Abstract
Introduction: Pediatric spinal deformity involves a complex 3-dimensional (3D) deformity that increases the risk of pedicle screw placement due to the close proximity of neurovascular structures. To increase screw accuracy, improve patient safety, and minimize surgical complications, the placement of pedicle screws is evolving from freehand techniques to computer-assisted navigation and to the introduction of robotic-assisted placement. Purpose: The aim of this review was to review the current literature on the use of robotic navigation in pediatric spinal deformity surgery to provide both an error analysis of these techniques and to provide recommendations to ensure its safe application. Methods: A narrative review was conducted in April 2021 using the MEDLINE (PubMed) database. Studies were included if they were peer-reviewed retrospective or prospective studies, included pediatric patients, included a primary diagnosis of pediatric spine deformity, utilized robotic-assisted spinal surgery techniques, and reported thoracic or lumbar pedicle screw breach rates or pedicle screw malpositioning. Results: In the few studies published on the use of robotic techniques in pediatric spinal deformity surgery, several found associations between the technology and increased rates of screw placement accuracy, reduced rates of breach, and minimal complications. All were retrospective studies. Conclusions: Current literature is of a low level of evidence; nonetheless, the findings suggest the accuracy and safety of robotic-assisted spinal surgery in pediatric pedicle screw placement. The introduction of robotics may drive further advances in less invasive pediatric spinal deformity surgery. Further study is warranted.
Keywords: spine, pediatrics, operative treatments, mini-incision surgery, scoliosis, robotics
Introduction
Pediatric spinal deformity often involves complex 3-dimensional (3D) deformities associated with small and frequently dysplastic pedicles [37,43]. One of the goals of surgical management is to obtain a stable and solid fusion after placement of spinal anchors followed by spinal deformity correction. The mainstay of operative pediatric spinal deformity management has been open posterior spinal instrumentation and fusion (PSIF), most frequently using pedicle screws inserted with freehand techniques [17,27,53].
In children with spinal deformity, the insertion of thoracic pedicle screws presents increased risk of malpositioned pedicle screws and other complications compared with lumbar pedicle screw insertion. Usually, this is caused by altered morphology in the thoracic pedicle, including dysplastic and narrow pedicles and altered location of neurovascular structures secondary to spinal deformity [11,15,17,37,43,47,55,58]. Screw malpositioning is a commonly documented implant-related complication; rates vary between 4.2% and 25% for placement with freehand techniques [15,17].
Multiple methods have been used to improve the accuracy and safety of thoracic pedicle screw placement, including anatomically based techniques (use of pedicle probes and pedicle wall palpation, visualization of cancellous starting points, and laminectomy with direct pedicle visualization and palpation), as well as preoperative and intraoperative use of various ionizing radiation techniques such as fluoroscopy, plain radiographs, and computed tomography (CT) scans. Several techniques for neuromonitoring have gained widespread acceptance as clinical standards for improving patient safety, including transcranial motor-evoked potentials and somatosensory evoked potentials [7,23,44,49,50], spontaneous electromyography (EMG) monitoring [7,23], triggered electromyography (t-EMG) screw stimulation [40,41], and pedicle probes with electroconductive tips [36]. Issues of safety, reliability, and accuracy have been crucial in the ongoing development and improvement of computer-assisted pedicle screw navigation in spine surgery.
First used in spine surgery in the late 1990s, computer navigation is proposed as a way to allow for real-time assessment of screw trajectory accuracy [15,20]. When comparing different pedicle screw insertion methods, computer-assisted surgical navigation of pedicle screws has lower rates of malpositioned screws and unplanned return to the operating room (OR) [5,6,22,24,25,31,39,52]. Additionally, surgical navigation has been associated with significantly lower rates of medial wall breach compared to freehand techniques (0%–7.9% vs 8.6%) [5,6,22]. The incidence of an unsafe, significant medial breach (50% of the screw diameter) was 7.6 times more likely to occur using freehand screw insertion compared with surgical navigation in one study [51].
Robotic-assisted spine surgery (RASS) is a natural evolution from computer-assisted surgical navigation, and it has several potential advantages over freehand techniques, including improved stability and maintenance of screw trajectory during insertion, opportunity for preoperative screw trajectory planning to find ideal trajectory, and potential for screw insertion without direct visualization of bony anatomy and screw entry site. Accurate, reliable, and safe pedicle screw insertion using robotically assisted techniques opens the possibility for lesser invasive surgical approaches for pediatric spinal deformity as less invasive robotic-assisted techniques have already been adopted by adult spine surgeons to assist in the correction of adult spinal deformity [2,8,9,26,38,54].
The first robotic system cleared for use in spine surgery by the US Food and Drug Administration (FDA) was the SpineAssist (Mazor Robotics LTD, Caesarea, Israel) in 2004 [13,16]. The SpineAssist was replaced by the Mazor Renaissance (Mazor Surgical Technologies Ltd, Caesarea, Israel) in 2011, and the first reports of the use of RASS in pediatric spinal deformity utilized this system [13,16,18,28]. This system was a mechanically driven bone-mounted system with a robotic manipulator, allowing for 6 degrees of freedom, attached to a bone-mounting frame on the patient’s spine.
There are now several FDA-cleared spine robots, including ROSA Spine Robot, which was subsequently replaced by the Rosa One Spine system (Zimmer Biomet Robotics, Montpelier, France), ExcelsiusGPS (Globus Medical, Inc, Audubon, PA), and Mazor X (Medtronic, Dublin, Ireland) [13]. The increasing interest in RASS has led to further FDA clearances, including the Cirq system (Brainlab AG, Munich, Germany) [14].
The safety, efficacy, and accuracy of these systems is an active area of research [11,21,52]. Increased adoption of robotically assisted insertion of pedicle screws has largely been driven by adult spine surgeons who have pioneered single-position lateral surgery for simultaneous anterior and posterior spinal fusion in the lumbar spine, as well as minimally invasive percutaneous screw insertion in the lumbar spine [19,21].
The current generation of spine robots facilitates pedicle screw placement through an end-effector that functions as a rigid drill sleeve that obtains and maintains planned screw insertion trajectory. The current generation of robotic spine systems provides for increased stability of the robotic arm, increased stability of spine region of interest via spine stabilization clamps, and improved overall system stability via direct connection of the robot to the skeletal anatomy of the patient. Simultaneous computer-assisted surgical navigation tracking the instruments and the skeleton also provides real-time visual feedback layered on top of the robotic navigation.
We aimed to review the current literature on the use of robotic navigation in pediatric spinal deformity. We sought to provide an error analysis of these techniques to include reported breach rates and surgical and patient factors that may lead to increased breach or other complications. In addition, we aimed to provide recommendations and best practices to ensure safe placement of pedicle screws and discuss further applications of robotic surgery in pediatric spine deformity correction based on our own experience.
Methods: Search Strategies and Criteria
A narrative review was conducted in April 2021 using the MEDLINE (PubMed) database. The search strategy consisted of the operators found in Appendix A. Studies were included if they were peer-reviewed, retrospective, or prospective studies; included pediatric patients and a primary diagnosis of pediatric spine deformity such as adolescent idiopathic scoliosis, neuromuscular scoliosis, congenital scoliosis, or Scheuermann’s kyphosis; utilized RASS techniques; and reported thoracic or lumbar pedicle screw breach rates or pedicle screw malpositioning.
The search identified 40 articles. Of the articles identified, 16 abstracts were screened, 12 of which were excluded, 3 for being a research design other than a prospective or retrospective study, 4 for not utilizing robotic navigation techniques, 3 for not including pediatric patients, 1 for not including thoracic or lumbar pedicle screw placement, and 1 for not providing breach rates. Four full-text articles were reviewed, 4 of which met inclusion criteria (Table 1) [16,29,34,45]. Study quality was graded using the Downs and Black checklist, a 27-question assessment with a maximum score of 28 [12]. Similar to other studies, question 27 was replaced with “Was a power analysis conducted?” with a score of 1 for yes and 0 for no [30,33].
Table 1.
Summary of articles identified studying robotic-assisted spine surgery (RASS) in the pediatric population.
| Author | Year published | Study type | Downs and Black score (x/28) | Robotic platform utilized | Number of patients | Diagnosis | Mean age at surgery (years) |
|---|---|---|---|---|---|---|---|
| Macke | 2016 | Retrospective review | 17 | Mazor Renaissance | 50 | AIS | N/A |
| Shaw | 2018 | Retrospective review | 18 | Mazor Renaissance | 19 | AIS | 14.5 ± 1.7 |
| Gonzalez | 2020 | Retrospective review | 16 | Mazor X Stealth Edition | 40 | Neuromuscular (N = 5), spondylolisthesis (N = 4), congenital scoliosis (N = 2), AIS (N = 26), other (N = 3) | 14.5 ± 2.6 |
| Morse | 2021 | Retrospective review | 18 | Mazor X Stealth Edition | 19 | AIS (N = 13), neuromuscular scoliosis (n = 3), Scheuermann’s kyphosis (n = 1), post radiation scoliosis (n = 1), congenital scoliosis (n = 1) | 14.6 ± 2.2 |
Results
Error Analysis in Pediatric Robotic Spine Surgery
Four studies were identified that have reviewed the potential complications with the use of both second-generation and third-generation robotic navigation in pediatric spinal deformity.
Macke et al reviewed 48 pediatric patients following screw placement with the second-generation Mazor Renaissance system and analyzed 662 screws [29]. The authors performed postoperative CT scans on all patients and found a total screw medial misplacement rate of 7.2%, with the majority (4.5%) having a medial breach between 2 and 4 mm. Additionally, they reported 1.5% of screws had a medial breach between 4 and 6 mm, and 1.2% were greater than 6 mm. Despite the medially placed screws, screws were more often misplaced laterally. While the authors did not evaluate time per screw to evaluate a learning curve, they reported a 9.6% breach rate in the first third of patients and a 7.4% breach rate in the last third. They also reported that patients who underwent preoperative CT scanning in the prone position were less likely to have a breach.
Shaw et al also examined pedicle screw accuracy with the Mazor Renaissance [45]. Following screw insertion, each pedicle screw was stimulated with t-EMG, with thresholds less than 8 mA deemed abnormal. These screws were subsequently removed and the tract was probed with a ball-tip probe. If a medial wall was not palpated, a new screw trajectory was created. The authors reviewed 49 patients and 844 instrumented pedicles and reported 28 (3.3%) screws with abnormal stimulation. All trajectories were found to have an intact medial wall and the screw was reinserted; 51% of these screws were periapical, 19 on the curve concavity, and in a logistic regression analysis, smaller pedicle width was found to be a significant predictor for t-EMG amplitude.
Recently, Gonazalez et al reviewed 40 patients with 314 screws placed robotically with the Mazor X [16]. The authors used a preoperative CT scan (12 patients) for surgical planning and then an intraoperative O-arm scan (Medtronic, Dublin, Ireland) for registration in lieu of fluoroscopy following spine exposure and facetectomies due to their belief in the difficulty of using fluoroscopy to register patients with significant apical rotation. The authors used either a spinous process clamp or a Schanz pin placed in the posterior superior iliac spine (PSIS) to attach the robotic arm. Screw position was assessed with intraoperative fluoroscopy and postoperative radiographs. They reported 4 (1.3%) screws that had difficult placement, 3 of which were lateral, and 1 which was unable to be placed due to a sclerotic pedicle. Overall, the authors reported a 98.7% screw success rate. Two patients in the series had wound drainage and returned to the OR for debridement.
Morse et al reviewed 19 patients and 194 pedicles, 168 (86.6%) of which were placed with the Mazor X [34]. A preoperative CT scan was performed for all patients and following instrumentation a 3D mobile fluoroscopic scan (Ziehm Imaging GmBH, Nuremberg, Germany) was used to assess screw position following placement in the OR. Standing biplanar low-dose whole-body slit radiographs (EOS Imaging, Paris, France), reconstructed to map individual vertebral body position, were used to assess the effect of vertebral body rotation on complications. The authors reported 29 (15.0%) screws that were abandoned or converted to freehand; the most common reason for freehand conversion was soft tissue impaction on the end effector (48.3%). There were 15 (8.9%) total medial breaches that occurred, the majority under 2 mm. There were 2 medial breaches between 2 and 4 mm, 1 of which was associated with a durotomy, which occurred due to inadequate tightening between the bone mount bridge and the spinous process clamp, causing medial skive from excess robotic arm motion. The durotomy did not produce any adverse symptoms postoperatively. Both of these breaches occurred in the first case, and excluding breaches less than 2 mm, robotically placed screws had a 98.8% accuracy rate. Most of the breaches occurred in the first case, with the majority occurring in the thoracic spine between T4 and T12. Risk of breach was associated with small pedicle diameter and vertebral body kyphosis.
In addition to reporting on errors, the authors presented a learning curve for screw time and reported a mean time per robotically placed screw of 3.6 ± 2.4 minutes. The time improved over the second half of cases and with experience. Slower screw placement times occurred in upper thoracic spine (T2–T3) and no difference was found between placement times of apical or peri-apical vertebrae and all other vertebrae [34]. The authors assessed screw accuracy, defined as a trajectory that matched preoperative templating and reported 76% of screws having a matched trajectory. Screws were most accurately placed in the lumbar spine and least accurately in the thoracic spine at the T4–T6 level. Additionally, cortically planned screws, those in which the mid-axis of the screw is placed on the pedicle cortex, were the least accurate. Accuracy improved over the second half of cases and was affected by smaller pedicle diameter, coronal rotation, and sagittal rotation. Screw insertion times are currently under 1 minute.
While the use of RASS in pediatric spinal deformity remains an underreported topic in the literature, these studies demonstrate the accuracy and safety of robotic screw placement. With the use of both second- and third-generation robotic techniques, there were no reported neurologic complications during pediatric spinal deformity correction [15,29,34,45]. These studies also highlight a limitation in assessing postoperative screw position in the pediatric population; only 1 study utilized a postoperative CT to assess screw malposition [29]. The use of imaging modalities other than CT may only detect dangerous breaches and not assess screw position, although these modalities are used to limit radiation exposure in children. Further study is required to determine the screw malpositioning rate. Of note, most reports utilized an open approach to the spine and screw placement could be visualized directly. Currently, the screw insertion technique has evolved and the transmuscular placement of proximal thoracic screws is being utilized.
Recommendations to Optimize Workflow and Ensure Safety
While RASS may offer further improved accuracy and reliability in placing pedicle screws, its adoption presents several challenges. First, the entire surgical team must learn to safely and efficiently implement a new robotically assisted workflow. This includes classroom, dry lab, cadaver lab, and mentorship-type technical training. Adequate training minimizes the duration of and id complications in the learning curve.
The use of the robot can be divided into 4 phases: preoperative planning, intraoperative setup, surgical execution, and postoperative assessment.
Planning may be performed in the OR with a plan and scan workflow or outside the OR with preoperative high-resolution spine imaging ahead of surgery. Preoperative imaging allows for optimal planning of screw trajectories, especially with abnormal or highly dysplastic anatomy. The preoperative scan is uploaded to the planning software and the surgeon can choose screw trajectory optimizing safety and screw purchase. The planning software maximizes the surgeon’s ability to select the correct trajectory and screw size as the screw’s trajectory, diameter, and length can be viewed in the axial, coronal, and sagittal planes. During templating, hypoplastic, sclerotic, and small diameter pedicles are noted, as pedicle morphology differences may suggest extra-pedicular (in-out-in) trajectories (Fig. 1). Decreased screw placement accuracy was noted when preoperative plan included screw placement directly overlying the pedicle cortex [34]. Additionally, when pedicle diameters were less than the smallest pedicle screw diameter, there was a significantly increased risk of breach [34,45]. Vertebral body kyphosis also correlated with increased rates of breach felt to be associated with soft-tissue pressure on the robotic end effector resulting in decreased accuracy of screw placement. Patient factors including height, weight, and length of deformity also impact deformity planning, because more than 1 registration may be required when more than 7 spinal levels are involved.
Fig. 1.
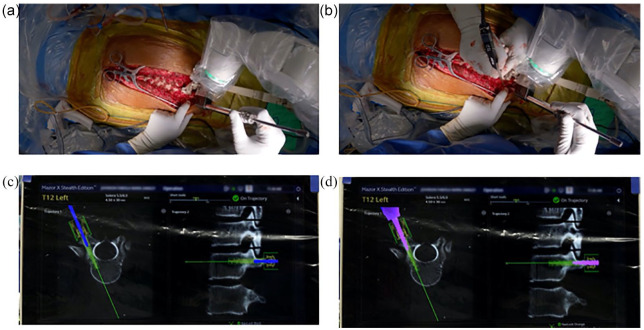
Planning software showing 2 screw trajectories: (a) both screws are planning completely within the pedicle and (b) both screws are planned as in-out-in with the mid-axis of the screw entering the pedicle, exiting, and then entering the vertebral body.
Setup of the robotic system can create ergonomic challenges for the surgeon and OR staff. We recommend placing the camera at the head of the table with a direct line of sight of the robotic arm and the operative field. Staff must be familiar with draping the robot, and all assistants must vigilantly maintain a sterile field. Monitors and the base station should be placed to allow visualization by the surgeon and assistants. The radiology technician must manipulate the fluoroscopic C-arm to capture the appropriate registration views while avoiding contact between the C-arm and the robot (Fig. 2). Failure to manipulate the C-arm correctly can result in registration failure requiring the registration to be completed again. Prior to registration, when obtaining the topographical optical scan, a blue towel is placed over the spine and the lights are turned away to best define the field of view. During registration, the targeting device can be brought close to the patient to obtain the largest field of view when taking fluoroscopic images. When moving between the anteroposterior (AP) view and the oblique view, the targeting device must be removed so that it does not inadvertently strike the patient. Following the registration, the surgeon must confirm that the registration produces an anatomic match to the preoperative imaging.
Fig. 2.
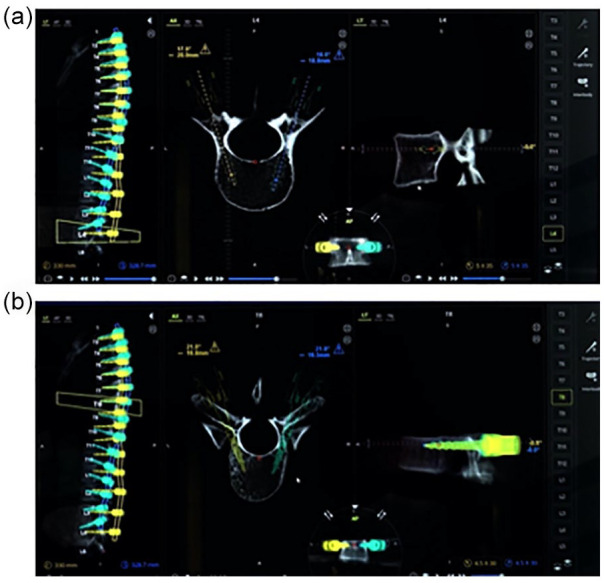
Intraoperative positioning of the fluoroscopic C-arm during registration. The surgeon guides the fluoroscopy technician to ensure appropriate placement during an anteroposterior (a) and a lateral (b) image.
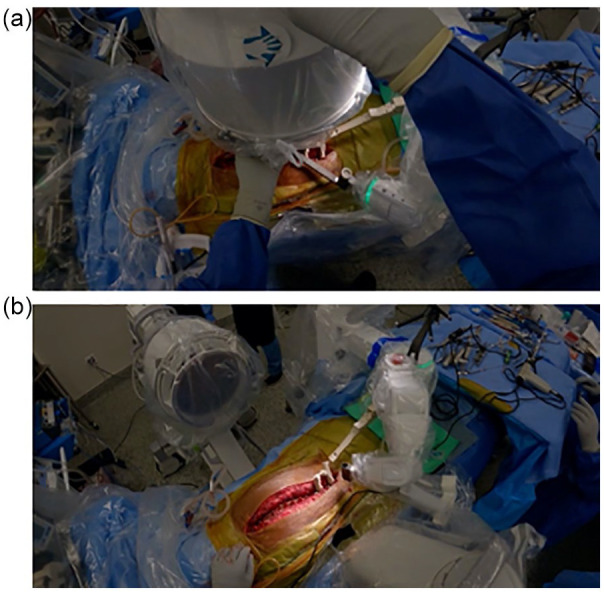
Although most robotic spine systems are freestanding on the OR floor, the Mazor X Stealth robotic platform is attached to the operating table and directly to the patient via threaded skeletal pins and/or spinous process clamps (Fig. 3). Direct patient attachment decreases the risk of inadvertent malalignment from patient or robot motion.
Fig. 3.
Direct attachment of Mazor X to the patient. Intraoperative imaging demonstrating adequate tightening of both the dual spinous process clamp (a) and connection to the bone mount bridge (b).
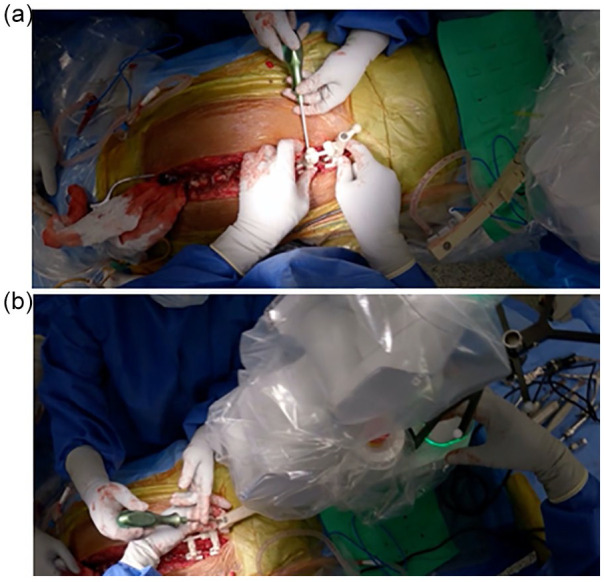
We advocate following traditional freehand pedicle screw steps including visual confirmation of starting points, palpation of pedicle tracks with ball-tip probe, and intraoperative fluoroscopy to confirm screw placement (Fig. 4). The surgeon must visually confirm that the anti-skive pin is properly seated on the pedicle. Occasionally, the anti-skive pin will not seat properly onto the pedicle and skive can occur. To prevent this, a burr can be used to create a pilot hole so that the pin does not slide off the pedicle and the trajectory can be maintained for drilling [16,34]. In addition, we found that the use of intraoperative 3D fluoroscopic scans is helpful to assess accuracy and modify screw planning.
Fig. 4.
Intraoperative screw placement. Direct visualization of a starting point (a). Given the potential of medial skive for this pedicle, a pilot hole was created for the start point with a burr (b). The simultaneous navigation showing drilling is occurring on the templated screw trajectory (c). Screw placement follows on the templated screw trajectory (d).
Future Applications: Minimally Invasive Pediatric Spinal Deformity Surgery
Minimally invasive surgery (MIS) has become an acceptable surgical approach for traumatic, neoplastic, degenerative, and even deformity spinal conditions in adults. These methods have demonstrated similar outcomes, with lower morbidity and mortality [3,4,35,48]. More recently, several authors have suggested that MIS has a role in the management of pediatric spinal deformity surgery [1,10,32,42,46,56,57]. When compared to open posterior spinal fusion (PSF), MIS has consistently been associated with lower estimated blood loss with no differences from open surgery in complication rates but with longer operative times [1,32,42,46,57].
Robotic surgical techniques facilitate a less invasive access to the pedicles via percutaneous or transmuscular approaches, without direct open exposure of the spine. Avoiding dissection of the paraspinal musculature may maintain better vascularity to the bone, thereby decreasing risk of infection, enhancing healing, and lessening pain. Compared to adults, pediatric patients have more flexible spines and excellent fusion rates. Less invasive approaches coupled with lower profile implant systems may result in similar safety and complication profiles as traditional open surgery. Rapid advancement in robotic techniques has resulted in faster operative times, more accurate pedicle screw placement, and equivalent results to computer-assisted freehand surgical navigation. Further research is required to confirm the use of these technologies in maintaining curve correction and measuring screw malpositioning.
Supplemental Material
Supplemental material, sj-docx-1-hss-10.1177_15563316211027828 for Less Invasive Pediatric Spinal Deformity Surgery: The Case for Robotic-Assisted Placement of Pedicle Screws by Kyle W. Morse, Hila Otremski, Kira Page and Roger F. Widmann in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery
Appendix A
Search Terms
“adolescences”[All Fields] OR “adolescency”[All Fields] OR “adolescent”[MeSH Terms] OR “adolescent”[All Fields] OR “adolescence”[All Fields] OR “adolescents”[All Fields] OR “adolescent s”[All Fields]) AND (“idiopathic”[All Fields] OR “idiopathically”[All Fields] OR “idiopathics”[All Fields]) AND (“scoliosis”[MeSH Terms] OR “scoliosis”[All Fields] OR “scolioses”[All Fields])) OR ((“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “paediatric”[All Fields] OR “pediatric”[All Fields]) AND (“spine”[MeSH Terms] OR “spine”[All Fields] OR “spines”[All Fields] OR “spine s”[All Fields])) OR ((“paediatrics”[All Fields] OR “pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “paediatric”[All Fields] OR “pediatric”[All Fields]) AND (“spine deform”[Journal] OR (“spine”[All Fields] AND “deformity”[All Fields]) OR “spine deformity”[All Fields])) OR (“Neuromuscular”[All Fields] AND (“scoliosis”[MeSH Terms] OR “scoliosis”[All Fields] OR “scolioses”[All Fields])) OR ((“congenital”[MeSH Subheading] OR “congenital”[All Fields] OR “congenitally”[All Fields]) AND (“scoliosis”[MeSH Terms] OR “scoliosis”[All Fields] OR “scolioses”[All Fields]))) AND (“robot”[All Fields] OR “robots”[All Fields] OR “robotically”[All Fields] OR “robotics”[MeSH Terms] OR “robotics”[All Fields] OR “robotic”[All Fields] OR “robotization”[All Fields] OR “robotized”[All Fields] OR “robots”[All Fields]).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Roger F. Widmann, MD, reports a relationship with Medtronic. The other authors declared no potential conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Alhammoud A, Alborno Y, Baco AM, et al. Minimally invasive scoliosis surgery is a feasible option for management of idiopathic scoliosis and has equivalent outcomes to open surgery: a meta-analysis [published online ahead of print February 9, 2021]. Global Spine J. 10.1177/2192568220988267. [DOI] [PMC free article] [PubMed]
- 2.Anand N, Baron EM, Khandehroo B, Kahwaty S.Long-term 2- to 5-year clinical and functional outcomes of minimally invasive surgery for adult scoliosis. Spine (Phila Pa 1976). 2013;38(18):1566–1575. 10.1097/BRS.0b013e31829cb67a. [DOI] [PubMed] [Google Scholar]
- 3.Anand N, Baron EM, Thaiyananthan G, Khalsa K, Goldstein TB.Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21(7):459–467. [DOI] [PubMed] [Google Scholar]
- 4.Anand N, Rosemann R, Khalsa B, Baron EM.Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus. 2010;28(3):E6. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Parent E, Narvacan K, San C, Lou E.Intraoperative image guidance compared with free-hand methods in adolescent idiopathic scoliosis posterior spinal surgery: a systematic review on screw-related complications and breach rates. Spine J. 2017;17:1215–1229. [DOI] [PubMed] [Google Scholar]
- 6.Chan A, Parent E, Wong J, Narvacan K, San C, Lou E.Does image guidance decrease pedicle screw-related complications in surgical treatment of adolescent idiopathic scoliosis: a systematic review update and meta-analysis. Eur Spine J. 2020;29:694–716. [DOI] [PubMed] [Google Scholar]
- 7.Charalampidis A, Jiang F, Wilson JRF, Badhiwala JH, Brodke DS, Fehlings MG.The use of intraoperative neurophysiological monitoring in spine surgery. Global Spine J. 2020;10(suppl 1):104S–114S. 10.1177/2192568219859314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou D, Mummaneni P, Anand N, et al. Treatment of the fractional curve of adult scoliosis with circumferential minimally invasive surgery versus traditional, open surgery: an analysis of surgical outcomes. Global Spine J. 2018;8(8):827–833. 10.1177/2192568218775069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou D, Mundis G, Wang M, et al. Minimally invasive surgery for mild-to-moderate adult spinal deformities: impact on intensive care unit and hospital stay. World Neurosurg. 2019;127:e649–e655. 10.1016/j.wneu.2019.03.237. [DOI] [PubMed] [Google Scholar]
- 10.de Bodman C, Ansorge A, Tabard-Fougère A, Amirghasemi N, Dayer R. Clinical and radiological outcomes of minimally-invasive surgery for adolescent idiopathic scoliosis at a minimum two years’ follow-up. Bone Joint J. 2020;102-B(4):506–512. [DOI] [PubMed] [Google Scholar]
- 11.Di Silvestre M, Parisini P, Lolli F, Bakaloudis G. Complications of thoracic pedicle screws in scoliosis treatment. Spine (Phila Pa 1976). 2007;32:1655–1661. [DOI] [PubMed] [Google Scholar]
- 12.Downs SH, Black N.The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Souza M, Gendreau J, Feng A, Kim LH, Ho AL, Veeravagu A.Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg. 2019;6:9–23. 10.2147/RSRR.S190720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration. 510 (k) Summary, November 17, 2020. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K202320.
- 15.Flynn JM, Sakai DS.Improving safety in spinal deformity surgery: advances in navigation and neurologic monitoring. Eur Spine J. 2013;22(suppl 2):S131–S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez D, Ghessese S, Cook D, Hedequist D. Initial intraoperative experience with robotic-assisted pedicle screw placement with stealth navigation in pediatric spine deformity: an evaluation of the first 40 cases [published online ahead of print October 22, 2020]. J Robot Surg. 10.1007/s11701-020-01159-3. [DOI] [PubMed]
- 17.Hicks JM, Singla A, Shen FH, Arlet V.Complications of pedicle screw fixation in scoliosis surgery: a systematic review. Spine (Phila Pa 1976). 2010;35(11):E465–E470. [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Ohnmeiss DD, Lieberman IH.Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J. 2013;22(3):661–666. 10.1007/s00586-012-2499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huntsman KT, Riggleman JR, Ahrendtsen LA, Ledonio CG.Navigated robot-guided pedicle screws placed successfully in single-position lateral lumbar interbody fusion. J Robot Surg. 2020;14(4):643–647. 10.1007/s11701-019-01034-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karkenny AJ, Mendelis JR, Geller DS, Gomez JA.The role of intraoperative navigation in orthopaedic surgery. J Am Acad Orthopaedic Surgeons. 2019;27(19):e849–e858. [DOI] [PubMed] [Google Scholar]
- 21.Keric N, Doenitz C, Haj A, et al. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus. 2017;42(5):E11. 10.3171/2017.2.FOCUS16552. [DOI] [PubMed] [Google Scholar]
- 22.Kwan MK, Chiu CK, Gani SMA, et al. Accuracy and safety of pedicle screw placement in adolescent idiopathic scoliosis patients: a review of 2020 screws using computed tomography assessment. Spine (Phila Pa 1976). 2017;42:326–335. [DOI] [PubMed] [Google Scholar]
- 23.Laratta JL, Ha A, Shillingford JN, et al. Neuromonitoring in spinal deformity surgery: a multimodality approach. Global Spine J. 2018;8(1):68–77. 10.1177/2192568217706970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson AN, Polly DW, Jr, Guidera KJ, et al. The accuracy of navigation and 3D image-guided placement for the placement of pedicle screws in congenital spine deformity. J Pediatr Orthop. 2012;32(6):e23–e29. 10.1097/BPO.0b013e318263a39e. [DOI] [PubMed] [Google Scholar]
- 25.Larson AN, Santos ER, Polly DW, Jr, et al. Pediatric pedicle screw placement using intraoperative computed tomography and 3-dimensional image-guided navigation. Spine (Phila Pa 1976). 2012;37(3):E188–E194. 10.1097/BRS.0b013e31822a2e0a. [DOI] [PubMed] [Google Scholar]
- 26.Lee KY, Lee JH, Kang KC, et al. Minimally invasive multilevel lateral lumbar interbody fusion with posterior column osteotomy compared with pedicle subtraction osteotomy for adult spinal deformity. Spine J. 2020;20(6):925–933. 10.1016/j.spinee.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Lenke LG, Kuklo TR, Ondra S, Polly DW., Jr.Rationale behind the current state-of-the-art treatment of scoliosis (in the pedicle screw era). Spine (Phila Pa 1976). 2008;33:1051–1054. [DOI] [PubMed] [Google Scholar]
- 28.Lovecchio F, Qureshi SA.The current state of minimally invasive approaches to adult spinal deformity. Curr Rev Musculoskelet Med. 2019;12(3):318–327. 10.1007/s12178-019-09570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macke JJ, Woo R, Varich L.Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg. 2016;10(2):145–150. 10.1007/s11701-016-0587-7. [DOI] [PubMed] [Google Scholar]
- 30.Mehin R, Burnett RS, Brasher PM.Does the new generation of high-flex knee prostheses improve the postoperative range of movement? a meta-analysis. J Bone Joint Surg Br. 2010;92(10):1429–1434. [DOI] [PubMed] [Google Scholar]
- 31.Meng XT, Guan XF, Zhang HL, He SS.Computer navigation versus fluoroscopy-guided navigation for thoracic pedicle screw placement: a meta-analysis. Neurosurg Rev. 2016;39(3):385–391. [DOI] [PubMed] [Google Scholar]
- 32.Miyanji F, Samdani A.Minimally invasive surgery for AIS: an early prospective comparison with standard open posterior surgery. J Spine. 2013;S5:1–4. [Google Scholar]
- 33.Morse KW, Su EP.Hip resurfacing arthroplasty for patients with inflammatory arthritis: a systematic review. Hip Int. 2018;28(1):11–17. [DOI] [PubMed] [Google Scholar]
- 34.Morse KW, Heath M, Avrumova F, et al. Comprehensive error analysis for robotic-assisted placement of pedicle screws in pediatric spinal deformity: the initial learning curve [published online ahead of print April 29, 2021]. J Pediatr Orthop. 10.1097/BPO.0000000000001842. [DOI] [PubMed] [Google Scholar]
- 35.Othman YA, Alhammoud A, Aldahamsheh O, Vaishnav AS, Gang CH, Qureshi SA.Minimally invasive spine lumbar surgery in obese patients: a systematic review and meta-analysis. HSS J. 2020;16(2):168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ovadia D, Korn A, Fishkin M, Steinberg DM, Wientroub S, Ofiram E.The contribution of an electronic conductivity device to the safety of pedicle screw insertion in scoliosis surgery. Spine (Phila Pa 1976). 2011;36(20):E1314–E1321. 10.1097/BRS.0b013e31822a82ec. [DOI] [PubMed] [Google Scholar]
- 37.Parent S, Labelle H, Skalli W, Latimer B, de Guise J.Morphometric analysis of anatomic scoliotic specimens. Spine (Phila Pa 1976). 2002;27:2305–2311. [DOI] [PubMed] [Google Scholar]
- 38.Patel PD, Canseco JA, Houlihan N, Gabay A, Grasso G, Vaccaro AR.Overview of minimally invasive spine surgery. World Neurosurg. 2020;142:43–56. 10.1016/j.wneu.2020.06.043. [DOI] [PubMed] [Google Scholar]
- 39.Rajasekaran S, Vidyadhara S, Ramesh P, Shetty AP.Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976). 2007;32(2):E56–E64. 10.1097/01.brs.0000252094.64857.ab. [DOI] [PubMed] [Google Scholar]
- 40.Raynor BL, Lenke LG, Bridwell KH, Taylor BA, Padberg AM.Correlation between low triggered electromyographic thresholds and lumbar pedicle screw malposition: analysis of 4857 screws. Spine (Phila Pa 1976). 2007;32(24):2673–2678. 10.1097/BRS.0b013e31815a524f. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Olaverri JC, Zimick NC, Merola A, et al. Using triggered electromyographic threshold in the intercostal muscles to evaluate the accuracy of upper thoracic pedicle screw placement (T3-T6). Spine (Phila Pa 1976). 2008;33(7):E194–E197. 10.1097/BRS.0b013e3181696094. [DOI] [PubMed] [Google Scholar]
- 42.Sarwahi V, Horn JJ, Kulkarni PM, et al. Minimally invasive surgery in patients with adolescent idiopathic scoliosis: is it better than the standard approach? a 2-year follow-up study. Clin Spine Surg. 2016;29(8):331–340. [DOI] [PubMed] [Google Scholar]
- 43.Sarwahi V, Sugarman EP, Wollowick AL, Amaral TD, Lo Y, Thornhill B. Prevalence, distribution, and surgical relevance of abnormal pedicles in spines with adolescent idiopathic scoliosis vs. no deformity: a CT-based study. J Bone Joint Surg Am. 2014;96:e92. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89(11):2440–2449. 10.2106/JBJS.F.01476. [DOI] [PubMed] [Google Scholar]
- 45.Shaw KA, Murphy JS, Devito DP.Accuracy of robot-assisted pedicle screw insertion in adolescent idiopathic scoliosis: is triggered electromyographic pedicle screw stimulation necessary? J Spine Surg. 2018;4(2):187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Si G, Li T, Wang Y, Liu X, Li C, Yu M.Minimally invasive surgery versus standard posterior approach for Lenke Type 1-4 adolescent idiopathic scoliosis: a multicenter, retrospective study. Eur Spine J. 2021;30(3):706–713. 10.1007/s00586-020-06546-w. [DOI] [PubMed] [Google Scholar]
- 47.Sucato DL, Duchene C.The position of the aorta relative to the spine a comparison of patients with and without idiopathic scoliosis. J Bone Joint Surg Am. 2003;85-A:1461–1469. [PubMed] [Google Scholar]
- 48.Than KD, Mummaneni PV, Bridges KJ, et al. Complication rates associated with open versus percutaneous pedicle screw instrumentation among patients undergoing minimally invasive interbody fusion for adult spinal deformity. Neurosurg Focus. 2017;43(6):E7. [DOI] [PubMed] [Google Scholar]
- 49.Thirumala PD, Bodily L, Tint D, et al. Somatosensory-evoked potential monitoring during instrumented scoliosis corrective procedures: validity revisited. Spine J. 2014;14(8):1572–1580. 10.1016/j.spinee.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 50.Thirumala PD, Huang J, Thiagarajan K, Cheng H, Balzer J, Crammond DJ.Diagnostic accuracy of combined multimodality somatosensory evoked potential and transcranial motor evoked potential intraoperative monitoring in patients with idiopathic scoliosis. Spine (Phila Pa 1976). 2016;41(19):E1177–E1184. 10.1097/BRS.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 51.Ughwanogho E, Patel NM, Baldwin KD, Sampson NR, Flynn JM.Computed tomography-guided navigation of thoracic pedicle screws for adolescent idiopathic scoliosis results in more accurate placement and less screw removal. Spine (Phila Pa 1976). 2012;37(8):E473–E478. [DOI] [PubMed] [Google Scholar]
- 52.Vardiman AB, Wallace DJ, Crawford NR, Riggleman JR, Ahrendtsen LA, Ledonio CG.Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J Robot Surg. 2020;14(3):409–413. 10.1007/s11701-019-00994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA.Adolescent idiopathic scoliosis. Lancet. 2008;371(9623):1527–1537. 10.1016/S0140-6736(08)60658. [DOI] [PubMed] [Google Scholar]
- 54.Wewel JT, Godzik J, Uribe JS.The utilization of minimally invasive surgery techniques for the treatment of spinal deformity. J Spine Surg. 2019;5(suppl 1):S84–S90. 10.21037/jss.2019.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter RB.Neurologic safety in spinal deformity surgery. Spine (Phila Pa 1976). 1997;22:1527–1533. [DOI] [PubMed] [Google Scholar]
- 56.Yang JH, Chang DG, Suh SW, et al. Safety and effectiveness of minimally invasive scoliosis surgery for adolescent idiopathic scoliosis: a retrospective case series of 84 patients. Eur Spine J. 2020;29(4):761–769. 10.1007/s00586-019-06172-1. [DOI] [PubMed] [Google Scholar]
- 57.Zhu W, Sun W, Xu L, et al. Minimally invasive scoliosis surgery assisted by O-arm navigation for Lenke Type 5C adolescent idiopathic scoliosis: a comparison with standard open approach spinal instrumentation. J Neurosurg Pediatr. 2017;19(4):472–478. [DOI] [PubMed] [Google Scholar]
- 58.Zindrick MR, Knight GW, Sartori MJ, Carnevale TJ, Patwardhan AG, Lorenz MA.Pedicle morphology of the immature thoracolumbar spine. Spine (Phila Pa 1976). 2000;25:2726–2735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hss-10.1177_15563316211027828 for Less Invasive Pediatric Spinal Deformity Surgery: The Case for Robotic-Assisted Placement of Pedicle Screws by Kyle W. Morse, Hila Otremski, Kira Page and Roger F. Widmann in HSS Journal®: The Musculoskeletal Journal of Hospital for Special Surgery