Abstract
Objectives:
The main objective of this study was to compare the effectiveness of empiric treatment with narrow-spectrum therapy versus broad-spectrum therapy for children hospitalized with community-acquired pneumonia (CAP) at the University of Gondar Referral Hospital, Gondar, Ethiopia.
Methods:
Institutional-based retrospective chart review was conducted at the University of Gondar Referral Hospital (GURH) pediatrics ward from 1 February 2016 to 30 April 2016. The collected data were entered and analyzed using Statistical Package for Social Sciences (SPSS) version 20. Descriptive statistics were done to present the basic features and summary of the data set. In addition, binary logistics and multivariable logistic regression analysis were conducted to test for an association between the dependent and independent variables. A P value of <0.05 was taken to declare statistical significance at a 95% confidence interval.
Result:
A total of 147 patients with CAP were included in the study. Seven different treatment regimens were employed for the 147 children hospitalized. About 63 (42.9%) of the study participants received a narrow-spectrum antibiotic and 84 (57.1%) received a broad-spectrum antibiotic. There was no significant difference between the broad and narrow spectrum treatment groups in main treatment outcomes. The median length of stay (LOS) for the study population was 3 days. The median LOS was shorter among those receiving narrow-spectrum therapy compared with those receiving broad-spectrum therapy. Treatment dose and duration of therapy were significantly associated with treatment outcome (P < 0.0001 and P = 0.003), respectively.
Conclusion:
The effectiveness of narrow-spectrum therapy is similar to that of broad-spectrum therapy for children hospitalized with CAP. Treatment regimens for children with community-acquired pneumonia should be selected based on their safety profile and their tendency for antibiotic resistance.
Keywords: Antibiotics, narrow-spectrum, broad-spectrum, children, pneumonia
Introduction
Community-acquired pneumonia (CAP) is one of the most common and serious causes of hospitalization among both adults and children worldwide. In the year 2014 alone more than 800,000 hospitalizations and more than 400,000 emergency department visits were recorded in the United States alone.1,2 It also took a significant amount of health care budget in many countries in which the United States hospitals alone took around $9.5 billion national aggregate costs for the treatment of pneumonia in 2013.3 Antimicrobial selection for the treatment of childhood CAP is nearly always made without direct knowledge of the causative microorganism. Empirical treatment with broad-spectrum antibiotics is common, even when clinical signs of infection were absent.4
Antibiotics are classified as “narrow-spectrum” or “broad-spectrum” depending on the range of bacterial types they affect. Narrow-spectrum antibiotics are effective against a certain group of bacterial types while broad-spectrum antibiotics are effective against a broader number of bacterial types and, thus, can be used to treat several infectious diseases. Broad-spectrum antibiotics are mainly useful when the infecting pathogen (bacteria) is unknown. Narrow-spectrum therapy can also be explained by the exclusive use of parenteral penicillin or ampicillin, and broad-spectrum therapy can be defined by the exclusive use of parenteral ceftriaxone or cefotaxime.5 Optimizing the utilization of antimicrobials is important for minimizing the spread of antimicrobial resistance on a global scale.
Antimicrobial resistance is said to occur when a certain bacterial species changes in response to the use of those medicines. Antibiotic resistance is enhanced by the improper utilization of antibiotics, as well as poor infection prevention and control. Intravenous penicillin or ampicillin is recommended as first-line treatment of hospitalized children with CAP by recent treatment guidelines.6,7 These guidelines were developed to establish a standard of care for children with CAP. Their promotion of the use of narrow-spectrum antibiotics was intended to minimize the development of bacterial resistance in the community, which has been found to rise with the increased use of broad-spectrum antibiotics.8
Many studies were conducted to investigate the effectiveness of narrow versus broad-spectrum antibiotics in the treatment of infections in children. A randomized trial conducted among children hospitalized with severe CAP in Brazil found that empirical therapy with parenteral amoxicillin/clavulanic acid was as efficacious as combination therapy with oxacillin and ceftriaxone.9 The finding proposed that routine use of penicillin or ampicillin does not contribute to considerable increases in hospitalization costs despite the increased dosing frequency of these medications compared with some third-generation cephalosporins, such as once-daily ceftriaxone.9
Another study conducted in the United States reported that there was no difference in length of stay (LOS), cost, need for intensive care, or readmissions between those children with CAP treated with parenteral ampicillin or penicillin and those treated with broader spectrum third-generation cephalosporin therapy.10 Similar findings were reported by a randomized trial conducted on hospitalized children in Finland, which showed that there were no differences in outcomes (time to recovery, normalization of laboratory parameters, or treatment failure) between those children receiving parenteral penicillin and cefuroxime treatment.11
Treatment with narrow-spectrum antimicrobials was also recommended for most hospitalized children with community-acquired pneumonia (CAP) by the Pediatric Infectious Diseases Society/Infectious Diseases Society of America (PIDS/IDSA).7 However, there is no such scientific evidence to support that this consensus-based recommendation was as effective as the more adopted broad-spectrum antibiotics therapy. Recent guidelines for the management of children infected with pneumonia recommend narrow-spectrum antimicrobial agents (e.g. ampicillin) for most children; however, only a few studies have directly compared the effectiveness of narrow-spectrum agents to the broader spectrum third-generation antibiotics commonly used among children hospitalized with pneumonia. Therefore, the current study aimed to assess the effectiveness of narrow-spectrum to that of the broader spectrum antibiotics for the treatment of childhood pneumonia.
Study methodology
Study period and setting
The study was conducted at the pediatric ward of the University of Gondar Referral Hospital, Gondar, Northwest Ethiopia. The hospital is located in Gondar town, Amhara National Regional State, which is about 727 km far from Addis Ababa (the capital city of the country). According to the 2007 population and housing census report, the total population size of Gondar was estimated to be around 206,987.12 At the time of the current study, Gondar has one referral hospital and five government Health Centers. The University of Gondar Referral Hospital is a teaching Hospital established in 1954 and serves more than five million people of the North Gondar zone and peoples of the neighboring areas. The study was conducted from 1 February 2016 to 30 April 2016.
Study design
Institutional based retrospective chart review was conducted at the University of Gondar Referral Hospital (UoGRH) pediatric ward.
Source population
The source population for the study was all children in the pediatric ward of the University of Gondar Referral Hospital.
Study population
All children who fulfill the inclusion criteria to the study.
Inclusion and exclusion criteria
Inclusion criteria
Children less than 18 years old who were on pneumonia treatment at the pediatric ward of the University of Gondar Referral Hospital.
Exclusion criteria
Children who were diagnosed with pneumonia but does not begin any treatment.
Study variables
Dependent variable
Improvement of signs and symptoms of pneumonia.
Independent variables
These include socio-demographic variables (age, sex, weight, breastfeeding, etc.), clinical factors (severity of disease, presence of fever, fast breathing, etc.), known medical co-morbidity, multiple medications intake, duration of therapy, the dose of drugs, and vaccination status.
Sample size and sampling procedure
Sample size determination
All children who fulfilled the inclusion criteria and that were admitted during the study period were included. As such, no sample size calculation and/or formula was needed.
Data collection procedure
A review of patient medical records (chart review) was conducted using a semi-structured questionnaire. The questionnaire was adapted from previously conducted similar studies.5,10 It was divided into two parts in which the first part consisted of patient’s socio-demographic data (age, weight, vaccination status, breastfeeding, etc.) and the second part consisted of the clinical characteristics of patients (exposure to antibiotics, type of antibiotics taken, duration of therapy, duration of hospitalization, the total cost of treatment, improvement of sign and symptoms, etc.). The data were collected by two of the investigators.
The classification of the disease severity and type of pneumonia were documented (in the medical charts) based on the recommended guidelines.13 In such classification, “typical (S. pneumonia, H. influenza)” was defined as acute onset of fever, cough with purulent sputum, dyspnea, consolidation on Chest X-Ray (CXR) while the “atypical (Mycoplasma, Chlamydia, Legionella, viral)” was defined with its presentation of insidious onset of dry cough, extrapulmonary symptoms (Nausea/Vomiting, diarrhea, headache, myalgias, sore throat), a patchy interstitial pattern on CXR, and elevated transaminases & low serum sodium with Legionella. The investigations used also include Sputum Gram stain, Sputum bacterial culture, Blood cultures, CXR (PA & lateral), and pleural fluid analysis, among others.
Data quality assurance
The data were collected by the investigators themselves. A pretest was done on 22 participants before the actual data collection to check for the reliability and applicability of the questionnaire and in case if corrections were important. Those 22 participants were excluded from the actual study. The collected data were checked for its completeness and accuracy each day before entering into software for analysis.
Data processing and analysis
The collected data were entered and analyzed using SPSS version 20.14 Descriptive statistics were conducted and to test for association between the dependent and independent variables, binary logistic and multivariable logistic regression analysis was conducted. A P value of <0.05 was taken to declare statistical significance at a 95% confidence interval.
Operational definition
Exposure to antibiotic: Patients taking antibiotics within 7 days before hospitalization.
Moderate pneumonia: Patients who have signs and symptoms of moderate fever, cough, and fast breathing.
Severe pneumonia: Patients who have a sign and symptoms of fever, cough fast, breathing, nasal flaring, respiratory distress, chest indrawing, and difficulty of breastfeeding.
Relief of signs and symptom: Breathing is slower, less indrawing of the lower chest wall, less fever (<38°C), feeding (well-improved ability to eat and drink), and better oxygen saturation.
Ethical consideration
Ethical clearance was secured from the ethical review board of the School of Pharmacy, College of medicine and health sciences, the University of Gondar with an approval number of SoP 826/08. The caretakers were the legally authorized representative of minor subjects. Data were collected anonymously so that there were no personal identifiers. Furthermore, the collected data were kept confidential and used strictly for study purpose.
Result
A total of 147 patients with CAP were included in the study. The majority of them were male 91 (61.9%) and 107 (72.8%) were under 5 years of age (Table 1). The majority of the study participants 84 (57.1%) received a broad-spectrum antibiotic while the remaining 63 (42.9%) received a narrow-spectrum antibiotic.
Table 1.
Socio-demographic characteristic of the study participants.
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Sex | Male | 91 | 61.9% |
| Female | 56 | 38.1% | |
| Age | ⩽5 years old | 107 | 72.8% |
| >5 years old | 40 | 27.2% | |
| Weight | ⩽10 kg | 91 | 61.9% |
| >10 kg | 56 | 38.1% | |
| Religion | Orthodox Christian | 128 | 87.1% |
| Muslim | 17 | 11.6% | |
| Protestant | 2 | 1.4% | |
| Residence | Rural | 87 | 59.18% |
| Urban | 60 | 40.82% | |
| Breastfeeding | Yes | 85 | 57.1% |
| No | 62 | 42.2% | |
| Vaccinated | Yes | 100 | 68% |
| No | 47 | 32% |
Clinical outcomes
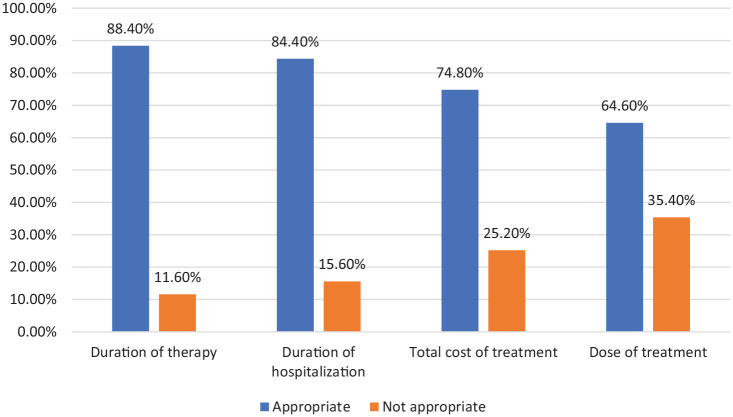
From the total of 147 children admitted to the hospital with pneumonia, 127 (86.4) had severe pneumonia while the remaining 20 (13.6%) had moderate pneumonia. The different treatment parameters of CAP for children admitted to the pediatric ward were analyzed based on the standard treatment guideline. The result showed that 88.4% of children were receiving the appropriate duration of therapy while only 64.6% of them were receiving the recommended dose of drugs (Figure 1).
Figure 1.
Appropriateness of duration of hospitalization, duration of therapy, total cost, and dose of drugs among children hospitalized with CAP at the University of Gondar Referral Hospital.
About 22.44% of the study participants were exposed to antibiotics before admission to the hospital while 77.6% were not exposed. Seven different treatment regimens had been employed for the 147 children hospitalized with pneumonia. Crystalline penicillin (44.9%) followed by ceftriaxone (25.9%), and Ampicillin with gentamycin (11.6%) were the most frequently prescribed medications (Figure 2).
Figure 2.
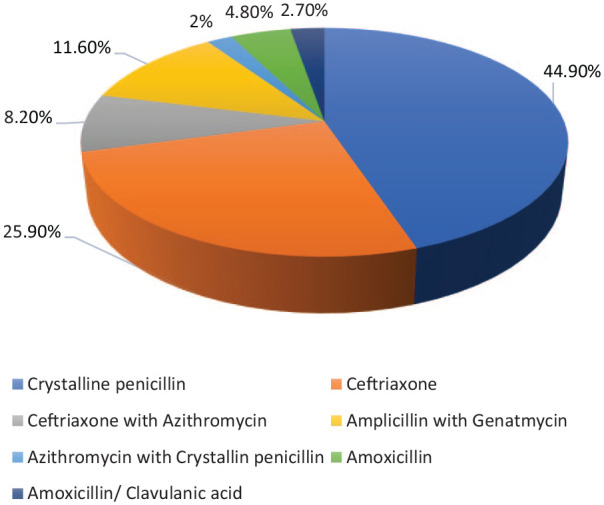
Most prescribed medications for the treatment of CAP at the University of Gondar Referral Hospital.
Sub-group analysis
The sub-group analysis based on the type of antibiotic treatment in the community before hospitalization revealed that patients receiving either crystalline penicillin/ or a macrolide antibiotic had significantly no better outcomes if they were treated with broad-spectrum antibiotics during their hospital stay. There was also no significant difference between the broad and narrow spectrum treatment groups in main treatment outcomes regardless of the type of antibiotic therapy administered after admission to the hospital.
In the binary logistic regression analysis: duration of therapy (crude odds ratio (COR) = 0.143) and weight of the patient (COR = 0.5) were significantly associated with improvement of signs and symptoms at P < 0.05% and 95% confidence interval (CI). These variables were then entered into the multivariable logistic regression model to check for possible confounding factors. The multivariable logistic regression analysis also showed that duration of therapy (adjusted odds ratio (AOR) = 0.134, P = 0.002) and weight of the patient (AOR = 0.204, P = 0.015) were significantly associated with relief of signs and symptoms (Table 2).
Table 2.
Association between relief of signs and symptoms and the different independent variables.
| Variables | N (%) | COR (95% CI) |
AOR (95% CI) | Significance | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age | ⩽5 years | 107 (72.8) | 0.864 | 1.797 | 0.409 | 0.448 | 7.214 |
| >5 years | 40 (17.2) | ||||||
| Weight | 10 kg | 91 (61.9) | 0.5 | 0.204 | 0.045** | 0.043 | 0.966 |
| >10 kg | 56 (38.1) | ||||||
| Breastfeeding | Yes | 85 (57.8) | 0.628 | 1.425 | 0.612 | 0.363 | 5.602 |
| No | 62 (42.2) | ||||||
| Appropriateness of dose of treatment | Appropriate | 95 (64.6) | 0.242 | 0.267 | 0.12 | 0.95 | 0.75 |
| Not-appropriate | 52 (33.4) | ||||||
| Type of therapy | Narrow | 63 (42.8) | 0.498 | 0.458 | 0.137 | 0.164 | 1.280 |
| Broad | 84 (57.2) | ||||||
| Duration of therapy | Appropriate | 130 (88.4) | 0.143 | 0.134 | 0.002** | 0.038 | 0.470 |
| Not-appropriate | 17 (11.6) | ||||||
| Severity of pneumonia | Sever | 127 (86.4) | 0.885 | 0.827 | 0.806 | 0.182 | 3.754 |
| Moderate | 20 (13.6) | ||||||
| Previous exposure to drugs | Yes | 114 (77.5) | 2.000 | 2.638 | 0.089 | 0.863 | 8.061 |
| No | 33 (22.5) | ||||||
| Vaccination status | Yes | 100 (68) | 0.758 | 1.126 | 0.836 | 0.364 | 3.482 |
| No | 47 (32) | ||||||
COR: crude odds ratio; AOR: adjusted odds ratio; CI: confidence interval.
P < 0.05.
The median LOS for the study participants was 3 days. The median LOS was shorter among those receiving narrow-spectrum therapy compared with those receiving broad-spectrum therapy. However, the difference was not significant at P < 0.05. In the standard cost analysis, the total cost of treatment was higher with an increased dose of the drug and longer duration of therapy (AOR = 0.073, P < 0.001, AOR = 0.122, P = 0.003), respectively (Table 3).
Table 3.
Cost-analysis.
| Categorical | COR | Significance | AOR | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 1.487 | 0.493 | 1.660 | 0.390 | 7.067 |
| Weight | 0.75 | 0.436 | 0.533 | 0.109 | 2.595 |
| Breastfeeding | 0.774 | 0.842 | 1.161 | 0.267 | 5.046 |
| Dose | 0.073 | 0.001** | 0.073 | 0.025 | 0.211 |
| Therapy | 0.758 | 0.808 | 1.129 | 0.425 | 2.997 |
| Duration of therapy | 0.183 | 0.003* | 0.122 | 0.031 | 0.484 |
| Pneumonia | 3.424 | 0.238 | 3.234 | 0.460 | 22.742 |
| Exposure to drugs | 0.595 | 0.280 | 0.474 | 0.122 | 1.836 |
| Vaccination | 0.731 | 0.241 | 2.014 | 0.625 | 6.489 |
COR: crude odds ratio; AOR: adjusted odds ratio; CI: confidence interval.
P value < 0.05; **P value < 0.001.
Discussion
In the current study, there was no significant difference between the broad and narrow spectrum treatment groups in main treatment outcomes regardless of the type of antibiotic therapy administered after admission to the hospital. This finding was in line with previous studies conducted in the United States in which clinical outcomes and associated costs for children hospitalized with CAP were not different when treatment was using narrow-spectrum compared with broad-spectrum antibiotic therapy.5 A similar finding was also reported by a study conducted in Helsinki, Finland, to compare narrow versus broad-spectrum parenteral antimicrobials against common childhood infections. The study revealed that Procaine penicillin was as effective and safe as cefuroxime for common community-acquired infections in immunocompetent children.10 Similar results were also reported by other studies.15
In the present study, Crystalline penicillin (44.9%) followed by ceftriaxone (25.9%), and Ampicillin with gentamycin (11.6%) were the most frequently prescribed medications. This finding was different from a study conducted in Bangladesh, in which parenteral ceftriaxone was the most commonly prescribed antibiotic 40 (50%), followed by cefotaxime plus amikacin 14 (17.5%), and cefuroxime 7 (8.8%).16 This difference could be due to differences in standard treatment guidelines in the two areas or attributed to the difference in antibiotic resistance patterns in the two areas. It could also be due to differences in the prescriber’s prescribing practice and following the standard treatment guidelines. The response to treatment with Crystalline Penicillin was well investigated and it was found to be very effective and, thus, can be used as the first-line drug in the treatment of children with CAP.17
The current study showed that 88.4% of the study participants were receiving the appropriate duration of therapy and the median LOS was 3 days. Evidence shows that for severe pneumonia, at least 3 days of treatment were recommended.18,19 Studies also show that although shorter courses than 10 days may be just as effective, particularly for more mild disease managed on an outpatient basis, infections caused by certain pathogens, particularly CA-MRSA, may require longer treatment than those caused by S. pneumonia.7 Shortening the duration of antibiotic therapy for CAP may result in fewer adverse events, decreased antimicrobial resistance, and decreased costs for both individuals and the health care system as a whole. The overall goal of treatment should be to treat children with the shortest duration of therapy possible to minimize the adverse consequences of antibiotics without compromising clinical outcomes.
The overall cost of hospitalization did not differ between children treated with narrow- versus broad-spectrum antimicrobial therapy in the current study. This finding suggests that routine use of penicillin or ampicillin does not contribute to substantial increases in hospitalization costs.20
In the present study, improvement of patient conditions was significantly associated with duration of therapy and weight of the child. This finding was in line was previously conducted studies.21–24 The appropriate duration of therapy is an important factor that determines the effectiveness of a particular treatment regimen. It determines the period in which a particular drug reaches its therapeutic dose to show its therapeutic effect. A shorter duration of therapy may hinder a particular drug to reach its maximum plasma level and show its maximum effect. The weight of the child (especially low birth weight) is also a single significant factor that determines the disease-fighting capacity and survival of a child after a particular infection.23
Limitation of the study
As the study was a retrospective chart review of patient records, important patient information may be missing due to poor documentation, and/ or recording error/bias. The study was also devoid of making a temporal relationship (cause and effect relationship) between the outcome variable (improvement of signs and symptoms) and the different independent variables. The other limitation of the current study could be the lack of microbiology data, and long enough clinical follow-up of patients to ensure the absence of late treatment failures, readmissions, and so on, which makes it difficult to compare the long-term clinical outcomes as well as associated costs between the treatment alternatives.
Conclusion
The effectiveness of narrow-spectrum therapy was similar to that of broad-spectrum therapy for children hospitalized with CAP. Improvement of patient signs and symptoms was significantly associated with the duration of therapy and weight of the child. A significant number of patients were not receiving the recommended dose of therapy and also there were patients treated for lower than the recommended duration of treatment.
Recommendation
Treatment regimens for children with community-acquired pneumonia should be selected based on their safety profile and their tendency for antibiotic resistance. Prescribers should strictly follow approved standard treatment guidelines for the treatment of CAP in children and focus on minimizing the adverse consequences of drug therapy without compromising clinical outcomes. The hospital management should regularly check the prescribing pattern of antibiotics for treatment of childhood infections and monitor standard treatment guidelines are strictly followed.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121211044379 for Comparative analysis of the effectiveness of narrow-spectrum versus broad-spectrum antibiotics for the treatment of childhood pneumonia by Chilot Abiyu Demeke, Getnet Mequanent Adinew, Tamrat Befekadu Abebe, Abebech Tewabe Gelaye, Sisay G/Hana Gemeda and Dawit Kumilachew Yimenu in SAGE Open Medicine
Acknowledgments
The authors acknowledge the University of Gondar for its support and facilitating the study and all the study participants for their collaboration and participation in the study.
Footnotes
Author contributions: CA performed the data collection and carried out the statistical analysis and interpretation. GM and TB participated in the methodological selection, sequence alignment, and revision of the work. AT and SG participated in the data collection and statistical analysis. DK participated in the sequence alignment and performed the revision and draft of the article. All authors read and approved the final article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical clearance was secured from the ethical review board of the School of Pharmacy, College of Medicine and Health Sciences, the University of Gondar with an approval number of SoP 826/08.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from legally authorized representative before the study.
Informed written consent was obtained from each of the children’s caretakers before data collection, and the data were collected anonymously with no personally traceable information on the questionnaire. The caretakers were the legally authorized representative of minor subjects.
ORCID iDs: Chilot Abiyu Demeke  https://orcid.org/0000-0002-4995-8063
https://orcid.org/0000-0002-4995-8063
Dawit Kumilachew Yimenu  https://orcid.org/0000-0002-0699-1840
https://orcid.org/0000-0002-0699-1840
Availability of data and materials: The data sets generated and/or analyzed during the current study are not available in public due to the requirement of confidentiality upon which the study was approved by the Ethical review committee and consent was secured from the legally authorized representative but is available from the corresponding author on reasonable request.
Supplemental material: Supplemental material for this article is available online.
References
- 1.McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005-2014 (HCUP Statistical Brief #225). Rockville, MD: Agency for Healthcare Research and Quality, 2017. [Google Scholar]
- 2.Nawar EW, Niska RW, Xu J. National hospital ambulatory medical care survey: 2005 emergency department summary. Adv Data 2007; 386: 1–32. [PubMed] [Google Scholar]
- 3.Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013 (HCUP Statistical Brief #204). Rockville, MD: Agency for Healthcare Research and Quality, 2016. [PubMed] [Google Scholar]
- 4.Braykov NP, Morgan DJ, Schweizer ML, et al. Assessment of empirical antibiotic therapy optimisation in six hospitals: an observational cohort study. Lancet Infect Dis 2014; 14(12): 1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DJ, Hall M, Shah SS, et al. Narrow vs broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics 2013; 132(5): e1141–e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66(Suppl. 2): ii1–ii23. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53: e25–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellit TH, Owens RC, McGowan JE, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159–177. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro CF, Ferrari GF, Fioretto JR. Antibiotic treatment schemes for very severe community-acquired pneumonia in children: a randomized clinical study. Rev Panam Salud Publica 2011; 29(6): 444–450. [PubMed] [Google Scholar]
- 10.Vuori-Holopainen E, Peltola H, Kallio MJT; SE-TU Study Group. Narrow- versus broad-spectrum parenteral antimicrobials against common infections of childhood: a prospective and randomised comparison between penicillin and cefuroxime. Eur J Pediatr 2000; 159(12): 878–884. [DOI] [PubMed] [Google Scholar]
- 11.Breuer O, Blich O, Cohen-Cymberknoh M, et al. Antibiotic treatment for children hospitalized with community-acquired pneumonia after oral therapy. Pediatr Pulmonol 2015; 50(5): 495–502. [DOI] [PubMed] [Google Scholar]
- 12.Federal Democratic Republic of Ethiopia Population Census Commission. Summary and statistical report of the 2007 population and housing census. Addis Ababa, Ethiopia: Federal Democratic Republic of Ethiopia Population Census Commission, 2008. [Google Scholar]
- 13.Food, Medicine and Healthcare Administration and Control Authority (FMHACA) of Ethiopia. Standard treatment guidelines for general hospitals. 3rd ed.Addis Ababa, Ethiopia: FMHACA, 2014. [Google Scholar]
- 14.IBM Corporation. IBM SPSS statistics for Windows, version 20.0. Armonk, NY: IBM Corporation, 2011. [Google Scholar]
- 15.Juvén T, Mertsola J, Waris M, et al. Clinical response to antibiotic therapy for community-acquired pneumonia. Eur J Pediatr 2004; 163(3): 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid MM, Chisti MJ, Akter D, et al. Antibiotic use for pneumonia among children under-five at a pediatric hospital in Dhaka city, Bangladesh. Patient Prefer Adherence 2017; 11: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aryal N, Neopane AK, Thapa M, et al. Crystalline penicillin for community acquired pneumonia: does it still work? Med J Shre Birend Hosp 2012; 11(2): 36–39. [Google Scholar]
- 18.World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva: World Health Organization, 2005. [Google Scholar]
- 19.Food, Medicine and Healthcare Administration and Control Authority (FMHACA) of Ethiopia. Standard treatment guidelines for general hospitals. 2nd ed.Addis Ababa, Ethiopia: FMHACA, 2010. [Google Scholar]
- 20.Ferech M, Coenen S, Malhotra-Kumar S, et al. European surveillance of antimicrobial consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother 2006; 58: 401–407. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi S, Malik GK, Jain A, et al. Study of ventilator associated pneumonia in neonatal intensive care unit: characteristics, risk factors and outcome. Intern J Med Update 2010; 5(1): 12–19. [Google Scholar]
- 22.Tan B, Zhang F, Zhang X, et al. Risk factors for ventilator-associated pneumonia in the neonatal intensive care unit: a meta-analysis of observational studies. Eur J Pediatr 2014; 173(4): 427–434. [DOI] [PubMed] [Google Scholar]
- 23.Victora CG, Fuchs SC, Flores JA, et al. Risk factors for pneumonia among children in a Brazilian metropolitan area. Pediatrics 1994; 93(6): 977–985. [PubMed] [Google Scholar]
- 24.Ramachandran P, Nedunchelian K, Vengatesan A, et al. Risk factors for mortality in community-acquired pneumonia among children aged 1–59 months admitted in a referral hospital. Indian Pediatr 2012; 49(11): 889–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121211044379 for Comparative analysis of the effectiveness of narrow-spectrum versus broad-spectrum antibiotics for the treatment of childhood pneumonia by Chilot Abiyu Demeke, Getnet Mequanent Adinew, Tamrat Befekadu Abebe, Abebech Tewabe Gelaye, Sisay G/Hana Gemeda and Dawit Kumilachew Yimenu in SAGE Open Medicine