Abstract
The paramount importance of synthetic organic chemistry in the pharmaceutical industry arises from the necessity to physically prepare all designed molecules to obtain key data to feed the design–synthesis–data cycle, with the medicinal chemist at the center of this cycle. Synthesis specialists accelerate the cycle of medicinal chemistry innovation by rapidly identifying and executing impactful synthetic methods and strategies to accomplish project goals, addressing the synthetic accessibility bottleneck that often plagues discovery efforts. At AbbVie, Discovery Synthesis Groups (DSGs) such as Centralized Organic Synthesis (COS) have been deployed as embedded members of medicinal chemistry teams, filling the gap between discovery and process chemistry. COS chemists provide synthetic tools, scaffolds, and lead compounds to fuel the pipeline. Examples of project contributions from neuroscience, cystic fibrosis, and virology illustrate the impact of the DSG approach. In the first ten years of innovative science in pursuit of excellence in synthesis, several advanced drug candidates, including ABBV-2222 (galicaftor) for cystic fibrosis and foslevodopa/foscarbidopa for Parkinson’s disease, have emerged with key contributions from COS.
Keywords: Centralized Organic Synthesis, Pharmaceutical, Process chemistry, Discovery Synthesis Group
In recent years, organic synthesis at AbbVie and the pharmaceutical industry at large has experienced a renaissance.1,2 In stark contrast to past industry trends toward downsizing and outsourcing,3−6 we and others continue to embrace the critical role of internal innovation, technology development, and expertise within the “synthesis” component of the design–synthesis–data cycle. The ability to rapidly prepare any molecule to effectively probe a scientific hypothesis is central to all aspects of Medicinal Chemistry, bridging the gap between structure and function to profoundly impact science and society.7 New synthesis methods and strategies also significantly influence the design of new molecules. Strong perspectives from diverse companies have unified around the theme of organic synthesis as a transformative discipline in need of increased emphasis and investment for drug discovery to continue to positively impact human health,1,2 and a variety of specialist synthesis and technology groups have been deployed to this end across the industry. We also fully embrace synthesis as a key driver of innovation and agility in drug discovery with a full appreciation for the necessity of continually expanding the Medicinal Chemistry toolbox8 to more effectively navigate the vast realms of chemical space9 through the pursuit of excellence in synthesis. Fundamentally, enhancing synthesis capabilities allows AbbVie to accelerate the design–synthesis–data cycle by making the best molecules faster.
Recently approved drugs from AbbVie exemplify this philosophy (Figure 1). All in the far-beyond-rule-of-five chemical space,10 rich with heterocycles, and with stereochemical complexity rivaling challenging natural products, Venetoclax,11 Pibrentasvir,12 and Glecaprevir13 represent the crown jewels of AbbVie’s synthesis-embracing culture. Since chemists were undaunted by the complexity of the many molecules that had to be made to discover and develop these medicines, patients now enjoy access to these best-in-class therapies for cancer and HCV. This historic excellence in synthesis philosophy must continue and evolve as we hunt for the next wave of novel therapeutics.
Figure 1.

Examples of structurally complex recently approved drugs from AbbVie.
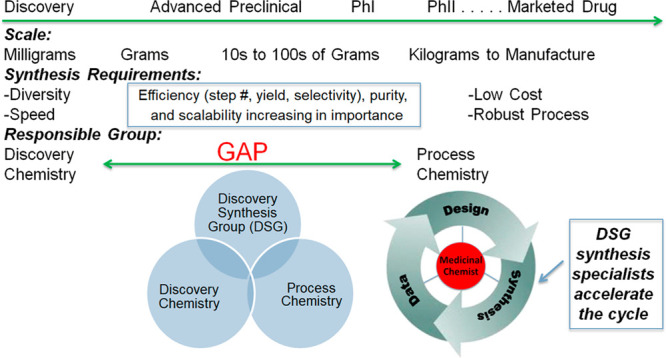
In recognition of the centrality of synthesis, in 2012, we began and quickly expanded upon the concept of dedicated Discovery Synthesis Groups (DSGs) across the organization (Figure 2). At this time, a gap was recognized between discovery and process chemistry roles. In this space, there is a rapidly evolving need for new synthesis methods and scaffolds for advanced lead optimization along with identification of more scalable routes for rapid transition to early process research. Therefore, we devised a new model to bridge this gap with DSG chemists, overlapping with both discovery and process chemistry, to bring these functions closer together and ideally shorten the time required between project launch and new drug identification. For example, chemists in the Centralized Organic Synthesis (COS) group in Centralized Medicinal Chemistry (CMeC) serve as embedded members of Discovery teams who specialize in solving synthesis problems to accomplish team goals. COS discovers and applies new synthetic methods and strategies, uses these to create key intermediates and lead molecules, and seeks to smooth project transitions to Process Chemistry. DSGs have also been formed within other departments at AbbVie. In contrast to alternative models, AbbVie made a conscious decision to keep DSGs within their respective Discovery organizations to align reporting structures with project assignments. This allows each DSG to focus on the science rather than resource allocation/prioritization. Members of each DSG, together with chemists from our technology and Process Chemistry groups, serve on the Excellence in Synthesis (EiS) committee, championing internal and external initiatives to influence all chemists at AbbVie toward excellence in synthesis and to engage with the external synthesis community, where the vast majority of synthetic organic chemistry research occurs.
Figure 2.
Models of chemistry strategy.
As we approach the 10 year anniversary of AbbVie’s DSG experiment, assessing pipeline impact is crucial as a measure of success. Many complex factors have contributed to encouraging recent trends in pharmaceutical R&D productivity in a challenging industry.14,15 Fueling the pipeline with high-quality drug candidates across a diverse portfolio of therapeutic modalities is an important component of this improvement in productivity. Since its inception, COS has contributed to more than 30 clinical candidates across the AbbVie portfolio. Here, we describe key contributions to several projects in cystic fibrosis, neuroscience, and virology to demonstrate pipeline impact via innovations in organic synthesis.
With a functional role lying somewhere between that of a traditional process and medicinal chemist, members of DSGs at AbbVie help accelerate medicinal chemistry programs by effectively viewing synthetic challenges through the eyes of both. Frequent communication with medicinal chemistry teams, particularly in the early stages of a Discovery campaign, helps embedded members to identify key chemistry challenges with the highest potential for broad impact. By enabling new SAR directions through identification of methods suitable for rapid analog synthesis or scaling up novel building blocks for late-stage functionalization, embedded synthesis specialists can help accelerate the lead optimization process. As Discovery campaigns progress and potential candidate lists narrow, DSG members shift to a role more aligned with that of a process chemist, with an eye on optimization of individual steps and the overall scalability of a route. Ideally, early embedded synthesis support can help craft the synthetic routes initially used to access chemical matter, thereby shortening the time required to effectively scale up an API prior to its advancement into development. Soon after its inception at AbbVie, the COS group initiated a collaboration with our medicinal chemistry team targeting cystic fibrosis, and the engagement between these two groups illustrates the most common role of an embedded COS chemist.
Cystic fibrosis is a life-shortening autosomal recessive disorder which continues to present an unmet clinical need. With multiorgan effects caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, treatment of the disease presents a particular challenge. Numerous recent advances have shown the dramatic impact that can be made to the lives of the nearly 75,000 worldwide afflicted with this devastating disorder through even slight restoration of lung function.16,17 The COS group has been deeply engaged in advancing the CFTR program at AbbVie since 2014. Following the group model of having embedded chemists/synthesis leads within medicinal chemistry projects, there have been opportunities for broad impact across multiple chemical series (potentiator, C1-corrector, C2-corrector), including work leading to several clinical candidates and publications.18−20,23−25 On the C1-corrector program,18 COS efforts initially focused on SAR-enabling asymmetric syntheses of diverse chromanamines through two highly diastereoselective steps (Stoltz-Hayashi addition and oxime reduction). Unselective early routes to these types of compounds hindered the ability of the medicinal chemistry team to focus on improving their biological properties, and the development of an asymmetric route by COS enabled rapid optimization and subsequent scaleup, culminating in the development of an enabling synthetic route to ABBV-2222 (galicaftor, Figure 3).19 A key phenol demethylation/difluoromethylation strategy accommodated a late-stage request for target substitution by the project team and enabled delivery of >130 g of API to support advanced preclinical studies. ABBV-2222 is currently in Phase II clinical trials as a C1-corrector for the treatment of Cystic Fibrosis.
Figure 3.

Enabling asymmetric synthesis of ABBV-2222 (galicaftor).
Interest in the cyclopropane carboxylate structure contained in ABBV-2222 led to the discovery of a general and highly efficient Reformatsky Negishi coupling reaction to generate these in a single step (Figure 4). This method was amenable to a wide variety of functionalized aromatic, vinyl, and heteroaromatic halides.20 The coupling reaction also offered significant improvements to the extant syntheses of multiple known compounds.21,22 The ability to rapidly synthesize fragments of this class via cross-coupling has proven beneficial in a variety of subsequent medicinal chemistry campaigns at AbbVie: a search of AbbVie notebooks indicated its use in >600 experiments to date across many diverse projects, highlighting the broad impact of this method.
Figure 4.
Reformatsky Negishi methodology.
COS involvement in the CFTR program continued with the C2-corrector project, which required the development of an efficient asymmetric [3 + 2]-cycloaddition reaction to enable access to N- and O-linked pyrrolidine analogues via the nitro functionality (Figure 5). Early synthetic routes to this complex chemical matter relied on late-stage separation of stereoisomers via chiral SFC, which significantly hindered the rapid synthesis of analogues. By identifying an asymmetric method to afford densely functionalized pyrrolidines at an early stage in the program, COS support helped enable the rapid synthesis of >1600 structurally complex analogues by obviating the need for isomer separation in a series containing 4 contiguous stereocenters.23 The remarkably broad substrate scope in the cycloaddition highlights the application of this method to the synthesis of a variety of tetrasubstituted pyrrolidines which were well-suited for multivector optimization (Figure 5).24,26 Additional route development by COS helped to deliver advanced intermediates suitable for library synthesis of analogues by the medicinal chemistry team, an example of DSG support driving SAR studies.
Figure 5.
Asymmetric [3 + 2] cycloaddition for the CFTR C2-corrector project.
Collaboration with AbbVie’s Process Chemistry Discovery Support Group was critical to delivering over 300 g of ABBV-3221 to support advanced preclinical studies with a COS group member serving as a member of the API team (Figure 6). The cycloaddition method identified by COS was scaled successfully to 900 g in the first campaign and subsequently to multikilogram scale by Process Chemistry.25 A rapid delivery of a dog dose range finding (DRF) lot in <3 months was facilitated by the early involvement of COS in designing the synthetic routes to the chiral pyrrolidine fragments. The collaborative efforts described here culminated in the selection of ABBV-3221 as a C2-corrector clinical candidate.
Figure 6.

Enabling synthesis of ABBV-3221.
As embedded members of CFTR medicinal chemistry teams and collaborators with Process Chemistry, COS has contributed to numerous successful campaigns since the program began in 2014. Various complex and richly functionalized chemical series have offered frequent opportunities for innovative synthesis solutions. In addition to helping to enable the selection of several clinical candidates, the methodologies and enabling synthetic routes discovered throughout these efforts have led to numerous publications and serve to highlight the key roles DSGs can play in accelerating drug discovery efforts. Industry averages for Discovery campaigns typically range from 4 to 5 years from project launch to candidate selection,14 and organic synthesis is often the rate-limiting factor in lead optimization.2 By investing in synthesis at an early stage in a Discovery campaign, however, the drug discovery timeline can be significantly shortened. Notably, the discovery phases of the CFTR projects described here were both completed in under 2 years from project initiation to candidate selection, a greater than 2-fold acceleration made possible in part by strategic investment in synthesis support.
Among neurological disorders, Parkinson’s disease (PD) affects the most rapidly increasing number of patients with movement problems including tremor, bradykinesia, and rigidity.27 Due to the lack of disease-modifying agents, dopamine replacement therapy remains the most effective method to treat PD.28 This is often achieved through administration of levodopa (LD), a dopamine prodrug, together with carbidopa (CD), a peripheral dopamine decarboxylase inhibitor. Fluctuating drug levels resulting from oral dosing become less effective as PD progresses, so LD/CD intestinal gel (Duodopa) was developed to sustain optimal DA levels.29 Unfortunately, the poor solubility of LD/CD leads to a large dose volume, requiring surgery for Duodopa administration. We sought a new treatment for PD via a minimally invasive and convenient mode of delivery, culminating in the discovery of Foslevodopa/Foscarbidopa (FLD/FCD).30
Unlike a traditional medicinal chemistry program where thousands of molecules must be prepared to find the optimal drug, finding an LD and CD compound with improved solubility had its own challenges. Our investigations found that a phosphate prodrug on one or both phenols of LD and CD could sufficiently improve aqueous solubility to enable subcutaneous dosing of a highly concentrated aqueous solution to treat PD (Figure 7).31 However, despite >50 years of patient experience with LD, no selective LD monophosphate synthesis had been reported, and there was no precedent for CD phosphates, so this was seen as an opportunity for synthesis innovation.
Figure 7.

Levodopa, carbidopa, and their phosphate prodrugs.
Following the initial syntheses of LD/CD phosphates by nonselective routes to access monophosphate and diphosphate isomers on a small scale for preliminary studies,32 we sought a regioselective route to fuel advanced preclinical studies (Figure 8). To selectively prepare FLD,32 a mild Negishi cross-coupling was employed, which tolerated a readily available free phenol.33 Efficient phosphate formation with an unprecedented tetrabenzylpyrophosphate/DBU combination, which was also utilized for diphosphate syntheses, gave an intermediate that was converted to FLD on decagram scale upon benzyl hydrogenolysis. For FCD, a nonselective phosphorylation of CD was initially achieved in a low 27% yield following prep HPLC due to stability challenges, giving a stable crystalline trihydrate form upon concentration of HPLC fractions for preclinical assessment.32 This solid form isolation was a key step forward in building confidence to pursue alternative routes to FCD. Regioselective larger-scale routes to both FLD and FCD were subsequently developed in collaboration with process chemistry.34
Figure 8.
Selective FLD synthesis and stable crystalline form of FCD.
With FLD/FCD in hand, we assessed their stability, solubility, and effectiveness in continuous SC dosing studies both preclinically and in PhI clinical trials.30 We were surprised to find excellent stability (<2% change for >1 year) at high concentration (e/g/, 240/12 and 360/18 mg/mL) across a wide pH range (6.5–9.2) with excellent PK and tolerability in preclinical species. In PhI clinical trials, administration of a concentrated FLD/FCD solution continuously over 72 h achieved comparable levels to those of oral 4:1 LD/CD, supporting further clinical assessment. FLD/FCD is currently in PhIII clinical trials for the treatment of advanced PD. The first selective preparation of FLD and first preparation of FCD made this new treatment a possibility for the millions suffering from PD.
Infection with the hepatitis C virus (HCV) is a major global public health concern, with associated chronic liver disease often developing asymptomatically over many years which can eventually progress to serious conditions such as cirrhosis and liver cancer.35 Mavyret, a combination of pibrentasvir and glecaprevir (Figure 1), is the only 8-week pan-genotype cure for patients suffering from HCV and is currently the most prescribed HCV medicine.36 The discovery of NS5A inhibitor pibrentasvir was a remarkable achievement from AbbVie chemistry.12 Due to the challenging physicochemical properties of this beyond-rule-of-five molecule,10 enabling formulations were required for improving bioavailability, so a solubility-enhancing prodrug strategy was pursued,20 resulting in the identification of three promising prodrug candidates with critical support from embedded COS group members (Figure 9).37
Figure 9.

Pibrentasvir, challenging features, structures of lead prodrug candidates, and comparison of original route to optimized route via a key desymmetrized intermediate.
In contrast to earlier prodrug projects involving highly water-soluble low-MW molecules (e.g., FLD/FCD, Figure 2), the uniquely challenging structural features presented by pibrentasvir, including high MW, six heterocycles, and a C2-symmetric structure with homotopic benzimidazoles, made our original nonselective alkylation route particularly inefficient (6–11% yield, Figure 9).37 Considering the high value of pibrentasvir, demonstration of a route with ∼50% yield was deemed a requirement for further development of these candidate compounds.37 As another opportunity for synthesis innovation, we applied the Horeau principle of statistical amplification,38 coupled with solubility-driven selective transformations,39 to achieve a remarkably efficient desymmetrization of pibrentasvir.40 The two-pot desymmetrization sequence used only Boc2O, DMAP, n-BuNH2, and aqeuous formaldehyde as reagents to prepare a key intermediate (Figure 9) from which all three prodrugs could be prepared in good overall yield (44–56% from pibrentasvir).40 Despite no precedent for C2-desymmetrization without a steric proximity or internal functionalization opportunity,41 our approach to dibenzimidazole desymmetrization allowed these PIB-prodrug candidates to be advanced.
Specialized DSGs at AbbVie, such as COS, accelerate the design–synthesis–data cycle by enabling and executing efficient syntheses of important molecules as embedded members of discovery teams and key collaborators with process chemistry to fuel the pipeline via innovations in organic synthesis. Examples from three therapeutic areas (cystic fibrosis, neuroscience, and virology) demonstrate diverse contributions to projects. These range from the synthesis of phosphate prodrugs for discovery of FLD/FCD for the treatment of PD to enabling the preparation of >1600 pyrrolidines with four contiguous stereocenters via expansion of asymmetric [3 + 2] methodology to facilitate the discovery and scale-up of ABBV-3221 for CF. Notably, attention to physical properties of molecules, such as isolation of a crystalline form of FCD and exploiting isomer solubility differences to enable desymmetrization of pibrentasvir, is a common feature of COS project contributions. While only a very limited number of projects are discussed here, the cystic fibrosis section illustrates the most common mode of COS chemist embedded, collaborative interactions with medicinal chemistry teams. This facilitates communication, smooths frequent and inevitable changes of priority, allows straightforward exchange of materials, and provides rapid hands-on assistance with scale-up when needed. Close collaboration with process chemistry has also been described, with COS members serving as temporary members of process teams at times and acting as ongoing internal consultants when past chemistry expertise provides value. Measurable pipeline impact over time is considered the most significant measure of new model success, with each project described here completed in <2 years vs the industry average of 4–5 years from project initiation to candidate selection,14 in part due to an enhanced focus on synthesis. Beyond the first productive decade of AbbVie’s DSG experiment, we anticipate additional exciting opportunities for DSGs and continued emphasis on excellence in synthesis within AbbVie medicinal chemistry.
Acknowledgments
The authors acknowledge AbbVie project team chemists and COS group members for their contributions to the projects described. All authors are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare the following competing financial interest(s): All authors are employees of AbbVie. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
References
- Campos K. R.; Coleman P. J.; Alvarez J. C.; Dreher S. D.; Garbaccio R. M.; Terrett N. K.; Tillyer R. D.; Truppo M. D.; Parmee E. R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363 (6424), 1–8. 10.1126/science.aat0805. [DOI] [PubMed] [Google Scholar]
- Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Woode A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- McMeekin P.; Lendrem D. W.; Lendrem B. C.; Pratt A. G.; Peck R.; Isaacs J. D.; Jones D. Schrödinger’s pipeline and the outsourcing of pharmaceutical innovation. Drug Discovery Today 2020, 25, 480–484. 10.1016/j.drudis.2019.11.015. [DOI] [PubMed] [Google Scholar]
- Ball P. Chemistry: Why synthesize?. Nature 2015, 528, 327–329. 10.1038/528327a. [DOI] [PubMed] [Google Scholar]
- Whitesides G. M. Reinventing chemistry. Angew. Chem., Int. Ed. 2015, 54, 3196–3209. 10.1002/anie.201410884. [DOI] [PubMed] [Google Scholar]
- Laird T. Is there a Future for Organic Chemists in the Pharmaceutical Industry outside China and India?. Org. Process Res. Dev. 2010, 14, 749. 10.1021/op1001676. [DOI] [Google Scholar]
- Nicolaou K. C. Catalyst: Synthetic Organic Chemistry as a Force for Good. Chem. 2016, 1, 331–334. 10.1016/j.chempr.2016.08.006. [DOI] [Google Scholar]
- Bostrom J.; Brown D. G.; Young R. J.; Keseru G. M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discovery 2018, 17, 709–727. 10.1038/nrd.2018.116. [DOI] [PubMed] [Google Scholar]
- a Lipinski C.; Hopkins A. Navigating chemical space for biology and medicine. Nature 2004, 432, 855–861. 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]; b Walters W. P. Virtual Chemical Libraries. J. Med. Chem. 2019, 62, 1116–1124. 10.1021/acs.jmedchem.8b01048. [DOI] [PubMed] [Google Scholar]
- DeGoey D. A.; Chen H.-J.; Cox P. B.; Wendt M. D. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J. Med. Chem. 2018, 61, 2636–2651. 10.1021/acs.jmedchem.7b00717. [DOI] [PubMed] [Google Scholar]
- Souers A. J.; Leverson J. D.; Boghaert E. R.; Ackler S. L.; Catron N. D.; Chen J.; Dayton B. D.; Ding H.; Enschede S. H.; Fairbrother W. J.; Huang D. C. S.; Hymowitz S. G.; Jin S.; Khaw S. L.; Kovar P. J.; Lam L. T.; Lee J.; Maecker H. L.; Marsh K. C.; Mason K. D.; Mitten M. J.; Nimmer P. M.; Oleksijew A.; Park C. H.; Park C.-M.; Phillips D. C.; Roberts A. W.; Sampath D.; Seymour J. F.; Smith M. L.; Sullivan G. M.; Tahir S. K.; Tse C.; Wendt M. D.; Xiao Y.; Xue J. C.; Zhang H.; Humerickhouse R. A.; Rosenberg S. H.; Elmore S. W. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Wagner R.; Randolph J. T.; Patel S. V.; Nelson L.; Matulenko M. A.; Keddy R.; Pratt J. K.; Liu D.; Krueger A. C.; Donner P. L.; Hutchinson D. K.; Flentge C.; Betebenner D.; Rockway T.; Maring C. J.; Ng T. I.; Krishnan P.; Pilot-Matias T.; Collins C.; Panchal N.; Reisch T.; Dekhtyar T.; Mondal R.; Stolarik D. F.; Gao Y.; Gao W.; Beno D. A.; Kati W. M. Highlights of the Structure-Activity Relationships of Benzimidazole Linked Pyrrolidines Leading to the Discovery of the Hepatitis C Virus NS5A Inhibitor Pibrentasvir (ABT-530). J. Med. Chem. 2018, 61, 4052–4066. 10.1021/acs.jmedchem.8b00082. [DOI] [PubMed] [Google Scholar]
- Wang G.; Ma J.; Jiang L.-J.; Gai Y.; Long J.; Wang B.; McDaniel K. F; Or Y. S.. Discovery and Development of the Next-Generation HCV NS3 Protease Inhibitor Glecaprevir. In HCV: The Journey from Discovery to a Cure. Topics in Medicinal Chemistry, Sofia M., Ed.; Springer: Cham, 2019; Vol 31. [Google Scholar]
- Paul S.; Mytelka D.; Dunwiddie C.; Persinger C. C.; Munos B. H.; Lindborg S. R.; Schacht A. L. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discovery 2010, 9, 203–214. 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Pammolli F.; Righetto L.; Abrignani S.; Pani L.; Pelicci P. G.; Rabosio E. The endless frontier? The recent increase of R&D productivity in pharmaceuticals. J. Transl. Med. 2020, 18, 162–175. 10.1186/s12967-020-02313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin E.; Pastor A.; Semeraro M.; Golec A.; Hayes K.; Chevalier B.; Berhal F.; Prestat G.; Hinzpeter A.; Gravier-Pelletier C.; Pranke I.; Sermet-Gaudelus I. Modulators of CFTR. Updates on clinical development and future directions. Eur. J. Med. Chem. 2021, 213, 113195. 10.1016/j.ejmech.2021.113195. [DOI] [PubMed] [Google Scholar]
- Laselva O.; Bartlett C.; Popa A.; Ouyang H.; Gunawardena T. N. A.; Gonska T.; Moraes T. J.; Bear C. E. Emerging preclinical modulators developed for F508del-CFTR have the potential to be effective for ORKAMBI resistant processing mutants. J. Cystic Fibrosis 2021, 20, 106–119. 10.1016/j.jcf.2020.07.015. [DOI] [PubMed] [Google Scholar]
- Wang X.; Liu B.; Searle X.; Yeung C.; Bogdan A.; Greszler S.; Singh A.; Fan Y.; Swensen A. M.; Vortherms T.; Balut C.; Jia Y.; Desino K.; Gao W.; Yong H.; Tse C.; Kym P. Discovery of 4-[(2R,4R)-4-({[1-(2,2-Difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-7-(difluoromethoxy)-3,4-dihydro-2H-chromen-2-yl]benzoic Acid (ABBV/GLPG-2222), a Potent Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Corrector for the Treatment of Cystic Fibrosis. J. Med. Chem. 2018, 61, 1436–1449. 10.1021/acs.jmedchem.7b01339. [DOI] [PubMed] [Google Scholar]
- Greszler S. N.; Shelat B.; Voight E. A. Enabling Synthesis of ABBV-2222, A CFTR Corrector for the Treatment of Cystic Fibrosis. Org. Lett. 2019, 21, 5725–5727. 10.1021/acs.orglett.9b02099. [DOI] [PubMed] [Google Scholar]
- Greszler S. N.; Halvorsen G. T.; Voight E. A. Synthesis of Substituted Cyclopropanecarboxylates via Room Temperature Palladium-Catalyzed α-Arylation of Reformatsky Reagents. Org. Lett. 2017, 19, 2490–2493. 10.1021/acs.orglett.7b00707. [DOI] [PubMed] [Google Scholar]
- Sabbatini F. M.; Fabio R. D.; Griffante C.; Pentassuglia G.; Corsi M. Bioorg. Med. Chem. Lett. 2010, 20, 623–627. 10.1016/j.bmcl.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Molinaro C.; Gauvreau D.; Hughes G.; Lau S.; Lauzon S.; Angelaud R.; O’Shea P. D.; Janey J.; Palucki M.; Hoermer S. R.; Raab C. E.; Sidler R. R.; Belley M.; Han Y. J. Org. Chem. 2009, 74, 6863–6866. 10.1021/jo901267x. [DOI] [PubMed] [Google Scholar]
- Scanio M. J. C.; Searle X. B.; Liu B.; Koenig J. R.; Altenbach R.; Gfesser G. A.; Bogdan A.; Greszler S.; Zhao G.; Singh A.; Fan Y.; Swensen A. M.; Vortherms T.; Manelli A.; Balut C.; Jia Y.; Gao W.; Yong H.; Schrimpf M.; Tse C.; Kym P.; Wang X. Discovery of ABBV/GLPG-3221, a Potent Corrector of CFTR for the Treatment of Cystic Fibrosis. ACS Med. Chem. Lett. 2019, 10, 1543–1548. 10.1021/acsmedchemlett.9b00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greszler S. N.; Zhao G.; Buchman M.; Searle X. B.; Liu B.; Voight E. A. General Asymmetric Synthesis of Densely Functionalized Pyrrolidines via Endo-Selective [3 + 2] Cycloaddition of β-Quaternary-Substituted Nitroalkenes and Azomethine Ylides. J. Org. Chem. 2020, 85, 7620–7632. 10.1021/acs.joc.0c00820. [DOI] [PubMed] [Google Scholar]
- Hartung J.; Greszler S. N.; Klix R. C.; Kallemeyn J. M. Development of an Enantioselective [3 + 2] Cycloaddition To Synthesize the Pyrrolidine Core of ABBV-3221 on Multikilogram Scale. Org. Process Res. Dev. 2019, 23, 2532–2537. 10.1021/acs.oprd.9b00292. [DOI] [Google Scholar]
- Garner P.; Cox P. B.; Rathnayake U.; Holloran N.; Erdman P. Design and Synthesis of Pyrrolidine-based Fragments That Sample Three-dimensional Molecular Space. ACS Med. Chem. Lett. 2019, 10, 811–815. 10.1021/acsmedchemlett.9b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey E. R.; Elbaz A.; Nichols E.; Abd-Allah F.; Abdelalim A.; Adsuar J. C.; Ansha M. G.; Brayne C.; Choi J. J.; Collado-Mateo D.; Dahodwala N.; Do H. P.; Edessa D.; Endres M.; Fereshtehnejad S.; Foreman K. J.; Gankpe F. G.; Gupta R.; Hankey G. J.; Hay S. I.; Hegazy M. I.; Hibstu D. T.; Kasaeian A.; Khader Y.; Khalil I.; Khang Y.; Kim Y. J.; Kokubo Y.; Logroscino G.; Massano J.; Ibrahim N. M.; Mohammed M. A.; Mohammadi A.; Moradi-Lakeh M.; Naghavi M.; Nguyen B. T.; Nirayo Y. L.; Ogbo F. A.; Owolabi M. O.; Pereira D. M.; Postma M. J.; Qorbani M.; Rahman M. A.; Roba K. T.; Safari H.; Safiri S.; Satpathy M.; Sawhney M.; Shafieesabet A.; Shiferaw M. S.; Smith M.; Szoeke C. E. E.; Tabarés-Seisdedos R.; Truong N. T.; Ukwaja K. N.; Venketasubramanian N.; Villafaina S.; Weldegwergs K. G.; Westerman R.; Wijeratne T.; Winkler A. S.; Xuan B. T.; Yonemoto N.; Feigin V. L.; Vos T.; Murray C. J. L. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the global burden of disease study. Lancet Neurol. 2018, 17, 939–953. 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong M. J.; Okun M. S. Diagnosis and Treatment of Parkinson Disease: A Review. J. Am. Med. Assoc. 2020, 323, 548–560. 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- Fernandez H. H.; Standaert D. G.; Hauser R. A.; Lang A. E.; Fung V. S. C.; Klostermann F.; Lew M. F.; Odin P.; Steiger M.; Yakupov E. Z.; Chouinard S.; Suchowersky O.; Dubow J.; Hall C. M.; Charamra K.; Robieson W. Z.; Benesh J. A.; Espay A. J. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, openlabel results. Mov. Disord. 2015, 30, 500–509. 10.1002/mds.26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosebraugh M.; Voight E. A.; Moussa E. M.; Jameel F.; Lou X.; Zhang G. G. Z.; Mayer P. T.; Stolarik D.; Carr R. A.; Enright B. P.; Liu W.; Facheris M. F.; Kym P. R. Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease. Ann. Neurol. 2021, 90, 52. 10.1002/ana.26073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review, see:; Sanches B. M. A.; Ferreira E. I. Is prodrug design an approach to increase water solubility?. Int. J. Pharm. 2019, 568, 118498. 10.1016/j.ijpharm.2019.118498. [DOI] [PubMed] [Google Scholar]
- Cardinal-David B.; Chan V. S.; Dempah K. E.; Enright B. P.; Henry R. F.; Ho R.; Huang Y.; Huters A. D.; Klix R. C.; Krabbe S. W.; Kym P. R.; Lao Y.; Lou X.; Mackey S. E.; Matulenko M. A.; Mayer P. T.; Miller C. P.; Stambuli J.; Voight E. A.; Wang Z.; Zhang G. G.; Stella V. J.. Carbidopa and L-dopa prodrugs and methods of use. US patent US 9446059B2, 2016.
- Ross A. J.; Lang H. L.; Jackson R. F. W. Much Improved Conditions for the Negishi Cross-Coupling of Iodoalanine Derived Zinc Reagents with Aryl Halides. J. Org. Chem. 2010, 75 (1), 245–248. 10.1021/jo902238n. [DOI] [PubMed] [Google Scholar]
- Huters A. D.; Stambuli J.; Klix R. C.; Matulenko M. A.; Chan V. S.; Simanis J.; Hill D. R.; Reddy R. E.; Towne T. B.; Bellettini J. R.; Kotecki B. J.; Cardinal-David B.; Ji J.; Voight E. A.; Shou M.; Balaraman S.; Ashok A.; Ghosh S. Scalable Asymmetric Syntheses of Foslevodopa and Foscarbidopa Drug Substances for the Treatment of Parkinson’s Disease. J. Org. Chem. 2021, 1. 10.1021/acs.joc.1c00905. [DOI] [PubMed] [Google Scholar]
- Ferri C.; Sebastiani M.; Giuggioli D.; Colaci M.; Fallahi P.; Piluso A.; Antonelli A.; Zignego A. L. HCV syndrome: A constellation of organ- and non-organ specific autoimmune disorders, B-cell non-Hodgkin’s lymphoma, and cancer. World Journal of Hepatology. 2015, 7 (7), 327–343. 10.4254/wjh.v7.i3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See www.mavyret.com.
- Randolph J. T.; Voight E. A.; Greszler S. N.; Uno B. E.; Newton J. N.; Gleason K. M.; Stolarik D.; Van Handel C.; Bow D. A. J.; DeGoey D. A. Prodrug Strategies to Improve the Solubility of the HCV NS5A Inhibitor Pibrentasvir (ABT-530). J. Med. Chem. 2020, 63, 11034–11044. 10.1021/acs.jmedchem.0c00956. [DOI] [PubMed] [Google Scholar]
- For a review, see:; Harned A. M. From determination of enantiopurity to the construction of complex molecules: The Horeau principle and its application in synthesis. Tetrahedron 2018, 74, 3797–3841. 10.1016/j.tet.2018.05.056. [DOI] [Google Scholar]
- For a review, see:; Brands K. M. J.; Davies A. J. Crystallization-Induced Diastereomer Trasformations. Chem. Rev. 2006, 106, 2711–2733. 10.1021/cr0406864. [DOI] [PubMed] [Google Scholar]
- Voight E. A.; Greszler S. N.; Hartung J.; Ji J.; Klix R. C.; Randolph J. T.; Shelat B. H.; Waters J. E.; DeGoey D. A. Desymmetrization of Pibrentasvir for Efficient Prodrug Synthesis. Chem. Sci. 2021, 12, 10076. 10.1039/D1SC02396A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a review, see:; Magnuson S. R. Two-Directional Synthesis and its Use in Natural Product Synthesis. Tetrahedron 1995, 51, 2167–2213. 10.1016/0040-4020(94)01070-G. [DOI] [Google Scholar]






