with respiratory events.
The detection of LMM associated with awakenings is an important point because they might lead to more severe sleep disturbances. Therefore, assessing them will potentially allow a better definition of the clinical relevance of LMM in future studies. The association of LMM with respiratory events is intriguing and needs to be explored because it might shed light onto sleep disruption in sleep disordered breathing.
Conclusion
This position article establishes new criteria to detect and score LMM in PSG studies following standard recording procedures, with the aim to fill a gap in the current scoring criteria and in the literature. While a number of motor activities during sleep, such as PLMS, are already well defined, LMM are not currently considered other than for the sleep epoch staging, as a secondary element. However, LMM are considered to be important by the taskforce because they are often accompanied by sleep stage changes, arousals, awakenings, and autonomic changes (heart rate rises). The absence of clear and detailed rules defining them has likely impeded the development of studies that might disclose their clinical relevance.
Acknowledgments
The authors would like to thank the IRLSSG Executive Committee for their input and support.
Appendix—Analysis of LMM Duration by Video-PSG
An analysis of the duration of LMM was carried out on data available from two previous studies. The first [11] included three groups of children: 15 with RLS (12 males and 3 females, mean age 11.9 ± 3.52 years), 15 with RSD (11 males and 4 females, mean age of 9.5 ± 3.18 years), and 15 controls (9 males and 6 females, mean age 10.5 ± 3.16 years). Movement parameters were assessed by video observation and characterized according to: (1) arm movements, (2) leg movements (not meeting the criteria for PLMS), (3) both arm and leg movements without body repositioning, (4) body position change, and (5) head movement without limb movement.
All clearly visible movements and lasting for at least 1 second were included. In particular, among the data collected, the duration of each movement was carefully assessed and annotated.
Only 10 young normal controls were used from the second study [24] (7 males and 3 females, mean age 24.6 ± 2.50 years). Also, in this study, movements were assessed by video-PSG; however, the presence of electromyographic potentials or movement artifacts in the PSG signals was considered and used to determine the movement onset (which most often corresponded to the onset of movement on video) for the measurement of its duration. Movements were classified into three categories: (1) physiological movements (body position changes, adjusting position, scratching, stretching, and fixing dresses or blankets), (2) movements codified in the International Classification of Sleep Disorders, 3rd edition (ICSD-3) [27] (limb movements, PLMS, alternating leg muscle activation, hypnic jerks, rhythmic masticatory muscle activity, hypnagogic foot tremor, and rhythmic movements), and (3) movements not codified or described in the previous literature studies (neck myoclonus, oro-alimentary automatisms). For the purposes of the present study, only the movements in the first category were analyzed and considered to be LMM.
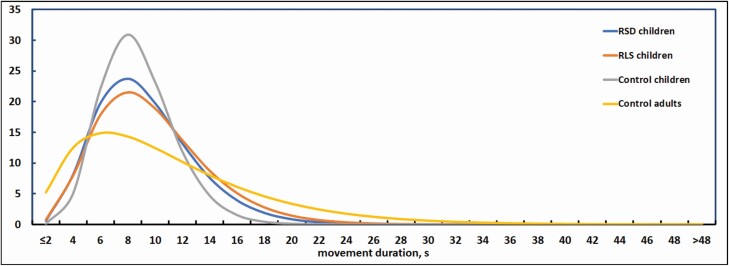
Figure 7 shows the distribution of movement duration in all groups of subjects considered. The real data distributions were best modeled by a gamma distribution fitting, reported in the figure. It is possible to notice that control children and adults show a similar distribution, but children have a more pronounced tendency to have movements within the main peak ranging from approximately from 4 to 15 seconds, with a rapid drop of movements after this peak and reaching values close to zero after 20–25 seconds. Adults present a main peak in the same duration range as control children, but the decrease is less steep and values approach zero at around 40 seconds. In both RSD and RLS children, the main peak seems to extend on a slighter wider range than control children.
Figure 7.
Distribution modeling (gamma function) of movement duration in all groups of subjects considered in this study. Data from two previous studies [11, 24].
Table 1 further details statistically the visual analysis of the distribution of LMM duration and shows that the 99th percentile for duration in children ranges between 15.7 seconds and 17.9 seconds, while it is 40.9 seconds in adults.
Table 1.
Statistical properties of the upper limit of the distribution of movement duration in all groups of subjects considered in this study
| 95th centile | 99th centile | |
|---|---|---|
| Control children | 12.5 | 15.7 |
| Control adults | 26.8 | 40.9 |
| RSD children | 14.5 | 16.9 |
| RLS children | 14.6 | 17.9 |
The results of this analysis of data obtained in different studies might be biased by differences in method; however, it is noteworthy that control subjects (children and young adults) recorded separately for different studies and different purposes showed a similar distribution of the duration of movements and that the distribution of LMM duration in patients with a sleep-related movement disorder (RSD or RLS) also showed a similar distribution and different from that of controls. In addition, the results of this analysis are valuable because this type of information is very rarely found in the literature.
Funding
Dr. Walters received funding for research on RLS from the NIH, Mundipharma, Xenoport and Arbor. The other authors report no financial disclosure.
Conflict of interest statement. None declared.
Disclaimer
SLEEP, the official journal of the Sleep Research Society, occasionally publishes position statements from other scientific and medical organizations. Such papers are subject to the standard, rigorous peer-review process of the journal. The Sleep Research Society does not necessarily endorse the positions stated in such papers.
References
- 1. Berry RB, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Ver. 2.6. Darien, IL: American Academy of Sleep Medicine; 2020. [Google Scholar]
- 2. Ferri R, et al. ; International and European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG). Sleep Med. 2016;26:86–95. [DOI] [PubMed] [Google Scholar]
- 3. Ferri R, et al. Peculiar lifespan changes of periodic leg movements during sleep in restless legs syndrome. J Sleep Res. 2020;29(3):e12896. [DOI] [PubMed] [Google Scholar]
- 4. Ferri R, et al. Short-interval leg movements during sleep entail greater cardiac activation than periodic leg movements during sleep in restless legs syndrome patients. J Sleep Res. 2017;26(5):602–605. [DOI] [PubMed] [Google Scholar]
- 5. Ferri R, et al. Data-driven approaches to define the upper limit of the intermovement interval of periodic leg movements during sleep. Sleep. 2018;41(3). doi: 10.1093/sleep/zsy008 [DOI] [PubMed] [Google Scholar]
- 6. Stefani A, et al. Diagnostic criteria, differential diagnosis, and treatment of minor motor activity and less well-known movement disorders of sleep. Curr Treat Options Neurol. 2019;21(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolfensberger B, et al. From physiological neck myoclonus to sleep related head jerk. J Sleep Res. 2019;28(5):e12831. [DOI] [PubMed] [Google Scholar]
- 8. Montagna P, et al. Propriospinal myoclonus at sleep onset. Neurophysiol Clin. 2006;36(5-6):351–355. [DOI] [PubMed] [Google Scholar]
- 9. Shimohira M, et al. Video analysis of gross body movements during sleep. Psychiatry Clin Neurosci. 1998;52(2):176–177. [DOI] [PubMed] [Google Scholar]
- 10. DelRosso LM, et al. Restless sleep disorder in children: a pilot study on a tentative new diagnostic category. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy102 [DOI] [PubMed] [Google Scholar]
- 11. DelRosso LM, et al. Video-polysomnographic characterization of sleep movements in children with restless sleep disorder. Sleep. 2019;42(4). doi: 10.1093/sleep/zsy269 [DOI] [PubMed] [Google Scholar]
- 12. DelRosso LM, et al. ; International Restless Legs Syndrome Study Group (IRLSSG). Consensus diagnostic criteria for a newly defined pediatric sleep disorder: restless sleep disorder (RSD). Sleep Med. 2020;75:335–340. [DOI] [PubMed] [Google Scholar]
- 13. Rechtschaffen A, et al. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Public Health service; US Government Printing Office; 1968. [Google Scholar]
- 14. Frauscher B, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20(4):514–521. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez-Arcos A, et al. Clinical and video-polysomnographic analysis of rapid eye movement sleep behavior disorder and other sleep disturbances in dementia with Lewy bodies. Sleep. 2019;42(7). doi: 10.1093/sleep/zsz086. [DOI] [PubMed] [Google Scholar]
- 16. Puligheddu MC, et al. The electromyographic diagnosis of REM sleep without atonia and REM sleep behavior disorder. In: Schenck CHH, Videnovic A, eds. Rapid-Eye-Movement Sleep Behavior Disorder. Cham: Springer; 2019:447–464. [Google Scholar]
- 17. Fukumizu M, et al. Central respiratory pauses, sighs, and gross body movements during sleep in children. Physiol Behav. 2004;82(4):721–726. [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto T, et al. Increased body movements during sleep in Gilles de la Tourette syndrome. Brain Dev. 1981;3(1):31–35. [DOI] [PubMed] [Google Scholar]
- 19. Laihinen A, et al. Sleep movements and associated autonomic nervous activities in patients with Parkinson’s disease. Acta Neurol Scand. 1987;76(1):64–68. [DOI] [PubMed] [Google Scholar]
- 20. Aaronson ST, et al. Brain state and body position. A time-lapse video study of sleep. Arch Gen Psychiatry. 1982;39(3):330–335. [DOI] [PubMed] [Google Scholar]
- 21. Montagna P. Physiological body jerks and movements at sleep onset and during sleep. In: Chokroverty S, Hening W, Walters AS, eds. Sleep and Movement Disorders. Boston, MA: Butterworth/Elsevier; 2003:247–259. [Google Scholar]
- 22. Wilde-Frenz J, et al. Rate and distribution of body movements during sleep in humans. Percept Mot Skills. 1983;56(1):275–283. [DOI] [PubMed] [Google Scholar]
- 23. Stefani A, et al. A prospective video-polysomnographic analysis of movements during physiological sleep in 100 healthy sleepers. Sleep. 2015;38(9):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loddo G, et al. Seizures with paroxysmal arousals in sleep-related hypermotor epilepsy (SHE): Dissecting epilepsy from NREM parasomnias. Epilepsia. 2020;61(10):2194–2202. [DOI] [PubMed] [Google Scholar]
- 25. Frauscher B, et al. A descriptive analysis of neck myoclonus during routine polysomnography. Sleep. 2010;33(8):1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Calandra-Buonaura G, et al. Hypnic jerks: neurophysiological characterization of a new motor pattern. Sleep Med. 2014;15(6):725–727. [DOI] [PubMed] [Google Scholar]
- 27. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]