Abstract
Study Objectives
Sleep quantity and continuity vary across the lifespan. Actigraphy is a reliable and widely used behavioral measure of sleep in research and personal health monitoring. This meta-analysis provides a novel examination of whether age (in years) is associated with actigraphy-assessed sleep across the lifespan.
Methods
A systematic search of PubMed, Embase.com, Cochrane CENTRAL, and PsycINFO using “actigraphy” and “sleep” terms provided 7079 titles/abstracts; studies of individuals with known psychiatric or medical comorbidities were excluded. Ninety-one articles (N = 23 365) provided data for six meta-analyses examining sleep duration (k = 89), sleep efficiency (k = 58), bedtime (k = 19) and waketime (k = 9) for individuals ages 6–21, and bedtime (k = 7) and waketime (k = 7) for individuals ages 22 and older.
Results
At older ages, sleep duration was shorter (r = −0.12) and sleep efficiency was lower (r = −0.05). Older age was associated with later bedtime (r = 0.37) and wake-up time (r = 0.24) from ages 6–21, whereas older age was associated with earlier bedtime (r = −0.66) and wake-up time (r = −0.59) for ages 22 and above. The strength of these associations was modified by study continent, but not by any other moderator.
Conclusions
Age was negatively associated with actigraphy-assessed sleep duration and efficiency, but the effects were small in magnitude. On the other hand, large associations were observed between age and sleep timing, despite a smaller literature and the absence of analyzable data for ages 30–60. Changes in sleep timing, rather than changes in sleep duration or continuity, may better characterize the effects of age on human sleep.
Keywords: meta-analysis, age, sleep timing, sleep duration, sleep efficiency
Statement of Significance.
Sleep changes across the lifespan. The extent to which this is true for multiple sleep characteristics assessed by actigraphy, collected in the home environment across multiple nights, has not been examined using meta-analysis. Additionally, key demographic moderators of the age-sleep association have not been explored. The current meta-analysis addresses these limitations using 91 articles and 23 365 participants with no known comorbidities. We report small effects of age (in years) on sleep duration and efficiency, but large effects of age for sleep timing. Changes in sleep timing, rather than changes in sleep duration or continuity, may better characterize the effects of age on human sleep.
Introduction
Multiple dimensions of optimal sleep, such as adequate duration, continuity, timing, and regularity, are associated with health and well-being [1]. Compelling evidence from previous meta-analyses demonstrates differences in these sleep characteristics as a function of age (in years). In child and adolescent samples, self-reported sleep duration was shorter and bedtimes were later at older ages [2]. In adult samples, polysomnography-assessed sleep duration was shorter, wake after sleep onset was higher, and sleep latency was longer at older ages [3–5]. Assessing sleep using actigraphy has several advantages including reliability [6], data collection in the participant’s habitual environment (rather than the laboratory), and widespread use as a behavioral measure of sleep in research and personal health monitoring [7]. To our knowledge, only two meta-analyses have examined age-related differences in actigraphy-assessed sleep. One meta-analysis of individuals aged 0–18 indicated that actigraphy-assessed sleep duration was shorter and bedtimes were later at older ages, but wake-up time and sleep efficiency were not associated with age [8]. Another meta-analysis reported that among individuals over the age of 5, actigraphy-assessed sleep duration was negatively associated with age, but other actigraphy measures were not examined [5]. Thus, the current study sought to examine age-related differences in actigraphy-assessed sleep duration, continuity, timing, and regularity across the lifespan.
Identifying individuals or groups with the strongest age-sleep associations could inform future data analytic strategies (e.g. stratifying analyses) and may identify relevant demographic characteristics. Previous review and meta-analytic evidence have demonstrated that there are stronger associations among age and sleep for: studies that screened for and excluded participants with mental health disorders, sleep disorders, and physical illnesses [5]; males when objective sleep measures are used, females when subjective sleep is assessed [9]; and Asian adolescents compared to North American adolescents [2]. Though there are replicable racial/ethnic [10] differences in sleep, previous meta-analyses have had insufficient data to test whether this is a moderator of the age-sleep association [3]. Moreover, the number of nights of data collection [11] and the actigraphy device type [12] can affect study results, but have yet to be tested as moderators of the age-sleep association.
In the current study, we evaluated the strength of the meta-analytic associations between age and actigraphy-assessed sleep duration, efficiency, timing, and regularity. Age is assessed in years throughout this study. We included participants who were ages 6 and older, who were not specifically recruited to the study for a medical, psychiatric, or sleep disorder comorbidity, and whose data were collected before any intervention or manipulation in their habitual environment. Any study design (e.g. cross-sectional, longitudinal) that met these aforementioned criteria was included. We hypothesized that sleep duration and sleep efficiency would be negatively associated with age, that sleep timing would be positively associated with age (i.e. delayed) across ages 6–21, and negatively associated with age (i.e. advanced) thereafter [13]. We also hypothesized that regularity of sleep timing would be positively associated with age (i.e. greater regularity at older ages [14]). Finally, we evaluated the effects of the following moderators on age-sleep associations: whether studies screened for and excluded participants with comorbid mental health, medical, and sleep disorders, sex, race/ethnicity, continent of the study, number of nights of data collection, and actigraphy device type.
Methods
This meta-analysis was pre-registered on the international prospective register of systematic reviews, PROSPERO (CRD42019137424). This meta-analysis was written per the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [15].
Study retrieval strategy
We searched PubMed, EMBASE, and Cochrane CENTRAL, according to recommendations from the Cochrane handbook [16], as well as PsychINFO. We did not restrict the dates of publication or the language. Search terms (Supplementary Table S1) were developed and tested by a medical librarian with expertise in systematic reviews and meta-analyses (JF). The search strategy was completed on June 5, 2019.
Inclusion and exclusion criteria
Inclusion criteria were studies that: recruited participants ages 6 and older, collected nocturnal sleep characteristics in the participants’ habitual environment, and reported on the association between age and at least one actigraphy-assessed sleep characteristic: duration, efficiency, timing, or regularity. Sleep duration was operationalized as the number of minutes a participant was asleep. Sleep efficiency was operationalized as sleep duration divided by time in bed and multiplied by 100 to render percent values. Sleep timing was measured by bedtime, wake-up time, or sleep midpoint. Bedtime was operationalized as the time that the participant pressed an event marker on the actigraph to indicate bedtime or the actigraphy-defined sleep onset. Wake-up time was operationalized as the time that the participant pressed an event marker on the actigraph to indicate wake-up time or the actigraphy-defined sleep offset. Sleep midpoint was operationalized as the middle of the sleep period, halfway between sleep onset and sleep offset. Sleep regularity was operationalized as the standard deviation of sleep midpoint, standard deviation of sleep duration, standard deviation of bedtime, and/or standard deviation of wake-up time.
Exclusion criteria were studies that were not peer-reviewed, studies of non-human animals, conference abstracts or summaries, unpublished data, narrative reviews, systematic reviews, meta-analyses, commentaries, and study protocol descriptions. We also excluded articles that only recruited individuals with a specifically assessed comorbidity (mental health disorder, medical condition, or sleep disorder) but did not have a control group. Our rationale for this exclusion criterion is that, while specific comorbidities may moderate age-sleep associations, a control group is necessary to test such moderation. We included studies of individuals with a specifically assessed comorbidity if they included a control group. Studies of shift workers without a comparison group of day workers were also excluded, as shift work systematically alters sleep-wake patterns. We excluded articles that manipulated sleep without data for baseline, assessed sleep only in a novel environment (e.g. in the laboratory), and intervention studies without data for baseline. Studies of daytime naps without nocturnal sleep were excluded. Finally, we excluded studies of children from birth through age 5, given the rapid and complex changes observed in sleep-wake cycles across this age range [17].
Consistent with language traditionally used in meta-analyses [18], we use the term “effect size” for the statistical associations extracted from the articles, as well as for the overall effects observed in our meta-analyses. However, it is worth noting that there may be residual confounding present at the study and meta-analytic level.
Study selection
Records were processed using DistillerSR (Evidence Partners, Ottawa, Canada). Data processing included three stages (flow chart in Figure 1). First, the title and abstract of 7,079 records were screened for eligibility by two independent raters (MAE screened all title/abstracts; AJ, RM, or SS served as second rater, each rating one-third of the total abstracts; weighted Cohen’s kappa = 94%). Records were excluded at this stage if the study design or participants were ineligible, or if at least one actigraphy-assessed sleep measure of interest was not included. Articles were included if both raters voted to include. If there was a discrepancy between raters (n = 424 records), a third independent rater (AJ, RM, or SS) evaluated the record, and the title/abstract was included if two out of three raters voted for inclusion. A total of 5410 records were excluded at the screening stage.
Figure 1.
PRISMA Flow Diagram.
Second, we located the full-text articles for records that were deemed eligible at stage one (n = 1,669). Full-text articles were deemed ineligible at the second stage if they did not report on the association between age and at least one actigraphy-assessed measure of sleep; if the effect size was already captured from the same dataset in another article with a larger sample size; or if the sample represented hunter-gatherer tribes (excluded post-hoc due to a small number of studies and lack of generalizability). We emailed authors that reported that the age-sleep association was significant or nonsignificant to request the effect size (n = 21), and excluded studies if the authors did not respond to our request (n = 8). A total of 1578 articles were excluded at this stage (see Figure 1 for the breakdown of exclusions).
Third, data extraction was entered into forms created by MAE in the software DistillerSR. Data extraction was conducted by two independent raters (AJ, LC, NK, RM, or SS; weighted Cohen’s kappa = 73%) from eligible studies for the meta-analysis (n = 91). A third independent rater (MAE) evaluated and resolved all discrepancies between the two raters.
For the primary aim of examining the association of age and sleep, we extracted sample size, effect size of associations between age and sleep parameters, mean and standard deviation of the age of participants, and mean and standard deviation of the sleep variables. We extracted data on the following moderators: continent (North America, Asia, or Europe), sex (male or female), race/ethnicity (Black, Hispanic, Asian, or Non-Caucasian), actigraphy device type (Philips, AMI, ActiGraph, Other), number of nights of actigraphy recording (e.g. seven nights), and whether the study excluded participants for mental health disorders, medical conditions, and/or sleep disorders.
Race/ethnicity analyses were only conducted for studies in the United States. Specifically, we evaluated the percent of the sample that was African American, Hispanic, Asian, or non-Caucasian. Meta-analysis accounts for sample size and precision of estimates with weighting [18], so we included both community and convenience samples to cast a wider net of potential studies. However, it is plausible that estimates may vary as a function of study design, so we compared effect sizes for representative samples vs. convenience samples.
We had insufficient case-control studies to evaluate age-sleep associations among control samples compared to participants with a comorbidity (1 study of individuals with dementia, 1 study of individuals with diabetes). We therefore only included the control groups from these studies. Instead, we tested three dichotomous (yes/no) moderators of whether the study specifically screened for (e.g. clinical interview) and excluded participants for mental health disorders, medical conditions, or sleep disorders. A previous meta-analysis of age and polysomnography-assessed sleep evaluated similar moderators and reported larger effect sizes for studies that screened for and excluded participants with mental health disorders, sleep disorders, and physical illnesses [5].
Analytic strategy
Meta-analysis, which evaluates aggregated data from published studies, was selected due to its systematic, quantifiable, efficient, and reproducible properties. This approach allowed aggregation of data from 23 365 individuals between 6 and 92 years of age from studies conducted in multiple laboratories. Descriptive statistics for the overall meta-analysis were calculated, including the average values for each sleep characteristic across each decade of life. Scatter plots were created to visualize the association between age and sleep characteristics. R version 3.4.3 [19] was used for descriptive statistics and the package ggplot 2 was used for scatter plots [20].
We chose a priori to conduct a meta-analysis if five or more effect sizes were available; while a meta-analysis can be conducted with a minimum of two studies, the median number of studies included in meta-analyses on Cochrane Database of Systematic Reviews Central is six [18]. Thus, we had insufficient studies to conduct meta-analyses for the association of age and any operationalization of sleep regularity: standard deviation of sleep duration (n = 4), standard deviation of sleep midpoint (n = 0), standard deviation of bedtime (n = 1), or standard deviation of wake-up time (n = 0). Other definitions of sleep regularity were observed, such as the coefficient of variation for sleep duration (n = 1), and the coefficient of variation combining bedtime and wake-up time (n = 1), but these definitions also had insufficient effect sizes. We also did not have sufficient studies to conduct a meta-analysis for age and sleep midpoint (n = 3), one operationalization of sleep timing. We evaluated post-hoc power analyses for all of the meta-analyses that we conducted [18].
Data were analyzed using Comprehensive Meta-Analysis (CMA) software, version 3.3.07 [21]. All effect sizes were initially converted to Pearson correlation coefficients (r), and transformed into Fisher’s z (to separate the effect size from its variance).
Random effects meta-analyses were then used to evaluate the strength of the association between age and sleep characteristics with sufficient data—sleep duration, sleep efficiency, bedtime, and wake-up time. The effect sizes were transformed back to Pearson correlations for ease of interpretation, and forest plots were created to visualize the associations across studies. The 95% confidence intervals surrounding Pearson’s r in forest plots may be non-symmetrical because they are based on the r-to-z then z-to-r transformation [18]. Heterogeneity across studies was assessed using both T2, which estimates the variance of the true effect sizes (between-studies variance), and I2, which can be interpreted as the proportion of observed variance that reflects true differences in effect size [18].
We evaluated the risk of publication bias by removal of one study, funnel plots, and Duval and Tweedie’s trim and fill method [22]. Removal of one study systematically removes each individual study from the meta-analysis to ensure that one effect size is not unduly affecting the meta-analytic estimate. Funnel plots include the studies’ effect size on the x-axis and the standard error of the study on the y-axis; if no publication bias is present, studies are symmetrically distributed around the meta-analytic effect size [21]. Duval and Tweedie’s trim and fill method imputes unpublished studies to the left and the right of the meta-analytic effect size, and evaluates the extent to which the imputed studies change the meta-analytic effect size [22].
Moderation analyses were conducted to probe heterogeneity in effect sizes. Random effects models with separate estimates of T2 for each subgroup were used. We restricted moderator analyses to those with more than ten effect sizes available per subgroup (per the guidelines of the Cochrane handbook [16]). For categorical moderators (continent, exclusion of comorbidities, actigraphy device type), we used the Z-test, which is comparable to the t-test used in an empirical study to test whether a regression coefficient is significantly different from zero [18]. For continuous moderators (sex, race, number of nights of actigraphy, and mean age of participants), we used meta-regression. This technique is similar to linear regression, but the covariates are at the level of the study and the dependent variable is the effect size [18]. Given the significant number of categorical and continuous moderators (n = 36), we applied Bonferroni corrections to adjust alpha to <.001, and only interpret moderators that were statistically significant at this level and/or that had a medium effect size (R2 ≥ 13% [23]). All of these moderation analyses were pre-registered.
We then evaluated the risk of bias (ROB) at the level of individual studies, specifically, sample bias (exclusion of mental health disorders, exclusion of medical conditions, exclusion of sleep disorders) and bias in outcome measurement (heterogeneity in actigraphy device and number of nights of actigraphy). We reported the number of studies that had ROB in these categories and evaluated whether these factors modified meta-analytic associations.
Finally, the quality of the body of evidence was evaluated using a modified version of the Community Preventive Services methods [24]. We chose not to use GRADE methodology as an assessment tool, as GRADE considers RCTs to be the gold-standard and meta-analyses with observational studies are automatically demoted to the category of “low” quality evidence. Our research question could not be answered by an RCT, as it is impossible to randomly assign participants to ages. Table 1 shows the details of how this methodology was applied to the current study.
Table 1.
Guidelines for evaluating the quality of the evidence—Based on a modification of the Community Preventive Services methods
| Domains | Operationalization |
|---|---|
| Execution | |
| Was the study population well-described? | Included the mean and standard deviation of age |
| Did the authors specify their screening criteria? | Specified how participants were included and/or excluded |
| Were the outcome measures reliable? | At least 7 days of actigraphy data |
| Was attrition low? | Less than 10% of missingness |
| Good, fair, or limited execution | Good: at least 70% of studies met criteria in 4 categories |
| Fair: at least 70% of studies met criteria in 2–3 categories | |
| Limited: at least 70% of studies met criteria 0–1 | |
| Greater or lesser design suitability | Greater: at least 70% of studies were longitudinal |
| Sufficient number of studies | “Yes” indicates > 5 studies for all meta-analyses (a priori rule) |
| Consistent or inconsistent effect sizes | Consistent: at least 70% of studies fall into the negative (effect size less than -0.10) or positive (effect size greater than 0.10) association category |
| Small, sufficient, or large effect sizes | Small: r = 0–0.29 |
| Sufficient: r = 0.3–0.49 | |
| Large: r ≥ 0.5 | |
| Strong, sufficient, or insufficient quality of evidence | Strong: execution was good or fair, greater design suitability, 5 studies, had a consistent association, and had a sufficient or large effect size |
| Sufficient: execution was good or fair, design suitability was of greater or lesser suitability, ≥5 studies, had a consistent association, and had a sufficient or large effect size |
Results
Study characteristics
Ninety-one articles [25–116] met inclusion criteria. These studies contained 189 effect sizes in a total of 23 365 participants. We conducted six meta-analyses, which included 89 effect sizes for sleep duration, 58 effect sizes for sleep efficiency, 19 effect sizes for bedtime ages 6–21, 7 effect sizes for bedtime ages 22 and older, 9 effect sizes for wake-up time ages 6–21, and 7 effect sizes for wake-up time ages 22 and older.
Descriptive statistics for each meta-analysis are presented in Table 2 (sample size, mean age, percent female, and number of nights of actigraphy data); we do not report average percent of non-Caucasian individuals included in studies, as less than half of the studies reported race/ethnicity composition. Supplementary Table S2 shows each individual study’s characteristics, including the study name, country, sample size, mean age and standard deviation of age, and which sleep characteristic(s) the study contributed. Three studies evaluated within-person change in sleep, four studies compared sleep between distinct age groups (e.g. 23-year-olds compared to 66-year-olds), and 84 studies examined between-person associations. Overall, 63% of the studies were conducted in North America, 19.3% in Europe, 5.6% in Asia, 5.6% in South Africa, 4.5% in the Middle East, and 2.2% in Australia.
Table 2.
Descriptive statistics for each meta-analysis of age and actigraphy-assessed sleep
| Sleep duration | Sleep efficiency | Bedtime, ages 6–21 | Bedtime, ages 22 and older | Wake-up time, ages 6–21 | Wake-up time, ages 22 and older | |
|---|---|---|---|---|---|---|
| M (range) | M (range) | M (range) | M (range) | M (range) | M (range) | |
| Sample size | 247.0 (12–3055) | 202.2 (14–3055) | 290.9 (21–1231) | 323.4 (11–3055) | 186.2 (21–417) | 503.3 (15–3055) |
| Mean age | 31.8 (6.5–91.9) | 36.4 (6.5–91.9) | 11.2 (7.6–17) | 44.6 (22.5–91.9) | 10.9 (9.23–15.5) | 38.7 (22.5–91.9) |
| % Female | 59.8 (0–100) | 60.9 (0–100) | 49.75 (28.6–77) | 42.7 (0–68.2) | 50.5 (28.6–68.2) | 35.9 (0–63.9) |
| Number of nights | 6.6 (1–29) | 6.4 (2–20) | 5.8 (2–7.1) | 7.2 (5–13.6) | 6.0 (2–7) | 6.6 (5.2–7) |
M indicates mean. Number of nights indicates number of nights of actigraphy data collected.
Descriptive associations of age and sleep characteristics
We created a table with the average values for each sleep characteristic by decade of life, using mean age and mean sleep characteristic for each study included in the meta-analyses (Table 3). Table 3 shows the limited data available for sleep efficiency between ages 30–40 and 50–60, and the absence of analyzable data for bedtime and wake-up time between ages 30–60 and 80–90.
Table 3.
Averages of sleep characteristics across each assessed decade of the lifespan
| 6–9.99 | 10–19.99 | 20–29.99 | 30–39.99 | 40–49.99 | 50–59.99 | 60–69.99 | 70–70.99 | 80–89.99 | 90–99.99 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sleep duration, hour | 8.63 | 7.18 | 7.20 | 6.42 | 6.78 | 6.83 | 6.28 | 6.80 | 6.35 | 8.97 |
| Number of studies | 16 | 25 | 11 | 7 | 8 | 7 | 5 | 5 | 5 | 1 |
| Sleep efficiency, % | 84.10 | 87.20 | 87.79 | 77.88 | 86.77 | 85.00 | 74.52 | 85.36 | 86.86 | 89.70 |
| Number of studies | 8 | 12 | 9 | 3 | 5 | 2 | 5 | 8 | 5 | 1 |
| Bedtime, hh:mm | 21:34 | 22:35 | 0:23 | 23:03 | 23:24 | 21:34 | ||||
| Number of studies | 9 | 9 | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 1 |
| Wake-up time, hh:mm | 7:05 | 7:10 | 8:07 | 6:58 | 6:55 | |||||
| Number of studies | 5 | 3 | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 1 |
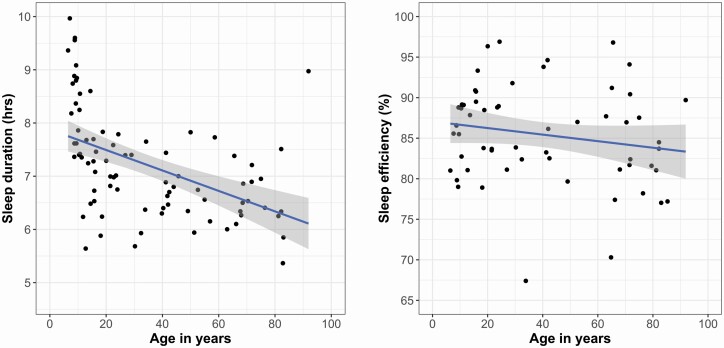
We characterized the associations between mean age and mean sleep duration and mean sleep efficiency using scatter plots, where each point represents a study (Figure 2). It is important to interpret these figures with caution, because they are descriptive and not the results of the meta-analyses. The association of age and sleep duration was better characterized by a quadratic than a linear regression (R2 = 39.5% and 23.2%, respectively, p < .001), with an inflection point at age 50. We conducted an exploratory moderation analysis to further probe this observation. Consistent with our hypotheses, the descriptive scatterplots suggest that the association of age and sleep efficiency is linear (R2 = 5.1%). We did not create scatterplots for sleep timing, given the absence of available data for ages 30–60 and the limited data for other age ranges, which makes it impossible to accurately assess whether associations are linear or quadratic.
Figure 2.
Scatterplots of the associations between age and actigraphy-assessed sleep duration and sleep efficiency.
Because we could not determine a precise age at which the relationship between age and sleep timing changes from delayed to advanced due to data availability, and because we hypothesize based on prior literature that the association is positive during adolescence and negative in adulthood, we conducted meta-analyses separately for individuals ages 6–21 and 22 and older.
Table 4 summarizes the results of the six meta-analyses, which are presented in detail below. Using the sample sizes, effect sizes, and variance for post-hoc power analyses, we found that all six meta-analyses achieved 100% power [18].
Table 4.
Results of meta-analyses of the association of age and actigraphy-assessed sleep characteristics
| k | Participants | Meta-analytic effect size [95% CI], p-value | |
|---|---|---|---|
| Overall | 189 | 23 365 | |
| Sleep duration | 89 | 21 242 | r = −0.12 [−0.16, −0.08], p < .001 |
| Efficiency | 58 | 11 525 | r = −0.05 [−0.10, −0.001], p = .05 |
| Bedtime, ages 6–21 | 19 | 3055 | r = 0.37 [0.23, 0.49], p < .001 |
| Bedtime, ages 22 and older | 7 | 3681 | r = −0.66 [−0.87, −0.24], p = .004 |
| Wake-up time, ages 6–21 | 9 | 748 | r = 0.24 [0.05, 0.41], p = .01 |
| Wake-up time, ages 22 and older | 7 | 3625 | r = −0.59 [−0.83, −0.15], p = .01 |
k indicates number of effect sizes.
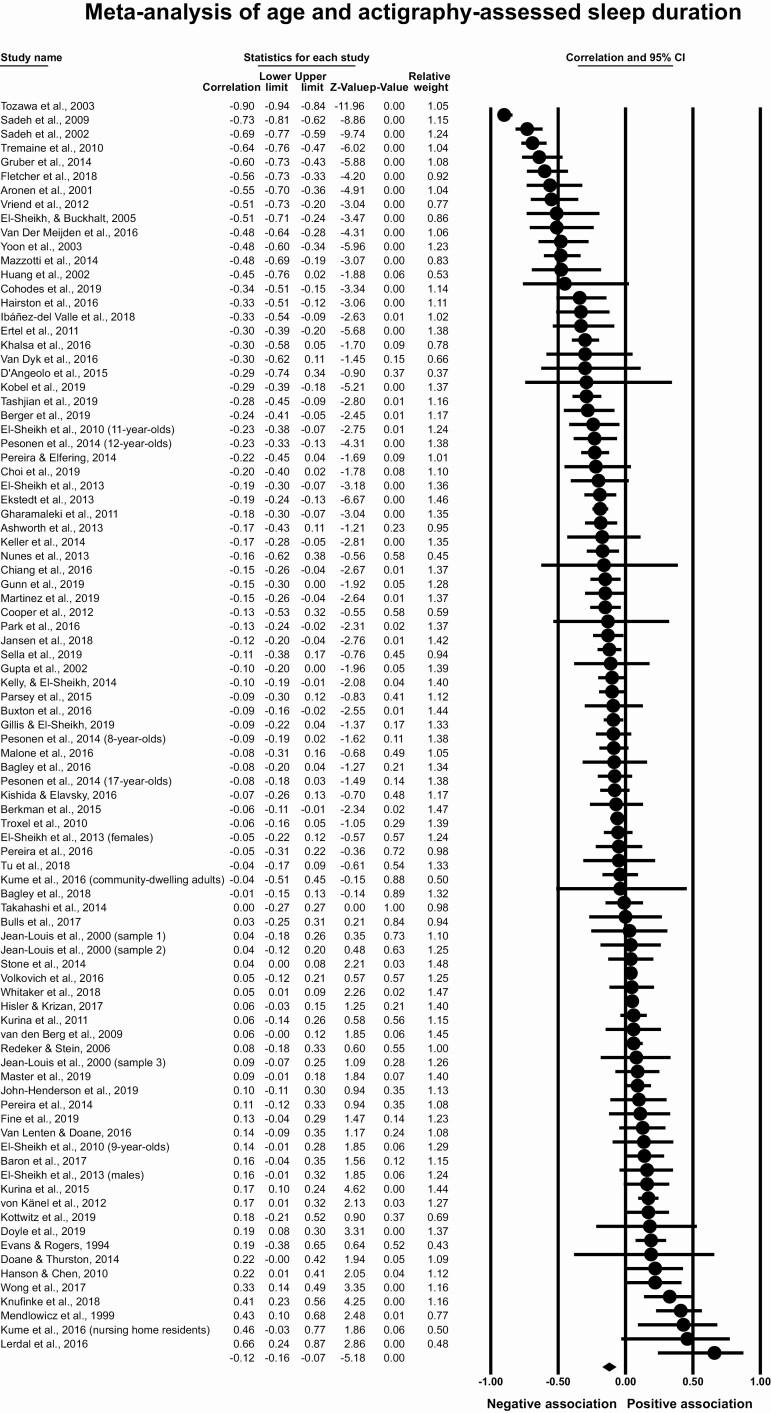
Meta-analysis of age and actigraphy-assessed sleep duration
This meta-analysis included 78 articles with 89 effect sizes and 21 242 participants. Although the scatterplot for age and sleep duration suggested a quadratic association (Figure 2), in this section, we evaluated the linear association based on our a priori hypothesis. In our section on “Moderators of meta-analyses,” we probe the quadratic association. Figure 3 shows a forest plot of effect sizes, confidence intervals, and the relative weights of each study. The overall effect size was r = −0.12, 95% CI [−0.16, −0.08], p < .001, which indicates a small magnitude correlation between older age and lower actigraphy-assessed sleep duration. The T2 of 0.05 suggests that the estimated amount of between-studies variation in the effect size is small. There was moderate heterogeneity across studies, with the I2 indicating that 48.5% of the variation in effect size was due to true differences in effect size and not sampling error. Removal of any one published effect size did not change the meta-analytic association (range of estimates r = −0.11 to −0.13). Based on examination of the funnel plot (Supplementary Figure S1) and Duval and Tweedie’s trim and fill method (imputed point estimate of r = −0.18, 95% CI [−0.23, −0.13]), it seems implausible that unpublished studies substantially affected the meta-analytic estimate.
Figure 3.
Meta-analysis of age and actigraphy-assessed sleep duration.
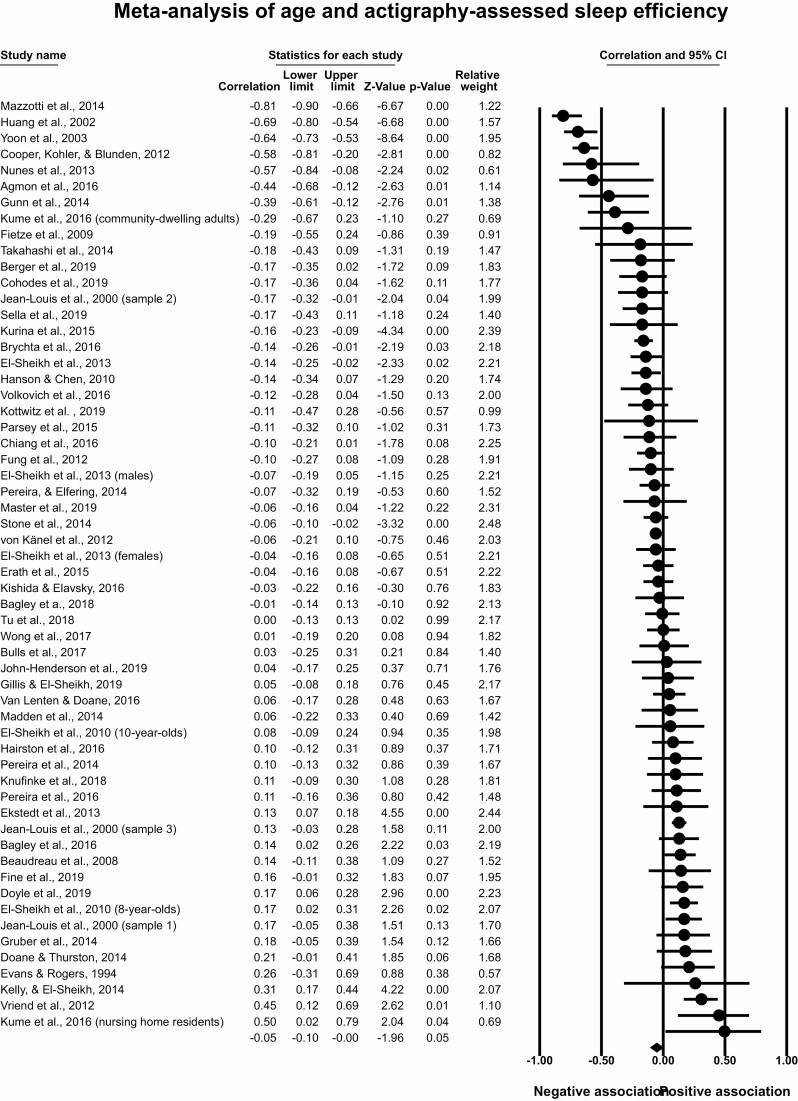
Meta-analysis of age and actigraphy-assessed sleep efficiency
This meta-analysis included 53 articles with 58 effect sizes and 11 525 participants. The overall effect size was r = −0.05, 95% CI [−0.10, −0.001], p = .05, T2 = 0.02, I2 = 42.24% (Figure 4), indicating a small correlation between older age and lower sleep efficiency. Removal of any one of 31 published effect sizes included in the meta-analysis rendered the overall association nonsignificant (p > .05), with a range of the point estimate after removal of individual studies from −0.04 to −0.06. These findings indicate a small and inconsistent correlation between older age and lower actigraphy-assessed sleep efficiency. To assess publication bias, we evaluated the funnel plot (Supplementary Figure S2) with the trim and fill method and found that the inclusion of seven imputed studies did not substantially change the overall effect size (r = −0.09, 95% CI [−0.13, −0.04]).
Figure 4.
Meta-analysis of age and actigraphy-assessed sleep efficiency.
Meta-analysis of age and actigraphy-assessed bedtime
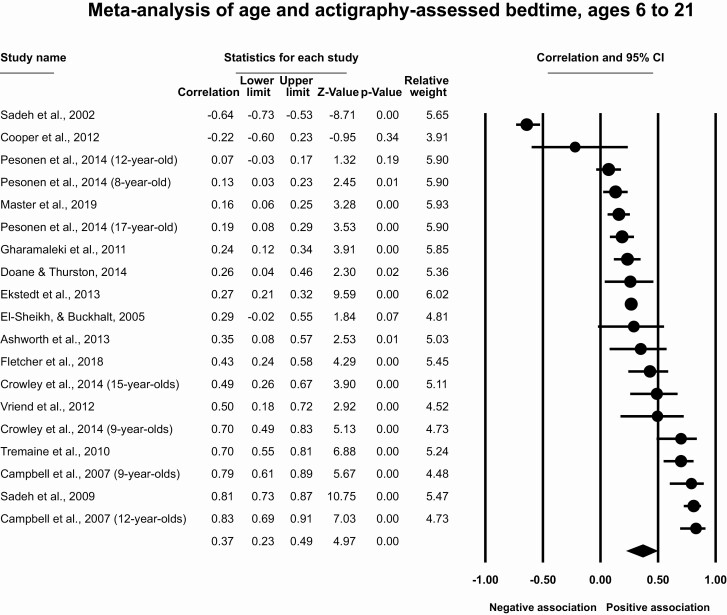
Results for participants ages 6–21
This meta-analysis included 15 articles with 19 effect sizes and 3055 participants. The overall effect size was r = 0.37 (95% CI [0.23, 0.49], p < .001, T2 = 0.11, I2 = 48.7%) indicating a moderate correlation between older age and later actigraphy-assessed bedtimes (Figure 5). Removal of individual studies does not substantially change the estimated meta-analytic effect size (r = 0.33 to 0.42). The funnel plot (Supplementary Figure S3) and the trim and fill method (which did not impute any studies) suggest no impact of publication bias.
Figure 5.
Meta-analysis of age and actigraphy-assessed bedtime, ages 6–24.
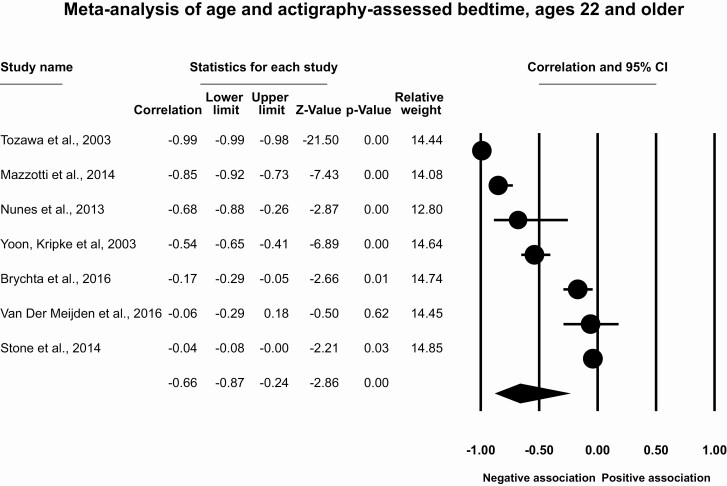
Results for participants ages 22 and older
This meta-analysis included seven articles with seven effect sizes and 3681 participants. The overall effect size for the association of age and bedtime for participants aged 22 and older was r = −0.66 (95% CI [−0.87, −0.24], p = .004, T2 = 0.34, I2 = 38.3%, Figure 6). This indicates a large correlation between older age and earlier actigraphy-assessed bedtime. Removal of individual studies indicates that the strength of the effect size may be moderate to large (range of r = −0.42 to −0.73), but in all cases remained statistically significant. There is little evidence for publication bias (Supplementary Figure S4), with only one imputed study using the trim and fill method (r = −0.73, 95% CI [−0.94, −0.12]).
Figure 6.
Meta-analysis of age and actigraphy-assessed bedtime, ages 60 and older.
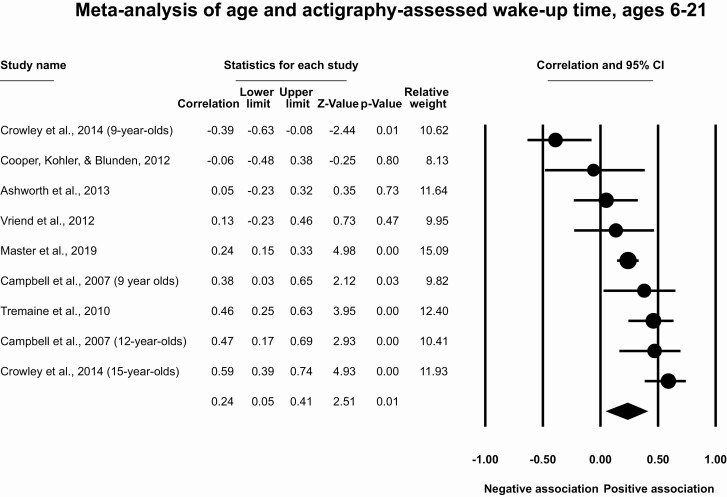
Meta-analysis of age and actigraphy-assessed wake-up time
Results for participants ages 6–21
This meta-analysis included seven studies with nine effect sizes and 748 participants. The meta-analytic effect size for age and wake-up time for ages 6–21 is r = 0.24 (95% CI [0.05, 0.41], p = .01, T2 = 0.06, I2 = 24.3%, Figure 7), which indicates a moderate correlation between older ages and later actigraphy-assessed wake-up times. Removal of individual effect sizes suggests that the association ranges from r = 0.18 to 0.31, with removal of one effect size (15-year-olds in ref. [34]) rendering the meta-analytic association nonsignificant. The funnel plot (Supplementary Figure S5) and the trim and fill method (which did not impute any studies) suggest no impact of publication bias.
Figure 7.
Meta-analysis of age and actigraphy-assessed wake-up time, ages 6–24.
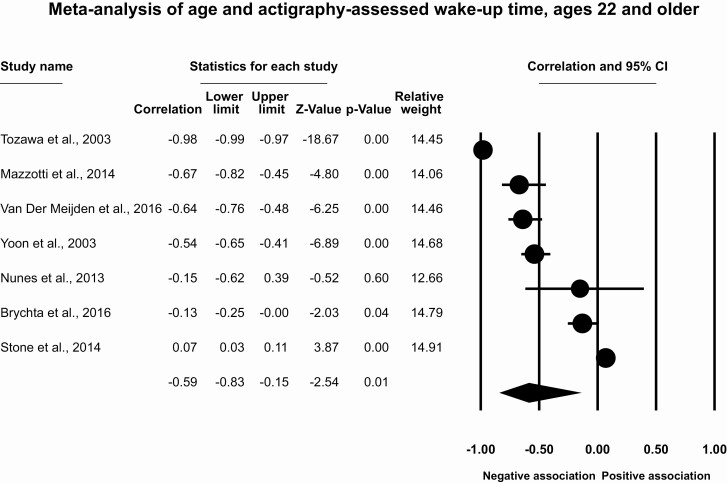
Results for participants ages 22 and older
This meta-analysis included seven studies with seven effect sizes and 3625 participants. The meta-analytic effect size for age and wake-up time for ages 22 and older is r = −0.59 (95% CI [−0.83, −0.15], p = .01, T2 = 0.15, I2 = 23.2%; Figure 8), which indicates a large association between older ages and earlier actigraphy-assessed wake-up times. Removal of individual studies suggests that the association ranges from r = 0.01 to 0.53, with removal of one effect size [101] rendering the meta-analytic association nonsignificant. There is little evidence of publication bias (Supplementary Figure S6), with two imputed studies using the trim and fill method (r = −0.73, 95% CI [−0.93, −0.10]).
Figure 8.
Meta-analysis of age and actigraphy-assessed wake-up time, ages 60 and older.
Moderators of meta-analyses
We evaluated moderators for three meta-analyses: sleep duration, sleep efficiency, and bedtime for individuals ages 6–21. We could not conduct moderation analyses for the other three meta-analyses because there were fewer than 10 studies in each moderator category. For categorical moderators (Table 5), we report the effect size within each subgroup of the moderator, test whether the moderator is statistically significant, and test the variance explained in the meta-analytic effect size by the moderator, or the R2. For continuous moderators (Table 6), we report the strength of the association as an unstandardized beta with standard error, as well as the R2. While we report all moderation analyses in tables, we only discuss in-text the moderators that were statistically significant (at the Bonferroni-corrected alpha of <.001) and/or had a medium effect size (R2 ≥ 13%).
Table 5.
Categorical moderators of meta-analytic associations of age and actigraphy-assessed sleep characteristics
| Sleep duration | Sleep efficiency | Bedtime, ages 6–21 | ||||
|---|---|---|---|---|---|---|
| Moderators | k | ES [95% CI] | k | ES [95% CI] | k | ES [95% CI] |
| Continent | R 2 = 10%, p < .001 | R 2 = 16%, p < .001 | R 2 = 0%, p = .20 | |||
| North America | 53 | −0.06 [−0.10, −0.02] | 37 | −0.006 [−0.06, 0.05] | 8 | Insufficient N |
| Europe | 19 | −0.13 [−0.22, −0.03] | 9 | Insufficient N | 5 | Insufficient N |
| Asia | 7 | Insufficient N | 5 | Insufficient N | 0 | Insufficient N |
| Exclusion criteria | ||||||
| Mental health | R 2 = 4%, p = .05 | R 2 = 0%, p = .57 | R 2 = 15%, p = .008 | |||
| Excluded | 34 | −0.20 [−0.30, −0.09]* | 23 | −0.08 [−0.22, 0.07] | 7 | Insufficient N |
| Included | 55 | −0.08 [−0.12, −0.03] | 35 | −0.04 [−0.08, 0.008] | 12 | 0.22 [0.05, 0.38] |
| Medical | R 2 = 2%, p = .03 | R 2 = 0%, p = .12 | R 2 = 0%, p = .60 | |||
| Excluded | 41 | −0.18 [−0.26, −0.09]* | 33 | −0.10 [−0.19, −0.005]* | 8 | Insufficient N |
| Included | 48 | −0.07 [−0.12, −0.02] | 25 | −0.01 [−0.07, 0.04] | 11 | 0.32 [0.19, 0.44]* |
| Sleep disorders | R 2 = 2%, p = .09 | R 2 = 0%, p = .61 | R 2 = 0%, p = .23 | |||
| Excluded | 26 | −0.19 [−0.28, −0.09]* | 18 | −0.03 [−0.13, 0.07] | 8 | Insufficient N |
| Included | 63 | −0.09 [−0.15, −0.04]* | 40 | −0.06 [−0.12, 0.001] | 11 | 0.28 [0.06, 0.48] |
| Actigraphy device | R 2 = 0%, p = .34 | R 2 = 0%, p = .39 | R 2 = 0%, p = .91 | |||
| Philips | 37 | −0.11 [−0.17, −0.04] | 18 | −0.12 [−0.22, −0.01]* | 10 | 0.38 [0.22, 0.52]* |
| AMI | 29 | −0.17 [−0.27, −0.07] | 25 | −0.04 [−0.10, 0.03] | 5 | Insufficient N |
| ActiGraph | 8 | Insufficient N | 3 | Insufficient N | 1 | Insufficient N |
| Other | 15 | −0.05 [−0.15, 0.06] | 12 | −0.01 [−0.17, 0.15] | 3 | Insufficient N |
k, number of effect sizes; ES, effect size, 95% CI, 95% confidence interval; R2, variance explained in the meta-analytic effect size by the categorical moderator; AMI, Ambulatory Monitoring, Inc.
*p < .001, which is the Bonferroni-corrected threshold for significance.
Table 6.
Continuous moderators of meta-analytic associations of age and actigraphy-assessed sleep characteristics
| Sleep duration | Sleep efficiency | Bedtime, ages 6–21 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariate | k | B (SE) | 95% CI | R 2 | k | B (SE) | 95% CI | R 2 | k | B (SE) | 95% CI | R 2 |
| Female | 87 | 0.004 (0.001) | 0.001, 0.006* | 0% | 57 | 0.003 (0.001) | 0.001, 0.005 | 0% | 19 | 0.005 (0.009) | −0.006, 0.02 | 0% |
| Mean Age | 83 | 0.005 (0.009) | 0.003, 0.007* | 24% | −0.012 (0.005) | −0.02, −0.002 | 0% | 16 | −0.01 (0.02) | −0.05, 0.03 | 0% | |
| Black | 40 | 0.001 (0.002) | −0.003, 0.006 | 0% | 27 | 0.001 (0.002) | −0.003, 0.005 | 0% | 4 | Insufficient N | ||
| Hispanic | 33 | −0.002 (0.002) | −0.003, 0.003 | 0% | 19 | −0.001 (0.002) | −0.006, 0.004 | 0% | 4 | Insufficient N | ||
| Asian | 26 | −0.001 (0.001) | −0.004, 0.002 | 0% | 17 | −0.002 (0.001) | −0.005, 0.001 | 12% | 3 | Insufficient N | ||
| Non-Caucasian | 47 | −0.001 (0.001) | −0.003, 0.001 | 3% | 37 | −0.003 (0.001) | −0.005, −0.001 | 2% | 7 | Insufficient N | ||
| Nights | 82 | −0.007 (0.006) | −0.02, 0.005 | 0% | 55 | −0.006 (0.009) | −0.02, 0.01 | 0% | 18 | 0.04 (0.04) | −0.04, 0.11 | 1% |
B (SE), unstandardized beta and standardized error; k, number of studies; R2, total proportion of between-study variance explained by the model; CI, confidence interval; Non-Caucasian indicates the percentage of participants who are not Caucasian (Black, Hispanic, Asian, American Indian, or “minority” variously defined); Nights indicates number of nights of actigraphy.
*p < .001, which is the Bonferroni-corrected threshold for significance.
Table 5 shows that 39% of effect sizes were from studies that excluded mental health disorders, 35% from studies that excluded medical conditions, and 31% from studies that excluded sleep disorders. In the 91 included articles, 38 studies used Philips Respironics devices, 33 used Ambulatory Monitoring Inc, 8 used ActiGraph, 12 used “other” devices (four used Sensewear Pro3 Armband, four used Actillume, one used Actiwatch Score, one used Actiwatch Mini, one used Actiheart, and one used Actigraph from Gaewhiler), and none used consumer wearables. Table 5 shows the number of studies that used each device type in each meta-analysis.
Continent of study was a significant moderator for actigraphy-assessed sleep duration and sleep efficiency, such that the strength of the association was stronger for studies conducted in Asia compared to North America or Europe (ps < .001, R2 = 10–16%, Table 5). The association between age and sleep duration was moderated by mean age of the participants (R2 = 24%, p < .001, Table 6), which is consistent with the scatterplot suggesting a quadratic association (Figure 2). Further testing indicated that the association between age and sleep duration was negative for participants ages 6–49 (k = 69; r = −0.17, 95% CI [−0.22, −0.12], p < .001) but weakly positive for individuals over the age of 50 (k = 20; r = 0.06, 95% CI [0.01, 0.10], p = .02). These findings suggest that the age-sleep duration association weakens across the lifespan.
Though not statistically significant at the Bonferroni-corrected alpha, exclusion of mental health disorders (R2 = 15%, p = .008) contributed substantially to the variance in the effect size for actigraphy-assessed bedtime (Table 5). The association between age and actigraphy-assessed sleep duration was weaker in samples with more females (p < .001), although this did not contribute to the variance (R2 = 0%).
No other moderators were statistically significant or contributed substantially to the variance in the effect size.
ROB within studies
ROB within each study was assessed by evaluating sample bias (studies that did not screen for and exclude individuals with mental health, medical conditions, or sleep disorders) and bias in outcome measurement (heterogeneity in actigraphy device or less than 7 days of actigraphy data). Of the 91 studies, low sample bias was present in 37 studies that excluded mental health conditions, 52 studies that excluded medical conditions, and 26 studies that excluded sleep disorders. We tested the impact of sample bias on results of the meta-analyses by evaluating each of these as moderators (Table 5). Exclusion of medical conditions or sleep disorders did not modify observed meta-analytic effect sizes. Exclusion of mental health disorders impacted the variance of the effect size for actigraphy-assessed bedtime (R2 = 15%), but did not impact the effect size for actigraphy-assessed sleep duration or sleep efficiency.
Low bias in outcome measurement was present for the 32 studies that included at least seven nights of actigraphy data. Although there were differences in actigraphy device used across studies, the type of actigraphy device (Table 5) and the number of nights of actigraphy data (Table 6) did not contribute significantly to variance in the meta-analytic effect sizes for actigraphy-assessed sleep duration, sleep efficiency, or bedtime.
Evaluating the quality of the evidence
Using a modified version of the Community Preventive Services methods [24], we evaluated the quality of the body of evidence included in these meta-analyses (Table 7). Because so few studies (0–28.6%) were longitudinal, we could not categorize the quality of evidence as strong for any meta-analysis. The quality of the evidence was sufficient for bedtime ages 6–21, and bedtime and wake-up time ages 22 and older, indicating that there was consistency in the direction of effects across studies, fair execution within studies, and sufficient to large effect sizes. The quality of the evidence was considered insufficient for sleep duration, sleep efficiency, and wake-up time ages 6–21. Although there were the largest number of effect sizes for sleep duration and efficiency, the direction of the associations were inconsistent and effect sizes were small.
Table 7.
Quality of the evidence for meta-analyses of age and actigraphy-assessed sleep
| Sleep duration | Sleep efficiency | Bedtime, ages 6–24 | Bedtime, ages 60 and older | Wake-up time, ages 6–24 | Wake-up time, ages 60 and older | |
|---|---|---|---|---|---|---|
| Execution | ||||||
| Study well-described, % | 85.9 | 86.8 | 80.0 | 85.7 | 85.7 | 85.7 |
| Specified screening criteria, % | 67.9 | 75.5 | 66.7 | 85.7 | 71.4 | 100.0 |
| ≥7 days data, % | 52.6 | 62.3 | 53.3 | 85.7 | 71.4 | 85.7 |
| <10% missingness, % | 33.3 | 34.0 | 53.3 | 14.3 | 57.1 | 14.3 |
| Good, fair, or limited execution | Limited | Fair | Fair | Fair | Fair | Fair |
| Design suitability | ||||||
| Longitudinal studies, % | 1.3 | 0 | 20 | 0 | 28.6 | 0 |
| Cross-sectional studies, % | 98.7 | 100 | 80 | 100 | 71.4 | 100 |
| Greater or lesser suitability | Lesser | Lesser | Lesser | Lesser | Lesser | Lesser |
| Number of studies | 89 | 58 | 20 | 6 | 10 | 6 |
| At least 2, 3, or 5 | At least 5 | At least 5 | At least 5 | At least 5 | At least 5 | At least 5 |
| Consistency | ||||||
| Negative associations, % | 44.9 | 39.6 | 13.3 | 71.4 | 28.6 | 85.7 |
| Neutral associations (−0.10, + 0.10), % | 34.8 | 30.2 | 6.7 | 28.6 | 14.3 | 0.0 |
| Positive associations, % | 20.0 | 30.2 | 80.0 | 0.0 | 57.1 | 14.3 |
| Consistent or inconsistent | Inconsistent | Inconsistent | Consistent | Consistent | Inconsistent | Consistent |
| Effect size, Pearson’s r | −0.12 | −0.05 | 0.35 | −0.66 | 0.24 | −0.59 |
| Small, sufficient, or large | Small | Small | Sufficient | Large | Small | Large |
| Evidence of association | ||||||
| Strong, sufficient, or insufficient | Insufficient | Insufficient | Sufficient | Sufficient | Insufficient | Sufficient |
Discussion
This review evaluated the associations among age and actigraphy-assessed sleep characteristics across the lifespan. We evaluated the published evidence using meta-analyses based on 189 effect sizes and 23 365 participants. We demonstrated that actigraphy-assessed sleep duration is shorter (r = −0.12) and sleep efficiency is lower (r = −0.05) at older ages, though these associations were small and inconsistent across studies. We found larger effect sizes for sleep timing, such that bedtime and wake-up time were later at older ages for individuals aged 6–21 (r = 0.37, 0.24, respectively), and earlier for individuals aged 22 and older (r = −0.66, −0.59, respectively). These findings may suggest that changes in sleep timing—rather than changes in sleep duration or efficiency—are the most robust hallmarks of age-related differences in sleep.
These meta-analyses demonstrate an association between age and later sleep timing among individuals ages 6–21, and an association between age and earlier sleep timing among adults. While this is the first meta-analysis to examine age and actigraphy-assessed sleep timing among adults, these findings are consistent with a previous meta-analysis of actigraphy-assessed sleep among individuals aged 3–18, which reported later bedtime and wake-up times at older ages [8]. Laboratory evidence suggests that adolescents delay bedtime and wake-up time due to changes in both the biological clock and social demands [117], and that middle-aged and older adults demonstrate earlier timing of circadian rhythms compared to younger adults [118]. The meta-analyses for sleep timing included fewer effect sizes compared to analyses for duration and efficiency, and we had no analyzable data of age and sleep timing associations for individuals aged 30–60. This lack of data precludes an analysis of the age at which sleep timing shifts from delaying to advancing. Importantly, for three of these meta-analyses (bedtime ages 6–21, bedtime ages 22 and older, and wake-up time ages 22 and older), the quality of evidence was deemed sufficient for strong conclusions, which means that these associations are statistically significant, reliable, and substantial.
The largest number of studies allowed examination of the association between age and actigraphy-assessed sleep duration. Consistent with previous meta-analyses based on various measurement modalities [3–5, 8], we observed that actigraphy-assessed sleep duration is shorter at older ages, though the effect was small in magnitude. Laboratory evidence has suggested that, compared to younger adults, older adults have shorter sleep with a 12-hour nocturnal sleep opportunity [119] and have a lower propensity for falling asleep during a daytime sleep window [119–121], which may be due to age-related changes in sleep propensity. However, this study’s exploratory meta-analyses revealed that actigraphy-assessed sleep duration is shorter at older ages only until approximately age 50, and is slightly longer at ages above 50 years. This could be due to differences between sleep propensity in a controlled laboratory environment compared to sleep duration assessed in the field. The finding of a U-shaped association between age and sleep duration should be interpreted cautiously due to the exploratory nature of the analysis (based on our descriptive scatterplot and test of linearity with 89 effect sizes), but warrants examination in future studies. We choose to focus our discussion of results primarily on our linear association because of this exploratory nature. These inconsistent associations, as well as small effect sizes, led to the quality of the evidence for this meta-analysis being deemed insufficient for drawing strong conclusions. Overall, the actigraphy-assessed sleep duration results suggest that inadequate sleep quantity should not be interpreted only as a consequence of aging. Therefore, older adults should be encouraged that their current sleep quantity is not unchangeable due to their age.
Though we report that sleep efficiency is lower at older ages, it was determined to be weakly linear, the meta-analytic effect size was small, and removal of individual studies rendered the association nonsignificant. These small effect sizes and the inconsistency of directions of associations means that the quality of the evidence for this meta-analysis is insufficient for drawing strong conclusions. While a previous meta-analysis reported that polysomnography-assessed sleep efficiency is lower at older ages [5], these discrepant results may be due to difference in measurement modality [122], and/or study design, as actigraphy allows for a greater number of nights studied and increased environmental validity of home-based assessment. Another possibility is that older adults are more sensitive to nocturnal awakenings in the laboratory compared to the home environment. The present meta-analytic results suggest that age is an unlikely confound in research examining correlates and consequences of actigraphy-assessed sleep efficiency. Additionally, these results suggest that interventions to improve sleep efficiency such as cognitive behavioral therapy for insomnia may be useful across the lifespan; though our review did not include studies of diagnosed insomnia patients, the average sleep efficiency was below 85% (a common threshold for insomnia [123]) for half of the included studies.
In our analysis of demographic moderators, continent of the study (North America, Asia, or Europe; insufficient studies from other regions) was the only consistent moderator. A previous meta-analysis found that continent of study was a modifier of age and subjective sleep associations among adolescent participants [2], and a recent analysis of worldwide commercial devices showed that sleep duration and timing were different across continents [124]. These results could be a macro-level indicator of differences in social environments (e.g. socioeconomic status, race/ethnicity distributions), social demands (e.g. work and school [125]), as well as cultural attitudes and values regarding sleep and its importance. These data suggest that continent of study should be included as a moderator in cross-continental and cross-cultural studies of sleep in relation to age.
Associations among age and sleep were similar in studies that excluded individuals with mental health disorders, medical conditions, or sleep disorders compared to studies that did not evaluate, screen for, or exclude these populations. In contrast, a previous meta-analysis [5] found that the strength of the age-sleep association was stronger for studies that excluded individuals with mental health disorders, medical conditions, or sleep disorders. Their results suggested that these comorbidities may mask the effects of age on sleep across the lifespan. In contrast, the present results suggest that age-related differences in actigraphy-assessed sleep across the lifespan may not be a function of comorbidities. Discrepant results across studies may be a function of modality differences, as Ohayon and colleagues [5] evaluated studies using either polysomnography or actigraphy (13 effect sizes for actigraphy-assessed sleep duration). Alternatively, differences may be related to exclusion criteria at the level of the meta-analysis. Ohayon and colleagues [5] included all studies that evaluated the association between age and sleep, whereas we excluded articles at the title/abstract stage if there was no control group, as these studies would not allow for a direct evaluation of the impact of comorbidities. In both the Ohayon [5] meta-analysis and the present meta-analysis, comparison of age-sleep associations for specific conditions (e.g. depression) to controls was not possible, given the lack of published studies reporting results stratified by comorbidity.
Though all meta-analyses obtained 100% statistical power (determined by number of studies and sample sizes), it is important to note that some of the meta-analyses are limited by the quality of the individual studies that were included. Previous conclusions from sleep clinical practice guidelines have similarly been tempered by the limitations of the extant literature. For example, the use of pharmacological therapy for adults with chronic insomnia disorder is quantified as a weak recommendation based on low-quality evidence [125]. As documented in the present study, the limited number of published longitudinal studies diminished the strength of the evidence regarding the association between age and sleep; though our requirement that greater than 70% of included studies be longitudinal for “greater design suitability” was a high threshold, we observed that only 0%–29% of included studies in our meta-analyses were longitudinal, which is certainly a minority of effect sizes.
Limitations and strengths
Limitations of the present study deserve consideration when evaluating the association between age and key sleep characteristics. First, lack of attention to the issue of age and sleep in published studies continues to limit our understanding of sleep across the lifespan. In the present study, limited published data regarding actigraphy-assessed sleep midpoint and regularity precluded meta-analytic evaluation of these important characteristics. Second, moderators (as well as confounders) have been overlooked in studies of age and sleep, which limited our ability to fully evaluate key putative moderators such as race/ethnicity, sex, and comorbidities. For example, approximately two-thirds of the studies evaluated in the present meta-analysis provided no detail regarding screening for comorbidities. While increasing the generalizability of the present findings, the lack of detail regarding comorbidities limits understanding of the extent to which comorbidities influence the age-sleep link. A greater understanding of the extent to which these factors influence sleep across the lifespan is important both to fundamental questions about sleep and well as the identification of risk factors for and treatment of disturbed sleep. Third, we were limited to the published data available. This means that we were unable to characterize age-sleep associations across the full lifespan because published data were not available for each decade of life and/or each actigraphy-assessed sleep outcome. This limitation was especially evident for sleep timing outcomes, with no studies available for individuals aged 30 to 60 and too few effect sizes to precisely capture the age at which sleep timing profiles shift from delay to advances. Additionally, across individual studies there were inconsistencies in the direction of the association between age and all sleep characteristics, and this was especially pronounced for the associations among age and sleep duration and sleep efficiency. These inconsistencies resulted in smaller meta-analytic effect sizes as well as a lower quality of evidence for the individual studies.
Finally, associations of age and actigraphy-assessed sleep across the lifespan were based primarily on between- versus within-persons analyses due to the limited number of longitudinal sleep studies and the absence of consortia organized to support aggregation, harmonization, and evaluation of individual-level data across studies. Reliance on study- versus individual-level data may obscure our understanding of changes in sleep characteristics across the lifespan due to the influence of study characteristics (e.g. sample selection) on measurement error. Notably, recent comparisons of the performance of meta-analysis compared to individual-patient data show comparable magnitude and direction of effects, which provides greater confidence in the results of the current report [126, 127].
These limitations are offset by several notable strengths. This is the first meta-analysis to evaluate the association of age and key indices of actigraphy-assessed sleep, including duration, efficiency, and timing in studies of children and adults. The present meta-analytic approach affords the opportunity to systematically and quantitatively assess actigraphy data from 23,365 participants ages 6–92. Evaluation of actigraphy-assessed sleep is important given its superior ability to measure sleep over a greater number of days and circumstances compared to polysomnography-assessed sleep. Moreover, the present meta-analysis was pre-registered on PROSPERO, allowing for increased transparency of aims, a priori hypotheses, and study design, thereby enhancing reproducibility. We systematically searched for articles with the assistance of a highly experienced medical librarian, and we used a software for screening articles designed for systematic reviews and meta-analyses (DistillerSR). Clear selection criteria were identified and implemented according to state-of-science methods. Two independent raters conducted title/abstract review and data extraction, with a third independent rater to resolve any discrepancies. Planned evaluation of methods demonstrated good inter-rater reliability. Finally, we evaluated a variety of demographic and methodological moderators chosen for their relevance for research and/or clinical questions.
Conclusion
There are age-related differences in actigraphy-assessed sleep across the lifespan. Actigraphy-assessed sleep duration is shorter and efficiency is lower at older ages, but the effect sizes are small. In contrast, patterns in sleep timing, including bedtime and wake time, differ as a function of age group with small to large effect sizes. Among individuals between 6 and 21 years of age, older age is associated with earlier bed- and wake times, whereas older age is associated with later bed- and wake times in those 22 years of age and older. Moderation analyses suggest that associations among age and actigraphy-assessed sleep may differ as a function of study continent (North America, Asia, or Europe) but are not related to comorbidities, sex, race/ethnicity, number of study nights, or device type, although these results should be interpreted with caution given limitations to the extant literature. Changes in sleep timing, rather than indices of sleep duration or efficiency, may be the most robust hallmark marker of age effects on actigraphy-assessed sleep.
Supplementary Material
Acknowledgments
We thank Alicia Bill, Devika Dewan, and Priyanka Purohit for their contributions to data processing. We thank the researchers who provided us with additional information about their study for inclusion in the meta-analysis.
Funding
Support and training for M. Bowman was provided by T32 HL07560 and HL082610. Partial support for investigator effort by AG047139 (DJB and MHH). The funders were not involved in this systematic review. We included data from the Osteoporotic Fractures in Men (MrOS) Study, and the following institutes provide support for this study: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Conflict of interest statement. Dr. Buysse has served as a paid consultant to Bayer, BeHealth Solutions, Cereve/Ebb Therapeutics, Emmi Solutions, National Cancer Institute, Pear Therapeutics, Philips Respironics, Sleep Number, and Weight Watchers International. He has served as a paid consultant for professional educational programs developed by the American Academy of Physician Assistants and CME Institute, and received payment for a professional education program sponsored by Eisai (content developed exclusively by Dr. Buysse). Dr. Buysse is an author of the Pittsburgh Sleep Quality Index, Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A), Brief Pittsburgh Sleep Quality Index (B-PSQI), Daytime Insomnia Symptoms Scale, Pittsburgh Sleep Diary, Insomnia Symptom Questionnaire, and RU_SATED (copyright held by University of Pittsburgh). These instruments have been licensed to commercial entities for fees. He is also co-author of the Consensus Sleep Diary (copyright held by Ryerson University), which is licensed to commercial entities for a fee. Dr. Hall has served as a paid consultant to Eisai and is an author of the Insomnia Symptom questionnaire, what has been licensed to commercial entities for fees. We have no non-financial conflicts of interest to disclose.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gradisar M, et al. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–118. [DOI] [PubMed] [Google Scholar]
- 3. Boulos MI, et al. Normal polysomnography parameters in healthy adults: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(6):533–543. [DOI] [PubMed] [Google Scholar]
- 4. Floyd JA, et al. Age-related changes in initiation and maintenance of sleep: a meta-analysis. Res Nurs Health. 2000;23(2):106–117. [DOI] [PubMed] [Google Scholar]
- 5. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 6. Acebo C, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. [DOI] [PubMed] [Google Scholar]
- 7. Goldstone A, et al. Actigraphy in the digital health revolution: Still asleep? Sleep. 2018;41(9):1–2. [DOI] [PubMed] [Google Scholar]
- 8. Galland BC, et al. Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep. 2018;41(4):1–16. [DOI] [PubMed] [Google Scholar]
- 9. Carrier J, et al. Sex differences in age-related changes in the sleep-wake cycle. Front Neuroendocrinol. 2017;47:66–85. [DOI] [PubMed] [Google Scholar]
- 10. Ruiter ME, et al. Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep Med. 2011;12(3):209–214. [DOI] [PubMed] [Google Scholar]
- 11. Soreca I, et al. Sleep dimensions in depressed and controls: How many study nights do we need? Sleep. 2010;33:A243–A244. [Google Scholar]
- 12. Kubala AG, et al. Field-based measurement of sleep: agreement between six commercial activity monitors and a validated accelerometer. Behav Sleep Med. 2020;18(5):637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roenneberg T, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. [DOI] [PubMed] [Google Scholar]
- 14. The National Sleep Foundation. 2003 Sleep in America Poll. 2003. https://sleepfoundation.org/ask-the-expert/sleep-hygiene. Accessed November 25, 2019.
- 15. Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019. https://training.cochrane.org/handbook. Accessed September 1, 2019.
- 17. Galland BC, et al. Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Med Rev. 2012;16(3):213–222. [DOI] [PubMed] [Google Scholar]
- 18. Borenstein M, et al. Introduction to Meta-analysis. West Sussex, United Kingdom: Wiley; 2009. [Google Scholar]
- 19. RStudio Team. RStudio: Integrated Development for R. RStudio. Boston, MA: PBC; 2020. http://www.rstudio.com/. [Google Scholar]
- 20. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer-Verlag; 2016. Accessed July 15, 2020. [Google Scholar]
- 21. Borenstein M, et al. Comprehensive Meta-Analysis Version 3. Englewood, NJ: Biostat; 2013. [Google Scholar]
- 22. Duval S, et al. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 23. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Revised Edition. New York, NY: Academic Press, Inc; 1977. [Google Scholar]
- 24. Briss PA, et al. Developing an evidence-based Guide to Community Preventive Services–methods. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):35–43. [DOI] [PubMed] [Google Scholar]
- 25. Agmon M, et al. Sleep quality is associated with walking under dual-task, but not single-task performance. Gait Posture. 2016;49:127–131. [DOI] [PubMed] [Google Scholar]
- 26. Aronen ET, et al. Associations of age and gender with activity and sleep. Acta Paediatr. 2001;90(2):222–224. [DOI] [PubMed] [Google Scholar]
- 27. Bagley EJ, et al. Community violence concerns and adolescent sleep. Sleep Health. 2016;2(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bagley EJ, et al. Neighborhood economic deprivation and social fragmentation: associations with children’s sleep. Behav Sleep Med. 2018;16(6):542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baron KG, et al. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond). 2017;41(2):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beaudreau SA, et al. The relationship between objectively measured sleep disturbance and dementia family caregiver distress and burden. J Geriatr Psychiatry Neurol. 2008;21(3):159–165. [DOI] [PubMed] [Google Scholar]
- 31. Berger RH, et al. The association between home chaos and academic achievement: the moderating role of sleep. J Fam Psychol. 2019;33(8):975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berkman LF, et al. Work-family conflict, cardiometabolic risk and sleep duration in nursing employees. J Occup Heal Psychol. 2015;20(4):420–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brychta RJ, et al. Influence of day length and physical activity on sleep patterns in older Icelandic men and women. J Clin Sleep Med. 2016;12(2):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bulls HW, et al. Depressive symptoms and sleep efficiency sequentially mediate racial differences in temporal summation of mechanical pain. Ann Behav Med. 2017;51(5):673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Buxton OM, et al. Work-Family conflict and employee sleep: evidence from IT workers in the work, family and health study. Sleep. 2016;39(10):1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Campbell IG, et al. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. Sleep. 2007;30(12):1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiang JJ, et al. Daily family stress and HPA axis functioning during adolescence: the moderating role of sleep. Psychoneuroendocrinology. 2016;71:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi H, et al. Relationship between sedentary time and sleep duration among Korean adolescents. J Sch Nurs. 2020;36(6):423–429. [DOI] [PubMed] [Google Scholar]
- 39. Cohodes EM, et al. Novel insights from actigraphy: anxiety is associated with sleep quantity but not quality during childhood. Clin Child Psychol Psychiatry. 2020;25(1):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cooper P, et al. Sleep and academic performance in Indigenous Australian children from a remote community: an exploratory study. J Paediatr Child Health. 2012;48(2):122–127. [DOI] [PubMed] [Google Scholar]
- 41. Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. D’Angelo V, et al. Cushing’s syndrome is associated with sleep alterations detected by wrist actigraphy. Pituitary. 2015;18(6):893–897. [DOI] [PubMed] [Google Scholar]
- 43. Doane LD, et al. Associations among sleep, daily experiences, and loneliness in adolescence: evidence of moderating and bidirectional pathways. J Adolesc. 2014;37(2):145–154. [DOI] [PubMed] [Google Scholar]
- 44. Doyle CY, et al. Associations between objective sleep and ambulatory blood pressure in a community sample. Psychosom Med. 2019;81(6):545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ekstedt M, et al. Sleep, physical activity and BMI in six to ten-year-old children measured by accelerometry: a cross-sectional study. Int J Behav Nutr Phys Act. 2013;10:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. El-Sheikh M, et al. Vagal regulation and emotional intensity predict children’s sleep problems. Dev Psychobiol. 2005;46(4):307–317. [DOI] [PubMed] [Google Scholar]
- 47. El-Sheikh M, et al. Children’s sleep and adjustment over time: the role of socioeconomic context. Child Dev. 2010;81(3):870–883. [DOI] [PubMed] [Google Scholar]
- 48. El-Sheikh M, et al. Economic adversity and children’s sleep problems: multiple indicators and moderation of effects. Health Psychol. 2013;32(8):849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El-Sheikh M, et al. Quick to berate, slow to sleep: interpartner psychological conflict, mental health, and sleep. Health Psychol. 2013;32(10):1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Erath SA, et al. Associations between children’s intelligence and academic achievement: the role of sleep. J Sleep Res. 2015;24(5):510–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ertel KA, et al. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep. 2011;34(4):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Evans BD, et al. 24-hour sleep/wake patterns in healthy elderly persons. Appl Nurs Res. 1994;7(2):75–83. [DOI] [PubMed] [Google Scholar]
- 53. Fietze I, et al. Sleep quality in professional ballet dancers. Chronobiol Int. 2009;26(6):1249–1262. [DOI] [PubMed] [Google Scholar]
- 54. Fine L, et al. Sleep disruption explains age-related prospective memory deficits: implications for cognitive aging and intervention. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2019;26(4):621–636. [DOI] [PubMed] [Google Scholar]
- 55. Fletcher FE, et al. The association between anxiety symptoms and sleep in school-aged children: a combined insight from the children’s sleep habits questionnaire and actigraphy. Behav Sleep Med. 2018;16(2):169–184. [DOI] [PubMed] [Google Scholar]
- 56. Fung CH, et al. Sleep disturbance among older adults in assisted living facilities. Am J Geriatr Psychiatry. 2012;20(6):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gharamaleki AS, et al. Sleep patterns in 6–9 years old students living in Tehran City. J Isfahan Med Sch. 2011;29(154):1–9. [Google Scholar]
- 58. Gillis BT, et al. Sleep and adjustment in adolescence: physical activity as a moderator of risk. Sleep Health. 2019;5(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gruber R, et al. Sleep efficiency (but not sleep duration) of healthy school-age children is associated with grades in math and languages. Sleep Med. 2014;15(12):1517–1525. [DOI] [PubMed] [Google Scholar]
- 60. Gunn DG, et al. Actigraphically-defined sleep disturbance in Parkinson’s disease is associated with differential aspects of cognitive functioning. J Clin Neurosci. 2014;21(7):1112–1115. [DOI] [PubMed] [Google Scholar]
- 61. Gunn HE, et al. Young adolescent sleep is associated with parental monitoring. Sleep Health. 2019;5(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gupta NK, et al. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14(6):762–768. [DOI] [PubMed] [Google Scholar]
- 63. Hairston IS, et al. Sleep mediates the link between resiliency and behavioural problems in children at high and low risk for alcoholism. J Sleep Res. 2016;25(3):341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hanson MD, et al. Daily stress, cortisol, and sleep: the moderating role of childhood psychosocial environments. Health Psychol. 2010;29(4):394–402. [DOI] [PubMed] [Google Scholar]
- 65. Hisler G, et al. Anger tendencies and sleep: Poor anger control is associated with objectively measured sleep disruption. J Res Pers. 2017;71:17–26. [Google Scholar]
- 66. Huang YL, et al. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav. 2002;76(4-5):597–603. [DOI] [PubMed] [Google Scholar]
- 67. Ibáñez-del Valle V, et al. Subjective and objective sleep quality in elderly individuals: the role of psychogeriatric evaluation. Arch Gerontol Geriatr. 2018;76(January):221–226. [DOI] [PubMed] [Google Scholar]
- 68. Jansen EC, et al. Adiposity in adolescents: the interplay of sleep duration and sleep variability. J Pediatr. 2018;203:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jean-Louis G, et al. Circadian sleep, illumination, and activity patterns in women: influences of aging and time reference. Physiol Behav. 2000;68(3):347–352. [DOI] [PubMed] [Google Scholar]
- 70. John-Henderson NA, et al. Life stress, sense of belonging and sleep in American Indian college students. Sleep Health. 2019;5(4):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keller PS, et al. Longitudinal relations between maternal depressive symptoms and child sleep problems: the role of parasympathetic nervous system reactivity. J Child Psychol Psychiatry. 2014;55(2):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kelly RJ, et al. Reciprocal relations between children’s sleep and their adjustment over time. Dev Psychol. 2014;50(4):1137–1147. [DOI] [PubMed] [Google Scholar]
- 73. Khalsa S, et al. Variability in cumulative habitual sleep duration predicts waking functional connectivity. Sleep. 2016;39(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kishida M, et al. An intensive longitudinal examination of daily physical activity and sleep in midlife women. Sleep Health. 2016;2(1):42–48. [DOI] [PubMed] [Google Scholar]
- 75. Knufinke M, et al. Train hard, sleep well? Perceived training load, sleep quantity and sleep stage distribution in elite level athletes. J Sci Med Sport. 2018;21(4):427–432. [DOI] [PubMed] [Google Scholar]
- 76. Kobel S, et al. Cross-sectional associations of objectively assessed sleep duration with physical activity, BMI and television viewing in German primary school children. BMC Pediatr. 2019;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kottwitz MU, et al. Sleep, work stress and headache in printing business: an actigraphy study. Sleep Vigil. 2019;3(1):9–15. [Google Scholar]
- 78. Kume Y, et al. Sleep/awake status throughout the night and circadian motor activity patterns in older nursing-home residents with or without dementia, and older community-dwelling people without dementia. Int Psychogeriatr. 2016;28(12):2001–2008. [DOI] [PubMed] [Google Scholar]
- 79. Kurina LM, et al. Loneliness is associated with sleep fragmentation in a communal society. Sleep. 2011;34(11):1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kurina LM, et al. Actigraphic sleep characteristics among older Americans. Sleep Health. 2015;1(4):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lerdal A, et al. Sleep among bereaved caregivers of patients admitted to hospice: a 1-year longitudinal pilot study. BMJ Open. 2016;6(1):e009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Madden KM, et al. Sedentary behavior and sleep efficiency in active community-dwelling older adults. Sleep Sci. 2014;7(2):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Malone SK, et al. Characteristics associated with sleep duration, chronotype, and social jet lag in adolescents. J Sch Nurs. 2016;32(2):120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Martinez SM, et al. Temporal associations between circadian sleep and activity patterns in Mexican American children. Sleep Health. 2019;5(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Master L, et al. Bidirectional, daily temporal associations between sleep and physical activity in adolescents. Sci Rep. 2019;9(1):7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mazzotti DR, et al. Human longevity is associated with regular sleep patterns, maintenance of slow wave sleep, and favorable lipid profile. Front Aging Neurosci. 2014;6:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mendlowicz MV, et al. Actigraphic predictors of depressed mood in a cohort of non-psychiatric adults. Aust N Z J Psychiatry. 1999;33(4):553–558. [DOI] [PubMed] [Google Scholar]
- 88. Nunes DM, et al. Actigraphic assessment of sleep in chronic obstructive pulmonary disease. Sleep Breath. 2013;17(1):125–132. [DOI] [PubMed] [Google Scholar]
- 89. Park H, et al. Sleep and inflammation during adolescence. Psychosom Med. 2016;78(6):677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Parsey CM, et al. Sleep and everyday functioning in older adulthood. J Appl Gerontol. 2015;34(1):48–72. [DOI] [PubMed] [Google Scholar]
- 91. Pereira D, et al. Illegitimate tasks and sleep quality: an ambulatory study. Stress Health. 2014;30(3):209–221. [DOI] [PubMed] [Google Scholar]
- 92. Pereira D, et al. Social stressors at work, sleep quality and psychosomatic health complaints—a longitudinal ambulatory field study. Stress Heal. 2014;30(1):43–52. [DOI] [PubMed] [Google Scholar]
- 93. Pereira D, et al. Daily impaired detachment and short-term effects of impaired sleep quality on next-day commuting near-accidents - an ambulatory diary study. Ergonomics. 2016;59(8):1121–1131. [DOI] [PubMed] [Google Scholar]
- 94. Pesonen AK, et al. Continuity and change in poor sleep from childhood to early adolescence. Sleep. 2014;37(2):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Redeker NS, et al. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 2006;35(4):252–261. [DOI] [PubMed] [Google Scholar]
- 96. Sadeh A, et al. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73(2):405–417. [DOI] [PubMed] [Google Scholar]
- 97. Sadeh A, et al. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32(12):1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sella E, et al. The influence of metacognitive beliefs on sleeping difficulties in older adults. Appl Psychol Health Well Being. 2019;11(1):20–41. [DOI] [PubMed] [Google Scholar]
- 99. Stone KL, et al. ; Osteoporotic Fractures in Men Study Group. Sleep disturbances and risk of falls in older community-dwelling men: the outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study. J Am Geriatr Soc. 2014;62(2):299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Takahashi M, et al. Occupational and socioeconomic differences in actigraphically measured sleep. J Sleep Res. 2014;23(4):458–462. [DOI] [PubMed] [Google Scholar]
- 101. Tashjian SM, et al. Bedtime autonomy and cellphone use influence sleep duration in adolescents. J Adolesc Health. 2019;64(1):124–130. [DOI] [PubMed] [Google Scholar]
- 103. Tozawa T, et al. Stability of sleep timing against the melatonin secretion rhythm with advancing age: clinical implications. J Clin Endocrinol Metab. 2003;88(10):4689–4695. [DOI] [PubMed] [Google Scholar]
- 103. Tremaine RB, et al. Subjective and objective sleep in children and adolescents: measurement, age, and gender differences. Sleep Biol Rhythms. 2010;8(4):229–238. [Google Scholar]
- 104. Troxel WM, et al. Marital/cohabitation status and history in relation to sleep in midlife women. Sleep. 2010;33(7):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tu KM, et al. The link between maternal sleep and permissive parenting during late adolescence. J Sleep Res. 2018;27(5):e12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. van den Berg JF, et al. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32(10):1367–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. van der Meijden WP, et al. Individual differences in sleep timing relate to melanopsin-based phototransduction in healthy adolescents and young adults. Sleep. 2016;39(6):1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Van Dyk TR, et al. Daily bidirectional relationships between sleep and mental health symptoms in youth with emotional and behavioral problems. J Pediatr Psychol. 2016;41(9):983–992. [DOI] [PubMed] [Google Scholar]
- 109. Van Lenten SA, et al. Examining multiple sleep behaviors and diurnal salivary cortisol and alpha-amylase: within- and between-person associations. Psychoneuroendocrinology. 2016;68:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Volkovich E, et al. Objective and subjective sleep during pregnancy: links with depressive and anxiety symptoms. Arch Womens Ment Health. 2016;19(1):173–181. [DOI] [PubMed] [Google Scholar]
- 111. von Känel R, et al. Sleep in spousal Alzheimer caregivers: a longitudinal study with a focus on the effects of major patient transitions on sleep. Sleep. 2012;35(2):247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vriend J, et al. Sleep quantity and quality in relation to daytime functioning in children. Child Heal Care. 2012;41(3):204–222. [Google Scholar]
- 113. Whitaker KM, et al. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41(11):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wong M, et al. Interaction of sleep and regular exercise in adolescents’ and young adults’ working memory. Int J Sport Psychol. 2017;48(1):1–17. [Google Scholar]
- 115. Yoon IY, et al. Actigraphy suggests age-related differences in napping and nocturnal sleep. J Sleep Res. 2003;12(2):87–93. [DOI] [PubMed] [Google Scholar]
- 116. Ashworth A, et al. Cross syndrome comparison of sleep problems in children with Down syndrome and Williams syndrome. Res Dev Disabil. 2013;34(5):1572–1580. [DOI] [PubMed] [Google Scholar]
- 117. Crowley SJ, et al. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. [DOI] [PubMed] [Google Scholar]
- 118. Carrier J, et al. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neurosci Lett. 2002;320(1-2):1–4. [DOI] [PubMed] [Google Scholar]
- 119. Klerman EB, et al. Age-related reduction in the maximal capacity for sleep–implications for insomnia. Curr Biol. 2008;18(15):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dijk DJ, et al. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33(2):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Buysse DJ, et al. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28(11):1365–1376. [DOI] [PubMed] [Google Scholar]
- 122. Marino M, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Spielman AJ, et al. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 124. Kuula L, et al. Using big data to explore worldwide trends in objective sleep in the transition to adulthood. Sleep Med. 2019;62:69–76. [DOI] [PubMed] [Google Scholar]
- 125. Qaseem A, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2016;165(2):125–133. [DOI] [PubMed] [Google Scholar]
- 126. Burke DL, et al. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36(5):855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Boedhoe PSW, et al. ; ENIGMA-OCD Working-Group. An empirical comparison of meta- and mega-analysis with data from the ENIGMA obsessive-compulsive disorder working group. Front Neuroinform. 2018;12:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.