Abstract
Age-related macular degeneration (AMD) is a global health problem. Lycium barbarum polysaccharide (LBP), a traditional Chinese herbal medicine, has been proven to be effective against several eye diseases. However, only a few studies have investigated the effectiveness of LBP for AMD. In the present study, the human retinal epithelial cell line, ARPE-19, was pretreated with LBP for 24 h before exposure to H2O2 (500 µM). Cell viability was assessed, and a series of oxidative and antioxidant indicators were evaluated to determine the influence of LBP on H2O2-triggered oxidative stress. The present study also determined the apoptosis status, as well as the expression levels of apoptotic proteins and nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway proteins. The present study aimed to determine the protective role for LBP pretreatment and its underlying molecular mechanism. The results of the present study suggest that pretreatment of ARPE-19 cells with LBP exhibit high efficacy at reducing oxidative damage and inhibiting cell apoptosis. Furthermore, LBP may modulate the expression of proteins involved in the apoptotic pathway and activate the Nrf2 signaling pathway.
Keywords: age-related macular degeneration, oxidative stress, Lycium barbarum polysaccharide, retinal pigment epithelium, nuclear factor erythroid 2-related factor 2/heme oxygenase-1 pathway
Introduction
Patients presenting with age-related macular degeneration (AMD) suffer from progressive visual degeneration due to a damaged macular area (1). It is estimated that ~300 million people will be diagnosed with AMD by 2040 (1). There are two prominent pathological features associated with AMD, the formation and accumulation of drusen, and damage to retinal pigment epithelial (RPE) cells (2). Furthermore, the decrease of neuronal nitric oxide synthase in RPE cell nuclei may be associated with the redox status of the RPE in patients with AMD (3). Therefore, protecting these cells from injury is important to prevent AMD pathology.
As a critical part of the blood-retinal barrier, the RPE plays an essential role in supporting the neural retina and visual cycle, by protecting the fundus tissue from oxidation (4). The RPE cell layer is easily damaged by reactive oxygen species (ROS) compared with other cells, due to the high oxygen consumption of the retina (5,6). In addition, ROS-induced damage to RPE cells is an irreversible process and is an early sign of AMD (7). Thus, therapies against oxidative stress (OxS) should be effective in protecting the RPE and may help prevent the development of AMD.
The antioxidant, nuclear factor erythroid 2-related factor 2 (Nrf2) plays an essential role in the immune defense system (8). For example, in the cytoplasm, Nrf2 combines with the inhibitor epichlorohydrin-related protein 1 (Keap1) like Kelch (9) under physiological conditions. However, when the cell is damaged, Nrf2 disassociates from Keap1 and translocates to the nucleus, triggering the downstream gene expression of heme oxygenase-1 (HO-1) (10). Recent studies have reported that Nrf2 and HO-1 participate in the etiology of AMD (11), and that they are involved in maintaining the dynamic balance of the retina under stress or trauma (12). Therefore, the activation of Nrf2 may represent a potentially useful therapy for the treatment of AMD.
Lycium barbarum polysaccharide (LBP) has been reported to exhibit several biological functions, including immunomodulation, neuroprotection, anti-aging and antioxidative capabilities (13). Furthermore, LBP has been reported to reduce the levels of ROS and the extent of apoptosis in human lens epithelial cells (14). In addition, ischemia-induced retinal damage in diabetic rats is ameliorated by LBP (15). However, the protective effect of LBP on AMD has not yet been studied; thus, the present study aimed to investigate the inhibitory effect of LBP on H2O2-induced OxS and apoptosis in RPE cells, as well as to investigate its effect on the Nrf2/HO-1 pathway, and employed an in vitro AMD model by exposing human retinal epithelial cell lines, ARPE-19, to H2O2, which may help to provide an alternative potential neoteric strategy for AMD therapy.
Materials and methods
Materials and chemicals
LBP (purity >90%; cat. no. SP9311), RIPA lysis buffer (cat. no. R0010) and Annexin V-FITC/PI double staining kit (cat. no. CA1020) were purchased from Beijing Solarbio Science & Technology Co., Ltd. DMEM/F12 medium (cat. no. PM150312) and fetal bovine serum (FBS, cat. no. 164210) were purchased from Procell Life Science & Technology Co., Ltd. Primary antibodies against histone H3 (cat. no. ab1791), Bcl2 (cat. no. ab32124), Caspase-3 (cat. no. ab13585), Cleaved caspase-3 (cat. no. ab214430), Bax (cat. no. ab3191) and β-actin (cat. no. ab6276) were purchased from Abcam (dilutions 1:500 or 1:1,000). Antibodies against Nrf2 (cat. no. 12721) and HO-1 (cat. no. 5853) were purchased from Cell Signaling Technology, Inc. (dilution 1:500). Commercial kits for the detection of 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, cat. no. S0033), malondialdehyde (MDA, cat. no. A003-1-2), superoxide dismutase (SOD, cat. no. A001-1-2), GSH-peroxidase (GSH-Px, cat. no. A005-1-2) and catalase (CAT, cat. no. A007-1-1) were purchased from Nanjing Jiancheng Bioengineering Institute. Horseradish peroxidase secondary antibody (1:500, cat. no. A0216), PBS (cat. no. ST447-5L) and enhanced chemiluminescence (ECL, cat. no. P0018S) reagent were purchased from Beyotime Institute of Biotechnology, and all other chemicals were purchased from Sigma-Aldrich; Merck KGaA.
Cell culture and treatments
ARPE-19 cells (Procell Life Science & Technology Co., Ltd., certified by STR) were maintained in DMEM/F-12 supplemented with 10% FBS, streptomycin (100 mg/ml) and penicillin (100 U/ml), at 37°C with 5% CO2. All treatments were performed when the cells reached ~80% confluence.
Cell viability assay
ARPE-19 cells were seeded into 96-well plates at a density of 1×104 cells/well, with six replicates for each group. Following incubation overnight at 37°C, the cells were incubated with different concentrations of H2O2 (0, 125, 250, 500 and 1,000 µM) for 2 h at 37°C to determine the optimal concentration. ARPE-19 cells were also pretreated with different concentrations of LBP (0, 0.25, 0.5, 1 and 2 mg/ml) for 24 h at 37°C to optimize the dose of LBP to be used in the present study. Pretreatment with LBP was preceded by co-incubation with H2O2 (500 µM) for 2 h at 37°C to assess the protective effect of LBP on H2O2-triggered cell death.
Cell viability was assessed via the Cell Counting Kit-8 (CCK-8, cat. no. HY-K0301; MedChemExpress) assay. Briefly, CCK-8 solution was added into each well and incubated for 2 h at 37°C, in the dark. Cell viability was determined using a microplate reader (BioTek Instruments, Inc.) and calculated as follows: Cell viability (%)=[(absorbance of the test sample-absorbance of the control sample)/mean absorbance of the control] ×100.
Measurement of intracellular ROS
The DCFH-DA method was used to measure ROS levels. Briefly, ARPE-19 cells (1×106 cells/well) were cultured in the presence or absence of different concentrations of LBP in 6-well plates for 24 h at 37°C, prior to treatment with 500 µM H2O2. Cells were subsequently cultured in the presence of DCFH-DA (10 mM) at room temperature in the dark for 20 min. Cells were washed three times with cold PBS and the fluorescence intensity of the harvested cells was determined using a FACSCalibur flow cytometer (Beckman Coulter, Inc.). All experimental results are presented as percentages relative to that of the control sample.
Measurement of MDA, SOD, CAT and GSH-Px
Following the different treatments, ARPE-19 cells (1×106 cells/well) in 1.5 ml Eppendorf tubes (Thermo Fisher Scientific, Inc.) were co-incubated with 100 µl RIPA lysis buffer and 10% protease inhibitor (cat. no. HY-K0010; MedChemExpress) at 4°C for 30 min. Following lysis and centrifugation at 12,000 × g for 15 min at 4°C, the proteins in the lysate were quantified using the BCA kit (cat. no. P0009; Beyotime Institute of Biotechnology). The intracellular activities of MDA and SOD, and levels of CAT and GSH-Px were determined spectrophotometrically using the relevant commercial kits. SOD, CAT and GPX-Px activities are presented as units/mg protein, while MDA levels are presented as nmol/g of protein. All experimental results are presented as percentages of the control value.
Quantification of apoptosis
After collecting cells from different treatment groups, the centrifuged ARPE-19 cells were resuspended in 100 µl binding buffer at a density of 1×106 cells/ml. Subsequently, 5 µl Annexin V-FITC and 5 µl of PI were added and gently mixed into the cell suspension. Following incubation at room temperature for 15 min in the dark, FACSVerse flow cytometer (BD Biosciences) and FACSuite software (version 1.0.4.2650; BD Biosciences) were used for the quantitation of apoptotic cells. The non-apoptotic, early and late apoptotic cells are presented as Annexin−/PI−, Annexin V−FITC+/PI− and Annexin+/PI+ cell populations, respectively.
Western blotting
Following the different treatments, ARPE-19 cells (1×106 cells/well) in 1.5 ml Eppendorf tubes were co-incubated with 100 µl RIPA lysis buffer and 10% protease inhibitor at 4°C for 30 min. Following lysis and centrifugation at 12,000 × g for 15 min at 4°C, the proteins in the lysate were quantified using the BCA kit. Following protein quantitation, 10 or 12% SDS-PAGE was used to resolve the proteins (30 µg/lane), which were transferred onto PVDF membranes (MilliporeSigma) and subsequently blocked with 5% skimmed milk for 2 h at room temperature. The membranes were incubated with primary antibodies overnight at 4°C. After washing three times with PBS, the membranes were incubated with secondary antibodies for 2 h at room temperature. Protein bands were visualized using ECL reagent and analyzed using Image Lab software (version 4.0, Bio-Rad Laboratories, Inc.). β-actin and histone H3 were used as the internal controls.
Small interfering (si)RNA
ARPE-19 cells (1×105 cells/well) were transfected with 100 µM negative control (NC, 5′-CACACTGGATGGCCTAGGAGGATAT-3′) siRNA or 100 µM siRNA Nrf2 (5′-CACACTGGATCAGACAGGAGGATAT-3′) (Shanghai GenePharma Co., Ltd.), using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 12 h of transfection at 37°C, ARPE-19 cells were pretreated with LBP for 24 h and then exposed to 500 µM H2O2 for 2 h. Next, western blotting and the CCK-8 assay were performed to assess the effect of LBP on the protection of ARPE-19 cells, and determine its underlying molecular mechanism.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 8.0 (GraphPad Software, Inc.). All experiments were performed in triplicate and data are presented as the mean ± SEM. Unpaired Student's t-test was used to compare differences between two groups, while one-way ANOVA and Tukey's post hoc test was used to compare differences between multiple groups. P<0.05 was considered to indicate a statistically significant difference.
Results
LBP reduces H2O2-induced cell damage
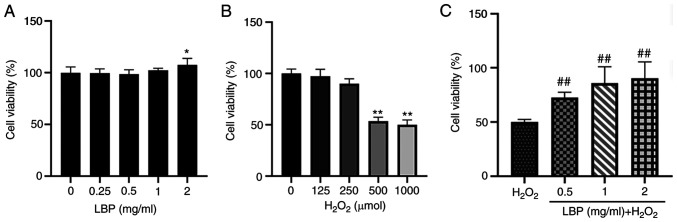
The toxicity of LBP against ARPE-19 cells was assessed. Following 24 h of pretreatment with LBP (0, 0.25, 0.5, 1 or 2 mg/ml), cell viability was assessed via the CCK-8 assay. The results demonstrated that cell viability was retained before and after 24 h of pretreatment, suggesting that the assessed concentrations of LBP were safe and did not affect the cells (Fig. 1A). To evaluate the potential impact of H2O2, ARPE-19 cells were incubated with 0, 125, 250, 500 and 1,000 µM H2O2 for 2 h, and the resulting cell toxicity was assessed. As expected, cell viability significantly decreased following treatment with H2O2 compared with the control group, in a dose-dependent manner (Fig. 1B). Notably, cell viability significantly decreased by 53.6% (P<0.01) at a concentration of 500 µM H2O2. Thus, this concentration was selected for subsequent experimentation. The antioxidant effect of LBP pretreatment was subsequently evaluated. ARPE-19 cells were treated with different concentrations of LBP (0.5, 1 or 2 mg/ml) and 500 µM H2O2, and cell viability was assessed via the CCK-8 assay. The results demonstrated that ARPE-19 cell viability increased up to 90.33% following pretreatment with 2 mg/ml LBP (P<0.001; Fig. 1C). Taken together, these results suggested that 24 h of pretreatment with LBP (0.5–2 mg/ml) effectively reduced the H2O2-induced damage in these cells.
Figure 1.
LBP reduces H2O2-induced cytotoxicity. (A) Effect of LBP on the viability of ARPE-19 cells treated with different concentrations of LBP (0, 0.25, 0.5, 1 and 2 mg/ml) for 24 h. (B) Effect of H2O2 on the viability of ARPE-19 cells. ARPE-19 cells were treated with different concentrations of H2O2 (0, 125, 250, 500 and 1,000 µM) for 2 h. (C) Effect of LBP on H2O2-induced cytotoxicity in ARPE-19 cells. ARPE-19 cells were pretreated with different concentrations of LBP (0.5, 1 and 2 mg/ml) for 24 h followed by 500 µM H2O2 for 2 h. Data are presented as the mean ± SEM (n=3). *P<0.05, **P<0.01 vs. control; ##P<0.001 vs. H2O2-treated cells with no LBP pretreatment. LBP, Lycium barbarum polysaccharide.
LBP ameliorates H2O2-induced OxS
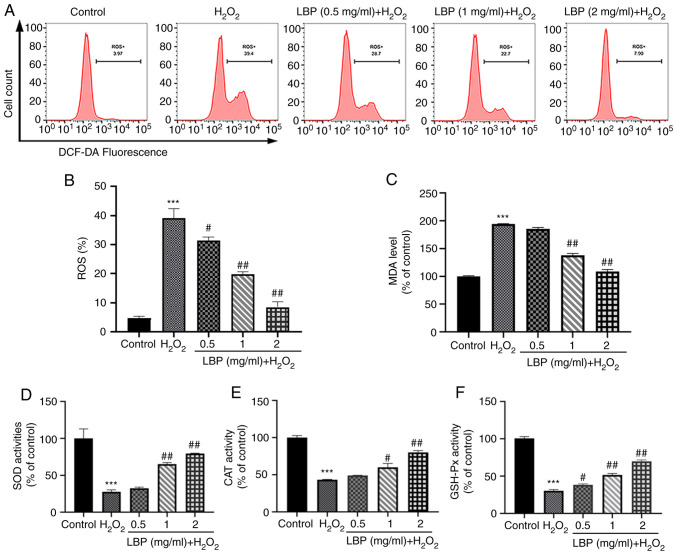
To determine the mechanism by which LBP exerts its cellular protection, indicators of intracellular OxS levels were used along with the evaluation of antioxidant enzyme levels. The DCFH-DA assay was performed to measure ROS levels, as well as the ability of LBP to scavenge H2O2-induced ROS. As presented in Fig. 2A-C, in comparison with the controls, both ROS and MDA levels in ARPE-19 cells significantly increased following treatment with H2O2 (P<0.001). However, pretreatment with LBP significantly decreased both ROS and MDA levels (P<0.01 or P<0.001). These results suggest the critical influence of LBP on the inhibition of H2O2-induced OxS in ARPE-19 cells. In addition, antioxidant stress markers (SOD, CAT and GSH-Px) were monitored both in the presence and absence of LBP and H2O2. As presented in Fig. 2D and E, H2O2 significantly decreased the activities of these antioxidant enzymes (P<0.001), while LBP pretreatment effectively restored the activities of SOD and CAT to normal levels (P<0.01 or P<0.001). Similarly, the GSH-Px ratio significantly enhanced following pretreatment with LBP compared with cells undergoing H2O2 treatment alone (P<0.01 or P<0.001; Fig. 2F), suggesting that LBP pretreatment is a potent inhibitor of OxS damage.
Figure 2.
LBP ameliorates H2O2-induced oxidative stress. (A and B) Effect of LBP on ROS levels in H2O2-treated ARPE-19 cells. (C) Effect of LBP on MDA levels in H2O2-treated ARPE-19 cells. (D) Effect of LBP on SOD activity in H2O2-treated ARPE-19 cells. (E) Effect of LBP on CAT activity in H2O2-treated ARPE-19 cells. (F) Effect of LBP on GSH-Px activity in H2O2-treated ARPE-19 cells. Data are presented as the mean ± SEM (n=3). ***P<0.001 vs. control; #P<0.01, ##P<0.001 vs. H2O2-treated cells with no LBP pretreatment. LBP, Lycium barbarum polysaccharide; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, GSH-peroxidase.
LBP prevents H2O2-induced apoptosis
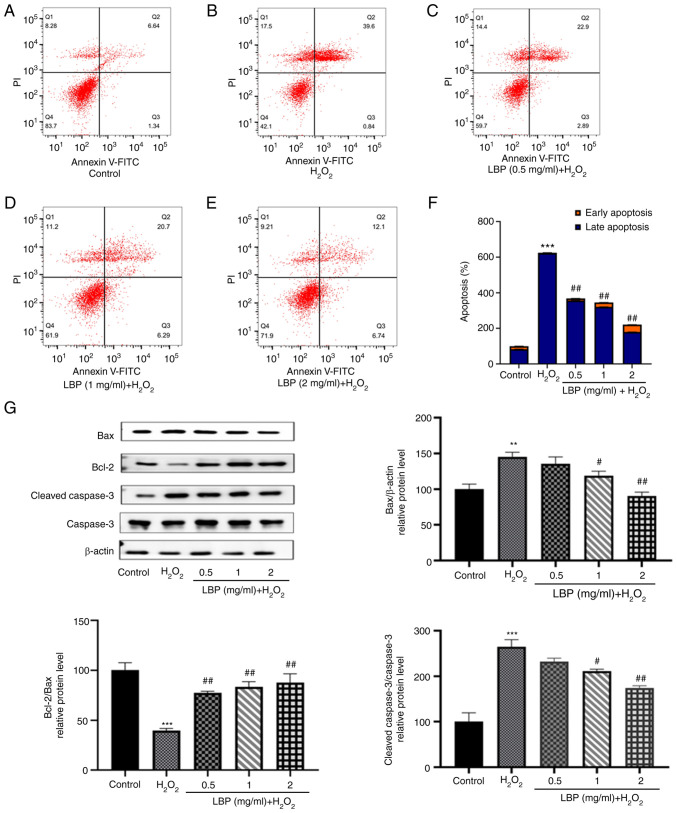
Flow cytometry was performed to determine the extent of apoptosis occurring in ARPE-19 cells under different experimental conditions. As presented in Fig. 3A and B, the extent of apoptosis in the H2O2 group was markedly higher compared with the control group. Notably, H2O2-induced apoptosis in ARPE-19 cells was inhibited following treatment with different concentrations of LBP (Fig. 3C-E). The percentage of apoptotic cells of each experimental group are shown in Fig. 3F, differences were significant (P<0.001). To identify the cause of this anti-apoptotic effect at the protein level, the present study detected the expression levels of apoptosis-related proteins, including pro-apoptotic Bax and cleaved caspase-3 and the anti-apoptotic protein Bcl-2 via western blotting (Fig. 3G). Compared with the controls, cells treated with 500 µM H2O2 exhibited higher levels of Bax and cleaved caspase-3 expression (P<0.01 or P<0.001), which is consistent with the results obtained from flow cytometry. Furthermore, an increased expression of Bcl-2 with a concomitant decrease in Bax and cleaved caspase-3 (P<0.01 or P<0.001) was observed 24 h after pretreatment with LBP, suggesting that LBP exhibits a significant dose-dependent reversal of H2O2-induced apoptosis (Fig. 3G). In addition, the Bcl-2/Bax ratio markedly increased in the LBP pretreatment group (P<0.001), the effects of which were reversed following treatment with H2O2, suggesting its effective protection against H2O2-induced apoptosis in ARPE-19 cells.
Figure 3.
LBP protects cells from H2O2-induced apoptosis. (A-E) Apoptosis was detected via staining with Annexin V-FITC and PI. Flow cytometric analysis of ARPE-19 cells in each group. (F) Quantification of the extent of apoptosis in each group. (G) Western blot analysis was performed to detect the levels of apoptosis-related proteins (cleaved caspase-3, Bax and Bcl-2). Data are presented as the mean ± SEM (n=3). **P<0.01, ***P<0.001 vs. control; #P<0.01, ##P<0.001 vs. H2O2-treated cells with no LBP pretreatment. LBP, Lycium barbarum polysaccharide; PI, propidium iodide.
LBP alleviates H2O2-induced cell damage via the Nrf2/HO-1 pathway
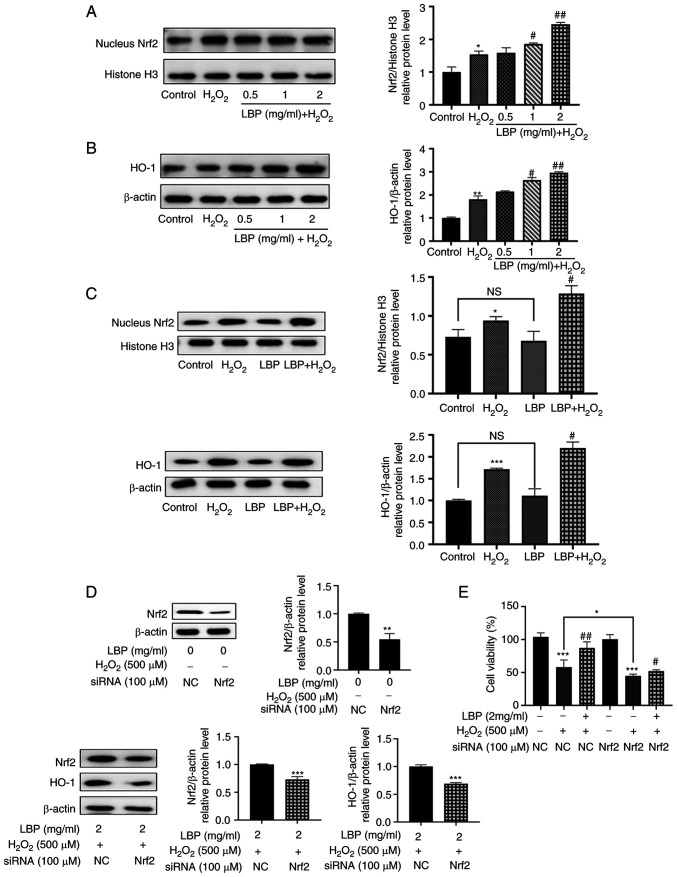
To determine the molecular mechanism involved in this protection against H2O2-induced oxidative damage and apoptosis, a potential signaling effect induced by LBP upon Nrf2/HO-1 was investigated. Western blot analysis demonstrated that treatment with H2O2 increased the nuclear transcriptional expression of Nrf2 protein (P<0.05; Fig. 4A), the main regulator of the cellular antioxidant response (8). Compared with the H2O2 group, pretreatment with LBP increased this nuclear transcriptional expression of Nrf2 protein, in a dose-dependent manner (P<0.01 or P<0.001; Fig. 4A). In addition, the downstream gene, HO-1 also exhibited a similar trend in expression to that of Nrf2 (P<0.01 or P<0.001; Fig. 4B). To further investigate the molecular mechanism of LBP on H2O2-induced ARPE-19 cell damage, treatment with LBP exhibited a negligible influence on the expression of nuclear Nrf2 and HO-1 (Fig. 4C). However, a statistically significant increase was observed in nuclear Nrf2 and HO-1 upon induction of damage by H2O2 (P<0.01; Fig. 4C), suggesting that the combination of LBP and OxS contributed to increasing the nuclear translocation of the Nrf2 protein in a synergistic manner.
Figure 4.
LBP alleviates H2O2-induced RPE cell damage via the Nrf2/HO-1 pathway. ARPE-19 cells were incubated in the presence or absence of LBP for 24 h, and subsequently treated with 500 µM H2O2 for 2 h. (A) The relative protein expression levels of nuclear Nrf2 were determined via western blotting. (B) The relative protein expression levels of HO-1 were determined via western blotting. (C) Western blot analysis was performed to detect the protein expression levels of nuclear Nrf2 and HO-1. (D) ARPE-19 cells were transfected with siRNA (NC or Nrf2) for 12 h. Protein expression levels of Nrf2 were analyzed via western blotting. ARPE-19 cells were transfected with siRNA (NC or Nrf2) for 12 h, incubated with LBP for 24 h and subsequently treated with H2O2 for 2 h. Protein expression levels of Nrf2 and HO-1 were analyzed via western blotting. (E) ARPE-19 cells were transfected with siRNA (NC or Nrf2) for 12 h, incubated in the presence or absence of LBP for 24 h and subsequently treated with H2O2 for 2 h. The cytoprotective effect of LBP was analyzed via the Cell Counting Kit-8 assay. +, presence of H2O2 or LBP; -, absence of H2O2 or LBP. *P<0.05, **P<0.01, ***P<0.001 vs. control; #P<0.01, ##P<0.001 vs. H2O2-treated cells with no LBP pretreatment. LBP, Lycium barbarum polysaccharide; RPE, retinal pigment epithelium; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; si, small interfering; NC, negative control.
To determine the molecular mechanisms involved in this process, siRNA transfection was performed to silence Nrf2 expression. The results demonstrated that Nrf2 protein expression significantly decreased in ARPE-19 cells (P<0.01; Fig. 4D) and LBP-mediated expression of HO-1 was almost eliminated (P<0.001; Fig. 4D). In addition, transfection with Nrf2-siRNA enhanced H2O2-induced cell death, thereby offsetting the protection by LBP (P<0.05; Fig. 4E). Taken together, these results suggest that LBP activates the Nrf2/HO-1 pathway, and thus protects ARPE-19 cells from H2O2-induced cell damage (Fig. 5).
Figure 5.
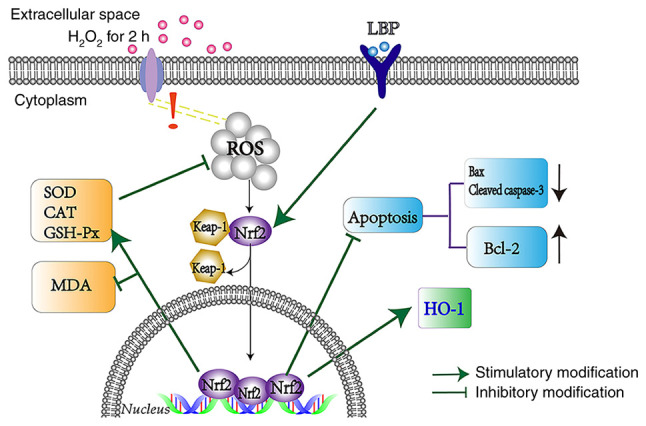
LBP protects ARPE-19 cells against H2O2-induced oxidative stress via the Nrf2/HO-1 pathway. LBP, Lycium barbarum polysaccharide; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; SOD, superoxide dismutase; CAT, catalase; GSH-Px, GSH-peroxidase; MDA, malondialdehyde; ROS, reactive oxygen species.
Discussion
RPE cells are critical for maintaining the structural integrity of the retina (16,17) and are particularly susceptible to the negative effects of OxS, and are generally exposed to high levels of ROS (18) due to the high oxygen demands of the retina. Previous studies (7,19,20) have reported the association between ROS-induced damage in RPE cells and AMD, suggesting that early intervention is important for the prevention of OxS-induced damage. The anti-oxidative and anti-apoptotic functions of LBP have been reported in several eye diseases, including glaucoma (21), retinal ischemia-reperfusion injury (22) and diabetic retinopathy (23). Studies on the chemical composition of LBP have revealed that glycopeptides within its structure can alleviate lipid peroxidation (24–26). Thus, the present study aimed to investigate how LBP prevents OxS and apoptosis in ARPE-19 cells and its potential mechanism of action.
The present study used H2O2 to mimic the pathogenesis of AMD to determine the influence of LBP on OxS (27–29). The results of the CCK-8 assay demonstrated that treatment with 500 µM H2O2 significantly reduced the viability of ARPE-19 cells, the effects of which were reversed following pretreatment with LBP, in a concentration-dependent manner.
It has been reported that H2O2-induced OxS is associated with increased ROS levels, which can be eliminated by enhancing the activity of antioxidant enzymes, thereby reducing the apoptotic state of aging RPE cells (30,31). The present study performed DCFH-DA staining to detect ROS levels, and flow cytometric analysis demonstrated that the fluorescence intensity of ROS in the H2O2 group significantly increased. Conversely, pretreatment with LBP reduced H2O2-triggered ROS enhancement. The level of MDA was also consistent with the level of ROS, and the antioxidant levels in ARPE-19 cells, including SOD, CAT and GSH-Px, were maintained at high levels in response to LBP pretreatment.
Previous studies have demonstrated that the activation of apoptosis triggered by ROS represents a contributing factor for AMD pathogenesis (32,33). The effect of H2O2 exposure resulted in an increase in pro-apoptotic proteins (Bax and cleaved caspase-3), and a decrease in the anti-apoptotic protein, Bcl-2. However, 24 h of LBP pretreatment before H2O2 addition reversed the previously observed phenomenon, as shown by the reduced expression of Bax and cleaved caspase-3 and increased expression of Bcl-2.
Furthermore, Nrf2 is heavily involved in the process of cell redox homeostasis, which serves to reduce OxS by promoting the expression of antioxidant enzymes (34,35). However, few studies have focused on the association between LBP and the Nrf2 pathway during oxidative damage (36,37). It has been reported that once stimulated by OxS, Nrf2 becomes dissociated from Keap1 and translocates to the nucleus where it activates the HO-1 gene (38,39). The results of the present study demonstrated that LBP pretreatment alone did not increase nuclear translocation of Nrf2, and as a result, HO-1 expression was not affected. However, when OxS was induced, LBP increased the nuclear translocation of Nrf2 and HO-1 expression. Thus, H2O2 is essential for Nrf2 translocation and the expression of antioxidant proteins; these results are consistent with previous findings (40). The results of the present study demonstrated that transfection with Nrf2 siRNA partially reversed the protective effect of LBP on H2O2-induced cell death. However, only one retinal cell line was assessed in the present study. Thus, other retinal cell lines and in vivo studies are required to verify the results presented here.
In conclusion, the results of the present study suggest that LBP exerts a protective effect on ARPE-19 cells, particularly by inhibiting H2O2-induced OxS and apoptosis, enhancing antioxidant enzymes and activating the Nrf2/HO-1 pathway. Thus, LBP, a nutritional supplement (41), has the ability to reduce the risk of AMD and OxS-associated retinal disorders.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AMD
age-related macular degeneration
- LBP
Lycium barbarum polysaccharide
- RPE
retinal pigment epithelium
- OxS
oxidative stress
- ROS
reactive oxygen species
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
QZhao and RL designed the present study. RL, MG and QZhu performed the experiments. XH analyzed the data. YW helped perform the analysis with constructive discussions. XH and YW confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2. [DOI] [PubMed] [Google Scholar]
- 2.Yang M, So KF, Lo ACY, Lam WC. The effect of Lycium barbarum polysaccharides on pyroptosis-associated amyloid β1-40 oligomers-induced adult retinal pigment epithelium 19 cell damage. Int J Mol Sci. 2020;21:4658. doi: 10.3390/ijms21134658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhutto IA, Baba T, Merges C, McLeod DS, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD) Exp Eye Res. 2010;90:155–167. doi: 10.1016/j.exer.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RE, Rapp LM, Wiegand RD. Lipid peroxidation and retinal degeneration. Curr Eye Res. 1984;3:223–227. doi: 10.3109/02713688408997203. [DOI] [PubMed] [Google Scholar]
- 6.Catalá A. An overview of lipid peroxidation with emphasis in outer segments of photoreceptors and the chemiluminescence assay. Int J Biochem Cell Biol. 2006;38:1482–1495. doi: 10.1016/j.biocel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/S1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 8.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 10.Shelton LM, Kevin Park B, Copple IM. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013;84:1090–1095. doi: 10.1038/ki.2013.248. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, Chen F, Yan A, Xia X. Madecassoside protects retinal pigment epithelial cells against hydrogen peroxide-induced oxidative stress and apoptosis through the activation of Nrf2/HO-1 pathway. Biosci Rep. 2020;40:BSR20194347. doi: 10.1042/BSR20194347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron BD, Sekhar KR, Ofori M, Freeman ML. The role of Nrf2 in the response to normal tissue radiation injury. Radiat Res. 2018;190:99–106. doi: 10.1667/RR15059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Liu Y, Zhu R, Yu J, Lu W, Pan C, Yao W, Gao X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr Polym. 2016;147:114–124. doi: 10.1016/j.carbpol.2016.03.087. [DOI] [PubMed] [Google Scholar]
- 14.Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G, Wang G, Zhong J. Lycium barbarum polysaccharides protect human lens epithelial cells against oxidative stress-induced apoptosis and senescence. PLoS One. 2014;9:e110275. doi: 10.1371/journal.pone.0110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan H, Shi Z, Yang TG, Yu LM, Xu AL. The protective effects of lycium barbarum polysaccharides on retinal neurons in diabetic rats and its mechanism. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2019;35:55–59. doi: 10.12047/j.cjap.5706.2019.014. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 16.Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:BIO211–BIO226. doi: 10.1167/iovs.17-21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopitz J, Holz FG, Kaemmerer E, Schutt F. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Gaur U, Chong CM, Lin S, Fang J, Zeng Z, Wang H, Zheng W. Berberine protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of AMPK. Int J Mol Sci. 2018;19:1736. doi: 10.3390/ijms19061736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golestaneh N, Chu Y, Cheng SK, Cao H, Poliakov E, Berinstein DM. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J Transl Med. 2016;14:344. doi: 10.1186/s12967-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi XS, Chiu K, Van G, Leung JW, Lo AC, Chung SK, Chang RC, So KF. Effect of Lycium barbarum Polysaccharides on the expression of endothelin-1 and its receptors in an ocular hypertension model of rat glaucoma. Neural Regen Res. 2012;7:645–651. doi: 10.3969/j.issn.1673-5374.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SY, Yang D, Yeung CM, Yu WY, Chang RC, So KF, Wong D, Lo AC. Lycium barbarum polysaccharides reduce neuronal damage, blood-retinal barrier disruption and oxidative stress in retinal ischemia/reperfusion injury. PLoS One. 2011;6:e16380. doi: 10.1371/journal.pone.0016380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Q, Yang Y, Lu X, Zhang Q, Luo M, Li PA, Pan Y. Lycium barbarum polysaccharides improve retinopathy in diabetic sprague-dawley rats. Evid Based Complement Alternat Med. 2018;2018:7943212. doi: 10.1155/2018/7943212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varoni MV, Pasciu V, Gadau SD, Baralla E, Serra E, Palomba D, Demontis MP. Possible antioxidant effect of Lycium barbarum polysaccharides on hepatic cadmium-induced oxidative stress in rats. Environ Sci Pollut Res Int. 2017;24:2946–2955. doi: 10.1007/s11356-016-8050-x. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Li W, Qi D, Wang D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress, and inflammation in pulmonary endothelial cells. Free Radic Res. 2018;52:480–490. doi: 10.1080/10715762.2018.1447105. [DOI] [PubMed] [Google Scholar]
- 26.Schoppet M, Tailhades J, Kulkarni K, Cryle MJ. Precursor manipulation in glycopeptide antibiotic biosynthesis: Are β-amino acids compatible with the oxidative cyclization cascade? J Org Chem. 2018;83:7206–7214. doi: 10.1021/acs.joc.8b00418. [DOI] [PubMed] [Google Scholar]
- 27.Kaczara P, Sarna T, Burke JM. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic Biol Med. 2010;48:1064–1070. doi: 10.1016/j.freeradbiomed.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiger RC, Waters CM, Kamp DW, Glucksberg MR. KGF prevents oxygen-mediated damage in ARPE-19 cells. Invest Ophthalmol Vis Sci. 2005;46:3435–3442. doi: 10.1167/iovs.04-1487. [DOI] [PubMed] [Google Scholar]
- 29.Zareba M, Raciti MW, Henry MM, Sarna T, Burke JM. Oxidative stress in ARPE-19 cultures: Do melanosomes confer cytoprotection? Free Radic Biol Med. 2006;40:87–100. doi: 10.1016/j.freeradbiomed.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhao H, Wang R, Ye M, Zhang L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int J Mol Med. 2019;43:936–944. doi: 10.3892/ijmm.2018.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintea A, Rugină DO, Pop R, Bunea A, Socaciu C. Xanthophylls protect against induced oxidation in cultured human retinal pigment epithelial cells. J Food Compos Anal. 2011;24:830–836. doi: 10.1016/j.jfca.2011.03.007. [DOI] [Google Scholar]
- 32.Musat O, Ochinciuc U, Gutu T, Cristescu TR, Coman C. Pathophysiology and treatment of ARMD. Oftalmologia. 2012;56:45–50. (In Romanian) [PubMed] [Google Scholar]
- 33.Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, Xu GT, Liu F, Zhang J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxid Med Cell Longev. 2020;2020:1740943. doi: 10.1155/2020/1740943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai E, Shimada-Sugawara M, Yamaguchi Y, Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K, Tsukuba T. Fisetin inhibits osteoclastogenesis through prevention of RANKL-induced ROS production by Nrf2-mediated up-regulation of phase II antioxidant enzymes. J Pharmacol Sci. 2013;121:288–298. doi: 10.1254/jphs.12243FP. [DOI] [PubMed] [Google Scholar]
- 35.Jiang P, Chen L, Sun J, Li J, Xu J, Liu W, Feng F, Qu W. Chotosan ameliorates cognitive impairment and hippocampus neuronal loss in experimental vascular dementia via activating the Nrf2-mediated antioxidant pathway. J Pharmacol Sci. 2019;139:105–111. doi: 10.1016/j.jphs.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Xiong GF, Li DW, Zheng MB, Liu SC. The effects of Lycium barbarum polysaccharide (LBP) in a mouse model of cerulein-induced acute pancreatitis. Med Sci Monit. 2019;25:3880–3886. doi: 10.12659/MSM.913820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Zhou F, Shen C, Wang H, Xiao Y. LBP reduces theinflammatory injuryof kidney in septic rat and regulates the Keap1-Nrf2/ARE signaling pathway1. Acta Cir Bras. 2019;34:e20190010000003. doi: 10.1590/s0102-865020190010000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen B, Lu Y, Chen Y, Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 39.Hiramatsu K, Tsuneyoshi T, Ogawa T, Morihara N. Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells. Nutr Res. 2016;36:143–149. doi: 10.1016/j.nutres.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Hao Y, Liu J, Wang Z, Yu LL, Wang J. Piceatannol protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis through modulating PI3K/Akt signaling pathway. Nutrients. 2019;11:1515. doi: 10.3390/nu11071515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao S, Du J, Hei Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp Ther Med. 2017;14:4919–4927. doi: 10.3892/etm.2017.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.