Abstract
Artesunate (ART) is a derivative of artemisinin that is extracted from the wormwood plant Artemisia annua. ART is an antimalarial drug that has been shown to be safe and effective for clinical use. In addition to its antimalarial properties, ART has been attracting attention over recent years due to its reported inhibitory effects on cancer cell proliferation, invasion and migration. Therefore, ART has a wider range of potential clinical applications than first hypothesized. The aim of the present review was to summarize the latest research progress on the possible anticancer effects of ART, in order to lay a theoretical foundation for the further development of ART as a therapeutic option for cancer.
Keywords: artesunate, artemisinin, antitumor, apoptosis, proliferation
1. Introduction
Cancer is a major health concern worldwide (1,2). According to GLOBOCAN 2020, which presented the latest estimates of cancer incidence and mortality (3), there were ~19.3 million new cases of cancer and 10 million cancer-related deaths worldwide in 2020. As such, the number of cancer cases worldwide is expected to reach 28.4 million by 2040, a 47% increase from 2020 (4). Asia, Latin America, the Caribbean and Africa are expected to experience particularly large increases in cancer morbidity and mortality rates (3). Therefore, it is crucial to develop novel anticancer agents.
Artesunate (ART) is a derivative of artemisinin that is characterized by high efficacy, rapid effects, low toxicity and reduced susceptibility to drug resistance (5,6). At present, ART is commonly used for the treatment of mild to severe malaria worldwide (7). However, accumulating evidence has shown that ART also displays anticancer properties, in addition to its antimalarial effect (8). For instance, ART has been reported to induce apoptosis and autophagy in human bladder cancer cells (9,10). Moreover, it can induce cell cycle arrest, reactive oxygen species (ROS) generation and ferroptosis in renal cell carcinoma (11). In the present review, the potential anticancer effects of ART and the underlying mechanism of action involved are summarized. The aim was to provide a theoretical basis for the further development of ART and its derivatives for the treatment of cancer.
2. Source and activity of ART
ART is a semi-synthetic, monomeric derivative of artemisinin isolated from Artemisia annua in the 1970s (12–14). The conversion from artemisinin to ART is a two-step process, starting with reduction of dihydroartemisinin with diisobutylaluminium hydride, followed by esterification with succinic anhydride (14). The chemical name of ART is dihydroartemisinin-1,2-α-succinate monoester, with the chemical formula of C24H39O8 and a molecular weight of 455.56 g/mol (15).
ART has a hydrophilic group, and the 1,2,4-endoperoxide bridge is responsible for the antimalarial activity of the drug. ART acts on all stages of malaria parasite circulation. ART also may penetrate the cell membranes and generate ROS, and a small amount of ART reaches the mitochondria of the parasite, where ART and ROS react with each other, leading to mitochondrial dysfunction (8). ART is the only artemisinin derivative with high water solubility, due to the addition of the hemisuccinate group. ART is metabolized to docosahexaenoic acid (DHA) as it enters the body (16–18). ART induces the generation of ROS, increasing malondialdehyde levels and decreasing the levels antioxidants such as superoxide dismutase and catalase, thereby causing alkylation of the proteins of the Plasmodium parasite (19). At present, ART is mainly used for the treatment of malaria of all types (20), for immune regulation (in type 1 diabetes in NOD mice) (21), as well as for liver (22), breast (23) and lung (24–26) cancer.
3. Anticarcinogenic mechanism of ART
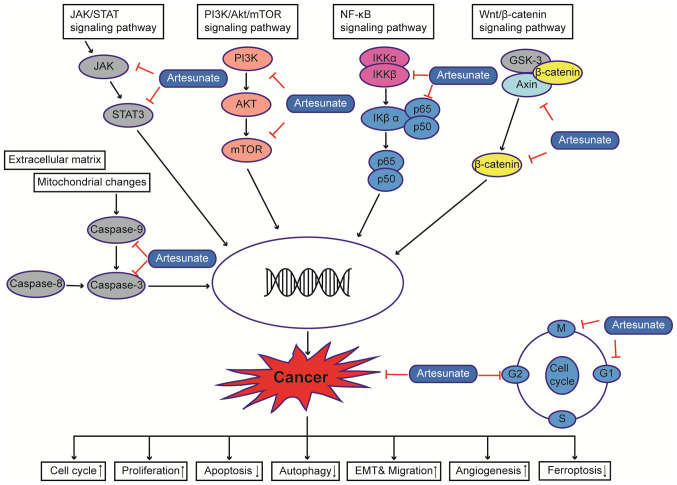
There is considerable evidence that ART can exert anticancer effects on several types of cancer cells (6,15). ART has been reported to induce apoptosis, differentiation and autophagy in colorectal cancer cells by impairing angiogenesis (27), inhibiting cell invasion and migration (28), inducing cell cycle arrest (11), upregulating ROS levels, regulating signal transduction [for example, activating the AMPK-mTOR-Unc-51-like autophagy activating kinase (ULK1) pathway in human bladder cancer cells] (9) and blocking immune escape (29). In addition, ART has been shown to restore the sensitivity of a number of cancer types to chemotherapeutic drugs by modulating various signaling pathways; for example, ART can improve the apoptosis of HCC by inhibiting the PI3K/AKT/mTOR pathway (30), and can increase liver cancer cell sensitivity to sorafenib via suppression of the MEK/ERK pathway (31) (Fig. 1).
Figure 1.
Anticarcinogenic mechanism of artesunate. Multiple molecular and signaling pathways regulate abnormal cell proliferation and migration, such as the NF-κB signaling pathway, the PI3K/Akt/mTOR signaling pathway and the JAK/STAT signaling pathway, among others, ultimately leading to tumorigenesis. Artesunate may affect the development of cancer by interfering with the cell cycle, proliferation, invasion, angiogenesis and apoptosis of cancer cells by acting on different sites. EMT, epithelial-mesenchymal transition.
Apoptosis
Apoptosis is a type of programmed cell death that does not elicit inflammatory responses (32). A number of studies have shown that ART can induce apoptosis by activating the mitochondria-dependent pathway, specifically by mediating the activation of caspase-3 and −9 and the release of cytochrome c into the cytosol after permeabilization of the mitochondrial membrane (33). Additionally, ART can induce HL-60 human acute promyelocytic leukemia cell and KG1a acute myeloid leukemia cell death by regulating antiapoptotic proteins, such as Bcl-2, as well as proapoptotic proteins, such as Bid and Bak, through inhibition of the MEK/ERK and PI3K/Akt pathways (34). ART has also been demonstrated to induce T helper 1 cell differentiation and promote apoptosis in ovarian cancer cells via the microRNA (miR)-142/sirtuin 1 pathway (35).
Autophagy
Autophagy is a conserved, self-degrading system that is essential for maintaining cell homeostasis under stress conditions, and which has been demonstrated to serve an important role in cancer in association with a family of autophagy-related proteins (LC3B) (36). ART can induce autophagy and increase the levels of CD155 in uterine corpus endometrial carcinoma (UCEC) cells. Moreover, it also regulates the interaction between CD155 and its receptor on the NK92 natural killer cell line by upregulating the co-stimulator CD226 and downregulating the co-inhibitor TIGIT, thereby enhancing the cytotoxicity of these cells. Thus, ART has a dual anticancer effect on UCEC cells (37). ART also induces autophagy by upregulating ROS production and activating the AMP-activated protein kinase/mTOR/ULK1 pathway in human bladder cancer cells (9).
ROS
ROS have a dual role in cellular metabolism (38). Their production is impaired during normal cellular homeostasis, whilst excessive production can lead to oxidative stress (OS), a process that can lead to damage to cellular structure (39). A study has shown that higher levels of ROS are important for the initiation, progression, angiogenesis and metastasis of cancer (40). Dysregulation of ROS has been found to promote tumorigenesis through activation of various oncogenic, signaling pathways such as MAPK, PI3K/AKT/mTOR and NF-κB (18,40), DNA damage (41,42), immune escape, metastasis, angiogenesis and telomere elongation (40). ROS production has been demonstrated to play an important role in ART-induced apoptosis in various tumor cell lines, including glioblastoma (43), lymphoma (44), breast cancer cells (45). Yao et al (46) suggested that ART could increase ROS levels in the hepatocellular carcinoma (HCC) cell lines Huh7 and Hep3B. In addition, the combination of sorafenib and ART treatment was found to synergistically produce antiproliferative effects in HCC cells and induce apoptosis.
Inhibition of angiogenesis
Blood vessels provide oxygen and a nutrient supply for the growth of tumors, which also facilitate the proliferation, migration and subsequent invasion of malignant tumor cells in the long term (47). Angiogenesis is a dynamic and complex process that is regulated by a variety of mechanisms. Inhibition of angiogenesis has become a therapeutic strategy for pancreatic cancer (48), breast cancer (49) and ovarian cancer (50). Chen et al (51) demonstrated that ART could downregulate the expression of VEGF and angiopoietin-1 in RPMI8226 myeloma cells, decrease the activation of ERK1and inhibit angiogenesis. Their study indicated that ART possessed a potential anti-myeloma effect, which was mediated by the inhibition of angiogenesis.
Cell cycle arrest
Aberrant cell division is one of the characteristic features of cancer cells (52). ART inhibits the proliferation of bladder cancer cells (RT4, RT112, T24 and TCCSup), which is associated with G0/G1-phase cell cycle arrest and downregulation of cell cycle regulatory proteins [cyclin D1 and CDK4 (required for entry into the G1 phase); CDK1 and cyclinA/B (essential during the late S phase and early M phase)] (10). ART can block cell cycle progression and lead to a significant reduction in the levels of the cell cycle activating proteins cyclin A, cyclin B, and CDK1, evoking G0/G1 phase arrest and inhibiting growth of the cells in renal cell carcinoma (11). In breast cancer cells (MCF-7 and MDA-MB-231), ART can block G2/M progression by upregulating beclin-1 expression, which promotes autophagy (53). In glioblastoma cells (A172, U251 and U87), ART also increases the proportion of cells in the G0/G1 phase, reduces the proportion of cells in the S phase and inhibits proliferation by downregulating the expression levels of the cell cycle-related proteins CDK2, CDK4, cyclin D1 and cyclin B1 (54).
Ferroptosis
Ferroptosis is a recently identified form of regulated cell death, which is characterized by iron overload, lipid ROS accumulation and lipid peroxidation (55). Evidence suggests that ferroptosis is closely associated with the occurrence, development and inhibition of cancer (56). Zhang et al (26) demonstrated that ART could upregulate the mRNA levels of transferrin receptor (a positive regulator of ferroptosis), thus inducing apoptosis and ferroptosis in A549 non-small cell lung cancer (NSCLC) cells. Li et al (57) showed that ART enhanced the anticancer effects of low-dose sorafenib (a novel multi-targeted oral drug for the treatment of gastroenteric tumors) against Huh7, SNU-449, and SNU-182 HCC cell lines in vitro and against a Huh7 cell xenograft model in BALB/c nude mice. In addition, ART-induced lysosome activation synergizes with the pro-oxidative effects of sorafenib to sequentially promote lysosomal cathepsin B/L activation, ferritin degradation, lipid peroxidation and ferroptosis (57).
4. Potential role of ART in human malignancies
Previous studies have reported that ART exerted minimal toxicity, was cost-effective and was effective for treating different types of cancer (Table I) (58–78). The potential anticancer properties of ART in different types of cancer are discussed below.
Table I.
Antitumor activity of ART in different cancer types.
| First author/s, year | Cancer type | Model/cell line | Mechanism/results | (Refs.) |
|---|---|---|---|---|
| Ishikawa et al, 2020 | ATLL | HTLV-1 | Cyclin-dependent kinase 1, 2, 4 and 6 ↑, cyclin B1, D2 and E ↓; p21 ↑; intracellular reactive oxygen species ↑; JunB↓; JunD↓ | (5) |
| Chen et al, 2020 | Leukemia | HL-60 and KG1a cells | Induced cell apoptosis and inhibited cell proliferation and stemness in a dose-dependent manner via the suppression of the MEK/ERK and PI3K/Akt pathways | (34) |
| Hu et al, 2019 | Leukemia | MV4-11 | Caspase-3 ↑; autophagy-related protein LC3B ↑; Bcl-2 ↓ | (58) |
| Kim et al, 2015 | CML | KBM-5 | Antiproliferative and proapoptotic effects through suppression of multiple signaling cascades | (59) |
| Kumar et al, 2017 | AML | AML MV4-11 and MOLM-13 | Cellular and mitochondrial ROS accumulation, double-stranded DNA damage, loss of mitochondrial membrane potential and induction of the intrinsic mitochondrial apoptotic cascade | (60) |
| Wang et al, 2017 | Pituitary adenoma | GH3 and MMQ | ART and BRC used in combination exert synergistic apoptotic and antitumor effects by suppressing miR-200c and stimulating PTEN expression | (61) |
| Karpel-Massler et al, 2014 | Glioblastoma | U87MG and A172 | A combination of ART and temozolomide resulted in increased cytotoxicity | (62) |
| Berte et al, 2016 | Glioblastoma | LN229 and A172 | Downregulation of RAD51 protein expression and HR activity. Inhibition of senescence induced by TMZ | (63) |
| Lian et al, 2016 | Glioma | SHG44 | Inhibition of cell proliferation, migration and invasion, and increase of cell apoptosis | (64) |
| Berdelle et al, 2011 | Glioblastoma | LN-229 | Oxidative DNA damage and DNA double-strand breaks, leading to tumor cell death | (65) |
| Button et al, 2014 | Schwannoma | RT4 | Combination with the autophagy inhibitor chloroquine potentiated cell death | (66) |
| Wei et al, 2020 | Glioma | U251, U87, U138 and SK-N-SH | Impairing the nuclear localization of protein SREBP2 and the expression of target genes HMGCR through the mevalonate pathway, further affecting the metabolism of glioma cells | (67) |
| Greenshields et al, 2019 | Breast cancer | MDA-MB-468 and SK-BR-3 cells | Inhibition of breast cancer cell proliferation via a ROS-dependent G2/M arrest and ROS-independent G1 arrest | (68) |
| Wen et al, 2018 | Breast cancer | MCF7 cells | Inhibition of cell proliferation and increased G2/M arrest through ATM activation and the ‘ATM-Chk2-CDC25C’ pathway | (69) |
| Greenshields et al, 2017 | Ovarian cancer | Ovarian cancer cells | Induction of ROS; reduced proliferation; altered expression of cell cycle regulatory proteins, including cyclin D3, E2F-1 and p21; inhibition of mTOR signaling | (70) |
| Chen et al, 2019 | Ovarian cancer | ID8 | miR-142 expression in peripheral CD4+ T cells ↑; Sirt1 levels ↓; Th1 differentiation from CD4+ T cells ↑ | (35) |
| Li et al, 2018 | Ovarian cancer | SKOV3 and primary EOC | Induction of autophagy; cell cycle arrest; inhibition of EOC growth | (71) |
| Liu et al, 2015 | Esophageal cancer | Eca109 and Ec9706 | By downregulating mitochondrial membrane potential, Bcl-2 and CDC25A, upregulating Bax and caspase-3, induction of cell apoptosis and cell cycle arrest; concentration-dependent inhibitory activity in vivo and in vitro | (72) |
| Fei et al, 2018 | Esophageal cancer | Irradiated TE-1 cells in vitro and in vivo | p21 ↑; cyclin D1, RAD51, RAD54, Ku70 and Ku86 protein ↓ | (42) |
| Wang et al, 2018 | Esophageal cancer | Eca109/ABCG2, xenograft tumor mouse model | Suppression of esophageal cancer drug resistance through the regulation of ABCG2 expression | (73) |
| Wang et al, 2017 | Gastric cancer | SGC-7901 | Inhibition of the cell growth; induction of apoptosis; may be related to the regulation of CDC25A, Bcl-2, Bax, caspase-3 and mitochondrial membrane potential | (74) |
| Zhang et al, 2015 | Gastric cancer | HGC-27 cells | COX-2 ↓; Bax↑; Bcl-2 ↓; caspase-3 ↓; caspase-9 ↓ | (75) |
| Jiang et al, 2018 | Colon cancer | HCT116; in vitro and in vivo | Mitochondrial cleaved caspase 3, PARP, caspase-9 and Bcl-2-associated X protein ↑; Bcl-2 ↓ | (76) |
| Kumar et al, 2019 | Colorectal cancer | Rat model | Inhibition of cellular influx; decreased the levels of oxidative stress and inflammatory markers; cyclooxygenase-2, inducible nitric oxide synthase, NF-κB, and IL-1β ↓ | (77) |
| Verma et al, 2017 | Colon carcinogenesis | Rat model | β-catenin signaling ↓; angiogenic markers (VEGF, MMP-9) ↓; inhibition of cell proliferation | (27) |
| Li et al, 2019 | Liver cancer | HepG2 and Huh7 | Increased the expression levels of cleaved caspase-9 and cleaved poly ADP ribose polymerase, reduced VEGFR2 protein expression and reduced cell migration | (22) |
| Ilamathi et al, 2016 | Hepatocellular carcinoma | HepG2 | Suppression of STAT3; increased apoptosis | (78) |
| Wang et al, 2020 | Lung cancer | A549 | Reduced cell clone numbers; cell cycle arrest at the G2/M phase; cell cycle and apoptosis-related proteins BAX, p21, p53 and caspase-3 ↑; Bcl-2 and cyclin B1 expression ↓ | (24) |
| Zhao et al, 2020 | Lung cancer | A549 | Induction cell apoptosis and cell cycle arrest, Bcl-2 protein ↓; mitochondrial membrane potential ↓; Bax protein ↑ | (25) |
ART, artesunate; ATLL, adult T-cell leukemia/lymphoma; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; ROS, reactive oxygen species; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase; SREBP2, sterol regulatory element-binding protein 2; Chk2, checkpoint kinase 2; CDC25, cell division cycle 25C; miR, microRNA; Sirt1, sirtuin 1; ABCG2, ATP binding cassette subfamily G member 2; COX-2, cyclo-oxygenase-2; PARP, poly(ADP-ribose) polymerase; BRC, bromocriptine; TMZ, temozolomide.
Leukemia
Leukemia is a clonal hematopoietic stem cell malignancy (79). ART can induce leukemic T cell apoptosis by promoting the generation of mitochondrial ROS (80). Previous studies have suggested that ART induces caspase-3/9-mediated apoptosis by targeting the outer mitochondrial membrane, leading to the activation and nuclear translocation of mitochondrial pro-apoptotic factors in human SKM-1 myelodysplastic syndrome cells (81). Nuclear translocation of apoptosis-inducing factors and endonuclease G were accompanied by low levels of ROS and increased mitochondrial production of superoxide, which occur prior to apoptosis and appear to be associated with the intracellular levels of divalent iron (59,60,82–84). Chen et al (34) found that ART may inhibit the levels of phosphorylated (p)-PI3K, p-AKT, p-MEK1 and p-ERK1/2 and promote the apoptosis of leukemia cells (HL-60 and KG1a cells) by inactivating PI3K/Akt and MEK/ERK signaling, ART also significantly reduced the expression of Ki67 and survivin, inhibited growth and stemness in KG1 ×enograft models (34). In the MV4-11 cell line, ART combined with bortezomib (which is commonly used for the treatment of patients with multiple myeloma) resulted in significantly higher proliferation inhibition and reduced apoptotic rates compared with ART or bortezomib alone in the same concentration gradient. After the combination of the two drugs for 24 h, the expression of the pro-apoptotic proteins BIM and cleaved activated caspase-3 and the autophagy-related protein LC3B was upregulated in MV4-11 cells, whereas that of the anti-apoptotic protein Bcl-2 was downregulated (58).
Nervous system tumors
Central nervous system tumors comprise a group of malignancies that originate from tissues or structures of the central nervous system and exhibit an incidence of 5.6 per 100,000 person-years in children under the age of 19 (85–87). ART can selectively downregulate the expression of survivin and induce the DNA damage response in glial cells to increase cell apoptosis and cell cycle arrest, resulting in increased sensitivity to radiotherapy (88). Previously, Wei et al (67) found that ART affected the nuclear localization of sterol regulatory element-binding protein 2 (SREBP2) by decreasing the expression of 3-hydroxy-3-methylglutaryl-CoA reductase and inhibited the mevalonate pathway, which in turn influenced the metabolism of glioma cells. In addition, ART disrupted the interaction between P53 and SREBP2 (which negatively regulates P53 and inhibits senescence), upregulated the expression of P21 and induced senescence in the U251, U87, U138 and SK-N-SH human glioma cell lines (67). The combination of ART and rapamycin (a specific inhibitor of mTOR) has been shown to synergistically decrease translation-controlled tumor protein (TCTP) expression and enhance the cytotoxicity of malignant peripheral nerve sheath tumor (MPNST) cells via mTOR-TCTP positive feedback loop, the results also suggested that TCTP may be a new target for the treatment of neurofibromatosis type 1-associated tumors and MPNSTs (89).
Thyroid carcinoma
Thyroid cancer is the most common cancer in the endocrine system, and its incidence is increasing worldwide (90). Anaplastic thyroid carcinoma (ATC) is an aggressive malignancy that is almost always fatal and lacks effective systemic treatment options. It is highly resistant to chemotherapy due to its undifferentiated and aggressive characteristics (91,92). Ma and Fei (91) showed that ART could inhibit growth and induce apoptosis in ATC cells (8505C, 8505C-r, KAT-4-r and KAT-4), by suppressing mitochondrial respiration and acting synergistically with chemotherapy drug doxorubicin without affecting glycolysis. Thus, ART led to oxidative stress and damage in ATC cells. Their work suggested that ART was a potential complement to the treatment of ATC, particularly cases with chemoresistance (91).
Breast cancer
Breast cancer is one of the most common malignancies among women (93). Despite decades of laboratory, epidemiological and clinical research, breast cancer rates continue to rise. Breast cancer remains the leading cancer-related cause of the burden of disease among women, affecting 1 in 20 women globally and 1 in 8 women in high-income countries (94,95). Systemic treatment (chemotherapy and endocrine therapy) of breast cancer is initially effective; however, after a period of time, drug resistance typically develops (96). ART has also been found to block the cell cycle progression of MCF-7 and MDA-MB-231 cells at the G2/M phase and upregulate the expression of p21 and Beclin-1, thereby inhibiting the proliferation of breast cancer cells by inducing autophagy (53). ART treatment was also revealed to inhibit the proliferation of the triple-negative breast cancer cell line MDA-MB-468 and the human epidermal growth factor-2-enriched breast cancer cell line SK-BR-3 in a dose- and time-dependent manner. The proliferation of MDA-MB-468 and SK-BR-3 cells was inhibited by ROS-dependent G2/M cell cycle arrest and ROS-independent G1 cell cycle arrest (68). Furthermore, ART can inhibit breast cancer MCF-7 cell proliferation and promote G2/M arrest by activating the ataxia-telangiectasia mutated/checkpoint kinase 2/cell division cycle 25 (CDC25) C pathway (69). By loading ART into the lipid core of a polymer-lipid hybrid carrier, the anticancer activity and physical stability of ART were found to be significantly increased and can be used for chemotherapy (97–99). Raza et al (100) found that ART generated the reactive oxygen species (ROS), resulted in DNA damage and enhanced the apoptosis of neighboring cells (Cx43-MCF7 cells) in breast cancer MCF-7 cells. In addition, the dose-dependent cytotoxicity of ART could be reduced by the gap junction (GJ) protein connexin-43 (Cx43). Li et al (101) found that ART could inhibit lysosomal function and clear dysfunctional mitochondria, and induce breast cancer cell apoptosis. In addition, ART was found to have a stronger inhibitory effect on drug-resistant breast cancer cells (A549/TAX and MCF-7/ADR) with higher lysosomal functional activity (101).
Ovarian cancer
Ovarian cancer is the seventh most common type of malignant neoplasm in women and the eighth cause of mortality (102–104). Most patients with ovarian cancer are typically diagnosed at an advanced stage of the disease (105). Ovarian cancer is treated with platinum chemotherapy following surgical resection (106). However, the recurrence rate is high (107,108) and the survival rates of ovarian cancer with International Federation of Gynecology and Obstetrics stage III and IV are only 10–30% (109). ART has been found to significantly reduce the expression of VEGF in the HO-8910 human ovarian cancer cell line, as well as that of KDR/flk-1 (VEGF receptor) in endothelial cells and HO-8910 cells, thereby significantly inhibiting angiogenesis in a dose-dependent form. Additionally, ART resulted in reduced xenograft tumor growth in nude mice, with no clear toxicity to the animal (110). ART could reduce the total amount of RAD51 and the formation of RAD51 foci in ovarian cancer cells sustaining DNA damage. Moreover, the downregulation of RAD51 conferred ovarian cancer cells an increased sensitivity to cisplatin (111). ART combined with cisplatin can synergistically induce DNA double-strand breaks and inhibit the proliferation of the HO8910 and SKOV-3 human ovarian cancer cell lines (111). ART induced the production of ROS and reduced proliferation in HEY1, HEY2 and SKOV-3 ovarian cancer cells, which were associated with downregulation in the expression levels of regulatory proteins of the cell cycle, including cyclin D3, CDKs (CDK4, CDK2, and CDK1), Rb, E2F-1 and CDC25C, while the tumor suppressor p21WAF1/CIP1, as well as phosphorylated Chk2 kinase which is important in the DNA damage response and an inhibitor of the CDC25 phosphatases were upregulated (70).
Esophageal cancer
Esophageal cancer (EC) is a common malignancy and has a high incidence rate in China (112). Although therapeutic approaches have improved, the 5-year survival of EC is <20% (113). ART can induce apoptosis and cell cycle arrest in the Eca109 and Ec9706 EC cell lines by upregulating Bax and caspase-3 and reducing mitochondrial membrane potential, as well as Bcl-2 and CDC25A expression in a concentration-dependent manner (72). In addition, an in vivo study showed that ART produced a dose-dependent Eca109-transplanted tumor regression in Balb/c nude mice, with little side effects. These results revealed that CDC25A was a molecular target of ART and that ART could inhibit the growth of EC cells by inducing apoptosis and G0/G1cell cycle arrest (72). Fei et al (42) demonstrated that ART inhibited the proliferation of EC cells, enhanced radiosensitivity of TE-1 cells in vitro and enhanced the effect of apoptosis induced by irradiation in TE-1 cells by upregulating P21 and downregulating the expression of cyclin D1, RAD51, RAD54, Ku70 and Ku86 protein of irradiated TE-1 cells. Moreover, ART also could aggravate DNA damage of EC cells and prolong the formation of γ-H2AX foci induced by IR in TE-1 cells. The results indicated that ART may be a promising radiosensitizer for the treatment of EC. In another study, Wang et al (73) found that ART can reverse doxorubicin resistance in EC by downregulating the expression of ATP-binding cassette G2 in Eca109 cells. ART was reported to inhibit the proliferation, migration and invasion of KYSE-150 esophageal squamous cell carcinoma cells by suppressing cell elasticity and increasing adhesion; ART also may increase the apoptosis rate by altering the cytoskeleton of KYSE-150 cells (114).
Gastric cancer
Gastric cancer is the fourth leading cause of cancer-related mortality in the world, with a 5-year survival rate of <40% (115,116). ART can inhibit the proliferation of the gastric cancer cell lines SGC-7901, BGC-823 and AGS in a concentration-dependent manner, BGC-823 cells treated with ART exhibited calcium overload, downregulated expression levels of VEGF and upregulated expression levels of calpain-2 (117). ART treatment can also inhibit the proliferation of the SGC-7901 gastric adenocarcinoma cell line and induce apoptosis; the mechanism may be associated with Bax and caspase-3 upregulation and CDC25A and Bcl-2 downregulation (74). In addition, ART could prevent the growth of Helicobacter pylori and gastric cancer cells, inhibit the adhesion of Helicobacter pylori to these cells and reduce Helicobacter pylori-enhanced ROS production. Moreover, ART significantly reduces the number of tumor nodules and tumor size in a gastric cancer mouse model by inhibiting the NF-κB signaling pathway (118).
Colorectal cancer
Colorectal cancer (CRC) is one of the most common types of cancer worldwide and has incidence and mortality rates globally (119,120). ART was found to inhibit CRC proliferation and promote apoptosis in a dose-dependent manner to significantly suppress the growth of colorectal tumors, decrease the physiological activity of cancer and delay spontaneous liver metastasis in the CLY CRC cell line. These anticancer effects were associated with the membrane translocation of β-catenin and the inhibition of unrestricted Wnt/β-catenin signaling (121). In addition, ART can reverse the immunosuppression by downregulating the concentrations of TGF-β1 and IL-10 in Colon26 and RKO CRC cells (122). Jiang et al (76) found that ART induced apoptosis by increasing the protein levels of cleaved caspase-3, poly-ADP ribose polymerase (PARP), caspase-9 and Bax protein levels, while decreasing the levels of LC3 and beclin-1 in HCT116 colon cancer cells. ART can reduce the levels of oxidative stress and inflammatory markers, downregulate cyclo-oxygenase-2, induce nitric oxide (NO) synthase, NF-κB and IF-1β and reduce the risk of colon cancer (77).
Lung cancer
Lung cancer is the most common cancer in the world and the leading cause of cancer death (123), which has an overall 5-year survival rate of ~15% (124). Despite advances in treatment, progressive NSCLC still severely limits survival and requires new therapeutic compounds (125). ART can significantly inhibit the invasion and migration of NSCLC cells (H1395, A549, LXF289 and H460 cells) by downregulating the transcription of urokinase-type plasminogen activator, MMP-2 and MMP-7, whilst inhibiting AP-1 and NF-κB-transactivation (126). In addition, ART promotes radiosensitivity in A549 cells in vitro and in vivo, possibly by inducing cell cycle arrest at the G2/M phase through the NO signaling pathway (127). Wang et al (128) found that ART could inhibit cell migration by upregulating the expression of the epithelial marker E-cadherin in A549 and H1975 NSCLC cell lines. In another study, ART could inhibit the invasion of A549 cells, and the mechanism may be associated with the reduced expression of intercellular adhesion molecule-1 and MMP-9 (129). Furthermore, ART inhibits the proliferation of A549 and H1299 cells by arresting the cell cycle at the G1 phase and suppresses lung tumor progression by inhibiting the Wnt/β-catenin pathway (130). In A549 cells, ART combined with cisplatin blocks the cell cycle at the G2/M phase and induces apoptosis by upregulating the expression of Bax, p53, p21, caspase-3, caspase-7 and caspase-9, whilst synergistically regulating the activity of the MAPK pathway by downregulating p-P38, p-JNK and p-ERK levels, which results in potentiated effects against cancer cell proliferation on A549 cells (131).
Liver cancer
Liver cancer is highly malignant and insensitive to cytotoxic chemotherapy, and is associated with a very poor patient prognosis (132,133). ART can activate caspase-3, increase the Bax/Bcl-2 ratio and PARP, whilst downregulating mouse double minute 2, which leads to induced apoptosis on human hepatocellular carcinoma (HCC) cells but had little effect on normal cells (134). The anticancer effects of ART nanoliposomes on human HepG2 cells was stronger than those mediated by ART active pharmaceutical ingredient at the same concentration (135). ART may function as a potential inhibitor of STAT3 in HCC cells to regulate STAT3 targets, including caspase-3, Bcl-xl and survivin, interfere with STAT3 dimerization and inhibition of both constitutive and IL-6-inducible STAT3, leading to cell apoptosis in vitro (78). Jing et al (30) also revealed that ART could inhibit phosphorylation of AKT and mTOR significantly, and induce apoptosis in HCC (SK-hep1 and SM-7721 cell lines) by inhibiting the PI3K/AKT/mTOR pathway. In addition, ART combined with sorafenib (which is a novel multi-targeted oral drug for the treatment of cancer) further increased the apoptosis of HCC cells by dual inhibition of both RAF/RAF/MAPK pathway and PI3K/AKT/mTOR pathway. Thus, the study identified a potential treatment strategy combining ART with sorafenib for the treatment of advanced HCC.
Other tumors
In a previous study, ART has been reported to induce lactate dehydrogenase release and cell death in necrosis-sensitive cholangiocarcinoma (136). Wang et al (137) found that ART could significantly inhibit proliferation in the Burkitt lymphoma Raji cell line, where it induced apoptosis and autophagy. The combination of ART and bromocriptine can synergistically promote apoptosis by inhibiting miR-200c expression and increasing that of PTEN in lactinomas (61). Chauhan et al (138) found that ART induced ROS production and subsequent cell death in a receptor-interacting protein 1-dependent manner in human renal carcinoma. ART exerted a potent antiproliferative effect on polyomavirus-positive Merkel cell carcinoma (MCC) cells with good overall tolerance and induced ferroptosis (139). In addition, ART also significantly suppressed the growth of established MCC tumors in xenotransplanted mice, suggesting that ART may be used for the treatment of MCC (138). In another study, ART blocked the Wnt/catenin pathway to inhibit the proliferation, migration and invasion of uveal melanoma cells (primary 92.1 and metastatic Omm2.3 UM cells), mainly by suppressing the phosphorylation of GSK3β at Ser9 and decreasing the protein levels of β-catenin and its downstream targets (c-Myc and cyclin D1) (140). Wang et al (141) found that ART decreased androgen receptor (AR) expression, increased the expression and the catalytic activity of DNA methyltransferase3b (DNMT3b) in 22rv1 cells either in transplanted mice or in vitro. ART can suppress tumor growth of prostatic cancer cells through AR-DNMT3b pathway, suggesting it may be used for the treatment of prostate cancer in the future. Yang et al (142) found that ART induced mitochondrial dysfunction and cell apoptosis in the WERI-Rb1 and Y79 human retinoblastoma cell lines and in the ARPE-19 human retinal pigment epithelium cell line by upregulating Kruppel-like factor 6 expression, increasing the Bax/Bcl-2 ratio, promoting the release of cytochrome c and stimulating the cleavage of caspase-9 and −3. Roh et al (143) demonstrated that ART could induce ferroptosis in head and neck (HNC) cells via cellular glutathione depletion and ROS accumulation, and ART sensitivity decreased in some cisplatin-resistant HNCs as a result of Nrf2-ARE pathway activation. Berköz et al (144) suggested that ART treatment could decrease cell migration, invasion and colony formation in the A375 human melanoma cell line, possibly by inhibiting STAT3, Src activation and the protein expression of STAT3-associated molecules, including MMP-2, MMP-9, myeloid-cell leukemia 11, Bcl-xl, VEGF and Twist.
5. Summary and perspectives
Cancer is one of the most life-threatening diseases. With the increasing prevalence of cancer, the development of anticancer agents has become a key field of clinical and scientific research. Developments in medical science and technology have enabled the extraction of bioactive components from Traditional Chinese medicines for research due to their reported anticancer effects and lack of adverse reactions. ART has been demonstrated to be effective against leukemia, breast cancer, gastrointestinal tumors and other types of cancer (8,23,145). Importantly, since it is a drug that is already being used for the treatment of malaria, ART has a reliable safety record for clinical use. Although the amount of clinical data regarding the use of ART as an anticancer drug remains limited, preliminary results have been encouraging in terms of efficacy and tolerance (22). Combination therapy should be a key consideration in the future. In addition, development of modified derivatives of ART after structural modifications or modifying the treatment regimen to optimize the efficacy and toxicity profile are also possible directions for future research.
To conclude, existing information provides evidence supporting the use of ART as an anticancer agent. However, data from systematic in vivo animal and human studies are required to improve our understanding of the anticancer effects and mechanism of action of ART in the future.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ART
artesunate
- LDH
lactate dehydrogenase
- ROS
reactive oxygen species
- HCC
hepatocellular carcinoma
- TCTP
translation-controlled tumor protein
- NSCLC
non-small cell lung cancer
- ATC
anaplastic thyroid carcinoma
- HNC
head and neck cancer
- MPNST
malignant peripheral nerve sheath tumor
- EC
esophageal cancer
- CRC
colorectal cancer
- AR
androgen receptor
Funding Statement
The present study was supported by the Health Commission of Hubei Province scientific research project (grant no. WJ2021Q015), the College Students Innovation Program of Yangtze University (grant no. Yz2020338) and the Health Commission of Hubei Province Scientific Research Project (grant no. WJ2019-17).
Funding
The present study was supported by the Health Commission of Hubei Province scientific research project (grant no. WJ2021Q015), the College Students Innovation Program of Yangtze University (grant no. Yz2020338) and the Health Commission of Hubei Province Scientific Research Project (grant no. WJ2019-17).
Availability of data and materials
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the present study.
Authors' contributions
LL designed and supervised the study. JH, YZ and FW reviewed the references. XY wrote the manuscript. YZ, FW and JZ contributed to the table and figure. XY and LL revised the manuscript. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing of interests
The authors declare that they have no competing interests.
References
- 1.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 3.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa C, Senba M, Mori N. Evaluation of artesunate for the treatment of adult T-cell leukemia/lymphoma. Eur J Pharmacol. 2020;872:172953. doi: 10.1016/j.ejphar.2020.172953. [DOI] [PubMed] [Google Scholar]
- 6.Slezakova S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives. Anticancer Res. 2017;37:5995–6003. doi: 10.21873/anticanres.12046. [DOI] [PubMed] [Google Scholar]
- 7.Cen YY, Zao YB, Li P, Li XL, Zeng XX, Zhou H. Research progress on pharmacokinetics and pharmacological activities of artesunate. Zhongguo Zhong Yao Za Zhi. 2018;43:3970–3978. doi: 10.19540/j.cnki.cjcmm.20180726.010. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 8.Khanal P. Antimalarial and anticancer properties of artesunate and other artemisinins: Current development. Monatsh Chem. 2021 Mar 30; doi: 10.1007/s00706-021-02759-x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Chen Y, Wang F, Wu H, Zhang Y, Liu J, Cai Y, Huang S, He N, Hu Z, Jin X. Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem Biol Interact. 2020;331:109273. doi: 10.1016/j.cbi.2020.109273. [DOI] [PubMed] [Google Scholar]
- 10.Zhao F, Vakhrusheva O, Markowitsch SD, Slade KS, Tsaur I, Cinatl J, Jr, Michaelis M, Efferth T, Haferkamp A, Juengel E. Artesunate impairs growth in cisplatin-resistant bladder cancer cells by cell cycle arrest, apoptosis and autophagy induction. Cells. 2020;9:2643. doi: 10.3390/cells9122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markowitsch SD, Schupp P, Lauckner J, Vakhrusheva O, Slade KS, Mager R, Efferth T, Haferkamp A, Juengel E. Artesunate inhibits growth of sunitinib-resistant renal cell carcinoma cells through cell cycle arrest and induction of ferroptosis. Cancers (Basel) 2020;12:3150. doi: 10.3390/cancers12113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaunig JE. Oxidative stress and cancer. Curr Pharm Des. 2018;24:4771–4778. doi: 10.2174/1381612825666190215121712. [DOI] [PubMed] [Google Scholar]
- 13.Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19:3533. doi: 10.3390/ijms19113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chekem L, Wierucki S. Extraction of artemisinin and synthesis of its derivates artesunate and artemether. Med Trop (Mars) 2006;66:602–605. (In French) [PubMed] [Google Scholar]
- 15.Efferth T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin Cancer Biol. 2017;46:65–83. doi: 10.1016/j.semcancer.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Wei T, Liu J. Anti-angiogenic properties of artemisinin derivatives (review) Int J Mol Med. 2017;40:972–978. doi: 10.3892/ijmm.2017.3085. [DOI] [PubMed] [Google Scholar]
- 17.Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: Pharmacological actions beyond anti-malarial. Pharmacol Ther. 2014;142:126–139. doi: 10.1016/j.pharmthera.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Sun X, Wang L, Wong YK, Lee YM, Zhou C, Wu G, Zhao T, Yang L, Lu L, et al. Artesunate-induced mitophagy alters cellular redox status. Redox Biol. 2018;19:263–273. doi: 10.1016/j.redox.2018.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alagbonsi AI, Salman TM, Sulaiman SO, Adedini KA, Kebu S. Possible mechanisms of the hypoglycaemic effect of artesunate: Gender implication. Metabol Open. 2021;10:100087. doi: 10.1016/j.metop.2021.100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venturini E, Zammarchi L, Bianchi L, Montagnani C, Tersigni C, Bortone B, Chiappini E, Galli L. Efficacy and safety of intravenous artesunate in children with severe imported malaria. Pediatr Infect Dis J. 2020;39:e220. doi: 10.1097/INF.0000000000002694. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Shi X, Liu J, Shao F, Huang G, Zhou Z, Zheng P. Artesunate prevents type 1 diabetes in NOD mice mainly by inducing protective IL-4-producing T cells and regulatory T cells. FASEB J. 2019;33:8241–8248. doi: 10.1096/fj.201900146R. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Xu K, Pian G, Sun S. Artesunate and sorafenib: Combinatorial inhibition of liver cancer cell growth. Oncol Lett. 2019;18:4735–4743. doi: 10.3892/ol.2019.10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirali M, Taheri M, Zarei S, Majidi M, Ghafouri H. Artesunate, as a HSP70 ATPase activity inhibitor, induces apoptosis in breast cancer cells. Int J Biol Macromol. 2020;164:3369–3375. doi: 10.1016/j.ijbiomac.2020.08.198. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Wang Q, He T, Li W, Liu Y, Fan Y, Wang Y, Wang Q, Chen J. The combination of artesunate and carboplatin exerts a synergistic anti-tumour effect on non-small cell lung cancer. Clin Exp Pharmacol Physiol. 2020;47:1083–1091. doi: 10.1111/1440-1681.13287. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Liu J, Liu L. Artesunate inhibits lung cancer cells via regulation of mitochondrial membrane potential and induction of apoptosis. Mol Med Rep. 2020;22:3017–3022. doi: 10.3892/mmr.2020.11341. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, Yi H, Yao H, Lu L, He G, Wu M, Zheng C, Li Y, Chen S, Li L, et al. Artemisinin derivatives inhibit non-small cell lung cancer cells through induction of ROS-dependent apoptosis/ferroptosis. J Cancer. 2021;12:4075–4085. doi: 10.7150/jca.57054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma S, Das P, Kumar VL. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact. 2017;278:84–91. doi: 10.1016/j.cbi.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Ma JD, Jing J, Wang JW, Yan T, Li QH, Mo YQ, Zheng DH, Gao JL, Nguyen KA, Dai L. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther. 2019;21:153. doi: 10.1186/s13075-019-1935-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian P, Zhang YW, Zhou ZH, Liu JQ, Yue SY, Guo XL, Sun LQ, Lv XT, Chen JQ. Artesunate enhances γδ T-cell-mediated antitumor activity through augmenting γδ T-cell function and reversing immune escape of HepG2 cells. Immunopharmacol Immunotoxicol. 2018;40:107–116. doi: 10.1080/08923973.2017.1386212. [DOI] [PubMed] [Google Scholar]
- 30.Jing W, Shuo L, Yingru X, Min M, Runpeng Z, Jun X, Dong H. Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;519:41–45. doi: 10.1016/j.bbrc.2019.08.115. [DOI] [PubMed] [Google Scholar]
- 31.He W, Huang X, Berges BK, Wang Y, An N, Su R, Lu Y. Artesunate regulates neurite outgrowth inhibitor protein B receptor to overcome resistance to sorafenib in hepatocellular carcinoma cells. Front Pharmacol. 2021;12:615889. doi: 10.3389/fphar.2021.615889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci Rep. 2019;39:BSR20180992. doi: 10.1042/BSR20180992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong YK, Xu C, Kalesh KA, He Y, Lin Q, Wong WSF, Shen HM, Wang J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med Res Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 34.Chen S, Gan S, Han L, Li X, Xie X, Zou D, Sun H. Artesunate induces apoptosis and inhibits the proliferation, stemness, and tumorigenesis of leukemia. Ann Transl Med. 2020;8:767. doi: 10.21037/atm-20-4558. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Chen X, Zhang XL, Zhang GH, Gao YF. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz J Med Biol Res. 2019;52:e7992. doi: 10.1590/1414-431x20197992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng J, Yuan C, Wu Z, Wang Y, Yin W, Lin Y, Zhou L, Lu J. Upregulation of microRNA-1 inhibits proliferation and metastasis of breast cancer. Mol Med Rep. 2020;22:454–464. doi: 10.3892/mmr.2020.11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Zhou L, Xiang JD, Jin CS, Li MQ, He YY. Artesunate-induced ATG5-related autophagy enhances the cytotoxicity of NK92 cells on endometrial cancer cells via interactions between CD155 and CD226/TIGIT. Int Immunopharmacol. 2021;97:107705. doi: 10.1016/j.intimp.2021.107705. [DOI] [PubMed] [Google Scholar]
- 38.Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohan R, Collin A, Guizzardi S, Tolosa de Talamoni N, Picotto G. Reactive oxygen species in cancer: A paradox between pro- and anti-tumour activities. Cancer Chemother Pharmacol. 2020;86:1–13. doi: 10.1007/s00280-020-04103-2. [DOI] [PubMed] [Google Scholar]
- 40.Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77:4459–4483. doi: 10.1007/s00018-020-03536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fei Z, Gu W, Xie R, Su H, Jiang Y. Artesunate enhances radiosensitivity of esophageal cancer cells by inhibiting the repair of DNA damage. J Pharmacol Sci. 2018;138:131–137. doi: 10.1016/j.jphs.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Petricciuolo M, Davidescu M, Fettucciari K, Gatticchi L, Brancorsini S, Roberti R, Corazzi L, Macchioni L. The efficacy of the anticancer 3-bromopyruvate is potentiated by antimycin and menadione by unbalancing mitochondrial ROS production and disposal in U118 glioblastoma cells. Heliyon. 2020;6:e05741. doi: 10.1016/j.heliyon.2020.e05741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, Wang X, Wang X, Zhou Y, Li Y, Wang A, Wang T, An Y, Sun W, Du J, et al. TEOA inhibits proliferation and induces DNA damage of diffuse large b-cell lymphoma cells through activation of the ROS-dependent p38 MAPK signaling pathway. Front Pharmacol. 2020;11:554736. doi: 10.3389/fphar.2020.554736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Li M, Zhu X, Zhang Z, Huang H, Hou L. Artemisinin co-delivery system based on manganese oxide for precise diagnosis and treatment of breast cancer. Nanotechnology. 2021 Apr 28; doi: 10.1088/1361-6528/abfc6f. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 46.Yao X, Zhao CR, Yin H, Wang K, Gao JJ. Synergistic antitumor activity of sorafenib and artesunate in hepatocellular carcinoma cells. Acta Pharmacol Sin. 2020;41:1609–1620. doi: 10.1038/s41401-020-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 48.Li S, Xu HX, Wu CT, Wang WQ, Jin W, Gao HL, Li H, Zhang SR, Xu JZ, Qi ZH, et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis. 2019;22:15–36. doi: 10.1007/s10456-018-9645-2. [DOI] [PubMed] [Google Scholar]
- 49.Cao J, Liu X, Yang Y, Wei B, Li Q, Mao G, He Y, Li Y, Zheng L, Zhang Q, et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/BAI1 signaling pathway. Angiogenesis. 2020;23:325–338. doi: 10.1007/s10456-020-09707-z. [DOI] [PubMed] [Google Scholar]
- 50.Singh N, Badrun D, Ghatage P. State of the art and up-and-coming angiogenesis inhibitors for ovarian cancer. Expert Opin Pharmacother. 2020;21:1579–1590. doi: 10.1080/14656566.2020.1775813. [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Shi L, Yang X, Li S, Guo X, Pan L. Artesunate inhibiting angiogenesis induced by human myeloma RPMI8226 cells. Int J Hematol. 2010;92:587–597. doi: 10.1007/s12185-010-0697-3. [DOI] [PubMed] [Google Scholar]
- 52.Andrade-Tomaz M, de Souza I, Rocha CRR, Gomes LR. The role of chaperone-mediated autophagy in cell cycle control and its implications in cancer. Cells. 2020;9:2140. doi: 10.3390/cells9092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen K, Shou LM, Lin F, Duan WM, Wu MY, Xie X, Xie YF, Li W, Tao M. Artesunate induces G2/M cell cycle arrest through autophagy induction in breast cancer cells. Anticancer Drugs. 2014;25:652–662. doi: 10.1097/CAD.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 54.Weng X, Zhu SQ, Cui HJ. Artesunate inhibits proliferation of glioblastoma cells by arresting cell cycle. Zhongguo Zhong Yao Za Zhi. 2018;43:772–778. doi: 10.19540/j.cnki.cjcmm.20171121.002. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Wei Z, Pan K, Li J, Chen Q. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25:786–798. doi: 10.1007/s10495-020-01638-w. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z, Zhao Q, Zuo ZX, Yuan SQ, Yu K, Zhang Q, Zhang X, Sheng H, Ju HQ, Cheng H, et al. Systematic analysis of the aberrances and functional implications of ferroptosis in cancer. iScience. 2020;23:101302. doi: 10.1016/j.isci.2020.101302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH, Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al. Artesunate synergizes with sorafenib to induce ferroptosis in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:301–310. doi: 10.1038/s41401-020-0478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu LJ, Jiang T, Wang FJ, Huang SH, Cheng XM, Jia YQ. Effects of artesunate combined with bortezomib on apoptosis and autophagy of acute myeloid leukemia cells in vitro and its mechanism. Zhonghua Xue Ye Xue Za Zhi. 2019;40:204–208. doi: 10.3760/cma.j.issn.0253-2727.2019.03.008. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim C, Lee JH, Kim SH, Sethi G, Ahn KS. Artesunate suppresses tumor growth and induces apoptosis through the modulation of multiple oncogenic cascades in a chronic myeloid leukemia xenograft mouse model. Oncotarget. 2015;6:4020–4035. doi: 10.18632/oncotarget.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar B, Kalvala A, Chu S, Rosen S, Forman SJ, Marcucci G, Chen CC, Pullarkat V. Antileukemic activity and cellular effects of the antimalarial agent artesunate in acute myeloid leukemia. Leuk Res. 2017;59:124–135. doi: 10.1016/j.leukres.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Du Q, Mao Z, Fan X, Hu B, Wang Z, Chen Z, Jiang X, Wang Z, Lei N, et al. Combined treatment with artesunate and bromocriptine has synergistic anticancer effects in pituitary adenoma cell lines. Oncotarget. 2017;8:45874–45887. doi: 10.18632/oncotarget.17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karpel-Massler G, Westhoff MA, Kast RE, Dwucet A, Nonnenmacher L, Wirtz CR, Debatin KM, Halatsch ME. Artesunate enhances the antiproliferative effect of temozolomide on U87MG and A172 glioblastoma cell lines. Anticancer Agents Med Chem. 2014;14:313–318. doi: 10.2174/18715206113136660340. [DOI] [PubMed] [Google Scholar]
- 63.Berte N, Lokan S, Eich M, Kim E, Kaina B. Artesunate enhances the therapeutic response of glioma cells to temozolomide by inhibition of homologous recombination and senescence. Oncotarget. 2016;7:67235–67250. doi: 10.18632/oncotarget.11972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lian S, Shi R, Huang X, Hu X, Song B, Bai Y, Yang B, Dong J, Du Z, Zhang Y, et al. Artesunate attenuates glioma proliferation, migration and invasion by affecting cellular mechanical properties. Oncol Rep. 2016;36:984–990. doi: 10.3892/or.2016.4847. [DOI] [PubMed] [Google Scholar]
- 65.Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 66.Button RW, Lin F, Ercolano E, Vincent JH, Hu B, Hanemann CO, Luo S. Artesunate induces necrotic cell death in schwannoma cells. Cell Death Dis. 2014;5:e1466. doi: 10.1038/cddis.2014.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei S, Liu L, Chen Z, Yin W, Liu Y, Ouyang Q, Zeng F, Nie Y, Chen T. Artesunate inhibits the mevalonate pathway and promotes glioma cell senescence. J Cell Mol Med. 2020;24:276–284. doi: 10.1111/jcmm.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greenshields AL, Fernando W, Hoskin DW. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp Mol Pathol. 2019;107:10–22. doi: 10.1016/j.yexmp.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Wen L, Liu L, Wen L, Yu T, Wei F. Artesunate promotes G2/M cell cycle arrest in MCF7 breast cancer cells through ATM activation. Breast Cancer. 2018;25:681–686. doi: 10.1007/s12282-018-0873-5. [DOI] [PubMed] [Google Scholar]
- 70.Greenshields AL, Shepherd TG, Hoskin DW. Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog. 2017;56:75–93. doi: 10.1002/mc.22474. [DOI] [PubMed] [Google Scholar]
- 71.Li B, Bu S, Sun J, Guo Y, Lai D. Artemisinin derivatives inhibit epithelial ovarian cancer cells via autophagy-mediated cell cycle arrest. Acta Biochim Biophys Sin (Shanghai) 2018;50:1227–1235. doi: 10.1093/abbs/gmy125. [DOI] [PubMed] [Google Scholar]
- 72.Liu L, Zuo LF, Zuo J, Wang J. Artesunate induces apoptosis and inhibits growth of Eca109 and Ec9706 human esophageal cancer cell lines in vitro and in vivo. Mol Med Rep. 2015;12:1465–1472. doi: 10.3892/mmr.2015.3517. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Liu L, Chen Y, Du Y, Wang J, Liu J. Correlation between adenosine triphosphate (ATP)-binding cassette transporter G2 (ABCG2) and drug resistance of esophageal cancer and reversal of drug resistance by artesunate. Pathol Res Pract. 2018;214:1467–1473. doi: 10.1016/j.prp.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Liu L, Wang J, Chen Y. Inhibitory effect of artesunate on growth and apoptosis of gastric cancer cells. Arch Med Res. 2017;48:623–630. doi: 10.1016/j.arcmed.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 75.Zhang P, Luo HS, Li M, Tan SY. Artesunate inhibits the growth and induces apoptosis of human gastric cancer cells by downregulating COX-2. Onco Targets Ther. 2015;8:845–854. doi: 10.2147/OTT.S81041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang F, Zhou JY, Zhang D, Liu MH, Chen YG. Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunate-induced apoptosis. Int J Mol Med. 2018;42:1295–1304. doi: 10.3892/ijmm.2018.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kumar VL, Verma S, Das P. Artesunate suppresses inflammation and oxidative stress in a rat model of colorectal cancer. Drug Dev Res. 2019;80:1089–1097. doi: 10.1002/ddr.21590. [DOI] [PubMed] [Google Scholar]
- 78.Ilamathi M, Santhosh S, Sivaramakrishnan V. Artesunate as an anti-cancer agent targets stat-3 and favorably suppresses hepatocellular carcinoma. Curr Top Med Chem. 2016;16:2453–2463. doi: 10.2174/1568026616666160212122820. [DOI] [PubMed] [Google Scholar]
- 79.Wojcicki AV, Kasowski MM, Sakamoto KM, Lacayo N. Metabolomics in acute myeloid leukemia. Mol Genet Metab. 2020;130:230–238. doi: 10.1016/j.ymgme.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Efferth T, Giaisi M, Merling A, Krammer PH, Li-Weber M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One. 2007;2:e693. doi: 10.1371/journal.pone.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y, Yang J, Chen L, Wang J, Wang Y, Luo J, Pan L, Zhang X. Artesunate induces apoptosis through caspase-dependent and -independent mitochondrial pathways in human myelodysplastic syndrome SKM-1 cells. Chem Biol Interact. 2014;219:28–36. doi: 10.1016/j.cbi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 82.Tan M, Rong Y, Su Q, Chen Y. Artesunate induces apoptosis via inhibition of STAT3 in THP-1 cells. Leuk Res. 2017;62:98–103. doi: 10.1016/j.leukres.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Li Y, Feng L, Li Y, Jiang W, Shan N, Wang X. Artesunate possesses anti-leukemia properties that can be enhanced by arsenic trioxide. Leuk Lymphoma. 2014;55:1366–1372. doi: 10.3109/10428194.2013.829573. [DOI] [PubMed] [Google Scholar]
- 84.Papanikolaou X, Johnson S, Garg T, Tian E, Tytarenko R, Zhang Q, Stein C, Barlogie B, Epstein J, Heuck C. Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis. Oncotarget. 2014;5:4118–4128. doi: 10.18632/oncotarget.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blessing MM, Alexandrescu S. Embryonal tumors of the central nervous system: An update. Surg Pathol Clin. 2020;13:235–247. doi: 10.1016/j.path.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Alegría-Loyola MA, Galnares-Olalde JA, Mercado M. Tumors of the central nervous system. Rev Med Inst Mex Seguro Soc. 2017;55:330–340. (In Spanish) [PubMed] [Google Scholar]
- 87.Francis SS, Wang R, Enders C, Prado I, Wiemels JL, Ma X, Metayer C. Socioeconomic status and childhood central nervous system tumors in California. Cancer Causes Control. 2021;32:27–39. doi: 10.1007/s10552-020-01348-3. [DOI] [PubMed] [Google Scholar]
- 88.Reichert S, Reinboldt V, Hehlgans S, Efferth T, Rödel C, Rödel F. A radiosensitizing effect of artesunate in glioblastoma cells is associated with a diminished expression of the inhibitor of apoptosis protein survivin. Radiother Oncol. 2012;103:394–401. doi: 10.1016/j.radonc.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 89.Kobayashi D, Hirayama M, Komohara Y, Mizuguchi S, Wilson Morifuji M, Ihn H, Takeya M, Kuramochi A, Araki N. Translationally controlled tumor protein is a novel biological target for neurofibromatosis type 1-associated tumors. J Biol Chem. 2014;289:26314–26326. doi: 10.1074/jbc.M114.568253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao J, Wen J, Wang S, Yao J, Liao L, Dong J. Association between adipokines and thyroid carcinoma: A meta-analysis of case-control studies. BMC Cancer. 2020;20:788. doi: 10.1186/s12885-020-07299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma L, Fei H. Antimalarial drug artesunate is effective against chemoresistant anaplastic thyroid carcinoma via targeting mitochondrial metabolism. J Bioenerg Biomembr. 2020;52:123–130. doi: 10.1007/s10863-020-09824-w. [DOI] [PubMed] [Google Scholar]
- 92.Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PA, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38:2620–2627. doi: 10.1200/JCO.19.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breast Cancer Expert Committee of National Cancer Quality Control Center; Breast Cancer Expert Committee of China Anti-Cancer Association; Cancer Drug Clinical Research Committee of China Anti-Cancer Association, corp-author. Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2020 edition) Zhonghua Zhong Liu Za Zhi. 2020;42:781–797. doi: 10.3760/cma.j.cn112152-20200817-00747. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 94.Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E, Farahmand L. Breast cancer: Biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535. doi: 10.1016/j.intimp.2020.106535. [DOI] [PubMed] [Google Scholar]
- 95.Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20:417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 96.Dong X, Bai X, Ni J, Zhang H, Duan W, Graham P, Li Y. Exosomes and breast cancer drug resistance. Cell Death Dis. 2020;11:987. doi: 10.1038/s41419-020-03189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tran TH, Nguyen TD, Poudel BK, Nguyen HT, Kim JO, Yong CS, Nguyen CN. Development and evaluation of artesunate-loaded chitosan-coated lipid nanocapsule as a potential drug delivery system against breast cancer. AAPS PharmSciTech. 2015;16:1307–1316. doi: 10.1208/s12249-015-0311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tran TH, Nguyen AN, Kim JO, Yong CS, Nguyen CN. Enhancing activity of artesunate against breast cancer cells via induced-apoptosis pathway by loading into lipid carriers. Artif Cells Nanomed Biotechnol. 2016;44:1979–1987. doi: 10.3109/21691401.2015.1129616. [DOI] [PubMed] [Google Scholar]
- 99.Zhang S, Yuan H, Guo Y, Wang K, Wang X, Guo Z. Towards rational design of RAD51-targeting prodrugs: platinumIV-artesunate conjugates with enhanced cytotoxicity against BRCA-proficient ovarian and breast cancer cells. Chem Commun (Camb) 2018;54:11717–11720. doi: 10.1039/C8CC06576D. [DOI] [PubMed] [Google Scholar]
- 100.Raza A, Ghoshal A, Chockalingam S, Ghosh SS. Connexin-43 enhances tumor suppressing activity of artesunate via gap junction-dependent as well as independent pathways in human breast cancer cells. Sci Rep. 2017;7:7580. doi: 10.1038/s41598-017-08058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z, Zhu YT, Xiang M, Qiu JL, Luo SQ, Lin F. Enhanced lysosomal function is critical for paclitaxel resistance in cancer cells: Reversed by artesunate. Acta Pharmacol Sin. 2021;42:624–632. doi: 10.1038/s41401-020-0445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kujawa KA, Lisowska KM. Ovarian cancer-from biology to clinic. Postepy Hig Med Dosw (Online) 2015;69:1275–1290. doi: 10.5604/17322693.1184451. (In Polish) [DOI] [PubMed] [Google Scholar]
- 103.Aziz NB, Mahmudunnabi RG, Umer M, Sharma S, Rashid MA, Alhamhoom Y, Shim YB, Salomon C, Shiddiky MJA. MicroRNAs in ovarian cancer and recent advances in the development of microRNA-based biosensors. Analyst. 2020;145:2038–2057. doi: 10.1039/C9AN02263E. [DOI] [PubMed] [Google Scholar]
- 104.Gaona-Luviano P, Medina-Gaona LA, Magaña-Pérez K. Epidemiology of ovarian cancer. Chin Clin Oncol. 2020;9:47. doi: 10.21037/cco-20-34. [DOI] [PubMed] [Google Scholar]
- 105.Yang Y, Qi S, Shi C, Han X, Yu J, Zhang L, Qin S, Gao Y. Identification of metastasis and prognosis-associated genes for serous ovarian cancer. Biosci Rep. 2020;40:BSR20194324. doi: 10.1042/BSR20194324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. doi: 10.1136/bmj.m3773. [DOI] [PubMed] [Google Scholar]
- 107.Rooth C. Ovarian cancer: Risk factors, treatment and management. Br J Nurs. 2013;22:S23–S30. doi: 10.12968/bjon.2013.22.Sup17.S23. [DOI] [PubMed] [Google Scholar]
- 108.Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA, III, Bidziński M, Kim JW, Nam JH, Madry R, Hernández C, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): A randomized phase III trial. J Clin Oncol. 2020;38:1164–1174. doi: 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yadav G, Vashisht M, Yadav V, Shyam R. Molecular biomarkers for early detection and prevention of ovarian cancer-a gateway for good prognosis: A narrative review. Int J Prev Med. 2020;11:135. doi: 10.4103/ijpvm.IJPVM_75_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen HH, Zhou HJ, Wu GD, Lou XE. Inhibitory effects of artesunate on angiogenesis and on expressions of vascular endothelial growth factor and VEGF receptor KDR/flk-1. Pharmacology. 2004;71:1–9. doi: 10.1159/000076256. [DOI] [PubMed] [Google Scholar]
- 111.Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang X, Zou Y, Liu Z, Liu J, Wei J, et al. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol Ther. 2015;16:1548–1556. doi: 10.1080/15384047.2015.1071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr) 2020;43:195–209. doi: 10.1007/s13402-019-00488-2. [DOI] [PubMed] [Google Scholar]
- 113.Fan J, Liu Z, Mao X, Tong X, Zhang T, Suo C, Chen X. Global trends in the incidence and mortality of esophageal cancer from 1990 to 2017. Cancer Med. 2020;9:6875–6887. doi: 10.1002/cam4.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi R, Cui H, Bi Y, Huang X, Song B, Cheng C, Zhang L, Liu J, He C, Wang F, et al. Artesunate altered cellular mechanical properties leading to deregulation of cell proliferation and migration in esophageal squamous cell carcinoma. Oncol Lett. 2015;9:2249–2255. doi: 10.3892/ol.2015.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eusebi LH, Telese A, Marasco G, Bazzoli F, Zagari RM. Gastric cancer prevention strategies: A global perspective. J Gastroenterol Hepatol. 2020;35:1495–1502. doi: 10.1111/jgh.15037. [DOI] [PubMed] [Google Scholar]
- 116.Niu PH, Zhao LL, Wu HL, Zhao DB, Chen YT. Artificial intelligence in gastric cancer: Application and future perspectives. World J Gastroenterol. 2020;26:5408–5419. doi: 10.3748/wjg.v26.i36.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhou X, Sun WJ, Wang WM, Chen K, Zheng JH, Lu MD, Li PH, Zheng ZQ. Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anticancer Drugs. 2013;24:920–927. doi: 10.1097/CAD.0b013e328364a109. [DOI] [PubMed] [Google Scholar]
- 118.Su T, Li F, Guan J, Liu L, Huang P, Wang Y, Qi X, Liu Z, Lu L, Wang D. Artemisinin and its derivatives prevent Helicobacter pylori-induced gastric carcinogenesis via inhibition of NF-κB signaling. Phytomedicine. 2019;63:152968. doi: 10.1016/j.phymed.2019.152968. [DOI] [PubMed] [Google Scholar]
- 119.La Vecchia S, Sebastián C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63–70. doi: 10.1016/j.semcdb.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 120.Johdi NA, Sukor NF. Colorectal cancer immunotherapy: Options and strategies. Front Immunol. 2020;11:1624. doi: 10.3389/fimmu.2020.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li LN, Zhang HD, Yuan SJ, Tian ZY, Wang L, Sun ZX. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int J Cancer. 2007;121:1360–1365. doi: 10.1002/ijc.22804. [DOI] [PubMed] [Google Scholar]
- 122.Cui C, Feng H, Shi X, Wang Y, Feng Z, Liu J, Han Z, Fu J, Fu Z, Tong H. Artesunate down-regulates immunosuppression from colorectal cancer Colon26 and RKO cells in vitro by decreasing transforming growth factor β1 and interleukin-10. Int Immunopharmacol. 2015;27:110–121. doi: 10.1016/j.intimp.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 123.Bei Y, Chen X, Raturi VP, Liu K, Ye S, Xu Q, Lu M. Treatment patterns and outcomes change in early-stage non-small cell lung cancer in octogenarians and older: A SEER database analysis. Aging Clin Exp Res. 2021;33:147–156. doi: 10.1007/s40520-020-01517-z. [DOI] [PubMed] [Google Scholar]
- 124.Huo KG, D'Arcangelo E, Tsao MS. Patient-derived cell line, xenograft and organoid models in lung cancer therapy. Transl Lung Cancer Res. 2020;9:2214–2232. doi: 10.21037/tlcr-20-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, Sun L, Wang N, Jiang X, Zhang Y. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi: 10.1016/j.biopha.2020.111111. [DOI] [PubMed] [Google Scholar]
- 126.Rasheed SA, Efferth T, Asangani IA, Allgayer H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer. 2010;127:1475–1485. doi: 10.1002/ijc.25315. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y, Jiang W, Li B, Yao Q, Dong J, Cen Y, Pan X, Li J, Zheng J, Pang X, Zhou H. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int Immunopharmacol. 2011;11:2039–2046. doi: 10.1016/j.intimp.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 128.Wang JS, Wang MJ, Lu X, Zhang J, Liu QX, Zhou D, Dai JG, Zheng H. Artesunate inhibits epithelial-mesenchymal transition in non-small-cell lung cancer (NSCLC) cells by down-regulating the expression of BTBD7. Bioengineered. 2020;11:1197–1207. doi: 10.1080/21655979.2020.1834727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen X, Han K, Chen F, Wu C, Huang W. Effects of artesunate on the invasion of lung adenocarcinoma A549 cells and expression of ICAM-1 and MMP-9. Zhongguo Fei Ai Za Zhi. 2013;16:567–571. doi: 10.3779/j.issn.1009-3419.2013.11.01. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tong Y, Liu Y, Zheng H, Zheng L, Liu W, Wu J, Ou R, Zhang G, Li F, Hu M, et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget. 2016;7:31413–31428. doi: 10.18632/oncotarget.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W, Ma G, Deng Y, Wu Q, Wang Z, Zhou Q. Artesunate exhibits synergistic anti-cancer effects with cisplatin on lung cancer A549 cells by inhibiting MAPK pathway. Gene. 2021;766:145134. doi: 10.1016/j.gene.2020.145134. [DOI] [PubMed] [Google Scholar]
- 132.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng Z, Wei-Qi J, Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188382. doi: 10.1016/j.bbcan.2020.188382. [DOI] [PubMed] [Google Scholar]
- 134.Hou J, Wang D, Zhang R, Wang H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin Cancer Res. 2008;14:5519–5530. doi: 10.1158/1078-0432.CCR-08-0197. [DOI] [PubMed] [Google Scholar]
- 135.Jin M, Shen X, Zhao C, Qin X, Liu H, Huang L, Qiu Z, Liu Y. In vivo study of effects of artesunate nanoliposomes on human hepatocellular carcinoma xenografts in nude mice. Drug Deliv. 2013;20:127–133. doi: 10.3109/10717544.2013.801047. [DOI] [PubMed] [Google Scholar]
- 136.Guragain D, Seubwai W, Kobayashi D, Silsinivanit A, Vaeteewoottacharn K, Sawanyawisuth K, Wongkham C, Wongkham S, Araki N, Cha'on U. Artesunate and chloroquine induce cytotoxic activity on cholangiocarcinoma cells via different cell death mechanisms. Cell Mol Biol (Noisy-le-grand) 2018;64:113–118. doi: 10.14715/cmb/2018.64.10.18. [DOI] [PubMed] [Google Scholar]
- 137.Wang ZC, Liu Y, Wang H, Han QK, Lu C. Research on the relationship between artesunate and raji cell autophagy and apoptosis of burkitt's lymphoma and its mechanism. Eur Rev Med Pharmacol Sci. 2017;21:2238–2243. [PubMed] [Google Scholar]
- 138.Chauhan AK, Min KJ, Kwon TK. RIP1-dependent reactive oxygen species production executes artesunate-induced cell death in renal carcinoma caki cells. Mol Cell Biochem. 2017;435:15–24. doi: 10.1007/s11010-017-3052-7. [DOI] [PubMed] [Google Scholar]
- 139.Sarma B, Willmes C, Angerer L, Adam C, Becker JC, Kervarrec T, Schrama D, Houben R. Artesunate affects T antigen expression and survival of virus-positive merkel cell carcinoma. Cancers (Basel) 2020;12:919. doi: 10.3390/cancers12040919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zheng L, Pan J. The anti-malarial drug artesunate blocks Wnt/β-catenin pathway and inhibits growth, migration and invasion of uveal melanoma cells. Curr Cancer Drug Targets. 2018;18:988–998. doi: 10.2174/1568009618666180425142653. [DOI] [PubMed] [Google Scholar]
- 141.Wang Z, Wang C, Wu Z, Xue J, Shen B, Zuo W, Wang Z, Wang SL. Artesunate suppresses the growth of prostatic cancer cells through inhibiting androgen receptor. Biol Pharm Bull. 2017;40:479–485. doi: 10.1248/bpb.b16-00908. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y, Wu N, Wu Y, Chen H, Qiu J, Qian X, Zeng J, Chiu K, Gao Q, Zhuang J. Artesunate induces mitochondria-mediated apoptosis of human retinoblastoma cells by upregulating Kruppel-like factor 6. Cell Death Dis. 2019;10:862. doi: 10.1038/s41419-019-2084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol. 2017;11:254–262. doi: 10.1016/j.redox.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berköz M, Özkan-Yılmaz F, Özlüer-Hunt A, Krośniak M, Türkmen Ö, Korkmaz D, Keskin S. Artesunate inhibits melanoma progression in vitro via suppressing STAT3 signaling pathway. Pharmacol Rep. 2021;73:650–663. doi: 10.1007/s43440-021-00230-6. [DOI] [PubMed] [Google Scholar]
- 145.Mancuso RI, Foglio MA, Olalla Saad ST. Artemisinin-type drugs for the treatment of hematological malignancies. Cancer Chemother Pharmacol. 2021;87:1–22. doi: 10.1007/s00280-020-04170-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the present study.