Abstract
Background:
This study aims at describing the therapeutic outcome of patients carrying the R92Q variant in the TNFRSF1A gene treated with anakinra (ANA) or canakinumab (CAN) and identifying any factors predictive of complete response to IL-1 inhibition.
Methods:
Clinical data of patients treated with ANA or CAN for recurrent inflammatory attacks due to the presence of the R92Q variant were retrospectively collected and analysed.
Results:
Data about 20 treatment courses with IL-1 inhibitors (16 with ANA and 4 with CAN) from 19 patients were collected. Mean age at disease onset was 20.2 ± 14.8 years. In 5 cases (26%) the R92Q variant was found in a family member affected by recurrent fever. The therapeutic response was complete in 13(68%) and partial in 2 patients (11%); treatment failure was observed in 4 cases (21%). Median AIDAI decreased from 10 (interquartile range [IQR] = 28) to 0 (IQR = 1) at the 12-month follow-up visit (p < 0.001). Mean ESR and median CRP dropped respectively from 40.8 ± 24.8 to 9.1 ± 4.5 mm/h (p < 0.001) and from 3.0 (IQR = 1.9) to 0.3 (IQR = 0.3) mg/dl (p < 0.001) after 12 months of treatment. A steroid-sparing effect was observed from the third month of treatment (p < 0.01). Thirteen patients (65%) were still on treatment at the last follow-up visit (median duration of treatment 17 (IQR = 38) months). The presence of R92Q mutation in a symptomatic relative (p = 0.022), the relapsing remitting disease course (p < 0.001) and the presence of migratory erythematous skin rashes during fever attacks (p = 0.005) were associated with complete efficacy of IL-1 inhibitors.
Conclusions:
R92Q patients showed a favourable response to ANA and CAN, particularly when the mutation segregated in a family member and when a relapsing-remitting disease course or TNF-α receptor-associated periodic syndrome (TRAPS) typical skin rash were observed. In the subgroup of patients not taking advantage of IL-1 blockage different molecular mechanisms underlying the autoinflammatory picture are likely to exist.
Keywords: anakinra, biologic therapy, canakinumab, innovative biotechnologies, interleukin-1 inhibition, personalized medicine, R92Q variant, TNF-α receptor associated periodic syndrome
Introduction
Mutations in the TNFRSF1A gene encoding for tumour necrosis factor-α (TNF-α) receptor type 1 are known to cause the autosomal dominant autoinflammatory syndrome named TNF-α receptor-associated periodic syndrome (TRAPS). The syndrome is characterized by a relapsing-remitting course with recurrent fever attacks lasting several days associated with skin rash, myalgia, abdominal pain, periorbital edema, articular involvement and other inflammatory symptoms; otherwise the course of the disease can be chronic with continuous symptoms and/or steadily elevated inflammatory markers.1,2 A wide spectrum of phenotypic variability has been described in patients carrying TNFRSF1A variants, ranging from the asymptomatic to the most severe forms of TRAPS, which might lead to development of systemic amyloidosis.3 Structural mutations are often localized on exons 2, 3 and 4 and affect highly preserved cysteine residues, necessary for the stability of disulphide bonds within the extracellular domain of the protein. Interrupting the folding of the extracellular portion of the receptor, these mutations generally lead to a severe disease phenotype with higher risk of AA amyloidosis.4 Among low-penetrance variants, R92Q located within exon 4 represents one of the most common mutations found in TRAPS patients. It is associated with a variable but usually mild autoinflammatory phenotype, with late disease onset, weaker familial association, negligible risk of amyloidosis, shorter fever attacks and higher rate of spontaneous resolution compared to structural mutations.1,5 Oligosymptomatic or atypical phenotypes, including idiopathic recurrent acute pericarditis, have been described as R92Q carriers as well.6,7 Moreover, R92Q frequency is around 1–5.2% in different populations, and in some cases the mutation does not even segregate within the TRAPS phenotype, to the point that it has been considered a functional polymorphism.3,8–10 Also, it has been identified at a higher frequency in patients with multifactorial inflammatory conditions such as periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome, early arthritis, juvenile idiopathic arthritis, vasculo-Behçet’s disease, idiopathic recurrent pericarditis and multiple sclerosis, compared to healthy subjects.10–15 For these reasons, the R92Q mutation is still classified among variants of uncertain significance (Infevers: an online database for autoinflammatory mutations. Available at https://infevers.umai-montpellier.fr/ Accessed (2020.12.13)).16
The gold standard treatment of TRAPS is the inhibition of interleukin (IL)-1, a pro-inflammatory cytokine which plays a pleiotropic role in innate immunity. Although key aspects of the pathogenesis of the disease still have to be clarified, IL-1 has been recognized as the final effector of the stress-response mechanisms activated by the deregulated cellular homeostasis in TRAPS patients.17–20 Indeed, several studies reported a striking efficacy of the IL-1 blockers anakinra (ANA) and canakinumab (CAN) in the treatment of TRAPS, including phase II and III trials leading to the registration of CAN by the European Medicines Agency (EMA) for this indication.21–24 Given the uncertainty about the molecular mechanism and pathogenic significance of the R92Q variant in TRAPS, the therapeutic response to IL-1 inhibitors in patients with recurrent inflammatory attacks due to the presence of this specific mutation is worth an extensive investigation.
Aims of the study
Primary aim of this study was to describe the therapeutic outcome of patients carrying the R92Q variant in the TNFRSF1A gene treated with ANA or CAN for their autoinflammatory condition.
Secondary aim was to identify among clinical and laboratory features at disease onset any factors predictive of complete response to IL-1 inhibition.
Patients and methods
This is an observational retrospective multicentre study involving 13 tertiary referral centres in Italy, Spain, Poland, Belgium and Egypt contributing to the AutoInflammatory Disease Alliance (AIDA) Network.
Adult and paediatric patients ever treated with ANA or CAN for inflammatory attacks due to the presence of the R92Q variant in the TNFRSF1A gene were included in the study, disregarding the fulfilment of the classification criteria for TRAPS.25 The presence of other gene mutations better explaining the clinical phenotype was an exclusion criterion. The diagnosis of a defined multifactorial autoinflammatory disease according to the relative diagnostic criteria, including PFAPA syndrome, systemic juvenile idiopathic arthritis, adult-onset Still’s disease, Behçet’s syndrome and others, was considered an exclusion criterion as well.
Demographic, clinical, laboratory, clinimetric and therapeutic data of each patient were collected retrospectively based on clinical charts by the treating physician. Timepoints for data collection were set at the time of disease onset, at the time of diagnosis, at the start of treatment with IL-1 inhibitors, at 1-, 3-, 6- and 12 months of follow-up, and at the last assessment. Classification scores for TRAPS were derived by the authors on the basis of available data.25
Disease activity was measured through the AIDAI score, as recommended internationally to standardize outcome measurements, and thus improve comparisons between studies on autoinflammatory diseases.26 The AIDAI score is a numeric index resulting from the sum of the symptoms reported day by day (as present or absent) by the patient over a 29-day period.27 It is the only validated disease activity index for TRAPS, since it has been demonstrated capable of discriminating clinically active (score ⩾ 9) from inactive (score < 9) disease. However, not all the clinical charts included the AIDAI score, which has been developed in 2011. Therefore, we decided to include further outcome measures in this study. In detail, the following outcome measures have been assessed: (1) reduction in the AIDAI score at 1-, 3-, 6- and 12 months of treatment;27 (2) reduction in the level of serum inflammatory markers (C-reactive protein (CRP), erythrocyte sedimentation rate (ESR)) at 1-, 3-, 6- and 12 months of treatment; (3) steroid-sparing effect of the therapy at 1-, 3-, 6- and 12 months (expressed as prednisone equivalent); (4) a physician global assessment of the therapeutic response, defined as follows. The global therapeutic response was considered ‘complete’ if the treatment induced a total control of symptoms, associated with the normalization of inflammatory markers (CRP, ESR and serum amyloid-A (SAA)); the response was defined ‘partial’ if (1) a reduction of symptom severity occurred during attacks or between flares for patients with relapsing-remitting or chronic disease course, respectively, or if (2) a reduction of the level of inflammatory markers during febrile attacks was observed, without reaching normal values; the therapeutic response was considered ‘absent’ (primary failure) if none of the previous criteria was satisfied. Therapeutic failure was defined ‘secondary’ if a clinical relapse was observed during maintenance treatment, several months after complete remission had been achieved.
As for the posology of the IL-1 inhibitors, we defined ‘standard dosages’ the following ones: ANA 100 mg/day (weight > 50 kg) or 1–2 mg/kg/day (weight < 50 kg); CAN 150 mg (weight > 40 kg) or 2 mg/kg (weight > 40 kg) every 4 weeks.
The study protocol conformed to the tenets of the Declaration of Helsinki and was approved by the local Ethics Committee of the University of Siena (Reference No. 14951). Written informed consent for using clinical data for research purposes was obtained according to the local Institutional review board guidelines. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies were followed.28
Statistical analysis
Data were analysed using JASP open-source statistics package. Descriptive statistics included sample sizes, mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Shapiro-Wilk test was used to assess normality distribution of data. Paired categorical variables were analysed using 2 x 2 contingency tables with Fisher exact test or McNemar’s Chi-Square test with Yates’ continuity correction, as appropriate. Analysis of means of paired samples was investigated through repeated measures ANOVA test with Greenhouse-Geisser sphericity correction and Bonferroni post hoc analysis, or Friedman’s test and Conover’s post hoc comparisons, as appropriate. Univariate logistic regression analysis was performed to detect potential factors predictive of complete response to the therapy. The threshold for statistical significance was set to p < 0.05 and all p-values were two-sided.
Results
Data about 20 treatment courses from 19 patients treated with ANA or CAN were collected. Mean age at disease onset was 20.2 ± 14.8 years (range: 1–52). In 5 cases (26%), the R92Q variant was found in a family member affected by recurrent fever. In 15 cases (79%), the disease could be classified as TRAPS according to the Eurofever classification criteria. As for the 5 patients not classified as TRAPS according to the current classification criteria, they have been treated with IL-1 inhibitors before 2019, when the Eurofever classification criteria that are currently used were not available yet. More in detail, two patients suffered from recurrent pericarditis, which was not controlled by colchicine prophylaxis, leading to the introduction of ANA; one of them had very high levels of serum amyloid A (>1000 mg/L) during attacks. Two further patients had spontaneous febrile attacks associated with abdominal pain, chest pain, myalgia and arthralgia, in one case, and erythematous rash and abdominal pain in the other one; these two patients had a chronic disease course, with steadily elevated inflammatory markers even between febrile attacks, and they were treated with ANA as steroid-sparing agent, since they needed daily use of moderate to high dose prednisone. On the contrary, the last patient was treated with CAN for relapsing-remitting inflammatory attacks of long-lasting fever, myalgia, oral aphthosis, chest and abdominal pain, with elevated ESR (> 100 mm/h) recurring despite colchicine therapy.
Main characteristics of the cohort are summarized in Table 1.
Table 1.
Demographic characteristics of the cohort and details of the therapeutic courses with IL-1 inhibitors.
| Number of treatment courses (ANA: CAN) | 20 (16:4) |
|---|---|
| Male: female | 8:11 |
| Current age, years | |
| mean ± SD | 36.5 ± 15.6 |
| (range) | (19–66) |
| Detection of R92Q variant in relatives affected by recurrent fever | 5 (26%) |
| Age at onset, years | |
| mean ± SD | 20.2 ± 14.8 |
| (range) | (1–52) |
| Age at diagnosis, years | |
| median (IQR) | 25 (17.5) |
| (range) | (12–57) |
| Diagnostic delay, years | |
| median (IQR) | 5.0 (6.5) |
| (range) | (1–28) |
| Disease course before the start of IL-1 inhibitors | |
| Relapsing-remitting | 14 (70%) |
| Chronic | 6 (30%) |
| Classification as TRAPS according to | |
| Eurofever criteria for TRAPS25 | 15 (79%) |
| Eurofever clinical criteria for TRAPS25 | 9 (47%) |
| Duration of the therapy with IL-1 inhibitors, months | |
| median (IQR) | 17.0 (38) |
| (range) | (1–120) |
| Duration of follow-up, years | |
| median (IQR) | 5 (9.5) |
| (range) | (2–36) |
| Line of biologic treatment | |
| First | 15 (75%) |
| Second (or further) | 5 (25%) |
| Previous therapies | |
| NSAIDs | 18 (90%) |
| Oral glucocorticoids | 16 (80%) |
| Colchicine | 13 (65%) |
| Anakinra | 3 (15%) |
| Etanercept | 3 (15%) |
| Adalimumab | 1 (5%) |
| Tocilizumab | 2 (10%) |
ANA, anakinra; CAN, canakinumab; IL, interleukin; IQR, interquartile range; NSAIDs, nonsteroidal anti-inflammatory drugs; SD, standard deviation; TRAPS, TNF-α receptor-associated periodic syndrome.
Sixteen patients were treated with ANA and 4 with CAN. One patient stopped ANA therapy after 3 months due to primary failure and was subsequently treated with adalimumab, tocilizumab and CAN. The median duration of therapy was 17 (IQR = 38) months (1–120). The IL-1 inhibitor was administered as first biologic agent in 15 cases (75%) and as second to fourth biologic line of treatment in 5 cases (25%). Patients had been previously treated with nonsteroidal anti-inflammatory drugs (NSAIDs) in 18 (90%), oral glucocorticoids in 16 (80%), colchicine in 13 (65%), ANA in 3 (15%), etanercept in 3 (15%), adalimumab in 1 (5%) and tocilizumab in 2 (10%) cases.
The IL-1 inhibitors were employed with standard posology in 19 patients (95%); 1 patient was treated with ANA at a lower attack dose. In 9 cases (45%) the initial dose was adjusted during follow-up based on the individual response, by increasing or decreasing the dose (in 1 and 2 cases, respectively) or by shortening or increasing intervals between administrations (in 2 and 4 cases, respectively).
The overall response to the biologic therapy was complete in 13 (68%), partial in 2 (11%) and absent in 4 patients (21%); for 1 patient (5%) the follow-up on CAN was too short to reliably assess the global therapeutic outcome. Among the 15 patients who satisfied the classification criteria for TRAPS, a complete therapeutic response was achieved in 11 (79%), a partial response in 1 (7%) and no response in 2 cases (14%); among the 5 patients who did not satisfy the classification criteria for TRAPS, a complete therapeutic response was achieved in 2 (40%), a partial response in 1 (20%) and no response in 2 cases (40%) (p = 0.28).
The frequency of autoinflammatory features in the cohort at the start of treatment with ANA or CAN and at 1, 3, 6 and 12 months of follow-up is summarized in Table 2.
Table 2.
Frequency of clinical manifestations at the start of treatment with IL-1 inhibitors and after 1, 3, 6 and 12 months of therapy.
| Chest pain | Pharyngitis | Oral aphthosis | Skin rash | Pericarditis | Lymphoadenitis | Abdominal pain | Myalgia | Arthralgia | Arthritis | Conjunctivitis | Periorbital edema | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8 (40%) | 8 (40%) | 7 (35%) | 9 (45%) | 6 (30%) | 6 (30%) | 11 (55%) | 14 (70%) | 15 (75%) | 6 (30%) | 3 (15%) | 0 |
| Month-1 | 2* (10%) | 0* | 0* | 2 (10%) | 1 (5%) | 2 (10%) | 4 (20%) | 3** (15%) | 7 (35%) | 1 (5%) | 0 | 0 |
| Month-3 | 0* | 0* | 0* | 1* (5%) | 0* | 1 (5%) | 2* (10%) | 0** | 4* (20%) | 0 | 0 | 0 |
| Month-6 | 1 (5%) | 2 (10%) | 0* | 1 (5%) | 0* | 1 (5%) | 1 (5%) | 1** (5%) | 4* (20%) | 0 | 0 | 1 (5%) |
| Month-12 | 2 (10%) | 3 (15%) | 0* | 1 (5%) | 1 (5%) | 2 (10%) | 1 (5%) | 2* (10%) | 4* (20%) | 0 | 0 | 1 (5%) |
Statistical significance is referred to the comparison of each timepoint with the baseline assessment: *p < 0.05 **p < 0.01.
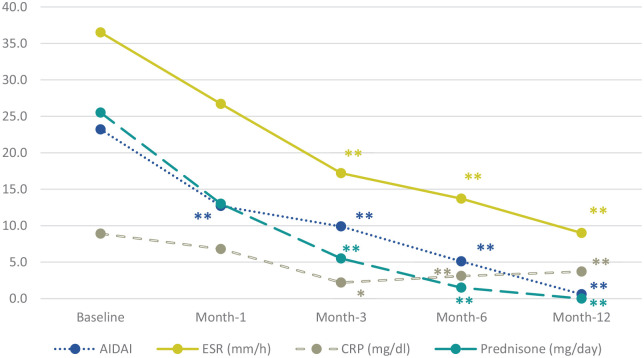
The median AIDAI27 decreased from 10 (IQR 28) to 3 (IQR 8) after 1 month of treatment (p < 0.001), to 0.5 (IQR 3.3) after 3 months (p < 0.001), and to 0 (IQR 2.5) after 6 months (p < 0.001) (Figure 1). The mean ESR and the median CRP decreased during 12 months of treatment from 40.8 ± 24.8 to 9.1 ± 4.5 mm/h (p < 0.001) and from 3.0 (IQR 1.9) to 0.3 (IQR 0.3) mg/dl (p < 0.001), respectively. A significant steroid sparing effect was observed since the third month of biologic therapy, with a mean prednisone dosage of 19.1 ± 19.3 and 4.0 ± 5.7 mg/day at the start of therapy and at 3-month follow-up, respectively (p = 0.004) (Figure 1).
Figure 1.
Time variation over 12 months of the AIDAI score, glucocorticoid need and serum values of inflammatory markers during IL-1 inhibitor therapy.
Statistical significance is referred to the comparison of each timepoint with the baseline assessment: *p < 0.05 **p < 0.01. AIDAI, autoinflammatory disease activity index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
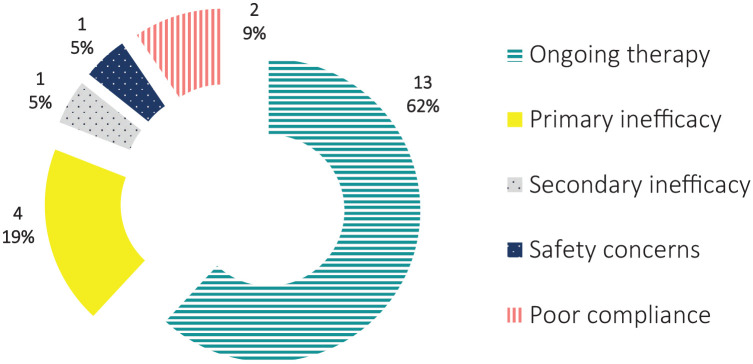
The therapy with ANA or CAN was ongoing at the last follow-up visit in 13 patients (65%) and had been withdrawn in 7 cases (35%). Withdrawal was due to primary failure in 3 (15%), primary failure and safety concern in 1 (5%), secondary inefficacy in 1 (5%), and poor compliance despite complete efficacy in 2 cases (10%) (Figure 2). Adverse events were classified as mild in all cases: 2 patients (10%) reported injection site reactions with ANA; 1 patient (5%) had chest tightness leading to ANA discontinuation; 1 further patient (5%) had amnesia with ANA; recurrent cystitis was reported by 1 patient (5%) treated with CAN.
Figure 2.
Number and percentage of patients still on therapy or having discontinued IL-1 inhibitors due to primary inefficacy, secondary inefficacy, safety concern or poor compliance at the last follow-up visit.
The presence of the R92Q mutation in a symptomatic relative, the relapsing-remitting disease course, and the presence of migratory erythematosus skin rashes during attacks were identified as factors associated with a complete response to the therapy with IL-1 inhibitors (p = 0.022, p < 0.001 and p = 0.023, respectively). No pre-treatment clinical and laboratory features were predictive of the therapeutic response with any statistical significance.
Discussion
TRAPS management is more challenging than in other monogenic autoinflammatory disorders, due to a frank genetic heterogeneity giving rise to protean and complex clinical sceneries. The identification of TNFRSF1A mutations as the genetic cause of TRAPS raised the possibility that blocking TNF-α could potentially represent the primary therapeutic strategy,29 though etanercept, the soluble form of the p75 TNF-α receptor, gave conflicting results in real-life experience, differently from the success obtained in other autoinflammatory disorders.30–32 More recently, anti-IL-1 agents have proved to be reasonable options to prevent TRAPS relapses both in the short- and long-term.33,34
The present study describes the therapeutic outcome of patients treated with two IL-1 inhibitors, ANA and CAN, to control febrile relapses in consequence of the R92Q variant in the TNFRSF1A gene. The pathogenic significance of this mutation in the context of TRAPS is still unclear.
It has been demonstrated that both the structure and functional behaviour of the protein affected by the R92Q variant is similar to the wild-type TNF-α receptor. Differently from what is demonstrated for structural mutations, neither accumulation of misfolded receptors in the cytoplasm, nor altered expression in cell membranes, nor defective shedding of the receptor–which are the mechanisms responsible of the increased production of IL-1 and the downstream pro-inflammatory cascade in TRAPS patients–have been observed.17,18,35,36 According to this view, patients with an autoinflammatory phenotype due to the presence of R92Q may be expected to exhibit a different response rate to IL-1 blockade than that documented in patients with structural TRAPS-related mutations.
It has been observed that R92Q-positive patients are successfully treated with NSAIDs, colchicine or glucocorticoids as monotherapy more frequently than those with different mutations of the same gene, probably due to the reduced expressivity of this variant.37–39 Nevertheless, recent data gathered from the AIDA cohort disclose that the percentage of R92Q patients requiring cytokine blockers is up to 53%, surprisingly higher than previously reported.39–43 In the present study, the efficacy rate of ANA and CAN was 68%, being ‘complete efficacy’ defined as the resolution of symptoms and normalization of ESR, CRP and SAA, which should demonstrate the absence of both manifest and subclinical inflammation. Accordingly, the median AIDAI score, which measures the clinical activity of the disease, was significantly lower at month-1 follow-up visit, while mean values of laboratory markers of inflammation decreased more slowly reaching the statistical significance after 3 months of treatment. Both clinical and serological parameters were adequately controlled up to 12 months of follow-up; moreover, more than two-thirds of our cohort were still on IL-1 inhibition at their last visit.
Few heterogeneous data about the therapeutic outcome of IL-1 inhibitors in R92Q-positive patients can be derived from most studies available in the medical literature. The ‘umbrella’ trial leading to the registration of CAN for TRAPS involved 15 patients carrying the R92Q mutation (equal to 33% of the study group); CAN displayed a complete efficacy at week 16 in 45% of patients (73% including those treated with increased dosage), but the genotype of responders/not responders was not detailed in the paper.22 Among the 5 patients affected by TRAPS successfully treated with ANA in a 2008 prospective, one carried the R92Q variant showing a chronic disease course with fluctuating symptoms and persistent elevation of acute-phase reactants; he had a favourable response to ANA, leading to discontinuation of the concomitant anti-inflammatory therapy.21 Data from case reports and case series of R92Q-positive patients undergoing ANA or CAN are controversial, reporting both success and failure of IL-1 inhibition;44–48 moreover, this level of evidence is usually affected by publication bias which may negatively impact on the number of therapeutic failures reported in the literature.
In the cohort described by the present study, the presence of the R92Q mutation in a relative affected by recurrent fever, the presence of migratory erythematosus skin rashes during attacks and the relapsing-remitting disease course were associated with a complete therapeutic response to ANA and CAN with statistical significance. The percentage of patients inheriting R92Q from a relative affected by recurrent fever was consistent with that previously reported in other TRAPS cohorts, although no data about the possible association between familial segregation and response to IL-1 inhibition are available in the literature to date.5,8 It is worth mentioning that two out of three of the variables associated to ANA and CAN complete efficacy in this study are included in the TRAPS classification score, although a direct association between the fulfilment of classification criteria and therapeutic outcome was not found. In a recent retrospective study based on the Eurofever cohort, ANA was efficacious in 7 patients out of 10 (70%) carrying variants of uncertain significance or not classified (VUS/NC) according to International Study Group for Systemic Autoinflammatory Diseases (INSAID) classification. The study group included both well-known low penetrance mutations such as R92Q and non classified variants, involving cysteine residues on the exons 3 and 4, making data too heterogeneous to compare. Nevertheless, of particular relevance to our results, this paper argues that the fulfilment of TRAPS classification criteria within the group of subjects carrying VUS/NC mutations would identify a subset of patients who display a better response to biologics.49 Therefore, our data support the increasing evidence that, when R92Q is detected in patients whose clinical phenotype clearly resembles TRAPS, IL-1 inhibitors can be considered the therapy of choice, if a therapeutic step up is needed due to the frequency and intensity of flares. However, these considerations should be taken with caution given the retrospective design of the study, the low number of data and the absence of a clear predictive value of the variables associated with therapeutic success.
From a different point of view, the 32% failure rate of IL-1 inhibitors in our cohort (either complete inefficacy or partial improvement of symptoms and laboratory parameters) discloses the predominance of molecular pathways not directly related to IL-1 as main pathogenic drivers of autoinflammation in the non-responder group. In the clinical setting, these patients should be carefully reassessed and eventually undergo further investigations to rule out diagnoses other than TRAPS. It is the authors’ opinion that both extended genetic analysis and gene expression profile studies may be warranted after failure of IL-1 inhibitors in R92Q-positive patients showing a clear autoinflammatory phenotype. In silico data suggest that the R92Q variant modifies the energy of interaction between different domains of the amino acid chain;50 this would affect the configuration and the molecular dynamics of the mutated receptor after binding to its ligand, inducing an increased or decreased bend angle, according to different studies.50,51 Studies on gene expression profiles of cells transfected with the R92Q variant revealed clustering of transcripts specifically altered in mutants compared with wild-type transfectants, and with a clear separation also from the cysteine mutant transfectants.52 Functional analysis of blood samples from R92Q carriers found an increased plasmatic level of monocyte chemoattractant protein-1 (MCP-1/CCL2) and TNF-induced IL-12p70 release.11 Whether such molecular effects are sufficient to cause an autoinflammatory picture by themselves is still being discussed and the phenotypic variability of R92Q patients does not yet offer a clear pathophysiological explanation. A recent concept hypothesizes a synergic pro-inflammatory function of R92Q in a context of oligogenic transmission, intermediate in the continuum from monogenic to multifactorial polygenic inheritance: the affected individual would inherit multiple low-frequency allele variants that act synergistically in determining the clinical picture.53
Conclusion
This study describes the therapeutic outcome of the largest cohort of R92Q patients treated with IL-1 inhibitors so far in the literature. More than two-thirds of them showed complete response to the therapy and retained the drugs up to the last follow-up visit, with a median treatment duration of 17 months. In the responder group, the presence of clinical manifestations typical of TRAPS (i.e. the erythematous migrating skin rash), the relapsing-remitting disease course and the segregation of the mutation in the family of the proband were significantly more frequent, although no predictive value was observed for any of these factors. In the subgroup of patients not taking advantage of IL-1 blockade different molecular mechanisms underlying the autoinflammatory picture are likely to exist. Gene expression analysis and functional studies performed in this cluster of patients may shed light on the molecular patterns set off by the R92Q mutation and guide the future therapeutic directions.
Acknowledgments
Carla Gaggiano and Donato Rigante equally contributed to this work.
Footnotes
Author contributions: C.G. and A.V. designed the study and performed statistical analysis with support of J.S.. C.G. carried out the main writing of the manuscript, with support from M.T. for literature selection. J.H.R., A.S., G.L., F.I., M.A.J., R.G., E.W.S., M.C., M.F., M.P., G.R., F.Z., A.F., V.S., M.T.H., O.A., L.P., A.F., C.F. and B.F. enrolled patients for the study, collected data and critically reviewed the manuscript for important intellectual content. J.S., A.R. and S.G. critically reviewed the manuscript for important intellectual content. L.C. and D.R. supervised the study, contributed to the interpretation of results and to the final version of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Florenzo Iannone  https://orcid.org/0000-0003-0474-5344
https://orcid.org/0000-0003-0474-5344
Ewa Wiȩsik-Szewczyk  https://orcid.org/0000-0001-8509-4453
https://orcid.org/0000-0001-8509-4453
Mohamed Tharwat Hegazy  https://orcid.org/0000-0002-2939-5611
https://orcid.org/0000-0002-2939-5611
Luca Cantarini  https://orcid.org/0000-0002-7352-1275
https://orcid.org/0000-0002-7352-1275
Contributor Information
Carla Gaggiano, Research Center of Systemic Autoinflammatory Diseases and Behçet’s Disease, and Rheumatology-Ophthalmology Collaborative Uveitis Center, Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy; Clinical Pediatrics, Department of Molecular Medicine and Development, University of Siena, Siena, Italy.
Donato Rigante, Department of Life Sciences and Global Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy; Rare Diseases and Periodic Fevers Research Centre, Università Cattolica del Sacro Cuore, Rome, Italy.
José Hernández-Rodríguez, Vasculitis Research Unit and Autoinflammatory Diseases Clinical Unit, Department of Autoimmune Diseases, Hospital Clinic of Barcelona, IDIBAPS, University of Barcelona, Barcelona, Spain.
Antonio Vitale, Research Center of Systemic Autoinflammatory Diseases and Behçet’s Disease, and Rheumatology-Ophthalmology Collaborative Uveitis Center, Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy.
Maria Tarsia, Clinical Pediatrics, Department of Molecular Medicine and Development, University of Siena, Siena, Italy.
Alessandra Soriano, Department of Internal Medicine, Arcispedale Santa Maria Nuova-IRCCS, Reggio Emilia, Italy.
Giuseppe Lopalco, Rheumatology Unit, Department of Emergency and Organ Transplantation, University of Bari, Bari, Italy.
Florenzo Iannone, Rheumatology Unit, Department of Emergency and Organ Transplantation, University of Bari, Bari, Italy.
Masen Abdel Jaber, Rheumatology Unit, Santa Chiara Hospital, Trento, Italy.
Roberto Giacomelli, Rheumatology Unit, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy.
Ewa Wiȩsik-Szewczyk, Department of Internal Medicine, Pulmonology, Allergy and Clinical Immunology, Central Clinical Hospital of the Ministry of National Defense, Military Institute of Medicine, Warsaw, Poland.
Marco Cattalini, Paediatric Clinic, University of Brescia and Spedali Civili di Brescia, Brescia, Italy.
Micol Frassi, Rheumatology and Clinical Immunology, Spedali Civili, Brescia, Italy; Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy.
Matteo Piga, Rheumatology Unit, Department of Medical Sciences, University and AOU of Cagliari, Cagliari, Italy.
Gaafar Ragab, Rheumatology and Clinical Immunology Unit, Internal Medicine Department, Faculty of Medicine, Cairo University, Cairo, Egypt.
Jurgen Sota, Research Center of Systemic Autoinflammatory Diseases and Behçet’s Disease, and Rheumatology-Ophthalmology Collaborative Uveitis Center, Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy.
Fiammetta Zunica, Paediatric Clinic, University of Brescia and Spedali Civili di Brescia, Brescia, Italy.
Alberto Floris, Rheumatology Unit, AOU University Clinic, Cagliari, Italy.
Vito Sabato, Faculty of Medicine and Health Sciences, Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerpen, Belgium.
Mohamed Tharwat Hegazy, Rheumatology and Clinical Immunology Unit, Internal Medicine Department, Faculty of Medicine, Cairo University, Cairo, Egypt.
Olga Araújo, Vasculitis Research Unit and Autoinflammatory Diseases Clinical Unit, Department of Autoimmune Diseases, Hospital Clinic of Barcelona, IDIBAPS, University of Barcelona, Barcelona, Spain.
Laura Pelegrín, Clinical Institute of Ophthalmology, Hospital Clinic of Barcelona, IDIBAPS, University of Barcelona, Barcelona, Spain.
Alessandra Fabbiani, Medical Genetics, University of Siena, Siena, Italy.
Alessandra Renieri, Medical Genetics, University of Siena, Siena, Italy; Medical Genetics, Azienda Ospedaliera Universitaria Senese, Siena, Italy.
Salvatore Grosso, Clinical Pediatrics, Department of Molecular Medicine and Development, University of Siena, Siena, Italy.
Claudia Fabiani, Ophthalmology Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy.
Bruno Frediani, Research Center of Systemic Autoinflammatory Diseases and Behçet’s Disease, and Rheumatology-Ophthalmology Collaborative Uveitis Center, Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy.
Luca Cantarini, Research Center of Systemic Autoinflammatory Diseases, Behçet’s Disease Clinic and Rheumatology-Ophthalmology Collaborative Uveitis Center, Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Policlinico ‘Le Scotte’, viale Bracci n. 1, 53100 Siena, Italy.
References
- 1.Lachmann HJ, Papa R, Gerhold K, et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: a series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann Rheum Dis 2014; 73: 2160–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rigante D.A systematic approach to autoinflammatory syndromes: a spelling booklet for the beginner. Expert Rev Clin Immunol 2017; 13: 571–597. [DOI] [PubMed] [Google Scholar]
- 3.Dodé C, André M, Bienvenu T, et al. The enlarging clinical, genetic, and population spectrum of tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum 2002; 46: 2181–2188. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999; 97: 133–144. [DOI] [PubMed] [Google Scholar]
- 5.Ruiz-Ortiz E, Iglesias E, Soriano A, et al. Disease phenotype and outcome depending on the age at disease onset in patients carrying the R92Q low-penetrance variant in TNFRSF1A gene. Front Immunol 2017; 278: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantarini L, Lucherini OM, Cimaz R, et al. Idiopathic recurrent pericarditis refractory to colchicine treatment can reveal tumor necrosis factor receptor-associated periodic syndrome. Int J Immunopathol Pharmacol 2009; 22: 1051–1058. [DOI] [PubMed] [Google Scholar]
- 7.Cantarini L, Lucherini OM, Baldari CT, et al. Familial clustering of recurrent pericarditis may disclose tumour necrosis factor receptor-associated periodic syndrome. Clin Exp Rheumatol 2010; 28: 405–407. [PubMed] [Google Scholar]
- 8.Ravet N, Rouaghe S, Dodé C, et al. Clinical significance of P46L and R92Q substitutions in the tumour necrosis factor superfamily 1A gene. Ann Rheum Dis 2006; 65: 1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aganna E, Hammond L, Hawkins PN, et al. Heterogeneity among patients with tumor necrosis factor receptor-associated periodic syndrome phenotypes. Arthritis Rheum 2003; 48: 2632–2644. [DOI] [PubMed] [Google Scholar]
- 10.Aksentijevich I, Galon J, Soares M, et al. The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers. Am J Hum Genet 2001; 69: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandemange S, Cabasson S, Sarrabay G, et al. Clinical dose effect and functional consequences of R92Q in two families presenting with a TRAPS/PFAPA-like phenotype. Mol Genet Genomic Med 2017; 5: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aganna E, Hawkins PN, Ozen S, et al. Allelic variants in genes associated with hereditary periodic fever syndromes as susceptibility factors for reactive systemic AA amyloidosis. Genes Immun 2004; 5: 289–293. [DOI] [PubMed] [Google Scholar]
- 13.Amoura Z, Dodé C, Hue S, et al. Association of the R92Q TNFRSF1A mutation and extracranial deep vein thrombosis in patients with Behçet’s disease. Arthritis Rheum 2005; 52: 608–611. [DOI] [PubMed] [Google Scholar]
- 14.Cantarini L, Lucherini OM, Vitale A, et al. Expanding spectrum of TNFRSF1A gene mutations among patients with idiopathic recurrent acute pericarditis. Intern Med J 2013; 43: 725–727. [DOI] [PubMed] [Google Scholar]
- 15.Goris A, Fockaert N, Cosemans L, et al. TNFRSF1A coding variants in multiple sclerosis. J Neuroimmunol 2011; 235: 110–112. [DOI] [PubMed] [Google Scholar]
- 16.Van Gijn ME, Ceccherini I, Shinar Y, et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J Med Genet 2018; 55: 530–537. [DOI] [PubMed] [Google Scholar]
- 17.Bachetti T, Chiesa S, Castagnola P, et al. Autophagy contributes to inflammation in patients with TNFR-associated periodic syndrome (TRAPS). Ann Rheum Dis 2013; 72: 1044–1052. [DOI] [PubMed] [Google Scholar]
- 18.Lobito AA, Kimberley FC, Muppidi JR, et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS). Blood 2006; 108: 1320–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulua AC, Simon A, Maddipati R, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1- associated periodic syndrome (TRAPS). J Exp Med 2011; 208: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon A, Park H, Maddipati R, et al. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc Natl Acad Sci USA 2010; 107: 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattorno M, Pelagatti MA, Meini A, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum 2008; 58: 1516–1520. [DOI] [PubMed] [Google Scholar]
- 22.De Benedetti F, Gattorno M, Anton J, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med 2018; 378: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 23.Gattorno M, Obici L, Cattalini M, et al. Canakinumab treatment for patients with active recurrent or chronic TNF receptor-associated periodic syndrome (TRAPS): an open-label, phase II study. Ann Rheum Dis 2017; 76: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malcova H, Strizova Z, Milota T, et al. IL-1 inhibitors in the treatment of monogenic periodic fever syndromes: from the past to the future perspectives. Front Immunol 2021; 11: 619257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gattorno M, Hofer M, Federici S, et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis 2019; 78: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 26.ter Haar NM, Oswald M, Jeyaratnam J, et al. Recommendations for the management of autoinflammatory diseases. Ann Rheum Dis 2015; 74: 1636–1644. [DOI] [PubMed] [Google Scholar]
- 27.Piram M, Koné-Paut I, Lachmann HJ, et al. Validation of the auto-inflammatory diseases activity index (AIDAI) for hereditary recurrent fever syndromes. Ann Rheum Dis 2014; 73: 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 29.Cantarini L, Rigante D, Lucherini OM, et al. Role of etanercept in the treatment of tumor necrosis factor receptor-associated periodic syndrome: personal experience and review of the literature. Int J Immunopathol Pharmacol 2010; 23: 701–707. [DOI] [PubMed] [Google Scholar]
- 30.Federico G, Rigante D, Pugliese AL, et al. Etanercept induces improvement of arthropathy in chronic infantile neurological cutaneous articular (CINCA) syndrome. Scand J Rheumatol 2003; 32: 312–314. [DOI] [PubMed] [Google Scholar]
- 31.Hashem H, Kelly SJ, Ganson NJ, et al. Deficiency of adenosine deaminase 2 (DADA2), an inherited cause of polyarteritis nodosa and a mimic of other systemic rheumatologic disorders. Curr Rheumatol Rep 2017; 19: 70. [DOI] [PubMed] [Google Scholar]
- 32.Sagˇ E, Sönmez HE, Demir S, et al. Chronic recurrent multifocal osteomyelitis in children: a single center experience over five years. Turk J Pediatr 2019; 61: 386–391. [DOI] [PubMed] [Google Scholar]
- 33.Rigante D, Lopalco G, Vitale A, et al. Key facts and hot spots on tumor necrosis factor receptor-associated periodic syndrome. Clin Rheumatol 2014; 33: 1197–1207. [DOI] [PubMed] [Google Scholar]
- 34.Vitale A, Insalaco A, Sfriso P, et al. A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front Pharmacol 2016; 7: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jéru I, Charmion S, Cochet E, et al. Involvement of the same TNFR1 residue in mendelian and multifactorial inflammatory disorders. PLoS ONE 2013; 8: e69757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebelo SL, Bainbridge SE, Amel-Kashipaz MR, et al. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum 2006; 54: 2674–2687. [DOI] [PubMed] [Google Scholar]
- 37.Balci S, Kisla Ekinci RM, Melek E, et al. Phenotypic variability in two patients with tumor necrosis factor receptor associated periodic fever syndrome emphasizes a rare manifestation: immunoglobulin A nephropathy. Eur J Med Genet 2020; 63: 103780. [DOI] [PubMed] [Google Scholar]
- 38.Ter Haar N, Lachmann H, Özen S, et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann Rheum Dis 2013; 72: 678–685. [DOI] [PubMed] [Google Scholar]
- 39.Gaggiano C, Vitale A, Obici L, et al. Clinical features at onset and genetic characterization of pediatric and adult patients with TNF-α Receptor-Associated Periodic Syndrome (TRAPS): a series of 80 cases from the AIDA Network. Mediators Inflamm 2020; 2020: 8562485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lainka E, Neudorf U, Lohse P, et al. Incidence of TNFRSF1A mutations in German children: epidemiological, clinical and genetic characteristics. Rheumatology 2009; 48: 987–991. [DOI] [PubMed] [Google Scholar]
- 41.Pelagatti MA, Meini A, Caorsi R, et al. Long-term clinical profile of children with the low-penetrance R92Q mutation of the TNFRSF1A gene. Arthritis Rheum 2011; 63: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cantarini L, Rigante D, Merlini G, et al. The expanding spectrum of low-penetrance TNFRSF1A gene variants in adults presenting with recurrent inflammatory attacks: clinical manifestations and long-term follow-up. Semin Arthritis Rheum 2014; 43: 818–823. [DOI] [PubMed] [Google Scholar]
- 43.Hernández-Rodríguez J, Ruíz-Ortiz E, Tomé A, et al. Clinical and genetic characterization of the autoinflammatory diseases diagnosed in an adult reference center. Autoimmun Rev 2016; 15: 9–15. [DOI] [PubMed] [Google Scholar]
- 44.Lopalco G, Rigante D, Vitale A, et al. Adult-onset tumour necrosis factor receptor-associated periodic syndrome presenting with refractory chronic arthritis. Clin Exp Rheumatol 2015; 33: S171–S172. [PubMed] [Google Scholar]
- 45.Ozen S, Kuemmerle-Deschner JB, Cimaz R, et al. International retrospective chart review of treatment patterns in severe familial mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, and mevalonate kinase deficiency/hyperimmunoglobulinemia D syndrome. Arthritis Care Res 2017; 69: 578–586. [DOI] [PubMed] [Google Scholar]
- 46.Camprubí D, Mitjavila F, Arostegui JI, et al. Efficacy of anakinra in an adult patient with recurrent pericarditis and cardiac tamponade as initial manifestations of tumor necrosis factor receptor-associated periodic syndrome due to the R92Q TNFRSF1A variant. Int J Rheum Dis 2017; 20: 510–514. [DOI] [PubMed] [Google Scholar]
- 47.Mejías Trueba M, Alonso Moreno M, Puñal Garrido N, et al. An unusual case of allergic reaction to anakinra in a patient with tumor necrosis factor receptor-1 associated periodic syndrome (TRAPS) and subsequent canakinumab treatment. Eur J Case Rep Intern Med 2020; 7: 001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrés M, Pascual E.Anakinra for a refractory case of intermittent hydrarthrosis with a TRAPS-related gene mutation. Ann Rheum Dis 2013; 72: 155. [DOI] [PubMed] [Google Scholar]
- 49.Papa R, Lane T, Minden K, et al. INSAID variant classification and Eurofever criteria guide optimal treatment strategy in patients with TRAPS: data from the Eurofever registry. J Allergy Clin Immunol Pract 2020; 9: 783–791.e4. [DOI] [PubMed] [Google Scholar]
- 50.Agulló L, Malhotra S, Fissolo N, et al. Molecular dynamics and intracellular signaling of the TNF-R1 with the R92Q mutation. J Neuroimmunol 2015; 289: 12–20. [DOI] [PubMed] [Google Scholar]
- 51.Lewis AK, Valley CC, Sachs JN.TNFR1 signaling is associated with backbone conformational changes of receptor dimers consistent with overactivation in the R92Q TRAPS mutant. Biochemistry 2012; 51: 6545–6555. [DOI] [PubMed] [Google Scholar]
- 52.Rebelo SL, Amel-Kashipaz MR, Radford PM, et al. Novel markers of inflammation identified in tumor necrosis factor receptor-associated periodic syndrome (TRAPS) by transcriptomic analysis of effects of TRAPS-associated tumor necrosis factor receptor type I mutations in an endothelial cell line. Arthritis Rheum 2009; 60: 269–280. [DOI] [PubMed] [Google Scholar]
- 53.Schnappauf O, Aksentijevich I.Current and future advances in genetic testing in systemic autoinflammatory diseases. Rheumatology 2019; 58: vi44–vi55. [DOI] [PMC free article] [PubMed] [Google Scholar]