Abstract
Background
In North America, both messenger RNA (mRNA) vaccines, Pfizer-BioNTech BNT162b2, and Moderna mRNA-1273, each utilizing a 2-dose regimen, have started to be administered to individuals.
Methods
We evaluated the quantitative serologic antibody response following administration of either a single dose or both doses of an mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in a cohort of 98 participants (88 healthcare workers [HCW] and 10 solid organ transplant [SOT] recipients). Antibody levels were compared across 3 immunoassays: Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics), SARS-CoV-2 TrimericS IgG (DiaSorin), and SARS-CoV-2 IgG II Quant (Abbott).
Results
Among HCW, sensitivity ranged from 100% (Roche), 99% (Abbott) and 98% (DiaSorin). The SARS-CoV-2 IgG II Quant and SARS-CoV-2 TrimericS IgG assays showed good agreement with a Pearson correlation coefficient of R = 0.95. Pearson correlation coefficients of R = 0.82 and 0.83 were obtained for Elecsys Anti-SARS-CoV-2 S vs SARS-CoV-2 TrimericS IgG and SARS-CoV-2 IgG II Quant vs Elecsys Anti-SARS-CoV-2 S, respectively. Significant differences in antibody levels between HCW and SOT recipients were observed. A decrease in antibody levels from time of vaccine administration to blood draw was evident. Among those with a second dose, an increase in antibody levels with increased time between administration of the first and second dose was observed.
Conclusions
The absolute values generated from each of the assay platforms are not interchangeable. Antibody levels differed with increased time between vaccine administration and with increased time between administration of the first and second dose. Further, significant differences in antibody levels between HCW and SOT recipients were observed.
Keywords: covid-19, SARS-CoV-2, serology, vaccine, antibody
Impact statement
With increasing availability of vaccinations against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is important to compare the clinical performance of serological assays that detect antibodies to SARS-CoV-2. In this study, we compare the antibody levels from 98 participants across the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics), SARS-CoV-2 TrimericS IgG (DiaSorin), and SARS-CoV-2 IgG II Quant (Abbott) platforms. Good agreement was observed between Abbott and DiaSorin assays, and sensitivity ranged from 100% (95% CI 96–100%) for Roche, 99% (95% CI 94–100%) for Abbott, and 98% (95% CI 92–100%) for DiaSorin among the HCW cohort.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a single stranded RNA virus that emerged in late 2019 and is the causative agent of the current coronavirus disease 2019 (COVID-19) pandemic (1). The enveloped virus contains 4 primary structural proteins including the spike (S), membrane, envelope, and nucleocapsid (N) proteins, all encoded within the virus’s 3’ end (2). The receptor binding domain (RBD), located within the S1 region of the S protein, is key to infection as it binds the angiotensin-converting enzyme 2 receptor on host cells (3, 4). Upon infection, the host generates an immune response to the virus, including the production of IgG and IgM antibodies, which can be produced simultaneously (5).
Given the severity and rate at which the virus has spread throughout the globe, extensive efforts were quickly devoted to the development of a vaccine against SARS-CoV-2. Currently, the Food and Drug Administration has approved 2 messenger RNA (mRNA) vaccines for emergency use: BNT162b2 from Pfizer-BioNTech and mRNA-1273 from Moderna (6). These vaccine platforms utilize a 2-dose approach to produce high levels of binding and neutralizing antibodies against the virus’s S protein to generate an immune response (7). Serology immunoassays have been developed to detect the generation of such antibodies to detect past exposure and/or vaccine response; approaches include either the detection of differing antigens such as the N vs S protein or immunoglobulin isotypes such as IgM, IgG, or total antibody levels (8).
In response to challenges in vaccine supply and in an effort to maximize early impact, delayed administration of the second dose has been suggested and implemented by public health officials to increase the number of people receiving the first dose (9). In the current study, we evaluated the antibody response following administration of either a single dose or both doses of the mRNA vaccine in a cohort of 98 participants (healthcare workers [HCW] and solid organ transplant [SOT] recipients) across 3 different commercially available platforms, the Elecsys Anti-SARS-CoV-2 S total antibodies (Roche Diagnostics), SARS-CoV-2 TrimericS IgG (DiaSorin), and SARS-CoV-2 IgG II Quant (Abbott), all of which quantify antibodies directed against the RBD of the SARS-CoV-2 S protein.
Materials and Methods
Clinical Specimens
This study was approved by the Institutional Review Board at the University Health Network (Toronto, ON, Canada). Informed, written consent from all participants was obtained. A selection criterion was set such that only those who had received at least their first dose ≥3 weeks prior to blood draw were included in the study. In total, 98 serum samples from 88 HCW and 10 SOT recipients were collected. All 88 HCW received the Pfizer-BioNTech BNT162b2 vaccine with 39 individuals having their serum sample collected after their first dose (21 to 62 days post first dose) and 49 individuals provided samples after their second dose (26 to 51 days post second dose). Of the 10 transplant recipients included in the study, 5 received both doses of the Pfizer-BioNTech BNT162b2 vaccine (25–40 days post second dose), and 5 received both doses of the Moderna mRNA-1273 vaccine (samples were drawn on the date of the second dose).
Measurement of SARS-CoV-2 Quantitative Antibody Levels in Serum
All serum samples were analyzed using the Elecsys anti-SARS-CoV-2 S total antibodies assay on the cobas e411 (Roche Diagnostics), the LIAISON SARS-CoV-2 TrimericS IgG assay on the LIAISON XL (DiaSorin), and the SARS-CoV-2 IgG II Quant on the Architect i2000 (Abbott). The samples were measured in multiple batches across the same reagent and calibrator lots. All assays are referenced to the First World Health Organization International Standard for anti-SARS-CoV-2 immunoglobulin. The Elecsys anti-SARS-CoV-2 assay is a double-antigen sandwich electrochemiluminescence immunoassay intended for the quantitative detection of total antibodies to the RBD of the SARS-CoV-2 S protein. A concentration of <0.80 U/mL was considered negative and ≥0.80 U/mL was considered positive. The precision (%CV) ranged from 1.9% to 3.4%. The LIAISON SARS-CoV-2 TrimericS IgG assay uses chemiluminescent immunoassay technology for the quantitative detection of antitrimeric S protein IgG-specific antibodies. A concentration of <33.8 BAU/mL was considered negative and ≥33.8 BAU/mL was considered positive. The precision (%CV) ranged from 0% to 4.2%. The SARS-CoV-2 IgG II Quant assay is a chemiluminescent microparticle immunoassay for the quantitative determination of IgG antibodies against the RBD of SARS-CoV-2. A concentration of <50 AU/mL was considered negative, and ≥50 AU/mL was considered positive. In addition, samples were analyzed using the qualitative SARS-CoV-2 IgG assay that targets the N protein on the Architect i2000 (Abbott) to assess past exposure of participants to SARS-CoV-2. Results are reported as a signal-to-calibrator ratio (S/C), with <1.40 index (S/C) considered negative and ≥1.40 index (S/C) considered positive. The precision (%CV) ranged from 1.5% to 1.7%. For samples over the upper analytical measuring range, manual and/or onboard dilutions were performed with manufacturer recommended diluent according to the manufacturer’s instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 8.4.3 (GraphPad Software) and EP Evaluator version 11.3.0.23 (Data Innovations LLC). Normality was assessed using a Shapiro-Wilk test. Data were considered to follow a normal distribution if a P-value of >0.05 was obtained. Statistically significant differences were determined using a Mann-Whitney test or Kruskal-Wallis test for nonparametric data. P-values of <0.05 were considered statistically significant. Passing-Bablok regression was used to assess the correlation between platforms. A chi-square test was used to compare between-group proportions of values above a given threshold.
Results
A total of 98 participants provided samples between January 4 to April 20, 2021, as shown in Table 1. There were 65 participants who were female (66%) and 33 who were male (34%), with a median age of 45 years (range from 22–84 years of age). No statistically significant antibody concentration differences were observed between sex or age on either of the 3 assays following the first or second dose (data not shown). Of all participants, 3 were identified to have had a previous SARS-CoV-2 infection as identified by the Abbott SARS-CoV-2 IgG assay, which allows for the qualitative detection of antibodies to the N protein, thus indicating a recent or prior infection. Two of these participants had received the first dose, whereas 1 participant received both doses. These individuals are shown as the filled symbol in all figures. Correlation analysis of the absolute values indicated that the Abbott and DiaSorin assays are in good agreement with a Pearson correlation coefficient of R = 0.95 (data not shown), as both assays measure the IgG antibody levels. Conversely, Pearson correlation coefficients of R = 0.82 and 0.83 were obtained for Roche (total antibodies) vs DiaSorin and Abbott vs Roche (total antibodies), respectively.
Table 1.
Characteristics of study participants.
| Characteristics | All participants | HCWs | Transplant recipients |
|---|---|---|---|
| N | 98 | 88 | 10 |
| Female, n (%) | 65 (66) | 62 (70) | 3 (30) |
| Male, n (%) | 33 (34) | 26 (30) | 7 (70) |
| Age, median (range) | 45 (22–84) | 44 (22–82) | 61 (42–84) |
| Pfizer-BioNTech BNT162b2 | |||
| Dose 1 only, n | 39 | 39 | 0 |
| Dose 2, n | 54 | 49 | 5 |
| Moderna mRNA-1273 | |||
| Dose 1 only, n | 0 | 0 | 0 |
| Dose 2, n | 5 | 0 | 5 |
Among the 88 HCW, using the predefined assay-specific thresholds for reporting the test result as positive or negative, all 88 had antibody levels considered as positive by Roche (sensitivity = 100%, 95% CI 96%–100%), 86 were considered positive by DiaSorin (sensitivity = 98%, 95% CI 92%–100%), and 87 considered positive by Abbott (sensitivity = 99%, 95% CI 94%–100%) as shown in Table 2. Sensitivity was also calculated for HCW who received either a single dose or both doses of the Pfizer-BioNTech BNT162b2 vaccine (online Supplemental Table 1). Notably, all 3 assays correctly identified the 49 individuals who received the second dose as positive (100% sensitivity).
Table 2.
SARS-CoV-2 antibody titre interpretation on all healthcare workers analyzed on Roche, DiaSorin, and Abbott Anti-S assays.
| Positive | Negative | Sensitivity (95% CI) | |
|---|---|---|---|
| Roche Anti-S | 88 | 0 | 100 (96–100) |
| DiaSorin Anti-S | 86 | 2 | 98 (92–100) |
| Abbott Anti-S | 87 | 1 | 99 (94–100) |
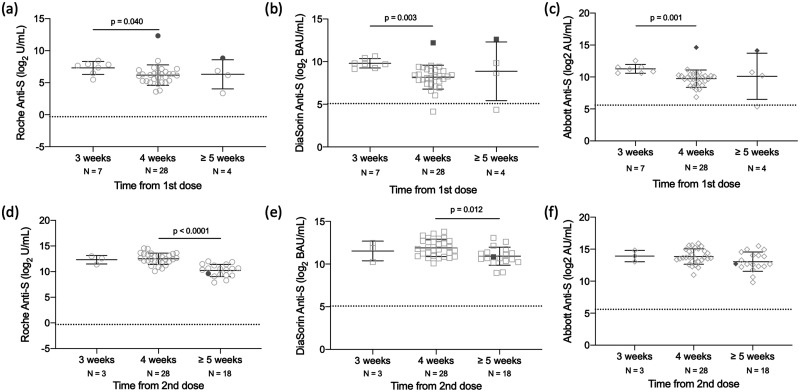
Among the HCW who received the first dose, the interquartile range (IQR) was 39.9 to 162.8 U/mL with a median of 81.4 U/mL (Roche anti-S), IQR 228.8 to 735.8 BAU/mL with a median of 403 BAU/mL (DiaSorin anti-S), and IQR 668.4 to 1662 AU/mL with a median of 1145 AU/mL (Abbott anti-S). In comparison, among the individuals who received both doses, IQR was 629.3 to 6270 U/mL with a median of 2678 U/mL (Roche anti-S), IQR 1204 to 4534 BAU/mL with a median of 2163 BAU/mL (DiaSorin anti-S), and IQR 6362 to 26443 AU/mL with a median of 12403 AU/mL (Abbott anti-S). As shown in Fig. 1, A–C, for those participants who received only the first dose, decreases in antibody levels were observed with increased time between first dose of the vaccine administration and blood draw, a trend that was observed across all 3 assays. Statistically significant differences are observed between weeks 3 and 4 on all 3 assays (Roche anti-S: P = 0.040; DiaSorin anti-S: P < 0.003; Abbott anti-S: P < 0.001). Given the small number of participants in the >5 weeks category, our study was underpowered to detect a decrease in antibody levels. However, similar results were obtained following dose 2 administration (Fig. 1, D–F), where statistically significant differences were observed between weeks 4 and ≥5 weeks on the Roche anti-S and DiaSorin anti-S as determined by Kruskal-Wallis (Roche anti-S: P < 0.0001; DiaSorin anti-S: P = 0.012). While antibody levels trend downward with Abbott anti-S, the difference was not statistically significant. Notably, similar to previous publications, participants with a past SARS-CoV-2 infection had antibody levels that were considerably elevated compared to those with no previous infection after receiving the first dose (Fig. 1, A–C). Conversely, this was not observed in the 1 participant with a previous SARS-CoV-2 infection who had received both doses of the vaccine (Fig. 1, D–F). Differences in antibody levels between HCWs who received either a single dose or both doses and date of blood draw were assessed. Significant differences were observed (online Supplemental Fig. 1) at the 4-week time point, with those with 2 doses having higher antibody levels than those with 1 dose.
Fig. 1.
Difference in SARS-CoV-2 antibody levels by time between dose and blood draw in 88 HCWs who received either a single dose (A–C) or both doses (D–F) of the Pfizer BNT162b2 vaccine. Samples were processed on the Roche anti-S (A, D), DiaSorin anti-S (B, E), and Abbott anti-S (C, F) assays. The dashed line represents the predefined assay-specific thresholds for reporting the test results as positive. Filled symbols are indicative of a participant with a prior infection and not included in statistical analysis. Statistical significance (P < 0.05) was determined using a Kruskal-Wallis test with Dunn’s multiple test correction.
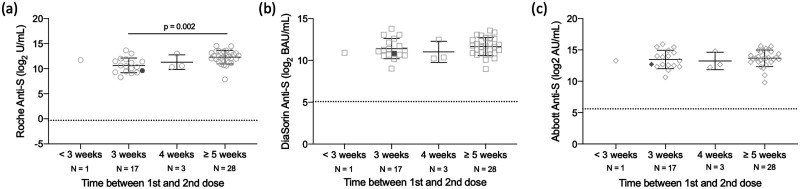
Among the 49 HCW who received both doses at time of blood draw, the time interval between their first and second dose of the Pfizer-BioNTech BNT162b2 vaccine was examined for antibody concentration. Figure 2 demonstrates that with increasing time interval between administration of the first and second dose, antibody levels increase. This trend was observed with all 3 assays; however, the difference was only statistically significant on the Roche assay, where the difference between 3 weeks and ≥5 weeks was significant by Kruskal-Wallis (P < 0.002). For the participants in the ≥5 weeks category, the days between the first and second doses ranged from 35 to 39 days.
Fig. 2.
Difference in SARS-CoV-2 antibody levels by time between the first and second dose in 49 HCWs who received both the first and second dose of the Pfizer-BioNTech BNT162b2 vaccine. Samples were processed on the Roche anti-S (A), DiaSorin anti-S (B), and Abbott anti-S (C) assays. The dashed line represents the predefined assay-specific thresholds for reporting the test results as positive. Filled symbols are indicative of a participant with a prior infection and not included in statistical analysis. Statistical significance (P < 0.05) was determined using a Kruskal-Wallis test with Dunn’s multiple test correction.
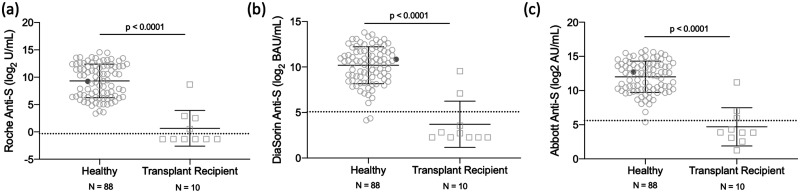
In addition to the studies in the HCW cohort, we also obtained blood collections from SOT recipients (n = 10). As shown in Fig. 3, statistically significant differences in antibody levels between the HCW cohort and SOT recipients can be observed across all 3 assays (Mann-Whitney test, P < 0.0001).
Fig. 3.
Antibody titers in HCWs (n = 88) versus transplant patients (n = 10) who received a SARS-CoV-2 vaccine. Samples were processed on Roche Anti-S (A), DiaSorin Anti-S (B), and Abbott Anti-S (C) assays. The dashed line represents the predefined assay-specific thresholds for reporting the test results as positive. Statistical significance (P < 0.05) was determined using a Mann-Whitney test.
Discussion
All 3 commercially available assays for quantitative SARS-CoV-2 IgG and/or total antibodies targeting the anti-S protein demonstrated a clinical sensitivity >90% for identifying post-vaccine response among the HCW and SOT cohort combined. Sensitivity >98% was obtained among all 3 assays within the HCW group and the sensitivity reached 100% when this cohort was restricted to those with both vaccine doses administered. The Abbott SARS-CoV-2 IgG II Quant and the LIAISON SARS-CoV-2 TrimericS IgG assays showed good correlation owing to the fact that both of these assays target the IgG antibodies. The absolute values generated from each of the assay platforms are not interchangeable as the test lacks standardization and harmonization. There was no statistically significant difference in antibody response between males and females following the first or second dose of the Pfizer-BioNTech BNT162b2 vaccine across all platforms in our cohort. Although there was no statistically significant difference in antibody response with age, a decrease in antibody response was observed with increasing age in HCWs who received either their first or second dose of the Pfizer-BioNTech BNT162b2 vaccine (data not shown). Our sample size was small when further sub-analyses were performed and part of not seeing a statistical significance could be due to insufficient power to detect a difference. In the current study, seropositivity with the SARS-CoV-2 IgG assay against N protein was interpreted as evidence of recent or prior SARS-CoV-2 infection. We identified 3 participants with previous SARS-CoV-2 infection. Of note, 2 individuals were found to have the highest antibody levels following the first dose of the Pfizer-BioNTech BNT162b2 vaccine across all platforms. This phenomenon has also been reported by several other studies (10, 11). Manisty et al. (12) showed that among HCWs with previous SARS-CoV-2 infection, vaccination increased anti-S levels, as detected using the Elecsys anti-SARS-CoV-2 S assay, more than 140-fold from peak pre-vaccine levels.
Presently, both mRNA vaccines are approved for use as a 2-dose schedule given either 21 days or 28 days apart (13). However, with limited vaccine supply, public health officials have extended dose intervals for both the Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines to optimize early vaccine rollout and population protection (9). In the current study, HCWs that had an extended period between their first and second dose of the Pfizer-BioNTech BNT162b2 vaccine had statistically significant higher antibody response (Roche anti-S) (Fig. 2). Moreover, a statistically significant decrease in antibody response was observed in HCWs whose antibody levels were measured 5 weeks after receiving their second dose compared to HCWs whose antibody levels were assessed 4 weeks after receiving their second dose of the Pfizer-BioNTech BNT162b2 vaccine (Roche anti-S and DiaSorin anti-S). A statistically significant decrease in antibody response was also observed in HCWs whose antibody levels were assessed 4 weeks after receiving their first dose of the Pfizer-BioNTech BNT162b2 vaccine compared to HCWs whose antibody levels were assessed 3 weeks post dose 1 across all platforms.
The safety of the SARS-CoV-2 mRNA vaccines in SOT recipients is unknown as these individuals were generally excluded from Phase 1 to 3 vaccine trials (14, 15). In the current study, we observed a significant difference in antibody response between HCWs and SOT recipients who received either the first or second dose of the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 vaccine across all platforms (Fig. 3). This was supported by Boyarsky et al., who showed that SOT recipients did not mount an appreciable anti-S antibody response after receiving the first dose of either the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 vaccines (16). This has also been observed in the context of other vaccine programs. Previous reports suggest that transplant recipients generate a less robust immune response to vaccines compared with nontransplant patients regardless of vaccine type (17). In fact, SOT recipients have relative humoral response rates that are approximately 50% to 70% of those seen in nontransplant populations (18, 19), which supports the findings of the current study. Interestingly, we found that 1 transplant recipient had an elevated antibody titer compared to the other transplant recipients. Boyarsky et al. showed that transplant patients receiving antimetabolite maintenance immunosuppression therapy were less likely to develop an antibody response than those not receiving such immunosuppression therapy. In addition, the authors found that older transplant recipients were less likely to develop an antibody response (16). This may explain why we observed different vaccine responses among transplant recipients.
This study has several limitations. First, the 3 serological assays examined are not measuring neutralizing antibody titers, and therefore we cannot comment on how these levels correlate with protective immunity. Next, the study lacks serial measurement post vaccination over a longer period of time. As such, we were unable to assess antibody response over time post first or second dose. In addition, we looked at a relatively small cohort of individuals; however, our objective was to compare the performance of these 3 commercially available assays in this specific cohort.
With increasing availability of vaccinations against SARS-CoV-2, it is important to compare the clinical performance of serological assays that detect antibodies to SARS-CoV-2. In this study, we compare the antibody levels from 98 participants across the Elecsys Anti-SARS-CoV-2 S (Roche Diagnostics), SARS-CoV-2 TrimericS IgG (DiaSorin), and SARS-CoV-2 IgG II Quant (Abbott) platforms. All 3 assays, while not interchangeable, showed sensitivity >90% in detecting vaccine response. We demonstrate that antibody levels decrease with increased time between vaccine administration and blood draw for the first and second dose. In addition, our results show an increase in antibody levels with increased time between administration of the first and second dose. We also analyzed antibody levels in a small cohort of SOT patients for comparison across the 3 assays. Our results show significant differences in antibody levels between our HCWs and transplant recipients.
Supplemental Material
Supplemental material is available at The Journal of Applied Laboratory Medicine online.
Supplementary Material
Nonstandard Abbreviations
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; S, spike; N, nucleocapsid; RBD, receptor binding domain; mRNA, messenger RNA; HCW, healthcare workers; IQR, inter-quartile range.
Author Contributions
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 4 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Authors’ Disclosures or Potential Conflicts of Interest
Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:Employment or Leadership: V. Kulasingam, The Journal of Applied Laboratory Medicine, AACC. Consultant or Advisory Role: None declared. Stock Ownership: None declared. Honoraria: None declared. Research Funding: Reagent kits were provided by Roche Diagnostics, DiaSorin and Abbott Laboratories. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. D. Kumar, grants from Roche outside the submitted work. Expert Testimony: None declared. Patents: None declared. Other Remuneration: V. Kulasingam, personal fees from Abbott outside the submitted work; D. Kumar, personal fees from Roche outside the submitted work.
Role of Sponsor
No sponsor was declared.
References
- 1.Hu B, Guo H, Zhou P, Shi Z-L.. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mousavizadeh L, Ghasemi S.. Genotype and phenotype of COVID-19: their roles in pathogenesis. J Microbiol Immunol Infect 2021;54:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sofi MS, Hamid A, Bhat SU.. SARS-CoV-2: A critical review of its history, pathogenesis, transmission, diagnosis and treatment. Biosaf Health 2020;2:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020;586: 572–7. [DOI] [PubMed] [Google Scholar]
- 5.Qu J, Wu C, Li X, Zhang G, Jiang Z, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020;71:2255–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 2021;384:1959–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. ; mRNA-1273 Study Group. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ainsworth M, Andersson M, Auckland K, Baillie JK, Barnes E, Beer S, et al. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021;397:1057–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callegaro A, Borleri D, Farina C, Napolitano G, Valenti D, Rizzi M, Maggiolo F.. Antibody response to SARS-CoV-2 vaccination is extremely vivacious in subjects with previous SARS-CoV-2 infection. J Med Virol 2021;93:4612–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis [Epub ahead of print 2021 Apr 5] as doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed]
- 12.Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021;397:1057–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Figueiredo JC, et al. Prior COVID-19 infection and antibody response to single versus double dose mRNA SARS-CoV-2 vaccination. Biochem Pharmacol 2021;24:1639–41. [Google Scholar]
- 14.Blumberg EA, Manuel O, Sester M, Ison MG.. The future of SARS-CoV-2 vaccines in transplant recipients: to be determined. Biochem Pharmacol 2021;24:1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D.. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol 2021;74:944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM.. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA 2021;325:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T.. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One 2013;8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danziger-Isakov L, Kumar D; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13563. [DOI] [PubMed] [Google Scholar]
- 19.Haddadin Z, Krueger K, Thomas LD, Overton ET, Ison M, Halasa N.. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am J Transplant 2021;21:938–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.