Abstract
Nicotinamide N-methyltransferase (NNMT) methylates nicotinamide (vitamin B3) to generate 1-methylnicotinamide (MNA). NNMT overexpression has been linked to a variety of diseases, most prominently human cancers, indicating its potential as a therapeutic target. The development of small-molecule NNMT inhibitors has gained interest in recent years, with the most potent inhibitors sharing structural features based on elements of the nicotinamide substrate and the S-adenosyl-l-methionine (SAM) cofactor. We here report the development of new bisubstrate inhibitors that include electron-deficient aromatic groups to mimic the nicotinamide moiety. In addition, a trans-alkene linker was found to be optimal for connecting the substrate and cofactor mimics in these inhibitors. The most potent NNMT inhibitor identified exhibits an IC50 value of 3.7 nM, placing it among the most active NNMT inhibitors reported to date. Complementary analytical techniques, modeling studies, and cell-based assays provide insights into the binding mode, affinity, and selectivity of these inhibitors.
Introduction
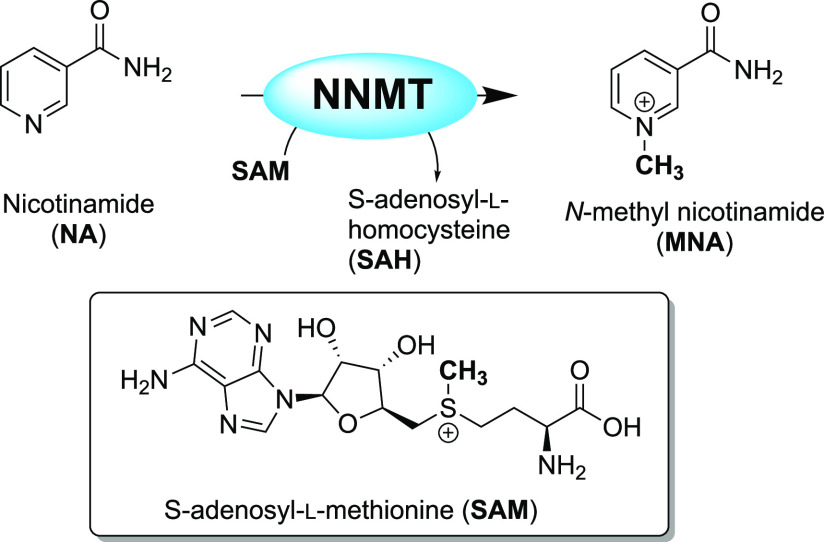
The enzyme nicotinamide N-methyltransferase (NNMT) catalyzes the methylation of nicotinamide using S-adenosyl-l-methionine (SAM) as a cofactor and produces S-adenosyl-l-homocysteine (SAH) as a byproduct (Figure 1). Since its discovery in 1952, its role was considered to be exclusively associated with cell detoxification through the metabolism of xenobiotics.1 This function is carried out thanks to NNMT’s broad substrate recognition that allows for the methylation of pyridines, quinolines, and other related heterocyclic metabolites, followed by their excretion.2 However, the view that NNMT is primarily involved in detoxification has recently changed as a result of numerous studies implicating NNMT in a variety of other critical metabolic pathways.3,4 For example, NNMT’s substrate nicotinamide is the precursor of NAD+, a compound heavily involved in redox processes and energy management.5 In addition, while NNMT does not play an epigenetic role per se, its influence on the SAM/SAH balance has an indirect impact on gene expression.6,7 The involvement of NNMT in epigenetic reprogramming and the cell’s energetic balance and detoxification pathways provides a broader appreciation of its role in the development and progression of cancer,3,6,8−12 diabetes,5,13,14 obesity,5,14 and neurodegenerative disorders.15−18
Figure 1.
Methylation of nicotinamide (NA) by NNMT using S-adenosyl-l-methionine (SAM) as the methyl donor, forming N-methyl nicotinamide (MNA) and S-adenosyl-l-homocysteine (SAH).
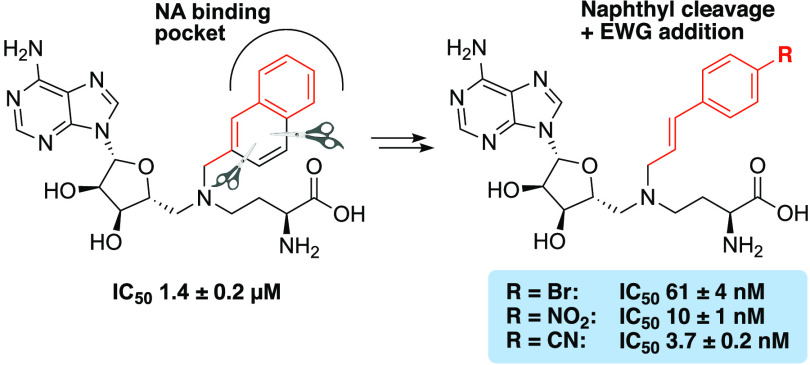
Improving our understanding of NNMT and its role in disease depends on the availability of potent, selective, and cell-active small-molecule inhibitors. Such chemical tools can lead the way to validating NNMT as a drug target and at the same time be used as templates for the development of new medicines for treating NNMT-driven conditions. At present, the most potent NNMT inhibitors described in the literature are bisubstrate analogues comprised of two covalently linked moieties that mimic the cofactor and substrate, SAM and nicotinamide, respectively. Following our initial reports describing such bisubstrate mimics as NNMT inhibitors,19,20 significant progress has been made by other groups also working in the field (Figure 2).21−24 Notably, the potency of bisubstrate NNMT inhibitors has improved from our first reported compounds with IC50 values in the micromolar range19,20,25 to those more recently described by the groups of Shair and Huang with IC50 values in the low nanomolar range.26,27 Collectively, these studies have shown that the bisubstrate inhibitor potency is heavily dependent on the relative spacing and spatial orientation of the adenosine-, amino acid-, and nicotinamide-mimicking moieties.19,20,25−27 Notable in this regard are the different linkers that have been used to connect the SAM and nicotinamide groups, among which alkynyl species have been shown to achieve the highest levels of inhibition (Figure 2). Building on our previous endeavors in the design of inhibitors for NNMT19,20 and bisubstrate inhibitors for other methyltransferase-containing alkene-based linkers,28,29 we here describe our most recent efforts at developing novel NNMT inhibitors characterized by an innovative design, an improved potency, and the ease of synthesis. These investigations have culminated in the discovery of a novel styrene scaffold with substitutions in the nicotinamide mimetic that move away from the amide functionality present in the majority of bisubstrate inhibitors that have been reported to date. Our results with this new scaffold also revealed interesting structure–activity relationships of electron-withdrawing substitutions, with the para-cyano compound 17u (Figure 2) being the most potent inhibitor identified with an IC50 value of 3.7 ± 0.2 nM. The extensive SAR results presented here were further corroborated by insights into the compound’s binding mode to NNMT, as predicted by molecular modeling. Compound 17u was further characterized by means of isothermal titration calorimetry (ITC) experiments, biochemical assays to assess its selectivity against other methyltransferases, and cell-based studies to assess its antiproliferative activity against several cancer cell lines.
Figure 2.
Chemical structures, inhibition data, and publication dates of bisubstrate inhibitors of NNMT.
Results and Discussion
Design
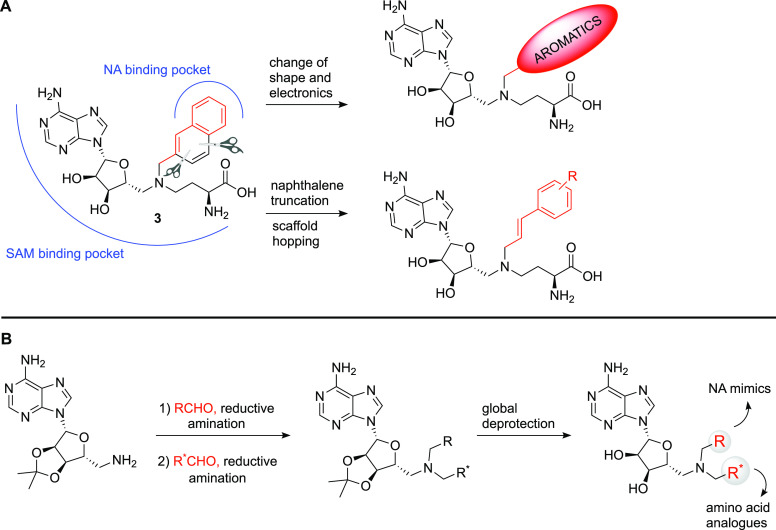
The crystal structures reported for NNMT consistently reveal π–π stacking interactions between the tyrosine residue Y204 and either the pyridine ring of the natural nicotinamide substrate30 or the aromatic group that mimics it in the bisubstrate inhibitors.25−27 To capitalize on these interactions and improve the potency of our previously disclosed NNMT ligand 3,20 we first undertook a systematic exploration of its naphthalene portion (Figure 3A), where a selection of bicyclic (hetero)aromatics was incorporated. In addition, prompted by the desire for an approach that would allow for the introduction of a wider range of nicotinamide mimics with different shapes and electronic features, a novel styrene-based scaffold was devised. This scaffold-hopping approach, which was based on a naphthalene truncation strategy (Figure 3A), presents two key advantages: (i) it allows for the expeditious synthesis of a diverse library of NNMT inhibitors starting from readily available building blocks and (ii) it provides insights into a novel alkenyl linker connecting the SAM-like portion and the nicotinamide mimic moiety. The latter feature is relevant because the resulting ligands complement the published bisubstrate inhibitors (Figure 2), which are generally linked by alkyl or alkynyl spacers.25−27 In addition, a selection of compounds were designed to assess the importance of both the amino acid and adenosine moieties for NNMT active-site binding.
Figure 3.
(A) Strategy for the modification and optimization of inhibitor 3 through the introduction of a variety of aromatics and the truncation of the naphthalene moiety, resulting in the introduction of the alkenyl linker. (B) General synthetic route for the preparation of NNMT inhibitors based on a double reductive amination approach, followed by a single deprotection step.
Synthesis
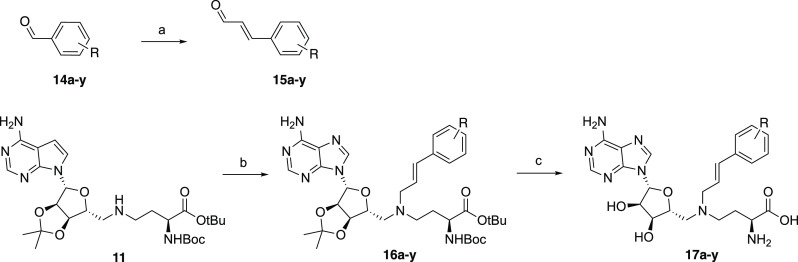
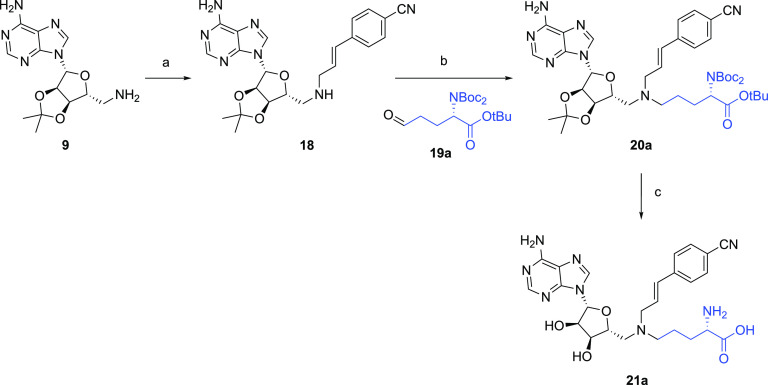
The synthesis of the NNMT inhibitors pursued here was based on a convenient modular strategy that provided access to a wide range of chemically different ligands. Starting from the known adenosine amine building block 9, all bisubstrate analogues were obtained via a sequential double-reductive amination process, followed by global deprotection (Figure 3B). The required bicyclic (hetero)aromatic aldehydes 8a–l used in the reductive amination steps were either commercially available or prepared through the formation of the Weinreb amide and a subsequent DIBAL-H reduction (Scheme 1). Phenylpropenaldehydes 15a–y were either commercially available or prepared through a Wittig reaction that coupled the corresponding benzaldehydes to (triphenyl-phosphoranylidene)acetaldehyde, as shown in Scheme 2. The aldehydes were subsequently coupled to compound 11 (which was prepared by the reductive amination of the adenosine amine building block 9 with the appropriate l-Asp-derived aldehyde building block 10). These reductive aminations were found to proceed cleanly using sodium triacetoxyborohydride and acetic acid, after which the final compounds were obtained by the global deprotection of the acid-labile protecting groups using TFA/CH2Cl2; the isopropylidene group cleavage was facilitated by the addition of water (Schemes 1 and 2).
Scheme 1. Representative Synthetic Scheme for the Preparation of Bicyclic Aromatic Compounds 13a–l.
Shown for the quinoline-containing compound 13a. Reagents and conditions: (a) CH3NHOCH3·HCl, BOP, Et3N, CH2Cl2, rt, 2 h (88%); (b) DIBAL-H in hexanes, THF, – 78 °C, 2 h (assumed quant.); (c) NaBH(OAc)3, AcOH, DCE, rt, overnight (47%); (d) TFA, CH2Cl2, H2O, rt, 2 h, (86%). The variable group for compounds 6b–l, 7b–l, 8b–l, 12b–l, and 13b–l is indicated in blue.
Scheme 2. Representative Synthetic Scheme for the Preparation of Substituted Cinnamaldehydes 15a–y and Resulting Alkenyl-Linked Aromatic Compounds 17a–y.
Reagents and conditions: (a) PPh3=CHCHO, toluene, 80 °C, overnight (45–77%); (b) aldehyde 15a-y, NaBH(OAc)3, AcOH, DCE, rt, overnight (43–81%); (c) TFA, CH2Cl2, H2O, rt, 2 h, (27–86%).
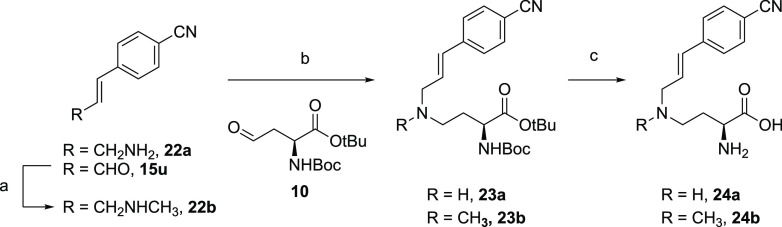
To investigate different substitutions of the amino acid moiety, building block 18 containing the para-cyano-substituted phenylpropenyl side chain was prepared through coupling of 4-cyano-phenylpropenaldehyde 15u to the adenosine amine starting material 9 (Scheme 3). A variety of aldehydes were then coupled to probe the amino acid pocket, as exemplified for compound 21a in which the amino acid linker was extended with an extra carbon. Compounds 24a and 24b lacking the adenosine unit were also synthesized in a similar fashion through the coupling of amino acid aldehyde 10 to either 4-cyano-phenylpropenylamine 22a or its methylated analogue 22b (Scheme 4). The crude products were purified by preparative high-performance liquid chromatography (HPLC) to yield the desired bisubstrate analogues.
Scheme 3. Representative Synthetic Scheme for the Preparation of 4-Cyano-phenylpropenyl Compounds with Different Substitutions of the Amino Acid Side Chain.
Shown for compound 21a bearing an extended linker to the amino acid moiety. Reagents and conditions are as follows: (a) aldehyde 15u, NaBH(OAc)3, AcOH, DCE, rt, overnight (81%); (b) aldehyde 19a, NaBH(OAc)3, AcOH, DCE, rt, overnight (81%); (c) TFA, CH2Cl2, H2O, rt, 2 h, (86%). The variable group in compounds 19b–k, 20b–k, and 21b–k is indicated in blue.
Scheme 4. Synthetic Scheme for the Preparation of 4-Cyano-phenylpropenyl Compounds 24a and 24b Lacking the Adenosine Unit.
Reagents and conditions are as follows: (a) methylamine in MeOH (33% w/w), NaBH(OAc)3, AcOH, DCE, rt, overnight (42%); (b) aldehyde 22, NaBH(OAc)3, AcOH, DCE, rt, overnight (48–77%); (c) TFA, CH2Cl2, H2O, rt, 2 h, (75–87%).
Inhibition Studies
All bisubstrate analogues prepared were tested for NNMT inhibitory activity using a method recently developed in our group.2 This assay employs hydrophilic liquid interaction chromatography (HILIC) coupled with tandem mass spectrometry (MS/MS) to rapidly and efficiently assess NNMT inhibition through the direct analysis of the formation of MNA. For each compound, NNMT inhibition was initially screened at a fixed inhibitor concentration of 25 μM. In cases where at least 50% inhibition was detected at this concentration, full inhibition curves were measured in triplicate to determine the corresponding half-maximal inhibitory concentration (IC50) values. As reference compounds, we included our previously described NNMT inhibitor 3 and the recently described NNMT inhibitor 5. The structures of these reference compounds are provided in Figure 2, and the IC50 values obtained in our assay were found to be in line with published values.20,27
Structure–Activity Relationships (SAR): β-Naphthalene Modification
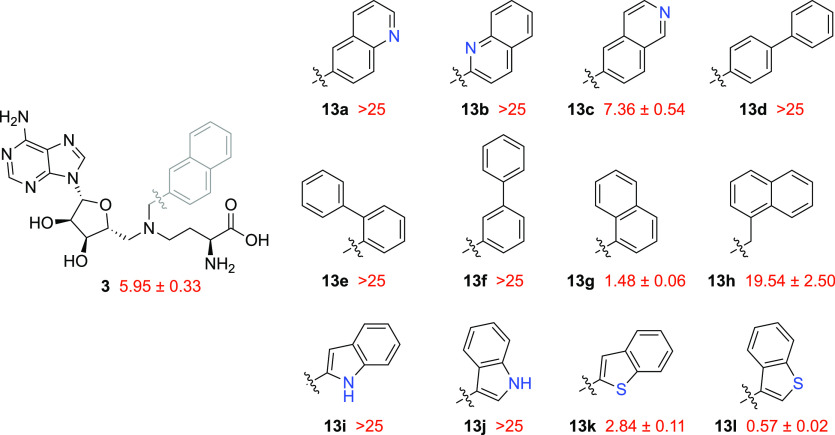
As previously mentioned, we aimed to improve the potency of our previously reported inhibitor 3 through further exploitation of the π–π stacking interactions between Y204 and the ligand’s nicotinamide-mimicking motif. To this end, a small library of compounds was made in which the naphthalene moiety of compound 3 was replaced with other (hetero)aromatic groups (compounds 13a–l, Figure 4). The introduction of electron-poor quinolines, which could potentially complement Y204 in a productive π–π stacking interaction, was met with poor results because the IC50 values of compounds 13a and 13b were above the 25 μM threshold, with only compound 13c showing moderate inhibition (IC50 = 7.36 μM). Although the incorporation of α-naphthalene led to good inhibition (13g, IC50 = 1.48 μM), the addition of an extra carbon to the linker portion abrogated it (13h, IC50 = 19.54 μM), and switching to biphenyl resulted in a considerable drop in potency (13d–f, IC50 > 25 μM). A similar trend was observed with the introduction of an indole moiety, with inhibitors 13i and 13j failing to display IC50 values below 25 μM. Improved potency was achieved when a benzothiophene ring was incorporated (13k and 13l), particularly when the branching point was at the C-3 position. Notable in this regard is compound 13l ,which was found to inhibit NNMT with an IC50 value of 0.57 μM (Figure 4).
Figure 4.
Structure–activity relationship (SAR) studies of bisubstrate NNMT inhibitors 13a–l carrying bicyclic (hetero)aromatic side chains to replace the naphthalene group of compound 3. IC50 values (μM) and s.e.m. values are shown in red.
Scaffold Hopping to Styrene Inhibitors
In light of the only moderate level of success obtained by introducing other bicyclic (hetero)aromatic groups, we next shifted our focus to a different approach. Specifically, we applied a scaffold-hopping and truncation strategy to compound 3 in which the naphthalene moiety was simplified to styrene derivatives 17a–y (Figure 3A). Notably, this structural alteration and accompanying synthetic route, along with the wide availability of substituted benzaldehydes, allowed for ready access to a wide range of novel bisubstrate analogues (Figure 5).
Figure 5.
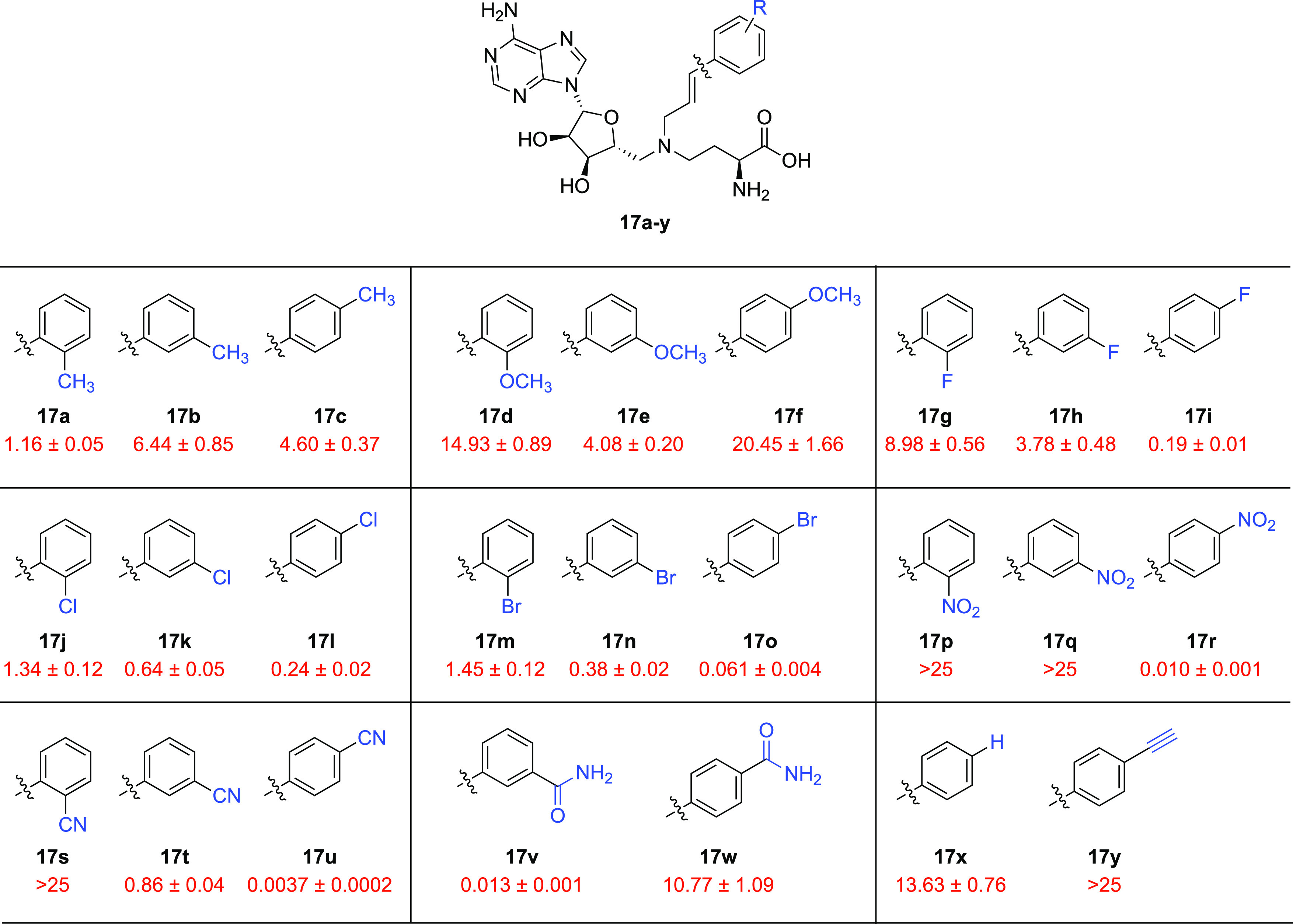
SAR studies of bisubstrate NNMT inhibitors 17a–y carrying alkenyl-linked substituted aromatics. IC50 values (μM) and s.e.m. values are shown in red, and the substitutions are highlighted in blue.
The various thus-prepared styrene analogues (17a–y) contained different electron-donating and electron-withdrawing substituents at the ortho-, meta-, and para-positions and were evaluated for their in vitro activity against NNMT. The ortho-methyl compound 17a (IC50 = 1.16 μM) showed a better activity than the corresponding meta- (17b, IC50 = 4.60 μM) and para-anaologues (17c, IC50 = 6.44 μM). Methoxy-substituted compounds 17d–f all showed somewhat lower potencies (IC50 ≥ 4 μM). A clear improvement was observed when electron-withdrawing substituents were introduced on the styrene ring. In addition, the orientation of the electron-withdrawing group was directly correlated to the activity, with the potency of the compounds increasing from ortho- to meta- to para-substitution. In the case of fluorinated ligands 17g–i, the activity increased from an IC50 value of 8.98 μM for ortho-F to that of 3.78 μM for meta-F, and the most potent activity was observed for the para-F-substituted compound, which displayed an IC50 value of 0.19 μM. The introduction of a chlorine atom in the same styrene scaffold resulted in a similar trend in NNMT inhibitory activity. In this instance, the IC50 values for the ortho-Cl and meta-Cl compounds were 1.34 μM and 0.64 μM, respectively (17j and 17k, Figure 5), while the para-analogue 17l (IC50 = 0.24 μM) was again the most active. Switching chlorine for bromine did not cause any major change in activity for the ortho-Br and meta-Br analogues (17m and 17n, IC50 = 1.45 and 0.38 μM, respectively) but positively impacted NNMT inhibition in the case of the para-Br compound 17o, which displayed a nanomolar activity (IC50 = 0.061 μM, Figure 5). Even more striking was the case of nitro-substituted compounds 17p–r. While the para-nitro-substituted analogue was found to be a highly potent inhibitor (17r, IC50 = 0.010 μM), both the ortho-nitro and meta-nitro compounds failed to show any appreciable activity (17p and 17q, IC50 > 25 μM). Finally, the introduction of a nitrile functionality on the styrene core caused yet further improvements in the potency, especially when situated at the para-position. While the ortho-cyano analogue 17s did not show inhibition at 25 μM, the meta-cyano analogue 17t displayed good inhibition with an IC50 of 0.86 μM. There was another leap in activity for the para-cyano compound 17u, which exhibited the most potent inhibition of all compounds prepared in the present study with a single-digit nanomolar IC50 value (IC50 = 3.7 nM).
We next assessed the potential for combining structural features of these new NNMT inhibitors with known potent inhibitors 4 and 5 (Figure 2). In doing so, we generated two styrene-based compounds inspired by 17u in which the nitrile functionality was replaced by a meta- or para-substituted primary amide (17v and 17w, respectively). Notably, the para-amide showed a marked decrease in potency (IC50 = 10.77 μM), while the meta-amide proved to be an active NNMT inhibitor (IC50 = 0.013 μM). The behavior of these two analogues highlighted an interesting trend. Whereas for the cyano substituent the para-arrangement is superior to the meta-one, for amides the contrary holds true. Interestingly, the unsubstituted compound 17x exhibited only a very modest potency (IC50 = 13.63 μM). Finally, it is worth noting that the para-alkynyl-substituted compound 17y, where the nitrile group of 17u was replaced by an acetylene group, was completely inactive with an IC50 > 25 μM. This result clearly indicates a specific role for the nitrile functionality in facilitating productive binding interactions between the inhibitor and the NNMT active site.
From the data presented above, it can be inferred that a strongly electron-rich styrene moiety is not beneficial for NNMT inhibition. Also, it is clear that electron-withdrawing substituents like nitro or cyano are most effective when located at the para-position on the aromatic ring. The origin of these trends is likely a combination of structural complementarity and electronics. For example, the geometric constraints of the binding pocket could be favoring the para-substitution pattern, while a particularly effective π–π stacking between NNMT’s tyrosine residue Y204 and the electron-poor styrene of compounds 17o, 17r, and 17u might lie behind these ligands’ potency.
Linker Modifications
After establishing compound 17u as our lead inhibitor, we turned our attention to the role of the linker bridging the SAM-derived motif and the nicotinamide-mimicking moiety. Our own work in the field had already highlighted the importance of the correct spacing for achieving potent NNMT inhibition.20 Moreover, reports by other groups have reinforced the notion that a carefully judged linker, in terms of both length and rigidity, is required for potency (see compounds 2, 4, and 5, Figure 2).25−27 To compare our own alkenyl linker with the alternatives devised by others, a series of analogues of inhibitor 17u were designed, featuring a truncated linker (25), a fully saturated linker (26), and a propargylic linker (27 and 28, Figure 6). Additionally, compound 29 was prepared to assess the impact of replacing the core amine functionality with an amide linkage.
Figure 6.
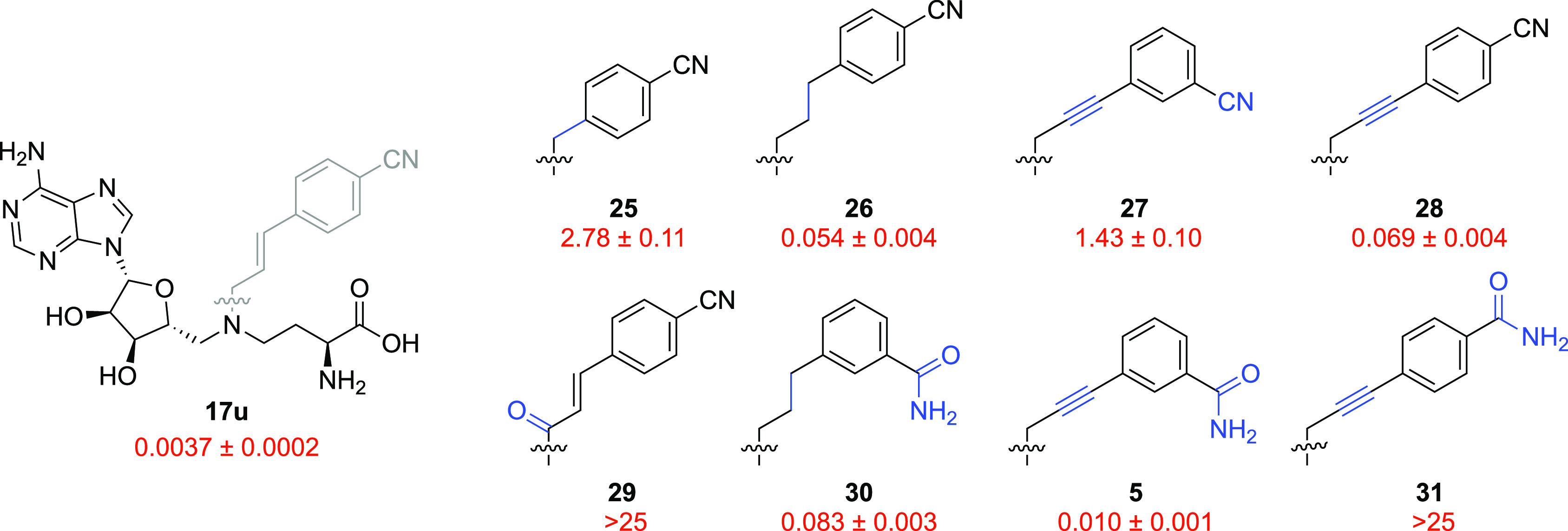
SAR studies of bisubstrate NNMT inhibitors 5 and 25–31 carrying different linkers. IC50 values (μM) and s.e.m. values are shown in red. Changes introduced relative to the lead inhibitor 17u are indicated in blue.
Both the truncated analogue 25 and the amide-linked compound 29 displayed a clear drop in activity against NNMT (IC50 = 2.78 and >25 μM, respectively). When the C=C double bond of inhibitor 17u was reduced to a saturated three-carbon linker, the IC50 value increased more than 10-fold (26, IC50 = 0.054 μM); however, the resulting compound still showed high potency. A similar outcome was observed when a propargyl spacer was introduced (28, IC50 = 0.069 μM).
In recently reported studies involving propargyl-linked bisubstrate inhibitors of NNMT, the benzamide fragment featured prominently as the favored nicotinamide mimic.25−27 Of note in this regard is the importance of the position of the amide group on the aromatic ring, with the para-substituted amide (31) displaying a clear lack of potency (IC50 > 25 μM) relative to the meta-compound (527), which was measured to have an IC50 value of 0.010 μM in our assay. Notably, a similar effect was also observed for the alkenyl-linked amides 17v and 17w reported in our present study (Figure 5), with the meta-substituted analogue displaying a nearly 1000-fold increase in NNMT inhibition. Also of note was the observation that this trend is reversed for the corresponding propargyl-linked meta- and para-cyano analogues; in this case the meta isomer 27 was a much weaker inhibitor (IC50 = 1.43 μM) than the para-isomer 28 (IC50 = 0.069 μM, Figure 6). Finally, as also observed for the fully reduced para-cyano analogue 26, replacing the unsaturated linker in the potent literature inhibitor 5 with a fully saturated alkyl linker led to compound 30, which exhibited a reduced activity but retained a nanomolar inhibition (IC50 = 0.083 μM).
The exploration of different linkers in conjunction with optimized nicotinamide-mimicking moieties revealed that nitrile- and amide-substituted aromatics confer a high level of NNMT inhibition, with the former narrowly outperforming the latter in our hands. Similarly, our newly developed unsaturated linker compared favorably to the alkyne-based linkers previously described.26,27 Taking a closer look at this finding, the potency of tight-binding alkenyl- and alkynyl-linked para-cyano (17u and 28, respectively) and meta-amide (17v and 5) inhibitors was reevaluated in the presence of elevated concentrations of cofactor SAM to increase their IC50 values, magnifying their differences in potency.31 The four compounds tested had the same SAM-mimicking motif and were assumed to be equally SAM-competitive and thus similarly affected by increased levels of the cofactor. Increasing the concentration of SAM to 85 μM (10× its KM value) in the biochemical assay resulted in a two- to fourfold increase in the IC50 values, confirming the trend observed under standard assay conditions. In addition, the apparent KI values were calculated using Morrison’s equation for tight-binding inhibitors32 and were found to be similar under both SAM concentrations tested (see Tables S2 and S3 in the Supporting Information). These studies confirm that compound 17u is the most potent NNMT inhibitor evaluated in the present study.
Amino Acid and Adenosine Modifications
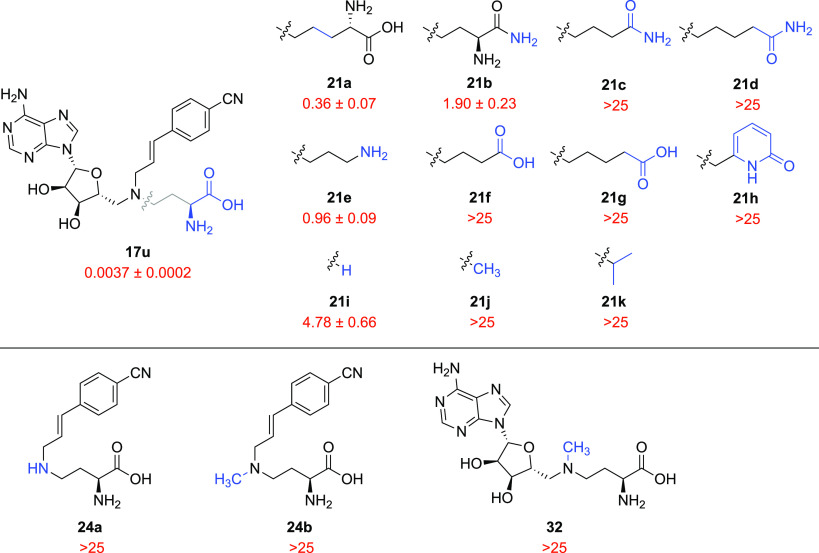
After identifying an optimal nicotinamide mimic and linker combination for potent NNMT inhibition, a small selection of ligands with modifications to other parts of the scaffold was next investigated. Structural alterations of the amino acid portion of 17u (Figure 7) revealed a very steep SAR, with all analogues exhibiting IC50 values several orders of magnitude higher than that of the parent compound. Compound 21a, an extended three-carbon homologue of 17u, was significantly less active compared to the parent compound but still showed a submicromolar potency (IC50 = 0.36 μM). It is also clear that the amino group of the amino acid moiety is critical for inhibition, as compounds 21f and 21g lost all activity. The removal of the carboxylic acid was tolerated slightly better, with amine 21e showing an IC50 value in the low micromolar range (0.96 μM). The amino amide analogue 21b showed a strong decrease in potency (1.90 μM), which was further diminished upon the removal of the primary amine (21c and 21d, IC50 > 25 μM). Replacing the amino acid moiety with a pyridinone mimic33 (21h) was also not tolerated. When the entire amino acid chain was swapped for a lipophilic methyl or isopropyl group, as in compounds 21j and 21k, all activity against NNMT was lost (both IC50 > 25 μM). Notable, however, is the fully truncated secondary amine 21i that was surprisingly found to be active, albeit in the low micromolar range. Taken together, the results presented here demonstrate the crucial role the amino acid motif plays in the interaction of these bisubstrate inhibitors in the NNMT binding pocket. Similarly, two truncated analogues of inhibitor 17u that lacked the adenosine unit (24a and 24b, see Figure 7) and the analogue that lacked nicotinamide-mimicking side-chain (AzaAdoMet 32) displayed a complete loss of potency (IC50 > 25 μM).
Figure 7.
SAR studies of bisubstrate NNMT inhibitors 21a–k bearing different amino acid substitutions and compounds 24a, 24b, and 32 lacking either the adenosine unit or the nicotinamide-mimicking aromatic side chain. IC50 values (μM) and s.e.m. values are shown in red. Changes introduced relative to the lead inhibitor 17u are indicated in blue.
NNMT Inhibitor Binding Studies
The binding of the most potent inhibitor 17u with NNMT was further characterized using isothermal titration calorimetry (ITC) (Figure 8). The dissociation constant (KD) thus obtained for compound 17u was determined to be 21.23 ± 6.12 nM, demonstrating a strong binding affinity as reflected by the potent NNMT inhibition measured in the biochemical assay. Furthermore, in keeping with the bisubstrate inhibitor’s capacity to simultaneously compete with both the cofactor SAM and the substrate NA, the ITC experiment also confirmed a 1:1 stoichiometry between the ligand and the enzyme. Details and additional thermograms of compound 17u and NNMT as well as control titrations are provided in the Supporting Information.
Figure 8.
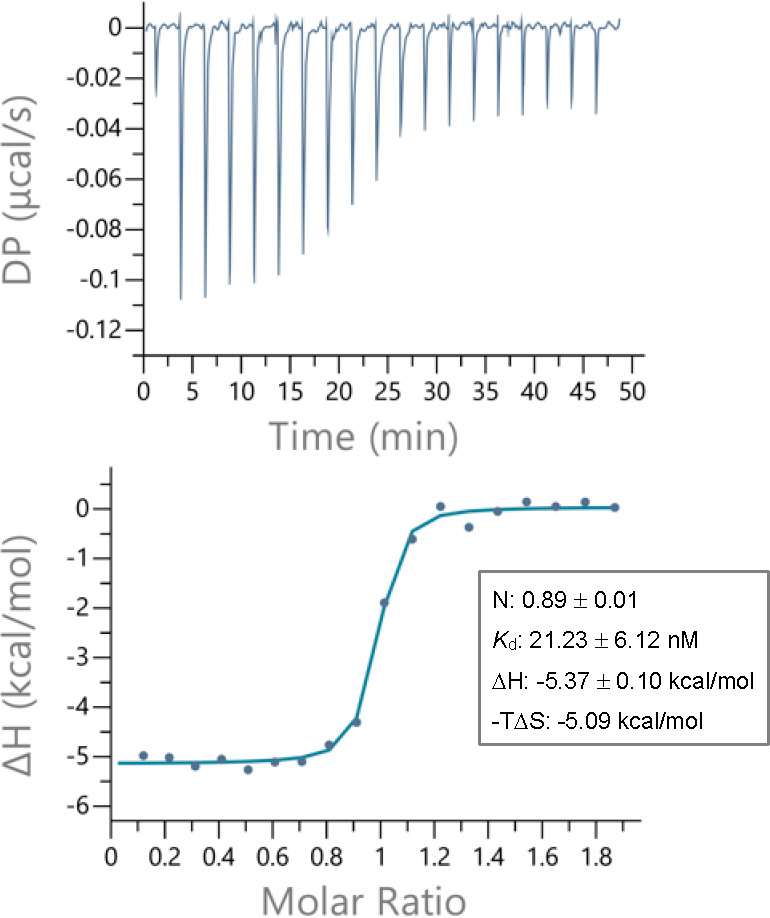
ITC thermogram of compound 17u, including the thermodynamic binding parameters obtained from three independent titration experiments with human wild-type NNMT.
NNMT Inhibitor Modeling
To learn more about the driving force of the ortho–meta–para effect observed for the electron-withdrawing group (EWG) substitutions in the styrene compounds, computational studies were performed on nitrile-substituted compounds 17s, 17t, and 17u. These studies were specifically aimed at estimating the relative binding affinity shifts, via free energy perturbation (FEP), due to the inclusion of the ortho-, meta-, or para-nitrile substituent in the unsubstituted reference compound 17x (Figure 9 and Table 1). From these calculations it becomes apparent that serine residues S201 and S213 in the nicotinamide binding pocket of NNMT play a crucial role in the potency of compound 17u. The model predicts hydrogen bonding interactions with the para-cyano substituent of compound 17u that would involve the side chains of both S201 and S213. These interactions result in an estimated improvement of the binding affinity due to the p-CN substitution of more than 4 kcal/mol relative to that of the unsubstituted analogue 17x, which is in agreement with the experimental data. For the meta-cyano compound 17t, these interactions seem to be much weaker (less frequent), resulting in only a moderate improvement of the predicted affinity shift that arises from the introduction of the meta-cyano substitution, which is again in line with the biochemical experiments. Conversely, the ortho-cyano compound 17s cannot reach the serine residues and instead seems to introduce counterproductive steric hindrance in the binding site, as reflected by the weaker predicted binding affinity predicted to that of the unsubstituted compound 17x. When modeling the meta-amide compound 17v, similar interactions with the hydroxyl side chains of S201 and S213 were also predicted (see Figure S7 in the Supporting Information), providing a possible explanation for the potency similar to that of compound 17u.
Figure 9.
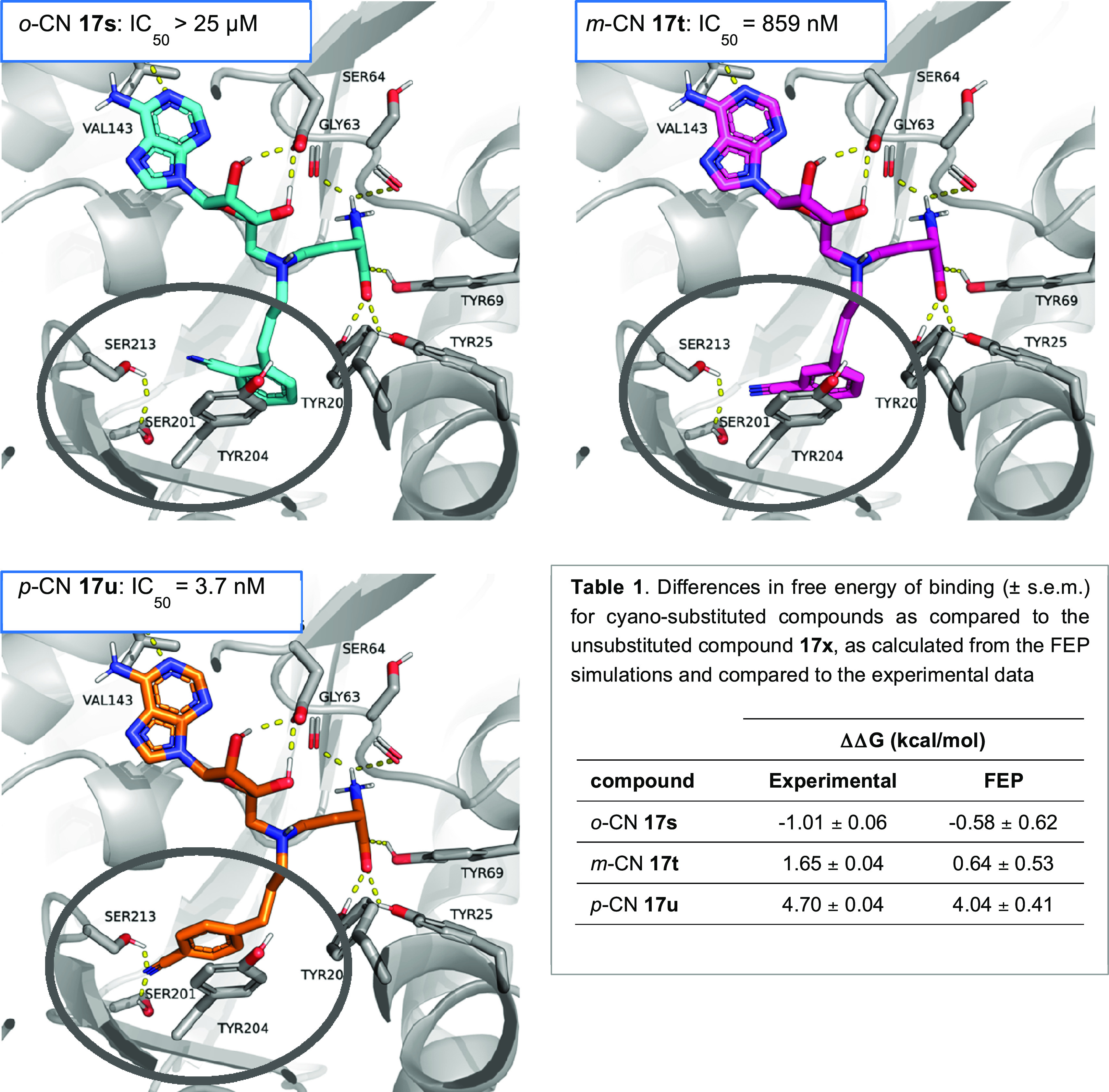
Results of the modeling of compounds 17s–u bearing the ortho-, meta-, and para-cyano substituents in the active site of NNMT (PDB ID 6PVE). The results indicate the strong hydrogen bonding of the para-cyano compound 17u with serine residues S201 and S213, which are not present in the models of compounds 17s and 17t. The modeled predictions are supported by the similarity of the difference in the Gibbs free energy (ΔΔG) compared to that of unsubstituted compound 17x from the biochemical assay and the MD simulations as displayed in Table 1.
Inhibitor Selectivity Studies and Cell-Based Assays
To evaluate the NNMT selectivity of the most potent bisubstrate inhibitor, compound 17u was tested for its activity against a panel of 12 different SAM-dependent methyltransferases (see Table S4 in the Supporting Information). For the selectivity study, we selected protein methyltransferases G9a, SETDB1, SETD2, MLL1, SMYD2, PRMT1, CARM1, PRMT5, PRMT7, DNMT1, and DOT1L as well as the small-molecule methyltransferase phenylethanolamine N-methyltransferase (PNMT). Notably, PNMT has a high structural similarity to NNMT, sharing 39% of its sequence identity.30 Compound 17u showed good selectivity against all the methyltransferases tested. Against PNMT, the moderate inhibitory activity observed for compound 17u was more than 3000-fold lower than that measured against NNMT. Against PRMT5 and DOT1L, 17u exhibited more than 50% inhibition at 10 μM, but no appreciable activity was detected at 1 μM. The highest percentage inhibition was observed against the lysine methyltransferase SMYD-2, with 19% and 39% activity remaining at the concentrations of 10 and 1 μM, respectively. Nevertheless, compound 17u still exhibits more than a 100-fold higher potency toward NNMT, indicating the compound’s good selectivity profile.
To investigate whether the potent activity observed in the biochemical inhibition assays translates to cellular activity, compound 17u was also tested against human cancer cell lines. In addition to the human oral cancer cell line HSC-2 that was previously used to assess the cell-based activity of naphthalene compound 3,20 here we also tested compound 17u against a human lung cancer cell line (A549) and a bladder cancer cell line (T24). The results of these studies reveal a clear inhibition of the cell viability for the different cancer cell lines upon treatment with compound 17u at a concentration of 100 μM (see Figure S7 in the Supporting Information for details). However, this effect was absent at the lower concentrations tested. As the difference between the biochemical inhibition and the cellular activity spans several orders of magnitude, we investigated the cell permability of compound 17u by means of a parallel artificial membrane permeability assay (PAMPA). The data revealed the very poor cell permeability of 17u, which was likely the explanation for the discrepancy between the nanomolar potency in the biochemical assay and the poor potency in the cellular assay (see Table S5 in the Supporting Information for details).
Conclusion
To date, the majority of bisubstrate NNMT inhibitors have logically employed benzamide groups to mimic the nicotinamide moiety. In addition, recent reports have highlighted the benefit of utilizing alkyne-based linkers to connect the benzamide group to the SAM-mimicking moiety. We here report notable departures from both these strategies to generate novel and potent NNMT inhibitors that (a) include nonbenzamide aromatic mimics of the nicotinamide group and (b) employ a three-carbon trans-alkene linker to connect these aromatic groups to the SAM unit. This approach was enabled by a convenient and robust synthetic route that utilized a double-reductive amination procedure, which allowed for the preparation of a number of novel bisubstrate inhibitors. Biochemical evaluation of the thus-prepared inhibitors revealed a striking effect for EWGs present on the aromatic ring, predominantly when introduced at the position para to the linker. Among these compounds, the para-cyano-substituted styrene-based inhibitor 17u was identified as the most potent NNMT inhibitor with an IC50 value of 3.7 nM. This compound was subsequently used to further investigate the possibility of altering or replacing the amino acid and adenosine moieties. These studies showed that subtle changes in the amino acid side chain resulted in dramatic decreases in activity. While the removal of the carboxylic acid moiety still yielded a low micromolar inhibitor, the elimination of the primary amine led to inactive compounds. Similarly, the novel para-cyano side chain could not compensate for the loss of binding interactions when the adenosine moiety was eliminated. The results from the ITC experiments confirm that compound 17u is a tight binder of NNMT with a dissociation constant of 21 nM and a 1:1 stoichiometry. In addition, modeling studies predict the presence of hydrogen bonding interactions of the para-cyano group with two active site serine residues in the substrate pocket of NNMT, providing a plausible explanation for the potency of compound 17u. The low nanomolar potency exhibited in biochemical assays was not reflected in cell-based assays, and a significant decrease in cell viability was observed only when compound 17u was tested at a 100 μM concentration against oral, lung, and bladder cancer cell lines. This discrepancy is likely explained by the poor cell permeability of compound 17u, which was found in the PAMPA assay. Taken together, our findings provide valuable new insights toward the design and further optimization of potent NNMT inhibitors.
Experimental Procedures
General Procedures
All reagents employed were of American Chemical Society grade or finer and were used without further purification unless otherwise stated. For compound characterization, 1H NMR spectra were recorded at 400, 500, or 600 MHz, and chemical shifts are reported in parts per million downfield relative to H2O (δ 4.79), CH3OH (δ 3.31), CHCl3 (δ 7.26), or DMSO (δ 2.50). 1H NMR data are reported in the following order: multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; and m, multiplet), coupling constant (J) in hertz (Hz), and the number of protons. Where appropriate, the multiplicity is preceded by br, indicating that the signal was broad. 13C NMR spectra were recorded at 101, 126, or 151 MHz, and chemical shifts are reported relative to CDCl3 (δ 77.16), methanol (δ 49.00), or DMSO (δ 39.52). The 13C NMR spectra of the compounds recorded in D2O could not be referenced. Compounds 5,279,3410,2019a,2019b,3519c–d,2019e,3619f–g,2022a,3730,27 and 32(38) were prepared as previously described and had NMR spectra and mass spectra consistent with the assigned structures. Purity was confirmed to be ≥95% by LCMS performed on a Shimadzu LC-20AD system with a Shimadzu Shim-Pack GISS-HP C18 column (3.0 × 150 mm, 3 μm) at 30 °C; the system was equipped with a UV detector, which monitored wavelengths at 214 and 254 nm. The following solvent system, at a flow rate of 0.5 mL/min, was used: solvent A, 0.1% formic acid in water; solvent B, acetonitrile. Gradient elution was as follows: 95:5 (A/B) for 2 min, from 95:5 to 0:100 (A/B) over 13 min, 0:100 (A/B) for 2 min, then a reversal to 95:5 (A/B) over 1 min, 95:5 (A/B) for 2 min. This system was connected to a Shimadzu 8040 triple-quadrupole mass spectrometer (ESI ionization).
The final compounds were purified via preparative HPLC that was performed on a BESTA-Technik system with a Dr. Maisch Reprosil Gold 120 C18 column (25 × 250 mm, 10 μm); the system was equipped with a ECOM Flash UV detector, which monitored wavelengths at 214 nm. The following solvent system, at a flow rate of 12 mL/min, was used: solvent A, 0.1% TFA in water/acetonitrile 95/5; solvent B, 0.1% TFA in water/acetonitrile 5/95. Gradient elution was as follows: 95:5 (A/B) for 5 min, from 95:5 to 0:100 (A/B) over 40 min, 0:100 (A/B) for 5 min, then a reversal to 95:5 (A/B) over 2 min, 95:5 (A/B) for 8 min.
HRMS analyses were performed on a Shimadzu Nexera X2 UHPLC system with a Waters Acquity HSS C18 column (2.1 × 100 mm, 1.8 μm) at 30 °C; the system was equipped with a diode array detector. The following solvent system, at a flow rate of 0.5 mL/min, was used: solvent A, 0.1% formic acid in water; solvent B, 0.1% formic acid in acetonitrile. Gradient elution was as follows: 95:5 (A/B) for 1 min, from 95:5 to 15:85 (A/B) over 6 min, 15:85 to 0:100 (A/B) over 1 min, 0:100 (A/B) for 3 min, then a reversal to 95:5 (A/B) for 3 min. This system was connected to a Shimadzu 9030 QTOF mass spectrometer (ESI ionization), which was calibrated internally with Agilent’s API-TOF reference mass solution kit (5.0 mM purine, 100.0 mM ammonium trifluoroacetate, and 2.5 mM hexakis(1H,1H,3H-tetrafluoropropoxy)phosphazine) that was diluted to achieve a mass count of 10000.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (11)
9-((3aR,4R,6R,6aR)-6-(Aminomethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-amine 9 (7.3 g, 24 mmol), tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoate 10 (5.5 g, 20 mmol), NaBH(OAc)3 (6.4 g, 30 mmol), and AcOH (1 mL) were added to 1,2-dichloroethane (DCE, 100 mL) in a 250 mL round-bottom flask (RBF), and the mixture was stirred at room temperature under a N2 atmosphere overnight. The reaction was quenched by adding 1 N NaOH (20 mL), and the product was extracted with CH2Cl2. The combined organic layers were washed with brine and dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography (10% MeOH in EtOAc) to give compound 11 as a white powder (6.4 g, 57% yield). 1H NMR (400 MHz, CDCl3): δ 8.31 (s, 1H), 7.90 (s, 1H), 6.04–5.76 (m, 4H), 5.49 (s, 1H), 5.29 (s, 1H), 5.09–5.05 (m, 1H), 4.36 (s, 1H), 4.28 (s, 1H), 2.95 (d, J = 9.5 Hz, 1H), 2.85–2.70 (m, 2H), 2.63 (s, 1H), 1.93 (br s, 1H), 1.81 (br, 1H), 1.60 (s, 3H), 1.41 (br d, J = 26.4 Hz, 21H). 13C NMR (101 MHz, CDCl3): δ 170.8, 156.0, 155.1, 153.0, 149.2, 140.4, 120.2, 113.3, 90.9, 84.9, 83.0, 82.1, 81.5, 79.2, 77.9, 77.3, 77.1, 76.8, 52.9, 50.3, 46.2, 32.1, 28.2, 27.8, 27.2, 25.4. HRMS (ESI): calculated for C26H42N7O7 [M + H]+ 564.3146, found 564.3150.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(quinolin-6-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (12a)
Compound 11 (112 mg, 0.20 mmol), 1-quinoline-6-carbaldehyde 8a (38 mg, 0.24 mmol), NaBH(OAc)3 (11 mg, 0.30 mmol), and AcOH (one drop) were added to 1,2-dichloroethane (DCE, 10 mL) in a 50 mL round-bottom flask (RBF), and the mixture was stirred at room temperature under a N2 atmosphere overnight. The reaction was quenched by adding 1 N NaOH (10 mL), and the product was extracted with CH2Cl2. The combined organic layers were washed with brine and dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography (5% MeOH in EtOAc) to give compound 12a as a white powder (66 mg, 47% yield). 1H NMR (400 MHz, CDCl3): δ 8.81 (d, J = 3.9 Hz, 1H), 8.02 (s, 1H), 7.95 (t, J = 9.2 Hz, 2H), 7.78 (s, 1H), 7.62 (d, J = 8.5 Hz, 1H), 7.55 (s, 1H), 7.29 (dd, J = 8.1, 4.2 Hz, 1H), 6.50 (s, 2H), 5.97 (s, 1H), 5.67 (d, J = 7.8 Hz, 1H), 5.28 (d, J = 5.4 Hz, 1H), 4.85–4.80 (m, 1H), 4.30 (d, J = 6.0 Hz, 1H), 4.20–4.12 (m, 1H), 3.78 (d, J = 8.1, 1H), 3.59 (br d, J = 12.0 Hz, 2H), 2.81–2.75 (m, 1H), 2.68–2.59 (m, 2H), 2.54–2.48 (m, 1H), 1.96 (br, 1H), 1.77 (br, 1H), 1.51 (s, 3H), 1.33–1.27 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.8, 155.4, 152.8, 150.0, 148.9, 139.7, 137.2, 135.7, 130.6, 129.2, 121.1, 120.1, 114.3, 90.6, 85.3, 83.3, 81.6, 58.9, 55.8, 52.8, 50.8, 29.4, 28.3, 27.8, 27.0, 25.3. HRMS (ESI): calculated for C36H49N8O7 [M + H]+ 705.3724, found 705.3728.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(quinolin-2-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (12b)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with quinoline-2-carbaldehyde 8b (38 mg, 0.24 mmol) to afford compound 12b, which was used in the next step without further purification.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(isoquinolin-6-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12c)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with isoquinoline-6-carbaldehyde 8c (38 mg, 0.24 mmol) to afford compound 12c as a white powder (77 mg, 55% yield). 1H NMR (400 MHz, CDCl3): δ 8.07 (s, 1H), 7.98 (dd, J = 8.3, 2.0 Hz, 2H), 7.86 (s, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.66–7.62 (m, 1H), 7.55 (d, J = 8.5 Hz, 1H), 7.49–7.45 (m, 1H), 6.04 (br, 3H), 5.57 (d, J = 7.7 Hz, 1H), 5.34 (d, J = 5.6 Hz, 1H), 4.94–4.89 (m, 1H), 4.43–4.36 (m, 1H), 4.20–4.16 (br, 1H), 3.96 (br, 1H), 3.86 (s, 1H), 2.92–2.84 (m, 1H), 2.81–2.66 (m, 2H), 2.61 (br, 1H), 2.06–1.92 (m, 1H), 1.77 (br, 1H), 1.56 (s, 3H), 1.41–1.31 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 159.9, 155.6, 152.9, 149.1, 147.4, 139.9, 136.2, 129.4, 129.0, 127.5, 127.3, 126.2, 124.8, 121.1, 120.2, 114.3, 90.7, 85.5, 83.9, 83.4, 81.6, 79.4, 77.3, 61.6, 56.4, 52.8, 51.2, 30.3, 28.4, 27.9, 27.2, 25.5. HRMS (ESI): calculated for C36H49N8O7 [M + H]+ 705.3724, found 705.3733.
tert-Butyl (2S)-4-(([1,1′-Biphenyl]-4-ylmethyl)(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12d)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with [1,1′-biphenyl]-4-carbaldehyde 8d (44 mg, 0.24 mmol) to afford compound 12d as a white powder (103 mg, 71% yield). 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 1H), 7.85 (s, 1H), 7.55 (d, J = 7.6 Hz, 2H), 7.46 (d, J = 7.9 Hz, 2H), 7.40 (t, J = 7.6 Hz, 2H), 7.30 (d, J = 7.9 Hz, 3H), 6.36 (s, 2H), 6.03 (s, 1H), 5.75 (d, J = 7.7 Hz, 1H), 5.37 (d, J = 5.4 Hz, 1H), 4.92–4.87 (m, 1H), 4.41–4.34 (m, 1H), 4.24–4.16 (m, 1H), 3.72 (br d, J = 12.0 1H), 3.49 (br d, J = 12.0 1H), 2.81 (br d, J = 19.7 Hz, 1H), 2.71–2.60 (m, 2H), 2.52 (d, J = 7.0 Hz, 1H), 2.06–1.93 (m, 1H), 1.86–1.74 (m, 1H), 1.59 (s, 3H), 1.41–1.36 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 155.5, 153.1, 149.3, 140.9, 137.6, 129.4, 128.8, 127.2, 127.0, 120.4, 58.7, 55.8, 53.0, 50.7, 30.4, 29.8, 29.4, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C39H52N7O7 [M + H]+ 730.3928, found 730.3956.
tert-Butyl (2S)-4-(([1,1′-Biphenyl]-2-ylmethyl)(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12e)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with [1,1′-biphenyl]-2-carbaldehyde 8e (44 mg, 0.24 mmol) to afford compound 12e as a white powder (99 mg, 69% yield). 1H NMR (400 MHz, CDCl3): δ 8.22 (s, 1H), 7.79 (s, 1H), 7.57–7.51 (m, 1H), 7.39–7.11 (m, 8H), 5.97 (br d, J = 12.0 Hz, 3H), 5.34 (br, 2H), 4.75 (dd, J = 6.4, 3.3 Hz, 1H), 4.22–4.17 (m, 1H), 4.07–3.98 (m, 1H), 3.61 (br d, J = 12.0, 1H), 3.44 (br d, J = 16.0 1H), 2.64–2.59 (m, 1H), 2.50–2.44 (m, 2H), 2.37–2.30 (m, 2H), 1.83–1.72 (m, 1H), 1.57 (s, 3H), 1.42–1.36 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 155.4, 153.1, 141.3, 136.1, 130.0, 129.7, 129.4, 128.1, 127.3, 127.0, 126.8, 114.3, 90.8, 85.4, 83.8, 83.3, 56.2, 55.9, 52.8, 50.8, 29.3, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C39H51N7O7Na [M + Na]+ 752.3748, found 730.3759.
tert-Butyl (2S)-4-(([1,1′-Biphenyl]-3-ylmethyl)(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12f)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with [1,1′-biphenyl]-3-carbaldehyde 8f (44 mg, 0.24 mmol) to afford compound 12f as a white powder (108 mg, 74% yield). 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 1H), 7.81 (s, 1H), 7.59–7.51 (m, 3H), 7.44 (d, J = 7.6 Hz, 1H), 7.38 (t, J = 7.5 Hz, 2H), 7.33–7.27 (m, 2H), 7.22 (d, J = 7.4 Hz, 1H), 6.51 (s, 2H), 6.02 (s, 1H), 5.68 (d, J = 6.6 Hz, 1H), 5.35 (d, J = 5.3 Hz, 1H), 4.93–4.89 (m, 1H), 4.39–4.32 (m, 1H), 4.22–4.15 (m, 1H), 3.75 (br, 1H), 3.52 (br, 1H), 2.84–2.79 (m, 1H), 2.71–2.60 (m, 2H), 2.59–2.49 (m, 1H), 2.06–1.94 (m, 1H), 1.83 (br s, 1H), 1.57 (s, 3H), 1.39–1.32 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.5, 153.1, 141.2, 141.1, 128.8, 127.9, 127.8, 127.3, 127.2, 126.0, 114.4, 90.8, 85.4, 83.9, 83.5, 59.1, 55.7, 52.9, 50.8, 29.5, 28.4, 28.0, 27.2, 25.4. HRMS (ESI): calculated for C39H52N7O7 [M + H]+ 730.3928, found 730.3938.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(naphthalen-1-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12g)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 1-naphthaldehyde 8g (37 mg, 0.24 mmol) to afford compound 12g as a white powder (94 mg, 67% yield). 1H NMR (600 MHz, CDCl3): δ 8.14 (d, J = 7.7 Hz, 1H), 8.10 (s, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.69–7.60 (m, 2H), 7.40–7.34 (m, 2H), 7.27–7.19 (m, 2H), 6.24 (br s, 2H), 5.88 (s, 1H), 5.32 (d, J = 7.8 Hz, 1H), 5.06 (d, J = 5.1 Hz, 1H), 4.54 (s, 1H), 4.30 (s, 1H), 4.10–4.05 (m, 2H), 3.78–3.73 (m, 1H), 2.72–2.64 (m, 2H), 2.60–2.56 (m, 1H), 2.53–2.47 (m, 1H), 2.02–1.93 (m, 1H), 1.86–1.73 (m, 1H), 1.46 (s, 3H), 1.33–1.29 (br m, 18H), 1.13 (s, 3H). 13C NMR (151 MHz, CDCl3): δ 171.7, 155.7, 155.4, 153.0, 149.1, 139.6, 134.2, 133.76, 132.2, 128.5, 128.1, 127.7, 125.8, 125.6, 125.0, 124.57, 120.18, 91.0, 85.10, 83.5, 83.3, 81.7, 57.6, 55.4, 53.5, 52.8, 51.0, 29.1, 28.4, 27.9, 27.0, 25.1. HRMS (ESI): calculated for C37H50N7O7 [M + H]+ 704.3772, found 704.3775.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(2-(naphthalen-2-yl)ethyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12h)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 2-(naphthalen-2-yl)acetaldehyde 8h (38 mg, 0.24 mmol) to afford compound 12h as a white powder (99 mg, 69% yield). 1H NMR (400 MHz, CDCl3): δ 8.36 (s, 1H), 7.90 (s, 1H), 7.79–7.69 (m, 3H), 7.52 (s, 1H), 7.45–7.36 (m, 2H), 7.21 (dd, J = 8.4, 1.5 Hz, 1H), 6.15 (s, 2H), 6.03 (d, J = 1.7 Hz, 1H), 5.68 (d, J = 8.0 Hz, 1H), 5.48–5.46 (d, J = 8.0, 1H) 4.96–4.93 (m, 1H), 4.39–4.31 (m, 1H), 4.20–4.15 (m, 1H), 2.90–2.50 (m, 8H), 2.05–1.97 (m, 1H), 1.70–1.75 (m, 1H), 1.59 (s, 3H), 1.43 (d, J = 3.4 Hz, 18H), 1.33 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 172.4, 156.6, 153.1, 147.1, 140.2, 138.8, 134.4, 132.5, 128.0, 127.6, 127.4, 126.9, 126.0, 125.3, 120.4, 114.4, 90.2, 85.7, 83.8, 83.3, 81.7, 79.5, 52.9, 50.1, 28.4, 28.1, 27.2, 25.4. HRMS (ESI): calculated for C38H52N7O7 [M + H]+ 718.3928, found 718.3932.
tert-Butyl (2S)-4-(((1H-Indol-2-yl)methyl)(((3aR,4R,6R,6aR)-6-(6-amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (12i)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 1H-indole-2-carbaldehyde 8i (35 mg, 0.18 mmol) to afford compound 12i as a white powder (77 mg, 56% yield). 1H NMR (600 MHz, CDCl3): δ 9.41 (s, 1H), 8.20 (s, 1H), 7.81 (s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.28–7.23 (m, 1H), 7.10 (t, J = 7.5 Hz, 1H), 7.03 (t, J = 7.4 Hz, 1H), 6.25 (s, 1H), 6.00 (s, 3H), 5.46 (d, J = 8.4 Hz, 1H), 5.30 (d, J = 5.3 Hz, 1H), 4.90 (d, J = 4.9 Hz, 1H), 4.44–4.37 (m, 1H), 4.2 (m 1H), 3.76 (dd, J = 8.0, 2H), 2.87–2.84 (m, 1H), 2.78–2.75 (m, 6.8 Hz, 1H), 2.72–2.60 (m, 2H), 2.02–1.94 (m, 1H), 1.79–1.75 (m, 1H), 1.54 (s, 3H), 1.47–1.32 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 172.1, 155.6, 153.0, 149.1, 139.8, 136.4, 128.2, 121.3, 120.2, 120.0, 119.2, 114.6, 110.8, 101.0, 90.2, 84.8, 83.9, 83.4, 82.0, 79.8, 55.9, 52.4, 52.1, 51.2, 30.5, 28.4, 27.9, 27.1, 25.5. HRMS (ESI): calculated for C35H49N8O7 [M + H]+ 693.3724, found 693.3732.
tert-Butyl 3-(((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((S)-4-(tert-butoxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutyl)amino) methyl)-1H-indole-1-carboxylate (12j)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with tert-butyl 3-formyl-1H-indole-1-carboxylate 8j (58 mg, 0.24 mmol) to afford compound 12j as a white powder (79 mg, 50% yield). 1H NMR (600 MHz, CDCl3): δ 8.24 (s, 1H), 8.09 (s, 1H), 7.82 (s, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.44 (s, 1H), 7.28 (d, J = 7.4 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 5.97 (br d, J = 39.0 Hz, 3H), 5.37–5.32 (m, 2H), 4.81 (dd, J = 6.4, 3.2 Hz, 1H), 4.40–4.37 (m, 1H), 4.19–4.10 (m, 1H), 3.82 (br d, J = 13.7 Hz, 1H), 3.61–3.57 (br d, J = 13.8 Hz, 1H), 2.85–2.82 (br m, 1H), 2.71–2.58 (m, 2H), 2.52–2.48 (m, 1H), 2.02–1.99 (br m, 1H), 1.89–1.79 (m, 1H), 1.66 (s, 9H), 1.57 (s, 3H), 1.38 (br d, J = 27.7 Hz, 18H), 1.29 (s, 3H).13C NMR (151 MHz, CDCl3): δ 170.7, 154.8, 154.4, 152.0, 148.6, 148.1, 138.7, 134.6, 129.3, 123.7, 123.4, 121.5, 119.2, 119.1, 114.1, 113.3, 89.7, 84.2, 82.6, 82.3, 80.6, 54.6, 52.4, 51.7, 49.7, 49.0, 28.5, 27.3, 27.2, 26.9, 26.1, 24.2. HRMS (ESI): calculated for C40H57N8O9 [M + H]+ 793.4249, found 793.4256.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(benzo[b]thiophen-2-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (12k)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with benzo[b]thiophene-2-carbaldehyde 8k (39 mg, 0.24 mmol) to afford compound 12k as a white powder (89 mg, 63% yield). 1H NMR (400 MHz, CDCl3): δ 8.10 (s, 1H), 7.86 (s, 1H), 7.72 (d, J = 7.7 Hz, 1H), 7.61 (d, J = 7.4 Hz, 1H), 7.28 (d, J = 7.1 Hz, 1H), 7.22 (d, J = 7.2 Hz, 1H), 6.99 (s, 1H), 6.27 (s, 2H), 6.04 (s, 1H), 5.61 (d, J = 7.8 Hz, 1H), 5.40 (d, J = 5.4 Hz, 1H), 5.00 (br s, 1H), 4.42–4.36 (m, 1H), 4.23–4.15 (m, 1H), 3.95–3.91 (br d, J = 16.0 Hz, 1H), 3.85–3.81 (br d, J = 16.0 Hz, 1H), 2.89–2.84 (m, 1H), 2.76–2.64 (m, 2H), 2.60–2.52 (m, 1H), 2.02–1.99 (br d, J = 12.0 Hz, 1H), 1.83–1.81 (d, J = 8.0 Hz, 1H), 1.59 (s, 3H), 1.40–1.36 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.8, 155.4, 153.0, 149.1, 143.1, 139.9, 139.5, 124.1, 123.9, 123.1, 122.2, 120.2, 114.4, 90.6, 85.5, 83.8, 83.2, 81.7, 79.4, 55.3, 54.0, 52.7, 50.3, 29.6, 28.3, 27.9, 27.1, 25.4. HRMS (ESI): calculated for C35H48N7O7S [M + H]+ 710.3336, found 710.3348.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(benzo[b]thiophen-3-ylmethyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (12l)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with benzo[b]thiophene-3-carbaldehyde 8l (39 mg, 0.24 mmol) to afford compound 12l as a white powder (79 mg, 50% yield). 1H NMR (400 MHz, CDCl3): δ 8.63 (s, 1H), 8.30–8.23 (br d, J = 28.0 Hz, 3H), 7.73–7.67 (br d, J = 24.0 Hz, 3H), 6.94 (s, 2H), 6.44 (s, 1H), 6.06 (s, 1H), 5.72 (s, 1H), 5.20 (s, 1H), 4.81 (s, 1H), 4.63 (s, 1H), 4.35–4.32 (br d, J = 8.0 Hz, 1H), 4.16–4.13 (br d, J = 12.0 Hz, 1H), 3.34–2.89 (m, 4H), 2.46 (s, 1H), 2.27 (s, 1H), 2.00 (s, 3H), 1.85–1.81 (br d, J = 16.0 Hz, 18H), 1.71 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.9, 155.4, 153.0, 149.0, 140.5, 139.6, 138.6, 133.4, 124.6, 124.3, 123.9, 122.6, 122.5, 120.2, 114.2, 90.8, 85.2, 83.6, 83.3, 81.7, 79.4, 77.4, 77.3, 77.1, 76.8, 55.7, 52.9, 52.8, 50.9, 29.3, 28.3, 27.9, 27.0, 25.2. HRMS (ESI): calculated for C35H48N7O7S [M + H]+ 710.3336, found 710.3355.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(quinolin-6-ylmethyl)amino)butanoic Acid (13a)
To a solution of compound 12a (50 mg, 0.071 mmol) in 1 mL of CH2Cl2 was added a mixture of 9 mL of TFA and 1 mL of H2O, and the solution was stirred for 2 h at room temperature. The mixture was concentrated, and the crude product was purified by preparative HPLC, affording compound 13a as a white powder (33 mg, 74% yield). 1H NMR (400 MHz, D2O): δ 8.34 (d, J = 1.2 Hz, 1H), 8.10 (s, 1H), 7.79 (s, 1H), 7.39 (s, 2H), 7.28 (d, J = 8.2 Hz, 1H), 7.06 (t, J = 7.6 Hz, 1H), 6.92 (s, 1H), 6.05 (d, J = 5.0 Hz, 1H), 4.79 (t, J = 5.0 Hz, 1H), 4.56–4.49 (m, 2H), 4.38 (d, J = 9.9 Hz, 1H), 3.76–3.69 (m, 1H), 3.60–3.50 (m, 4H), 3.25 (t, J = 7.1 Hz, 1H), 2.43–2.34 (m, 1H), 2.24 (br s, 1H), 2.14–2.08 (m, 1H). 13C NMR (101 MHz, D2O): δ 169.9, 146.8, 143.6, 126.8, 122.8, 122.7, 120.3, 118.6, 109.0, 108.8, 73.5, 71.7, 52.2, 24.8. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2266.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(quinolin-2-ylmethyl)amino)butanoic Acid (13b)
Following the procedure described for compound 13a, compound 12b (50 mg, 0.071 mmol) was deprotected and purified, affording compound 13b as a white powder (8 mg, 17% yield over two steps). 1H NMR (400 MHz, D2O): δ 8.45 (d, J = 8.6 Hz, 1H), 8.14 (s, 1H), 7.88–7.81 (m, 1H), 7.62–7.56 (m, 3H), 7.53 (s, 1H), 7.40 (d, J = 9.7 Hz, 1H), 5.93 (d, J = 4.5 Hz, 1H), 4.58–4.48 (m, 3H), 4.46–4.41 (m, 1H), 4.29 (t, J = 5.1 Hz, 1H), 4.06 (dd, J = 7.8, 5.3 Hz, 1H), 3.48–3.28 (m, 4H), 2.37–2.18 (m, 2H). 13C NMR (101 MHz, D2O): δ 145.8, 142.4, 133.2, 132.1, 131.6, 129.8, 127.2, 123.0, 120.4, 92.3, 81.3, 80.4, 76.5, 74.4, 71.2, 54.2, 53.1, 27.5. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2265.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(isoquinolin-6-ylmethyl)amino)butanoic Acid (13c)
Following the procedure described for compound 13a, compound 12c (50 mg, 0.071 mmol) was deprotected and purified, affording compound 13c as a white powder (21 mg, 47% yield). 1H NMR (400 MHz, D2O): δ 8.00 (s, 1H), 7.83–7.70 (m, 3H), 7.49–7.32 (m, 3H), 6.99 (s, 1H), 5.81 (s, 1H), 4.88 (br d, J = 13.7 Hz, 1H), 4.64 (br d, J = 14.1 Hz, 1H), 4.44 (dd, J = 7.2, 5.7 Hz, 1H), 4.32 (dd, J = 5.4, 2.0 Hz, 2H), 3.94 (dd, J = 9.1, 4.1 Hz, 1H), 3.71 (t, J = 7.0 Hz, 2H), 3.59 (br d, J = 12.9 Hz, 1H), 2.50–2.45 (m, 1H), 2.38–2.28 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.5, 163.0, 162.6, 153.9, 148.9, 146.8, 144.2, 143.3, 142.9, 139.6, 133.3, 129.1, 128.3, 121.9, 120.8, 118.6, 117.7, 114.8, 90.4, 80.4, 72.9, 71.6, 56.8, 56.5, 51.0, 50.6, 25.9. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2273.
(S)-4-(([1,1′-Biphenyl]-4-ylmethyl)(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl)amino)-2-aminobutanoic Acid (13d)
Following the procedure described for compound 13a, compound 12d (50 mg, 0.068 mmol) was deprotected and purified, affording compound 13d as a white powder (30 mg, 68% yield). 1H NMR (400 MHz, D2O): δ 8.13 (br s, 1H), 7.94 (s, 1H), 7.40–7.29 (m, 5H), 7.19 (br s, 4H), 5.88 (s, 1H), 4.53–4.48 (m, 1H), 4.31 (s, 3H), 4.06 (dd, J = 8.3, 4.8 Hz, 1H), 3.69–3.49 (m, 4H), 2.49–2.37 (br d, J = 48.0 Hz, 2H). 13C NMR (101 MHz, D2O): δ 171.1, 163.0, 162.6, 162.2, 143.6, 140.2, 137.8, 131.1, 129.2, 128.4, 126.0, 118.4, 117.7, 114.8, 111.9, 90.5, 77.7, 73.9, 71.4, 51.0, 24.6. HRMS (ESI): calculated for C27H33N7O5 [M + H]+ 534.2465, found 534.2474.
(S)-4-(([1,1′-Biphenyl]-2-ylmethyl)(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl)amino)-2-aminobutanoic Acid (13e)
Following the procedure described for compound 13a, compound 12e (50 mg, 0.068 mmol) was deprotected and purified, affording compound 13e as a white powder (35 mg, 79% yield). 1H NMR (400 MHz, D2O): δ 8.31 (s, 1H), 8.24 (s, 1H), 7.51–7.29 (m, 6H), 7.25–7.17 (m, 3H), 5.98 (d, J = 3.4 Hz, 1H), 4.63–4.53 (m, 2H), 4.48 (d, J = 13.8 Hz, 1H), 4.40 (s, 1H), 4.27–4.21 (m, 1H), 3.71 (s, 1H), 3.48–3.23 (m, 4H), 2.19–2.11 (m, 1H), 2.03–1.95 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.2, 149.9, 147.6, 144.2, 143.6, 138.9, 131.2, 130.9, 130.1, 129.32, 128.9, 128.3, 126.2, 119.3, 117.7, 114.8, 90.3, 77.9, 73.3, 71.7, 55.3, 51.1, 24.3. HRMS (ESI): calculated for C27H33N7O5 [M + H]+ 534.2465, found 534.2472.
(S)-4-(([1,1′-Biphenyl]-3-ylmethyl)(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl)amino)-2-aminobutanoic Acid (13f)
Following the procedure described for compound 13a, compound 12f (50 mg, 0.068 mmol) was deprotected and purified, affording compound 13f as a white powder (34 mg, 77% yield). 1H NMR (400 MHz, D2O): δ 7.98 (s, 1H), 7.67 (s, 1H), 7.23–7.14 (m, 8H), 7.03 (d, J = 6.9 Hz, 2H), 5.86 (s, 1H), 4.38–4.32 (br m, 3H), 4.25–4.13 (m, 2H), 3.96 (dd, J = 8.6, 4.6 Hz, 1H), 3.61–3.39 (m, 4H), 2.48–2.42 (m, 1H), 2.39–2.23 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.9, 163.3, 162.9, 162.2, 149.0, 146.9, 143.6, 143.0, 139.6, 137.6, 129.5, 129.0, 128.1, 127.2, 125.5, 120.7, 118.5, 117.8, 90.4, 73.6, 71.5, 51.8, 24.7. HRMS (ESI): calculated for C27H33N7O5 [M + H]+ 534.2465, found 534.2468.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(naphthalen-2-ylmethyl)amino)butanoic Acid (13g)
Following the procedure described for compound 13a, compound 12g (50 mg, 0.071 mmol) was deprotected and purified, affording compound 13g as a white powder (33 mg, 74% yield). 1H NMR (400 MHz, D2O): δ 7.94 (s, 1H), 7.55 (d, J = 8.3 Hz, 4H), 7.39 (d, J = 6.9 Hz, 1H), 7.24 (s, 3H), 5.79 (s, 1H), 4.56 (br d, J = 12.0, 1H), 4.42–4.37 (m, 1H), 4.36–4.21 (m, 2H), 3.97 (dd, J = 8.6, 4.4 Hz, 1H), 3.76–3.42 (m, 4H), 2.53–2.25 (m, 2H). 13C NMR (101 MHz, D2O): δ 171.4, 163.0, 162.7, 149.3, 146.7, 143.4, 143.3, 132.8, 130.1, 128.4, 126.4, 122.2, 118.5, 117.7, 90.7, 73.5, 71.6, 51.4. HRMS (ESI): calculated for C25H30N7O5 [M + H]+ 508.2308, found 508.2314.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(2-(naphthalen-2-yl)ethyl)amino)butanoic Acid (13h)
Following the procedure described for compound 13a, compound 12h (50 mg, 0.069 mmol) was deprotected and purified, affording compound 13h as a white powder (33 mg, 76% yield). 1H NMR (400 MHz, CD3OD): δ 8.45 (s, 1H), 8.23 (s, 1H), 7.78–7.63 (m, 3H), 7.58 (s, 1H), 7.46–7.39 (m, 2H), 7.26 (d, J = 8.4 Hz, 1H), 6.13 (d, J = 4.6 Hz, 1H), 4.71 (d, J = 9.6 Hz, 1H), 4.62–4.55 (m, 1H), 4.44 (t, J = 5.1 Hz, 1H), 4.11 (dd, J = 8.3, 4.7 Hz, 1H), 3.86–3.54 (m, 6H), 3.21 (t, J = 8.1 Hz, 2H), 2.56–2.46 (m, 1H), 2.36–2.28 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.3, 161.6, 161.2, 151.5, 148.1, 133.5, 133.1, 132.5, 119.7, 118.0, 115.1, 90.6, 79.8, 74.2, 68.7, 54.8, 52.0, 51.0, 29.4, 24.5. HRMS (ESI): calculated for C26H32N7O5 [M + H]+ 522.2465, found 522.2477.
(S)-4-(((1H-Indol-2-yl)methyl)(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl)amino)-2-aminobutanoic Acid (13i)
Following the procedure described for compound 13a, compound 12i (50 mg, 0.072 mmol) was deprotected and purified, affording compound 13i as a white powder (27 mg, 61% yield). 1H NMR (400 MHz, D2O): δ 8.30 (s, 1H), 7.68 (s, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.13 (t, J = 7.5 Hz, 1H), 7.06 (t, J = 6.9 Hz, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.08 (s, 1H), 4.69–4.64 (m, 1H), 4.61–4.45 (m, 4H), 4.03–4.00 (m, 2H), 3.70 (t, J = 7.3 Hz, 2H), 3.63–3.60 (br d, J = 12.0, 1H), 2.57–2.45 (m, 1H), 2.38–2.33 (m, 1H). 13C NMR (101 MHz, D2O): δ 170.7, 149.2, 146.7, 143.9, 143.0, 123.0, 120.4, 120.1, 111.0, 91.2, 73.8, 72.0, 25.0. HRMS (ESI): calculated for C23H29N8O5 [M + H]+ 497.2261, found 497.2263.
(S)-4-(((1H-Indol-3-yl)methyl)(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxy-tetrahydrofuran-2-yl)methyl)amino)-2-aminobutanoic Acid (13j)
Following the procedure described for compound 13a, compound 12j (50 mg, 0.063 mmol) was deprotected and purified, affording compound 13j as a pink powder (23 mg, 61% yield). 1H NMR (500 MHz, CD3OD): δ 8.56–8.31 (m, 1H), 7.64 (d, J = 7.0 Hz, 1H), 7.55 (s, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.18 (t, J = 8.2 Hz, 1H), 7.07 (t, J = 7.5 Hz, 1H), 6.14 (dd, J = 9.4, 4.3 Hz, 1H), 4.75–4.56 (m, 3H), 4.51–4.38 (m, 1H), 4.02 (dd, J = 8.4, 4.7 Hz, 1H), 3.81–3.74 (m, 1H), 3.71–3.59 (m, 2H), 3.56–3.49 (m, 1H), 3.37 (s, 4H), 2.58–2.48 (m, 1H), 2.42–2.31 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 170.3, 160.4, 150.5, 148.1, 134.7, 128.0, 127.2, 122.2, 120.1, 116.8, 111.7, 101.8, 91.5, 90.3, 81.0, 78.8, 74.6, 66.4, 49.9, 48.5, 44.6, 26.1, 23.1. HRMS (ESI): calculated for C23H29N8O5 [M + H]+ 497.2261, found 497.2268.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(benzo[b]thiophen-2-ylmethyl)amino)butanoic Acid (13k)
Following the procedure described for compound 13a, compound 12k (50 mg, 0.070 mmol) was deprotected and purified, affording compound 13k as a white powder (34 mg, 78% yield). 1H NMR (400 MHz, D2O): δ 8.26 (s, 1H), 7.69 (s, 1H), 7.64–7.58 (m, 1H), 7.42–7.35 (m, 1H), 7.34–7.27 (m, 2H), 7.13 (s, 1H), 6.04 (d, J = 2.3 Hz, 1H), 4.70–4.57 (m, 3H), 4.49–4.42 (m, 2H), 4.07 (dd, J = 8.7, J = 4.5 Hz, 1H), 3.92–3.86 (br t, J = 12.0 Hz, 1H), 3.73–3.67 (m, 2H), 3.63–3.59 (br d, J = 16.0, 1H), 2.57–2.47 (m, 1H), 2.41–2.33 (m, 1H). 13C NMR (101 MHz, D2O): δ 172.7, 162.7, 144.0, 143.0, 128.7, 125.7, 125.0, 123.7, 122.1, 91.2, 78.0, 73.9, 71.9, 53.1, 51.45, 24.1. HRMS (ESI): calculated for C23H28N7O5S [M + H]+ 514.1873, found 514.1875.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(benzo[b]thiophen-3-ylmethyl)amino)butanoic Acid (13l)
Following the procedure described for compound 13a, compound 12l (50 mg, 0.070 mmol) was deprotected and purified, affording compound 13l as a white powder (29 mg, 67% yield). 1H NMR (400 MHz, CD3OD): δ 8.37 (s, 1H), 8.06 (s, 1H), 7.93 (s, 1H), 7.85–7.80 (m, 2H), 7.35–7.26 (m, 2H), 6.12 (d, J = 3.0 Hz, 1H), 4.72 (s, 2H), 4.61–4.53 (m, 2H), 4.50–4.46 (m, 1H), 4.00 (dd, J = 8.5, 4.4 Hz, 1H), 3.84–3.60 (m, 4H), 2.55–2.46 (m, 1H), 2.37–2.31 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.8, 162.1, 161.8, 161.4, 161.1, 151.1, 147.8, 140.0, 137.8, 124.8, 120.9, 119.5, 118.0, 115.1, 112.2, 54.7, 51.80, 25.1. HRMS (ESI): calculated for C23H28N7O5S [M + H]+ 514.1873, found 514.1877.
(E)-3-(4-((Trimethylsilyl)ethynyl)phenyl)acrylaldehyde (15y)
To a solution of 4-((trimethylsilyl)ethynyl)benzaldehyde 14y (1.81 g, 8.0 mmol) in THF (40 mL) was added (triphenyl phosphoramylidene)acetaldehyde (2.20 g, 7.2 mmol). The suspension was stirred at 50 °C under N2 for overnight and concentrated to dryness under vacuum. The crude product was purified by flash chromatography on silica gel (0–90% CH2Cl2 in petroleum ether) to give compound 15y (1.2 g, 73%) as a white solid. 1H NMR (400 MHz, CDCl3): δ 9.72 (d, J = 7.7 Hz, 1H), 7.54–7.50 (m, 4H), 7.45 (br d, J = 12.0 Hz, 1H), 6.75–6.69 (m, 1H), 0.28 (s, 9H). 13C NMR (101 MHz, CDCl3): δ 193.5, 151.6, 132.9, 132.6, 128.3, 126.1, 104.3, 97.6. HRMS (ESI): calculated for C14H17OSi [M + H]+ 229.3740, found 229.3744.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(o-tolyl)allyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (16a)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(o-tolyl)acrylaldehyde 15a (35 mg, 0.24 mmol) to afford compound 16a as a white powder (100 mg, 72% yield) 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H), 7.95 (s, 1H), 7.41–7.35 (m, 1H), 7.29 (s, 1H), 7.14 (dd, J = 5.3, 3.9 Hz, 3H), 6.6–6.64 (br d, J = 12.0 Hz, 1H), 6.27 (s, 2H), 6.13–6.03 (m, 2H), 5.73 (d, J = 8.1 Hz, 1H), 5.48 (d, J = 5.1 Hz, 1H), 5.05–4.96 (m, 1H), 4.43–4.39 (m, 1H), 4.25–4.21 (m, 1H), 3.42–3.33 (m, 1H), 3.31–3.23 (m, 1H), 2.89–2.84 (m, 1H), 2.72–2.55 (m, 3H), 2.30 (s, 3H), 2.07–1.91 (m, 1H), 1.86–1.74 (m, 1H), 1.63 (s, 3H), 1.44–1.41 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.9, 155.8, 153.1, 149.3, 140.0, 136.0, 135.2, 130.9, 127.4, 126.1, 125.7, 120.4, 114.5, 90.8, 85.5, 83.9, 83.4, 81.7, 57.2, 55.9, 52.9, 50.6, 29.5, 28.4, 28.0, 27.2, 25.5, 19.9. HRMS (ESI): calculated for C36H52N7O7 [M + H]+ 694.3928, found 694.3935.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(m-tolyl)allyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (16b)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(m-tolyl)acrylaldehyde 15b (35 mg, 0.24 mmol) to afford compound 16b as a white powder (104 mg, 75% yield). 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 1H), 7.92 (s, 1H), 7.18–7.09 (m, 3H), 7.01 (d, J = 7.3 Hz, 1H), 6.40–6.36 (br d, J = 16.0 Hz, 1H), 6.20–6.05 (m, 4H), 5.66 (d, J = 7.9 Hz, 1H), 5.44 (d, J = 6.1 Hz, 1H), 4.96 (d, J = 5.8 Hz, 1H), 4.38 (s, 1H), 4.24–4.08 (m, 1H), 3.39–3.14 (m, 2H), 2.84–2.79 (m, 1H), 2.71–2.50 (m, 3H), 2.31 (s, 3H), 2.00–1.93 (m, 1H), 1.82–1.73 (m 1H), 1.60 (s, 3H), 1.41–1.38 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 155.8, 153.1, 149.3, 141.0, 140.0, 135.1, 128.5, 126.1, 123.5, 120.4, 114.5, 90.8, 85.4, 83.3, 57.0, 55.9, 52.9, 50.6, 29.5, 28.4, 28.0, 27.2, 25.5. 21.4. HRMS (ESI): calculated for C36H52N7O7 [M + H]+ 694.3928, found 694.3938.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(p-tolyl)allyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (16c)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(p-tolyl)acrylaldehyde 15c (35 mg, 0.24 mmol) to afford compound 16c as a white powder (109 mg, 79% yield). 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 1H), 7.92 (s, 1H), 7.19 (d, J = 8.1 Hz, 2H), 7.07 (d, J = 8.0 Hz, 2H), 6.39–6.35 (br J = 16.1 Hz, 1H), 6.25–5.98 (m, 4H), 5.66 (d, J = 8.1 Hz, 1H), 5.43 (d, J = 6.1 Hz, 1H), 4.96 (d, J = 6.1 Hz, 1H), 4.36 (br s, 1H), 4.21–4.17 (m, 1H), 3.33–3.16 (m, 2H), 2.84–2.79 (m, 1H), 2.67–2.53 (m, 3H), 2.30 (s, 3H), 1.98–1.93 (m, 1H), 1.84–1.71 (m, 1H), 1.60 (s, 3H), 1.44–1.37 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 155.8, 153.1, 149.3, 140.03, 136.8, 133.0, 128.6, 127.5, 126.3, 120.4, 114.5, 90.8, 85.5, 83.9, 83.4, 81.7, 57.0, 55.9, 52.9, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H52N7O7 [M + H]+ 694.3928, found 694.3940.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-methoxyphenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16d)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-methoxyphenyl)acrylaldehyde 15d (39 mg, 0.24 mmol) to afford compound 16d as a white powder (75 mg, 53% yield). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 7.95 (s, 1H), 7.38 (d, J = 7.6 Hz, 1H), 7.24–7.17 (m, 1H), 6.93–6.75 (m, 3H), 6.33 (br s, 2H), 6.25–6.14 (m, 1H), 6.07 (d, J = 2.2 Hz, 1H), 5.74 (d, J = 8.2 Hz, 1H), 5.44 (d, J = 6.5 Hz, 1H), 5.06–4.93 (m, 1H), 4.43–4.39 (m, 1H), 4.26–4.16 (m, 1H), 3.83 (s, 3H), 3.39–3.22 (m, 2H), 2.88–2.83 (m, 1H), 2.75–2.50 (m, 3H), 2.03–1.98 (m, 1H), 1.85–1.78 (m, 1H), 1.62 (s, 3H), 1.42–1.40 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.3, 156.5, 155.8, 155.1, 153.6, 149.9, 141.4, 130.3, 126.8, 125.9, 120.7, 119.7, 114.5, 111.3, 90.8, 85.8, 84.0, 82.7, 81.7, 79.4, 57.4, 55.9, 55.4, 52.9, 49.9, 29.4, 28.4, 27.2, 25.5. HRMS (ESI): calculated for C36H52N7O8 [M + H]+ 710.3877, found 710.3882.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-methoxyphenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16e)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-methoxyphenyl)acrylaldehyde 15e (39 mg, 0.24 mmol) to afford compound 16e as a white powder (82 mg, 58% yield). 1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H), 7.96 (s, 1H), 7.23 (t, J = 7.9 Hz, 1H), 6.97–6.88 (m, 2H), 6.80 (dd, J = 8.2, 2.4 Hz, 1H), 6.44–6.40 (br d, J = 16.0 Hz, 1H), 6.28–6.17 (m, 1H), 6.09–6.03 (br d, J = 24.0 Hz, 3H), 5.66–5.48 (br m, 2H), 5.05–4.97 (m, 1H), 4.48–4.36 (m, 1H), 4.23 (d, J = 4.7 Hz, 1H), 3.83 (s, 3H), 3.43–3.18 (m, 2H), 2.88–2.83 (m, 1H), 2.75–2.53 (m, 3H), 2.00–1.97 (m, 1H), 1.88–1.73 (m, 1H), 1.64 (s, 3H), 1.45–1.42 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 159.8, 155.7, 151.9, 149.3, 140.1, 138.3, 133.5, 130.0, 127.6, 120.4, 119.9, 114.5, 113.3, 110.8, 93.1, 89.5, 81.7, 83.4, 81.72, 57.0, 55.9, 55.3, 50.6, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H52N7O8 [M + H]+ 710.3877, found 710.3885.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-methoxyphenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16f)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-methoxyphenyl)acrylaldehyde 15f (39 mg, 0.24 mmol) to afford compound 16f as a white powder (86 mg, 61% yield). 1H NMR (400 MHz, CDCl3): δ 8.29 (s, 1H), 7.96 (s, 1H), 7.26 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.8 Hz, 2H), 6.45–6.21 (m, 3H), 6.09–6.04 (m, 2H), 5.72 (d, J = 8.2 Hz, 1H), 5.47(d, J = 8.1 Hz, 1H), 5.01–4.99 (m, 1H), 4.41–4.40 (br d, J = 8.2 Hz, 1H), 4.25–4.20 (m, 1H), 3.81 (s, 3H), 3.36–3.30 (m, 1H), 3.25–3.17 (m, 1H), 2.87–2.82 (m, 1H), 2.71–2.53 (m, 3H), 2.03–1.96 (m, 1H), 1.86–1.75 (m, 1H), 1.63 (s, 3H), 1.47–1.41 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 159.1, 155.8, 155.6, 153.1, 149.3, 140.1, 132.5, 129.7, 127.5, 124.9, 120.3, 114.5, 114.0, 90.9, 85.5, 83.95, 83.4, 81.7, 57.1, 55.8, 55.3, 52.9, 29.5, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H52N7O8 [M + H]+ 710.3877, found 710.3887.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-fluorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16g)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-fluorophenyl)acrylaldehyde 15g (36 mg, 0.24 mmol) to afford compound 16g as a white powder (96 mg, 69% yield). 1H NMR (600 MHz, CDCl3): δ 8.26 (s, 1H), 7.91 (s, 1H), 7.37 (t, J = 7.1 Hz, 1H), 7.19–7.15 (m, 1H), 7.04 (t, J = 7.9 Hz, 1H), 7.01–6.97 (m, 1H), 6.58 (m, 1H), 6.30–6.21 (m, 1H), 6.04 (s, 1H), 5.90 (s, 2H), 5.58 (d, J = 8.0 Hz, 1H), 5.44 (d, J = 5.4 Hz, 1H), 5.01–4.92 (m, 1H), 4.38 (s, 1H), 4.22–4.15 (m, 1H), 3.35 (d, J = 6.2 Hz, 1H), 3.29–3.19 (m, 1H), 2.86–2.80 (m, 1H), 2.71–2.51 (m, 3H), 1.99–1.96 (m, 1H), 1.82–1.72 (m, 1H), 1.60 (s, 3H), 1.40–1.38 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 171.5, 168.8, 159.4, 156.6, 152.5, 147.4, 145.2, 141.6, 137.1, 127.2, 123.5, 121.5, 119.8, 116.2, 112.4, 91.7, 85.9, 83.3, 81.7, 79.9, 57.1, 54.8, 52.4, 51.8, 49.6, 28.1, 26.1, 24.4. HRMS (ESI): calculated for C35H49FN7O7 [M + H]+ 698.3678, found 698.3690.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-fluorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16h)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(3-fluorophenyl)acrylaldehyde 15h (36 mg, 0.24 mmol) to afford compound 16h as a white powder (93 mg, 67% yield). 1H NMR (600 MHz, CDCl3): δ 8.26 (s, 1H), 7.91 (s, 1H), 7.25–7.18 (m, 1H), 7.03 (d, J = 7.7 Hz, 1H), 7.01–6.98 (m, 1H), 6.91–6.87 (m 1H), 6.36 (d, J = 8.1 Hz, 1H), 6.20–6.15 (m, 1H), 6.04 (s, 1H), 5.91 (s, 2H), 5.57 (d, J = 8.0 Hz, 1H), 5.45 (d, J = 5.5 Hz, 1H), 4.97 (d, J = 5.7 Hz, 1H), 4.42–4.34 (m, 1H), 4.19 (d, J = 4.9 Hz, 1H), 3.32–3.28 (m, 1H), 3.23–3.19 (m, 1H), 2.82–2.79 (m, 1H), 2.70–2.50 (m, 3H), 2.03–1.91 (m, 1H), 1.79–1.75 (m, 1H), 1.60 (s, 3H), 1.42–1.38 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 170.70, 162.85, 161.22, 154.58, 154.49, 152.06, 148.19, 139.03, 138.13, 130.67, 128.94, 128.88, 127.12, 121.17, 119.29, 113.45, 113.28, 113.14, 111.76, 111.61, 89.75, 84.52, 82.87, 82.26, 80.69, 55.83, 54.96, 51.81, 49.61, 28.54, 27.33, 26.95, 26.14, 24.42. HRMS (ESI): calculated for C35H49FN7O7 [M + H]+ 698.3678, found 698.3682.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-fluorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16i)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-fluorophenyl)acrylaldehyde 15i (36 mg, 0.24 mmol) to afford compound 16i as a white powder (86 mg, 62% yield). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.89 (s, 1H), 7.21–7.16 (m, 2H), 6.90 (t, J = 8.6 Hz, 2H), 6.51 (s, 2H), 6.32–6.29 (br d, J = 16.1 Hz, 1H), 6.08–5.99 (m, 2H), 5.74 (d, J = 8.1 Hz, 1H), 5.42 (d, J = 7.9 Hz, 1H), 4.96 (d, J = 3.5 Hz, 1H), 4.36–4.32 (m, 1H), 4.22–4.14 (m, 1H), 3.27–3.22 (m, 1H), 3.18–3.12 (m, 1H), 2.80–2.75 (m, 1H), 2.66–2.57(m, 2H), 2.54–2.46 (m, 1H), 1.99–1.88 (m, 1H), 1.78–1.69 (m, 1H), 1.56 (s, 3H), 1.36–1.34 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 163.3, 160.9, 155.9, 155.5, 153.0, 149.1, 139.9, 132.9, 131.5, 127.7, 126.2, 120.2, 115.4, 115.2, 114.3, 90.7, 85.5, 83.8, 83.2, 81.7, 79.3, 56.9, 55.8, 52.8, 50.5, 29.4, 28.3, 27.9, 27.1, 25.4. HRMS (ESI): calculated for C35H49FN7O7 [M + H]+ 698.3678, found 698.3694.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-chlorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16j)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-chlorophenyl)acrylaldehyde 15j (40 mg, 0.24 mmol) to afford compound 16j as a white powder (84 mg, 59% yield). 1H NMR (400 MHz, CDCl3): δ 8.25 (s, 1H), 7.91 (s, 1H), 7.44–7.41 (m, 1H), 7.29 (dd, J = 7.5, 1.7 Hz, 1H), 7.17–7.09 (m, 2H), 6.80 (d, J = 15.9 Hz, 1H), 6.22–6.10 (m, 3H), 6.04 (s, 1H), 5.65 (d, J = 8.0 Hz, 1H), 5.44 (d, J = 5.6 Hz, 1H), 4.98 (d, J = 9.5 Hz, 1H), 4.41–4.33 (m, 1H), 4.23–4.16 (m, 1H), 3.38–3.30 (m, 1H), 3.28–3.20 (m, 1H), 2.86–2.81 (m, 1H), 2.62 (br s, 2H), 2.56 (d, J = 12.9 Hz, 1H), 12.01–1.92 (m, 1H), 1.79–1.75 (m, 1H), 1.59 (s, 3H), 1.39–1.37 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 174.1, 158.8, 158.6, 156.1, 152.2, 135.8, 132.6, 132.0, 131.5, 129.9, 129.8, 123.4, 117.5, 93.8, 88.5, 86.9, 86.3, 82.4, 59.0, 32.6, 31.4, 31.0, 30.2, 28.5. HRMS (ESI): calculated for C35H49ClN7O7 [M + H]+ 714.3382, found 714.3389.
tert-butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-chlorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16k)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(3-chlorophenyl)acrylaldehyde 15k (40 mg, 0.24 mmol) to afford compound 16k as a white powder (79 mg, 65% yield). 1H NMR (400 MHz, CDCl3): δ 8.22 (s, 1H), 7.88 (s, 1H), 7.17–7.09 (m, 3H), 6.32–6.28 (br d, J = 16.0 Hz, 1H), 6.16 (d, J = 5.8 Hz, 3H), 6.01 (s, 1H), 5.62 (d, J = 7.9 Hz, 1H), 5.42 (d, J = 5.6 Hz, 1H), 4.98–4.91 (m, 1H), 4.37–4.30 (m, 1H), 4.18 (s, 1H), 3.29–3.24 (m, 1H), 3.19–3.14 (m, 1H), 2.81–2.76 (m, 1H), 2.66–2.60 (m, 2H), 2.53–2.47 (m, 1H), 1.99–1.88 (m, 1H), 1.79–1.67 (m, 1H), 1.56 (s, 3H), 1.36 (d, J = 6.8, 21H). 13C NMR (101 MHz, CDCl3): δ 174.0, 158.9, 158.7, 157.0, 150.5, 143.2, 141.2, 137.2, 135.1, 132.9, 131.5, 130.5, 129.4, 127.7, 124.1, 118.9, 95.0, 88.7, 87.6, 86.5, 84.8, 84.3, 60.7, 59.1, 53.8, 32.7, 31.6, 31.1, 28.6. HRMS (ESI): calculated for C35H49ClN7O7 [M + H]+ 714.3382, found 714.3408.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-chlorophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16l)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-chlorophenyl)acrylaldehyde 15l (40 mg, 0.24 mmol) to afford compound 16l as a white powder (79 mg, 56% yield). 1H NMR (400 MHz, CDCl3): δ 8.22 (s, 1H), 7.89 (s, 1H), 7.19–7.13 (m, 4H), 6.43 (s, 2H), 6.31–6.28 (br, J = 16.0 Hz, 1H), 6.12–6.05 (m, 1H), 6.02 (d, J = 4.1 Hz, 1H), 5.70 (d, J = 8.1 Hz, 1H), 5.42 (d, J = 5.9 Hz, 1H), 4.97–4.94(m, 1H), 4.37–4.32 (m, 1H), 4.22–4.14 (m, 1H), 3.28–3.22 (m, 1H), 3.19–3.13 (m, 1H), 2.80–2.76 (m, 1H), 2.68–2.58 (m, 2H), 2.54–2.47 (m, 1H), 2.00–1.89 (m, 1H), 1.75 (d, J = 9.4 Hz, 1H), 1.57 (s, 3H), 1.37–1.35 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.8, 155.5, 152.9, 149.1, 139.9, 135.2, 132.9, 131.5, 128.6, 127.4, 120.2, 114.3, 90.7, 85.5, 83.8, 83.3, 81.6, 79.4, 56.9, 55.9, 52.8, 50.5, 29.5, 28.3, 27.1, 25.4. HRMS (ESI): calculated for C35H49ClN7O7 [M + H]+ 714.3382, found 714.3403.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-bromophenyl)allyl)amino)-2-((tert-butoxycarbonyl)amino) butanoate (16m)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-bromophenyl)acrylaldehyde 15m (51 mg, 0.24 mmol) to afford compound 16m as a white powder (80 mg, 53% yield). 1H NMR (400 MHz, CDCl3): δ 8.33–8.22 (m, 1H), 7.93 (s, 1H), 7.50 (dd, J = 7.9, 3.9 Hz, 1H), 7.47–7.38 (m, 1H), 7.28 (t, J = 4.4 Hz, 1H), 7.22 (d, J = 7.3 Hz, 1H), 7.06 (d, J = 7.4 Hz, 1H), 6.77 (d, J = 15.2 Hz, 1H), 6.19 (s, 2H), 6.16–6.01 (m, 2H), 5.67 (s, 1H), 5.46 (s, 1H), 5.01 (s, 1H), 4.40 (s, 1H), 4.22 (s, 1H), 3.32 (br d, J = 22.6 Hz, 2H), 2.84 (s, 1H), 2.63 (br d, J = 42.6 Hz, 3H), 1.98 (s, 1H), 1.79 (s, 1H), 1.61 (d, J = 3.8 Hz, 3H), 1.42 (d, J = 2.1 Hz, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.6, 155.5, 152.9, 149.0, 139.9, 136.3, 133.9, 132.3, 128.1, 128.0, 126.3, 124.0, 120.2, 90.9, 85.5, 83.8, 83.5, 81.7, 59.2, 56.0, 53.5, 52., 50.9, 29.5, 28.4, 27.9, 27.1, 25.4. HRMS (ESI): calculated for C35H49BrN7O7 [M + H]+ 758.2877, found 758.2882.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-bromophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16n)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(3-bromophenyl)acrylaldehyde 15n (51 mg, 0.24 mmol) to afford compound 16n as a white powder (94 mg, 62% yield). 1H NMR (600 MHz CDCl3): δ 8.25 (s, 1H), 7.90 (s, 1H), 7.43 (s, 1H), 7.30 (d, J = 7.8 Hz, 1H), 7.18–7.10 (m, 2H), 6.33–6.30 (br d, J = 12.0 Hz, 1H), 6.19–6.14 (m, 1H), 6.05 (d, J = 8.1 Hz, 2H), 5.61 (d, J = 7.7 Hz, 1H), 5.44 (d, J = 5.3 Hz, 1H), 4.98 (s, 1H), 4.36 (s, 1H), 4.19 (s, 1H), 3.31–3.18 (m, 2H), 2.82–2.78 (m, 1H), 2.70–2.50 (m, 3H), 1.96(br d, J = 4.0 Hz, 1H), 1.78 (br d, J = 4.1 Hz, 1H), 1.59 (s, 3H), 1.39 (d, J = 10.7 Hz, 21H). 13C NMR (151 MHz, CDCl3): δ 170.2, 156.2, 153.1, 147.5, 138.5, 131.3, 130.3, 130.0, 129.1, 122.7, 124.5, 122.7, 90.7, 85.2, 84.6, 83.9, 83.3, 81.3, 79.5, 56.0, 52.8, 49.9, 29.58, 29.6, 28.4, 27.2, 24.4. HRMS (ESI): calculated for C35H49BrN7O7 [M + H]+ 758.2877, found 758.2881.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-bromophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16o)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-bromophenyl)acrylaldehyde 15o (51 mg, 0.24 mmol) to afford compound 16o as a white powder (122 mg, 81% yield). 1H NMR (400 MHz, CDCl3): δ 8.22 (s, 1H), 7.89 (s, 1H), 7.33 (d, J = 8.4 Hz, 2H), 7.08 (d, J = 8.4 Hz, 2H), 6.41 (s, 2H), 6.26 (s, 1H), 6.14–6.07 (m, 1H), 6.03 (d, J = 1.6 Hz, 1H), 5.70 (d, J = 8.1 Hz, 1H), 5.43 (d, J = 5.8 Hz, 1H), 4.96 (dd, J = 6.3 Hz, 3.6 Hz, 1H), 4.37–4.31 (m, 1H), 4.22–4.14 (m, 1H), 3.27–3.22 (m, 1H), 3.18–3.13 (m, 1H), 2.80–2.75 (m, 1H), 2.69–2.57 (m, 2H), 2.54–2.47 (m, 1H), 1.99–1.88 (m, 1H), 1.79–1.69 (m, 1H), 1.57 (s, 3H), 1.37–1.35 (br d, J = 8.3 Hz, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.8, 155.5, 153.0, 149.1, 139.9, 135.7, 131.5, 127.7, 127.5, 121.1, 120.2, 114.3, 90.7, 85.5, 83.8, 83.3, 81.6, 79.4, 56.89 55.9, 52.80 50.5, 29.5, 28.3, 27.9, 27.1, 25.4. HRMS (ESI): calculated for C35H49BrN7O7 [M + H]+ 758.2877, found 758.2895.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-nitrophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16p)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(2-nitrophenyl)acrylaldehyde 15p (42 mg, 0.24 mmol) to afford compound 16p as a white powder (69 mg, 47% yield). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.91 (s, 1H), 7.82 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 4.1 Hz, 2H), 7.29 (dd, J = 8.3, 4.2 Hz, 1H), 6.85–6.81 (br d, J = 16.1 Hz, 1H), 6.46 (s, 2H), 6.15–6.06 (m, 1H), 6.03 (d, J = 2.0 Hz, 1H), 5.73 (d, J = 8.1 Hz, 1H), 5.41 (d, J = 5.7 Hz, 1H), 4.96 (dd, J = 6.4, 3.6 Hz, 1H), 4.37–4.31 (m, 1H), 4.21–4.14 (m, 1H), 3.33–3.28 (m, 1H), 3.24–3.19 (m, 1H), 2.83–2.78 (m, 1H), 2.72–2.60 (m, 2H), 2.56–2.50 (m, 1H), 1.98–1.91 (m, 1H), 1.79–1.68 (m, 1H), 1.55 (s, 3H), 1.42–1.29 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.7, 155.8, 155.5, 153.0, 149.1, 139.9, 135.7, 131.5, 127.7, 121.1, 120.2, 114.3, 90.7, 85.5, 83.8, 83.3, 81.6, 79.4, 56.9, 55.9, 53.4, 52.8, 50.6, 29.5, 28.3, 27.1, 25.4. HRMS (ESI): calculated for C35H49N8O9 [M + H]+ 725.3633, found 725.3632.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-nitrophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16q)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(3-nitrophenyl)acrylaldehyde 15q (42 mg, 0.24 mmol) to afford compound 16q as a white powder (63 mg, 43% yield). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.91 (s, 1H), 7.82 (d, J = 8.1 Hz, 1H), 7.44 (d, J = 4.1 Hz, 2H), 7.29 (dd, J = 8.3, 4.2 Hz, 1H), 6.85–6.81 (br d, J = 15.7 Hz, 1H), 6.46 (s, 2H), 6.15–6.06 (m, 1H), 6.03 (d, J = 2.0 Hz, 1H), 5.73 (d, J = 8.1 Hz, 1H), 5.41 (d, J = 5.7 Hz, 1H), 4.96 (dd, J = 6.4, 3.6 Hz, 1H), 4.37–4.31 (m, 1H), 4.21–4.14 (m, 1H), 3.33–3.19 (m, 2H), 2.83–2.78 (m, 1H), 2.72–2.60 (m, 2H), 2.56–2.50 (m, 1H), 1.98–1.93 (m, 1H), 1.79–1.68 (m, 1H), 1.55 (s, 3H), 1.42–1.29 (m, 21H). 13C NMR (151 MHz, CDCl3): δ 170.7, 154.8, 154.4, 152.0, 148.6, 148.1, 138.7, 134.6, 129.3, 123.7, 123.4, 121.5, 119.2, 119.1, 114.1, 113.3, 89.73, 84.2, 82.6, 82.3, 80.6, 54.6, 52.4, 51.7, 49.7, 49.0, 28.5, 27.3, 27.2, 26.9, 26.1, 24.2. HRMS (ESI): calculated for C35H49N8O9 [M + H]+ 725.3633, found 725.3634.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-nitrophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16r)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-nitrophenyl)acrylaldehyde 15r (42 mg, 0.24 mmol) to afford compound 16r as a white powder (74 mg, 51% yield). 1H NMR (600 MHz, CDCl3): δ 8.24 (s, 1H), 8.10 (d, J = 7.7 Hz, 2H), 7.93 (s, 1H), 7.34 (d, J = 7.9 Hz, 2H), 6.45–6.43 (br d, J = 15.9 Hz, 1H), 6.36–6.31 (m, 1H), 6.24 (s, 2H), 6.08 (s, 1H), 5.62 (d, J = 7.7 Hz, 1H), 5.46 (d, J = 5.0 Hz, 1H), 5.02 (s, 1H), 4.40 (s, 1H), 4.24 (s, 1H), 3.37–3.23 (m, 2H), 2.87–2.81 (m, 1H), 2.78 (br d, J = 19.4 Hz, 1H), 2.66 (s, 1H), 2.61–2.57 (m, 1H), 2.03–1.99 (br d, J = 20.7 Hz, 1H), 1.84–1.73 (m, 1H), 1.61 (s, 3H), 1.42–1.40 (br d, J = 15.3 Hz, 21H). 13C NMR (151 MHz, CDCl3): δ 171.6, 155.7, 153.0, 149.1, 146.7, 143.2, 140.1, 126.6, 123.9, 120.3, 114.43, 90.7, 85.7, 84.0, 83.3, 81.8, 79.5, 56.9, 56.1, 52.8, 50.8, 29.7, 28.4, 28.0, 27.2, 25.4. HRMS (ESI): calculated for C35H49N8O9 [M + H]+ 725.3633, found 725.3639.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(2-cyanophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16s)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-2-(3-oxoprop-1-en-1-yl)benzonitrile 15s (58 mg, 0.24 mmol) to afford compound 16s as a white powder (82 mg, 68% yield). 1H NMR (600 MHz, CDCl3): δ 8.26 (s, 1H), 7.97 (s, 1H), 7.58 (d, J = 7.7 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 6.78 (br d, J = 11.6 Hz, 1H), 6.54 (s, 2H), 6.43–6.34 (m, 1H), 6.09 (s, 1H), 5.78 (d, J = 8.2 Hz, 1H), 5.47 (d, J = 5.6 Hz, 1H), 5.03 (dd, J = 6.2, 3.6 Hz, 1H), 4.42–4.39 m, 1H), 4.26–4.23 (m, 1H), 3.40–3.37 (m, 1H), 3.32–3.28 (m, 1H), 2.89–2.85 (m, 1H), 2.77–2.73 (m, 1H), 2.70–2.66 (m, 1H), 2.62–2.56 (m, 1H), 2.06–1.93 (m, 1H), 1.85–1.73 (m, 1H), 1.62 (s, 3H), 1.49–1.34 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 171.8, 155.9, 155.5, 153.0, 149.1, 132.7, 128.2, 127.5, 125.6, 120.2, 117.9, 114.4, 110.7, 90.6, 85.4, 83.9, 83.2, 81.6, 57.0, 56.0, 53.5, 52.8, 50.8, 29.6, 28.3, 27.9, 27.2, 25.4. HRMS (ESI): calculated for C36H49N8O7 [M + H]+ 705.3724, found 705.3734.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-cyanophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16t)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(3-oxoprop-1-en-1-yl)benzonitrile 15t (58 mg, 0.24 mmol) to afford compound 16t as a white powder (69 mg, 49% yield). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.90 (s, 1H), 7.51 (s, 1H), 7.49–7.41 (m, 2H), 7.33 (t, J = 7.7 Hz, 1H), 6.33 (d, J = 16.0 Hz, 1H), 6.27–6.15 (m, 3H), 6.04 (d, J = 1.9 Hz, 1H), 5.62 (d, J = 8.0 Hz, 1H), 5.43 (d, J = 5.9 Hz, 1H), 4.97 (dd, J = 6.3, 3.6 Hz, 1H), 4.39–4.32 (m, 1H), 4.23–4.14 (m, 1H), 3.31–3.18 (m, 2H), 2.82–2.77 (m, 1H), 2.72–2.58 (m, 2H), 2.56–2.49 (m, 1H), 2.02–1.90 (m, 1H), 1.77–1.70 (br, 1H), 1.58 (s, 3H), 1.40–1.36 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 171.7, 155.8, 155.5, 153.0, 149.1, 140.0, 138., 130.7, 130.3, 129.7, 129.3, 120.3, 118.8, 114.4, 112.7, 90.7, 85.6, 83.9, 83.3, 81.7, 79.5, 56.8, 56.0, 52.8, 50.7, 29.6, 28.3, 27.2, 25.4. HRMS (ESI): calculated for C36H49N8O7 [M + H]+ 705.3724, found 705.3732.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16u)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-4-(3-oxoprop-1-en-1-yl)benzonitrile 15u (58 mg, 0.24 mmol) to afford compound 16u as a white powder (93 mg, 66% yield). 1H NMR (400 MHz, CDCl3): δ 8.23 (s, 1H), 7.90 (s, 1H), 7.53 (d, J = 8.4 Hz, 2H), 7.30 (d, J = 8.3 Hz, 2H), 6.40–6.36 (br d, J = 16.0, 1H), 6.33–6.22 (m, 1H), 6.08–5.91 (m, 3H), 5.53 (d, J = 8.0 Hz, 1H), 5.44 (d, J = 6.0 Hz, 1H), 5.03–4.94 (m, 1H), 4.42–4.32 (m, 1H), 4.20 (d, J = 4.9 Hz, 1H), 3.36–3.20 (m, 2H), 2.84–2.79 (m, 1H), 2.72 (d, J = 5.2 Hz, 1H), 2.68–2.59 (m, 1H), 2.58–2.50 (m, 1H), 2.03–1.91 (m, 1H), 1.75 (d, J = 9.6 Hz, 1H), 1.59 (s, 3H), 1.42–1.37 (br m, 21H). 13C NMR (151 MHz, CDCl3): δ 171.6, 155.6, 153.0, 149.1, 140.1, 132.3, 126.7, 120.3, 119.0, 114.5, 110.6, 90.7, 85.7, 83.9, 81.8, 79.5, 56.9, 56.1, 53.4, 52.8, 50.7, 29.1, 28.3, 27.2, 25.5. HRMS (ESI): calculated for C36H49N8O7 [M + H]+ 705.3724, found 705.3738.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(3-carbamoylphenyl)allyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (16v)
To a solution of compound 16t (0.21 mmol, 150 mg) in DMSO (10 mL) was added KOH (0.25 mmol, 14 mg). The mixture was cooled to 0 °C and treated with H2O2 (30% w/w) in H2O (0.5 mL). The reaction mixture was warmed to room temperature and stirred for 3 h at room temperature. The reaction mixture was diluted with water and extracted with EtOAc (3×). The combined organic layers were dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography (5% MeOH in EtOAc) to give compound 16v as a white powder (127 mg, 82% yield). 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 1H), 7.94 (s, 1H), 7.78 (s, 1H), 7.70 (d, J = 7.4 Hz, 1H), 7.37–7.24 (m, 3H), 7.03 (s, 1H), 6.64 (s, 2H), 6.32 (d, J = 15.8 Hz, 1H), 6.23–6.12 (m, 1H), 6.06 (d, J = 1.5 Hz, 1H), 5.89 (s, 1H), 5.43 (d, J = 6.2 Hz, 1H), 4.97 (dd, J = 6.2, 3.4 Hz, 1H), 4.39–4.35 (m, 1H), 4.22–4.17 (m, 1H), 3.26–3.13 (m, 2H), 2.78–2.55 (m, 4H), 1.97 (dd, J = 13.5, 6.0 Hz, 1H), 1.81–1.70 (m, 1H), 1.58 (s, 3H), 1.40–1.36 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 172.0, 170.1, 156.0, 155.7, 153.0, 149.0, 137.1, 134.0, 128.7, 128.0, 126.6, 120.1, 114.3, 90.7, 85.8, 84.0, 83.4, 81.8, 79.5, 57.0, 50.6, 45.9, 29.7, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H51N8O8 [M + H]+723.3830, found 723.3838.
tert-Butyl (S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-carbamoylphenyl)allyl)amino)-2-((tert-butoxycarbonyl) amino)butanoate (16w)
Following the procedure described for compound 16v, compound 16u was oxidized to afford compound 16w as a white powder (118 mg, 77% yield). 1H NMR (400 MHz, CDCl3): δ 8.09 (s, 1H), 7.92 (s, 1H), 7.75 (d, J = 8.0 Hz, 2H), 7.20 (d, J = 8.3 Hz, 2H), 6.72 (s, 2H), 6.34–6.30 (br d, J = 16 Hz, 1H), 6.20–6.10 (m, 1H), 6.04 (d, J = 1.6 Hz, 1H), 5.79 (d, J = 8.0 Hz, 1H), 5.41 (d, J = 6.2 Hz, 1H), 4.98 (dd, J = 6.1, 3.7 Hz, 1H), 4.38–4.34 (m, 1H), 4.21–4.17 (m, 1H), 3.22 (d, J = 5.6 Hz, 2H), 3.07–3.02 (br m, 1H), 2.80–2.67 (m, 2H), 2.61–2.55 (m, 2H), 1.58 (s, 3H), 1.44–1.36 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.9, 169.9, 156.0, 155.6, 152.9, 149.0, 140.2, 132.3, 131.7, 127.9, 126.2, 90.7, 85.7, 84.0, 83.3, 81.8, 79.5, 56.9, 55.8, 52.9, 45.9, 30.3, 29.7, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H51N8O8 [M + H]+ 723.3830, found 723.3832.
tert-Butyl (2S)-4-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(cinnamyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (16x)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with cinnamaldehyde 15x (32 mg, 0.24 mmol) to afford compound 16x as a white powder (110 mg, 50% yield). 1H NMR (400 MHz, CDCl3): δ 8.29 (s, 1H), 7.96 (s, 1H), 7.34–7.21 (m, 5H), 7.28 (s, 1H), 6.46–6.42 (br d, J = 16.0 Hz, 1H), 6.31–6.16 (m, 3H), 6.08 (d, J = 1.7 Hz, 1H), 5.71 (d, J = 8.1 Hz, 1H), 5.48 (d, J = 5.1 Hz, 1H), 5.02–5.00 (m, 1H), 4.50–4.35 (m, 1H), 4.22 (d, J = 7.4 Hz, 1H), 3.38–3.22 (m, 2H), 2.88–2.83 (m, 1H), 2.77–2.51 (m, 3H), 2.06–1.92 (m, 1H), 1.84–1.79 (m, 1H), 1.64 (s, 3H), 1.44–1.42 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 155.8, 153.1, 149.3, 140.0, 137.3, 134.1, 133.0, 129.3, 126.3, 125.3, 120.3, 114,5, 90.8, 85.5, 83.9, 83.4, 52.9, 50.6, 29.5, 28.41, 28.0, 27.2, 25.5, 21.2. HRMS (ESI): calculated for C35H50N7O7 [M + H]+ 680.3772, found 680.3780.
tert-butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-((trimethylsilyl)ethynyl)phenyl)allyl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (16y)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with (E)-3-(4-((trimethylsilyl)ethynyl)phenyl)acrylaldehyde 15y (55 mg, 0.24 mmol) to afford compound 16y as a white powder (98 mg, 63% yield). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 7.93 (s, 1H), 7.38 (d, J = 8.4 Hz, 2H), 7.22 (d, J = 8.4 Hz, 2H), 6.40–6.36 (br d, J = 16.0, 1H), 6.25–6.15 (m, 1H), 6.19–6.06 (m, 2H), 5.68–5.66 (br d, J = 8.2 Hz, 1H), 5.47–5.45 (br d, J = 8.6 Hz, 1H), 5.04–4.94 (m, 1H), 4.39 (d, J = 4.9 Hz, 1H), 4.24–4.19 (m, 1H), 3.35–3.30 (br m, 1H), 3.25–3.19 (br m, 1H), 2.86–2.81 (m, 1H), 2.74–2.47 (m, 4H), 2.01–1.96 (br, 1H), 1.81–1.78 (br, 1H), 1.61 (s, 3H), 1.42–1.39 (br m, 21H), 0.25 (s, 9H). 13C NMR (101 MHz, CDCl3): δ 171.8, 155.6, 153.1, 149.2, 140.1, 137.0, 132.2, 126.1, 122.2, 120.3, 114.5, 105.2, 90.8, 85.5, 83.9, 83.3, 79.5, 57.0, 56.0, 52.9, 50.7, 29.5, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C40H58N7O7Si [M + H]+ 776.4167, found 776.4172.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(o-tolyl)allyl)amino)butanoic Acid (17a)
Following the procedure described for compound 13a, compound 16a (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17a as a white powder (31 mg, 71% yield). 1H NMR (400 MHz, CD3OD): δ 8.50 (s, 1H), 8.21 (s, 1H), 7.38 (d, J = 7.5 Hz, 1H), 7.23–7.11 (m, 3H), 6.99–6.5 (br d, J = 16.0 Hz, 1H), 6.29–6.23 (m, 2H), 4.69 (t, J = 4.2 Hz, 1H), 4.59 (d, J = 6.3 Hz, 2H), 4.19–4.08 (m, 3H), 3.91–3.85 (m, 1H), 3.75–3.55 (m, 3H), 2.59–2.49 (m, 1H), 2.38–2.32 (m, 1H), 2.20 (s, 3H). 13C NMR (101 MHz, CD3OD): δ 170.53, 151.1, 147.9, 144.7, 143.2, 138.8, 135.8, 134.1, 130.1, 128.7, 126.0, 125.4, 119.63, 116.9, 91.0, 78.9. 735.5, 72.3, 54.39, 51.2, 51.0, 25.02, 18.3. HRMS (ESI): calculated for C24H32N7O5 [M + H]+ 498.2465, found 498.2572.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(m-tolyl)allyl)amino)butanoic Acid (17b)
Following the procedure described for compound 13a, compound 16b (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17b as a white powder (32 mg, 73% yield). 1H NMR (400 MHz, CD3OD): δ 8.48 (s, 1H), 8.23 (s, 1H), 7.21 (t, J = 7.5 Hz, 1H), 7.11 (dd, J = 18.9, 8.4 Hz, 3H), 6.69 (d, J = 15.8 Hz, 1H), 6.24 (dt, J = 15.3, 7.3 Hz, 1H), 6.17 (d, J = 3.6 Hz, 1H), 4.66 (t, J = 4.1 Hz, 1H), 4.56 (d, J = 6.8 Hz, 2H), 4.10 (dd, J = 8.3, 5.0 Hz, 3H), 3.88 (dd, J = 13.9, 10.1 Hz, 1H), 3.70–3.52 (m, 3H), 2.55–2.46 (m, 1H), 2.33 (s, 3H), 2.31–2.27 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.3, 151.3, 148.0, 145.0, 143.0, 141.0, 138.3, 135.1, 129.5, 127.0, 123.6, 119.8, 115.3, 91.1, 78.9, 72.3, 54.4, 51.2, 50.9.5.0. 20.0. HRMS (ESI): calculated for C24H32N7O5 [M + H]+ 498.2465, found 498.2574.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(p-tolyl)allyl)amino)butanoic Acid (17c)
Following the procedure described for compound 13a, compound 16c (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17c as a white powder (31 mg, 70% yield). 1H NMR (400 MHz, D2O): δ 8.47 (s, 1H), 8.22 (s, 1H), 7.16–7.09 (m, 4H), 6.69–6.65 (br d, J = 16.0 Hz, 1H), 6.21–6.13 (m, 2H), 4.65 (t, J = 4.0 Hz, 1H), 4.54 (d, J = 6.2 Hz, 2H), 4.10–4.06 (m, 3H), 3.88–3.82 (m, 1H), 3.71–3.49 (m, 3H), 2.57–2.43 (m, 1H), 2.32–2.27 (m, 4H). 13C NMR (101 MHz, D2O): δ 168.7, 163.1, 162.8, 152.7, 149.4, 146.3, 144.5, 142.3, 140.5, 133.73, 127.8, 121.1, 119.4, 116.5, 115.7, 92.4, 80.4, 75.0, 73.7, 55.8, 52.3, 26.40, 21.4. HRMS (ESI): calculated for C24H32N7O5 [M + H]+ 498.2465, found 498.2570.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-methoxyphenyl)allyl)amino)butanoic Acid (17d)
Following the procedure described for compound 13a, compound 16d (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17d as a white powder (32 mg, 72% yield). 1H NMR (400 MHz, CD3OD): δ 8.46 (s, 1H), 8.16 (s, 1H), 7.33–7.24 (m, 2H), 6.96–6.85 (m, 3H), 6.27–6.21 (m, 1H), 6.15 (d, J = 3.4 Hz, 1H), 4.62 (dd, J = 4.8, 3.5 Hz, 1H), 4.59–4.50 (m, 2H), 4.15–4.02 (m, 3H), 3.90 (dd, J = 13.9, 9.7 Hz, 1H), 3.77 (s, 3H), 3.69–3.51 (m, 3H), 2.55–2.45 (m, 1H), 2.36–2.28 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.3, 157.0, 151.0, 147.9, 144.4, 143.2, 136.0, 130.1, 127.0, 123.56, 119.8, 115.8, 110.8, 91.2, 79.0, 73.6, 72.3, 55.9, 54.6, 54.2, 50.8, 25.0. HRMS (ESI): calculated for C24H32N7O6 [M + H]+ 514.2414, found 514.2422.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-methoxyphenyl)allyl)amino)butanoic Acid (17e)
Following the procedure described for compound 13a, compound 16e (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17e as a white powder (34 mg, 77% yield). 1H NMR (400 MHz, CD3OD): δ 8.46 (s, 1H), 8.21 (s, 1H), 7.21 (t, J = 7.9 Hz, 1H), 6.89–6.83 (m, 2H), 6.77 (s, 1H), 6.68–6.64 (br d, J = 16.0 Hz, 1H), 6.27–6.19 (m, 1H), 6.15 (d, J = 3.5 Hz, 1H), 4.66–4.61 (m, 1H), 4.56–4.52 (m, 2H), 4.12–4.02 (m, 3H), 3.89–3.83 (m, 1H), 3.78 (s, 3H), 3.69–3.50 (m, 3H), 2.54–2.44 (m, 1H), 2.34–2.27 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.3, 160.0, 151.2, 148.0, 144.8, 143.1, 140.8, 136.5, 129.5, 119.8, 118.8, 91.1, 78.9, 73.6, 72.3, 54.4, 50.9, 25.0. HRMS (ESI): calculated for C24H32N7O6 [M + H]+ 514.2414, found 514.2419.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-methoxyphenyl)allyl)amino)butanoic Acid (17f)
Following the procedure described for compound 13a, compound 16f (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17f as a white powder (35 mg, 80% yield). 1H NMR (400 MHz, D2O): δ 8.47 (s, 1H), 8.24 (s, 1H), 7.21 (d, J = 8.5 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 6.65 (d, J = 15.7 Hz, 1H), 6.15 (d, J = 3.6 Hz, 1H), 6.07–6.03 (m, 1H), 4.66 (t, J = 4.0 Hz, 1H), 4.54 (d, J = 6.4 Hz, 2H), 4.12–4.01 (m, 3H), 3.88–3.82 (m, 4H), 3.70–3.47 (m, 3H), 2.54–2.44 (m, 1H), 2.34–2.26 (m, 1H). 13C NMR (101 MHz, D2O): δ 169.0, 161.9, 152.7, 149.44, 146.3, 144.5, 142.0, 129.3, 129.1, 121.1, 115.2, 114.1, 92.4, 80.4, 75.0, 73.7, 55.8, 52.5, 52.1, 26.4. HRMS (ESI): calculated for C24H32N7O6 [M + H]+ 514.2414, found 514.2425.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-fluorophenyl)allyl)amino)butanoic Acid (17g)
Following the procedure described for compound 13a, compound 16g (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17g as a white powder (31 mg, 68% yield). 1H NMR (400 MHz, D2O): δ 8.30 (s, 1H), 8.04 (s, 1H), 7.56–7.52 (m, 1H), 7.38 (d, J = 5.4 Hz, 2H), 7.21 (s, 1H), 6.85–6.56 (m, 1H), 6.10–6.05 (m, 1H), 6.02–5.93 (m, 1H), 4.70 (dd, J = 7.1, 5.6 Hz, 1H), 4.57 (dd, J = 5.5, 2.4 Hz, 1H), 4.43 (s, 1H), 4.11–4.06 (m, 2H), 3.96 (s, 1H), 3.86–3.76 (m, 1H), 3.66–3.51 (m, 3H), 2.55–2.41 (m, 1H), 2.36–2.30 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.7, 163.2, 162.9, 162.5, 162.2, 149.0, 147.1, 144.0, 133.2, 130.9, 129.9, 128.7, 120.6, 118.7, 117.7, 114.8, 111.9, 91.1, 72.9, 72.3, 50.9, 25.0. HRMS (ESI): calculated for C23H29FN7O5 [M + H]+ 502.2214, found 502.2215.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-fluorophenyl)allyl)amino)butanoic Acid (17h)
Following the procedure described for compound 13a, compound 16h (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17h as a white powder (30 mg, 67% yield). 1H NMR (400 MHz, D2O): δ 8.30 (s, 1H), 8.04 (s, 1H), 7.20–7.15 (m, 1H), 6.96 (t, J = 8.4 Hz, 1H), 6.75 (d, J = 7.6 Hz, 1H), 6.68 (d, J = 9.4 Hz, 1H), 6.30 (s, 1H), 6.08 (s, 1H), 6.00–5.92 (m, 1H), 4.67 (d, J = 6.1 Hz, 1H), 4.54–4.48 (m, 1H), 4.39 (s, 1H), 4.07–3.76 (m, 4H), 3.46–3.45 (m, 3H), 2.42–2.36 (m, 1H), 2.30–2.15 (m, 1H). 13C NMR (101 MHz, D2O): δ 172.1, 149.3, 147.2, 144.1, 143.6, 137.0, 130.5, 122.3, 119.2, 115.8, 115.6, 112.42, 112.19, 91.4, 72.0, 51.8. HRMS (ESI): calculated for C23H29FN7O5 [M + H]+ 502.2214, found 502.2218.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-fluorophenyl)allyl)amino)butanoic Acid (17i)
Following the procedure described for compound 13a, compound 16i (50 mg, 0.072 mmol) was deprotected and purified, affording compound 17i as a white powder (34 mg, 76% yield). 1H NMR (400 MHz, D2O): δ 8.32 (s, 1H), 8.02 (s, 1H), 6.98–6.81 (m, 4H), 6.28 (br s, 1H), 6.09 (s, 1H), 5.89–5.82 (m, 1H), 4.70 (dd, J = 6.9, 5.5 Hz, 1H), 4.55–4.53 (m, 1H), 4.43 (br s, 1H), 4.11–3.79 (m, 4H), 3.65–3.47 (m, 3H), 2.52–2.38 (m, 1H), 2.31 (s, 1H). 13C NMR (101 MHz, D2O): δ 171.5, 163.8, 163.0, 161.3, 149.2, 147.1, 144.0, 143.5, 138.8, 131.0, 128.0, 127.9, 115.6, 115.3, 114.0, 112.0, 91.3, 73.6, 51.2, 24.9. HRMS (ESI): calculated for C23H29FN7O5 [M + H]+ 502.2214, found 502.2216.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-chlorophenyl)allyl)amino)butanoic Acid (17j)
Following the procedure described for compound 13a, compound 16j (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17j as a white powder (28 mg, 63% yield). 1H NMR (400 MHz, D2O): δ 8.31 (d, J = 10.5 Hz, 1H), 8.00 (br d, J = 12.0 Hz, 1H), 7.46–6.98 (m, 4H), 6.58–6.38 (br d, J = 80.0 Hz, 1H), 6.09 (d, J = 10.8 Hz, 1H), 6.00–5.88 (m, 1H), 4.74–4.68 (m, 1H), 4.50 (dd, J = 5.4, 2.2 Hz, 1H), 4.43 (s, 1H), 4.15–3.81 (m, 4H), 3.67–3.48 (m, 3H), 2.47 (s, 1H), 2.36–2.30 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.1, 163.3, 163.0, 162.6, 149.0, 147.0, 144.1, 143.4 138.5, 132.3, 130.3, 129.5, 127.3, 126.33, 125.4, 120.6, 119.1, 117.7, 114.9, 112.0, 91.4, 73.5, 72.0, 55.7, 50.9, 25.0. HRMS (ESI): calculated for C23H29ClN7O5 [M + H]+ 518.1919, found 518.1922.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-chlorophenyl)allyl)amino)butanoic Acid (17k)
Following the procedure described for compound 13a, compound 16k (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17k as a white powder (28 mg, 63% yield). 1H NMR (400 MHz, CD3OD): δ 8.48 (s, 1H), 8.27 (s, 1H), 7.34–7.23 (m, 4H), 6.73 (br d, J = 16.0 Hz, 1H), 6.37–6.29 (m, 1H), 6.16 (d, J = 3.8 Hz, 1H), 4.68–4.64 (m, 1H), 4.58–4.49 (m, 2H), 4.08 (dd, J = 8.3, 4.5 Hz, 3H), 3.86–3.80 (m, 1H), 3.63–3.53 (m, 3H), 2.54–2.44 (m, 1H), 2.33–2.25 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.5, 162.0, 161.2, 151.3, 148.1, 145.0, 143.0, 139.2, 137.3, 134.4, 130.0, 128.6, 126.2, 125.0, 119.6, 118.0, 117.6, 115.1, 54.66, 78.9, 73.6, 72.2, 54.7, 51.2, 51.0, 25.0, 22.9. HRMS (ESI): calculated for C23H29ClN7O5 [M + H]+ 518.1919, found 518.1928.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-chlorophenyl)allyl)amino)butanoic Acid (17l)
Following the procedure described for compound 13a, compound 16l (50 mg, 0.070 mmol) was deprotected and purified, affording compound 17l as a white powder (30 mg, 69% yield). 1H NMR (400 MHz, D2O): δ 8.33 (s, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.14 (d, J = 7.8 Hz, 2H), 6.87 (d, J = 8.3 Hz, 2H), 6.27 (br s, 1H), 6.10 (s, 1H), 5.97–5.89 (m, 1H), 4.72 (dd, J = 6.9, 5.6 Hz, 1H), 4.56–4.38 (m, 2H), 4.10–3.79 (m, 4H), 3.64–3.50 (m, 3H), 2.53–2.39 (m, 1H), 2.37–2.24 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.8, 163.0, 162.7, 149.1, 147.1, 144.0, 1435, 138.6, 134.0, 133.2, 128.6, 127.4, 119.0, 117.8, 114.9, 91.4, 73.6, 72.0, 25.0. HRMS (ESI): calculated for C23H29ClN7O5 [M + H]+ 518.1919, found 518.1925.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-bromophenyl)allyl)amino)butanoic Acid (17m)
Following the procedure described for compound 13a, compound 16m (50 mg, 0.066 mmol) was deprotected and purified, affording compound 17m as a white powder (26 mg, 59% yield). 1H NMR (400 MHz, D2O): δ 8.21 (s, 1H), 7.86 (s, 1H), 7.29 (d, J = 9.3 Hz, 1H), 7.03 (d, J = 7.2 Hz, 3H), 6.42 (s, 1H), 5.99 (s, 1H), 5.84–5.77 (m, 1H), 4.65–4.60 (m, 1H), 4.42 (dd, J = 5.4, 2.2 Hz, 1H), 4.34 (s, 1H), 4.02 (dd, J = 8.4, 4.9 Hz, 1H), 3.84–3.74 (m, 2H), 3.57–3.41 (m, 3H), 2.38 (br s, 1H), 2.29–2.20 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.1,163.3, 163.0, 162.2, 149.0, 146.9, 144.1, 143.4, 132.7, 130.5, 127.9, 126.5, 122.7, 120.6, 119.1, 117.7, 114.8, 111.9, 91.5, 73.5,72.0, 55.5, 50.8, 251. HRMS (ESI): calculated for C23H29BrN7O5 [M + H]+ 562.1414, found 562.1427.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-bromophenyl)allyl)amino)butanoic Acid (17n)
Following the procedure described for compound 13a, compound 16n (50 mg, 0.066 mmol) was deprotected and purified, affording compound 17n as a white powder (28 mg, 64% yield). 1H NMR (400 MHz, D2O): δ 8.33 (s, 1H), 8.05 (s, 1H), 7.38–7.35 (m, 1H), 7.09 (t, J = 7.8 Hz, 1H), 7.00 (s, 1H), 6.95 (d, J = 7.8 Hz, 1H), 6.10 (s, 2H), 5.99–5.92 (m, 1H), 4.75–4.69 (m, 1H), 4.52–4.43 (m, 2H), 4.09–3.91 (m, 4H), 3.66–3.51 (m, 3H), 2.53–2.45 (m, 1H), 2.36–2.30 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.3, 163.4, 162.7, 162.3, 149.0, 147.0, 144.0, 143.4, 138.4, 136.7, 131.6, 130.4, 128.5, 124.9, 122.2, 120.7, 119.1, 117.8, 114.9, 91.5, 73.7, 71.90, 51.1, 25.0. HRMS (ESI): calculated for C23H29BrN7O5 [M + H]+ 562.1414, found 562.1425.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-bromophenyl)allyl)amino)butanoic Acid (17o)
Following the procedure described for compound 13a, compound 16o (50 mg, 0.066 mmol) was deprotected and purified, affording compound 17o as a white powder (33 mg, 75% yield).1H NMR (400 MHz, D2O): δ 8.33 (s, 1H), 7.93 (s, 1H), 7.21–7.07 (m, 2H), 6.65 (d, J = 8.3 Hz, 2H), 6.00 (s, 2H), 5.86–5.78 (m, 1H), 4.62 (d, J = 6.3 Hz, 1H), 4.42–4.33 (m, 2H), 4.04–3.79 (m, 4H), 3.66–3.41 (m, 3H), 2.41–2.21 (m, 2H). 13C NMR (101 MHz, D2O): δ 171.3, 163.0, 162.6, 162.3 149.0, 147.0, 143.9, 143.4, 138.7, 135.5, 131.5, 127.6, 122.3, 120.7, 119.0, 117.8, 114.9, 91.4, 73.7, 71.9, 51.1, 25.0. HRMS (ESI): calculated for C23H29BrN7O5 [M + H]+ 562.1414, found 562.1421.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-nitrophenyl)allyl)amino)butanoic Acid (17p)
Following the procedure described for compound 13a, compound 16p (50 mg, 0.069 mmol) was deprotected and purified, affording compound 17p as a white powder (18 mg, 43% yield). 1H NMR (400 MHz, D2O): δ 8.29 (s, 1H), 8.06 (s, 1H), 7.83–7.78 (m, 1H), 7.47 (s, 1H), 7.41 (d, J = 9.2 Hz, 1H), 7.27 (s, 1H), 6.72 (d, J = 13.6 Hz, 1H), 6.07 (s, 1H), 6.00–5.93 (m, 1H), 4.67–4.59 (m, 2H), 4.49–4.40 (m, 1H), 4.13–3.91 (m, 3H), 3.80–3.73 (m, 1H), 3.68–3.49 (m, 3H), 2.50–2.43(s, 1H), 2.37–2.29 (m, 1H). 13C NMR (101 MHz, D2O): δ 170.8, 149.2, 147.3, 146.4, 143.9, 143.8, 136.1, 134.1,130.1, 129.8, 124.6, 117.7, 114.8, 111.9, 91.0, 73.2, 71.9, 67.9, 66.5, 50.6, 24.8, 17.9. HRMS (ESI): calculated for C23H29N8O7 [M + H]+ 529.2159, found 529.2166.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-nitrophenyl)allyl)amino)butanoic Acid (17q)
Following the procedure described for compound 13a, compound 16q (50 mg, 0.069 mmol) was deprotected and purified, affording compound 17q as a white powder (20 mg, 45% yield). 1H NMR (500 MHz, CD3OD): δ 8.51 (s, 1H), 8.27 (s, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 5.02 (s, 2H), 4.69 (t, J = 4.5 Hz, 1H), 4.08 (h, J = 7.7 Hz, 3H), 3.85–3.80 (m, 1H), 3.68 (6.10 (d, J = 8.1 Hz, 1H), 3.64–3.55 (m, 2H), 2.54–2.46 (m, 1H), 2.32–2.24 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 171.1, 161.9, 161.8, 161.6, 151.4, 148.2, 139.5, 134.3, 131.6, 128.3, 122.6, 119.5, 116.7. 90.5, 79.1, 73.6, 72.2, 54.7, 51.6, 51.2, 29.8, 25.0. HRMS (ESI): calculated for C23H29N8O7 [M + H]+ 529.2159, found 529.2162.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E/Z)-3-(4-nitrophenyl)allyl)amino)butanoic Acid (17r, Mixture of Isomers)
Following the procedure described for compound 13a, compound 16r (50 mg, 0.069 mmol) was deprotected and purified, affording compound 17r as a pink powder (mixture of (E)- and (Z)-isomers, 23 mg, 51% yield). 1H NMR (500 MHz, CD3OD): δ 8.48 (s, 1H), 8.38 (d, J = 6.2 Hz, 1H), 8.30 (s, 1H), 8.20–8.12 (m, 3H), 7.54 (s, 2H), 7.42 (d, J = 7.1 Hz, 1H), 7.01–6.85 (br m, 2H), 6.56–6.51 (m, 1H), 6.17 (d, J = 3.6 Hz, 1H), 6.12–5.99 (m, 1H), 4.70–4.66 (m, 1H), 4.61–4.53 (m, 3H), 4.42–4.35 (m, 1H), 4.30 (d, J = 6.3 Hz, 1H), 4.21–4.04 (m, 4H), 3.90–3.82 (m, 1H), 3.79–3.50 (m, 5H), 2.56–2.19 (m, 3H). ((E/Z)-mixture). 13C NMR (126 MHz, CD3OD): δ 171.1, 153.9, 148.4, 147.71, 141.6, 137.7, 134.5, 129.5, 123.6, 123.3, 121.6, 90.8, 79.3, 73.4, 73.3, 72.4, 72.1, 54.8,52.0, 25.0. HRMS (ESI): calculated for C23H29N8O7 [M + H]+ 529.2159, found 529.2178.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(2-cyanophenyl)allyl)amino)butanoic Acid (17s)
Following the procedure described for compound 13a, compound 16s (50 mg, 0.071 mmol) was deprotected and purified, affording compound 17s as a white powder (34 mg, 78% yield). 1H NMR (400 MHz, D2O): δ 8.33 (s, 1H), 8.03 (s, 1H), 7.52–7.42 (m, 2H), 7.33 (t, J = 7.2 Hz, 2H), 6.44 (s, 1H), 6.22–6.15 (m, 1H), 6.11 (d, J = 2.3 Hz, 1H), 4.70–4.65 (m, 1H), 4.45 (t J = 7.8, 1H), 4.14–4.04 (m, 3H), 3.86 (d, J = 10.2 Hz, 1H), 3.67–3.51 (m, 3H), 2.53–2.43 (m, 1H), 2.37–2.29 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.0, 163.2, 162.9, 162.5, 162.2, 149.1, 147.1, 144.2, 143.7, 137.3, 135.2, 133.6, 133.0, 129.4, 125.6, 120.6, 119.0, 117.7, 117.3, 114.8, 109.1, 91.4, 73.4, 72.0, 55.4, 50.8, 24.9. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2271.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-cyanophenyl)allyl)amino)butanoic Acid (17t)
Following the procedure described for compound 13a, compound 16t (50 mg, 0.071 mmol) was deprotected and purified, affording compound 17t as a white powder (34 mg, 77% yield). 1H NMR (400 MHz, D2O): δ 8.36 (s, 1H), 8.10 (s, 1H), 7.60–7.58 (m, 1H), 7.39–7.29 (m, 3H), 6.40 (br s, 1H), 6.13–6.05 (m, 2H), 4.71 (dd, J = 7.1, 5.5 Hz, 1H), 4.56–4.51 (m, 1H), 4.45 (s, 1H), 4.11–3.86 (m, 4H), 3.68–3.52 (m, 3H), 2.53–2.44 (m, 1H), 2.37–2.31 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.1, 149.2, 147.2, 144.1, 143.6, 137.8, 135.7, 132.3, 130.8, 129.7, 119.1, 118.9, 118.5, 117.7, 114.8, 111.5, 91.4, 71.8, 50.9, 24.9. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2264.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)butanoic Acid (17u)
Following the procedure described for compound 13a, compound 16u (50 mg, 0.071 mmol) was deprotected and purified, affording compound 17u as a white powder (35 mg, 80% yield). 1H NMR (400 MHz, D2O): δ 8.38 (s, 1H), 8.14 (s, 1H), 7.59 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.1 Hz, 2H), 6.47 (d, J = 8.1, 1H), 6.23–6.13 (m, 2H), 4.75–4.72 (m, 1H), 4.58–4.56 (m, 1H), 4.47 (br s, 1H), 4.09–4.01 (m, 4H), 3.67–3.54 (m, 3H), 2.52–2.42 (m, 1H), 2.35–2.29 (m, 1H). 13C NMR (101 MHz, D2O): δ 171.8, 149.3, 147.2, 144.1, 143.6, 138.1, 132.63, 126.6, 119.2, 114.9, 110.8, 91.4, 73.6, 71.9, 51.6, 20.5. HRMS (ESI): calculated for C24H29N8O5 [M + H]+ 509.2261, found 509.2266.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(3-carbamoylphenyl)allyl)amino)butanoic Acid (17v)
Following the procedure described for compound 13a, compound 16v (50 mg, 0.069 mmol) was deprotected and purified, affording compound 17v as a white powder (34 mg, 77% yield). 1H NMR (400 MHz, CD3OD): δ 8.46 (s, 1H), 8.26 (s, 1H), 7.91 (s, 1H), 7.82 (d, J = 7.6 Hz, 1H), 7.53–7.41 (m, 2H), 6.16 (d, J = 3.8 Hz, 1H), 4.72–4.68 (m, 1H), 4.60–4.49 (m, 2H), 4.17–4.02 (m, 3H), 3.86–3.80 (m, 1H), 3.71–3.52 (m, 3H), 2.51–2.45 (m, 1H), 2.30–2.20 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.7, 148.7, 144.6, 139.7, 137.0, 134.8, 129.8, 128.7, 127.5, 126.2, 118.0, 90.7, 79.5, 73.4, 71.9, 53.5, 52.4, 50.8, 25.0. HRMS (ESI): calculated for C24H31N8O6 [M + H]+ 527.2367, found 527.2378.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-carbamoylphenyl)allyl)amino)butanoic Acid (17w)
Following the procedure described for compound 13a, compound 16w (50 mg, 0.069 mmol) was deprotected and purified, affording compound 17w as a white powder (34 mg, 77% yield). 1H NMR (400 MHz, CD3OD): δ 8.45 (s, 1H), 8.26 (s, 1H), 7.85 (d, J = 8.4 Hz, 2H), 7.41 (s, 2H), 6.81 (br d, J = 15.8 Hz, 1H), 6.45–6.37 (m, 1H), 6.16 (s, 1H), 4.73–4.68 (m, 1H), 4.55 (dd, J = 5.6, 2.8 Hz, 2H), 4.16–4.01 (m, 3H), 3.88–3.79 (m, 1H), 3.69–3.57 (m, 4H), 2.51–2.45 (m, 1H), 2.29–2.20 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 171.9, 148.2, 138.5, 129.0, 126.5, 120.9, 118.4, 51.40, 90.9, 79.0, 72.9, 72.3, 51.7, 51.4, 25.0. HRMS (ESI): calculated for C24H31N8O6 [M + H]+ 527.2367, found 527.2373.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(cinnamyl)amino)butanoic Acid (17x)
Following the procedure described for compound 13a, compound 16x (50 mg, 0.074 mmol) was deprotected and purified, affording compound 17x as a white powder (35 mg, 79% yield). 1H NMR (500 MHz, CD3OD): δ 8.49 (s, 1H), 8.24 (s, 1H), 7.31–7.26 (s, 5H), 6.74–6.71 (br d, J = 12.0, 1H), 6.29–6.23 (m, 1H), 6.18 (d, J = 3.8 Hz, 1H), 4.70–4.68 (m, 1H), 4.60–4.56 (m, 2H), 4.14–4.10 (m, 3H), 3.90–3.85 (br m, 1H), 3.76–3.52 (m, 3H), 2.57–2.51 (m, 1H), 2.41–2.34 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 169.5, 162.0, 161.7, 161.4, 149.7, 151.0,148.0, 140.9, 135.1, 128.8, 128.5, 125.7, 120.0, 119.8, 117.7, 115.5, 115.4, 91.1, 79.4, 73.6, 72.3, 55.5, 54.5, 50.9, 50.6, 25.0. HRMS (ESI): calculated for C23H30N7O5 [M + H]+ 484.2308, found 484.2311.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-ethynylphenyl)allyl)amino)butanoic Acid (17y)
Following the procedure described for compound 13a, compound 16y (50 mg, 0.064 mmol) was deprotected and purified, affording compound 17y as a white powder (8 mg, 21% yield). 1H NMR (500 MHz, CD3OD): δ 8.41 (s, 1H), 8.28 (s, 1H), 7.43 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.3 Hz, 2H), 6.77–6.73 (br d, J = 12.0, 1H), 6.37–6.25 (m, 1H), 6.14 (d, J = 3.5 Hz, 1H), 4.70–4.66 (m, 1H), 4.53 (dd, J = 5.5, 2.5 Hz, 2H), 4.14–3.94 (m, 3H), 3.82–3.77 (m, 1H), 3.66–3.63 (br d, J = 16.0, 1H), 3.61 (s, 1H), 3.60–3.47 (m, 2H), 2.48–2.40 (m, 1H), 2.24–2.16 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 148.6, 139.6, 135.6, 132.0, 126.5, 91.1, 79.1, 78.9, 73.4, 71.7, 54.6, 51.5, 25.2. HRMS (ESI): calculated for C25H30N7O5 [M + H]+ 508.2308, found 508.2315.
4-((E)-3-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)amino)prop-1-en-1-yl)benzonitrile (18)
Following the procedure described for compound 12a, 9-((3aR,4R,6R,6aR)-6-(aminomethyl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-amine 9 (67 mg, 0.22 mmol) was coupled with (E)-4-(3-oxoprop-1-en-1-yl)benzonitrile 15u (31 mg, 0.20 mmol) to afford compound 18 as a yellow powder (49 mg, 55% yield). 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 1H), 7.92 (s, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.3 Hz, 2H), 6.51 (s, 1H), 6.41–6.36 (m, 1H), 6.15 (s, 2H), 6.01 (d, J = 3.3 Hz, 1H), 5.52–5.47 (m, 1H), 5.10 (dd, J = 6.4, 3.3 Hz, 1H), 4.45–4.41 (m, 1H), 3.46 (t, J = 5.5 Hz, 2H), 3.06–2.93 (m, 2H), 2.61 (s, 3H), 1.64 (s, 3H), 1.41 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 155.7, 153.1, 149.3, 141.5, 140.1, 132.5, 129.8, 126.8, 114.8, 110.6, 91.1, 85.5, 83.3, 82.3, 51.6, 50.9, 27.4, 25.5. HRMS (ESI): calculated for C23H26N7O3 [M + H]+ 448.2097, found 448.2106.
tert-Butyl (S)-5-((((3 3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)-2-(bis(tert-butoxycarbonyl)amino)pentanoate (20a)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with tert-butyl (S)-2-(bis(tert-butoxycarbonyl)amino)-5-oxopentanoate 19a (82 mg, 0.24 mmol) to afford the protected intermediate 20a as a white powder (113 mg, 69% yield). 1H NMR (400 MHz, CDCl3): δ 8.23 (s, 1H), 7.92 (s, 1H), 7.51 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 8.2 Hz, 2H), 6.44–6.21 (m, 4H), 6.04 (d, J = 1.9 Hz, 1H), 5.44 (dd, J = 6.4, 1.9 Hz, 1H), 4.97 (dd, J = 6.4, 3.6 Hz, 1H), 4.71 (dd, J = 9.6, 5.2 Hz, 1H), 4.38–4.34 (m, 1H), 3.26 (d, J = 6.0 Hz, 2H), 2.79–2.69 (m, 2H), 2.56–2.51 (m, 2H), 2.07–2.00 (m, 1H), 1.91–1.75 (m, 1H), 1.59 (s, 3H), 1.44 (br d, J = 21.0, 27H), 1.37 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 169.8, 155.8, 153.0, 152.6, 149.2, 141.4, 140.0, 132.4, 131.7, 130.6, 126.6, 119.1, 114.4, 110.4, 107.0, 90.8, 85.7, 83.9, 83.3,82.8, 81.2, 77.5, 77.2, 76.9, 58.6, 54.3, 28.1, 27.2, 26.9, 25.5, 23.9. HRMS (ESI): calculated for C42H59N8O9 [M + H]+ 819.4405, found 819.4410.
Methyl (S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)-2-((tert-butoxycarbonyl) amino)butanoate (20b)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with methyl (S)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoate 19b (82 mg, 0.24 mmol) to afford the protected intermediate 20b as a white powder (97 mg, 73% yield). 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.94 (s, 1H), 7.49 (d, J = 8.3 Hz, 2H), 7.27 (d, J = 8.3 Hz, 2H), 6.60 (s, 2H), 6.41–6.25 (m, 1H), 6.26–6.19 (m, 1H), 6.06 (s, 1H), 5.94 (d, J = 8.1 Hz, 1H), 5.45 (d, J = 6.2 Hz, 1H), 5.03–4.95 (m, 1H), 4.41–4.30 (m, 2H), 3.64 (s, 3H), 3.23 (d, J = 6.0 Hz, 2H), 2.79–2.69 (m, 2H), 2.58–2.50 (m, 2H), 2.07–2.00 (m, 1H), 1.86–1.79 (m, 1H), 1.58 (s, 3H), 1.41–1.33 (br m, 12H). 13C NMR (101 MHz, CDCl3): δ 173.3, 155.9, 155.6, 155.3, 153.0, 149.0, 141.3, 140.1, 132.3, 131.0, 126.7, 120.2, 119.0, 114.4, 110.5, 90.7, 85.7, 83.9, 83.3, 56.6, 56.1, 53.6, 52.2, 50.6, 44.8, 29.2, 28.4, 27.2, 25.4. HRMS (ESI): calculated for C33H43N8O7 [M + H]+ 663.3255, found 663.3258.
4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)-N-tritylbutanamide (20c)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with 4-oxo-N-tritylbutanamide 19c (82 mg, 0.24 mmol) to afford the protected intermediate 20c as a white powder (79 mg, 51% yield). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 7.90 (s, 1H), 7.54 (d, J = 6.6 Hz, 2H), 7.33–7.19 (m, 20H), 6.72 (br s, 1H), 6.43–6.39 (br d, J = 16.0 Hz, 1H), 6.32–6.23 (m, 1H), 6.04 (d, J = 2.1 Hz, 1H), 5.82 (s, 2H), 5.47 (dd, J = 6.4, 2.1 Hz, 1H), 4.99 (dd, J = 6.4, 3.5 Hz, 1H), 4.42–4.38 (m, 1H), 3.28 (t, J = 6.4 Hz, 2H), 2.77 (d, J = 6.7 Hz, 2H), 2.57–2.51 (m, 2H), 2.35–2.25 (m, 2H), 1.87–1.73 (m, 2H), 1.59 (s, 3H), 1.38 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 171.7, 154.9, 153.6, 149.2, 144.0, 140.8, 132.4, 128.7, 128.0, 127.0, 126.7, 120.8, 119.1, 114.4, 110.5, 90.9, 86.7, 83.9, 82.7, 69.1, 56.6, 56.0, 54.5, 35.6, 26.5, 25.4, 22.6. HRMS (ESI): calculated for C46H47N8O4 [M + H]+ 775.3720, found 775.3733.
5-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)-N-tritylpentanamide (20d)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with 5-oxo-N-tritylpentanamide 19d (86 mg, 0.24 mmol) to afford the protected intermediate 20d as a white powder (88 mg, 56% yield). 1H NMR (500 MHz, CDCl3): δ 8.15 (s, 1H), 7.97 (s, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.29–7.27 (m, 7H), 7.25–7.18 (m, 11H), 7.07 (s, 2H), 6.75 (s, 1H), 6.05 (d, J = 2.0 Hz, 1H), 5.40 (dd, J = 6.4, 2.0 Hz, 1H), 4.97 (dd, J = 6.4, 3.5 Hz, 1H), 4.45–4.41 (m, 1H), 3.30 (d, J = 6.5 Hz, 2H), 2.78 (d, J = 6.6 Hz, 2H), 2.54 (dd, J = 10.8, 4.0 Hz, 2H), 2.28–2.25 (m, 2H), 1.60 (s, 3H), 1.46–1.41 (m, 2H), 1.37 (s, 3H). 13C NMR (126 MHz, CDCl3): δ 175.9, 171.9, 155.8, 152.3, 148.9, 144.8, 141.2, 132.5, 131.5, 130.6, 128.8, 127.1, 126.8, 119.5, 119.1, 114.6, 110.7, 91.1, 86.1, 84.4, 83.3, 70.5, 60.5, 56.4, 55.8, 54.0, 37.2, 26.1, 23.2, 21.5. HRMS (ESI): calculated for C47H49N8O4 [M + H]+ 789.3877, found 789.3886.
tert-Butyl (3-((((3aR,4R,6R,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)propyl)carbamate (20e)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with tert-butyl (3-oxopropyl)carbamate 19e (41 mg, 0.24 mmol) to afford the protected intermediate 20e as a white powder (88 mg, 73% yield). 1H NMR (500 MHz, CDCl3): δ 8.09 (s, 1H), 7.97 (s, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.4 Hz, 2H), 6.98 (s, 2H), 6.40 (d, J = 15.9 Hz, 1H), 6.32–6.22 (m, 1H), 6.06 (s, 1H), 5.41 (d, J = 7.8 Hz, 1H), 4.40–4.37 (m, 1H), 3.26 (d, J = 6.5 Hz, 2H), 3.17–3.13 (m, 1H), 2.75 (d, J = 4.7 Hz, 1H), 2.56 (s, 1H), 1.42 (d, J = 8.6 Hz, 12H), 1.38 (s, 3H). 13C NMR (126 MHz, CDCl3): δ 175.8, 154.8, 152.4, 149.7, 141.2, 132.6, 126.8, 119.6, 119.0, 114.6, 112.1, 90.9, 86.4, 84.1, 57.0, 51.2, 39.2, 28.5, 27.2, 26.5, 25.4, 22.0. HRMS (ESI): calculated for C31H41N8O5 [M + H]+ 605.3200, found 605.3211.
tert-Butyl 4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)butanoate (20f)
Following the procedure described for compound 12a, compound 18 (89 mg, 0.20 mmol) was coupled with tert-butyl 4-oxobutanoate 19f (35 mg, 0.22 mmol) to afford the protected intermediate 20f as a white powder (84 mg, 71% yield). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 7.93 (s, 1H), 7.55 (d, J = 8.4 Hz, 2H), 7.32 (d, J = 8.4 Hz, 2H), 6.43 (d, J = 15.9 Hz, 1H), 6.33–6.26 (m, 1H), 6.10 (s, 2H), 6.07 (d, J = 2.1 Hz, 1H), 5.47 (dd, J = 6.5, 2.1 Hz, 1H), 5.01 (dd, J = 6.5, 3.6 Hz, 1H), 4.40–4.36 (m, 1H), 3.30 (d, J = 6.5 Hz, 2H), 2.83–2.73 (m, 2H), 2.54 (t, J = 7.3 Hz, 2H), 2.26–2.23 (m, 2H), 1.79–1.69 (m, 2H), 1.61 (s, 3H), 1.43–1.38 (br m, 12H). 13C NMR (101 MHz, CDCl3): δ 173.7, 157.1, 153.0, 150.2, 142.2, 141.7, 140.1, 138.9, 136.8, 134.2, 130.7, 127.0, 120.9, 119.0, 114.4, 111.2, 90.8, 85.8, 84.6, 83.2, 79.5, 56.8, 56.0, 52.4, 33.1, 28.7, 27.2, 25.4, 22.4. HRMS (ESI): calculated for C31H40N7O5 [M + H]+ 590.3091, found 590.3097.
tert-Butyl 5-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)pentanoate (20g)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with tert-butyl 5-oxopentanoate 19g (41 mg, 0.24 mmol) to afford the protected intermediate 20g as a white powder (82 mg, 68% yield). 1H NMR (500 MHz, CDCl3): δ 8.23 (s, 1H), 7.92 (s, 1H), 7.53 (d, J = 6.7 Hz, 2H), 7.29 (d, J = 8.4 Hz, 2H), 6.40 (d, J = 16.0 Hz, 1H), 6.31–6.25 (m, 1H), 6.20 (s, 2H), 6.05 (d, J = 2.1 Hz, 1H), 5.45 (dd, J = 6.4, 2.1 Hz, 1H), 4.99 (dd, J = 6.4, 3.6 Hz, 1H), 4.39–4.34 (m, 1H), 3.69–3.59 (m, 3H), 3.28 (d, J = 6.8 Hz, 2H), 2.75 (d, J = 6.7 Hz, 2H), 2.53–2.45 (m, 3H), 2.18 (t, J = 7.3 Hz, 2H), 1.76–1.68 (m, 1H), 1.65–1.48 (m, 8H), 1.47–1.43 (m, 2H), 1.41 (s, 9H), 1.37 (s, 3H). 13C NMR (126 MHz, CDCl3): δ 173.0, 155.7, 153.1, 149.2, 141.4, 132.4, 126.7, 120.3, 119.1, 114.4, 111.3, 90.9, 85.8, 84.0, 83.3, 80.2, 62.5, 62.0, 56.9, 56.0, 54.4, 35.3, 32.4, 28.2, 27.2, 25.5, 23.5. HRMS (ESI): calculated for C32H42N7O5 [M + H]+ 604.3247, found 604.3255.
4-((E)-3-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)((6-(tert-butoxy)pyridin-2-yl)methyl)amino)prop-1-en-1-yl)benzonitrile (20h)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with 6-(tert-butoxy)picolinaldehyde 19h (43 mg, 0.24 mmol) to afford the protected intermediate 20h as a white powder (73 mg, 60% yield). 1H NMR (400 MHz, CDCl3): δ 8.15 (s, 1H), 7.95 (s, 1H), 7.54 (d, J = 6.6 Hz, 2H), 7.45–7.41 (m, 1H), 7.30 (d, J = 6.5 Hz, 2H), 6.88 (d, J = 7.4 Hz, 1H), 6.59 (d, J = 6.8 Hz, 2H), 6.52 (dd, J = 8.2, 0.8 Hz, 1H), 6.44 (d, J = 16.0 Hz, 1H), 6.37–6.29 (m, 1H), 6.08 (d, J = 2.1 Hz, 1H), 5.42 (dd, J = 6.4, 2.1 Hz, 1H), 4.97 (dd, J = 6.4, 3.5 Hz, 1H), 4.50–4.56 (m, 1H), 3.75 (s, 2H), 3.46–3.32 (m, 2H), 2.90 (d, J = 6.6 Hz, 2H), 1.60 (s, 3H), 1.56 (s, 9H), 1.39 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 163.3, 156.0, 153.0, 149.2, 141.5, 139.9, 132.4, 131.8, 130.7, 126.6, 120.3, 119.1, 115.4, 114.3, 111.5, 110.5, 90.8, 85.8, 84.0, 83.2, 79.4, 60.2, 56.9, 56.0, 28.8, 27.2, 25.5. HRMS (ESI): calculated for C33H39N8O4 [M + H]+ 611.3094, found 611.3102.
4-((E)-3-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)amino)prop-1-en-1-yl)benzonitrile (20j)
Following the procedure described for compound 12a, 9-((3aR,4R,6R,6aR)-2,2-dimethyl-6-((methylamino)methyl)tetrahydrofuro[3,4-d][1,3]dioxol-4-yl)-9H-purin-6-amine39 (64 mg, 0.20 mmol) was coupled with 15u (34 mg, 0.22 mmol) to afford the protected intermediate 20j as a yellow powder (66 mg, 72% yield). 1H NMR (400 MHz, CDCl3): δ 8.20 (s, 1H), 7.97 (s, 1H), 7.52 (d, J = 8.1 Hz, 2H), 7.28 (d, J = 8.5 Hz, 2H), 6.57 (s, 2H), 6.42 (d, J = 16.0 Hz, 1H), 6.34–6.23 (m, 1H), 6.08 (s, 1H), 5.45 (d, J = 7.9 Hz, 1H), 4.98 (dd, J = 6.3, 3.7 Hz, 1H), 4.48–4.35 (m, 1H), 3.28–3.11 (m, 2H), 2.80–2.74 (m, 1H), 2.65–2.60 (br m, 1H), 2.33 (s, 3H), 1.61 (s, 3H), 1.38 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 155.8, 152.9, 149.1, 140.0, 132.4, 131.0, 126.6, 120.2, 119.1, 114.5, 110.6, 90.8, 85.2, 84.1, 83.2, 60.4, 59.0, 42.8, 29.7, 27.2, 25.4. HRMS (ESI): calculated for C24H28N7O3 [M + H]+ 462.2254, found 462.2259.
4-((E)-3-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetrahydro-furo[3,4-d][1,3]dioxol-4-yl)methyl)(isopropyl)amino)prop-1-en-1-yl)benzonitrile (20k)
Following the procedure described for compound 12a, compound 18 (112 mg, 0.20 mmol) was coupled with 2 mL of dry acetone afforded the protected intermediate 20k as a white powder (47 mg, 48% yield). 1H NMR (400 MHz, CDCl3): δ 8.29 (s, 1H), 7.92 (s, 1H), 7.54 (d, J = 8.4 Hz, 2H), 7.33 (d, J = 8.3 Hz, 2H), 6.49–6.38 (m, 3H), 6.37–6.29 (m, 1H), 6.06 (d, J = 2.0 Hz, 1H), 5.49 (dd, J = 6.4, 2.0 Hz, 1H), 5.04 (dd, J = 6.4, 3.5 Hz, 1H), 4.35–4.31 (m, 1H), 3.35–3.22 (m, 2H), 3.04–2.97 (m, 1H), 2.83–2.78 (m, 1H), 2.69–2.60 (m, 1H), 1.58 (s, 3H), 1.40 (s, 3H), 1.05 (d, J = 6.6 Hz, 3H), 0.93 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 155.8, 153.0, 149.2, 141.6, 140.2, 133.7, 132.4, 129.6, 126.6, 120.3, 119.1, 114.3, 110.3, 90.9, 86.6, 83.9, 83.1, 53.8, 51.7, 27.2, 25.5, 18.6, 17.9. HRMS (ESI): calculated for C26H32N7O3 [M + H]+ 490.2567, found 490.2573.
(S)-2-Amino-5-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)pentanoic Acid (21a)
Following the procedure described for compound 13a, compound 20a (50 mg, 0.061 mmol) was deprotected and purified, affording compound 21a as a white powder (24 mg, 63% yield). 1H NMR (400 MHz, CD3OD): δ 8.47 (s, 1H), 8.33 (s, 1H), 7.68 (d, J = 8.3 Hz, 2H), 7.48 (d, J = 8.2 Hz, 2H), 6.82–6.78 (br d, J = 15.8 Hz, 1H), 6.51–6.43 (m, 1H), 6.18 (d, J = 3.2 Hz, 1H), 4.71–4.65 (m, 1H), 4.62–4.51 (m, 2H), 4.19–4.00 (m, 3H), 3.89–3.84 (m, 1H), 3.69 (d, J = 8.9 Hz, 1H), 3.47–3.37 (m, 2H), 2.13–1.91 (m, 4H). 13C NMR (101 MHz, CD3OD): δ 170.0, 151.3, 148.1, 145.0, 143.1, 139.7, 138.5, 132.3, 127.2, 120.0, 119.7, 118.1, 118.0, 115.1, 111.9, 91.3, 73.5, 72.2, 55.3, 52.8, 27.1, 20.0. HRMS (ESI): calculated for C25H32N8O5 [M + H]+ 523.2417, found 523.2423.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)butanamide (21b)
Compound 20b (50 mg, 0.076 mmol) was added to ammonia in MeOH (33% w/w, 5 mL) in a sealed tube, and the mixture was stirred overnight at room temperature. The solvent was evaporated, and the crude intermediate was deprotected and purified following the procedure described for compound 13a, affording compound 21b as a white powder (33 mg, 71% yield, two steps). 1H NMR (400 MHz, CD3OD): δ 8.44 (s, 1H), 8.32 (s, 1H), 7.70 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 6.85–6.81 (br d, J = 15.8 Hz, 1H), 6.50–6.41 (m, 1H), 6.15 (d, J = 3.3 Hz, 1H), 4.68 (dd, J = 5.0, 3.4 Hz, 1H), 4.58–4.48 (m, 2H), 4.15–4.03 (m, 3H), 3.86–3.80 (m, 1H), 3.67–3.64 (br d, J = 9.0 Hz, 1H), 3.51–3.40 (m, 2H), 2.47–2.34 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 169.4, 163.1, 161.0, 152.6, 148.1, 140.1, 138.3, 132.7, 119.8, 118.0, 111.8, 91.1, 78.6, 73.4, 72.2, 55.4, 54.8, 49.3, 26.8. HRMS (ESI): calculated for C24H40N9O4 [M + H]+ 508.2421, found 508.2427.
4-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)butanamide (21c)
Following the procedure described for compound 13a, compound 20c (50 mg, 0.065 mmol) was deprotected and purified, affording compound 21c as a white powder (19 mg, 59% yield). 1H NMR (400 MHz, CD3OD): δ 8.46 (s, 1H), 7.70 (d, J = 8.1 Hz, 2H), 7.53 (s, 2H), 6.88–6.84 (br d, J = 9.0 Hz, 1H), 6.54–6.40 (m, 1H), 4.75 (br s, 1H), 4.57 (d, J = 6.1 Hz, 2H), 4.12 (dd, J = 7.4, 3.7 Hz, 2H), 3.88–3.82 (m, 1H), 3.68–3.64 (br d, J = 16.0 Hz, 1H), 3.37(s, 1H), 2.46 (t, J = 6.5 Hz, 2H), 2.10–2.04 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 174.8, 151.7, 149.1, 141.3, 137.9, 132.8, 127.2, 120.1, 117.0, 110.2, 91.0, 73.8, 71.1, 55.1, 30.1, 19.3. HRMS (ESI): calculated for C24H29N8O4 [M + H]+ 493.2312, found 493.2320.
5-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)pentanamide (21d)
Following the procedure described for compound 13a, compound 20d (50 mg, 0.063 mmol) was deprotected and purified, affording compound 21d as a white powder (22 mg, 57% yield). 1H NMR (400 MHz, CD3OD): δ 8.44 (s, 1H), 8.32 (s, 1H), 7.71 (d, J = 7.9 Hz, 2H), 7.51 (s, 2H), 6.87 (s, 1H), 6.52 (s, 1H), 6.16 (d, J = 3.5 Hz, 1H), 4.70 (s, 1H), 4.60–4.48 (m, 2H), 4.11 (d, J = 7.2 Hz, 2H), 3.87–3.81 (m, 1H), 3.66 (br d, J = 15.8 Hz, 1H), 2.31 (t, J = 7.0 Hz, 2H), 1.89–1.77 (m, 2H), 1.72–1.64 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 175.5, 140.8, 138.6, 132.3, 127.2, 118.0, 112.7, 91.1, 73.4, 72.3, 54.4, 31.4, 23.1, 21.8. HRMS (ESI): calculated for C25H31N8O4 [M + H]+ 507.2468, found 507.2479.
3-((E)-3-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(3-aminopropyl)amino)prop-1-en-1-yl)benzonitrile (21e)
Following the procedure described for compound 13a, compound 20e (50 mg, 0.083 mmol) was deprotected and purified, affording compound 21e as a white powder (41 mg, 72% yield). 1H NMR (500 MHz, CD3OD): δ 8.48 (s, 1H), 8.33 (s, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.47 (s, 2H), 6.18 (d, J = 3.3 Hz, 1H), 4.68 (d, J = 3.3 Hz, 1H), 4.60–4.55 (m, 2H), 4.14 (d, J = 7.3 Hz, 2H), 3.90–3.83 (m, 1H), 3.73–3.70 (br d, J = 9.0 Hz, 1H), 3.08 (t, J = 7.5 Hz, 2H), 2.26–2.20 (m, 2H). 13C NMR (126 MHz, CD3OD): δ 151.1, 148.1, 139.7, 138.6, 132.3, 127.2, 120.0, 119.8, 118.2, 111.8, 91.3, 73.6, 72.3, 55.4, 54.6, 50.6, 36.51, 48.6, 36.5, 22.2. HRMS (ESI): calculated for C23H29N8O3 [M + H]+ 465.2363, found 465.2372.
4-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)butanoic Acid (21f)
Following the procedure described for compound 13a, compound 20f (50 mg, 0.085 mmol) was deprotected and purified, affording compound 21f as a white powder (37 mg, 71% yield). 1H NMR (CD3OD): δ 8.44 (s, 1H), 8.34 (s, 1H), 6.87 (d, J = 15.8 Hz, 1H), 6.51–6.43 (m, 1H), 6.16 (d, J = 3.7 Hz, 1H), 4.74 (t, J = 4.1 Hz, 1H), 4.55 (d, J = 6.9 Hz, 2H), 4.13 (d, J = 7.4 Hz, 2H), 3.37 (dd, J = 9.5, 6.9 Hz, 2H), 2.47 (t, J = 6.8 Hz, 2H), 2.11–2.02 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 178.8, 151.1, 147.5, 139.7, 139.2, 132.3, 127.3, 120.5, 118.1, 112.3, 95.4, 78.3, 73.4, 72.3, 57.9, 52.9, 32.6, 22.2. HRMS (ESI): calculated for C24H28N7O5 [M + H]+ 494.2152, found 494.2160.
5-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((E)-3-(4-cyanophenyl)allyl)amino)pentanoic aAcid (21g)
Following the procedure described for compound 13a, compound 20g (50 mg, 0.083 mmol) was deprotected and purified, affording compound 21g as a white powder (39 mg, 75% yield). 1H NMR (400 MHz, CD3OD): δ 8.45 (s, 1H), 8.33 (s, 1H), 7.71 (d, J = 8.0 Hz, 2H), 7.52 (br s, 2H), 6.85 (br d, J = 15.7 Hz, 1H), 6.48–6.41 (m, 1H), 6.17 (d, J = 4.3 Hz, 1H), 4.59–4.48 (m, 2H), 4.12 (d, J = 7.3 Hz, 2H), 3.86–3.81 (br m, 1H), 3.68–3.65 (br d, J = 12.2 Hz, 1H), 2.38 (t, J = 7.0 Hz, 2H), 1.86 (t, J = 7.8 Hz, 1H), 1.70–1.63 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 171.7, 139.1, 138.6, 131.7, 119.9, 118.0, 111.9, 91.1, 73.4, 72.2, 55.1, 53.0, 31.7, 23.0, 20.7. HRMS (ESI): calculated for C25H30N7O5 [M + H]+ 508.2308, found 508.2317.
4-((E)-3-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)((6-oxo-1,6-dihydropyridin-2-yl)methyl)amino)prop-1-en-1-yl)benzonitrile (21h)
Following the procedure described for compound 13a, compound 20h (50 mg, 0.082 mmol) was deprotected and purified, affording compound 21h as a white powder (25 mg, 59% yield). 1H NMR (400 MHz, CD3OD): δ 8.43 (s, 1H), 8.26 (s, 1H), 7.64 (d, J = 8.4 Hz, 2H), 7.57 (dd, J = 8.8, 7.0 Hz, 1H), 7.46 (d, J = 8.4 Hz, 2H), 6.79–6.69 (m, 2H), 6.57–6.43 (m, 2H), 6.14 (d, J = 3.1 Hz, 1H), 4.63 (dd, J = 4.9, 3.1 Hz, 1H), 4.58–4.49 (m, 2H), 4.35 (s, 2H), 4.13–4.00 (m, 2H), 3.76–3.57 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 164.1, 150.9, 148.0, 144.3, 143.3, 141.4, 137.3, 132.3, 127.1, 122.2, 119.8, 118.2, 117.8, 115.8, 114.9, 112.3, 111.5, 91.2, 79.4, 73.7, 72.3, 56.1, 55.4, 55.1. HRMS (ESI):) calculated for C26H27N8O4 [M + H]+ 515.2155, found 515.2164.
4-((E)-3-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)amino)prop-1-en-1-yl)benzonitrile (21i)
Following the procedure described for compound 13a, compound 18 (50 mg, 0.11 mmol) was deprotected and purified, affording compound 21i as a white powder (30 mg, 52% yield). 1H NMR (500 MHz, CD3OD): δ 8.49 (s, 1H), 8.38 (s, 1H), 7.69 (d, J = 8.4 Hz, 2H), 7.58 (d, J = 8.4 Hz, 2H), 6.86 (d, J = 15.9 Hz, 1H), 6.49–6.40 (m, 1H), 6.15 (d, J = 4.6 Hz, 1H), 4.83 (d, J = 4.9 Hz, 2H), 4.50 (t, J = 5.1 Hz, 1H), 4.47–4.43 (m, 1H), 3.95 (d, J = 7.2 Hz, 2H), 3.66–3.61 (br m, 1H), 3.56–3.53 (br m, 1H). 13C NMR (126 MHz, CD3OD): δ 161.3, 150.8, 148.3, 140.3, 136.7, 132.5, 128.3, 122.2, 119.7, 118.6, 108.9, 90.6, 80.3, 73.7, 71.9, 50.7. HRMS (ESI): calculated for C20H22N7O3 [M + H]+408.1784, found 408.1792.
4-((E)-3-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl) (methyl)amino)prop-1-en-1-yl)benzonitrile (21j)
Following the procedure described for compound 13a, compound 20j (50 mg, 0.11 mmol) was deprotected and purified, affording compound 21j as a white powder (37 mg, 64% yield). 1H NMR (400 MHz, CD3OD): δ 8.46 (s, 1H), 8.37 (s, 1H), 7.73 (d, J = 8.3 Hz, 2H), 7.59 (d, J = 8.0 Hz, 2H), 6.94–6.90 (br d, J = 16.0, 1H), 6.53–6.45 (m, 1H), 6.16 (d, J = 4.2 Hz, 1H), 4.81–4.76 (m, 1H), 4.59–4.46 (m, 2H), 4.09 (d, J = 5.0 Hz, 2H), 3.84 (br t, J = 9.0 Hz, 1H), 3.63–3.61 (br d, J = 8.0 Hz, 1H), 3.00 (s, 3H). 13C NMR (101 MHz, CD3OD): δ 151.82, 148.9, 139.7, 138.7, 132.3, 127.8, 118.05, 111.1, 89.5, 78.7, 73.3, 72.2, 56.9. HRMS (ESI): calculated for C21H24N7O3 [M + H]+ 422.1941, found 422.1945.
4-((E)-3-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(isopropyl)amino)prop-1-en-1-yl)benzonitrile (21k)
Following the procedure described for compound 13a, compound 20k (50 mg, 0.10 mmol) was deprotected and purified, affording compound 21k as a white powder (32 mg, 69% yield). 1H NMR (500 MHz, CD3OD): δ 8.34 (s, 1H), 8.28 (s, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 6.9 Hz, 2H), 6.82 (d, J = 8.9 Hz, 1H), 6.44–6.38 (m, 1H), 6.12–6.10 (m, 1H), 4.64 (dd, J = 5.3, 3.2 Hz, 1H), 4.58–4.53 (m, 2H), 4.47–4.43 (m, 1H), 4.07 (d, J = 8.1 Hz, 2H), 3.89–3.81 (m, 1H), 3.69 (d, J = 4.2 Hz, 2H), 1.44 (d, J = 6.6 Hz, 3H), 1.41 (d, J = 6.6 Hz, 3H). 13C NMR (101 MHz, CD3OD): δ 161.9, 161.6, 161.2, 160.8, 142.9, 139.8, 132.3, 119.7, 118.1, 115.1, 111.7, 91.3, 73.7, 72.1, 51.7. HRMS (ESI): calculated for C23H28N7O3 [M + H]+ 450.2254, found 450.2262.
(E)-4-(3-(Methylamino)prop-1-en-1-yl)benzonitrile (22b)
Aldehyde 15u (157 mg, 1.0 mmol), 5 mL of methylamine in MeOH (33% w/w), NaBH(OAc)3 (57 mg, 1.5 mmol), and AcOH (one drop) were added to DCE (10 mL) in a sealed tube, and the mixture was stirred at room temperature overnight. The reaction was quenched by adding 1 N NaOH (10 mL), and the product was extracted with CH2Cl2. The combined organic layers were washed with brine and dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography (20% MeOH in EtOAc) to give compound 22b as a white powder (72 mg, 42% yield). 1H NMR (400 MHz, CD3OD): δ 7.76 (d, J = 8.6 Hz, 2H), 7.68 (d, J = 8.7 Hz, 2H), 6.97–6.93 (br d, J = 12.0 Hz, 1H), 6.52–6.45 (m, 1H), 3.85 (dd, J = 7.1, 1.3 Hz, 2H), 2.77 (s, 3H). 13C NMR (101 MHz, CD3OD): δ 140.1, 136.5, 132.4, 127.3, 122.2, 118.1, 111.7, 50.0, 31.5. HRMS (ESI): calculated for C11H13N2 [M + H]+ 173.1079, found 173.1084.
tert-Butyl (S,E)-2-((tert-Butoxycarbonyl)amino)-4-((3-(4-cyanophenyl)allyl)amino)butanoate (23a)
Following the procedure described for compound 12a, (E)-4-(3-aminoprop-1-en-1-yl)benzonitrile 22a (35 mg, 0.22 mmol) was coupled with tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoate 10 (55 mg, 0.20 mmol) to afford compound 23a as a white powder (40 mg, 48% yield).1H NMR (400 MHz, CDCl3): δ 7.57 (d, J = 8.3 Hz, 2H), 7.43 (d, J = 8.3 Hz, 2H), 6.56–6.52 (br d, J = 16.0 Hz, 1H), 6.45–6.35 (m, 1H), 5.57 (d, J = 8.1 Hz, 1H), 4.30–4.18 (m, 1H), 3.48–3.40 (m, 2H), 2.76–2.67 (m, 2H), 2.04–1.92 (m, 2H), 1.81–1.75 (m, 1H), 1.45–1.42 (m, 18H). 13C NMR (101 MHz, CDCl3): δ 171.9, 155.7, 141.7, 132.4, 129.5, 126.8, 119.0, 110.5, 81.9, 79.6, 52.6, 51.5, 45.4, 32.9, 28.4. HRMS (ESI): calculated for C23H34N3O4 [M + H]+ 416.2549, found 416.2563.
tert-Butyl (S,E)-2-((tert-Butoxycarbonyl)amino)-4-((3-(4-cyanophenyl)allyl)(methyl)amino)butanoate (23b)
Following the procedure described for compound 12a, (E)-4-(3-(methylamino)prop-1-en-1-yl)benzonitrile 22b (34 mg, 0.20 mmol) was coupled with tert-butyl (S)-2-((tert-butoxycarbonyl)amino)-4-oxobutanoate 10 (66 mg, 0.24 mmol) to afford compound 23b as a white powder (66 mg, 77% yield).1H NMR (400 MHz, CDCl3): δ 7.59 (d, J = 8.3 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 6.53 (d, J = 16.0 Hz, 1H), 6.46–6.34 (m, 1H), 5.83 (d, J = 7.9 Hz, 1H), 4.23 (d, J = 6.4 Hz, 1H), 3.26–3.21 (m, 1H), 3.14–3.09 (m, 1H), 2.58–2.49 (m, 1H), 2.44–2.38 (m, 1H), 2.26 (s, 3H), 2.07–1.97 (m, 1H), 1.87–1.79 (m, 1H), 1.46–1.42 (br m, 18H).13C NMR (101 MHz, CDCl3): δ 171.7, 156.9, 141.5, 132.4, 131.8, 130.8, 126.9, 119.0, 110.6, 81.7, 79.5, 60.2, 53.6, 53.3, 42.3, 28.4, 28.0. HRMS (ESI): calculated for C24H35N3O4 [M + H]+ 430.2706, found 430.2715.
(S,E)-2-Amino-4-((3-(4-cyanophenyl)allyl)amino)butanoic Acid (24a)
Following the procedure described for compound 13a, compound 23a (20 mg, 0.048 mmol) was deprotected and purified, affording compound 24a as a white powder (14 mg, 76% yield). 1H NMR (400 MHz, CD3OD): δ 7.75 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.4 Hz, 2H), 6.96 (d, J = 15.9 Hz, 1H), 6.54–6.47 (m, 1H), 4.10 (dd, J = 8.1, 5.3 Hz, 1H), 3.91 (d, J = 8.3 Hz, 2H), 3.43–3.35 (m, 1H), 2.45–2.21 (m, 2H). 13C NMR (101 MHz, CD3OD): δ 169.4, 144.8, 136.5, 131.3, 127.8, 126.2, 122.5, 118.0, 114.1, 111.7, 50.7, 48.7, 42.8, 28.7. HRMS (ESI): calculated for C14H18N3O2 [M + H]+ 260.1399, found 260.1408.
(S,E)-2-Amino-4-((3-(4-cyanophenyl)allyl)(methyl)amino)butanoic Acid (24b)
Following the procedure described for compound 13a, compound 23b (13 mg, 0.046 mmol) was deprotected and purified, affording compound 24b as a white powder (9 mg, 72% yield). 1H NMR (400 MHz, CD3OD δ 7.76 (d, J = 8.6 Hz, 2H), 7.71 (d, J = 8.5 Hz, 2H), 7.04–7.00 (br d, J = 15.8 Hz, 1H), 6.62–6.51 (m, 1H), 4.13 (dd, J = 8.0, 5.3 Hz, 1H), 4.06 (d, J = 7.3 Hz, 2H), 3.53–3.30 (br m, 2H), 2.96 (s, 3H), 2.53–2.42 (m, 1H), 2.41–2.30 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 171.7, 143.8, 140.9, 138.7, 132.3, 127.5, 120.3, 117.0, 115.1, 110.6, 54.9, 53.0, 50.8, 39.3, 23.8. HRMS (ESI): calculated for C15H20N3O2 [M + H]+ 274.1556, found 274.1561.
tert-Butyl (2S)-4-((((3 3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(4-cyanobenzyl)amino)-2-((tert-butoxycarbonyl) amino)butanoate (25a)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 4-formylbenzonitrile (31 mg, 0.24 mmol) to afford the protected intermediate 25a as a white powder (79 mg, 50% yield). 1H NMR (500 MHz, CDCl3): δ 8.14 (s, 1H), 7.86 (s, 1H), 7.46 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.2 Hz, 2H), 6.42 (s, 2H), 6.03 (s, 1H), 5.43–5.37 (m, 2H), 4.93 (dd, J = 6.5, 3.6 Hz, 1H), 4.38–4.33 (m, 1H), 4.21–4,17 (m, 1H), 3.69–3.53 (m, 2H), 2.82–2.67 (m, 2H), 2.65–2.48 (m, 2H), 2.00–1.96 (br m, 1H), 1.77–1.71 (br m, 1H), 1.59 (s, 3H), 1.43–1.37 (br m, 21H). 13C NMR (126 MHz, CDCl3): δ 175.4, 171.7, 155.7, 148.9, 132.1, 129.3, 120.1, 119.0, 115.8, 109.5, 90.8, 85.7, 84.0, 83.5, 59.4, 52.7, 50.73, 29.8, 28.4, 27.2, 25.5. HRMS (ESI): calculated for C34H47N8O7 [M + H]+ 679.3568, found 679.3571.
(S)-2-amino-4-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(4-cyanobenzyl)amino)butanoic Acid (25)
Following the procedure described for compound 13a, compound 25a (50 mg, 0.074 mmol) was deprotected and purified, affording compound 25 as a white powder (33 mg, 75% yield). 1H NMR (500 MHz, CD3OD): δ 8.40 (s, 1H), 8.34 (s, 1H), 7.69 (d, J = 8.5 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 6.11 (d, J = 3.2 Hz, 1H), 4.60 (dd, J = 5.3, 3.3 Hz, 1H), 4.48–4.40 (m, 3H), 4.33 (br d, J = 13.7 Hz, 1H), 3.98 (dd, J = 7.9, 5.1 Hz, 1H), 3.53–3.37 (m, 4H), 2.39–2.33 (m, 1H), 2.22–2.18 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 172.2, 161.2, 151.5, 147.8, 132.4, 128.0, 119.6, 118.3, 112.8, 90.8, 79.4, 73.1, 70.9, 57.3, 55.1, 51.7, 51.0, 39.1, 25.5. HRMS (ESI): calculated for C24H27N8O5 [M + H]+ 483.2104, found 483.2115.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(3-(4-cyanophenyl)propyl)amino)-2-((tert-butoxy carbonyl)amino)butanoate (26a)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 4-(3-oxopropyl)benzonitrile (38 mg, 0.24 mmol) to afford protected intermediate compound 26a as a white powder (80 mg, 57% yield). 1H NMR (400 MHz, CDCl3): δ 8.30 (s, 1H), 7.92 (s, 1H), 7.54 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.2 Hz, 2H), 6.24 (br s, 2H), 6.07 (d, J = 1.7 Hz, 1H), 5.76 (d, J = 8.0 Hz, 1H), 5.52 (d, J = 6.0 Hz, 1H), 5.02 (s, 1H), 4.32–4.28 (m, 1H), 4.20–4.14 (m, 2H), 3.01–2.73 (m, 2H), 2.67–2.57 (m, 4H), 2.50–2.34 (m, 3H), 2.16–1.88 (m, 2H), 1.75–1.64 (m, 3H), 1.62 (s, 3H), 1.48–1.38 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 171.9, 153.1, 149.2, 148.0, 140.3, 132.2, 129.2, 120.4, 119.2, 114.4, 109.6, 90.9, 85.7, 83.9, 83.4, 81.7, 79.5, 60.4, 54.0, 53.7, 53.0, 52.6, 50.9, 42.1, 33.6, 30.8, 29.3, 28.4, 28.2, 27.2, 25.5, 20.0, 14.3. HRMS (ESI): calculated for C36H51N8O7 [M + H]+ 707.3881, found 707.3882.
(S)-2-amino-4-((((2R,3S,4R,5R)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(3-(4-cyanophenyl)propyl)amino)butanoic Acid (26)
Following the procedure described for compound 13a, compound 26a (50 mg, 0.071 mmol) was deprotected and purified, affording compound 26 as a white powder (35 mg, 79% yield). 1H NMR (400 MHz, CD3OD): δ 8.47 (s, 1H), 8.38 (s, 1H), 7.61 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.2 Hz, 2H), 6.12 (d, J = 3.9 Hz, 1H), 4.70 (t, J = 4.3 Hz, 1H), 4.50–4.43 (m, 2H), 4.08 (dd, J = 8.2, 4.7 Hz, 1H), 3.84–3.78 (m, 1H), 3.71–3.67 (br d, J = 16.0 Hz, 1H), 3.64–3.47 (m, 2H), 2.81–2.68 (m, 2H), 2.47–2.38 (m, 1H), 2.30–2.22(m, 1H), 2.07 (h, J = 7.4 Hz, 2H). 13C NMR (101 MHz, CD3OD): δ 170.2, 161.9, 161.5, 161.2, 160.8, 151.6, 148.3, 146.1, 145.4, 142.8, 132.1, 129.1, 119.6, 118.4, 115.1, 109.9, 90.6, 78.7, 73.3, 72.2, 54.9, 51.1, 31.9, 24.7, 24.3. HRMS (ESI): calculated for C24H31N8O5 [M + H]+ 511.2417, found 511.2425.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(3-(3-cyanophenyl)prop-2-yn-1-yl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (27a)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 3-(3-oxoprop-1-yn-1-yl)benzonitrile (37 mg, 0.24 mmol) to afford the protected intermediate compound 27a as a white powder (90 mg, 64% yield). 1H NMR (400 MHz, CDCl3): δ 8.31 (s, 1H), 7.99 (s, 1H), 7.63 (s, 1H), 7.55 (dd, J = 7.7, 2.1 Hz, 2H), 7.39 (t, J = 7.8 Hz, 1H), 6.34 (d, J = 11.2 Hz, 2H), 6.09 (d, J = 2.3 Hz, 1H), 5.64–5.49 (br m, 2H), 5.08–5.00 (m 1H), 4.42–4.23 (m, 2H), 3.66 (s, 2H), 2.91–2.86 (m, 1H), 2.81–2.73 (br m, 1H), 2.65 (t, J = 6.9 Hz, 2H), 2.02–1.96 (m, 1H), 1.87–1.80 (m, 1H), 1.62 (s, 3H), 1.50–1.35 (br m, 21H). 13C NMR (101 MHz, CDCl3): δ 175.9, 171.7, 155.8, 155.5, 153.0, 149.2, 140.1, 135.83, 135.8, 135.1, 131.4, 129.2, 124.5, 120.2, 118.1, 114.5, 112.8, 86.8, 85.7, 83.9, 83.4, 55.6, 52.67,50.6, 43.5, 29.7, 28.4, 28.0, 27.2, 25.5. HRMS (ESI): calculated for C36H47N8O7 [M + H]+ 703.3568, found 703.3582.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(3-(3-cyanophenyl)prop-2-yn-1-yl)amino)butanoic Acid (27)
Following the procedure described for compound 13a, compound 27a (50 mg, 0.074 mmol) was deprotected and purified, affording compound 27 as a white powder (33 mg, 73% yield). 1H NMR (500 MHz, CD3OD): δ 8.48 (s, 1H), 8.38 (s, 1H), 7.83 (t, J = 1.3 Hz, 1H), 7.78–7.75 (m, 1H), 7.74–7.72 (m, 1H), 7.59–7.55 (m, 1H), 6.14 (d, J = 4.1 Hz, 1H), 4.74–4.71 (m, 1H), 4.50–4.46 (m, 1H), 4.44 (t, J = 5.4 Hz, 1H), 4.23 (s, 2H), 4.13 (t, J = 6.4 Hz, 1H), 3.60–3.49 (m, 2H), 3.40 (t, J = 6.9 Hz, 2H), 2.42–2.35 (m, 1H), 2.24–2.17 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 170.3, 161.0, 160.8, 151.2, 148.4, 135.8, 134.9, 132.4, 129.6, 123.1, 119.6, 117.4, 115.2, 112.8, 90.3, 86.1, 81.44, 80.2, 73.7, 72.2, 55.9, 51.7, 51.1, 42.7, 25.6. HRMS (ESI): calculated for C24H27N8O5 [M + H]+ 507.2104, found 507.2108.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(3-(4-cyanophenyl)prop-2-yn-1-yl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (28a)
Following the procedure described for compound 12a, compound 11 (112 mg, 0.20 mmol) was coupled with 4-(3-oxoprop-1-yn-1-yl)benzonitrile (37 mg, 0.24 mmol) to afford the protected intermediate compound 28a as a white powder (104 mg, 74% yield). 1H NMR (500 MHz, CDCl3): δ 8.32 (s, 1H), 7.96 (s, 1H), 7.56 (s, 2H), 7.40 (d, J = 8.4 Hz, 2H), 6.09 (d, J = 9.8 Hz, 3H), 5.57 (d, J = 8.2 Hz, 1H), 5.50 (d, J = 8.7 Hz, 1H), 5.09–4.99 (m, 1H), 4.43–4.36 (m, 1H), 4.25–4.23 (m, 1H), 3.66 (d, J = 2.9 Hz, 2H), 2.90–2.75 (m, 2H), 2.64 (t, J = 6.9 Hz, 2H), 2.01–1.97 (m, 1H), 1.85–1.80 (m, 1H), 1.61 (s, 3H), 1.45–1.38 (br m, 21H). 13C NMR (126 MHz, CDCl3): δ 171.7, 154.5, 153.1, 150.6, 132.3, 132.0, 129.0, 120.9, 118.5, 113.9, 110.2, 90.9, 89.0, 85.8, 83.2, 81.9, 80.2, 55.2, 50.6, 42.1, 30.1, 28.4, 28.1, 27.2, 25.5. HRMS (ESI): calculated for C36H47N8O7 [M + H]+ 703.3568, found 703.3577.
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(3-(4-cyanophenyl)prop-2-yn-1-yl)amino)butanoic Acid (28)
Following the procedure described for compound 13a, compound 28a (50 mg, 0.074 mmol) was deprotected and purified, affording compound 28 as a white powder (36 mg, 81% yield). 1H NMR (500 MHz, CD3OD): δ 8.48 (s, 1H), 8.37 (s, 1H), 7.73 (d, J = 8.6 Hz, 2H), 7.59 (d, J = 8.6 Hz, 2H), 6.13 (d, J = 4.1 Hz, 1H), 4.74–4.71 (m, 1H), 4.48–4.41 (m, 2H), 4.19 (s, 2H), 4.12 (t, J = 6.4 Hz, 1H), 3.53–3.42 (m, 2H), 3.35 (d, J = 6.5 Hz, 2H), 2.39–2.32 (m, 1H), 2.20–2.13 (m, 1H). 13C NMR (126 MHz, CD3OD): δ 170.5, 161.0, 151.50, 148.4, 132.1, 126.6, 117.8, 115.3, 112.2, 90.2, 86.2, 84.2, 73.7, 72.2, 55.9, 52.2, 51.1, 42.7, 25.8. HRMS (ESI): calculated for C24H27N8O5 [M + H]+ 507.2104, found 507.2113.
tert-Butyl (2S)-4-((E)-N-(((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyl-tetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)-3-(4-cyanophenyl)acrylamido)-2-((tert-butoxy carbonyl)amino)butanoate (29a)
To a stirred solution of (E)-3-(4-cyanophenyl)acrylic acid (35 mg, 0.20 mmol) in CH2Cl2 (10 mL) under a N2 atmosphere were added BOP (97 mg, 0.22 mmol), compound 11 (112 mg, 0.20 mmol), and Et3N (0.1 mL) sequentially. The resulting reaction mixture was then stirred for 16 h at room temperature. After washing with 5% KHSO4 (2 × 80 mL), 5% NaHCO3 (2 × 80 mL), and H2O (80 mL), the organic phase was dried over Na2SO4. The solvent was evaporated, and the crude product was purified by column chromatography (5% MeOH in EtOAc) to give the protected intermediate compound 29a as a white powder (83 mg, 58% yield). 1H NMR (400 MHz, CDCl3): δ 8.32 (d, J = 8.1 Hz, 1H), 7.93 (d, J = 43.7 Hz, 1H), 7.73–7.59 (m, 2H), 7.52–7.36 (m, 2H), 7.08–6.87 (m, 2H), 6.76 (s, 1H), 6.70–6.47 (m, 1H), 6.07 (d, J = 9.4 Hz, 1H), 5.63–5.47 (br m, 1H), 5.27 (d, J = 6.4 Hz, 1H), 5.17–5.12 (m, 1H), 4.27–3.80 (m, 3H), 3.70–3.66 (br d, J = 16.0 Hz, 1H), 3.60–3.13 (m, 2H), 2.11 (s, 1H), 1.61 (d, J = 10.2 Hz, 3H), 1.46–1.36 (br m, 21H) 13C NMR (101 MHz, CDCl3): δ 175.7, 141.4, 166.2, 155.0, 153.1, 140.2, 139.5, 132.5, 128.5, 127.6, 124.8, 121.5, 118.7, 90.6, 89.9, 84.7, 81.8, 52.2, 50.5, 43.4, 28.4, 28.0, 25.5. HRMS (ESI): calculated for C36H47N8O8 [M + H]+ 719.3517, found 719.3524.
(S)-2-Amino-4-((E)-N-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydro-furan-2-yl)methyl)-3-(4-cyanophenyl)acrylamido)butanoic Acid (29)
Following the procedure described for compound 13a, compound 29a (50 mg, 0.070 mmol) was deprotected and purified, affording compound 29 as a white powder (18 mg, 49% yield). 1H NMR (400 MHz, CD3OD): δ 8.40 (s, 1H), 8.32 (s, 1H), 7.68 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 6.7 Hz, 2H), 7.43–7.39 (br d, J = 16.0, 1H), 7.18 (br s, 1H), 6.09–6.04 (m, 1H), 4.69–4.63 (m, 2H), 4.39–4.25 (m, 1H), 4.07–4.01 (m, 2H), 3.97 (dd, J = 7.7, 5.4 Hz, 1H), 3.89–3.82 (m, 1H), 3.74–3.63 (m, 1H), 2.40–2.35 (m, 1H), 2.27–2.20(m, 1H). 13C NMR (101 MHz, CD3OD): δ 170.0, 168.4, 162.5, 151.6, 148.3, 140.1, 139.3, 133.2, 127.9, 121.2, 117.96, 112.52, 90.6, 82.5, 72.5, 70.9, 50.2, 42.6, 27.9. HRMS (ESI): calculated for C24H27N8O6 [M + H]+ 523.2054, found 523.2061.
tert-Butyl (2S)-4-((((3aR,3aR,4R,6R,6aR,6aR)-6-(6-Amino-9H-purin-9-yl)-2,2-dimethyltetra-hydrofuro[3,4-d][1,3]dioxol-4-yl)methyl)(3-(4-carbamoylphenyl)prop-2-yn-1-yl)amino)-2-((tert-butoxycarbonyl)amino)butanoate (31a)
Following the procedure described for compound 16v, compound 28a was oxidized to afford the protected intermediate compound 31a as a white powder (109 mg, 82% yield). 1H NMR (400 MHz, CDCl3): δ 8.31 (s, 1H), 7.99 (s, 1H), 7.76 (d, J = 8.3 Hz, 2H), 7.38 (d, J = 8.2 Hz, 2H), 6.47 (s, 2H), 6.18 (s, 2H), 6.10 (s, 1H), 5.62 (d, J = 8.0 Hz, 1H), 5.51 (d, J = 5.7 Hz, 1H), 5.12–5.03 (m, 1H), 4.43 (s, 1H), 4.32–4.21 (m, 1H), 3.67 (s, 2H), 2.92–2.80 (m, 2H), 2.69–2.63 (m, 2H), 1.99 (d, J = 5.5 Hz, 1H), 1.87–1.82 (br m, 1H), 1.64 (s, 3H), 1.44–1.42 (br d, J = 8.0 Hz, 21H). 13C NMR (101 MHz, CDCl3): δ 171.8, 169.0, 155.6, 153.0, 149.2, 140.2, 132.7, 131.8, 127.4, 120.3, 114.5, 90.9, 86.8, 86.0, 83.0, 83.3, 55.7, 52.8, 50.7, 28.4, 28.0, 27.2, 25.50. HRMS (ESI): calculated for C36H51N8O8 [M + H]+ 723.3830, found 723.3841
(S)-2-Amino-4-((((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)(3-(4-carbamoylphenyl)prop-2-yn-1-yl)amino)butanoic Acid (31)
Following the procedure described for compound 13a, compound 31a (50 mg, 0.074 mmol) was deprotected and purified, affording compound 31 as a white powder (34 mg, 80% yield). 1H NMR (400 MHz, CD3OD): δ 8.59 (s, 1H), 8.32 (s, 1H), 7.87 (d, J = 8.5 Hz, 2H), 7.53 (d, J = 8.4 Hz, 2H), 6.20 (d, J = 4.8 Hz, 1H), 4.79 (t, J = 4.8 Hz, 1H), 4.61 (dd, J = 7.9, 4.6 Hz, 1H), 4.50 (t, J = 4.9 Hz, 1H), 4.44 (s, 2H), 4.20 (dd, J = 8.1, 4.7 Hz, 1H), 3.85–3.68 (m, 2H), 3.67–3.53 (m, 2H), 2.57–2.48 (m, 1H), 2.35–2.22 (m, 1H). 13C NMR (101 MHz, CD3OD): δ 171.4, 169.9, 162.2, 161.8, 161.5, 161.1, 151.2, 148.3, 144.9, 148.3, 144.9, 143.0, 134.2, 127.5, 124.6, 121.0, 119.2, 118.1, 115.2, 112.3, 89.9, 88.4, 73.7, 72.2, 55.7,52.1, 51.8, 42.3, 25.3. HRMS (ESI): calculated for C24H28N8O6 [M + H]+ 525.2210, found 525.2223.
Enzymatic Activity Assay
The expression and purification of the full-length wild-type NNMT protein (NNMTwt) were performed as previously described.30 The purity of the enzyme was confirmed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie blue staining, and the NNMT identity was confirmed using SDS-PAGE and Western blotting.
Catalytic activity of the recombinant protein was evaluated with 1 unit of enzyme activity representing the formation of 1 nmol MNA per hour of incubation. The specific activity of the batch used in the inhibitory activity assays was 18060 units per milligram of protein at a protein concentration of 0.98 mg/mL. NNMT was used at a final concentration of 100 nM diluted in assay buffer (final concentrations of 50 mM Tris buffer (pH 8.4) and 1 mM dithiothreitol). The compounds were dissolved in DMSO and diluted with water to concentrations ranging from 1 nM to 500 μM (DMSO was kept constant at a 1.25% final concentration). The compounds were screened for activity at fixed concentrations of 25 and 5 μM. When at least 50% inhibition was observed at 25 μM, full IC50 curves were generated. The compounds were incubated with the enzyme for 10 min at room temperature before the reaction was initiated with a mixture of NA and SAM at their KM concentrations of 400 and 8.5 μM, respectively. The formation of MNA was measured after 30 min at room temperature. The reaction was quenched by adding 30 μL of the sample to 70 μL of acetonitrile, which contained 50 nM deuteromethylated nicotinamide (d3-MNA) as an internal standard. Sample analysis was performed using multiple reaction monitoring (MRM) on an LC-MS system as previously described with minor modifications.20 The LC-MS system consisted of a Shimadzu 8040 triple quadrupole mass spectrometer (ESI ionization). Isocratic elution was performed after 5 μL injections on a Waters Acquity BEH Amide HILIC column (3.0 × 100 mm, 1.7 μm particle size, Waters, Milford) using water that contained 300 mM formic acid and 550 mM NH4OH (pH 9.2) at 40% v/v and acetonitrile at 60% v/v with a runtime of 1.7 min. Calibration samples were prepared using 70 μL of the internal standard d3-MNA at a 50 nM concentration in acetonitrile and 30 μL of an aqueous solution of the reference standard MNA with concentrations ranging from 1 to 1024 nM. All compounds exhibiting IC50 values below 500 nM were considered tight-binding inhibitors and were retested using an enzyme concentration of 10 nM and a reaction time of 45 min. Full IC50 curves are presented in Figures S1–S4 in the Supporting Information.
Isothermal Titration Calorimetry
All binding experiments are performed using a MicroCal PEAQ-ITC automated microcalorimeter (Malvern). The samples are equilibrated to 20 °C prior to the measurement. The hNNMT enzyme (8.4 mg/mL in 50 mM NaH2PO4, pH 8, 300 mM NaCl, 200 mM imidazole, 0.5 mM DTT, 1 mM PMSF, and 20% glycerol) was diluted with 20 mM Tris HCl, pH 7.0, to reach a final concentration of 11.4 μM. Compound 17u was diluted to a final concentration of 114 μM in 20 mM Tris HCl, pH 7.0, with the addition of enzyme buffer to avoid any buffer mismatch during titration. Compound 17u (114 μM) was titrated into hNNMT (11.4 μM). The titrations are conducted at 25 °C under constant stirring at 750 rpm. Each binding experiment consisted of an initial injection of 0.4 μL followed by 18 separate injections of 2.0 μL into the 200 μL sample cell. The time between each injection is 150 s, the measurements are performed with the reference power set at 10 μcal/s, and the feedback mode was set at “high”. The calorimetric data obtained were analyzed using MicroCal PEAQ-ITC Analysis software ver. 1.20. ITC data fitting was made based on the “one set of sites” fitting model of the software. The best fit is defined by χ2-minimization. All thermodynamic parameters and thermograms of the measurements of the three independent experiments are reported in the Supporting Information (Table S3 and Figure S5).
Enzyme Assays for Selectivity
The PRMT4/CARM1 methyltransferase inhibition assay was performed as previously described29 using a commercially available chemiluminescent assay kit for PRMT4/CARM1 (purchased from BPS Bioscience). Compound 17u was tested at concentrations of 3.7, 11.1, 33.3, and 100 μM, and no inhibition was observed at the concentrations tested. The phenylethanolamine N-methyltransferase (PNMT) assay was developed using the Promega MTase-Glo Methyltransferase assay (purchased from Promega Corporation). Compound 17u was tested at concentrations of 1 and 10 μM, and less than 50% inhibition was observed at the concentrations tested. Full details of the PNMT assay are provided in the Supporting Information. All other methyltransferase assays were performed as previously described.25
Modeling
The structure of NNMT was taken from PDB entry 6PVE(27) and subsequently prepared using the Protein Preparation Wizard in Maestro (Schrodinger ver. 2020-3). Compounds were aligned to the cocrystallized ligand based on their chemical similarity using the flexible ligand alignment in Maestro. The generated protein–ligand complexes were used as starting point for molecular dynamics (MD) simulations performed in the software package Q.40 This software is tailored for different types of free energy calculations under spherical boundary conditions; in particular, we used the QligFEP utility as a free energy perturbation (FEP) protocol41 for the generation of all input files and their subsequent analysis. A 25 Å radius sphere was solvated based on the center of geometry of the ligand. Protein atoms in the boundary of the sphere (22–25 Å outer shell) had a positional restraint of 20 kcal/mol/Å2, while solvent atoms were subject to polarization and radial restrains using the surface-constrained all-atom solvent (SCAAS)42,43 model to mimic the properties of bulk water at the sphere surface. Atoms lying outside the simulation sphere were tightly constrained (200 kcal/mol/Å2 force constant) and excluded from the calculation of the nonbonded interactions. Long-range electrostatic interactions beyond a 10 Å cut off were treated with the local reaction field method,43 except for the atoms undergoing the FEP transformation, where no cutoff was applied. Solvent bonds and angles were constrained using the SHAKE algorithm.44 All titratable residues outside the sphere were neutralized, and histidine protonation states were assigned by the Protein Preparation Wizard. The OPLS-AA/M force field45 was adopted for the protein and solvent (TIP3P model) parameters, while compatible OPLS2005 ligand parameters were generated using the ffld_server46 and translated to Q format using QligFEP. The simulation sphere was warmed from 0.1 to 298 K during a first equilibration period of 0.61 ns, where an initial restraint of 25 kcal/mol/Å2 imposed on all heavy atoms was slowly released for all complexes. Thereafter, the system was subject to ten parallel replicates of unrestrained MD, starting in all cases with a 0.25 ns unbiased equilibration period using randomized initial velocities. Thereafter, the FEP protocol followed for every investigated ligand pair, which consisted of 101 FEP λ-windows where the coupling parameter λ was unevenly distributed using a sigmoidal function and each window was sampled for 10 ps. To close a thermodynamic cycle and calculate the relative binding free energies for each ligand pair, an analogous FEP transformation was run in parallel in a sphere of water. In these water simulations, the same parameters applied (i.e., sphere size, simulation time, etc.), and the relative binding free energy difference was estimated by solving the thermodynamic cycle utilizing the Bennett acceptance ratio (BAR).47 The corresponding experimental values were extracted from the herein-reported IC50 values for each ligand using eq 1
| 1 |
where R = 1.987–3 kcal/mol/K and T = 298 K.
Cell Culture and Treatment with Compounds
The HSC-2 human oral cancer cell line, the T24 human bladder cancer cell line, and the A549 human lung cancer line were purchased from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in a DMEM/F12 medium, which was supplemented with 10% fetal bovine serum and 50 μg/mL gentamicin, at 37 °C in a humidified 5% CO2 incubator. Compound 17u was dissolved in DMSO at a 100 mM concentration. This stock solution was then diluted in the culture medium to final concentration values ranging between 1 and 100 μM. For each sample, DMSO was kept constant at a 0.1% final concentration. The day before starting the treatment, cells were seeded in 96-well plates at a density of 2 × 103 cells/well. Cells were allowed to attach overnight and then incubated for 24, 48, and 72 h with either compound 17u at different final concentrations or DMSO only. All experiments were performed in triplicate. Cell proliferation was determined using a colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT). The MTT assay measures the conversion of MTT to insoluble formazan by the dehydrogenase enzymes of the intact mitochondria of living cells. Cell proliferation was evaluated by measuring the conversion of the tetrazolium salt MTT to formazan crystals upon treatment with either compound 17u or DMSO only for 24, 48, and 72 h. Briefly, cells were incubated for 2 h at 37 °C with 100 μL of the fresh culture medium that contained 5 μL of the MTT reagent (5 mg/mL in PBS). The medium was removed, and 200 μL isopropanol was added. The amount of formazan crystals that formed correlated directly with the number of viable cells. The reaction product was quantified by measuring the absorbance at 540 nm using an ELISA plate reader. Experiments were repeated three times. Results are expressed as a percentage of the control (control equals 100% and corresponds to the absorbance value of each sample at time zero) and presented as mean values ± the standard deviation of three independent experiments performed in triplicate. Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). Significant differences between groups were determined using the one-way analysis of variance (ANOVA). A p-value of <0.05 was considered statistically significant. Bar graphs are presented in Figure S8 in the Supporting Information.
Parallel Artificial Membrane Permeability Assay
The PAMPA assay was carried out with a Corning BioCoat precoated PAMPA Plate System (cat. 353015). The stock solutions were prepared at 10 mM concentration in DMSO and diluted with PBS to achieve a final sample concentration of 200 μM (2% DMSO (v/v)). The bottom plate (donor) was filled with 300 μL of the diluted sample solution, while the top plate (acceptor, containing the synthetic phospholipid membrane) was filled with 200 μL of PBS. The acceptor plate was then placed on the donor plate, and the system was incubated for 5 h at 25 °C. The plate sandwich was separated, and the concentrations of samples in both the donor and acceptor compartments were evaluated by means of UV spectrometry using a Tecan plate reader set at 280 nM.
Acknowledgments
We kindly thank Irene Chau and Masoud Vedadi (Structural Genomics Consortium, Toronto) for performing the methyltransferase selectivity assays.
Glossary
ABBREVIATIONS
- BOP
benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate
- DCE
1,2-dichloroethane
- DIBAL-H
diisobutylaluminum hydride
- DMAP
4-dimethylaminopyridine
- HILIC
hydrophilic liquid interaction chromatography
- hNNMT
human nicotinamide N-methyltransferase
- IC50
half-maximal inhibitory concentration
- ITC
isothermal titration calorimetry
- Kd
dissociation constant
- MNA
1-methyl-nicotinamide
- NA
nicotinamide
- NNMTwt
wild-type NNMT
- PDC
pyridinium dichromate
- PNMT
phenylethanolamine N-methyltransferase
- PRMT4
protein arginine N-methyltransferase 4
- SAH
S-adenosyl-l-homocysteine
- SAM
S-adenosyl-l-methionine
- Trt
triphenylmethyl (trityl)
- DNMT1
DNA (cytosine-5)-N-methyltransferase 1
- DOT1L
disruptor of telomeric silencing 1-like
- MLL1
mixed-lineage leukemia 1
- PRMT
protein arginine N-methyltransferase
- SETDB1
SET domain bifurcated 1
- SETD2
SET domain-containing 2
- SMYD2
SET and MYND domain-containing protein 2
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.1c01094.
Selectivity data, ITC thermograms, IC50 curves, comparative inhibition data for tight-binding compounds, modeling data, HPLC chromatograms of lead compounds, cell-based data, permeability data, and NMR spectra (PDF)
Molecular formula strings for all final compounds (CSV)
PDB coordinates for the NNMT—17s complex MD starting configuration (PDB)
PDB coordinates for the NNMT—17t complex MD starting configuration (PDB)
PDB coordinates for the NNMT—17u complex MD starting configuration (PDB)
PDB coordinates for the NNMT—17v complex MD starting configuration (PDB)
PDB coordinates for the NNMT—17x complex MD starting configuration (PDB)
Author Contributions
∇ These authors contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Financial support was provided by the European Research Council (ERC consolidator grant to N.I.M, Grant agreement 725523).
The authors declare no competing financial interest.
Special Issue
Published as part of the Journal of Medicinal Chemistry special issue “Epigenetics 2022”.
Supplementary Material
References
- Aksoy S.; Szumlanski C. L.; Weinshilboum R. M. Human Liver Nicotinamide N-Methyltransferase. CDNA Cloning, Expression, and Biochemical Characterization. J. Biol. Chem. 1994, 269 (20), 14835–14840. 10.1016/S0021-9258(17)36700-5. [DOI] [PubMed] [Google Scholar]
- van Haren M. J.; Sastre Toraño J.; Sartini D.; Emanuelli M.; Parsons R. B.; Martin N. I. A Rapid and Efficient Assay for the Characterization of Substrates and Inhibitors of Nicotinamide N -Methyltransferase. Biochemistry 2016, 55 (37), 5307–5315. 10.1021/acs.biochem.6b00733. [DOI] [PubMed] [Google Scholar]
- Pissios P. Nicotinamide N-Methyltransferase: More Than a Vitamin B3 Clearance Enzyme. Trends Endocrinol. Metab. 2017, 28 (5), 340–353. 10.1016/j.tem.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston T. A.; Abeles R. H. Substrate Specificity of Nicotinamide Methyltransferase Isolated from Porcine Liver. Arch. Biochem. Biophys. 1988, 260 (2), 601–608. 10.1016/0003-9861(88)90487-0. [DOI] [PubMed] [Google Scholar]
- Kraus D.; Yang Q.; Kong D.; Banks A. S.; Zhang L.; Rodgers J. T.; Pirinen E.; Pulinilkunnil T. C.; Gong F.; Wang Y.; Cen Y.; Sauve A. A.; Asara J. M.; Peroni O. D.; Monia B. P.; Bhanot S.; Alhonen L.; Puigserver P.; Kahn B. B. Nicotinamide N-Methyltransferase Knockdown Protects against Diet-Induced Obesity. Nature 2014, 508 (7495), 258–262. 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulanovskaya O. A.; Zuhl A. M.; Cravatt B. F. NNMT Promotes Epigenetic Remodeling in Cancer by Creating a Metabolic Methylation Sink. Nat. Chem. Biol. 2013, 9 (5), 300–306. 10.1038/nchembio.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber H.; Mathieu J.; Wang Y.; Ferreccio A.; Hesson J.; Xu Z.; Fischer K. A.; Devi A.; Detraux D.; Gu H.; Battle S. L.; Showalter M.; Valensisi C.; Bielas J. H.; Ericson N. G.; Margaretha L.; Robitaille A. M.; Margineantu D.; Fiehn O.; Hockenbery D.; Blau C. A.; Raftery D.; Margolin A. A.; Hawkins R. D.; Moon R. T.; Ware C. B.; Ruohola-Baker H. The Metabolome Regulates the Epigenetic Landscape during Naive-to-Primed Human Embryonic Stem Cell Transition. Nat. Cell Biol. 2015, 17 (12), 1523–1535. 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z.; Liu Y.; Liu X. Nicotinamide N-Methyltransferase Enhances the Progression of Prostate Cancer by Stabilizing Sirtuin 1. Oncol. Lett. 2018, 15 (6), 9195–9201. 10.3892/ol.2018.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X.; Yu H.; Wang Y.; Zhou Y.; Li G.; Ruan Z.; Li F.; Wang X.; Liu H.; Zhang J. Nicotinamide N-Methyltransferase Enhances the Capacity of Tumorigenesis Associated with the Promotion of Cell Cycle Progression in Human Colorectal Cancer Cells. Arch. Biochem. Biophys. 2014, 564, 52–66. 10.1016/j.abb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Eckert M. A.; Coscia F.; Chryplewicz A.; Chang J. W.; Hernandez K. M.; Pan S.; Tienda S. M.; Nahotko D. A.; Li G.; Blaženović I.; Lastra R. R.; Curtis M.; Yamada S. D.; Perets R.; McGregor S. M.; Andrade J.; Fiehn O.; Moellering R. E.; Mann M.; Lengyel E. Proteomics Reveals NNMT as a Master Metabolic Regulator of Cancer-Associated Fibroblasts. Nature 2019, 569 (7758), 723–728. 10.1038/s41586-019-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartini D.; Morganti S.; Guidi E.; Rubini C.; Zizzi A.; Giuliante R.; Pozzi V.; Emanuelli M. Nicotinamide N-Methyltransferase in Non-Small Cell Lung Cancer: Promising Results for Targeted Anti-Cancer Therapy. Cell Biochem. Biophys. 2013, 67 (3), 865–873. 10.1007/s12013-013-9574-z. [DOI] [PubMed] [Google Scholar]
- Tomida M.; Ohtake H.; Yokota T.; Kobayashi Y.; Kurosumi M. Stat3 Up-Regulates Expression of Nicotinamide N-Methyltransferase in Human Cancer Cells. J. Cancer Res. Clin. Oncol. 2008, 134 (5), 551–559. 10.1007/s00432-007-0318-6. [DOI] [PubMed] [Google Scholar]
- Brachs S.; Polack J.; Brachs M.; Jahn-Hofmann K.; Elvert R.; Pfenninger A.; Bärenz F.; Margerie D.; Mai K.; Spranger J.; Kannt A. Genetic Nicotinamide N-Methyltransferase (Nnmt) Deficiency in Male Mice Improves Insulin Sensitivity in Diet-Induced Obesity but Does Not Affect Glucose Tolerance. Diabetes 2019, 68 (3), 527–542. 10.2337/db18-0780. [DOI] [PubMed] [Google Scholar]
- Liu M.; Li L.; Chu J.; Zhu B.; Zhang Q.; Yin X.; Jiang W.; Dai G.; Ju W.; Wang Z.; Yang Q.; Fang Z. Serum N1-Methylnicotinamide Is Associated with Obesity and Diabetes in Chinese. J. Clin. Endocrinol. Metab. 2015, 100 (8), 3112–3117. 10.1210/jc.2015-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons R. B.; Smith M.-L.; Williams A. C.; Waring R. H.; Ramsden D. B. Expression of Nicotinamide N-Methyltransferase (E.C. 2.1.1.1) in the Parkinsonian Brain. J. Neuropathol. Exp. Neurol. 2002, 61 (2), 111–124. 10.1093/jnen/61.2.111. [DOI] [PubMed] [Google Scholar]
- Thomas M. G.; Saldanha M.; Mistry R. J.; Dexter D. T.; Ramsden D. B.; Parsons R. B. Nicotinamide N-Methyltransferase Expression in SH-SY5Y Neuroblastoma and N27 Mesencephalic Neurones Induces Changes in Cell Morphology via Ephrin-B2 and Akt Signalling. Cell Death Dis. 2013, 4 (6), e669–e669. 10.1038/cddis.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren M. J.; Thomas M. G.; Sartini D.; Barlow D. J.; Ramsden D. B.; Emanuelli M.; Klamt F.; Martin N. I.; Parsons R. B. The Kinetic Analysis of the N-Methylation of 4-Phenylpyridine by Nicotinamide N-Methyltransferase: Evidence for a Novel Mechanism of Substrate Inhibition. Int. J. Biochem. Cell Biol. 2018, 98 (March), 127–136. 10.1016/j.biocel.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Kocinaj A.; Chaudhury T.; Uddin M. S.; Junaid R. R.; Ramsden D. B.; Hondhamuni G.; Klamt F.; Parsons L.; Parsons R. B. High Expression of Nicotinamide N-Methyltransferase in Patients with Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1769. 10.1007/s12035-020-02259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren M. J.; Taig R.; Kuppens J.; Sastre Toraño J.; Moret E. E.; Parsons R. B.; Sartini D.; Emanuelli M.; Martin N. I. Inhibitors of Nicotinamide N-Methyltransferase Designed to Mimic the Methylation Reaction Transition State. Org. Biomol. Chem. 2017, 15 (31), 6656–6667. 10.1039/C7OB01357D. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Van Haren M. J.; Moret E. E.; Rood J. J. M.; Sartini D.; Salvucci A.; Emanuelli M.; Craveur P.; Babault N.; Jin J.; Martin N. I. Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity. J. Med. Chem. 2019, 62 (14), 6597–6614. 10.1021/acs.jmedchem.9b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelakantan H.; Wang H. Y.; Vance V.; Hommel J. D.; McHardy S. F.; Watowich S. J. Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase. J. Med. Chem. 2017, 60 (12), 5015–5028. 10.1021/acs.jmedchem.7b00389. [DOI] [PubMed] [Google Scholar]
- Kannt A.; Rajagopal S.; Kadnur S. V.; Suresh J.; Bhamidipati R. K.; Swaminathan S.; Hallur M. S.; Kristam R.; Elvert R.; Czech J.; Pfenninger A.; Rudolph C.; Schreuder H.; Chandrasekar D. V.; Mane V. S.; Birudukota S.; Shaik S.; Zope B. R.; Burri R. R.; Anand N. N.; Thakur M. K.; Singh M.; Parveen R.; Kandan S.; Mullangi R.; Yura T.; Gosu R.; Ruf S.; Dhakshinamoorthy S. A Small Molecule Inhibitor of Nicotinamide N-Methyltransferase for the Treatment of Metabolic Disorders. Sci. Rep. 2018, 8, 3660. 10.1038/s41598-018-22081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-Y.; Suciu R. M.; Horning B. D.; Vinogradova E. V.; Ulanovskaya O. A.; Cravatt B. F. Covalent Inhibitors of Nicotinamide N-Methyltransferase (NNMT) Provide Evidence for Target Engagement Challenges in Situ. Bioorg. Med. Chem. Lett. 2018, 28 (16), 2682–2687. 10.1016/j.bmcl.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Bach D.-H.; Kim D.; Bae S. Y.; Kim W. K.; Hong J.-Y.; Lee H.-J.; Rajasekaran N.; Kwon S.; Fan Y.; Luu T.-T.-T.; Shin Y. K.; Lee J.; Lee S. K. Targeting Nicotinamide N-Methyltransferase and MiR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung. Mol. Ther.--Nucleic Acids 2018, 11 (June), 455–467. 10.1016/j.omtn.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babault N.; Allali-Hassani A.; Li F.; Fan J.; Yue A.; Ju K.; Liu F.; Vedadi M.; Liu J.; Jin J. Discovery of Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT). J. Med. Chem. 2018, 61 (4), 1541–1551. 10.1021/acs.jmedchem.7b01422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Policarpo R. L.; Decultot L.; May E.; Kuzmič P.; Carlson S.; Huang D.; Chu V.; Wright B. A.; Dhakshinamoorthy S.; Kannt A.; Rani S.; Dittakavi S.; Panarese J. D.; Gaudet R.; Shair M. D. High-Affinity Alkynyl Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT). J. Med. Chem. 2019, 62 (21), 9837–9873. 10.1021/acs.jmedchem.9b01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.; Li L.; Diaz K.; Iyamu I. D.; Yadav R.; Noinaj N.; Huang R. Novel Propargyl-Linked Bisubstrate Analogues as Tight-Binding Inhibitors for Nicotinamide N -Methyltransferase. J. Med. Chem. 2019, 62 (23), 10783–10797. 10.1021/acs.jmedchem.9b01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren M.; van Ufford L. Q.; Moret E. E.; Martin N. I. Synthesis and Evaluation of Protein Arginine N-Methyltransferase Inhibitors Designed to Simultaneously Occupy Both Substrate Binding Sites. Org. Biomol. Chem. 2015, 13 (2), 549. 10.1039/C4OB01734J. [DOI] [PubMed] [Google Scholar]
- van Haren M. J.; Marechal N.; Troffer-Charlier N.; Cianciulli A.; Sbardella G.; Cavarelli J.; Martin N. I. Transition State Mimics Are Valuable Mechanistic Probes for Structural Studies with the Arginine Methyltransferase CARM1. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (14), 3625–3630. 10.1073/pnas.1618401114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y.; Sartini D.; Pozzi V.; Wilk D.; Emanuelli M.; Yee V. C. Structural Basis of Substrate Recognition in Human Nicotinamide N -Methyltransferase. Biochemistry 2011, 50 (36), 7800–7808. 10.1021/bi2007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Copeland R. A.; Lai Z. Defining Balanced Conditions for Inhibitor Screening Assays That Target Bisubstrate Enzymes. J. Biomol. Screening 2009, 14 (2), 111–120. 10.1177/1087057108328763. [DOI] [PubMed] [Google Scholar]
- Copeland R. A. Enzymes. A Practical Introduction to Structure, Mechanism, and Data Analysis, Second ed. Robert A. Copeland. Anal. Biochem. 2001, 291 (1), 172. 10.1006/abio.2001.5023. [DOI] [Google Scholar]
- Brooun A.; Gajiwala K. S.; Deng Y.-L.; Liu W.; Bolaños B.; Bingham P.; He Y.-A.; Diehl W.; Grable N.; Kung P.-P.; Sutton S.; Maegley K. A.; Yu X.; Stewart A. E. Polycomb Repressive Complex 2 Structure with Inhibitor Reveals a Mechanism of Activation and Drug Resistance. Nat. Commun. 2016, 7 (1), 11384. 10.1038/ncomms11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai K.; Fujii H.; Ikenaka R.; Akagawa M.; Hayashi H. An Acyl-SAM Analog as an Affinity Ligand for Identifying Quorum Sensing Signal Synthases. Chem. Commun. 2014, 50 (62), 8586–8589. 10.1039/C4CC03094J. [DOI] [PubMed] [Google Scholar]
- Hernández J. N.; Ramírez M. A.; Martín V. S. A New Selective Cleavage of N,N-Dicarbamoyl-Protected Amines Using Lithium Bromide. J. Org. Chem. 2003, 68 (3), 743–746. 10.1021/jo026300b. [DOI] [PubMed] [Google Scholar]
- Evans C. G.; Smith M. C.; Carolan J. P.; Gestwicki J. E. Improved Synthesis of 15-Deoxyspergualin Analogs Using the Ugi Multi-Component Reaction. Bioorg. Med. Chem. Lett. 2011, 21, 2587–2590. 10.1016/j.bmcl.2011.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennie K. M.; Banik S. M.; Reichert E. C.; Jacobsen E. N. Catalytic Diastereo- and Enantioselective Fluoroamination of Alkenes. J. Am. Chem. Soc. 2018, 140 (14), 4797–4802. 10.1021/jacs.8b02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. J.; Mekhalfia A.; Jakeman D. L.; Phillips S. E. V.; Phillips K.; Porter J.; Blackburn G. M. Homochiral Synthesis of an Aza Analogue of S-Adenosyl-L-Methionine (AdoMet) and Its Binding to the E. Coli Methionine Repressor Protein (MetJ). Chem. Commun. 1996, 6, 791–792. 10.1039/cc9960000791. [DOI] [Google Scholar]
- Joce C.; Caryl J.; Stockley P. G.; Warriner S.; Nelson A. Identification of Stable S-Adenosylmethionine (SAM) Analogues Derivatised with Bioorthogonal Tags: Effect of Ligands on the Affinity of the E. Coli Methionine Repressor, MetJ, for Its Operator DNA. Org. Biomol. Chem. 2009, 7 (4), 635–638. 10.1039/B816495A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelius J.; Kolmodin K.; Feierberg I.; Åqvist J. Q: A Molecular Dynamics Program for Free Energy Calculations and Empirical Valence Bond Simulations in Biomolecular Systems. J. Mol. Graphics Modell. 1998, 16 (4–6), 213–225. 10.1016/S1093-3263(98)80006-5. [DOI] [PubMed] [Google Scholar]
- Jespers W.; Esguerra M.; Åqvist J.; Gutiérrez-de-Terán H. QligFEP: An Automated Workflow for Small Molecule Free Energy Calculations in Q. J. Cheminf. 2019, 11 (1), 26. 10.1186/s13321-019-0348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G.; Warshel A. A Surface Constrained All-atom Solvent Model for Effective Simulations of Polar Solutions. J. Chem. Phys. 1989, 91 (6), 3647–3661. 10.1063/1.456845. [DOI] [Google Scholar]
- Lee F. S.; Warshel A. A Local Reaction Field Method for Fast Evaluation of Long-range Electrostatic Interactions in Molecular Simulations. J. Chem. Phys. 1992, 97 (5), 3100–3107. 10.1063/1.462997. [DOI] [Google Scholar]
- Ryckaert J.-P.; Ciccotti G.; Berendsen H. J. C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23 (3), 327–341. 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- Robertson M. J.; Tirado-Rives J.; Jorgensen W. L. Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field. J. Chem. Theory Comput. 2015, 11 (7), 3499–3509. 10.1021/acs.jctc.5b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J. L.; Beard H. S.; Cao Y.; Cho A. E.; Damm W.; Farid R.; Felts A. K.; Halgren T. A.; Mainz D. T.; Maple J. R.; Murphy R.; Philipp D. M.; Repasky M. P.; Zhang L. Y.; Berne B. J.; Friesner R. A.; Gallicchio E.; Levy R. M. Integrated Modeling Program, Applied Chemical Theory (IMPACT). J. Comput. Chem. 2005, 26 (16), 1752–1780. 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. H. Efficient Estimation of Free Energy Differences from Monte Carlo Data. J. Comput. Phys. 1976, 22 (2), 245–268. 10.1016/0021-9991(76)90078-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.