To the Editor:
The rapid and widespread administration of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) offers great promise for curbing the spread of infection and potentially reaching herd immunity. However, because immunocompromised individuals were excluded from the initial clinical trials of mRNA vaccinations, the humoral response in immunocompromised solid organ transplant (SOT) recipients that are completely vaccinated (2 doses of mRNA SARS-CoV-2 vaccines) is not fully characterized. Recent studies report that 15% and 54% of SOT participants had detectable antibody levels by serologic testing after the first and second dose of vaccine, respectively (1, 2). How quantitative antibody levels correlate with neutralization capacity and how antibody profiling varies between SOT recipients vs immunocompetent individuals and those who recovered from SARS-CoV-2 infection is not well understood.
We retrospectively studied 37 SOT recipients who received lung (n = 16), kidney (n = 6), heart (n = 13), liver (n = 1), or heart and lung (n = 1) transplants and had negative PCR tests for SARS-CoV-2, all of whom were on immunosuppressive medications (tacrolimus, cyclosporine, sirolimus, or everolimus). The SOT recipients had a male:female (M:F) ratio of 27:10 with a median age of 64 years [interquartile range (IQR) 50 to 69] and received 2 doses of mRNA vaccine (Pfizer or Moderna) within 21 days (median, IQR 19 to 25). In control groups, 10 immunocompetent individuals had a M:F ratio of 2:8 with a median age of 66 years (IQR 57 to 75) and received 2 doses of vaccine within 19 days (median, IQR 18 to 25). Individuals with past COVID-19 infection (n = 11) had a median age of 61 years (IQR 55 to 64), a M:F ratio of 8:3, and a median of 40 days since their first positive PCR test (Supplemental Table 1).
This study was conducted under an approved Institutional Review Board protocol at the University of Texas Southwestern Medical Center. Lithium heparin plasmas were stored at 4 °C after collection for up to 72 h and then at -80°C before analyses. Semiquantitative measurement of anti-SARS-CoV-2 spike protein antibody (anti-S IgG) was performed on an Abbott Alinity instrument (analytical measurement range: 50 to 50 000 AU/mL, maximum dilution 1:5). To assess the neutralizing capacity of plasma, the inhibitory effects on spike receptor-binding domain (RBD) and angiotensin converting enzyme 2 receptor interactions were quantified using an enzyme-linked immunosorbent assay (3).
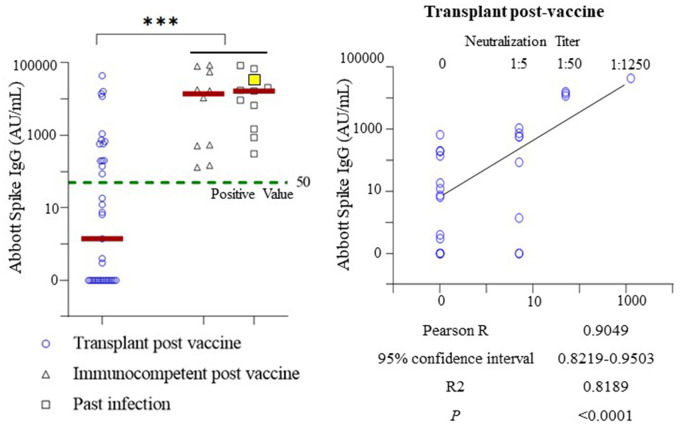
Quantitative serological assays of anti-S IgG (cutoff value 50 AU/mL) revealed a positive response in 15 of 37 fully vaccinated SOT recipients. The seroconversion rate of SOT recipients (40.5%) was significantly lower than the 100% positive antibody response in fully vaccinated immunocompetent individuals and patients with past COVID-19 infection (Fig. 1) (4). In addition, the median value of anti-S IgG in SOT recipients (1.4 AU/mL) was approximately 10 000-fold lower than median values observed in vaccinated immunocompetent individuals (13 951.5 AU/mL) and patients with past infection (16 532.5 AU/mL) (t-test; P < 0.005) (Fig. 1). We next assessed plasma antibody function in SOT recipients. Neutralization assays against spike RBD proteins revealed that anti-S IgG titers were precisely correlated with capacity to neutralize spike RBD proteins (Pearson correlation R = 0.905 vs 0.62 in immunocompetent, 0.71 in past infection group).
Fig. 1.
Serological profiling of SOT recipients following complete vaccination (2 doses of mRNA vaccine). Left. Anti-S IgG was positive in the plasma of 15 of 37 SOT recipients, with a seroconversion rate of 40.5%. In contrast, 100% seroconversion rates were seen in immunocompetent individuals with complete vaccination (n = 10) or past COVID-19 infection (n = 11). Spike IgG concentrations were plotted on a log scale on the y-axis with data points of zero manually plotted. The dashed green line indicates reported positive value of anti-S IgG. Solid red lines indicate the medians of individual study groups. The SOT recipient with prior complete vaccination and subsequent COVID-19 infection in the past infection group is highlighted in yellow. ***, P-value for student t-test <0.005 when comparing SOT to immunocompetent and past infection groups combined. Right. Pearson correlation analysis demonstrating correlation of anti-S IgG values in SOT recipients with their capacity to neutralize spike RBD proteins. Spike IgG concentrations and neutralization titers were plotted on a log scale with data points of zero manually plotted. The solid black line indicates the trend line of simple linear regression.
Because SOT recipients have poorer seroconversion rates and lower anti-S IgG levels, they may still be at high risk for SARS-CoV-2 infection after complete vaccination. Through PCR screening, a COVID-19–positive SOT recipient was identified 13 days after complete vaccination (grouped as individuals with past infection). Notably, a serological study revealed 39 391.2 AU/mL of anti-S IgG 17 days after the positive PCR test (30 days after complete vaccination), a value significantly higher than that of most SOT recipients (36 of 37) but comparable with those of fully vaccinated immunocompetent or previously infected control groups (Fig. 1) (4). This finding indicates that subsequent infection may function as an additional stimulus to boost antibody response and induce higher levels of anti-S IgG in SOT recipients.
Taken together, our findings demonstrate that SOT recipients have significantly poorer antibody response to the currently employed (2 dose) mRNA vaccination in both seroconversion rate [40.5% in our study and 54% by other reports (2)] and a median level of anti-S IgG 10 000-fold lower than immunocompetent individuals. The unexpectedly high level of anti-S IgG in a fully vaccinated SOT recipient with subsequent COVID-19 infection suggests that SOT recipients may benefit from additional vaccine dose(s) to boost seroconversion rate and increase anti-S IgG production. Because anti-S-IgG level precisely correlated with neutralization capacity in SOT recipients (Fig. 1), antibody profiling can be monitored using automated quantitative assays with cutoffs corresponding to certain neutralization titers (5). To determine whether SOT recipients and other immunocompromised patients may benefit from additional vaccine dose(s), large-scale clinical trials are needed.
Supplemental Material
Supplemental material is available at Clinical Chemistry online.
Supplementary Material
Nonstandard Abbreviations
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplant
- IQR
interquartile range
- COVID-19
coronavirus disease
- anti-S IgG
anti-SARS-CoV-2 spike protein antibody
- RBD
receptor-binding domain.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors' Disclosures or Potential Conflicts of Interest
No authors declared any potential conflicts of interest.
Acknowledgments
The authors thank the study participants and Drs. Sasha M. Pejerrey and Heather McConnell at Houston Methodist Hospital for editorial assistance. Department of Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX, USA.
References
- 1.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Wang Y-L, Wu J, Qi J, Zeng Z, Wan Q, et al. Neutralizing aptamers block S/RBD-ACE2 interactions and prevent host cell infection. Angewandte Chemie Int Ed Engl 2021;60: 10273–10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narasimhan M, Mahimainathan L, Araj E, Clark AE, Markantonis J, Green A, et al. Clinical evaluation of the Abbott Alinity SARS-CoV-2 spike-specific quantitative IgG and IgM assays among infected, recovered, and vaccinated groups. J Clin Microbiol 2021;59:e0038821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.