ABSTRACT
Takotsubo syndrome is a rare cause of systolic dysfunction and can be found as a clinical manifestation of pheochromocytoma. We present a case of rapid onset of systolic dysfunction with cardiogenic shock, which developed after the surgical excision of an adrenal gland tumor in a 60-year-old male. Coronary angiography excluded coronary artery disease. The echocardiography and ventriculography images suggested Takotsubo cardiomyopathy pattern. Following 2 weeks of inotropic and vasopressor therapy, the left ventricular function gradually improved, until complete resolution.
INTRODUCTION
Pheochromocytomas and paragangliomas are rare catecholamine-producing tumors (0.2–0.6% of hypertensive patients; [1]), which could appear sporadically or be part of a hereditary syndrome. An even rarer occurrence is the association between pheochromocytomas and Takotsubo syndrome. We discuss the case of a patient who developed Takotsubo syndrome and subsequent cardiogenic shock during surgery for an adrenal tumor.
CASE REPORT
A 60-year-old male patient was admitted to the Surgery Department for the removal of a recently diagnosed left adrenal gland tumor [2]. The patient’s medical history consisted of type 2 diabetes mellitus, dyslipidemia, moderate aortic regurgitation (regurgitant orifice area 0.2 cm2) and recently diagnosed secondary arterial hypertension. Three months prior to admission, the patient started experiencing specific spells, with anxiety, agitation, headaches, pallor, palpitations and hypertensive crises. The abdominal computed tomography (CT) scan described a left adrenal mass (Fig. 1A and B). The association between a CT image of a left adrenal tumor and elevated urinary normetanephrines and metanephrines pointed towards catecholamine-producing tumor [1]. Upon admission the patient was hemodynamically stable: blood pressure, heart rate and blood oxygen levels within normal range. The preoperative preparation of the patient consisted of 30-day treatment with alpha and beta adrenergic blockade: doxazosin 1 mg/day and bisoprolol 2.5 mg/day [1].
Figure 1 .
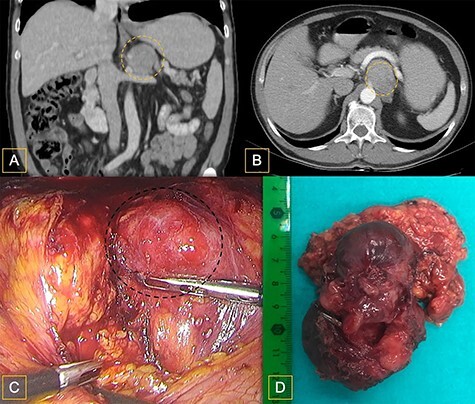
(A) Abdominal CT coronal view of a left adrenal nodule compressing the splenic vein; (B) Abdominal CT axial view of left adrenal nodule compressing the splenic vein; (C) Intraoperative aspect of the nodule; during retraction, pressure exerted on the nodule caused spikes of arterial blood pressure; (D) resection specimen.
Initial blood tests were within normal range. His electrocardiogram showed sinus tachycardia and negative T waves in the lateral leads. The echocardiography showed a left ventricular ejection fraction (LVEF) of 55%, no regional wall motion abnormalities and a moderate aortic regurgitation. The Revised Cardiac Risk Index [3] amounted to 1 point and showed a class II risk.
The patient underwent standard lateral transperitoneal laparoscopic adrenalectomy using four trocars [2]. During dissection, even gentle manipulation of the tumor caused spikes of arterial pressure up to 180 mmHg but all receded after control of the main left adrenal vein (Fig. 1C and D). During the surgery, after the removal of the tumor, the patient became hemodynamically unstable. He presented hypotension (50/30 mmHg) and new onset bundle branch block (LBBB). He was moved directly to the angiography room and emergency coronarography was performed. The ventriculography described circumferential midventricular akinesia (Fig. 2A and B). The coronarography showed no obstructive atherosclerotic disease of epicardial vessels, low flow TIMI 2 and no vasospasm (Fig. 2C and D). The echocardiography showed left ventricular circumferential midventricular and apical akinesia and a LVEF of 25% (Supplementary Video 1). The patient had important dynamic changes in troponin levels (a maximum value of 21.9 ng/ml on the second-postoperative day, with an upper reference limit [URL] of 0.2 ng/ml) and high NT-proBNP values (a maximum value of 7000 pg/ml on the second-postoperative day with an URL of 300 pg/ml). He stayed in intensive care unit for 12 days requiring inotropic and vasopressor support and for the first three days he required intubation and mechanical ventilation. The left ventricular systolic function improved gradually. On the 12th day after the surgery, the patient no longer required oxygen or vasopressors and was transferred to the Cardiology Department. On the 15th-postoperative day, the echocardiography showed a left ventricle with no regional motion abnormalities, a dramatically improved ejection fraction of 55% (Supplementary Video 2), normal troponin levels and the electrocardiogram no longer presented LBBB (Fig. 3). The pathology report described a pheochromocytoma of the adrenal gland scaled score (PASS) of seven, which rendered the tumor as biologically aggressive. Furthermore, one out of seven resected lymph nodes had similar tumor cells, thus the tumor was staged as pT3N1 (TNM). At the 1-month follow up, the patient had normal blood pressure values (120/75 mmHg) and preserved LVEF (55%).
Figure 2 .
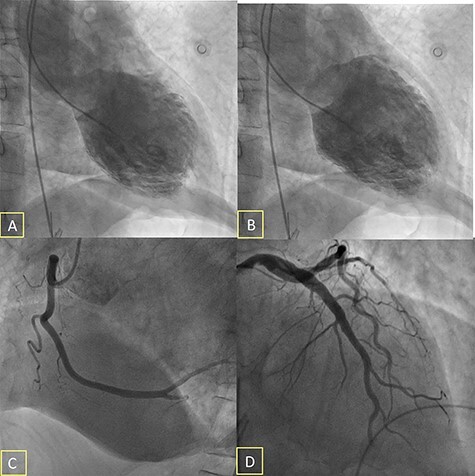
Ventriculogram showing left ventricular „ballooning”—midventricular akinesia (A) Telesystolic and (B) Teledyastolic; (C) Coronary angiography showing right coronary artery with no atherosclerotic significant stenosis; (D) Coronary angiography showing left main, left descending artery and circumflex artery with no atherosclerotic significant stenosis.
Figure 3 .
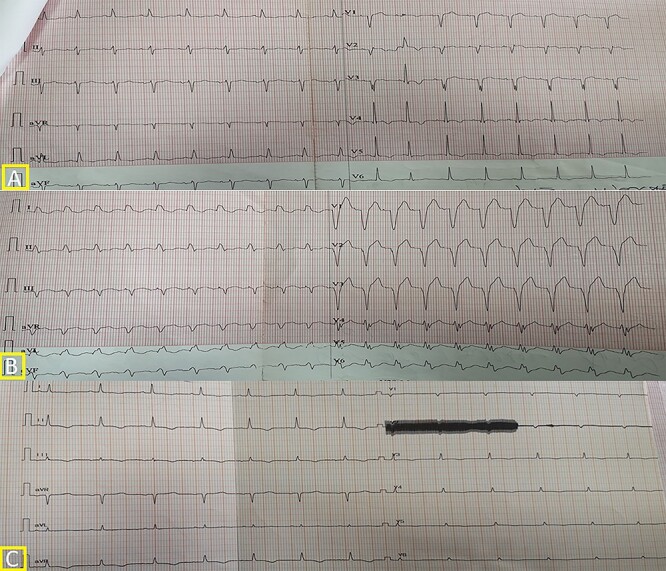
Electrocardiogram (A) Before the surgery; (B) Immediately after the surgery; (C) on the 15th-postoperative day.
DISCUSSION
We present the case of a patient with no history of systolic dysfunction who developed Takotsubo syndrome (TS) after the surgical removal of a pheochromocytoma, which raised complex issues regarding diagnosis and treatment. Pheochromocytomas are rare, catecholamine secreting tumors [1]. An even rarer occurrence is the coexistence of pheochromocytoma and TS, probably due to catecholamine excess [4]. The patient was monitored closely by the anesthesia care team and drugs which could promote catecholamine release were avoided. During the surgery, as the patient became hemodynamically unstable, the differential diagnosis consisted of acute coronary syndrome, hypovolemic shock and anesthesia induced hypotension. Because the condition manifested as acute heart failure with cardiogenic shock and electrocardiographic changes, coronary artery disease had to be ruled out. The patient’s recent history showed no indication of a viral infection, no inflammatory markers present and no pericardial effusion [5].
Following the diagnostic algorithm of TS, the patient started with an InterTAK Score of low/intermediate probability, the coronary angiography showed no coronary lesions, he had no red flags of acute myocarditis and after 15 days he had no wall motion abnormalities, which leads to the diagnosis of TS [5].
Sympathetic activation has a central role in the pathophysiology of TS, with high levels of circulating catecholamines [4, 6]. Therefore, the management of the patient was based on inotropic and vasopressors agents, while attempting to avoid any adrenergic agonists, as they could aggravate the condition [4–6]. When the patient became hemodynamically unstable, he was treated at first with dobutamine and noradrenaline. Then, he was started on levosimendane and vasopressin. But, as the blood pressure could not be elevated, he also received noradrenaline. Gathering all the arguments, we conclude that this case was a diagnostic and therapeutic challenge, a transient systolic dysfunction associated with the surgical stress of the removal of a catecholamine secreting tumor.
TS is a complex pathology, frequently associated with an excess of catecholamines, which requires careful consideration regarding diagnosis and treatment. We regard this case as a suddenly acquired, reversible systolic dysfunction associated with the perioperative surgical stress of the removal of a pheochromocytoma.
Supplementary Material
ACKNOWLEDGMENTS
Dr Irina Tudose, Dr Vlad Mageriu.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No funding was received for this work.
ETHICAL APPROVAL
No approval was required.
Consent has been obtained from the patient.
REFERENCES
- 1.Lenders JWM, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SKG, Murad MH et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915–42. [DOI] [PubMed] [Google Scholar]
- 2.Miron A, Giulea C, Nadragea M, Enciu O. Laparoscopic partial adrenalectomy. Chirurgia (Bucur) 2017;112:77–81. [DOI] [PubMed] [Google Scholar]
- 3.Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S et al. Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17–32. [DOI] [PubMed] [Google Scholar]
- 4.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ et al. International expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR et al. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


