Highlights
-
•
Cortical hypoactivity during inhibitory control predicted greater opioid use severity.
-
•
Frontoparietal brain networks significantly contributed to the prediction of severity.
-
•
Brain marker of severity predicted subsequent on-treatment opioid craving.
-
•
Brain marker predicted on-treatment craving better than clinical severity did.
Keywords: Opioid use disorder, Emotional inhibitory control, Drug use severity, Craving, Functional magnetic resonance imaging, Multivariate pattern analysis
Abstract
Opioid use disorder (OUD) is characterized by emotional and cognitive impairements that are associated with poor treatment outcomes. The present study investigated the neural mechanism underlying emotion evaluation and inhibitory control using an affective go/no-go (AGN) task and its association with drug use severity and craving in patients with OUD. Twenty-six recently detoxified patients with OUD underwent functional magnetic resonance imaging (fMRI) while performing the AGN task that required response to frequently presented appetitive stimuli (“go”) and inhibition of response to infrequently presented aversive stimuli (“no-go”). The fMRI session was immediately followed by an injection of extended-release opioid antagonist naltrexone (XR-NTX). Participants’ opioid craving was assessed immediately before fMRI and 10 ± 2 days after XR-NTX injection. Multivariate pattern analysis (MVPA) showed that drug use severity was associated with distributed brain hypoactivity in response to aversive no-go stimuli, with particularly large negative contributions from the cognitive control and dorsal attention brain networks. While drug use severity and its associated MVPA brain response pattern were both correlated with opioid craving at baseline, only the brain response pattern predicted craving during XR-NTX treatment. Our findings point to widespread functional hypoactivity in the brain networks underlying emotional inhibitory control in OUD. Such a distributed pattern is consistent with the multifaceted nature of OUD, which affects multiple brain networks. It also highlights the utility of the multivariate approach in uncovering large-scale cortical substrates associated with clinical severity in complex psychiatric disorders and in predicting treatment response.
1. Introduction
Opioid use disorder (OUD) is a chronic, relapsing disorder and a growing public health crisis. In 2018, approximately 10.3 million Americans misused opioids (Substance Abuse and Mental Health Services Administration, 2019), and over 48,000 deaths were linked to opioid-related overdoses (Wilson et al., 2020). Pharmacotherapies such as methadone, buprenorphine and naltrexone are highly effective at reducing opioid craving and relapse and can hence save lives (Volkow et al., 2014). However, impaired cognitive and emotional processing undermines patients’ ability to adhere to these treatments, while the individual variability in these impairments makes it difficult to personalize treatments and predict treatment outcomes. Therefore, there is an urgent need to research individual differences in brain function associated with opioid use and treatment success.
The ability to regulate prepotent behavioral responses to emotionally salient stimuli, i.e., emotional inhibitory control, is central to effective coping with real-life emotional and cognitive challenges. Poor emotional inhibitory control is characteristic of various types of psychopathology (Elliott et al., 2004, Erickson et al., 2005, Hummer et al., 2013, Köchel et al., 2012, Magnuson et al., 2019, Sætren et al., 2021) including addiction (Ely et al., 2020, Loeber and Duka, 2009, Shi et al., 2019). Patients with substance use disorders exhibit deficits in both behavioral response inhibition (Lubman et al., 2009, Salloum et al., 2007, Wilcox et al., 2016) and processing of naturally salient stimuli (Moeller et al., 2016, Smith et al., 2014). Using functional magnetic resonance imaging (fMRI), studies have shown that patients with OUD have blunted frontolimbic responses to non-drug emotional stimuli vs. drug-related stimuli (Shi et al., 2021, Shi et al., 2018) and lower prefrontal and parietal engagement on cognitive control (Fu et al., 2008). While most previous studies have used domain-specific behavioral paradigms (e.g., cue-reactivity and go/no-go tasks) to investigate emotional and cognitive deficits separately, they do not take into consideration that in real life, emotional and cognitive challenges often occur concurrently. For example, in order to achieve abstinence, it would require a patient to not only recognize aversive consequences of drug use (i.e., an emotional challenge) but also to be able to suppress compulsive drug seeking (i.e., a cognitive challenge). A paradigm that captures both emotional and cognitive components of emotional inhibitory control may more closely approximate patients’ real-life challenges and hence possess better external validity. Moreover, the conventional mass-univariate approach that remains the most widely used technique in the analysis of fMRI data has limited ability to integrate effects across multiple regions that are jointly linked to addiction severity. Multivariate pattern analysis (MVPA) offers a powerful alternative to the univariate approach that applies machine learning to the investigation of multivariate brain activity patterns (Kriegeskorte et al., 2006, Mahmoudi et al., 2012, Norman et al., 2006, Sundermann et al., 2014). It is well-suited to the study of multifaceted psychiatric disorders like addiction (Goldstein and Volkow, 2011, Kwako et al., 2016) and has strong potential for the identification of neural signatures of clinical diagnoses and treatment responses (Abi-Dargham and Horga, 2016, Garrison and Potenza, 2014, Orrù et al., 2012, Sundermann et al., 2014, Volkow et al., 2015, Woo et al., 2017).
The current study aimed to investigate the neural substrates of emotional inhibitory control and its association with individual differences in drug use severity and response to extended-release naltrexone (XR-NTX), an opioid antagonist treatment that blocks the effect of opioids for 30 days (Krupitsky et al., 2011). We used a previously reported affective go/no-go (AGN) paradigm, in which participants are asked to respond to frequently presented naturally positive stimuli (i.e., “go”), and to inhibit responses to infrequently presented naturally negative stimuli (i.e., “no-go”) (Goldman et al., 2015, Shi et al., 2019). The task is affectively congruent with the human predisposition to approach rewards and avoid harms (Chen and Bargh, 1999, Goldman et al., 2015), a capacity that is compromised by chronic preoccupancy with drug use (Kakoschke et al., 2019, Wiers et al., 2013, Wiers et al., 2014). We used MVPA with partial least squares regression (PLSR) (Krishnan et al., 2011) to uncover the multivariate brain activity pattern that best accounts for the individual differences in drug use severity measured by the Addiction Severity Index (ASI), an instrument widely used in the clinical research and treatment of substance use disorders (McLellan et al., 2006, McLellan et al., 1980). Furthermore, we explored the relative external validity of drug use severity and the multivariate brain activity pattern by examining their correlations with (1) opioid craving ratings on the same day as the fMRI scan, and (2) future residual opioid craving approximately 10 days after starting treatment with XR-NTX. First, we hypothesized that the severity of drug use would negatively covary with responses to aversive no-go signal in the frontoparietal cortices that subserve emotional inhibitory control (Brown et al., 2012). Second, we hypothesized that compared to drug use severity, the PLSR brain activity pattern would show greater associations with baseline opioid craving and future opioid craving during XR-NTX treatment.
2. Materials and methods
2.1. Participants
Twenty-nine patients with OUD who were recently detoxified in preparation for XR-NTX treatment (see Study Medications section) were enrolled. Two participants were excluded due to excessive errors of omission on the AGN task (>75% missed response on go trials), and one excluded due to excessive head motion during fMRI (>1 voxel), which was an a priori cut-off used previously (Shi et al., 2021, Shi et al., 2018). Results from analyses that included the participant with excessive head motion are reported in the Supplementary Information. The characteristics of the remaining 26 participants are summarized in Table 1. Benefits of participation included a free, medically supervised, 3-month treatment for OUD with XR-NTX, and referral to community providers after study completion. All participants gave written informed consent to participate in the protocol approved by the University of Pennsylvania Institutional Review Board.
Table 1.
Participant demographic and clinical characteristics.
| Variable | mean ± SD/count |
|---|---|
| Sex | 7 female, 19 male |
| Age (years) | 28.69 ± 9.90 |
| Race | 3 AA, 23 Caucasian |
| Ethnicity | 3 Hispanic |
| Education (years) | 14.04 ± 1.68 |
| COWS score | 3.57 ± 2.19 |
| HAM-A score | 6.92 ± 5.11 |
| HAM-D score | 7.96 ± 5.52 |
| Days of abstinence | 18.43 ± 24.95 |
| ASI drug use severity score | 0.30 ± 0.11 |
| Craving (baseline) | 3.24 ± 2.57 |
| Craving (on-treatment) | 2.14 ± 2.75 |
Abbreviations: COWS, Clinical Opiate Withdrawal Scale; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale; ASI, Addiction Severity Index; AA, African American.
2.2. Inclusion and exclusion criteria
DSM-IV-TR diagnosis of opioid dependence was established using the best estimate format, on the basis of all available sources of information including history and physical examination and the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Inclusion criteria were age between 18 and 59 years; a DSM-IV-TR diagnosis of opioid dependence confirmed by self-report and medical records documenting daily opioid use for >2 weeks in the past 3 months; evidence of detoxification from opioids before XR-NTX injections, established by urine drug screen (UDS) (Redwood Toxicology Laboratory) and a negative naloxone challenge test (Krupitsky et al., 2011); and good physical health ascertained by history and physical examination, blood chemistry and urinalysis. Exclusion criteria were current use of medications that could confound blood oxygen level-dependent (BOLD) fMRI response, such as anti-dopaminergic agents, anticonvulsants, and β-blockers; current psychosis, dementia, intellectual disability, or lifetime history of schizophrenia; clinically significant cardiovascular, hematologic, hepatic, renal, pulmonary, metabolic, gastrointestinal, neurologic, or endocrine abnormalities; pregnancy or breastfeeding; history of clinically significant head trauma; contraindications for XR-NTX treatment, including medical conditions requiring opioid analgesics such as chronic pain disorder, planned surgery, obesity, elevated liver enzymes >3 times the upper limit of normal, or failure to complete opioid detoxification; contraindications for MRI, such as indwelling magnetically active foreign bodies, or fear of enclosed spaces; and current use of illicit drugs (e.g., cocaine) except marijuana
2.3. Study medication
Participants were detoxified using non-opioid withdrawal management medications (e.g., clonidine) in combination with supportive measures, except for one participant who was already detoxified elsewhere upon enrollment, and his detoxification regimen included tapered doses of buprenorphine/naloxone (Suboxone®). To ensure completeness of opioid detoxification, XR-NTX was preceded by a challenge with 0.6 mg of naloxone hydrochloride IV. Participants were offered up to three monthly intramuscular injections of XR-NTX (380 mg of naltrexone-HCl gradually released from dissolvable polymer microspheres over a period of one month, manufactured by Alkermes Inc., Cambridge, MA, under the brand name Vivitrol®), free of charge. As part of consent procedure, participants were briefed about the expected loss of pharmacological effects of opioids resulting from the XR-NTX treatment and the dangers of attempting to overcome the opioid receptor blockade with higher than usual opioid doses. Medication was provided in the context of ongoing psychosocial support (two weekly sessions of professional drug counseling and anti-relapse strategies by trained clinical psychologists) and twice-weekly UDS monitoring.
2.4. Procedure
Participants completed the following clinical assessments after the informed consent procedure: the MINI (Sheehan et al., 1998), the ASI 5th edition (McLellan et al., 1980), the Clinical Opiate Withdrawal Scale (COWS) (Wesson and Ling, 2003), the Hamilton Anxiety Rating Scale (HAM-A) (Hamilton, 1959), and the 24-item version of the Hamilton Depression Rating Scale (HAM-D) (Guy, 1976). Drug use severity was indexed by the ASI drug composite score (McLellan et al., 1980). Opioid craving was assessed using a 10-point self-reported craving scale (0 = none; 9 = extremely), which has shown good external validity in prior studies (Franklin et al., 2015, Franklin et al., 2007, Shi et al., 2018). The craving score was missing in one participant. The COWS score was missing in three participants. The HAM-A and HAM-D scores were missing in two participants. Participants then completed the fMRI AGN task adopted from previous research (Goldman et al., 2015, Shi et al., 2019). The go trials included 122 positively valenced pictures depicting naturally rewarding stimuli (e.g., sweets), and the no-go trials were 38 negatively valenced pictures depicting naturally aversive stimuli (e.g., snakes). Each trial consisted of a stimulus displayed for 300 ms, followed by a 1700-ms baseline period during which a crosshair was displayed. We used an invariant inter-trial interval in order to facilitate automaticity of prepotent “go” response tendencies (Erickson et al., 2005, Wessa et al., 2007). Pseudo-random order of the stimuli and baseline periods was generated using optseq2 (surfer.nmr.mgh.harvard.edu/optseq). A subsample of 22 participants received an XR-NTX injection 2.22 (±7.54) days after the fMRI session and completed the on-treatment craving assessment 10.64 (±2.38) days after the injection.
2.5. fMRI data acquisition and processing
MRI data were collected using a Siemens Tim Trio 3-Tesla scanner. BOLD fMRI was performed, using a whole-brain, single-shot gradient-echo echo-planar sequence with the following parameters: repetition time (TR)/echo time (TE) = 2000/30 ms, field of view (FOV) = 220 × 220 mm2, matrix = 64 × 64, slice thickness/gap = 4.5/0 mm, 32 slices, effective voxel resolution of 3.4 × 3.4 × 4.5 mm3, flip angle (FA) = 90°. T1-weighted structural images were acquired using the magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following parameters: TR/TE = 1510/3.71 ms, FOV = 256 × 192 mm3, matrix = 256 × 192, slice thickness/gap = 1/0 mm, 160 slices, effective voxel resolution of 1 × 1 × 1 mm3, FA = 9°. An oblique acquisition, oriented along the anterior commissure–posterior commissure line, allowed coverage of the entire brain with the exception of the lower cerebellum.
Imaging data analysis was performed using MATLAB 2020a (MathWorks, Natick, MA) and SPM 12 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were adjusted for slice timing, realigned to the mean image and motion corrected, normalized into the stereotactic Montreal Neurological Institute (MNI) space with 3-mm cubic voxels by applying deformation field, and spatially smoothed by a Gaussian filter with full-width at half-maximum parameter (FWHM) set to 8 mm. Individual-level statistical analyses were performed by modeling go and no-go trials using a canonical hemodynamic response function and its temporal derivative. Multicollinearity was low for both the “go” and “no-go” regressors (variance inflation factor = 1.85 & 1.87). We focused on the “no-go vs. go” contrast which is the most common contrast used to examine inhibitory control in prior go/no-go studies (Criaud and Boulinguez, 2013).
2.6. Statistical analysis
MVPA was performed using the MATLAB Statistics and Machine Learning Toolbox’s implementation of PLSR (de Jong, 1993, Krishnan et al., 2011). PLSR finds a set of latent variables that simultaneously projects the independent variables to a low-dimensional space and predicts the dependent variable(s). In the case of p independent variables and q dependent variables in n observations, the underlying PLSR model that projects the data to k latent components can be expressed as X = TPT and Ŷ = TWQT, where X and Ŷ are the n × p and n × q independent and predicted dependent variable matrices, respectively; T is the n × k matrix for X’s latent components; P and Q are the p × k and q × k orthogonal loading matrices, respectively; W is the k × k diagonal matrix for regression weights. The PLSR approach is well suited for analyzing fMRI data where the number of brain regions is often larger than the number of participants (p≫n), and there is multicollinearity among brain activity across regions (Krishnan et al., 2011). Specifically, in the case of k = 1 and q = 1, we have Ŷ = XPT+WQT = XPTWQ/||P||2 (where PT+ is the Moore-Penrose pseudo-inverse of PT), which would allow vector P to be interpreted as a scaled indicator of activation pattern proposed by Haufe et al. (2014).
Before performing PLSR, we first subdivided the cortex into 1000 functionally homogeneous parcels using the atlas developed by Schaefer et al. (2018), with each parcel belonging to one of the 7 previously established brain networks: cognitive control network (CCN), dorsal attention network (DAN), ventral attention network (VAN, also known as the salience network), default mode network (DMN), visual network (VN), somatomotor network (SMN), and limbic network (LN) (Yeo et al., 2011). Contrast values were extracted from each parcel and entered into the PLSR as the independent variables, and drug use severity was entered as the dependent variable. To avoid overfitting, the PLSR model was validated using 10-fold cross-validation (CV) with 10 Monte-Carlo repetitions, resulting in 100 training–testing partitions (Varoquaux et al., 2017, Whelan and Garavan, 2014). Within each training set, nested 10-fold CV with 10 Monte-Carlo repetitions was used to determine the optimal value for hyperparameter k (i.e., the number of latent components) by grid search over k = [1, 2 … 10] (Varma and Simon, 2006, Varoquaux et al., 2017, Yip et al., 2020). Prediction accuracy was indexed by the cross-validated mean squared error (MSE). The statistical significance of MSE and that of the reliability of regional loadings (P) were determined by 5000-iteration permutation and bootstrap tests, respectively (McIntosh and Lobaugh, 2004). Due to a total of 1000 parcels being examined, the p-values for loadings were further corrected for false discovery rate (FDR) following the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995).
We evaluated the engagement of the 7 brain networks in the PLSR model using a procedure adapted from Kozák et al. (2017). Each network’s engagement metric was calculated as the average of supra-threshold PLSR loadings that achieved FDR-corrected p < 0.05 within that network. The metric was further normalized by first subtracting the minimum of supra-threshold loadings and then dividing it by the range of supra-threshold loadings across the brain (Kozák et al., 2017). The relative importance of each network was examined by comparing the normalized engagement metric of that network with the average of engagement metrics of the other 6 networks.
The external validity of the latent PLSR brain score was investigated by examining its association with baseline and on-treatment levels of opioid craving using Pearson correlation. Commonality analysis was performed to compare the relative contribution of the ASI drug use severity measure and the brain score in accounting for individual variability in craving (Seibold and McPhee, 1979). Given two predictors X1 and X2, their unique contributions were calculated as R122–R22 and R122–R12, respectively, and their common contribution was calculated as R12 + R22–R122. Here, R12, R22, and R122 stand for the coefficients of determination for the models with X1 only, with X2 only, and with both X1 and X2, as predictor(s), respectively. Using a 5000-iteration bootstrap test, we compared the unique contributions of drug use severity and the brain score to the prediction of baseline craving and that of on-treatment craving (Nimon and Oswald, 2013).
Exploratory whole-brain analysis using the conventional mass-univariate approach was performed to examine the effect of no-go vs. go stimuli as well as the association between brain response and drug use severity. Exploratory PLSR analysis used the raw contrast values from the 1000 cortical parcels to predict craving scores. Results of these exploratory analyses are reported in the Supplementary Information.
3. Results
The average errors of commission and omission during the fMRI AGN task were 39.17% (SD = 18.48%) and 3.69% (SD = 4.72%), respectively. Drug use severity was not associated with any of the demographic, behavioral or clinical variables listed in Table 1 (p’s > 0.052) except for a positive correlation with baseline opioid craving (r = 0.46, p = 0.021) and negative correlation with number of days of abstinence (r = –0.40, p = 0.045).
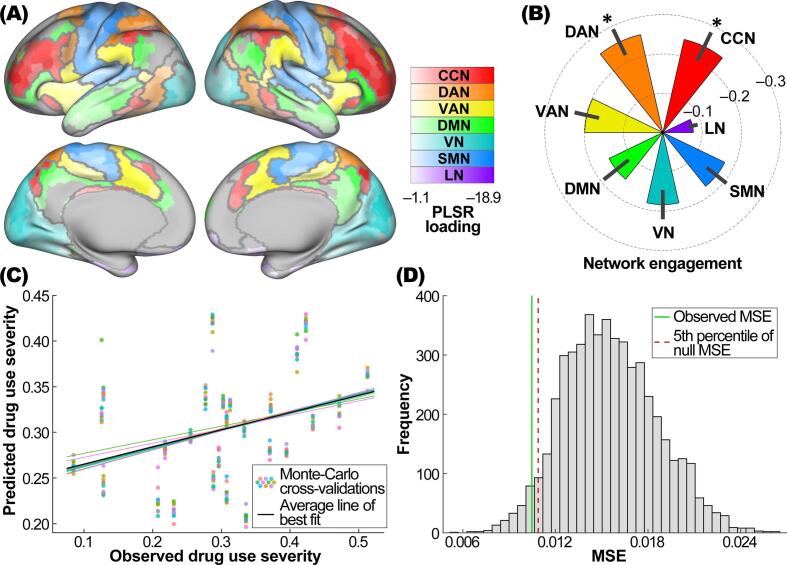
The multivariate PLSR model achieved optimal performance in predicting drug use severity when the number of latent components was set to one (i.e., k = 1) in most of the CV iterations (99%). Drug use severity was associated with distributed brain hypoactivity in response to the no-go vs. go stimuli, as evidenced by significantly negative loadings in 874 of the 1000 cortical parcels (FDR-corrected p’s < 0.05) (see Fig. 1A). No regions showed significantly positive loadings. Of the 7 brain networks, the CCN and DAN had significantly more negative normalized engagement metrics compared to the average of other networks (CCN vs. others, –0.24 vs. –0.18, 95% bootstrap confidence interval (CI) of difference = –0.10 to –0.02, FDR-corrected p = 0.024; DAN vs. others, –0.26 vs. –0.18, 95% bootstrap CI of difference = –0.12 to –0.02, FDR-corrected p = 0.033), while the LN engagement was significantly less negative than the average of other networks (–0.08 vs. –0.20, 95% bootstrap CI of difference = 0.06 to 0.16, FDR-corrected p = 0.003) (see Fig. 1B). The association between the observed and predicted drug use severity scores is shown in Fig. 1C. The cross-validated prediction error was significantly lower than those obtained from a 5000-iteration permutation test (observed MSE = 0.010, 5th percentile of null MSE distribution = 0.011, p = 0.035; see Fig. 1D).
Fig. 1.
Results of multivariate pattern analyses. (A) Brain parcels with significantly negative PLSR loadings (FDR-corrected p < 0.05), color-coded by brain network belongingness. Brain parcellation (1000 parcels) and network assignment were based on atlases developed by Schaefer et al., 2018, Yeo et al., 2011, respectively. Brain networks are outlined in grey. (B) Normalized engagement metric for each brain network. Error bars represent bootstrap standard errors. *: significantly more negative engagement than the average of other networks at FDR-corrected p < 0.05. (C) Observed drug use severity vs. its predicted values from 10 Monte-Carlo repetitions of 10-fold cross-validation of the PLSR model. (D) Cross-validated MSE of the PLSR model compared to the null distribution of MSE obtained from a 5000-iteration permutation test. Solid green line represents the actual MSE from the original model. Dashed red line represents the 5th percentile of the empirical MSE null distribution. Abbreviations: PLSR, partial least squares regression; CCN, cognitive control network; DAN, dorsal attention network; VAN, ventral attention network; DMN, default mode network; VN, visual network; SMN, somatomotor network; LN, limbic network; MSE, mean squared error; FDR, false discovery rate.
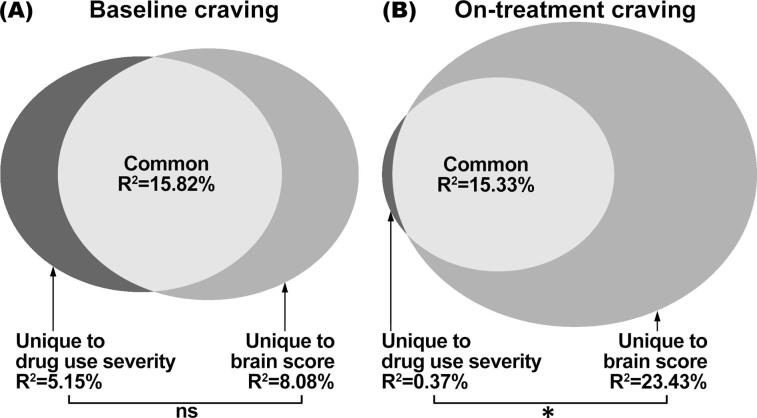
The PLSR brain score obtained from the multivariate neural response pattern was significantly correlated with both baseline opioid craving (r = 0.49, p = 0.013) and on-treatment opioid craving (r = 0.62, p = 0.002). It was not significantly associated with any other demographic, behavioral or clinical variables (p’s > 0.14). Commonality analyses showed that the brain score and drug use severity made comparable unique contributions in accounting for the variance in baseline craving (ΔR2 = 2.93%, 95% bootstrap CI = –23.05% to 25.76%, p = 0.74; see Fig. 2A). For on-treatment craving, the brain score made significantly greater unique contribution than drug use severity (ΔR2 = 23.06%, 95% bootstrap CI = 3.19% to 48.77%, p = 0.022; see Fig. 2B).
Fig. 2.
Results of commonality analyses. (A) Comparable amount of variance in baseline opioid craving was uniquely explained by drug use severity and by the PLSR brain score. ns: non-significant. (B) Significantly more variance in future on-treatment opioid craving was uniquely explained by PLSR brain score than by drug use severity. *: p < 0.05. Abbreviation: PLSR, partial least squares regression.
Neither drug use severity nor the MVPA brain score was correlated with craving reduction (baseline minus on-treatment, r = –0.06 & –0.25, p = 0.78 & 0.27). Considering that a higher level of baseline craving had more room for a subsequent reduction, we adjusted craving reduction for baseline craving. We found that the brain score, but not drug use severity, was significantly negatively correlated with adjusted craving reduction (r = –0.46 & –0.23, p = 0.034 & 0.31). Commonality analyses showed that the brain score made significantly greater unique contribution than drug use severity (ΔR2 = 16.28%, 95% bootstrap CI = 0.42% to 47.03%, p = 0.042) in accounting for the variance in adjusted craving reduction.
4. Discussion
Using an MVPA machine learning approach, we found that clinically measured drug use severity in OUD patients was associated with a multivariate brain hypoactivity pattern in response to aversive no-go stimuli. Such a pattern was characterized by contributions from largely distributed brain regions, with particularly prominent engagement of the CCN and DAN networks that span across the frontoparietal cortices. This finding is in line with previous studies indicating lower frontoparietal response to no-go signals in OUD patients compared to non-dependent controls (Fu et al., 2008). It also provides evidence for an association between functional brain hypoactivation during emotional inhibitory control and clinical severity in OUD. Moreover, while drug use severity and the brain pattern were similarly correlated with baseline opioid craving, the brain pattern accounted for a greater proportion of variance in future craving during XR-NTX treatment. It suggests that compared to clinically measured drug use severity, the brain activity pattern may have better external validity.
Addiction is a complex brain disease that impacts multiple psychological domains and brain regions. One of the well-established phenomena in addiction is abnormal incentive salience (Kwako et al., 2016). Chronic drug use results in emotional dysregulation that disrupts normal processing of natural, non-drug rewards and punishments and assigns high incentive value to drugs of abuse (Elman and Borsook, 2016, Gardner, 2011, Kenny et al., 2006, Keramati and Gutkin, 2013). Neuroimaging studies have shown blunted frontolimbic (Noori et al., 2016) and occipital (Hanlon et al., 2014) responses to non-drug stimuli compared to drug-related stimuli. In addition, those with substance use disorders exhibit various cognitive control deficits including inhibitory dysregulation (Kwako et al., 2016). The ability to inhibit prepotent but inappropriate behavioral responses is crucial for maintaining normal social and occupational functioning. Inhibitory control is governed by multiple frontoparietal regions including the medial and lateral prefrontal cortex, anterior cingulate cortex, and the superior and inferior parietal lobules (Swick et al., 2011, Zhang et al., 2017). Compared to healthy controls, patients with OUD show reduced neural response in these regions during inhibitory control (Fu et al., 2008). In the current study, we investigated emotional inhibitory control in OUD patients with the AGN task that involved both emotional and cognitive processing, i.e., natural saliency encoding and behavioral inhibitory control. Using PLSR, we found that clinical drug use severity was negatively associated with a multivariate pattern of neural activity across a large number of cortical regions. This is consistent with the fact that OUD affects a wide range of neuropsychological functions. Moreover, the PLSR model did not decompose the brain pattern into separate emotional and cognitive components. Instead, nested cross-validation identified a single-component solution that best accounted for individual differences in drug use severity. It suggests that the emotional and cognitive processes are interrelated during emotional inhibitory control and may jointly contribute to the maintenance of OUD.
ASI is a clinical instrument broadly used in research and treatment settings (McLellan et al., 2006). Specifically, the drug use composite score derived from ASI has been used as both a diagnostic tool (Rikoon et al., 2006) and an indicator for intervention outcome (Roy-Byrne et al., 2014). We found that baseline drug use severity was correlated with baseline but not subsequent, on-treatment opioid craving, which is consistent with the external validity of ASI that is often but not always satisfactory (Leonhard et al., 2000, Mäkelä, 2004). Here we made the first attempt to use ASI to guide the search for a multivariate brain signature for drug use severity in OUD. Interestingly, the brain signature, characterized by a large-scale pattern of cortical hypoactivity, correlated with opioid craving both at baseline and during subsequent treatment. This finding underscores a potentially greater prognostic value of the multivariate brain response pattern than clinically measured addiction severity. It also adds to a growing literature reporting the use of fMRI to predict other mental health outcomes such as smoking cessation (Falk et al., 2011, Shi et al., 2017) and OUD treatment adherence (Shi et al., 2019, Wang et al., 2015). In addition, we noted that the brain response obtained at baseline appeared to be more correlated with on-treatment craving (r = 0.62) than with baseline craving (r = 0.49). The difference may reflect the fact that the current sample comprised treatment-seeking patients whose brain activity during emotional inhibitory control may better indicate potential treatment benefit than current drug motivation.
Craving is an important characteristic of substance use disorders (American Psychiatric Association, 2013). Reduction in craving is one of the key objectives of substance use disorder treatments (Wise, 1988). Traditionally, measurement of craving has mainly relied on self-reports, which can be influenced by cognitive bias and poor insight (Sayette et al., 2000). Objective alternatives to self-reports have been proposed, ranging from implicit behavioral probes (e.g., attentional bias (Field et al., 2009), approach bias (Wiers et al., 2014)) to functional neuroimaging markers. The latter includes resting-state fMRI indices of cortical functional connectivity (Li et al., 2016) and task-based fMRI measures of regional neural response to drug cues (Kühn and Gallinat, 2011) and stress (Sinha et al., 2005). The association we found between the neural response during emotional inhibitory control and opioid craving contributes to the efforts for elucidating the neural mechanism of craving. A better understanding of neural mechanisms underlying drug craving may help uncover novel brain targets for addiction treatments that aim to reduce craving and prevent relapse (Wise, 1988).
Recent MVPA studies have reported neural indicators of abnormal incentive salience in smokers (Elton et al., 2019), treatment response in internet gamers (Wang et al., 2020), and problematic eating behavior (Cosme and Lopez, 2020). Our study is among the first to utilize MVPA to investigate individual differences in drug use severity among OUD patients. Compared to the conventional mass-univariate approach, MVPA and other multivariate methods provide greater sensitivity in detecting subtle intra- and inter-individual differences (Kriegeskorte et al., 2006, Mahmoudi et al., 2012, Norman et al., 2006, Sundermann et al., 2014). By studying brain response patterns across multiple regions instead of examining individual regions (e.g., voxels), multivariate analysis can also negate the need for excessive correction for multiple comparisons and thus improve statistical power (Cremers et al., 2017). Moreover, the use of cross-validation (and particularly nested cross-validation) prevents model overfitting and promotes generalizability (Yip et al., 2020). In the current study, we used PLSR to establish the multivariate brain signature for drug use severity. By taking a dimension reduction approach, PLSR projects features (e.g., neural response) to a low-dimensional space by accounting for their underlying covariance structure and produces one or more latent variables that best predict the outcomes (Krishnan et al., 2011, McIntosh and Lobaugh, 2004). These properties make PLSR well-suited for analyzing fMRI data, where brain regions with similar functions tend to have covarying fMRI signals. Applying PLSR to translational addiction fMRI research may advance the discovery of novel mechanistic neural signatures of clinical severity and treatment response.
In addition to its many practical benefits, the prospective within-subjects design of our study has several limitations. First, the relatively small sample size is prone to undetected effects (i.e., Type II error) and requires replication. Second, our study did not include a healthy control group, which would have enabled a normative comparison of the neuroimaging findings. Third, our study treatment was the antagonist XR-NTX. Hence, replication with the more commonly used opioid agonist methadone and partial agonist buprenorphine is required to show the generalizability of our findings. Fourth, the AGN task that we used was limited to investigating emotional inhibitory control. Other domains of neurobehavioral functioning such as attentional and approach bias (Field et al., 2009, Wiers et al., 2014), stress reactivity (MacLean et al., 2019), social cognition (McDonald et al., 2013) and decision making (Schoenbaum et al., 2006) could have yielded complementary results. Fifth, alternative multivariate regression methods exist. Unlike PLSR, methods such as regression trees (Kesler et al., 2017) and artificial neural networks (Yan et al., 2019) allow the study of non-linear relationships between predictors and outcomes. Other methods like stepwise regression (Loughead et al., 2015), supporter vector regression (Smyser et al., 2016) and elastic net regression (Marquand et al., 2012) eliminate features that make little contribution to outcome prediction. Future studies are needed to determine if non-linear and feature-elimination approaches yield similar or complementary findings. Lastly, fMRI is expensive and not widely available in the substance abuse treatment settings, limiting its direct clinical utility. Therefore, replication studies with electroencephalography or functional near-infrared spectroscopy are warranted.
In conclusion, our findings provide evidence for an association between drug use severity and the widespread functional neural hypoactivity during emotional inhibitory control in OUD. Such a distributed neural hypoactivity pattern is consistent with the multifaceted nature of OUD, whose severity may be attributed to impairments in multiple brain networks. Our findings also highlight the power of the multivariate approach in uncovering large-scale cortical substrates associated with clinical severity in complex psychiatric disorders and in predicting treatment response.
5. Funding and disclosure
This work was supported by the Commonwealth of Pennsylvania CURE grant SAP#4100055577 (PI: Anna Rose Childress) and the following National Institutes of Health grants: DA051709 (PI: Zhenhao Shi), DA024553 (PI: Charles P. O’Brien), DA028874 (PI: Anna Rose Childress), DA036028 (PI: Daniel D. Langleben), DA043983 (PI: Daniel D. Langleben), and AA026892 (PI: Corinde E. Wiers). The authors report no conflict of interest.
Acknowledgments
Acknowledgements
The authors wish to thank Dr. Kevin G. Lynch, Ms. Kanchana Jagannathan, Ms. Victoria P. Fairchild, and Mr. James H. Padley for their assistance in data analysis.
Author contributions
DDL, CPO and ARC designed the study. DDL and ARC collected the data. ZS and CEW performed the analyses, interpreted the results, and wrote the manuscript. All authors contributed to the revision of the manuscript for critical intellectual content. All authors approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102806.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abi-Dargham A., Horga G. The search for imaging biomarkers in psychiatric disorders. Nat. Med. 2016;22:1248–1255. doi: 10.1038/nm.4190. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Royal Stat. Soc.: Ser. B (Stat. Methodol.) 1995;57:289–300. [Google Scholar]
- Brown M.R.G., Lebel R.M., Dolcos F., Wilman A.H., Silverstone P.H., Pazderka H., Fujiwara E., Wild T.C., Carroll A.M., Hodlevskyy O. Effects of emotional context on impulse control. NeuroImage. 2012;63:434–446. doi: 10.1016/j.neuroimage.2012.06.056. [DOI] [PubMed] [Google Scholar]
- Chen M., Bargh J.A. Consequences of automatic evaluation: immediate behavioral predispositions to approach or avoid the stimulus. Pers. Soc. Psychol. Bull. 1999;25:215–224. [Google Scholar]
- Cremers H.R., Wager T.D., Yarkoni T. The relation between statistical power and inference in fMRI. PLoS ONE. 2017;12:e0184923. doi: 10.1371/journal.pone.0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M., Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci. Biobehav. Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- de Jong S. SIMPLS: an alternative approach to partial least squares regression. Chemometr. Intell. Lab. Syst. 1993;18:251–263. [Google Scholar]
- Elliott R., Ogilvie A., Rubinsztein J.S., Calderon G., Dolan R.J., Sahakian B.J. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol. Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Elman I., Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89:11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Elton A., Chanon V.W., Boettiger C.A. Multivariate pattern analysis of the neural correlates of smoking cue attentional bias. Pharmacol. Biochem. Behav. 2019;180:1–10. doi: 10.1016/j.pbb.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely A.V., Jagannathan K., Hager N., Ketcherside A., Franklin T.R., Wetherill R.R. Double jeopardy: comorbid obesity and cigarette smoking are linked to neurobiological alterations in inhibitory control during smoking cue exposure. Addict. Biol. 2020;25:e12750. doi: 10.1111/adb.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K., Drevets W.C., Clark L., Cannon D.M., Bain E.E., Zarate C.A., Jr., Charney D.S., Sahakian B.J. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am. J. Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Berkman E.T., Whalen D., Lieberman M.D. Neural activity during health messaging predicts reductions in smoking above and beyond self-report. Health Psychol. 2011;30:177–185. doi: 10.1037/a0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Munafò M.R., Franken I.H.A. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Jagannathan K., Wetherill R.R., Johnson B., Kelly S., Langguth J., Mumma J., Childress A.R. Influence of menstrual cycle phase on neural and craving responses to appetitive smoking cues in naturally cycling females. Nicotine Tob. Res. 2015;17:390–397. doi: 10.1093/ntr/ntu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T.R., Wang J., Sciortino N., Harper D., Li Y., Ehrman R., Kampman K., O'Brien C.P., Detre J.A., Childress A.R. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Fu L.P., Bi G.H., Zou Z.T., Wang Y., Ye E.M., Ma L., Ming F., Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci. Lett. 2008;438:322–326. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Gardner E.L. Addiction and brain reward and antireward pathways. Adv. Psychosom. Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K.A., Potenza M.N. Neuroimaging and biomarkers in addiction treatment. Curr. Psychiatry Rep. 2014;16:513. doi: 10.1007/s11920-014-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M., Ehrman R.N., Suh J.J., Hurt H., Marquez K., Franklin T.R., O’Brien C.P., Childress A.R. Brief report: “Spiders-No, Puppies-Go”, introducing a novel Go NoGo task tested in inner city adolescents at risk for poor impulse control. J. Adolesc. 2015;38:45–48. doi: 10.1016/j.adolescence.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R.Z., Volkow N.D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Department of Health, Education, and Welfare; Washington, DC, U.S: 1976. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hanlon C.A., Dowdle L.T., Naselaris T., Canterberry M., Cortese B.M. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend. 2014;143:206–212. doi: 10.1016/j.drugalcdep.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufe S., Meinecke F., Görgen K., Dähne S., Haynes J.-D., Blankertz B., Bießmann F. On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage. 2014;87:96–110. doi: 10.1016/j.neuroimage.2013.10.067. [DOI] [PubMed] [Google Scholar]
- Hummer T.A., Hulvershorn L.A., Karne H.S., Gunn A.D., Wang Y., Anand A. Emotional response inhibition in bipolar disorder: a functional magnetic resonance imaging study of trait-and state-related abnormalities. Biol. Psychiatry. 2013;73:136–143. doi: 10.1016/j.biopsych.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoschke N., Albertella L., Lee R.S.C., Wiers R.W. Assessment of automatically activated approach–avoidance biases across appetitive substances. Curr. Addict. Rep. 2019;6:200–209. [Google Scholar]
- Kenny P.J., Chen S.A., Kitamura O., Markou A., Koob G.F. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramati M., Gutkin B. Imbalanced decision hierarchy in addicts emerging from drug-hijacked dopamine spiraling circuit. PLoS ONE. 2013;8:e61489. doi: 10.1371/journal.pone.0061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R., Rao A., Blayney D.W., Oakley-Girvan I.A., Karuturi M., Palesh O. Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front. Hum. Neurosci. 2017;11:555. doi: 10.3389/fnhum.2017.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köchel A., Leutgeb V., Schienle A. Affective inhibitory control in adults with attention deficit hyperactivity disorder: abnormalities in electrocortical late positivity. Neurosci. Lett. 2012;530:47–52. doi: 10.1016/j.neulet.2012.09.053. [DOI] [PubMed] [Google Scholar]
- Kozák L.R., van Graan L.A., Chaudhary U.J., Szabó Á.G., Lemieux L. ICN_Atlas: automated description and quantification of functional MRI activation patterns in the framework of intrinsic connectivity networks. NeuroImage. 2017;163:319–341. doi: 10.1016/j.neuroimage.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Goebel R., Bandettini P. Information-based functional brain mapping. PNAS. 2006;103:3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A., Williams L.J., McIntosh A.R., Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. NeuroImage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Krupitsky E., Nunes E.V., Ling W., Illeperuma A., Gastfriend D.R., Silverman B.L. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–1513. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Kwako L.E., Momenan R., Litten R.Z., Koob G.F., Goldman D. Addictions Neuroclinical Assessment: a neuroscience-based framework for addictive disorders. Biol. Psychiatry. 2016;80:179–189. doi: 10.1016/j.biopsych.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C., Mulvey K., Gastfriend D.R., Shwartz M. The Addiction Severity Index: a field study of internal consistency and validity. J. Subst. Abuse Treat. 2000;18:129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- Li Q., Li Z., Li W., Zhang Y., Wang Y., Zhu J., Chen J., Li Y., Yan X., Ye J. Disrupted default mode network and basal craving in male heroin-dependent individuals: a resting-state fMRI study. J. Clin. Psychiatry. 2016;77:1211–1217. doi: 10.4088/JCP.15m09965. [DOI] [PubMed] [Google Scholar]
- Loeber S., Duka T. Acute alcohol decreases performance of an instrumental response to avoid aversive consequences in social drinkers. Psychopharmacology. 2009;205:577–587. doi: 10.1007/s00213-009-1565-9. [DOI] [PubMed] [Google Scholar]
- Loughead J., Wileyto E.P., Ruparel K., Falcone M., Hopson R., Gur R., Lerman C. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40:1311–1320. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman D.I., Yucel M., Kettle J.W., Scaffidi A., Mackenzie T., Simmons J.G., Allen N.B. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Arch. Gen. Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- MacLean R.R., Armstrong J.L., Sofuoglu M. Stress and opioid use disorder: a systematic review. Addict. Behav. 2019;98:106010. doi: 10.1016/j.addbeh.2019.05.034. [DOI] [PubMed] [Google Scholar]
- Magnuson J.R., Peatfield N.A., Fickling S.D., Nunes A.S., Christie G., Vakorin V., D’Arcy R.C.N., Ribary U., Iarocci G., Moreno S. Electrophysiology of inhibitory control in the context of emotion processing in children with autism spectrum disorder. Front. Hum. Neurosci. 2019;13:78. doi: 10.3389/fnhum.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi A., Takerkart S., Regragui F., Boussaoud D., Brovelli A. Multivoxel pattern analysis for fMRI data: a review. Comput. Math. Methods Med. 2012;2012:961257. doi: 10.1155/2012/961257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä K. Studies of the reliability and validity of the Addiction Severity Index. Addiction. 2004;99:398–410. doi: 10.1111/j.1360-0443.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- Marquand A.F., O'Daly O.G., De Simoni S., Alsop D.C., Maguire R.P., Williams S.C., Zelaya F.O., Mehta M.A. Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: a multi-class pattern recognition approach. NeuroImage. 2012;60:1015–1024. doi: 10.1016/j.neuroimage.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S., Darke S., Kaye S., Torok M. Deficits in social perception in opioid maintenance patients, abstinent opioid users and non-opioid users. Addiction. 2013;108:566–574. doi: 10.1111/add.12040. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Cacciola J.C., Alterman A.I., Rikoon S.H., Carise D. The Addiction Severity Index at 25: origins, contributions and transitions. Am. J. Addict. 2006;15:113–124. doi: 10.1080/10550490500528316. [DOI] [PubMed] [Google Scholar]
- McLellan A.T., Luborsky L., Woody G.E., O'Brien C.P. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J. Nerv. Mental Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Moeller S.J., Bederson L., Alia-Klein N., Goldstein R.Z. Neuroscience of inhibition for addiction medicine: from prediction of initiation to prediction of relapse. Prog. Brain Res. 2016;223:165–188. doi: 10.1016/bs.pbr.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimon K.F., Oswald F.L. Understanding the results of multiple linear regression: beyond standardized regression coefficients. Organ. Res. Methods. 2013;16:650–674. [Google Scholar]
- Noori H.R., Cosa Linan A., Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis. Eur. Neuropsychopharmacol. 2016;26:1419–1430. doi: 10.1016/j.euroneuro.2016.06.013. [DOI] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Orrù G., Pettersson-Yeo W., Marquand A.F., Sartori G., Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci. Biobehav. Rev. 2012;36:1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Rikoon S.H., Cacciola J.S., Carise D., Alterman A.I., McLellan A.T. Predicting DSM-IV dependence diagnoses from Addiction Severity Index composite scores. J. Subst. Abuse Treat. 2006;31:17–24. doi: 10.1016/j.jsat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P., Bumgardner K., Krupski A., Dunn C., Ries R., Donovan D., West I.I., Maynard C., Atkins D.C., Graves M.C. Brief intervention for problem drug use in safety-net primary care settings: a randomized clinical trial. JAMA. 2014;312:492–501. doi: 10.1001/jama.2014.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sætren S.S., Augusti E.-M., Hafstad G.S. Affective inhibitory control and risk for internalizing problems in adolescents exposed to child maltreatment: a population-based study. J. Abnorm. Psychol. 2021;130:113–125. doi: 10.1037/abn0000582. [DOI] [PubMed] [Google Scholar]
- Salloum J.B., Ramchandani V.A., Bodurka J., Rawlings R., Momenan R., George D., Hommer D.W. Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol. Clin. Exp. Res. 2007;31:1490–1504. doi: 10.1111/j.1530-0277.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- Sayette M.A., Shiffman S., Tiffany S.T., Niaura R.S., Martin C.S., Shadel W.G. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Kong R., Gordon E.M., Laumann T.O., Zuo X.N., Holmes A.J., Eickhoff S.B., Yeo B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28:3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G., Roesch M.R., Stalnaker T.A. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibold D.R., McPhee R.D. Commonality analysis: a method for decomposing explained variance in multiple regression analyses. Hum. Commun. Res. 1979;5:355–365. [Google Scholar]
- Cosme D., Lopez R.D. Neural indicators of food cue reactivity, regulation, and valuation and their associations with body composition and daily eating behavior. Soc. Cogn. Affect. Neurosci. 2020 doi: 10.1093/scan/nsaa155. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–33. (quiz 34–57) [PubMed] [Google Scholar]
- Shi Z., Jagannathan K., Fairchild V.P., Wang A.L., Suh J.J., Childress A.R., Langleben D.D. Behavioral and accumbal responses during an affective Go/No-go task predict adherence to injectable naltrexone treatment in opioid use disorder. Int. J. Neuropsychopharmacol. 2019;22:180–185. doi: 10.1093/ijnp/pyz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Jagannathan K., Padley J.H., Wang A.L., Fairchild V.P., O’Brien C.P., Childress A.R., Langleben D.D. The role of withdrawal in mesocorticolimbic drug cue reactivity in opioid use disorder. Addict. Biol. 2021;26:e12977. doi: 10.1111/adb.12977. [DOI] [PubMed] [Google Scholar]
- Shi Z., Wang A.L., Aronowitz C.A., Romer D., Langleben D.D. Individual differences in the processing of smoking-cessation video messages: an imaging genetics study. Biol. Psychol. 2017;128:125–131. doi: 10.1016/j.biopsycho.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Wang A.L., Jagannathan K., Fairchild V.P., O’Brien C.P., Childress A.R., Langleben D.D. Effects of extended-release naltrexone on the brain response to drug-related stimuli in opioid use disorder. J. Psychiatry Neurosci. 2018;43:254–261. doi: 10.1503/jpn.170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Lacadie C., Skudlarski P., Fulbright R.K., Rounsaville B.J., Kosten T.R., Wexler B.E. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–180. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- Smith J.L., Mattick R.P., Jamadar S.D., Iredale J.M. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Dosenbach N.U.F., Smyser T.A., Snyder A.Z., Rogers C.E., Inder T.E., Schlaggar B.L., Neil J.J. Prediction of brain maturity in infants using machine-learning algorithms. NeuroImage. 2016;136:1–9. doi: 10.1016/j.neuroimage.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2019. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD.
- Sundermann B., Herr D., Schwindt W., Pfleiderer B. Multivariate classification of blood oxygen level-dependent fMRI data with diagnostic intention: a clinical perspective. Am. J. Neuroradiol. 2014;35:848–855. doi: 10.3174/ajnr.A3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Varma S., Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinf. 2006;7:91. doi: 10.1186/1471-2105-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoquaux G., Raamana P.R., Engemann D.A., Hoyos-Idrobo A., Schwartz Y., Thirion B. Assessing and tuning brain decoders: cross-validation, caveats, and guidelines. NeuroImage. 2017;145:166–179. doi: 10.1016/j.neuroimage.2016.10.038. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Frieden T.R., Hyde P.S., Cha S.S. Medication-assisted therapies — tackling the opioid-overdose epidemic. N. Engl. J. Med. 2014;370:2063–2066. doi: 10.1056/NEJMp1402780. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Koob G., Baler R. Biomarkers in substance use disorders. ACS Chem. Neurosci. 2015;6:522–525. doi: 10.1021/acschemneuro.5b00067. [DOI] [PubMed] [Google Scholar]
- Wang A.L., Elman I., Lowen S.B., Blady S.J., Lynch K.G., Hyatt J.M., O'Brien C.P., Langleben D.D. Neural correlates of adherence to extended-release naltrexone pharmacotherapy in heroin dependence. Transl. Psychiatry. 2015;5:e531. doi: 10.1038/tp.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-L., Potenza M.N., Song K.-R., Fang X.-Y., Liu L., Ma S.-S., Xia C.-C., Lan J., Yao Y.-W., Zhang J.-T. Neural classification of internet gaming disorder and prediction of treatment response using a cue-reactivity fMRI task in young men. J. Psychiatric Res. 2020 doi: 10.1016/j.jpsychires.2020.11.014. In press. [DOI] [PubMed] [Google Scholar]
- Wessa M., Houenou J., Paillère-Martinot M.-L., Berthoz S., Artiges E., Leboyer M., Martinot J.-L. Fronto-striatal overactivation in euthymic bipolar patients during an emotional go/nogo task. Am. J. Psychiatry. 2007;164:638–646. doi: 10.1176/ajp.2007.164.4.638. [DOI] [PubMed] [Google Scholar]
- Wesson D.R., Ling W. The clinical opiate withdrawal scale (COWS) J. Psychoact. Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Whelan R., Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol. Psychiatry. 2014;75:746–748. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Wiers C.E., Kühn S., Javadi A.H., Korucuoglu O., Wiers R.W., Walter H., Gallinat J., Bermpohl F. Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology. 2013;229:187–197. doi: 10.1007/s00213-013-3098-5. [DOI] [PubMed] [Google Scholar]
- Wiers C.E., Stelzel C., Park S.Q., Gawron C.K., Ludwig V.U., Gutwinski S., Heinz A., Lindenmeyer J., Wiers R.W., Walter H., Bermpohl F. Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology. 2014;39:688–697. doi: 10.1038/npp.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C.E., Pommy J.M., Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. Am. J. Psychiatry. 2016;173:344–361. doi: 10.1176/appi.ajp.2015.15060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith Iv H., Davis N.L. Drug and opioid-involved overdose deaths—United States, 2017–2018. Morb. Mortal. Wkly Rep. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A. The neurobiology of craving: implications for the understanding and treatment of addiction. J. Abnorm. Psychol. 1988;97:118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- Woo C.W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Calhoun V., Song M., Cui Y., Yan H., Liu S., Fan L., Zuo N., Yang Z., Xu K. Discriminating schizophrenia using recurrent neural network applied on time courses of multi-site FMRI data. EBioMedicine. 2019;47:543–552. doi: 10.1016/j.ebiom.2019.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip S.W., Kiluk B., Scheinost D. Toward addiction prediction: an overview of cross-validated predictive modeling findings and considerations for future neuroimaging research. Biol. Psychiatry: Cogn. Neurosci. Neuroimag. 2020;5:748–758. doi: 10.1016/j.bpsc.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Geng X., Lee T.M.C. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct. Funct. 2017;222:3973–3990. doi: 10.1007/s00429-017-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.