Abstract
Background: Robotic-assisted total knee arthroplasty (rTKA) has emerged as a patient-specific customizable tool that enables 3-dimensional preoperative planning, intraoperative adjustment, robotic-assisted bone preparation, and soft-tissue protection. Haptic rTKA may enhance component positioning, but only a few small studies have examined patient satisfaction and clinical outcomes after haptic rTKA. Purpose: In patients who underwent haptic rTKA, we sought to evaluate (1) the discrepancy in alignment between the executed surgical plan and implanted alignment in the coronal and sagittal planes 1 year postoperatively and (2) patient-reported outcomes 2 years postoperatively. Methods: From a prospectively collected database, we reviewed 105 patients who underwent haptic rTKA from August 2016 to May 2017. Two fellowship-trained arthroplasty surgeons independently reviewed hip-to-ankle standing biplanar radiographs to measure overall limb alignment and individual tibial and femoral component alignment relative to the mechanical axis and compared this to the executed surgical plan. Patient-reported outcomes were collected preoperatively and at 2 years postoperatively using the Lower Activity Extremity Score (LEAS), Knee Injury and Osteoarthritis Outcome Score Junior (KOOS Jr.), and Numeric Pain Rating Scale (NPRS). Results: Mean patient age was 62.4 years, and mean body mass index was 30.6 kg/m2. Interobserver reliability was significant with a κ of 0.89. Absolute mean deviations in postoperative coronal alignment compared to intraoperative alignment were 0.625° ± 0.70° and 0.45° ± 0.50° for the tibia and femur, respectively. Absolute mean deviations in postoperative tibial sagittal alignment were 0.47° ± 0.76°. Overall mechanical alignment was 0.97° ± 1.79°. Outcomes in LEAS, KOOS Jr., and NPRS changed from 8 to 10, 78 to 88.3, and 8 to 1, respectively. Conclusions: Haptic rTKA demonstrated high reliability and accuracy (less than 1°) of tibial coronal, femoral coronal, and tibial sagittal component alignment postoperatively compared to the surgical plan. Patient-reported outcomes improved, as well. A more rigorous study on long-term outcomes is warranted.
Keywords: robotic-assisted, total knee arthroplasty, outcomes, femoral component alignment, tibial component alignment
Introduction
Many studies suggest that long-term function and survival rates improve when coronal plane alignment is within 3° of a neutral mechanical axis [2,4,14–16]. Others have reported on the early and midterm success of kinematic alignment [2,3,13]. Regardless of the desired goal, all surgeons have a target when performing total knee arthroplasty (TKA).
Robotic-assisted TKA (rTKA) has emerged as a patient-specific tool that assists in preoperative planning, intraoperative adjustment to customize the patient’s plan, and precise surgical execution that facilitates fewer outliers from the surgeon’s plan. Prior studies of non-haptic-based rTKA demonstrated no difference in outcomes compared to conventional TKA [5]. However, non-haptic-based rTKA uses a much different technology platform than haptic rTKA. While haptic rTKA has the potential of providing enhanced component positioning, only a few studies with small cohorts have examined subjective patient satisfaction and outcomes [7–11,16]. While the true, long-term clinical and functional significance of rTKA is yet to be determined, we can now report on executed alignment and 2-year outcomes.
In order to investigate a haptic-based rTKA technology and surgical technique, we analyzed the following in a cohort of patients: (1) the accuracy between the executed surgical plan and follow-up implant position in both coronal and sagittal planes 1 year postoperatively and (2) selected patient-reported outcome measures preoperatively and 2 years postoperatively.
Methods
Institutional Review Board approval was obtained prior to patient enrollment. During the initial release of a new rTKA system, 3 orthopedic surgeons at a single high-volume institution began enrolling patients. From August 2016 to April 2017, a total of 105 patients were prospectively enrolled to undergo rTKA using computed tomography (CT)-based haptic guidance with a single manufacturer and design including both cruciate retaining and posterior stabilized prostheses. Exclusion criteria were body mass index (BMI) over 40 kg/m2, previous knee surgery, global instability, and neuromuscular or neurosensory deficiency. All patients had hip-to-ankle standing biplanar radiographs (EOS, Paris, France) preoperatively and 1-year postoperatively.
The Mako system (Stryker, Mahwah, NJ) was used for all cases. The standard technique with this system requires a preoperative CT scan, which allows for 3-dimensional preoperative planning (Fig. 1). During surgery, femoral and tibial arrays were placed, and the patient’s femur and tibia were registered. Once this was completed—and before the bony cuts were finalized—the surgeon assessed the patient’s alignment, correctability, and laxity and adjusted the implant position to optimize balance and maintain alignment goals (Fig. 2). In general, the alignment goal for overall coronal limb alignment was within 3° of neutral, and the tibial slope was between 0° and 3°. However, 2 cases were planned in 4° of varus, one due to an extra-articular deformity and the other due to severe medial tibial erosion. The alignment of the intraoperative cuts, implant position, and overall limb alignment were recorded and compared to postoperative standing biplanar imaging 1 year following surgery.
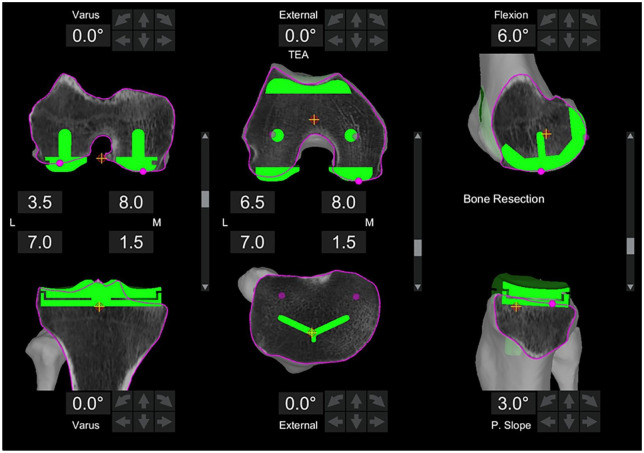
Fig. 1.
Preoperative three-dimensional plan prior to surgery. The default plan is in neutral mechanical alignment and rotation.
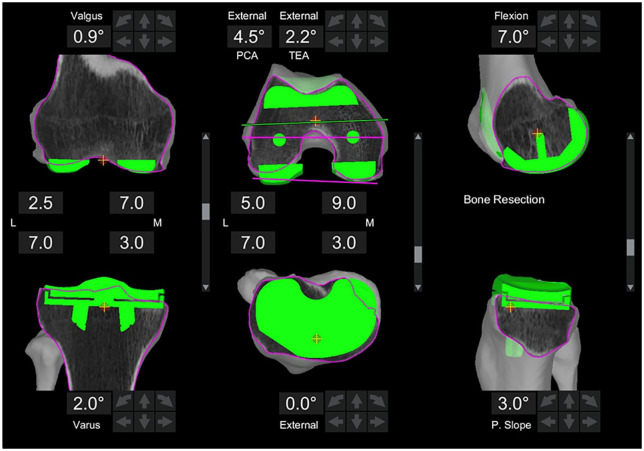
Fig. 2.
Adjusted three-dimensional plan based on an intraoperative assessment of the patient’s laxity and correctability. This adjusted surgical plan was executed during surgery and had 0.9 degrees of valgus in the femur, 2 degrees of varus in the tibia, and 3 degrees of posterior slope in the tibia.
Independently, 2 fellowship-trained arthroplasty surgeons measured the overall coronal mechanical alignment, the coronal and sagittal alignment of the tibial component, and the coronal alignment of the femoral component relative to the mechanical axis on the 1-year postoperative standing biplanar radiographs (Fig. 2). These values were then compared to the executed intraoperative plan. The measurements were assessed for interobserver reliability. The following patient-reported outcome measures were taken preoperatively and at 2 years postoperatively: Lower Activity Extremity Score (LEAS), Knee Injury and Osteoarthritis Outcome Score Junior (KOOS Jr.), and the Numeric Pain Rating Scale (NPRS).
Statistical Analysis
Continuous variables are reported as means and standard deviations in the descriptive analysis. Frequencies and percentages are used to report descriptive statistics of discrete variables. Longitudinal analysis of patient-reported outcomes using KOOS Jr., LEAS, and NPRS was performed using generalized estimating equation (GEE) modeling. This modeling technique was chosen due to its robust nature for handling data, regardless of whether or not it met the assumption of normality. Additionally, GEE allows for the clustered analysis of all observations that have been collected longitudinally and accounts for any missing data from patients who were lost to follow-up. All observations were analyzed using maximum likelihood estimations. Each model contained time as the sole predictor (treated as a fixed effect). All parameter estimates from the GEE models are reported as means, standard errors, and 95% confidence intervals (95% CIs). Bonferroni correction was used to adjust for multiple pairwise comparisons. Statistical significance was defined as P ≤ .05. All analyses were performed by our institutional statistician with SPSS, version 23.0 (IBM Corp., Armonk, NY). Interobserver reliability to determine radiographic alignment was calculated using a κ score.
Results
In this study cohort, the average age was 62.4 ± 8.5 years, and the average BMI was 30.6 ± 6.1 kg/m2 (Table 1). Executed intraoperative alignment and the radiographically measured alignment of the implants at 1 year are displayed in Figs. 3 to 5. Of note, the preoperative template component size was 100% accurate for both components and no intraoperative adjustment of implant sizing occurred. The overall mechanical alignment was 0.97° ± 1.79° [−2.4° to 3°]. The absolute mean deviation in postoperative coronal alignment compared to intraoperative alignment was −0.74° ± 1.23° and 0.01° ± 1.3° for the tibia and femur, respectively. The absolute mean deviation in postoperative tibial sagittal alignment was 0.47° ± 0.76°. Interobserver reliability was significant between both observers with a κ of 0.89 (P < .05), and intraobserver reliability was significant with a κ of 0.84 (P <.05). Of the 6 reoperations, 5 were manipulations under anesthesia and 1 was arthroscopic debridement of patella clunk syndrome (Table 1). No reoperations for implant failures were reported. Patient-reported outcome scores (Table 2) were markedly improved 2 years postoperatively. In comparing preoperative to 2-year postoperative scores, the following changes in outcome measures were reported: LEAS, 8 to 10 (P < .001); NPRS, 8 to 1 (P < .001); and KOOS Jr., 78 to 88.3 (P < .001).
Table 1.
Demographics.
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age at surgery | 62.4 | 8.5 | 45.0 | 84.0 |
| BMI at surgery | 30.6 | 6.1 | 18.0 | 38.7 |
| KOOS JR at 6 weeks | 81.0 | 6.8 | 61.6 | 100.0 |
| LEAS at 6 weeks | 8.5 | 2.0 | 3.0 | 14.0 |
| NPRS at 6 weeks | 2.2 | 1.6 | 0.0 | 7.0 |
| Laterality, n (%) | ||||
| Right | 54 | 51% | ||
| Left | 51 | 49% | ||
| 90-day adverse event, n (%) | ||||
| None | 105 | 100% | ||
| Reoperation, n (%) | ||||
| None | 99 | 94% | ||
| Yes | 6 | 6% | ||
BMI body mass index, KOOS JR Knee Injury and Osteoarthritis Outcome Score Junior, LEAS Lower Activity Extremity Score, NPRS Numeric Pain Rating Scale.
Fig. 3.
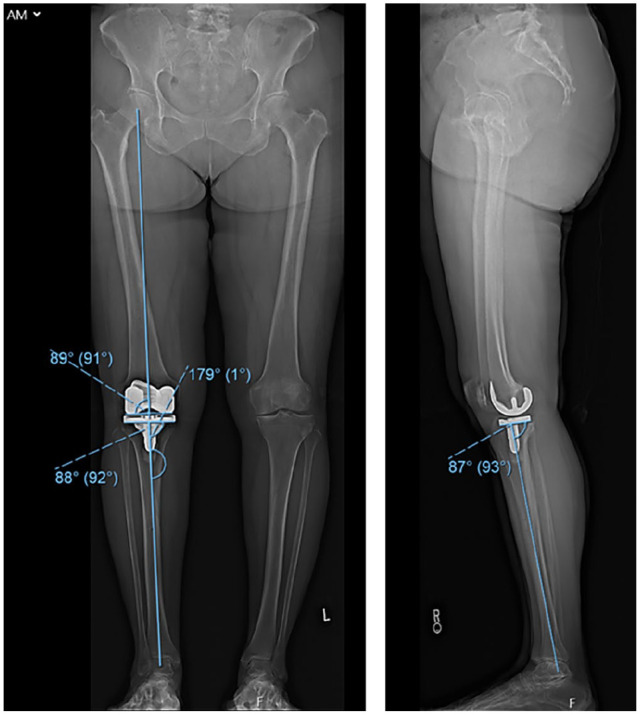
Postoperative standing biplanar images. In the coronal plane, the femur is in 1 degree of valgus, the tibia in 2 degrees of varus, and overall mechanical alignment of 1 degree of varus. In the sagittal plane, the tibia has 3 degrees of posterior slope. This matches the adjusted, executed plan in Figure 2.
Fig. 5.
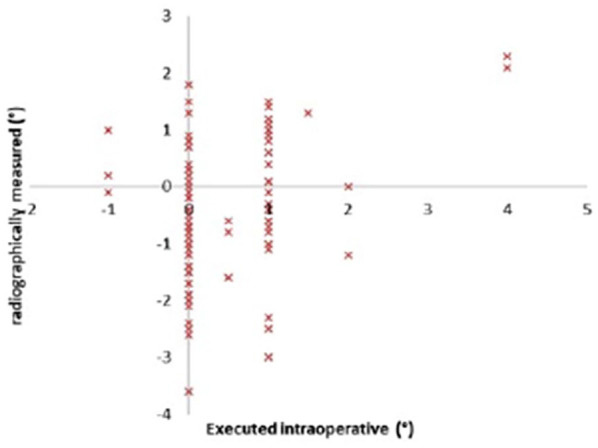
Tibial coronal alignment angles.
Table 2.
Patient-reported outcomes.
| PROM | Time | Mean | SE | 95% CI lower |
95% CI upper |
P value |
|---|---|---|---|---|---|---|
| KOOS JR | 6 weeks | 81.0 | 0.7 | 79.7 | 82.3 | <.001 |
| 3 months | 84.8 | 1.2 | 82.5 | 87.1 | ||
| 1 year | 89.6 | 1.1 | 87.5 | 91.7 | ||
| 2 years | 89.6 | 1.0 | 87.5 | 91.7 | ||
| LEAS | 6 weeks | 8.5 | 0.2 | 8.1 | 8.9 | <.001 |
| 3 months | 11.9 | 0.4 | 11.0 | 12.7 | ||
| 1 year | 11.9 | 0.3 | 11.4 | 12.5 | ||
| 2 years | 11.9 | 0.2 | 8.6 | 12.7 | ||
| NPRS | 6 weeks | 2.2 | 0.2 | 1.9 | 2.5 | <.001 |
| 3 months | 0.9 | 0.1 | 0.7 | 1.2 | ||
| 1 year | 0.7 | 0.1 | 0.5 | 0.9 | ||
| 2 years | 0.7 | 0.1 | 0.5 | 0.8 |
CI confidence interval, KOOS JR Knee Injury and Osteoarthritis Outcome Score Junior, LEAS Lower Activity Extremity Score, NPRS Numeric Pain Rating Scale, PROM patient-reported outcome measures, SE standard error.
Fig. 4.
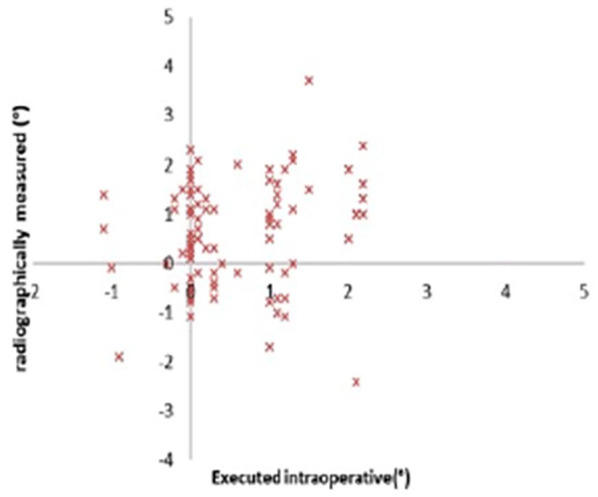
Femoral coronal alignment angles.
Discussion
To our knowledge, this is the first study to compare the executed intraoperative alignment to the 1-year follow-up alignment with standing biplanar imaging. We also assessed 2-year postoperative patient-reported outcome measures. The improvement in patient-reported outcomes and tight radiographic range relative to the executed plan suggest that subtle changes in implant position may provide better balance while eliminating outliers. Further study and follow-up are required to determine if haptic-guided rTKA with small intentional alterations in implant alignment will improve long-term outcomes and implant longevity.
There were several limitations to this study. In order to achieve the largest cohort of rTKA patients during the limited release period, we combined the results of 3 arthroplasty surgeons who were in the early stages of learning to use haptic-guided rTKA. Even though the study occurred during this period, the rTKAs performed were accurate to the executed plan. Other limitations included the following: (1) the study lacked a control group of manual/conventional TKAs; (2) the surgeons had not routinely used the specific knee replacement implanted by the robot, which added to the learning curve; and (3) the study looked only at short-term radiographic and functional outcomes.
The most recent reviews of non-haptic rTKA are that predicable alignment alone has not necessarily translated into improved clinical results [1,6,9,11]. Liow et al [10] randomized 60 knees (31 robotic) and at 6 months’ follow-up reported no overall difference in range of motion, Oxford knee score, Knee Society Score, or any Short Form Health Survey (SF-36) metrics (except vitality). Song et al [18] performed a prospective, randomized trial of 100 TKA procedures (50 robotic) and at minimum 41 months’ follow-up reported no difference in HSS knee score, range of motion, or Western Ontario and McMaster University Arthritis Index (WOMAC) scores, but did note improvement in flexion-extension gap balancing and reduction in alignment outliers. Yang et al [19] reported minimum 8-year follow-up outcomes for 71 robotic TKA versus 42 conventional procedures and found no difference between HSS, WOMAC, visual analogue scale, or range of motion scores. Specifically, several well-designed studies concluded computer-assisted TKA improves component alignment precision and accuracy [6], but future studies are required to determine if component alignment and short-term outcomes translate into improved long-term implant survivorship and patient satisfaction [8,12]. None of the above studies customized the plan to the patient intraoperatively or changed their alignment goals depending on the patients’ soft tissue balance. Several studies have examined non-haptic rTKA, but few have focused on haptic-based technology with long-term clinical results.
When a surgical plan can be accurately adjusted with haptic execution, it may be possible to individualize surgery for each patient while maintaining near neutral mechanical alignment and avoiding excess soft tissue stripping. Kayani et al [7] developed a soft tissue scoring system and found that haptic-based robotic-assisted technology led to less soft tissue damage than mechanical guides. They went on to report in-hospital early outcomes and found that haptic rTKA patients had a short length of stay, lower opioid requirements, lower pain scores, better range of motion, and fewer physical therapy sessions prior to discharge [8].
Marchand et al [12] investigated short-term patient satisfaction outcomes in 20 consecutive haptic rTKAs and 20 manual TKAs at 6 months postoperatively, reporting significantly improved outcomes with the haptic rTKA cohort in terms of WOMAC mean pain and overall satisfaction scores (P < .05).
While the previously mentioned studies [10,12,17,19] differ in clinical outcome measures, all reported improved alignment and reduction of outliers relative to the mechanical axis with the use of robotics. Component positioning in this study not only resulted in fewer outliers, but precise alignment was achieved within 0.45° to 0.63° of the final planned position. Having the freedom and confidence to reliably place implants within 1° of a plan is advantageous, regardless of the intended target.
Haptic-guided rTKA demonstrated high reliability and accuracy of coronal tibial, coronal femoral, tibial sagittal, and mechanical alignment when comparing the executed intraoperative plan to the 1-year postoperative biplanar hip-to-ankle radiographs. In addition, patient-reported outcomes were excellent 2 years after surgery. Long-term follow-up studies should investigate the clinical advantage of reliable alignment, decreased outliers, and ability to place implants in patient-specific, precise alignment. Further clinical evidence is needed to determine if this technology and technique of balancing will lead to improved long-term outcomes and survivorship.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kaitlin M. Carroll, BS, reports relationships with Canary Medical and Orthalign. Andrew D. Pearle, MD, reports relationships with Stryker, Exactech, Engage, Smith and Nephew, and Zimmer. David J. Mayman, MD, reports relationships with Stryker, Imagen, Insight, Smith and Nephew, and Wishbone. Geoffrey Westrich, MD, reports relationships with Stryker, Exactech, and Mallinckrodt Pharmaceuticals. Seth Jerabek, MD, reports relationships with Stryker and Imagen. Brian Nickel, MD, Laura J. Kleeblad, MD, and Joost Burger, DMed, declare no potential conflicts of interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported from Stryker, the manufacturer of the device used in this study, for the research, authorship, and/or publication of this article.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent: Informed consent was obtained from all patients included in this study.
Level of Evidence: Level IV: Therapeutic Study
Required Author Forms: Disclosure forms provided by the authors are available with the online version of this article as supplemental material.
References
- 1.Banerjee S, Cherian JJ, Elmallah RK, Jauregui JJ, Pierce TP, Mont MA.Robotic-assisted knee arthroplasty. Expert Rev Med Devices. 2015;12:727–735. [DOI] [PubMed] [Google Scholar]
- 2.Choong PF, Dowsey MM, Stoney JD.Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24:560–569. [DOI] [PubMed] [Google Scholar]
- 3.Courtney PM, Lee G-C. Early outcomes of kinematic alignment in primary total knee arthroplasty: a meta-analysis of the literature. J Arthroplasty. 2017;32:2028–2032.e1. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery RS, Morris RW, Denham RA.Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73:709–714. [DOI] [PubMed] [Google Scholar]
- 5.Jeon S-W, Kim K-I, Song SJ.Robot-assisted total knee arthroplasty does not improve long-term clinical and radiologic outcomes. J Arthroplasty. 2019;34:1656–1661. [DOI] [PubMed] [Google Scholar]
- 6.Jones CW, Jerabek SA.Current role of computer navigation in total knee arthroplasty. J Arthroplasty. 2018;33:1989–1993. [DOI] [PubMed] [Google Scholar]
- 7.Kayani B, Konan S, Pietrzak JRT, Haddad FS.Iatrogenic bone and soft tissue trauma in robotic-arm assisted total knee arthroplasty compared with conventional jig-based total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplasty. 2018;33:2496–2501. [DOI] [PubMed] [Google Scholar]
- 8.Kayani B, Konan S, Tahmassebi J, Pietrzak JRT, Haddad FS.Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital study. Bone Jt J. 2018;100-B:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khlopas A, Sodhi N, Sultan AA, Chughtai M, Molloy RM, Mont MA.Robotic arm-assisted total knee arthroplasty. J Arthroplasty. 2018;33:2002–2006. [DOI] [PubMed] [Google Scholar]
- 10.Liow MHL, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis: a prospective randomised study. J Arthroplasty. 2014;29:2373–2377. [DOI] [PubMed] [Google Scholar]
- 11.Lonner JH, Fillingham YA.Pros and cons: a balanced view of robotics in knee arthroplasty. J Arthroplasty. 2018;33:2007–2013. [DOI] [PubMed] [Google Scholar]
- 12.Marchand RC, Sodhi N, Khlopas A, et al. Patient satisfaction outcomes after robotic arm-assisted total knee arthroplasty: a short-term evaluation. J Knee Surg. 2017;30:849–853. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto T, Takayama K, Ishida K, Hayashi S, Hashimoto S, Kuroda R.Radiological and clinical comparison of kinematically versus mechanically aligned total knee arthroplasty. Bone Jt J. 2017;99-B:640–646. [DOI] [PubMed] [Google Scholar]
- 14.Parratte S, Pagnano MW, Trousdale RT, Berry DJ.Effect of postoperative mechanical axis alignment on the fifteen-year survival of modern, cemented total knee replacements. J Bone Jt Surg Am. 2010;92:2143–2149. [DOI] [PubMed] [Google Scholar]
- 15.Reed SC, Gollish J.The accuracy of femoral intramedullary guides in total knee arthroplasty. J Arthroplasty. 1997;12:677–682. [DOI] [PubMed] [Google Scholar]
- 16.Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA.The effect of alignment and BMI on failure of total knee replacement. J Bone Jt Surg Am. 2011;93:1588–1596. [DOI] [PubMed] [Google Scholar]
- 17.Song E-K, Seon J-K, Park S-J, Jung W, Bin Park H-W, Lee GW.Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19:1069–1076. [DOI] [PubMed] [Google Scholar]
- 18.Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL.Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA knee. Clin Orth Relat Res. 2013;471:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HY, Seon JK, Shin YJ, Lim HA, Song EK.Robotic total knee arthroplasty with a cruciate-retaining implant: a 10-year follow-up study. Clin Orthop Surg. 2017;9:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]