Abstract
The bed nucleus of the stria terminalis (BNST) is a compact but neurophenotypically complex structure in the ventral forebrain that is structurally and functionally linked to other limbic structures, including the amygdala nuclear complex, hypothalamic nuclei, hippocampus and related midbrain structures, to participate in emotion, emotional learning and stress-related responses. From a variety of sensory inputs, the BNST acts as a node for signal integration and coordination for information relay to downstream central neuroendocrine and autonomic centers for appropriate homeostatic physiological and behavioral responses. In distinction from amygdala roles in fear, the BNST has gained wide interest from work suggesting that it has main roles in mediating sustained responses to diffuse, unpredictable and/or long duration threats which are typically associated with anxiety-related responses. Notably, maladaptive BNST neuroplasticity and function have been implicated in chronic pain, depression and other psychopathologies including posttraumatic stress disorders (PTSD). The BNST circuits are predominantly GABAergic - the glutaminergic neurons represent a minor population – but the complexity of the system results from an overlay of diverse neuropeptide coexpression in these neurons. More than a dozen neuropeptides may be differentially coexpressed in BNST neurons, and from variable G protein-coupled receptor (GPCR) signaling, may inhibit or activate downstream circuit activities. The mechanisms and roles of these peptides in modulating intrinsic BNST neurocircuit signaling and BNST long distance target cell projections are still not well understood. Nevertheless, an understanding of some of the principal players may allow assembly of the circuit interactions.
Keywords: Bed nucleus of the stria terminalis, peptides, stress, anxiety, behavior, extended amygdala, GABA
Introduction
The bed nucleus of the stria terminalis (BNST) is a small, compact but complex heterogeneous limbic structure in the ventral forebrain of the central nervous system (CNS) important for the integration and coordination of stress-related behaviors. Although the BNST, from neurodevelopment, neurophenotypic expression and microcircuitry, is often described as a component of the central extended amygdala, many studies have suggested a dichotomy of behavioral function from the central nucleus of the amygdala (CeA). For example, while the CeA is classically associated with coordinating immediate responses to predictable fearful threats, BNST function has been interpreted to mediate responses to long duration or temporally unpredictable stimuli that are more akin to anxiety responding (Goode and Maren, 2017; Hammack et al., 2015 ; Waddell et al., 2006; Walker et al., 2009). The latter includes hypervigilance, arousal and sensitivity to environmental dynamics, and BNST maladaptations from chronic stress-induced neuroplasticity may lead to psychopathologies including posttraumatic stress disorder. In rodents, the BNST is located just anterior to bregma and bounded by the areas around the anterior commissure, the lateral ventricles and the internal capsule, extending from the nucleus accumbens (nAcb) and lateral septal nucleus to the anterior hypothalamic area. From mouse brain stereotaxic measurements, the BNST is approximately 0.4mm3 or something comparable in size to the head of a pin. Yet despite its small size, the BNST has been parsed into 12 – 20 subdivisions or nuclei, and to contain more than 15 different cell types (Dong et al., 2001; Dong and Swanson, 2004; Larriva-Sahd, 2006). Several approaches have been described in the literature to describe the subdivisions but the BNST can be divided broadly into the anterior and posterior BNST separated environs the posterior borders of the anterior commissure, the preoptic area and the vertical septum fibers of the stria terminalis (see below) (Ju and Swanson, 1989; Ju et al., 1989). The posterior BNST has three well-studied nuclei, the principal, interfascicular and traverse nucleus, and some of these nuclei express high levels of sex hormone receptors to regulate reproductive and defensive behaviors (Simerly, 2002; Lebow and Chen, 2016). The sexual dimorphism of the BNST has been reviewed previously (Segovia and Guillamón, 1993; Stefanova and Ovtscharoff, 2000). The anterior BNST can be parsed further into regions dorsal and ventral to the anterior commissure (dBNST and vBNST, respectively) although the 2 regions show ventrolateral continuity, and both areas can be divided into lateral and medial segments - i.e., the dBNST can be subdivided into dorsolateral (dlBNST; also anterolateral or alBNST) and dorsomedial (dmBNST, also anteromedial or amBNST) BNST based on position relative to the stria terminalis (below, Figure 1). Some well-defined nuclei in the dlBNST include the oval, juxtacapsular and rhomboid nucleus; those in the vBNST include the fusiform and the magnocellular nucleus. Among these, the oval nucleus (BNSTov) has particular note as a center for stress signal integration and relay to other limbic structures.
Figure 1.
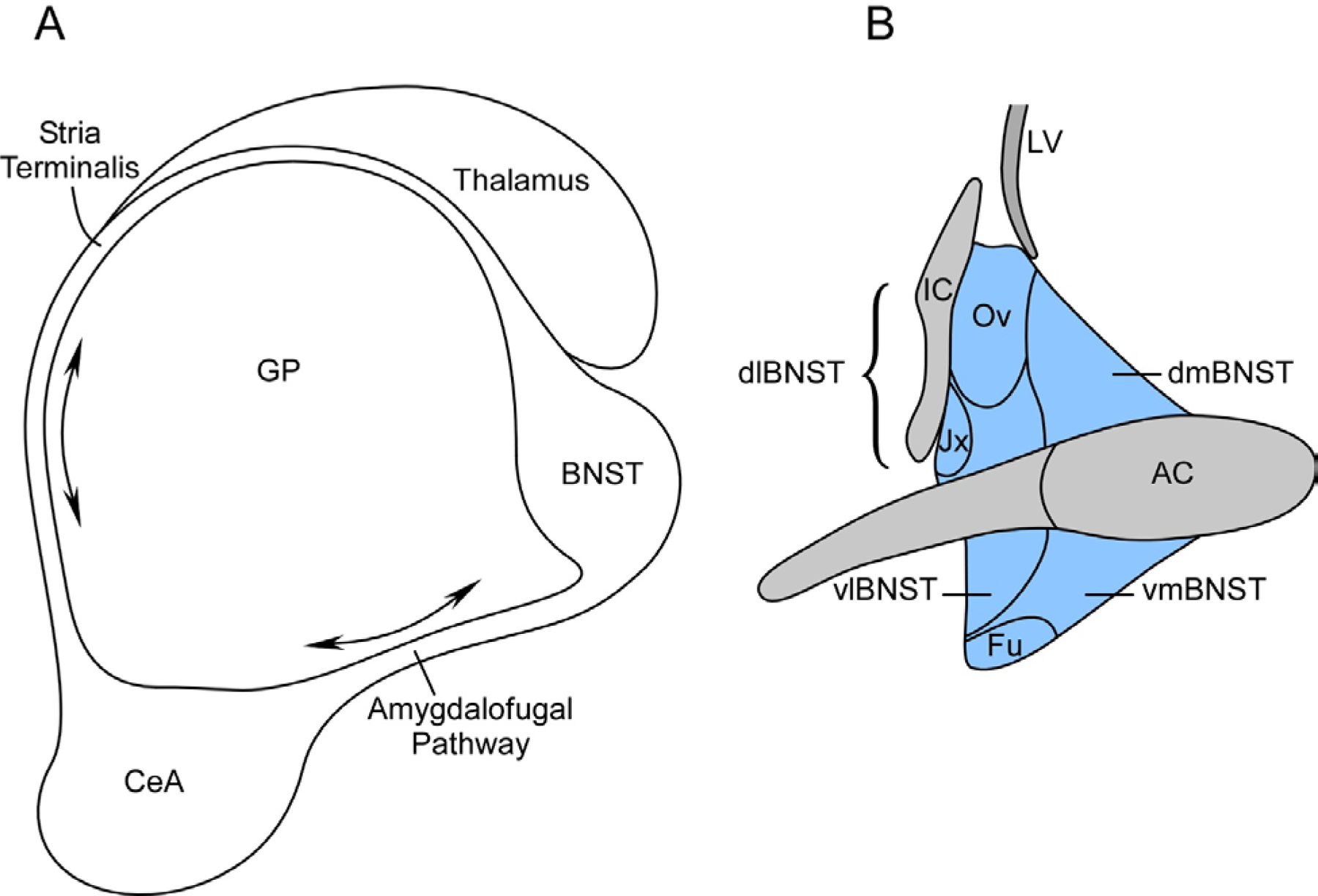
Schematic of BNST
Panels A and B depict the anatomical right rodent BNST. The BNST and central nucleus of the amygdala (CeA) are interconnected through two primary bidirectional fiber pathways (A). The stria terminalis fiber tract from the CeA arc in parallel with the fornix over the thalamus and target the BNST. The amygadalofugal pathway is ventral and more direct, interconnecting the CeA and BNST through hypothalamic areas. The BNST can be divided into many subdivisions (B). The anterior BNST is divided into dorsal and ventral areas by the anterior commissure; each area can be parceled further into lateral and medial divisions (highlighted in blue; dlBNST, dmBNST, vlBNST and vmBNST). The dorsolateral division (dlBNST; bracket blue area) contains the juxtacapsular (Jx) and the important oval nucleus (Ov; BNSTov in text) associated with stress-related responses. GP, globus pallidus; Fu, fusiform nucleus; AC, anterior commissure; LV, lateral ventricle; IC, internal capsule.
The CeA and the BNST are interconnected reciprocally through two fiber pathways (Figure 1). From the CeA in the temporal lobes, the stria terminalis fibers arc in parallel with the fornix over the lateral border of the thalamus to intersect with the BNST and direct major projections to the mediobasal forebrain, hypothalamus and brainstem nuclei. The second shorter and more direct CeA - BNST circuit is through the ventral amygdalofugal pathway. Hence from neuroanatomy and neurocircuitry, the BNST is a well situated relay nexus to receive and interpret sensory information for stress-related behavioral responses. Subregions of the BNST receive major afferent fibers from the medial prefrontal cortex (mPFC), hippocampus and lateral parabrachial nucleus (LPBn); some of the cortical and thalamic afferents may be indirect via the basolateral amygdala (BLA)/CeA. In addition to the reciprocal efferents from the BNST to the CeA via the stria terminalis and amygdalofugal tracts, the same pathways project to three main areas: anterior projections to target the nucleus accumbens and prelimbic cortex; projections to the diencephalon including the hypothalamic paraventricular nucleus (PVN), lateral hypothalamus (LH) and paraventricular nucleus of the thalamus (PVT); and long distance downstream efferents to brainstem areas such as the ventral tegmental area (VTA), periaqueductal gray (PAG), dorsal raphe (DR), substantia nigra (SN), red nucleus, pontine nuclei and the nucleus of the solitary tract (NST).
Neurotransmitters
The vast majority of BNST neurons are GABAergic although some glutaminergic neurons have been identified in the vBNST. While most BNST output are GABAergic, BNST output is also tightly regulated by local GABA activity. BNST glutaminergic neurons have been largely studied for their distal conntections to midbrain dopaminergic nuclei. In addition, some BNST output may be primarily peptidergic (Radley and Sawchenko, 2011). By contrast, the BNST receives a variety of neurotransmitter afferents. The dBNST receives heavy dopaminergic inputs from the PAG A10 cell groups and the VTA, with minor contributions from the substantia nigra. The BNST, and in particular the vBNST, receives heavy noradrenergic projections primarily from the A1 ventrolateral and A2 dorsal NTS medullary cell groups via the ventral noradrenergic bundle; a smaller dorsal noradrenergic projection may arise from the locus coeruleus (LC). The BNST also is heavily innervated by serotonergic projections from the medial and caudal aspects of the dorsal raphe nucleus. The roles of these neurotransmitter afferents are complex and not well understood given that the ligands can activate many different receptor subtypes at different cellular sites within local BNST circuits (Hammack et al., 2009b; Daniel and Rainnie, 2016). Dopamine can generate opposing D1/Gαs- or D2/Gαi-mediated signals and both systems appear to participate in the BNST. Dopamine D1/Gαs-mediated neuroplasticity appears to participate in addiction/reward mechanisms and to act on CRF neurons in a feedforward mechanisms to enhance glutaminergic transmission and stress responses. Among the adrenergic receptors, the β1, β2 and α1 receptors have postsynaptic functions and appear to facilitate anxiety-like behavior, stress-induced reinstatement of drug use and activation of stress pathways, whereas α2 receptor presynaptic Gαi signaling appear to block fear and reduce stress-induced reinstatement behaviors. Only a subset of serotonin receptors is expressed in the BNST but those present can generate both anxiogenic and anxiolytic responses. However, the primary actions of 5HT appear to be neuronal hyperpolarization and inhibition of pre- and postsynaptic BNST mechanisms to attenuate stress-induced anxiety responses. The 5HT dorsal raphe neurons appear to be innervated by CRF and the resulting increase in 5HT release in the BNST and other limbic structures have been suggested to represent a feedback mechanism to attenuate the maladaptive effects of stress (Hammack et al., 2009b).
Neuropeptides
Since the early 1980’s many different neuropeptides have been described to be expressed and modulate BNST function. Many of the initial localization and circuit mapping studies were dependent on immunocytochemical techniques and for several reasons, some of the early data should be interpreted with caution. Firstly, since not all of the related peptides within a family were recognized at the time, some of the available antisera at the time may not have been specific and consequently identified multiple peptide family members. Isolated a bit later, urocortins, for example share 45% sequence homology with corticotropin-releasing factor (CRF); similarly, the PACAP peptides share approximately 67% homology with VIP. Hence from antisera crossreactivity, some of the early staining data may not have been completely accurate. Secondly, for reasons that are still somewhat unclear, the immunocytochemical approaches are much better in labeling peptides in axonal fibers and terminals than soma. As peptide precursors predominate in soma and the endoproteolytic and posttranslational processing steps are completed in vesicles, these observations were interpreted to reflect the identification sites of fully processed and mature peptides. These results are often unsatisfying as the axons and terminals are often long distance fiber projections and cannot identify the nuclei of origin. Consequently, for the reasons above, many of the early studies were performed following intracerebroventricular infusions with colchicine to inhibit axonal transport and allow vesicular mature peptide retention in the soma. Although the colchicine approach resulted in intense somal peptide staining and was widely adopted, colchicine is now recognized to highly induce cellular stress resulting in neurochemical plasticity, i.e., neurons that do not normally express certain peptides for normal physiological functions, can be induced to express a plethora of peptides under cellular challenges (Cortés et al., 1990). Accordingly, under colchicine paradigms, the attribution of peptides to neuronal areas may not always be physiologically relevant under homeostatic conditions. The BNST expresses a diverse array of neuropeptides, many of which may be stress-sensitive, including corticotropin-releasing factor (CRF), urocortin (Ucn), pituitary adenylate cyclase activating poly peptides (PACAP), vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP), enkephalins (Enk)/dynorphin (Dyn), neuropeptide Y (NPY), neurotensin (NT), somatostatin (Sst), and oxytocin (Oxt)/vasopressin (Avp), among others. The peptides will be considered in turn and the summaries below will attempt to reflect consensus data from immunocytochemical, in situ hybridization and transgenic animal studies.
Corticotropin releasing factor (CRF)
Since its identification, the 41-amino acid α-amidated corticotropin releasing hormone (also corticotropin releasing hormone, CRH) has been the prototypic hypothalamic stress regulatory peptide (Spiess et al., 1981; Vale et al., 1981; Dedic et al., 2018). CRF and urocortin peptides signal via shared heptahelical G protein-coupled CRFR1 and CRFR2 receptors (see below) which are preferentially coupled to Gs to activate adenylyl cyclase (AC)/cAMP pathways, although receptor coupling to other G proteins (Gq/Gi/Go) has also been described (Deussing and Chen, 2018). CRF receptor AC/cAMP and internalization/endosomal mechanisms can also generate ERK activation. CRF has higher preference for CRFR1 than CRFR2 whereas most urocortin peptides (see below) have higher affinity to CRFR2. CRF and CRF receptors are widely and differentially distributed in the central nervous system (CNS) but notably, their high expression in hypothalamic and limbic structures has implicated CRF systems in the neuroendocrine, autonomic and behavioral consequences of stress. From cells in the hypothalamic paraventricular nuclei (PVN), stress-activated CRF axonal release at the median eminence enters the portal system to stimulate anterior pituitary corticotrope ACTH secretion for downstream adrenal cortisol (corticosterone in rodents) release and adaptive responses.
There are several extrahypothalamic sources of CRF in the CNS and the population of CRF neurons in limbic system is one of the highest in the rodent brain (Ju et al., 1989; Moga et al., 1989; Kono et al., 2017). Using immunocytochemical, in situ hybridization and tracing techniques in transgenic animals, the many BNST CRF studies have been largely coherent in demonstrating CRF neurons and dense CRF-immunoreactive fibers in the dorsolateral BNST region of the oval nucleus (BNSTov), with lower levels of expression in the ventral fusiform nucleus of the BNST (Choi et al., 2006; Cummings et al., 1983; Day et al., 1999; Ju et al., 1989; Miles and Maren, 2019; Roman et al., 2014; Uchida et al., 2019; Dabrowska et al., 2016; Daniel and Rainnie, 2016; Sakanaka et al., 1987). The BNSTov CRF fibers are complex in that they represent not only CRF afferent inputs from other brain regions, but fibers from intrinsic BNSTov CRF neurons that can form short intra-BNST connections and/or long axonal projections to distant target sites. The CRF afferents innervating the BNSTov have not been completely delineated but a major source include the central nucleus of the amygdala (CeA, especially the lateral division or CeL) (Pomrenze et al., 2015; Daniel and Rainnie, 2016; Sakanaka et al., 1986). Hence the CRF afferents may carry a variety of sensory signals including stress-related physiological and psychological inputs, fear and pain for BNST integration and relay. The CRF afferents appear to act presynaptically within the BNSTov to facilitate dopaminergic, noradrenergic and glutaminergic signaling. Whether these CRF afferents synapse in part on BNSTov CRF neurons is unclear. From the many exogenous peptide infusion, receptor antagonist and transgenic animal studies, there is consensus that BNSTov CRF/CRFR1 signaling induces stress-related behaviors.
A small fraction of the BNSTov CRF output fibers project rostrally to the nucleus accumbens and prelimbic cortex; but by far, most of the long distance projections are caudal to target the CeA, BLA, paraventricular (PVN) and lateral (LH) hypothalamic nuclei, and several brainstem nuclei including the substantia nigra, red nucleus, periaqueductal gray, pontine nuclei and dorsal raphe (DR); other brainstem structures include regions of the ventral tegmental area (VTA) and the nucleus of the solitary tract (NTS) (Dabrowska et al., 2016). Hence the diversity of these efferent projections implicate BNST roles in the activation of the HPA stress axis, metabolic regulation, motivation, aggression, motor activities, mood and affective behaviors. The BNSTov CRF neurons also form intra-BNST connections to modulate intrinsic functions, but whether these represent separate neuronal populations or axonal collaterals from long distance projecting BNSTov CRF neurons is also not well understood. Unlike the CRF afferents, the BNSTov CRF neurons are largely GABAergic; the complex consequences of BNSTov CRF activation remain unclear. Likely, the actions of GABA would predominate at low levels of stimulation, but higher levels would produce a more complex response involving both GABA and CRF receptor activation. A variety of acute and chronic stress paradigms, including footshock, have been shown to increase CRF transcript and peptide expression in the PVN and BNST. From adrenalectomy experiments, the expression of CRF from the PVN and BNST appear sensitive to classic glucocorticoid-mediated feedback inhibition; however chronic corticosterone administration to the CeA can increase amygdala and dorsolateral BNST CRF expression (Shepard 2006), suggesting a feedforward mechanism that can potentially facilitate maladaptive behavioral consequences. The BNST is a sexually dimorphic structure and the number of BNSTov CRF neurons is greater in females than males (Uchida et al., 2019).
Urocortin (Ucn)
Urocortins are CRF-related peptides that were isolated nearly 15 years after the identification of CRF. The 3 urocortin variants, urocortin-1, −2 and −3 (Ucn1, Ucn2 and Ucn3, respectively), demonstrate approximately 45% amino acid sequence homology with CRF and accordingly, CRF and UCN peptides have shared receptors (Deussing and Chen, 2018). Whereas CRFR1 preferentially binds both CRF and Ucn1, CRFR2 shows selectivity only for the Ucn peptides. Unlike CRF which has a wide distribution pattern in the CNS, the expression of Ucn peptides exhibit regional selectivity, and among the variants, Ucn3 is notable in that it is preferentially expressed in limbic structures, including the BNST and amygdala, and associated with stress-related physiology and behaviors (Deussing and Chen, 2018). Both Ucn3 neurons and fibers have been identified in the BNST and medial amygdala by in situ hybridization and immunocytochemical analyses, respectively (Lewis et al., 2001; Li et al., 2002; Venihaki et al., 2004; Wittmann et al., 2009); the other Ucn3 expression sites include the prefornical area, medial preoptic nucleus, parabrachial nucleus and the superior preolivary area (Deussing and Chen, 2018). The characterization of BNST Ucn3 fibers has not been extensive – although some of the BNST Ucn3 fibers have been suggested to represent projections from the medial amygdala (Li et al., 2002), whether some of the fiber are for intrinsic connections and/or whether the BNST Ucn3 neurons have long distance rostral or caudal projections has not been well established. CRFR2 is expressed in the dorsal and ventral BNST implicating Ucn3 activities within the nucleus; as high levels of CRFR2 expression are also apparently restricted in the CNS to the lateral septum, medial amygdala, dorsal raphe and nucleus of the solitary tract, BNST Ucn3 neurons may project to many of these areas (Deussing and Chen, 2018). As for CRF, the BNST Ucn3 neurons are thought to be GABAergic, but unlike the CRF/CRFR1 circuits, which mediate stress-related anxiogenic responses, the Ucn3/CRFR2 signaling, conversely, is thought to generate responses that are anxiolytic or facilitate coping mechanisms that can ameliorate the consequences of stress (Lebow and Chen, 2016). CRFR2 knockdown studies, for example, heightened arousal and vigilance, and increased anxiety- and depression-like responses (Lebow et al., 2012; Bale and Vale, 2003); in accord, optogenetic activation of CRFR2 receptors decreased the anxiety-related behaviors to stress (Henckens et al., 2017). However, CRFR2 activation in the dorsal raphe nucleus has been shown to produce an anxiogenic response in the learned helplessness paradigm, which is also dependent on BNST activation (Hammack et al., 2002; Hammack et al., 2003).
Neurotensin (NT)
The small 13-amino acid hypotensive peptide, neurotensin (Carraway and Leeman, 1973; Carraway and Leeman, 1975), is widely distributed in the CNS including the periventricular and paraventricular nuclei of the hypothalamus, central and medial amygdala, BNST, nucleus accumbens, thalamic nuclei, ventral tegmentum, specific dorsal raphe nuclei, locus coeruleus, parabrachial nucleus and the NTS (Jennes et al., 1982). The BNST receives heavy neurotensin innervation from the amygdala (Uhl and Synder, 1979) to implicate its transmitter roles in regulating BNST function; but importantly, approximately 80–90% of the CRF-containing neurons in the BNST was found to coexpress neurotensin (Shimada et al., 1989). This implied that CRF and neurotensin have synergistic actions on the same targets. Depolarization of select BNST neurons results in the corelease of CRF and neurotensin, and both neuropeptides act on their respective Gαs-coupled GPCRs, for synergistic AC/cAMP activities. In some studies, BNST CRF/NT acted presynaptically enhance BNST GABAergic neurotransmission. The inhibition of either peptidergic systems alone with specific receptor antagonists was insufficient in blocking the depolarization-induced inhibitory effects; however, the application of antagonist to block both receptor systems simultaneously inhibited the responses completely. Coordinately, the antagonists to the neurotensin receptor (NTR) attenuated stress-induced anxiety-like responses (Normandeau et al., 2018). Although these mechanisms are still not well defined, CRF and neurotensin from these studies appear to have closely coordinate actions.
Pituitary adenylate cyclase activating polypeptide (PACAP)
The pituitary adenylate cyclase activating polypeptides (PACAP) are important in regulating and maintaining the homeostasis of many physiological systems. The critical roles of PACAP in stress responses were not initially appreciated because unlike CRF, PACAP levels did not appear to be affected by acute stress (Hannibal et al., 1995; Lezak et al., 2014); subsequent studies, however, demonstrated that PACAP expression is regulated primarily by repeated or chronic stress (Lezak et al., 2014; Hammack et al., 2009a).
Rather than identification based on specific bioassays for anterior pituitary hormone secretion, PACAP was isolated from nearly 5000 ovine hypothalami using classical brute force biochemistry based on its abilities to stimulate anterior pituitary cell cAMP production (Kimura et al., 1990; Miyata et al., 1990). From the precursor molecule, two PACAP peptides may be generated from alternative posttranslational processing; PACAP38 is 38-amino acids long and PACAP27 is the 1–27 amino acid, C-terminally truncated, segment of PACAP38. As both PACAP38 and PACAP27 peptides are α-amidated, PACAP27 is not a degradation product of PACAP38. From amino acid sequence homology, PACAP belongs to the VIP/glucagon/secretin family of related peptides; PACAP27 and VIP demonstrate 67% amino acid homology (Sherwood et al., 2000; Vaudry et al., 2009). Accordingly, similar to CRF and urocortins, PACAP and VIP share GPCR subtypes. The PAC1 receptor is selective for PACAP38 and PACAP27; PACAP and VIP bind with near equal affinities to VPAC1 and VPAC2 receptors (Harmar et al., 2012; Blechman and Levkowitz, 2013). The two PACAP peptides are widely distributed in central and peripheral tissues, but PACAP38 represents the predominant form in all tissue regions examined to date (Arimura et al., 1991). The central distributions of PACAP and PAC1 receptors have been mapped (Hashimoto et al., 1996; Hannibal, 2002); the PACAP distribution patterns are comparable to those observed in the PACAP-EGFP mouse (Condro et al., 2016). Among diverse CNS regions, PACAP fibers and neurons have been identified in the hypothalamus, BNST and related limbic structures to participate in stress-related neuroendocrine and behavioral responses. Dense PACAP immunoreactive fibers were observed in the BNSTov, CeA and medial amygdala nucleus (Kozicz et al., 1997; Roman et al., 2014; Hannibal, 2002); the number of PACAP-immunoreactive soma in these regions appeared low (see comments above) but were better identified by in situ hybridization for PACAP transcripts. More than half of the PACAP fibers in the BNSTov and lateral capsular division of the CeA may have represented axonal projections from PACAP neurons in the lateral parabrachial nucleus (PBn) relaying nociceptive sensory information (Missig et al., 2014; Missig et al., 2017). The parabrachial (PBn) PACAP fibers are glutaminergic and approximately 70% of the PACAP fibers coexpress CGRP. Other PACAP afferent fibers to the BNSTov are likely projections from the peri/paraventriclar nuclei of the hypothalamus, CeA, supraoptic nucleus and/or the dorsal vagal nucleus. The remaining BNSTov PACAP fibers may be efferents representing components of intrinsic circuits and long distance axonal projections to distal target sites. Importantly, some of the PACAP stress regulatory effects appear to result in part from PACAP upstream circuit intersections with CRF pathways. BNSTov PACAP-immunoreactive fibers and terminals formed axodendritic and axosomatic synapses on CRF neurons, the heightened corticosterone response to chronic stress was blunted in PACAP knockout mice, and PACAP was shown to directly regulate CRF expression (Kozicz et al., 1997; Agarwal et al., 2005; Stroth et al., 2011; Stroth and Eiden, 2010). Hence, in addition to independent PACAP effects, some of the PACAP-mediated stress responses reflected PACAP actions on CRF neurons. Chronic stress increased BNSTov PACAP transcript expression and immunoreactivity, PACAP BNST signaling was anxiogenic, and the chronic stress-mediated anxiety-related responses that could be ameliorated with PAC1 receptor antagonists (Hammack et al., 2009a; Lezak et al., 2014; Roman et al., 2014). The anxiogenic effects of PACAP were consistent with data from PACAP and PAC1 receptor knockout mice which exhibited a diminished anxiety responses to stressors (Hashimoto et al., 2001; Otto et al., 2001).
Vasoactive intestinal peptide (VIP)
Vasoactive intestinal peptide (VIP) was first identified by Sami Said and Viktor Mutt from porcine intestinal tissues based on the vasodilatory actions of the peptide (Said and Mutt, 1972; Said and Mutt, 1974). As described above, PACAP and VIP are related peptides, but unlike many other peptide families, the expression and function of one peptide do not supplant or compensate for deficiences of the other (Girard et al., 2006). From PACAP and VIP amino acid sequence homology, the immunocytochemical studies for the two peptidergic systems have been stringent to demonstrate lack of crossreactivity. From VIP immunocytochemistry and in situ hybridization data, VIP neurons were not apparent in the BNST and only scattered neurons were observed in the BLA and hippocampus; higher levels of VIP mRNA expression were observed in the cortical layers, thalamic nuclei, suprachiasmatic nucleus (SCN) of the hypothalamus and the dorsal raphe (Hill et al., 1994). These data suggest that VIP-containing neurons do not originate in the BNST; however, like PACAP, VIP immunoreactive fibers were found in the BNST but different fiber patterns between these two peptides. Whereas PACAP-immunoreactive fibers were confined predominantly to the oval nucleus, VIP-immunoreactive fibers appeared scattered and more diffuse throughout the dorsolateral BNST (Kozicz et al., 1997). The BNST VIP fibers appeared to originate predominantly from the dorsal raphe and caudal linear raphe nuclei, although afferents from the amygdala, parabrachial nucleus and dorsal vagal complex were also implicated (Kozicz et al., 1997; Petit et al., 1995). Even though VIP-immunoreactive terminals have also been described to synapse onto BNST CRF neurons, similar to that observed for PACAP fibers, the roles of VIP in the BNST have not been fully established as BNST VIP infusions did not mimick the anxiogenic effects PACAP (Roman et al., 2014). Similarly, the anxiogenic and nociceptive hypersensitivity responses observed from CeA PACAP infusions were not recapitulated by VIP (Missig et al., 2017; Missig et al., 2014). In coherence, the anxiety and pain sensitivity responses of PACAP in the BNST and CeA were mimicked by infusions of the specific PAC1 receptor agonist maxadilan into the limbic structures, implicating the PACAPergic (and not the VIPergic) system in stress-related behaviors.
Opioid peptides
Opioid peptides are derived from the expression and processing of three different precursor molecules: proopiomelanocortin (POMC, also pro-ACTH/endorphin) in the synthesis if β-lipotropin/β-endorphin; pro-enkephalin (proEnk, also pro-enkephalin A) in the production of Met-/Leu-enkephalins and related peptides; and pro-dynorphin (proDyn, also pro-enkephalin B) in the synthesis of neo-endorphins and dynorphin molecules. The opioid peptides are endogenous ligands to three GPCRs – the mu (MOR), delta (DOR) and kappa (KOR) receptors - all coupled to Gi to inhibit adenylyl cyclase/cAMP pathways. Whereas the enkephalin peptides preferentially bind to the MOR and DORs, dynorphins appear to be ligands for KORs. POMC is preferentially expressed in the pituitary gland and arcuate nucleus of the hypothalamus, resulting in the tissue-specific biosynthesis of final bioactive peptide products in each region (i.e., ACTH/β-lipotropin in anterior pituitary gland vs αMSH/β-endorphin in hypothalamus). Pro-Enk and proDyn by contrast are more widely distributed in the CNS, including the BNST and amygdala, although the expression patterns of the two peptidergic systems within these regions maybe different. From in situ hybridization data, dynorphin expression appeared highly localized in many BNST subnuclei including the oval, anterolateral, rhomboid and fusiform nuclei; more moderate levels were observed in the anteromedial BNST (Poulin et al., 2009). In variance from dynorphin expression, the in situ distribution patterns for enkephalins appeared more extensive in the oval, anterolateral, anteromedial, juxtacapsular and posterior BNST; enkephalin expression was not apparent in either the rhomboid or fusiform nuclei (Poulin et al., 2009). As described above, essentially all neurons in the anterolateral BNST (>95%) are GABAergic and hence, these studies suggested that the dynorphin and enkephalin neurons in these BNST subnuclei regions represented largely separate populations of GABAergic cells. A larger fraction (>15%) of enkephalingergic neurons in the posterior BNST may be glutaminergic. Some studies have suggested that CRF and met-enkephalin immunoreactivities could be colocalized in BNST neurons (Sakanaka et al., 1989), but as the two peptidergic systems engage opposing signaling mechanisms, the implications of those observations are unclear. VIPergic fibers have also been described to synapse and regulate BNST met-enkephalin neurons (Kozicz et al., 1998).
Interestingly, the two opioid systems may have opposing functional roles. Dynorphin release and signaling within the BNST has been shown to produced dysphoria via KOR mechanisms which could be blocked with KOR antagonists (Land et al., 2008; McLaughlin et al., 2003). In this regards, the effects of dynorphin appear concerted with those for CRF and PACAP in facilitating anxiogenic responses. Indeed, some observations suggested that the dynorphin system may be downstream of CRF signaling.
In contrast to CRF/PACAP-mediated GPCR/Gs signaling in anxiety, BNST enkephalin GPCR/Gi signaling has been suggested to facilitate anxiolytic responses (Hebb et al., 2005; Ragnauth et al., 2001; Wilson et al., 2003). The lateral BNST enkephalinergic neurons project to the CEA and enkephalin release/signaling in the amygdala can diminish anxiety-like behaviors. These interpretations have been supported in general in knockout and peptide overexpression studies, and appear consistent with other data implicating the BNST in modulating anxiety behavioral levels.
Calcitonin gene-related peptide (CGRP)
The calcitonin gene can undergo tissue-specific alternative splicing to generate either calcitonin transcripts in thyroid follicular cells for calcium homeostasis, or CGRP mRNAs in neural tissues for sensory signaling. As for all bioactive peptides, calcitonin and CGRP are ligands for GPCRs, but unlike many other peptide receptor systems, the specificity of the receptors is dependent on the cell co-expression of receptor-associated membrane proteins (RAMP1, RAMP2 and RAMP3). The abilities of specific RAMPs to partner with the calcitonin receptor (CT) or calcitonin related-like receptor (CRLR) generate GPCR specificity to calcitonin, CGRP, amylin and adrenomedullin. Among bioactive peptides, CGRP appears particularly sensitive to colchicine-induced expression. But even from in situ hybridization data, CGRP expression in the CNS appears extensive in the diencephalon and brainstem structures. CGRP expression was high in the lateral hypothalamic areas, arcuate nucleus, posterior and peripeduncular thalamic nuclei, lateral olfactory tract, parabrachial nucleus (PBn) and cranial motor nuclei (Kresse et al., 1995). CGRP transcript expression was not apparent in the different amygdaloid nuclei; very few CGRP neurons were identified in the anterolateral and anteromedial BNST. However, there are significant levels of CGRP-immunoreactive fibers and terminals in the BNST and as described above, the majority of the fibers coexpressed PACAP which represented dense afferent projections from the parabrachial nucleus (Missig et al., 2017; Missig et al., 2014). Accordingly, consistent with current appreciations of BNST PACAP/CRF activities, recent studies have also implicated BNST CGRP signaling in stress-related anxiogenic responses. Bilateral CGRP BNST infusions increased baseline startle and decreased open arm entries and times on elevated plus maze tests; the responses could be attenuated with the CGRP8–37 receptor antagonist or with the CRF receptor antagonist GSK876008, suggesting that coordinate with PACAP/CGRP signaling is upstream of BNST CRF neurons (Sink et al., 2013a; Sink et al., 2013b; Sink et al., 2011). These interpretations are supported by the observation of apparent CGRP-immunoreactive fibers synapsing onto CRF and met-enkephalin neurons (Kozicz and Arimura, 2001). Parabrachial PACAP and CGRP peptides can carry a variety of sensory signals including nociceptive information and aversive taste responses from illness, and hence integrate sensory inputs within limbic structures to inform behavioral responses (Chen et al., 2018; Missig et al., 2017).
Neuropeptide Y (NPY)
The α-amidated 36 amino acid neuropeptide Y (NPY) has been well studied in the sympathetic autonomic nervous system as a potent vasoconstrictor coexpressed in many catecholaminergic neurons. NPY binds to at least four Gi-coupled GPCRs designated as Y1, Y2, Y4 and Y5; hence the receptors inhibit adenylyl cyclase and cAMP signaling. Y3 has not been identified definitively; Y6 is not expressed in rats and appears to be a pseudogene in humans. In the CNS, NPY is widely distributed in many regions including cortex, hippocampus, hypothalamus, brainstem nuclei and limbic structures. Both NPY neurons and fibers were identified in the CeA, BLA and BNST (Allen et al., 1984), and notably, the BNST and CeA/BLA appeared to have reciprocal NPY circuit connections. Some of the heavy NPYergic fibers in the BLA appeared to represent afferents from the BNST and the amygdalostriatal area, and conversely, lesions of the stria terminalis diminished BNST NPY fiber projections from the amygdaloid complex (Allen et al., 1984; Leitermann et al., 2016). The roles of NPY in the behavioral consequences of stress, however, have still remained enigmatic as differential responses have been described based on the actions of postsynaptic Y1 versus predominantly presynaptic Y2 receptor actions. Whereas NPY signaling at the Y1 has been shown to be anxiolytic and buffer the behavioral consequences of stress, Y2 receptor signaling has been associated with augmented anxiety-related responses. NPY Y1 agonist injections into the amygdala or cerebral ventricles produced anxiolytic responses and in coherence, Y1 receptor knockdown increased anxiety-like behaviors (Heilig et al., 1993; Broqua et al., 1995; Wahlestedt et al., 1993). This contrasted with intracerebroventricular infusions with Y2 receptor antagonists or Y2 receptor knockdown which resulted in anxiolytic responses (Bacchi et al., 2006; Tschenett et al., 2003; Tasan et al., 2010); however, these Y2 effects were attributed to signaling in the CeA and BLA, and not the BNST.
Somatostatin (Sst)
The 14- and 28-amino acid forms of somatostatin (Sst) bind to GPCRs that are coupled to Gi to potently inhibit adenylyl cyclase/cAMP signaling. From immunocytochemical, in situ hybridization and immunoassay data, Sst is widely distributed in the CNS (Palkovitz et al., 1982; Finley et al., 1981; Kiyama and Emson, 1990). High levels of Sst neurons and fibers were identified in the piriform and neocortex (especially in layers V and VI), hippocampal hilar and CA3 areas, and hypothalamic nuclei and median eminence; notably, some of the highest expression levels in the telencephalon were found in the amygdaloid nuclear complex, lateral septum, nucleus accumbens and BNST, and in fiber tracts such as the diagonal band of Broca, stria terminalis and the amygdalofugal pathways. In the brainstem, the localization of Sst neurons and fibers appeared extensive in many areas including the periaqueductal gray (PAG), locus coeruleus (LC), lateral parabrachial nucleus (PBn), superior/inferior colliculi, nucleus of the lateral lemniscus, nucleus ambiguus, nucleus of the solitary tract (NST), spinal trigeminal nucleus and regions within the reticular formation. The expression and function of BNST and amygdala Sst have been examined in many studies although its roles in stress and behavior are not fully understood. Variably, approximately 5 – 25% of the neurons in the all subdivisions of the BNST (including the oval nucleus) and the lateral/capsular divisions of the CeA have been described to express Sst; as for other peptides, BNST and CeA Sst neurons are GABAergic and some populations (up to 40 – 70% of Sst neurons) show apparent CRF or NPY coexpression. The afferent inputs to the BNST Sst neurons have not been identified, but interestingly, the heavy lateral parabrachial (LPBn) CGRP (and by inference PACAP) projections to the BNST do not innervate the Sst neurons (Ye and Veinante, 2019). As in the lateral CeA, the BNST Sst neuronal fibers are likely contributory to inhibitory local microcircuits and the long-range efferent projections to the parabrachial nucleus (PBn) and periaqueductal gray (PAG)/dorsal raphe (DR). The roles and mechanisms of Sst signaling in stress, mood, emotion and behavioral abnormalities have not been fully elucidated and some of the studies appear contradictory. Some studies suggest that the peptide is anxiolytic, anti-stress and anti-depressive; Sst knockout mice displayed increased anxiety-like behaviors and emotionality scores, high basal corticosterone levels and downregulated CNS BDNF and GAD67 gene expression profiles that appeared consistent with patterns associated with depression (Lin and Sibille, 2015). Although the studies did not utilize conditional/tissue-selective but instead global Sst knockouts in mice, intracerebroventricular Sst receptor agonist infusions were in coherence and decreased stress-related hormone levels and specific behavioral responses. However, rather than anxiolytic, studies have also shown that Sst signaling is associated with fear and fear-conditioning freezing responses, which could be dissociated from defensive flight behaviors (Fadok et al., 2017). Optogenetic activation of CeA Sst neurons heightened freezing and decreased flight, whereas CRF neurons had the opposing effects of inhibiting freezing and increasing flight. The dichotomous nature of the responses appeared unique and suggested that Sst and CRF neurons form reciprocal inhibitory local circuits. Although these studies were in the CeA, similarities in circuit organizations may suggest similar mechanisms in the BNST.
Oxytocin and Vasopressin
Oxytocin (Oxt) and vasopressin (arginine vasopressin; Avp) are related α-amidated nonapeptides that differ only by 2 amino acids. GPCR Oxt receptor signaling through Gαi/Gαo/Gαq is often related to uterus smooth muscle relaxation in childbirth and lactation processes; Avp V1 and V2 receptor signaling through Gαq and Gαs, respectively, has been best studied with respect to homeostatic water balance and blood pressure control mechanisms. In the CNS, both Oxt and Avp are now understood to have wide ranging behavioral effects (Jurek and Neumann, 2018; Caldwell et al., 2008). Oxt and Avp, with their respective carrier proteins neurophysin 1 and neurophysin 2, have been mapped extensively in the brain and the highest levels of expression are found in the supraoptic, paraventricular and suprachiasmatic nuclei of the hypothalamus. In colchicine treated preparations, a large number of Avp-immunoreactive neurons was identified in the BNST; by contrast, only a few scattered Oxt-immunoreactive neurons were apparent in the BNST which appeared to be confined largely to the posterior division associated with reproductive activities (Sofroniew, 1985; Bingham and Viau, 2008). These observations were largely corroborated by in situ hybridization studies for Oxt and Avp transcripts although the radiolabeled grain densities for BNST Oxt neurons were adjudged to be very low (Caldwell et al., 1989; Hallbeck et al., 1999). However, in situ hybridization data for Oxt and Avp receptor transcripts revealed moderate to high levels of OxtR and variant V1aR expression in the BNST (Yoshimura et al., 1993; Ostrowski, 1998); Avp V1b and V2 receptors were not apparent in this region.
The high levels of receptors suggest that Oxt and Avp may be able to regulate BNST function, yet as endogenous BNST Oxt expression levels are very low, these observations may implicate long distance Oxt fiber projections from other regions, especially the hypothalamus or CeA, as mechanisms of BNST OxtR activation. The roles of BNST Oxt and Avp receptor signaling are still unclear. The BNST is sexually dimorphic and some of the complexity may arise from gonadal steroid hormone regulation of Oxt and OxtR expression (Caldwell et al., 1989; Ostrowski, 1998); estrogen for example, can upregulate Oxt/OxtR levels. Broadly, in concert with its general pro-social, empathy, coping and anti-stress behavioral actions (Jurek and Neumann, 2018), Oxt/OxtR signaling in the BNST appears to be anxiolytic in pharmacological animal and human studies. However, these responses contrast with BNST Oxt/OxtR mechanisms that facilitate fear to acute discrete cues. Fear learning can be complex from changes in neurotransmitter/neuropeptide expression and function from stress experience and brain structural plasticity and one interpretation of these apparent divergent BNST Oxt actions is that Oxt may heighten acute fear responses as a rapid means of threat detection for survival and preservation but ameliorate responses to diffuse, undefined and long term threats associated with contextual fear and anxiety-related behaviors (Moaddab and Dabrowska, 2017; Janecek and Dabrowska, 2019).
The roles of BNST Avp signaling are similarly complex. Similar to gonadal steroid regulation of Oxy, Avp and Avp receptors are regulated by androgens; testosterone priming in neonates for example, can regulate the number of Avp expressing neurons in the BNST (Bingham and Viau, 2008) and gonadectomy can decrease BNST Avp fiber innervation of distal targets. Avp has been best studied with respect to facilitating offensive aggression, especially between males, but overlapping with Oxt effects, Avp can have pro-social/affiliative learning and memory, and anxiolytic behaviors (Caldwell et al., 2008). However, Avp-mediated effects can be variable highlighting again that the responses may be dependent on environment, experience and resulting neuroplasticity.
Human BNST
The human and rodent BNST share neuroanatomical and neurochemical similarities. As in rodents, the human BNST is bounded by the nucleus accumbens, anterior thalamus, inferior lateral ventricles and the internal capsule; it is approximately 190 mm3 or the approximate size of a sunflower seed (Avery et al., 2016) and hence not well resolved spatially even with the best MRI scanners (Shackman and Fox, 2016). The structure is also subdivided, but rather than the two broad dorsolateral/dorsomedial BNST regions described for rodents, the dorsal BNST in humans has been parsed into lateral, central and medial sectors (Lesur et al., 1989). In analogy to animal data, the human BNST is interconnected with the amygdala through the stria terminalis and ventral amygdalofugal pathways, and parcellated comparable to rodent subdivisions. For obvious reasons, the human BNST circuits cannot be mapped in fine structural detail using tracing techniques, but from recent neuroimaging techniques, the human and rodent BNST appear to share broad similarities in structural and functional connectivity. Diffusion tensor MRI and probabilistic tractography demonstrated that the human BNST, as in rodents, is structurally connected to other limbic structures including the centromedial amygdala and hippocampus, the nucleus accumbens, caudate, putamen and related regions of the basal ganglia, and the thalamus; a probable connection to the limen insulae of the insular cortex appears distinct to humans. Functional connectivity from resting state fMRI, which can be different from structural connectivity in that monosynaptic fiber tracts between regions may be absent, revealed comparable results in BNST connections with limbic, thalamic and basal ganglia structures. However, the paracingulate gyrus, which is distinct from the rodent medial prefrontal cortex and not identified as a BNST structural connection, was found to be a functional BNST-associated target in these studies. Although the human studies require additional work, these results suggest that the human and rodent BNST neurocircuits have broad parallels.
From these structural and functional similarities, the human BNST may be anticipated to demonstrate neuropeptide expression patterns comparable to those found in rodents. The immunocytochemical processing of human brain sections however, presents unique challenges related to the use of tissues of variable postmortem duration. Hence, for some studies, the human brain staining patterns can be complicated by protein/peptide degradation and artefacts from poor tissue preservation. Nevertheless, many of the peptides identified in rodent BNST have been found in humans, though not as comprehensively characterized as in rodents. Apparent Sst immunoreactivity, for example, was identified in scattered neurons and dense fiber/varicosity networks in the dBNST/vBNST of human and non-human primates (Bennett-Clarke and Joseph, 1986; Kovner et al., 2019; Lesur et al., 1989; Walter et al., 1991); as described previously, a substantial fraction of the Sst neurons may coexpress CRF. Similarily, NPY-, enkephalin-, neurotensin-, VIP- and substance P-immunoreactive fibers were found in the human BNST; notably, in departure from patterns observed in rodents, VIP-, somatostatin-, enkephalin- and neurotensin-immunoreactive neurons appeared in a discrete cluster within the central BNST (Lesur et al., 1989; Walter et al., 1991). Also, in contrast to rodents, Avp fibers and neurons were observed in the human and non-human primate BNST; no Oxt immunoreactive structures were apparent (Fliers et al., 1986; Caffe et al., 1989). Human BNST PACAP levels, were measured only by radioimmunoassay and found to be among the highest in the brain (Palkovitz et al., 1995), consistent with rodent data described above. Hence our understanding of the chemoarchitecture and neurocircuitry of the human BNST are still very limited and require additional study.
Overview and Conclusions
The BNST is a small and complex sexually dimorphic ventral forebrain structure important in integration of many sensory and regulatory inputs to coordinate the physiological and behavioral responses to stress. It is structurally and functionally associated with the amygdala nuclear complex but rather than the mediation of fear responses alone, the BNST also appears to respond to long-duration and/or temporally unpredictable threats, associated with anxiety. Hence many studies have suggested that sustained BNST activation from chronic stress may result in maladaptive neuroplasticity within the structure to facilitate psychopathologies such as PTSD. Understanding BNST neurocircuits driving the responses continues to be a challenge. The BNST is largely GABAergic with a small population of glutaminergic neurons, but the high and heterogenous coexpression of many neuropeptides in BNST neurons (Figure 2) obscures how these circuits actually work. The CRF/GABA neurons appear to represent the major output of the BNST, but how the intrinsic CRF/GABA interneurons within the BNST local circuits impact the long distance output neurons is unclear. Hence there are a number of outstanding questions. A diverse set of stimulatory and inhibitory peptides has been identified in the BNST; are they all GABAergic also or are some coexpressed in glutaminergic neurons? A similar set of peptides is found in afferents to the BNST; how are these peptides and transmitters integrated into the BNST circuits? And in particular, what does output from same stimulatory CRF but inhibitory GABAergic neuron mean to the downstream neuronal target? In one possible mechanism, the inhibitory peptides and transmitters directly innervate the CRF/GABA output neurons whereas stimulatory afferents target intermediary inhibitory interneurons which in turn innervate the inhibitory output cells. In this model, the networks disinhibit inhibitory neurons resulting in positive outputs from the BNST (Figure 2).
Figure 2.
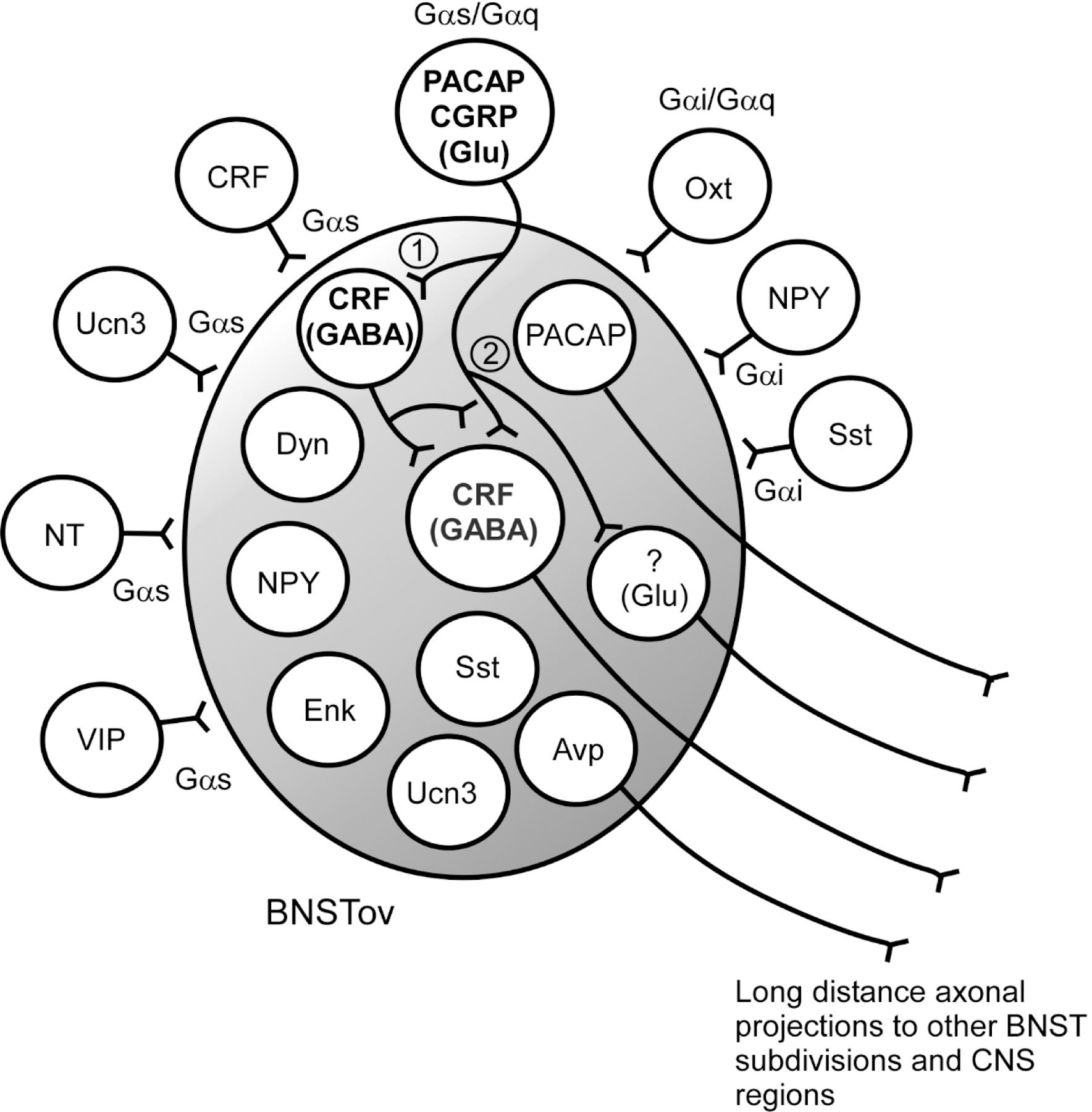
BNSTov phenotypic heterogeneity.
Diverse peptides in BNSTov neurons and fibers have been identified. The peptidergic fibers include afferents from extrinsic peptidergic neurons, and intrinsic neuronal networks and projection fibers to other BNST subdivisions or long distance CNS targets. The peptides can be coupled variably to Gαs, Gαq, Gαi and/or G protein dependent- or independent signaling cascades. Using the PACAP and CRF neurons to illustrate potential network mechanisms (bold font), the extrinsic PACAP/CGRP projections (i.e., from the lateral parabrachial nucleus) may synapse onto a BNSTov CRF/GABA interneuron to inhibit an output inhibitory CRF/GABA neuron (1). The activation of a local inhibitory neuron may inhibit GABAergic output of the BNST, leading to downstream disinhibition to net a facilitatory stress signal. Stimulatory extrinsic PACAP afferents may also directly stimulate intrinsic CRF and/or glutaminergic neurons, or BNSTov PACAP may have direct output projections (2) to affect stress responses. Mechanistic refinements and alternatives may be apparent from future work. Peptide abbreviations as in text.
Alternatively, the neuropeptides have dynamic functional roles in BNST function. Neuropeptide vs transmitter release is dependent on stimulation frequency. Peptidergic neurotransmission via dense core vesicle release at the terminals requires high frequency stimulation or bursting activity and one heuristic interpretation of CRF/GABA signaling may be that GABAergic signaling prevails under basal non-stress conditions and that high or chronic stress stimulatory conditions from peptide Gαs/Gαq presynaptic or postsynaptic neuronal activation (such as PACAP) may drive CRF signaling (Figure 2), to shift the circuit from a anxiolytic to anxiogenic state. The interacting peptide/interneuron circuits within the BNST may fine-tune the inputs to the CRF/GABA neurons so that the appropriate anxiolytic or anxiogenic responses may be generated to specific stressors or cues. In this model, chronic stress-induced peptidergic activation and neuroplasticity may also alter BNST neuronal cytoarchitecture, phenotypic expression, circuit organization or synaptic function to facilitate maladaptations that promote behavioral abnormalities. Variations in these and other models may become apparent from ongoing high resolution studies and from the many recent reviews, clear understandings of BNST circuits and mechanisms may provide insights for novel therapeutics to some rather intractable and devastating pain and behavioral disorders.
Acknowledgements:
This work was supported in part by National Institutes of Health (NIH) grant National Institute of General Medical Sciences (NIGMS) P30 GM103498 / National Center for Research Resources (NCRR) P30 RR032135 (RLP) and National Institute of Mental Health (NIMH) MH097988 (SEH and VM).
References
- Agarwal A, Halvorson LM & Legradi G (2005). Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res. 138: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen YS, Roberts GW, Bloom SR, et al. (1984). Neuropeptide Y in the stria terminalis: evidence for an amygdalofugal projection. Brain Res. 321: 357–362. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvári-Vigh A, Miyata A, et al. (1991). Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129: 2787–2789. [DOI] [PubMed] [Google Scholar]
- Avery SN, Clauss JA & Blackford JU (2016). The human BNST: funtional roles in anxiety and addiction. Neuropsychopharmacology Reviews 41: 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi F, Mathe AA, Jimenez P, et al. (2006). Anxiolytic-like effect of the selective neuropeptide Y-Y2 receptor antagonist BIIE0246 in the elevated plus-maze. Peptides 27: 3202–3207. [DOI] [PubMed] [Google Scholar]
- Bale TL & Vale WW (2003). Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 23: 5295–5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Clarke CA & Joseph SA (1986). Immunocytochemical localization of somatostatin in human brain. Peptides 7: 877–884. [DOI] [PubMed] [Google Scholar]
- Bingham B & Viau V (2008). Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology 149: 3581–3591. [DOI] [PubMed] [Google Scholar]
- Blechman J & Levkowitz G (2013). Alternative Splicing of the Pituitary Adenylate Cyclase-Activating Polypeptide Receptor PAC1: Mechanisms of Fine Tuning of Brain Activity. Front Endocrinol (Lausanne) 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broqua P, Wettstein JG, Rocher MN, et al. (1995). Behavioral effects of neuropeptide Y receptor agonists in the elevated plus-maze and fear-potentiated startle procedures. Behav Pharmacol 6: 215–222. [PubMed] [Google Scholar]
- Caffe AR, Van Ryen PC, Van de Woude TP, et al. (1989). Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaque fascicularis. J Comp Neurol. 287: 302–325. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee J-J, Macbeth AH, et al. (2008). Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 84: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JD, Brooks PJ, Jirikowski GF, et al. (1989). Estrogen alters oxytocin mRNA levels in the preoptic area. J Neuroendocrinol. 1: 273–278. [DOI] [PubMed] [Google Scholar]
- Carraway R & Leeman SE (1973). The isolation of a new hypotensive peptide neurotensin from bovine hypothalami. J Biol Chem 248: 6854–6861. [PubMed] [Google Scholar]
- Carraway R & Leeman SE (1975). The amino acid sequence of a hypothamlamic peptide neurotensin. J Biol Chem 250: 1907–1911. [PubMed] [Google Scholar]
- Chen JY, Campos CA, Jarvie BC, et al. (2018). Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 100: 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Nguyen MMN, Tamashiro KLK, et al. (2006). Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav 89: 301–310. [DOI] [PubMed] [Google Scholar]
- Condro MC, Matynia A, Foster NN, et al. (2016). High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol. 524: 3827–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés R, Ceccatelli S, Schalling M, et al. (1990). Differential effects of intracerebroventricular colchicine administration on the expression of mRNAs for neuropeptides and neurotransmitter enzymes, with special emphasis on galanin: an in situ hybridization study. Synapse 6. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, et al. (1983). Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 3: 1355–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Martinon D, Moaddab M, et al. (2016). Targeting corticotropin-releasing factor projections from the oval nucleus of the bed nucleus of the stria terminalis using cell-type specific neuronal tracing studies in mouse and rat brain. J Neuroendocrinol. 28: 10.1111/jne.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel SE & Rainnie DG (2016). Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology 41: 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJJ, et al. (1999). Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 413: 113–128. [PubMed] [Google Scholar]
- Dedic N, Chen A & Deussing JM (2018). The CRF family of neuropeptides and their receptors - mediators of the central stress response. Curr Mol Pharmacol. 11: 4–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussing JM & Chen A (2018). The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 98: 2225–2286. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD & Swanson LW (2001). Basic organization of prejections from theoval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 436: 430–455. [DOI] [PubMed] [Google Scholar]
- Dong HW & Swanson LW (2004). Organization of axonal projections fom the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 468: 277–298. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, et al. (2017). A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542: 96–100. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Maderdrut JL, Roger LJ, et al. (1981). The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience. 6: 2173–2192. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, et al. (1986). Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 375: 363–367. [DOI] [PubMed] [Google Scholar]
- Girard BA, Lelievre V, M. BK, et al. (2006). Noncompensation in peptide/receptor gene expression and distinct behavioral phenotypes in VIP- and PACAP-deficient mice. J Neurochem. 99: 499–513. [DOI] [PubMed] [Google Scholar]
- Goode TD & Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn Mem. 24: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, Hermanson O & Blomqvist A (1999). Distribution of preprovasopressin mRNA in the rat central nervous system. J Comp Neurol. 411: 181–200. [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, et al. (2009a). Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo J-D, Hazra R, et al. (2009b). The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuro-Psychopharmacol Biol Psych 33: 130–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Schmid MJ, et al. (2002). The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 22: 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Schmid MJ, LoPresti ML, et al. (2003). Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 23: 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Todd TP, Kocho-Schellenberg M, et al. (2015. ). Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav Neurosci. 129: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J (2002). Pituitary adenylate cyclase activating peptide in the rat central nervous system: an immunocytochemical and in situ hybridization study. J Comp Neurol. 453: 389–417. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Mikkelsen JD, Fahrenkrug J, et al. (1995). Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress. Endocrinology 136: 4116–4124. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, et al. (2012). Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 166: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, et al. (1996). Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 371: 567–577. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, et al. (2001). Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci U S A. 98: 13355–13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb AL, Poulin J-F, Roach SP, et al. (2005). Cholescystokinin and endogenous opioid peptides: interactive influence on pain, congnition and emotion. Prog Neuro-Psychopharmacol Biol Psychiatry 29: 1225–1238. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, et al. (1993). Anxiolytic-like actions of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology 8: 357–363. [DOI] [PubMed] [Google Scholar]
- Henckens MJAG, Printz Y, Shamgar U, et al. (2017). CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry 22: 1691–1700. [DOI] [PubMed] [Google Scholar]
- Hill JM, Agoston DV, Gressens P, et al. (1994). Distribution of VIP mRNA and two distinct VIP binding sites in the developing rat brain. relation to ontogenic events. J Comp Neurol. 342: 186–205. [DOI] [PubMed] [Google Scholar]
- Janecek M & Dabrowska J (2019). Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res 375: 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE & Kalivas PW (1982). Neurotensin: a topographical distribution in rat brain by immunocytochemistry. J Comp Neurol. 210: 211–224. [DOI] [PubMed] [Google Scholar]
- Ju G & Swanson LW (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. cytoarchitecture. J Comp Neurol. 280: 587–602. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW & Simerly RB (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 280: 603–621. [DOI] [PubMed] [Google Scholar]
- Jurek B & Neumann ID (2018). The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 98: 1805–1908. [DOI] [PubMed] [Google Scholar]
- Kimura C, Ohkubo S, Ogi K, et al. (1990). A novel peptide which stimulates adenylate cyclase: molecular cloning and characterization of the ovine and human cDNAs. Biochem Biophys Res Commun. 166: 81–89. [DOI] [PubMed] [Google Scholar]
- Kiyama H & Emson PC (1990). Distribution of somatostatin mRNA in the rat nervous system as visualized by a novel non-radioactive in situ hybridization histochemistry procedure. Neuroscience. 38: 223–244. [DOI] [PubMed] [Google Scholar]
- Kono J, Konno K, Talukder AH, et al. (2017). Distribution of corticotropin-releasing factor neurons in the mouse brain: a study using corticotropin-releasing factor-modified yellow fluorescent protein knock-in mouse. Brain Struct Funct. 222: 1705–1732. [DOI] [PubMed] [Google Scholar]
- Kovner R, Fox AS, French DA, et al. (2019). Somatostatin gene and protein expression in the non-human primate central extended amygdala. Neuroscience. 400: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozicz T & Arimura A (2001). Axon terminals containing CGRP-immunoreactivity form synapses with CRF- and met-enkephalin-immunopositive neurons in the laterodorsal division of the bed nucleus of the stria terminalis in the rat. Brain Res. 893: 11–20. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S & Arimura A (1997). Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis. Brain Res. 767: 109–119. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S & Arimura A (1998). Immunocytochemical evidence for PACAP and VIP interaction with met-enkephalin and CRF containing neurons in the bed nucleus of the stria terminalis. Ann NY Acad Sci 11: 523–528. [DOI] [PubMed] [Google Scholar]
- Kresse A, Jaobowitz DM & Skofitsch G (1995). Detailed mapping of CGRP mRNA expression in the rat central nervous sysem: comparison with previous immunocytochemical findings. Brain Res Bull. 36: 261–274. [DOI] [PubMed] [Google Scholar]
- Land BBB, M. R., Lemos JC, Xu M, et al. (2008). The dysphoric component of stress is encoded by activation of the dynorphin Kappa-opioid system. J Neurosci. 28: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larriva-Sahd J (2006). Histological and cytological study of the bed nucleus of the stria terminalis in adult rat. II Oval nucleus: extrinsic inputs, cell types, neuropil and neuronal modules. J Comp Neurol. 497: 772–807. [DOI] [PubMed] [Google Scholar]
- Lebow M, Neufeld-Cohen A, Kuperman Y, et al. (2012). Susceptibility to PTSD-like behavior is mediated by corticotropin-releasing factor receptor type 2 levels in the bed nucleus of the stria terminalis. J Neurosci. 32: 6906–6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebow MA & Chen A (2016). Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as a key to psychiatric disorders. Mol Psychiatry 21: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitermann RJ, Rostkowski AB & Urban JH (2016). Neuropeptide Y input t othe rat basolateral amygdala complex and modulation by conditioned fear. J Comp Neurol. 524: 2418–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesur A, Gaspar P, Alvarez C, et al. (1989). Chemoanatomic compartments in the human bed nucleus of the stria terminalis. Neuroscience. 32: 181–194. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, et al. (2001). Identification of urocortin IIIm an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 98: 7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak KR, Roman CW, Braas KM, et al. (2014). Regulation of bed nucleus of the stria terminalis PACAP expression by stress and corticosterone. J Mol Neurosci. 54: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, et al. (2002). Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 22: 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC & Sibille E (2015). Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 20: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovich M & Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disrupion block stress-induced behavioral responses. J Neurosci. 23: 5674–5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW & Maren S (2019). Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Frontiers Behav Neurosci 13: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Mei L, Vizzard MA, et al. (2017). Parabrachial pituitary adenylate cyclase-activating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol Psychiatry 81: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missig G, Roman CW, Vizzard MA, et al. (2014). Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology 86: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RR, et al. (1990). Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38). Biochem Biophys Res Commun 170: 643–648. [DOI] [PubMed] [Google Scholar]
- Moaddab M & Dabrowska J (2017). Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology 121: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Saper CB & Gray TS (1989). Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 283: 315–332. [DOI] [PubMed] [Google Scholar]
- Normandeau CP, Ventura-Silva AP, Hawken ER, et al. (2018). A key role for neurotensin in chronic-stress induced anxiety-like behavior in rats. Neuropsychopharmacology 43: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski NL (1998). Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology 23: 989–1004. [DOI] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, et al. (2001). Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 92: 78–84. [DOI] [PubMed] [Google Scholar]
- Palkovitz M, Somogyvari-Vigh A & Arimura A (1995). Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res. 699: 116–120. [DOI] [PubMed] [Google Scholar]
- Palkovitz M, Tapia-Arancibia L, Kordon C, et al. (1982). Somatostatin connections between the hypothalamus and the limbic system of the rat brain. Brain Res. 250: 223–228. [DOI] [PubMed] [Google Scholar]
- Petit JM, Luppi P, H., Peyron C, et al. (1995). VIP-like immunoreactivity projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosi Lett. 193: 77–80. [DOI] [PubMed] [Google Scholar]
- Pomrenze MB, Millan EZ, Hopf FW, et al. (2015). A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front Neurosci. 9: 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin J-F, Arbour D, Laforest S, et al. (2009). Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuro-Psychopharmacol Biol Psychiatry 33: 1356–1365. [DOI] [PubMed] [Google Scholar]
- Radley JJ & Sawchenko PE (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 31(26):: 9683–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Schuller A, Morgan M, et al. (2001). Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 98: 1958–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CW, Lezak KR, Hartsock MJ, et al. (2014). PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47: 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said SI & Mutt V (1972). Isolation from porcine-intestinal wall of a vasocative octacosapeptide related to secretin and to glucagon. Eur J Biochem 28: 199–204. [DOI] [PubMed] [Google Scholar]
- Said SI & Mutt V (1974). Structure of the porcine vasoactive intestinal octacosapeptide. The amino acid sequence. Use of kallikrein in its determination. Eur J Biochem 42: 581–589. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Magari S, Shibasaki T, et al. (1989). Colocalization of corticotropin-releasing factor- and enkephalin-like immunoreactivities in nerve cells of the rat hypothalamus and adjacent areas. Brain Res. 487: 357–362. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T & Lederis K (1986). Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 382: 213–238. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T & Lederis K (1987). Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 260: 256–298. [DOI] [PubMed] [Google Scholar]
- Segovia S & Guillamón A (1993). Sexual dimorphism in the vomeronasal pathway and sex differences in reproductive behaviors. Brain Res Brain Res Rev. 18: 51–74. [DOI] [PubMed] [Google Scholar]
- Shackman AJ & Fox AS (2016). Contribution of the central extended amygdala to fear and anxiety. J Neurosci. 36: 8050–8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL & McRory JE (2000). The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 21: 619–670. [DOI] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, et al. (1989). Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience. 30: 377–383. [DOI] [PubMed] [Google Scholar]
- Simerly RB (2002). Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 25: 507–536. [DOI] [PubMed] [Google Scholar]
- Sink KS, Chung A, Ressler KJ, et al. (2013a). Anxiogenic effects of CGRP within the BNST may be mediated by CRF acting at BNST CRFR1 receptors. Behav Brain Res. 243: 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Davis M & Walker DL (2013b). CGRP antagonist infused into the bed nucleus of the stria terminalis impairs the acquisition and expression of context but not discretely cued fear. Learning & Memory 20: 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Yang Y, et al. (2011). Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation of anxiety-related structures. J Neurosci. 31: 1802–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (1985). Vasopressin- and neurophysin-immunoreactive neurons in the septal region, medial amygdala and locus coeruleus in colchicine-treated rats. Neuroscience. 15: 347–358. [DOI] [PubMed] [Google Scholar]
- Spiess J, Rivier J, Rivier C, et al. (1981). Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 78: 6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N & Ovtscharoff W ( 2000). Sexual dimorphism of the bed nucleus of the stria terminalis and the amygdala. Adv Anat Embryol Cell Biol. 158: 1–78. [DOI] [PubMed] [Google Scholar]
- Stroth N & Eiden LE (2010). Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 165: 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Liu Y, Aguilera G, et al. (2011). Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. J Neuroendocrinol. 23: 944–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, et al. (2010). The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschenett A, Singewald N, Carli M, et al. (2003). Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 18: 143–148. [DOI] [PubMed] [Google Scholar]
- Uchida K, Otsuka H, Morishita M, et al. (2019). Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ. 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR & Synder SH (1979). Neurotensin: a neuronal pathway projecting from the amygdala through the stria terminalis. Brain Res. 161: 522–526. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, et al. (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213: 1394–1397. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, et al. (2009). Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 61: 283–357. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Skihara S, Subramanian S, et al. (2004). Urocortin III, a brain neuropeptide of corticotropin-releasing homrone family: modulation by stress and attenuation of some anxiety-like behaviors. J Neuroendocrinol. 16: 441–422. [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW & Bouton ME (2006). Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 120: 324–336. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C, Pich EM, Koob GF, et al. (1993). Modulation of anxiety and neuropeptide Y-Y1 receptors by antisense oligodeoxynucleotides. Science 259: 528–531. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA & Davis M (2009). Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 33: 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Mai JK, Lanta L, et al. (1991). Differential distribution of immunohistochemical markers in the bed nucleus of the stria terminalis in the human brain. J Chemical Neuroanatomy 4: 281–298. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burghardt PR, Lugo JNJ, et al. (2003). Effect of amygdalar opioids on the anxiolytic properties of ethanol. Ann NY Acad Sci 985: 472–475. [DOI] [PubMed] [Google Scholar]
- Wittmann G, Fuzesi T, Liposits Z, et al. (2009). Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. J Comp Neurol. 517: 825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J & Veinante P (2019). Cell-type specific parallel circuits in the bed nucleusof the stria terminalis and the central nucleus of the amygdala of the mouse. Brain Struct Funct. 224: 1067–1095. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kiyama H, Kimura T, et al. (1993). Localization of oxytocin receptor messenger ribonucleic acid in the rat brain. Endocrinology 133: 1239–1246. [DOI] [PubMed] [Google Scholar]


