Abstract
This post hoc analysis of the Adaptive Coronavirus Disease 2019 (COVID-19) Treatment Trial-1 (ACTT-1) shows a treatment effect of remdesivir (RDV) on progression to invasive mechanical ventilation (IMV) or death. Additionally, we create a risk profile that better predicts progression than baseline oxygen requirement alone. The highest risk group derives the greatest treatment effect from RDV.
Keywords: COVID-19, remdesivir, ACTT-1
The Adaptive Coronavirus Disease 2019 (COVID-19) Treatment Trial-1 (ACTT-1) identified remdesivir (RDV) as the first antiviral to benefit hospitalized COVID-19 patients, demonstrating a significant improvement in median time to recovery from 15 days with placebo to 10 days with RDV [1]. On post hoc subgroup analysis using an 8-point ordinal scale, the largest benefit of RDV was seen in subjects receiving supplemental oxygen at baseline, with no clear benefit in other subgroups.
ACTT-1 was not designed to evaluate RDV’s impact on progression to invasive mechanical ventilation (IMV) or death. Although deaths were numerically lower in the RDV arm, the difference was not statistically significant. Here we retrospectively explore RDV’s treatment effect within the data set as a whole and by defining a new risk profile for disease progression, not solely dependent upon baseline oxygen requirement.
METHODS
Data Set
ACTT-1 included 1062 subjects; 1051 had an ordinal score (OS) recorded. OS reflected the subject’s oxygen requirement at enrollment: OS4, not requiring supplemental oxygen; OS5, requiring supplemental oxygen; OS6, requiring noninvasive positive pressure ventilation (NIPPV) or high-flow oxygen (HFO); OS7, requiring IMV or extracorporeal membrane oxygenation (ECMO). Time to progression to IMV or death was defined as number of days until first occurrence of IMV or death, except for subjects requiring IMV at baseline, where the endpoint was time until death. Demographic characteristics, biomarkers, comorbidities, and temporal features were collected, as previously described [1]. Missing biomarker values, for those missing <5%, were imputed as the in-group median within OS (Supplementary Sections 2–3).
Risk Profile Development and RDV Treatment Effect
We developed a risk profile for progression to IMV or death by examining 13 features in addition to baseline oxygen requirement. Features were selected using the results of a model fit on half the placebo arm data. The risk profile was developed from the selected features on the remaining placebo recipients. We grouped participants into risk profile quartiles: “high,” “moderate,” “lower,” and “least” risk. We compared risk profile and OS accuracy using leave-one-out cross-validated area under the receiver operating characteristic curve (AUC) [2] in placebo recipients [2]. We fit separate Fine-Gray competing risk [3] (time to recovery vs progression to IMV/death) and logistic regression (binary day 29 outcomes) models to evaluate RDV efficacy within each quartile (not adjusted for multiplicity) (Supplementary Sections) [4–7].
RESULTS
Risk Profile
The risk profile included four baseline variables: (1) platelet count, (2) absolute lymphocyte count (ALC), (3) absolute neutrophil count (ANC), and (4) oxygen requirement. Independently, lower platelet count, lower ALC, and higher ANC were associated with greater risk of progression to IMV or death (Table 1). Each risk quartile included participants with a range of baseline oxygen requirements. For example, in the “high risk” quartile 34.0% (89/262) required IMV/ECMO, 18.7% (49/262) required NIPPV or HFO, 38.2% (100/262) required supplemental oxygen, and 9.2% (24/262) required no supplemental oxygen.
Table 1.
Risk Profile Variables and Comparison of Risk Profile With Ordinal Score (OS) in Predicting Progression to Invasive Mechanical Ventilation (IMV) or Death
| Variable | Risk Profile: High Risk (N = 262) |
Risk Profile: Moderate Risk (N = 263) |
Risk Profile: Lower Risk (N = 263) |
Risk Profile: Least Risk (N = 263) |
OS7 (N = 285) |
OS6 (N = 193) |
OS5 (N = 435) |
OS4 (N = 138) |
|---|---|---|---|---|---|---|---|---|
| Progression to IMV or death, n (% events per group) | 98 (37.4%) | 69 (26.2%) | 44 (16.7%) | 22 (8.3%) | 57 (20.0%) | 82 (42.5%) | 84 (19.3%) | 10 (7.2%) |
| Death, n (% all deaths) | 62 (45.6%) | 37 (27.2%) | 22 (16.2%) | 15 (11%) | 57 (41.9%) | 39 (28.7%) | 34 (25%) | 6 (4.4%) |
| Recovery, n (% all recoveries) | 145 (19.3%) | 180 (24%) | 200 (26.6%) | 226 (30.1%) | 140 (18.6%) | 118 (15.7%) | 362 (48.2%) | 131 (17.4%) |
| Baseline oxygen requirement, n OS7; OS6; OS5; OS4 (%) | 89; 49; 100; 24 (34.0, 18.7, 38.2, 9.2)% |
73; 56; 102; 32 (27.8, 21.3, 38.8, 12.2)% |
67; 48; 105; 43 (25.5, 18.3, 39.9, 16.3)% |
56; 40; 128; 39 (21.3, 15.2, 48.7, 14.8)% |
… | … | … | … |
| ANC, median (25th,75th percentile) | 8.1 (6–10.9) | 5.5 (4.1–7.4) | 4.8 (3.5–6) | 3.5 (2.5–4.8) | 7.1 (5.1–9.7) | 5.7 (3.7–8) | 4.5 (3.3–6.3) | 3.7 (2.5–4.9) |
| ALC, median (25th–75th percentile) | 0.6 (0.4–0.8) | 0.9 (0.7–1.1) | 1.1 (0.9–1.3) | 1.4 (1.1–1.8) | 0.9 (0.6–1.2) | 0.8 (0.6–1.2) | 1.0 (0.8–1.4) | 1.0 (0.8–1.4) |
| Platelets, median (25th–75th percentile) | 192.5 (152.2–251.0) | 215.0 (159.5–274.0) | 226.0 (171.0–283.5.0) | 254.0 (194.0–352.0) | 235.0 (181.0–295.0) | 229.0 (173.0–294.0) | 218.0 (166.5–283.0) | 183.0 (142.2–260.6) |
ALC, ANC, and platelets are measured in 10^9/L.
Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count.
Observed proportions of patients progressing to IMV or death by quartile were 37.4% (98/262) “high risk,” 26.2% (69/263) “moderate risk,” 16.7% (44/263) “lower risk,” and 8.3% (22/263) “least risk.” The risk profile AUC was higher than OS alone (0.73 vs 0.53; P < .0001), better predicting progression to IMV or death (Supplementary Figure 5.1). In this regard, the “high risk” quartile captured more deaths than OS7: 45.6% (62/136) versus 41.9% (57/136) all deaths, respectively. When compared to OS7, the “high risk” quartile had lower median baseline ALC (0.6 × 10^9/L vs 0.9 × 10^9/L), higher median baseline ANC (8.1 × 10^9/L vs 7.1 × 10^9/L), and lower median baseline platelets (192.5 × 10^9/L vs 235 × 10^9/L). Further highlighting the validity of the risk profile, 73.4% (69/94) of OS4 and OS5 subjects that progressed to IMV or death were captured in either the “high risk” or “moderate risk” quartiles (Supplementary Table 5.3).
Treatment Effect of RDV
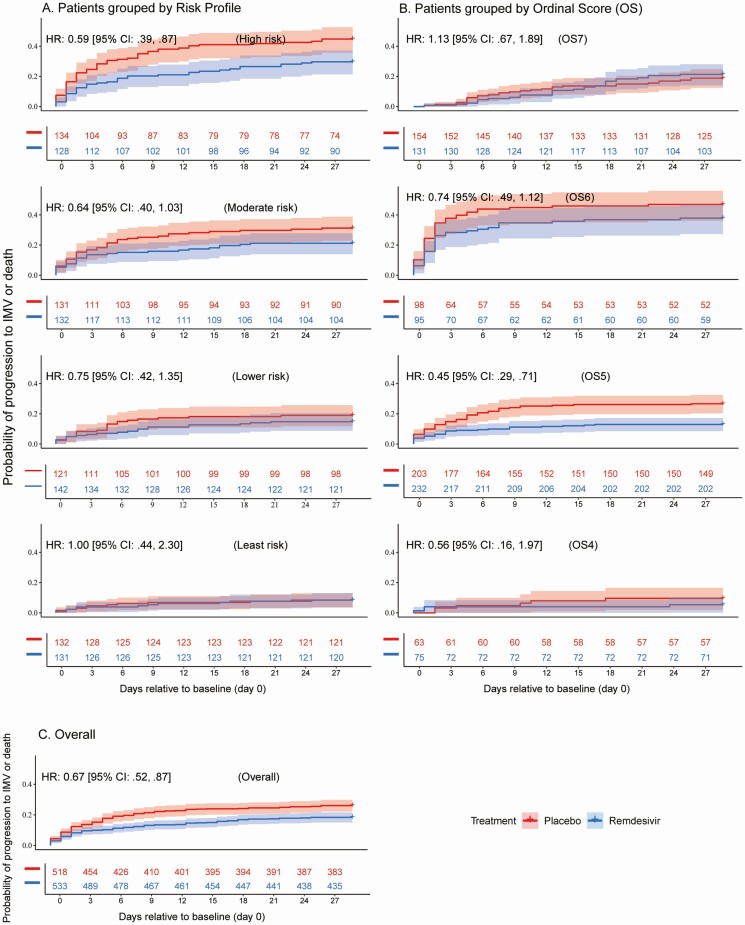
Treatment with RDV was associated with fewer progressions to IMV or death across the entire cohort (hazard ratio [HR] 0.67; [95% confidence interval {CI}: .52, .87] P = .0023), as well as in OS5 (HR 0.45; [95% CI: .29, .71] P = .0003). The “high risk” quartile also showed a significant RDV treatment effect (HR 0.59; [95% CI: .39, .87] P = .009) (Figure 1).
Figure 1.
Kaplan-Meier estimates of remdesivir (RDV) treatment effect for progression to invasive mechanical ventilation (IMV) or death: Probability of progression to IMV or death is shown in panel A for subjects receiving RDV (blue) and placebo (red) in risk profile quartiles defined by baseline oxygen requirement, ALC, ANC, and platelets. Quartiles from top to bottom are “high risk,” “moderate risk,” “lower risk,” and “least risk.” Probability of progression to IMV or death is shown in panel B for subjects receiving RDV (blue) and placebo (red) in each ACTT-1 ordinal score (OS group). Ordinal scores from top to bottom are OS7 (requiring IMV or extracorporeal membrane oxygenation [ECMO]). OS6 (requiring noninvasive positive pressure ventilation (NIPPV) or high-flow oxygen [HFO]), OS5 (requiring supplemental oxygen) and OS4 (not requiring supplemental oxygen). Probability of progression to IMV or death is shown in panel C for subjects receiving RDV (blue) and placebo (red) in the overall ACTT-1 data set. HR estimates with a value <1 indicate that treatment effect is associated with being less likely to progress to IMV or death. Number-at-risk table is provided for each plot with numbers colored by treatment group. Abbreviations: ACTT-1, Adaptive COVID-19 Treatment Trial-1; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; CI, confidence interval; HR. hazard ratio.
The risk quartiles were also assessed for time to one-point OS improvement, time to recovery (ACTT-1 endpoint), and death. Statistically significant effects for RDV treatment in the “high risk” quartile were observed for time to one-point improvement and time to recovery, with no impact seen in any of the other risk quartiles (Supplementary Table 6.1 and Supplementary Figure 6.1).
DISCUSSION
In the ACTT-1 cohort, combining baseline ALC, ANC, and platelets with baseline OS resulted in a more predictive risk profile of participant outcome. Low platelet count, low ALC, and high ANC have been shown to correlate with worsening disease severity in small cohorts of patients with COVID-194–6. Low ALC, in particular, has been linked to increased mortality [7]. By incorporating these commonly measured hematologic parameters, we improved the predicted risk of IMV or death beyond OS alone. Although our risk profile requires validation in large prospective studies, it lends credence to the hypothesis that OS groups are heterogeneous and that some patients within each OS are more likely to experience severe outcomes.
Our results highlight a need to reassess treatment guidelines regarding the use of RDV in hospitalized COVID-19 patients. Currently, for example, the World Health Organization (WHO) [8] recommends against routine RDV use, whereas COVID-19 treatment guidelines issued by US organizations generally endorse use. The WHO developed their recommendations based on data from several clinical trials, the largest of which was the Solidarity trial, which did not show a mortality benefit with RDV treatment [9]. However, our post hoc analysis of the ACTT-1 data set shows a clinically important salutary treatment effect of RDV on curtailing progression to IMV or death across the cohort.
Furthermore, the risk profile described in this report was able to identify patients more likely to progress to IMV or death, and this group had a substantial RDV treatment effect. These findings may help align published recommendations for the use of RDV within the United States. In this regard, the Infectious Diseases Society of America (IDSA) [10] recommends the general use of RDV in hospitalized COVID-19 patients, whereas the National Institutes of Health (NIH) [11] does not recommend for or against RDV use in patients who are not on supplemental oxygen or in patients requiring IMV. The Society for Critical Care Medicine Surviving Sepsis Campaign guidelines [12] suggest against RDV use in patients requiring IMV. Our analysis proposes no restriction should be placed on RDV use based solely on oxygen requirement. Including other patient-specific variables may be a better metric for RDV use. As described here, there is a subset of patients at highest risk for progression to IMV or death who may benefit from treatment with RDV, regardless of their baseline oxygen requirement. Patients in this “high risk” quartile, whose baseline oxygen requirements ranged from room air to IMV, receiving RDV had a hazard ratio of 0.59 [95% CI: .39, .87], P = .009 for IMV or death compared to patients receiving placebo. It should be noted that the extensive clinical and laboratory characterizations intrinsic to the ACTT-1 study design afforded the in-depth analyses described. This type of granularity may be needed to capture the presence of a RDV treatment effect if present in only a subgroup of participants.
Our study has several limitations. First, this is a post hoc analysis with an endpoint, time to progression to IMV or death, which differed from the primary endpoint for ACTT-1, time to recovery. Progression to IMV or death was chosen because it is a clinically meaningful endpoint being used increasingly in other trials. We used time-to-event models instead of binary outcome models, to improve statistical efficiency, although both models had similar results (Supplementary Section 7.3). Second, several key baseline variables were not available, including body mass index, inflammatory markers, and viral load (the latter 2 assessments are planned). The inclusion of these variables may influence the risk profile. Third, in baseline OS7 patients only 1 step in disease progression to the worst outcome, death, is possible, whereas in all risk profile quartiles, the worst outcome is either IMV or death. However, when assessing death alone the risk profile AUC was also higher than that of OS (AUC 0.69 vs 0.60 P = .006) (Supplemental Section 5.2). Finally, within the “high risk” quartile it is unclear whether subjects needing only supplemental oxygen are the key driver of the RDV treatment effect; however, each quartile contains a similar percentage of such patients (between 38% and 49%), suggesting against this.
Despite these limitations, ACTT-1 was a large, double-blind, randomized, placebo-controlled trial assessing RDV utility in the relative absence of competing therapies. Our post hoc analysis suggests that baseline oxygen requirements may be too blunt of an instrument to assess an individual’s risk of progression to IMV or death and response to RDV treatment. The impact of RDV is likely to differ based on individual patient characteristics and use should not be restricted solely based on oxygen requirements. Our findings have implications for clinical practice, development of COVID-19 treatment guidelines, and design of future COVID-19 treatment trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgement. Collaborating investigators involved with collection of data during the ACTT-1 trial are noted in the Supplemental Appendix.
Financial support. This analysis used data from the Adaptive COVID-19 Treatment Trial (ACTT-1) trial (DOI:10.1056/NEJMoa2007764). The ACTT-1 trial was sponsored and primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, Maryland. This trial has been funded in part with federal funds from the Department of Defense, Defense Health Program. This trial has been supported in part by the NIAID of the NIH under grant numbers UM1AI148684, UM1AI148576, UM1AI148573, UM1AI148575, UM1AI148452, UM1AI148685, UM1AI148450, and UM1AI148689. The trial has also been funded in part by the governments of Denmark, Japan, Mexico, and Singapore. The trial site in South Korea received funding from the Seoul National University Hospital. Support for the London International Coordinating Centre was also provided by the United Kingdom Medical Research Council (grant number MRC_UU_12023/23). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. 75N910D00024, Task order no. 75N91019F00130. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Potential conflicts of interest. C. I. P. serves as a consultant for Axle informatics for work not related to this manuscript. W. R. S. serves as a consultant for ViiV and Gilead for work not related to this manuscript and received payment or honoraria from ViiV. D. C. L. was on the Gilead global advisory board on Remdesivir 2020 but declined honorarium. C. A. B. has received grant/contract from Gilead to her institution for clinical trials not related to this manuscript, payment from IAS-USA for educational lectures/seminars not related to this manuscript, payment from GlaxoSmithKline for work not related to this manuscript, payment from IDSA as Deputy Editor Clinical Infectious Diseases, and serves as an unpaid volunteer for CROI and IAS-USA. C. R. W. received consulting fees from Enzychem Lifesciences for serving on the advisory board for COVID-19 therapeutics, and they have patents DSMB Biogen and DSMB Atea for COVID-19 therapeutics. N. A. H. had a contract with the NIH/NIAID via Leidos for adaptive COVID-19 Therapeutics Trial (ACTT), specifically ACTT-1. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med 2020; 383:994. [DOI] [PubMed] [Google Scholar]
- 2. Dodd LE, Pepe MS. Semiparametric regression for the area under the receiver operating characteristic curve. J Am Stat Assoc 2003; 98:409–17. [Google Scholar]
- 3. Austin PC, Fine JP. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 2017; 36:4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv Z, Wang W, Qiao B, et al. . The prognostic value of general laboratory testing in patients with COVID-19. J Clin Lab Anal 2021; 35:e23668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meizlish ML, Pine AB, Bishai JD, et al. . A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv 2021; 5:1164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao D, Zhou F, Luo L, et al. . Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 2020; 7:e671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee J, Park SS, Kim TY, Lee DG, Kim DW. Lymphopenia as a biological predictor of outcomes in COVID-19 patients: a nationwide cohort study. Cancers 2021;13. Doi: 10.3390/cancers13030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. COVID-19 Clinical management: living guidance. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. Accessed 25 March 2021.
- 9. Pan H, Peto R, Henao-Restrepo AM, et al. . Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med 2021; 384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhimraj MR, Shumaker AH, Lavergne V, et al. . Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Infectious Diseases Society of America 2021; Version 4.2.0. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 25 March 2021. [DOI] [PMC free article] [PubMed]
- 11. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 25 March 2021. [PubMed]
- 12. Society of Critical Care Medicine Surviving Sepsis Campaign. COVID-19 Guidelines. Available at: https://sccm.org/SurvivingSepsisCampaign/Guidelines/COVID-19. Accessed 25 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.