Abstract
We evaluated angiogenin as a prospective biomarker in peripheral artery disease (PAD) patients with and without claudication symptoms. A pilot study suggested an elevation of angiogenin in critical limb ischemia. However, in PAD patients, the predictive value of angiogenin has not yet been evaluated. For this purpose, 342 patients with PAD (age: 69 ± 10 years, 34.5% women) were followed-up for 7 years in a cross-sectional study. Angiogenin was measured by enzyme-linked immunosorbent assay. All-cause and cardiovascular mortality were analyzed by Cox regression. Angiogenin levels were higher in men (P = .001) and were associated with patient waist-to-hip ratio (P < .001), fasting triglycerides (P = .011), and inversely with estimated glomerular filtration rate (P = .009). However, angiogenin showed no association with age, characteristics of diabetes, markers of lipid metabolism, or C-reactive protein. Angiogenin did not correlate with markers of angiogenesis such as vascular endothelial growth factor, angiopoietin-2, or tie-2. Furthermore, angiogenin was not associated with PAD Fontaine stages or with patient ankle-brachial index in addition to all-cause mortality (hazard ratio [HR] = 1.09 [95% CI: 0.89-1.34]) or cardiovascular morality (HR = 1.05 [0.82-1.35]). These results suggest that angiogenin does not provide further information regarding outcome prediction in patients with PAD.
Keywords: angiogenin, mortality, peripheral artery disease, angiogenesis
Introduction
Angiogenin, a member of the pancreatic RNAse superfamily, has been suggested as a possible marker for a number of conditions which are associated with increased rates of angiogenesis. It represents the only member of the superfamily expressing that ability.1 First isolated and described in 1985 in tumor cells, it has been found to promote primary and metastatic tumor growth.2,3 Angiogenin is expressed by tumor-associated NK cells in colorectal cancer4 but has demonstrated a widespread expression pattern,5–8 including production in vascular endothelial cells in culture.9
Angiogenin has been implied to represent a general requirement for cell proliferation and angiogenesis when reduced tumor angiogenesis was observed despite elevated vascular endothelial growth factor (VEGF) expression in mice.10 Consecutively, angiogenin was reported to be involved in inflammation11 and tissue regeneration.12 Angiogenin was shown to sufficiently induce circulating angiogenic cells, regardless of VEGF involvement, when investigating the reason for greater propensity of neovascularization in deep burn wounds compared with superficial burn wounds.13 Interestingly, in patients with diabetes, a disease with implications in both micro- and macrovascular disease, systemic angiogenin levels were similar in patients with diabetes and healthy controls.3 In chronic kidney disease, angiogenin has been demonstrated to gradually increase with kidney disease progression assessed by chronic kidney disease stage in a small patient cohort.14 Experimental in vitro hypoxemia models in periodontal fibroblasts increased expression of angiogenin.15 Apart from angiogenesis, angiogenin has been shown to induce nitric oxide synthase activity.16 In coronary artery disease patients, higher angiogenin levels were associated with better development of coronary collateral circulation.17 It was shown that after revascularization procedures, angiogenin levels decrease,18 supporting an important role in angiogenesis. In cardiac ischemia, this increase in angiogenin levels could be shown both in an acute setting and in a chronic setting where higher levels predicted future adverse events.19
A positive therapeutic effect of an increase in serum levels has been shown in mammals, where stem cell transplantation of cells transfected with the angiogenin gene led to an improvement of heart perfusion and function.20 Additionally, angiogenin has been suggested as a possible biomarker for improvement after ischemic strokes.21
In peripheral artery disease (PAD), limited data are available. Angiogenin concentrations have been hypothesized to be associated with compensatory revascularization in Fontaine stage IV,22 and elevated angiogenin levels have been reported in a small sample of patients with PAD and critical limb ischemia with ulceration.3 Prospective data on patient with PAD and angiogenin is lacking. The present study aims to evaluate angiogenin as possible prospective biomarker in PAD patients with and without claudication symptoms- and outcome.
Materials and Methods
Study Population
Angiogenin was measured from frozen blood samples from the Vascular Medicine Center Vienna cohort. Frozen blood samples were available in 342 patients of 370 patients. Detailed inclusion and exclusion criteria have been published, previously.23 Briefly, all patients included exhibited stable PAD (asymptomatic or with claudication) without planned revascularization. Key exclusion criteria consisted of critical limb ischemia and/or ulceration, patients with known cancer or severe renal insufficiency (serum creatinine above 3 mg/dL [265.2 μmol/L]). The study was approved by the ethics committee of the Medical University of Vienna and complies with the Declaration of Helsinki including current revisions and the Good Clinical Practice guidelines.24,25 The procedures followed were in accordance with institutional guidelines and all participants gave their written informed consent before inclusion into the study.
Definition of Cardiovascular Comorbidities
Baseline demographic and clinical characteristics were recorded. Hypertension was defined as a systolic blood pressure (BP) ≥140 mm Hg and/or a diastolic BP ≥90 mm Hg in at least 2 measurements26 or active use of antihypertensive medication. Diabetes mellitus type 2 was defined as a fasting plasma glucose level >7.0 mmol/L, glucose level over 11.1 mmol/L after standardized oral glucose tolerance test,27 glycated hemoglobin (HbA1c) of at least 6.5%, or active use of an antidiabetic agent. Body mass index (BMI) was calculated as body weight in kilograms divided by squared body height in m (kg/m2). Fasting blood samples were drawn at baseline for glucose HbA1c, cholesterol, liver, and renal function parameter monitoring. Spot urinary albumin-to-creatinine ratio (UACR) with >30 mg/g were classified as microalbuminuria and >300 mg/g as macroalbuminuria. Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology (CKD-EPI) equation.28
Definition of PAD
Presence of PAD disease was detected by noninvasive ankle-brachial index (ABI) measurements and sphygmomanometric oscillography (ELCAT VL5000) by trained technicians. Systolic BP was measured in both arms (brachial arteries) and both ankles (dorsal pedal arteries and posterior tibial arteries). Ankle-brachial index was calculated according to the Trans-Atlantic Inter-Society Consensus criteria29 by dividing of the higher ankle pressure by the highest brachial pressure. In case of incompressible ankle arteries (ABI >1.4), patients were classified as media sclerosis.
Sample Collection and Measurement of Angiogenetic Proteins
Fasting blood samples were collected at study entry and stored at −80 °C. Plasma angiogenin levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems Inc) with an intra-assay and inter-assay variation of 4.7% and 8.4%, respectively. Angiopoietin-2, Tie-2, and VEGF have been already assessed in this patient cohort30 using bead-based multiplex assay for the Luminex platform with a sensitivity of 17.1, 16.0, and 2.1 pg/mL (R&D Systems). The intra-assay and inter-assay coefficient of variation for angiopoietin-2, Tie-2, and VEGF levels were 5.7 and 3.2%, 7.0 and 3.6%, and 9.7 and 5.0%, respectively.
Follow-Up and Outcome
Follow-up of patients was conducted as previously described to identify cardiovascular and PAD specific events.23 Mortality was assessed by queries of the Austrian central death registry managed by Statistics Austria, a department of the federal government of Austria. In case of survival, patients were additionally contacted by phone to ensure data quality. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes were retrieved from the central death registry and verified by hospital or autopsy reports as available to quantify cardiovascular mortality. During the study period, 86 patients within 7 (6.3-7) years died. ICD-10 mortality codes were classified as 60 cardiovascular, 21 oncological, and 5 other causes of death. Major cardiovascular events (MACE) including nonfatal stroke, nonfatal myocardial infarction, and all-cause death were available for the first 5 study years. Major cardiovascular events included 13 nonfatal myocardial infarctions, 11 nonfatal stroke, and 43 deaths. It has to be noted that patients with a history of cancer were not eligible to participate in this study; however, during the observation period, 24.4% died due to oncological cause reflecting the increased risk of the development of cancer in patients with PAD.31
Statistics
Data are presented as mean ± SD or median (25; 75 percentile). Angiogenin levels were log-transformed for statistical analyses due to skewed distribution. Student unpaired t test as well as χ2 test were used as appropriate. Pearson correlation coefficient was applied. Survival was analyzed by Cox-regression analysis to estimate effect size. Effect size for continuous parameters is given as hazard ratio (HR) per 1 SD and 95% CI. A 2-sided P < .05 was considered significant. All statistical analyses were performed with the statistical software package SPSS 24 (IBM).
Results
This study included 342 PAD patients (age 69 ± 10 years, 34.5% women). Angiogenin levels were higher in men compared with women (P = .001) as depicted in Figure 1. Typical cardiovascular risk factors such as hypertension (P = .079), type 2 diabetes mellitus (P = .368), statin intake (P = .218), or active smoking (P = .988) were similar in all angiogenin tertiles. Previous stroke (P = .546) or myocardial infarction (P = .339) was evenly distributed among angiogenin tertiles. Detailed baseline characteristics according to angiogenin tertiles are depicted in Table 1.
Figure 1.
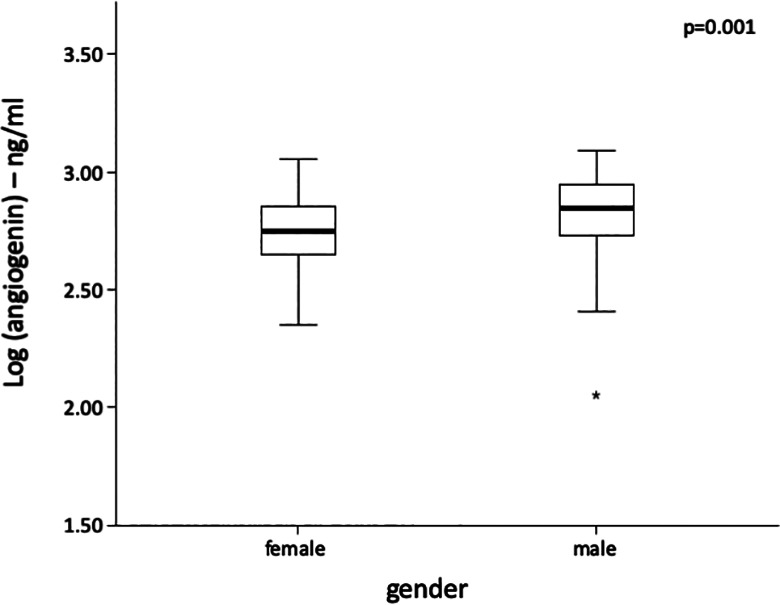
Box plot of angiogenin levels according to patient sex. Differences were analyzed by Student unpaired t test after log transformation of angiogenin.
Table 1.
Baseline Characteristics According to Angiogenin Level Tertiles.
| Low | Medium | High | P | |
|---|---|---|---|---|
| n | 114 | 114 | 114 | |
| Age (years) | 69 ± 10 | 69 ± 11 | 69 ± 10 | .966 |
| Female, n (%) | 55 (48.2) | 37 (32.5) | 26 (22.8) | <.001 |
| BP systolic (mm Hg) | 140 ± 23 | 142 ± 21 | 142 ± 20 | .683 |
| BP diastolic (mm Hg) | 77 ± 11 | 78 ± 12 | 79 ± 11 | .297 |
| Body mass index (kg/m2) | 27.4 ± 4.4 | 27.1 ± 3.9 | 28 ± 4.0 | .229 |
| HbA1c (mmol/mol) | 42 (38-48) | 42 (39-48) | 42 (39-51) | .273 |
| Triglycerides (mg/dL) | 127 (102-179) | 141 (106-200) | 151 (97-211) | .067 |
| HDL-C (mg/dL) | 51 (55-64) | 52 (43-63) | 50 (43-57) | .548 |
| LDL-C (mg/dL) | 106.4 (85.6-129.4) | 105.1 (82.8-131.4) | 96.9 (79.6-122.6) | .539 |
| Statin usage, n (%) | 92 (82.9) | 90 (80.4) | 84 (74.5) | .218 |
| C-reactive protein (mg/dL) | 0.31 (0.16-0.55) | 0.27 (0.14-0.54) | 0.30 (0.13-0.56) | .645 |
| eGFR (mL/min/1.73 m2) | 78.7 ± 15 | 67.3 ± 18.0 | 57.5 ± 19.5 | .052 |
| UACR (mg/g) | 7.5 (4-20) | 11 (5-34) | 12 (5-38) | .300 |
| Angiogenin (ng/mL) | 450 (385-495) | 650 (607-704) | 924 (829-980) | <.001 |
| Ankle-brachial index | 0.79 ± 0.23 | 0.78 ± 0.20 | 0.79 ± 0.21 | .908 |
| Hypertension, n (%) | 100 (87.7) | 106 (93) | 109 (95.6) | .079 |
| Type 2 DM, n (%) | 45 (39.8) | 50 (43.9) | 56 (49.1) | .368 |
| Smoking—active, n (%) | 38 (33.3) | 39 (34.2) | 42 (36.8) | .922 |
| Coronary artery disease, n (%) | 26 (22.8) | 35 (30.7) | 45 (39.5) | .025 |
| Carotid artery disease, n (%) | 48 (42.1) | 41 (36) | 43 (37.7) | .618 |
Abbreviations: ANOVA, analysis of variance; BP, blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate according to CKD-EPI equation; HbA1c, glycated hemoglobin A1c, HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; UACR, urine albumin-to-creatine ratio; suPAR, soluble urokinase plasminogen activator receptor.
a Data are mean ± SD or median (25; 75 percentile) or n (%). Differences were analyzed by ANOVA and χ2 test as appropriate. An α-level of P < .05 (2-tailed) was considered statistically significant.
Angiogenin and Anthropometric as Well as Laboratory Parameters
Angiogenin showed no association with patient age (r = −0.008, P = .878), body weight (r = 0.087, P = .110), or BMI (r = 0.045, P = .405). However, angiogenin levels were significantly linked with the waist-to-hip ratio (r = 0.206, P < .001). In univariate correlation analyses, angiogenin levels showed no association with fasting glucose (r = 0.057, P = .295) or HbA1c (r = 0.065, P = .234) as continuous markers of glucose metabolism. Patients with type 2 diabetes mellitus (2.81 ± 0.16 ng/mL) showed similar angiogenin levels as those without diabetes (2.79 ± 0.15 ng/mL, P = .192). Angiogenin was associated with fasting triglycerides (r = 0.137, P = .011) but showed no association with total cholesterol (r = 0.012, P = .829), low-density lipoprotein–cholesterol (r = −0.018, P = .746), or high-density lipoprotein-cholesterol (r = −0.033, P = .549) levels. Angiogenin levels were inversely associated with eGFR levels (r = −0.142, P = .009) but showed no association with UACR levels (r = 0.091, P = .100). Angiogenin levels were not linked to C-reactive protein levels (r = −0.074, P = .174).
Angiogenin and other Biomarkers for Angiogenesis
Angiogenin levels were not associated with other known biomarkers of angiogenesis such as VEGF (r = 0.059, P = .278, n = 338) and angiopoietin-2 (r = 0.070, P = .278, n = 341) levels or the corresponding receptor tie-2 (r = 0.048 P = .404, n = 304).
Angiogenin and PAD
Angiogenin levels were similar in patients with asymptomatic PAD and claudication symptoms assessed by the Fontaine classification (P = .705) as shown in Figure 2A. In addition, angiogenin levels showed no association with ABI decline (r = 0.075, P = .242) or ABI category (P = .188) as shown in Figure 2B. Angiogenin levels were not influenced by the presence of media sclerosis (P = .741).
Figure 2.
Box plot of angiogenin levels according to (A) Fontaine stage or (B) ankle-brachial index. Differences were analyzed by analysis of variance (ANOVA) after log transformation of angiogenin.
Angiogenin and Cardiovascular Outcome
Baseline angiogenin levels were not associated with all-cause mortality (HR = 1.09 [0.89-1.34], P = .393) or cardiovascular morality (HR = 1.05 [0.82-1.35], P = .709) over 7 years. Furthermore, angiogenin levels were not related with MACEs (HR = 1.01 [0.78-1.30], P = .973) over 5 years.
Discussion
This study showed that angiogenin levels were similar in varying degrees of stable PAD severity as assessed by the ABI. The presence of intermittent claudication as a symptom of recurrent local ischemia in the effected limb did not increase angiogenin levels.
Angiogenin levels both for symptomatic and asymptomatic stable PAD patients of this cohort were similar to those of a previous small cohort of intermittent claudication and healthy control patients.22 However, in this study,22 in patients with critical limb ischemia and ulceration (n = 38), angiogenin levels were elevated compared with stable PAD and healthy controls. Thus, with regard to the stronger power of our current study due to the larger sample size for stable PAD, elevated angiogenin levels might be due to local inflammation induced by ulcerations.
Furthermore, a possible association of C-reactive protein and angiogenin has not been studied in patients with critical limb ischemia up to date.22 In this study, the only available marker of systemic inflammation, C-reactive protein, was not linked to systemic angiogenin levels. This finding is in line with a previous study, in which systemic angiogenin levels were unchanged in relation to C-reactive protein elevation above normal limits in hemodialysis patients.32
In line with the published literature,3 angiogenin levels were unchanged by the presence of type 2 diabetes. Furthermore, HbA1c as a long-term marker of diabetes severity or fasting glucose as a short-term marker of diabetes control were not associated with angiogenin levels. In the literature, a trend for an association of diabetes and angiogenin has been reported.3 Since patients with diabetes mellitus often qualify for the term metabolic syndrome, this finding might actually be based on the observed association of the patient’s waist-to-hip ratio and angiogenin in the current study.
Angiogenin levels are increased in advanced chronic kidney disease14 and hemodialysis patients.32 The present study adds that angiogenin levels are linked to eGFR levels (CKD-EPI formula) in moderate kidney disease. Interestingly, angiogenin was not related to urinary albumin-to-creatine ratio, another marker of declining kidney function. This lacking association of angiogenin with albumin-to-creatine ratio is, however, biased by only 20 patients categorized as exhibiting macroalbuminuria and thus low statistical power.
In contrast to patients with acute coronary syndrome,33 angiogenin was not a predictor of cardiovascular events in patients with PAD. A possible explanation for this discrepancy might be that this study was restricted to stable PAD patients without planned peripheral or cardiac revascularization. However, the presence of additionally known coronary artery disease increased angiogenin levels significantly (2.79 ± 0.15 vs 2.83 ± 0.16 ng/mL, P = .016). Subgroup analyses of PAD patients with and without coronary artery disease showed no association with all-cause or cardiovascular mortality. It has to be considered that no invasive screening for coronary artery disease was performed in this study, thus it would be expected that some patients were misclassified.
Additionally, other angiogenic markers such as VEGF or angiopoetin-2, a marker known to be associated with increased mortality in PAD patients,30 were not associated with angiogenin levels. No association of angiogenin and VEGF has been reported in hemodialysis patients.32
Several limitations have to be considered. First, the study was restricted to stable PAD patients, thus we are not able to define a possible role of angiogenin in ischemia ulceration and signs of systemic inflammation. Second, angiogenin was measured once at baseline, thus no angiogenin levels contemporary to MACE events were available. Third, PAD patients have an abundance of comorbidities influencing their clinical outcome and raising the threshold for a biomarker to be of added benefit. In addition, according to current clinical practice and guidelines, no invasive screening for coronary artery disease was performed in asymptomatic patients.
In conclusion, the measurement of systemic angiogenin levels in stable PAD patients currently lacks additional value to foster patient care.
Footnotes
Authors’ Note: All authors contributed to (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content; and (3) final approval of the version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the Medical University Vienna, Vienna, Austria.
ORCID iD: Clemens Höbaus  https://orcid.org/0000-0002-9704-1452
https://orcid.org/0000-0002-9704-1452
References
- 1.Wiedlocha A. Following angiogenin during angiogenesis: a journey from the cell surface to the nucleolus. Archivum immunologiae et therapiae experimentalis. 1999;47:299–305. [PubMed] [Google Scholar]
- 2.Fett JW, Strydom DJ, Lobb RR, et al. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985;24:5480–5486. [DOI] [PubMed] [Google Scholar]
- 3.Yu D, Cai Y, Zhou W, Sheng J, Xu Z.The Potential of Angiogenin as a Serum Biomarker for Diseases: Systematic Review and Meta-Analysis. Dis Markers. 2018;2018:1984718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruno A, Bassani B, D’Urso DG, et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. 2018;32:5365–5377. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro R, Strydom DJ, Olson KA, Vallee BL. Isolation of angiogenin from normal human plasma. Biochemistry 1987;26:5141–5146. [DOI] [PubMed] [Google Scholar]
- 6.Sheng J, Xu Z. Three decades of research on angiogenin: a review and perspective. Acta Biochim Biophys Sin (Shanghai). 2016;48:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spong CY, Ghidini A, Sherer DM, Pezzullo JC, Ossandon M, Eglinton GS. Angiogenin: a marker for preterm delivery in midtrimester amniotic fluid. American journal of obstetrics and gynecology. 1997;176:415–418. [DOI] [PubMed] [Google Scholar]
- 8.Ilzecka J. Cerebrospinal fluid angiogenin level in patients with amyotrophic lateral sclerosis. Acta clinica Croatica. 2008;47:77–79. [PubMed] [Google Scholar]
- 9.Fischer S, Nishio M, Dadkhahi S, et al. Expression and localisation of vascular ribonucleases in endothelial cells. Thromb Haemost. 2011;105:345–355. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005;24:445–456. [DOI] [PubMed] [Google Scholar]
- 11.Koutroubakis IE, Xidakis C, Karmiris K, Sfiridaki A, Kandidaki E, Kouroumalis EA. Serum angiogenin in inflammatory bowel disease. Dig Dis Sci. 2004;49:1758–1762. [DOI] [PubMed] [Google Scholar]
- 12.King TV, Vallee BL. Neovascularisation of the meniscus with angiogenin. An experimental study in rabbits. The Journal of bone and joint surgery. British volume. 1991;73:587–590. [DOI] [PubMed] [Google Scholar]
- 13.Pan SC, Wu LW, Chen CL, Shieh SJ, Chiu HY. Angiogenin expression in burn blister fluid: implications for its role in burn wound neovascularization. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20:731–739. [DOI] [PubMed] [Google Scholar]
- 14.Choi HM, Kwon YE, Kim S, Oh DJ. Changes in FGF-23, Neutrophil/Platelet Activation Markers, and Angiogenin in Advanced Chronic Kidney Disease and Their Effect on Arterial Stiffness. Kidney Blood Press Res. 2019;44:1166–1178. [DOI] [PubMed] [Google Scholar]
- 15.Janjic K, Bauer P, Edelmayer M, et al. Angiogenin production in response to hypoxia and l-mimosine in periodontal fibroblasts. Journal of periodontology. 2019;90:674–681. [DOI] [PubMed] [Google Scholar]
- 16.Trouillon R, Kang DK, Chang SI, O’Hare D. Angiogenin induces nitric oxide release independently from its RNase activity. Chemical communications. 2011;47:3421–3423. [DOI] [PubMed] [Google Scholar]
- 17.Gurses KM, Yalcin MU, Kocyigit D, et al. The association between serum angiogenin and osteopontin levels and coronary collateral circulation in patients with chronic total occlusion. Anatol J Cardiol. 2019;22:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krecki R, Krzeminska-Pakula M, Peruga JZ, et al. Influence of treatment strategy on serum adiponectin, resistin and angiogenin concentrations in patients with stable multivessel coronary artery disease after one-year follow-up. Kardiol Pol. 2010;68:1313–1320. [PubMed] [Google Scholar]
- 19.Tello-Montoliu A, Marin F, Patel J, et al. Plasma angiogenin levels in acute coronary syndromes: implications for prognosis. Eur Heart J. 2007;28:3006–3011. [DOI] [PubMed] [Google Scholar]
- 20.Huang SD, Lu FL, Xu XY, et al. Transplantation of angiogenin-overexpressing mesenchymal stem cells synergistically augments cardiac function in a porcine model of chronic ischemia. J Thorac Cardiovasc Surg. 2006;132:1329–1338. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel-Salazar M, Morancho A, Rodriguez S, et al. Importance of Angiogenin and Endothelial Progenitor Cells After Rehabilitation Both in Ischemic Stroke Patients and in a Mouse Model of Cerebral Ischemia. Front Neurol. 2018;9:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgmann H, Hollenstein U, Maca T, et al. Increased serum laminin and angiogenin concentrations in patients with peripheral arterial occlusive disease. J Clin Pathol. 1996;49:508–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobaus C, Herz CT, Obendorf F, et al. Center-based patient care enhances survival of elderly patients suffering from peripheral arterial disease. Ann Med. 2017;49:291–298. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. <https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/> (last accessed 22.02.2021).
- 25.European Parliament C. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use <http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0020:EN:NOT> (last accessed 22.02.2021). [PubMed]
- 26.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 27.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 2003;26(Suppl 1):S5–20. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. [DOI] [PubMed] [Google Scholar]
- 30.Hobaus C, Pesau G, Herz CT, Wrba T, Koppensteiner R, Schernthaner GH. Angiopoietin-2 and Survival in Peripheral Artery Disease Patients. Thromb Haemost. 2018;118:791–797. [DOI] [PubMed] [Google Scholar]
- 31.Paraskevas KI, Mikhailidis DP, Veith FJ. Patients with peripheral arterial disease, abdominal aortic aneurysms and carotid artery stenosis are at increased risk for developing lung and other cancers. Int Angiol. 2012;31:404–405. [PubMed] [Google Scholar]
- 32.Eleftheriadis T, Antoniadi G, Liakopoulos V, Pissas G, Stefanidis I, Galaktidou G. Plasma angiogenin and vascular endothelial growth factor a among hemodialysis patients. Iran J Kidney Dis. 2012;6:209–215. [PubMed] [Google Scholar]
- 33.Tello-Montoliu A, Patel JV, Lip GY. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006;4:1864–1874. [DOI] [PubMed] [Google Scholar]



