ABSTRACT
Tularemia is a disease caused by Francisella tularensis—gram-negative coccobacillus. The ulceroglandular type characterized by skin ulcers and painful regional adenopathy is recognized as the most common.
A 1-year-old patient was admitted with severe normocytic anemia, high fever and hepatosplenomegaly. A nonspecific lesion in the axillary region with a homogenous nodal reaction was found, combined with a history of a tick-bite in the pectoral muscle. Primary differentiation included leukemia, lymphoma, mononucleosis, borrelial lymphoma and simple abscess. All of the above were excluded. A further search for diagnosis focused on tick-borne diseases: TIBOLa or anaplasmosis. The ulceroglandular tularemia was eventually confirmed serologically.
Besides the fact that tularemia is a rare diagnosis nowadays, it is still necessary to include this disease in the differentiation of a nonresolving tick-bite abscess with lymphadenopathy. Diagnostic vigilance is the key to effective treatment because other obvious symptoms such as severe anemia might delay the diagnosis.
INTRODUCTION
Tularemia is a potentially lethal disease, caused by gram-negative, facultative intracellular bacterium Francisella tularensis (F. tularensis) [1, 2]. Illness is transmitted to humans by multiple routes, such as arthropod bites, direct contact with infected animals, or their fluids and tissues, digestion of contaminated water or food, inhalation of infected aerosols, for instance during lawn mowing [3]. No human-to-human transmission was yet reported [4].
The most common form of tularemia is ulceroglandular [5], where ticks play a predominant role as vectors [6]. The incubation period varies between 1 and 21 days (usually 3–5 days) [4]. It is characterized by a triad of symptoms: primary lesion (soft, painless ulcer that evolves into a scar), regional lymphadenitis and fever. The nonspecific symptoms, such as prolonged fever, chills, headache, general malaise, sore throat or nausea, may delay proper diagnosis [2].
CASE REPORT
A 1-year-old male patient was admitted to the Emergency Department on account of a 4-day long fever, paleness of the skin and significant malaise. There was a history of a tick-bite 12 days ago, in the area of the left pectoral muscle with visible inflammation. No recent foreign travel was reported. Vaccinations were up todate.
On admission, an inflammatory reaction around the place of the tick-bite was visible. An axillary node on this side was palpable and painless. Hepatosplenomegaly was revealed during the physical examination. The patient exhibited severe normocytic anemia, slight leukocytosis with polymorphonuclear leukocytes (PMN) predominance. The ultrasonography indicated reactive enlargement of one lymph node in the left armpit (18 × 16 mm), several minor lymph nodes in this area and confirmed organomegaly (Fig. 1).
Figure 1 .
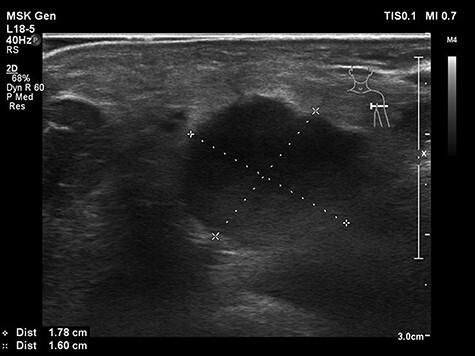
Ultrasonography of the lymph node in the left armpit on admission.
Due to significant paleness of the skin, considerable weakness and the alarming laboratory findings, a decision was made to admit this patient to the Department of Hematology, Oncology, Clinical Transplantology and Pediatrics, the Medical University of Warsaw.
Initially, efforts were made to rule out Epstein-Barr virus (EBV) infection which was suspected by the pediatric infectious diseases specialist due to the presence of nonspecific symptoms such as fever, malaise and hepatosplenomegaly. Serological tests presented trace levels of EBV-VCA-IgG. Besides serology, suspecting immunodeficiency, we performed the EBV-PCR test to rule out mononucleosis—test proved to be negative. Further diagnostics led to consideration of tick-borne diseases on account of history of a tick-bite. Due to his rapidly deteriorating clinical condition, a suspicion of Lyme disease, a decision was made to implement first-line antibiotic therapy—cefuroxime.
The fever subsided, but the hemoglobin and red blood cells level were low. Lymph nodes were still inflamed and the lesion still present. Borrelial lymphocytoma was excluded by serology and confirmed by westernblot.
The following suspicions were directed toward Rickettsia slovaca (tick-borne lymphadenopathy—TIBOLA) or Anaplasma phagocytophilum infection. These being intracellular bacteria, the antibiotic was changed to clarithromycin. The patient’s general status and blood tests stabilized. Nevertheless, the abscess-like structure and lymph nodes did not heal. Both serology and the PCR test did not confirm the suspicions mentioned above.
The disturbing course of disease, with nonhealing ulcerated lymph node, directed suspicions toward the other tick-borne infections. Tularemia became the most probable among them, regardless of its rarity and the child’s age. The ELISA test confirmed ulceroglandular tularemia.
According to the WHO guidelines, gentamicin was administered intravenously in three divided doses per day for 14 days. Following treatment, laboratory findings fully normalized and the ulcerative lesion started toheal.
Control ELISA tests, made in the first year after the diagnosis, did not reveal active tularemia infection. Nonetheless, a lesion was still present. Eventually, 2 more years later, there was no sign of abscess.
DISCUSSION
The presented case is an example of a potentially life-threatening infection with a hematological presentation. Worldwide, the incidence of tularemia is probably underestimated [5]. Sweden and Finland are the countries with the highest number of cases in Europe [7].
The exact cause for admission in this patient was severe anemia and fever accompanied by organomegaly, therefore the patient was hospitalized at the hematology department (Table 1).
Table 1.
Laboratory findings during hospitalization and follow-up appointments
| Laboratory findings during hospitalization: | ||||
|---|---|---|---|---|
| Day of hospitalization | Red blood cells count ×103/ul (4.3–5.5) |
Hemoglobin g/dl (11–14) |
Reticulocytes per mille (5–20) |
CRP mg/dl (<1) |
| 1 | 3.16 | 7.9 | 1.7 | 4 |
| 5 | 2.92 | 7.2 | 15.5 | 2.1 |
| 9 | 3.13 | 7.9 | 72.9 | <0.5 |
| 14 | 3.89 | 9.4 | 97.4 | 0.6 |
| 23 | 4.33 | 10.5 | 50.6 | <0.5 |
| 38 | 4.73 | 11.6 | 8.2 | <0.5 |
| 42 | 4.89 | 11.8 | 7.9 | <0.5 |
| Laboratory findings during follow-up appointments: | ||||
| 6 months after diagnosis | 4.23 | 10.7 | 3.0 | 2.8 |
| 1 year after diagnosis | 4.58 | 11.7 | – | 0.6 |
Severe anemia occurred because of transient erythroblastopenia of childhood (TEC), which in turn was caused by tularemia. TEC is a self-limiting disease, mostly occurring in children between 6 months and 3 years. Infectious disease has been suggested as a possible etiology. Normocytic and normochromic anemia with a low number of reticulocytes is a characteristic feature of TEC [8]. We conclude that tularemia triggered a systemic inflammatory response. Its manifestation was organomegaly and TEC. These factors, and the fact that the symptoms of tularemia are nonspecific, initially blurred the picture of tularemia.
The diagnosis of tularemia is mainly based on clinical manifestations, epidemiological data together with a positive serological test [9]. ELISA and western Blot show high sensitivity and specificity [4]. Antibodies against F. tularensis are detectable 10–20 days after infection [3]. Due to the mentioned data, an ELISA test was performed to confirm tularemia. IgA, IgM and IgG titers against tularemia antigens were positive.
The disease has various manifestations, from an asymptomatic course to a potentially lethal infection. Therefore, rapidly performed serological surveys (minimum 10 days after infection) could be helpful in defining the true prevalence of tularemia.
According to the WHO recommendations, treatment including aminoglycosides—streptomycin or gentamicin administered for 7–10 days is the first choice [10]. Gentamicin is a preferred drug in children and adolescents, in spite of the ototoxicity and nephrotoxicity as the lethal course of tularemia should always be considered [3].
The first broad-spectrum antibiotic of cefuroxime was implemented as an immediate treatment. Subsequently, clarithromycin was introduced due to the suspicion of intracellular Rickettsia and Anaplasma. However, none of these medications used were effective. Therefore, gentamicin implementation was a necessity for the ulcer toheal.
Summarizing, various overlapping symptoms can create an incoherent disease picture, prolonging the diagnostic process of the primary disease. Due to the high virulence of F. tularensis, its resistance and ability to cause potentially lethal disease, its course and possible treatment should be explored. Tularemia should always be considered as a possible etiology in patients presenting with an unusual course of the illness resulting from tick-bite.
ACKNOWLEDGMENTS
We would like to express our gratitude to Prof. Michał Matysiak for the support in the publication of this case report. This work was supported by the Medical University of Warsaw.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICAL APPROVAL
No ethical approval is required.
CONSENT
Consent for admission to hospital, examination and conduction of noninterventional research and scientific activities according to Polish law must be obtained from a legal representative of patients under the age of 16. Legal representative is the patient’s parent or guardian—legally mandated person, if the child does not have parents with parental authority. Polish law accepts the single parent permission. In our case of a 1-year-old boy, written informed consent was given by the patient’s mother who has the parental authority.
GUARANTOR
Marek W. Karwacki, MD,PhD.
REFERENCES
- 1.Putzova D, Senitkova I, Stulik J. Tularemia vaccines. Folia Microbiol (Praha) 2016;61:495–504. [DOI] [PubMed] [Google Scholar]
- 2.Rojko T, Korva M, Lotrič-Furlan S, Strle F, Avšič-Županc T. Cluster of ulceroglandular tularemia cases in Slovenia. Ticks Tick Borne Dis 2016;7:1193–7. [DOI] [PubMed] [Google Scholar]
- 3.Yeni DK, Büyük F, Ashraf A, Shah MSUD. Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol 2021;66:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imbimbo C, Karrer U, Wittwer M, Buettcher M. Tularemia in children and adolescents. Pediatr Infect Dis J 2020;39:e435–8. [DOI] [PubMed] [Google Scholar]
- 5.Sobolewska-Pilarczyk M, Pawłowska M, Halota W. Ulceroglandular tularemia complicated by pneumonia--a case report. Przegl Epidemiol 2014;68:421–4 531-424. [PubMed] [Google Scholar]
- 6.Desvars A, Furberg M, Hjertqvist M, Vidman L, Sjöstedt A, Rydén P, et al. Epidemiology and ecology of tularemia in Sweden, 1984–2012. Emerg Infec Dis J 2015;21:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ørbæk M, Lebech AM, Helleberg M. The clinical spectrum of tularemia-two cases. IDCases 2020;21:e00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Akker M, Dror Y, Odame I. Transient erythroblastopenia of childhood is an underdiagnosed and self-limiting disease. Acta Paediatr 2014;103:e288–94. [DOI] [PubMed] [Google Scholar]
- 9.Maurin M. Francisella tularensis, Tularemia and serological diagnosis. Front Cell Infect Microbiol 2020;10:512090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health O . WHO Guidelines on Tularaemia. Geneva: World Health Organization, 2007. [Google Scholar]


