Abstract
As an important medium of intercellular communication, exosomes play an important role in information transmission between tumor cells and their microenvironment. Tumor metastasis is a serious influencing factor for poor treatment effect and shortened survival. Lung cancer is a major malignant tumor that seriously threatens human health. The study of the underlying mechanisms of exosomes in tumor genesis and development may provide new ideas for early and effective diagnosis and treatment of lung cancer metastasis. Many studies have shown that tumor-derived exosomes promote lung cancer development through a number of processes. By promoting epithelial–mesenchymal transition of tumor cells, they induce angiogenesis, establishment of the pretransfer microenvironment, and immune escape. This understanding enables researchers to better understand the mechanism of lung cancer metastasis and explore new treatments for clinical application. In this article, we systematically review current research progress of tumor-derived exosomes in metastasis of lung cancer. Although positive progress has been made toward understanding the mechanism of exosomes in lung cancer metastasis, systematic basic research and clinical translational research remains lacking and are needed to translate our scientific understanding toward applications in the clinical diagnosis and treatment of lung cancer metastasis in the near future.
Keywords: Lung cancer, Exosomes, Metastases, Diagnosis, Therapeutic targets
Statement of significance
Tumor-derived exosomes promote lung cancer development through a number of processes.
In this article, we systematically review the current research progress of tumor-derived exosomes in metastasis of lung cancer.
These findings about exosomes in tumor genesis and development may provide new ideas for diagnosis and treatment of lung cancer metastasis.
Introduction
Extracellular vesicles (EVs) are lipid bilayer-enclosed extracellular structures which can be formed by outward budding of the plasma membrane or by an intracellular endocytic trafficking pathway involving fusion of multivesicular late endocytic compartments with the plasma membrane. These fusion events result in the extracellular release of the intraluminal vesicles of these compartments, generating a subtype of EVs termed ‘exosomes’ [1]. Information transmission between tumor cells and various cells in the microenvironment plays an important role in tumor metastasis, and exosomes are one of the important mediums of intercell communication [1, 2]. Exosomes are vesicles with a diameter of 30–100 nm secreted by different types of cells [3]. They carry many kinds of substances, such as lipids, nucleic acids, and proteins, and are widely distributed in body fluids, including urine, plasma, lavage fluid, serosal effusion, and cerebrospinal fluid [4]. Exosomes have important roles in multiple physiological and pathological processes, exerting biological functions. On the one hand, they are necessary to maintain normal physiological responses. On the other hand, in the pathological state, especially in the tumor environment, they promote carcinogenesis, proliferation, migration, invasion, immunosuppression, and angiogenesis as well as reshape the microenvironment [5].
In recent years, the study of exosomes in tumor has received enormous interest. Exosomes contain bio-macromolecules to participate in information exchange between cells [6]. They can increase the invasion ability of tumor cells and promote tumor metastasis, which has become a research hotspot in the field of cancer recently [7, 8]. Different types of tumor cells secrete different exosome contents. Additionally, factors affecting cell homeostasis, such as a hypoxic microenvironment, survival pressure, and chemotherapy drugs, induces tumor cells to secrete exosomes [9, 10]. Therefore, the volume of exosomes secreted by tumors is much higher than that of normal cells. Although the role of most exosomal compounds in cancer is unclear, previous studies have shown that tumor-derived exosomes promote tumor growth and metastasis by inducing epithelial–mesenchymal transformation (EMT) of tumor cells. They also promote angiogenesis, the transformation of cancer-associated fibroblasts, immunosuppression, and formation of a premetastatic microenvironment by acting on stromal cells in the microenvironment [11–15].
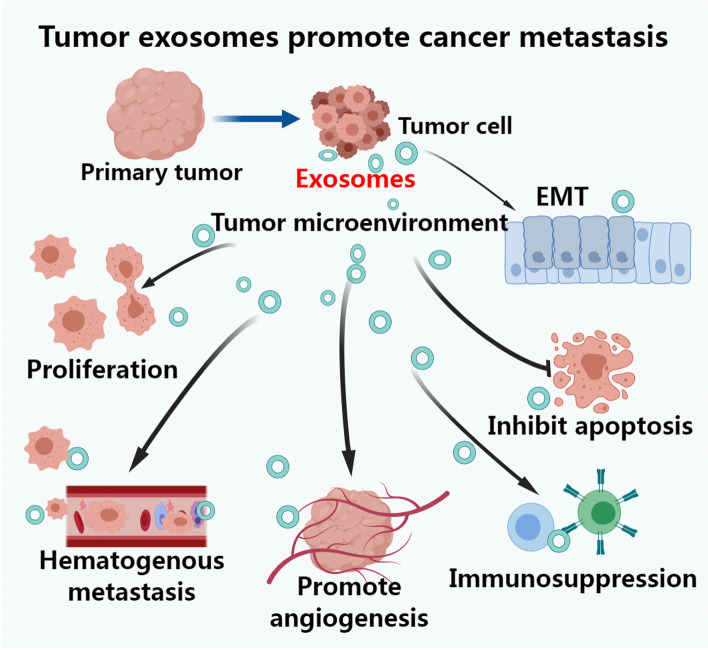
Several studies have shown that differential expression of exosome contents is closely related to lung cancer metastasis, playing an important role in the multilink and multistep process [16, 17]. The multiple mechanisms of tumor-derived exosomes promoting cancer metastasis are mainly summarized in Fig. 1. The abscission of cancer cells is essentially a manifestation of increased migration and invasion of tumor cells. Compared with those in healthy people, exosomes are more abundant in circulating body fluids of patients with lung cancer. A number of studies have found that exosomes promote the occurrence and development of lung cancer by promoting the formation of the lung cancer microenvironment, increasing the ability of tumor cell invasion and metastasis, mediating tumor immunosuppression, and participating in chemo-radiotherapy resistance [18]. The study of the underlying mechanisms of exosomes in tumor genesis and development may provide new ideas for early and effective diagnosis and treatment of lung cancer metastasis. Therefore, in this article, the relevant research status of the role of exosomes in lung cancer migration and invasion, immunosuppression and escape, angiogenesis, and other processes is reviewed.
Fig. 1.
Tumor-derived exosomes promote cancer metastasis. Tumor-derived exosomes through multiple mechanisms participate in cancer metastasis by reshaping the tumor microenvironment; promoting cellular epithelial–mesenchymal transformation (EMT); promoting cell proliferation, inhabiting apoptosis; immunosuppression; promoting hematogenous metastasis and angiogenesis of metastasitic tumor to promote cancer metastasis
Tumor exosomes influence lung cancer metastasis by promoting cellular EMT
Many studies have confirmed that lung cancer cell-derived exosomes have an important biological role in distant metastasis of lung cancer by promoting the EMT process in normal epithelial cells. Additionally, mesenchymal stem cells (MSCs), cancer stem cells (CSCs) and other exosome-producing cells can promote the EMT process of cells (summarized in Table 1).
Table 1.
Exosomes related with lung cancer metastasis by EMT
| Cancer type | Exosomes source | Related genes or pathway | Tissues and/or cells | Experimental data | Function | Refs |
|---|---|---|---|---|---|---|
| Lung cancer | Cell culture fluid | SNAI1 | Lung cancer cells | Promote cells EMT by CAFs deliver SNAI1 to recipient cancer cells via exosomes | Promote EMT | [19] |
| Lung cancer (NSCLC) | Cell culture fluid | ZEB1 | HBEC cell model | Exosomes transfer chemoresistance and mesenchymal phenotypes to recipient cells. | Promote EMT | [20] |
| Lung cancer (NSCLC) | Cell culture fluid | TRAF4 | Lung fibroblasts | By stabilize NOX complex to promote the proliferation and EMT of NSCLC cells | Promote EMT | [21] |
| Lung cancer | Cell culture fluid | TGF-β1; Smad2/3; Akt/GSK-3β/β-catenin; NF-κB; ERK; JNK and p38 MAPK | MSCs | Promotes EMT, invasion, and migration; enhance the anti-proliferation and pro-apoptotic effect of MSCs on lung cancer cells | Promote EMT | [22] |
| Lung cancer | Cell culture fluid | TGF-β1; specific miRNAs of exosomes | Lung cancer cells (A549 and H1299 cells) | Promote migration, invasion and expression of mesenchymal markers in the recipient cells | Promote EMT | [23] |
| Lung cancer | Hypoxic BMSCs fluid | miR-193a-3p; miR-210-3p; miR-5100; STAT3 signaling | Lung cancer cells | Exosomes miRNAs activate STAT3 signaling pathway | Promote EMT | [24] |
| Lung cancer | Cell culture fluid | miR-210-3p; FGFRL1 | Lung CSCs | Promotes EMT, and through miR-210-3p combining FGFRL1 enhance the metastatic ability of lung cancer cells | Promote EMT, | [25] |
| Lung cancer | Cell culture fluid; Serum | Lung cancer cells and human late stage lung cancer serum | Promote EMT | Promote EMT | [26] |
Abbreviations: BMSCs Bone marrow-derived mesenchymal stem cells, CAFs Cancer-associated fibroblasts, CSCs Cancer stem cells, EMT Epithelial-mesenchymal transition, FGFRL1 Fibroblast growth factor receptor-like 1, MSCs Mesenchymal stem cells, NSCLC Non-small cell lung cancer
EMT is an important part of the biological process of tumor metastasis. Cancer-associated fibroblasts (CAFs) contain SNAI1, which is delivered to recipient lung cancer cells via exosomes of CAFs to promote EMT. The level of SNAI1 in exosomes is critical for EMT induction in lung cancer cells [19]. Exosomes from lung cancer mesenchymal cells contain ZEB1 mRNA, which increases the expression of the EMT major transcription factor ZEB1 in recipient cells through the transfer of ZEB1 in exosomes, promoting its transformation from epithelial cells to mesenchymal phenotypes and transferring chemotherapeutic resistance to bronchial epithelial cells [20]. TRAF4 in normal fibroblasts surrounding non-small cell lung cancer (NSCLC) cells that can promote their proliferation and EMT. TRAF4 mediates phosphorylated p47-phox to form complexes with NOX2 or NOX4, which increase reactive oxygen species level in vivo and enter the cytoplasm, leading to NF-κB-mediated upregulation of ICAM1 in NSCLC cells [21].
MSCs derived from human umbilical cord can promote EMT and invasion and migration of lung cancer cells, inhibit cell proliferation, and promote cell apoptosis. TGF-β1 expression in MSCs enhances the role of MSCs in promoting EMT and enhances antiproliferative and proapoptotic effects of MSCs on lung cancer cells through MSC-derived exosomes [22].
Furthermore, specific exosomal microRNAs (miRNAs), including miR-193A-3p, miR-210-3p, and miR-5100, are also involved in promoting EMT of epithelial cells by exosomes. The miRNA profile of exosomes may be altered after cellular EMT, and these exosomal miRNAs may in turn promote EMT and the migration and invasion of cancer cells [23]. Therefore, miRNAs specifically expressed in exosomes are associated with EMT and metastasis and may serve as new biomarkers for the EMT process in lung cancer. Bone marrow-derived mesenchymal stem cell (BMSC)-derived exosomal miRNAs, including miR-193A-3p, miR-210-3p, and miR-5100, transferred to epithelial cancer cells under hypoxia promote lung cancer cell invasion and EMT by activating the STAT3 signaling pathway and increasing the expression of mesenchymal-related molecules [24]. Similarly, exosomes derived from lung CSCs promote EMT, migration, and invasion of lung cancer cells by upregulating the expression levels of N-cadherin, Vimentin, MMP-9, and MMP-1, and downregulating the expression of E-cadherin. Exosome-miR-210-3p also promotes lung cancer metastasis by binding FGFRL1 and downregulating its expression [25].
In a study on the effects of exosomes derived from human lung cancer serum, highly metastatic cells, and nonmetastatic cells on recipient human bronchial epithelial cells (HBECs), they found that exosomes from highly metastatic lung cancer cells and serum from patients with advanced lung cancer induce Vimentin expression and EMT in HBECs. The results also confirmed that exosomes from highly metastatic cancer cells and advanced lung cancer serum induce migration, invasion, and proliferation of noncancer receptor cells [26]. Thus, cancer-derived exosomes can be a potential mediator of EMT in recipient cells.
Inhibition of EMT process in recipient cells may be an effective treatment for lung cancer metastasis. Targeting specific genes (such as SNAI1, ZEB1, miR-193A-3p, miR-210-3p, etc.) in these tumor-associated exosomes can effectively inhibit the exosome pathway to promote lung cancer metastasis, which may provide a new method for the prevention and treatment of lung cancer metastasis. Based on the important findings of the above studies, how to inhibit EMT specifically and effectively to block lung cancer metastasis as well as how to conduct clinical research and translate it into clinical practice are important questions that will need to be addressed in key future research.
Tumor exosomes influence lung cancer metastasis by regulating cell proliferation, apoptosis, and migration
Studies have shown that exosomes from lung cancer or other tumor sources play important roles in lung cancer metastasis by promoting the proliferation of lung cancer cells, inhibiting apoptosis, and regulating the invasion and migration ability of lung cancer cells (summarized in Table 2). Wnt3a/β-catenin, circSATB2, HIF-1α/COX-2, KLF9, and LMO7 in tumor-derived exosomes regulate genes or signaling pathways to promote proliferation and migration of lung cancer cells. Further, specific miRNAs in tumor-derived exosomes, including miR-326, miR-135b, miR-210, miR-660-5p, and miR-96, play a stimulating role as important regulators in lung cancer cell proliferation and migration [27–31]. Wnt5b overexpression is associated with cancer aggressiveness. Exosomes derived from cells of the human pancreatic cancer cell line PANC-1 activate Wnt5b signaling in Chinese hamster ovary cells and stimulate the migration and proliferation of A549 lung adenocarcinoma (ADC) cells. Caco-2 colon cancer cells were used to establish Caco-2/Wnt5b cells with ectopic expression of Wnt5b, whose exosomes also stimulated the migration and proliferation of A549 cells [32]. These results indicate that Wnt5b-associated exosomes promote cell migration and proliferation in a paracrine manner.
Table 2.
Exosomes related with lung cancer metastasis by regulating cell proliferation, apoptosis and migration
| Cancer type | Exosomes source | Related genes or pathway | Tissues and/or cells | Experimental data | Functions | Refs | |
|---|---|---|---|---|---|---|---|
| Promote proliferation and migration | |||||||
| Lung cancer | Cell culture fluid | Wnt3a/β-catenin | Lung cancer cells | Exosomes containing high levels of Wnt3a activate β-catenin signaling | Promote proliferation | [27] | |
| Lung cancer (NSCLC) | Serum | circSATB2; miR-326 | Lung cancer cells | Regulate FSCN1 expression positively via miR-326 and be transferred by exosomes | Promote proliferation, migration and invasion | [28] | |
| Lung cancer (NSCLC) | Cell culture fluid | HIF-1α/COX-2; miR-135b; miR-210 | Lung cancer cells | Hypoxia enhance numbers of exosomes and up-regulate of exosomal HIF-1α/COX-2 and expression of exosomal miR-135b and miR-210 | Promote proliferation and migration | [29] | |
| Lung cancer (NSCLC) | Serum | miR-660-5p; KLF9 | Lung cancer cells | miR-660-5p in exosome may control NSCLC proliferation, viability, and metastasis by targeting KLF9 | Promote proliferation and migration | [30] | |
| Lung cancer | Serum | miR-96; LMO7 | Lung cancer patients | Exosomal miR-96 promotes lung cancer progression by targeting LMO7. | Promote proliferation, migration, and drug resistance | [31] | |
| Lung cancer (adenocarcinoma) | Cell culture fluid | Wnt5b | PANC-1 cells | Exosomes activate Wnt5b signaling in CHO cells and stimulate migration and proliferation of lung adenocarcinoma cells A549 | Promote proliferation and migration | [32] | |
| Promote invasion and or migration | |||||||
| Lung cancer (Adenocarcinoma) | Lung cancer | TNF-β; TNF-α; IL-6; IL-8; IL-10; CD163; iNOS; MMP2; MMP9 | Lung cancer cells | Exosomes derived from lung adenocarcinoma cells can activate macrophages, increase MMP2 and MMP9 levels, and enhance the invasion of lung adenocarcinoma cells. | Promote invasion | [33] | |
| Lung cancer (NSCLC) | Pleura exudates | GGT-1; LTC4; CysLT1 | Lung cancer cells | Exosomes contain GGT-1 and transform exogenous LTC4 to pro-tumorigenic LTD4, and elevate the level of endogenous CysLT | Promote survival and migration | [34] | |
| Lung cancer | BALF | E-cadherin | Lung cancer cells | In exosomes from lung cancer, BALF promote the migration and invasion by carrying E-cadherin | Promote migration and invasion | [35] | |
| Lung cancer (SCLC) | Cell culture fluid | FECR1; FECR2; miR584-3p; ROCK1 | Lung cancer cells | FECR1 and FECR2 up-regulated in SCLC tissues; exosomal FECRs promotes lung cancer cells metastasis through the miR584-ROCK1 pathway | Promote invasion and migration | [36] | |
| Lung cancer | Serum | miR-106b; PTEN; MMP-2; MMP-9 | Lung cancer patients | Exosomal miR-106b target PTEN, increase the MMP-2 and MMP-9 expression and promote lung cancer cell migration and invasion | Promote invasion and migration | [37] | |
| Lung cancer | Cell culture fluid | ALDOA; ALDH3A1 | Lung cancer cells | Exosomes carrying ALDOA and ALDH3A1 from irradiated lung cancer cells enhance migration and invasion of recipient cells by accelerating glycolysis | Promote invasion and migration | [38] | |
| Lung cancer | Cell culture fluid | MMP3; MMP9 | Adipocytes cells | Adipocyte-derived exosomes promote lung cancer cells metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells | Promote invasion and migration | [39] | |
| Promote proliferation and or inhibit apoptosis | |||||||
| Lung cancer (NSCLC) | Cell culture fluid | ASMA | Lung cancer cells; Human lung fibroblasts cell line HLF1 cells | Promote cell proliferation and inhibit cell apoptosis in both normal lung fibroblasts and NSCLC cells by delivering ASMA | Promote proliferation and inhibit apoptosis | [40] | |
| Lung cancer (NSCLC) | Cell culture fluid | MALAT-1 | Lung cancer cells | Serum exosome-derived long noncoding RNA MALAT-1 promotes the tumor growth and migration, and prevents tumor cells from apoptosis | Promote proliferation and migration; Inhibit apoptosis | [41] | |
| Lung cancer (SCLC) | HBMECs | S100A16 | Lung cancer cells | Elevation of S100A16 prevent the loss of mitochondrial membrane potential and enhance resistance to apoptosis of SCLC cells | Inhibit apoptosis | [42] | |
| Lung cancer | Macrophages cell culture fluid | Let-7a-5p; BCL2L1; PI3Kγ | Macrophages cells | Exogenous let-7a-5p induces lung cancer cell death through BCL2L1-mediated PI3Kγ signaling pathway | Promote autophagic cell death | [43] | |
| Regulate or inhibit migration | |||||||
| Lung cancer | Cell culture fluid | TGF-β; lnc-MMP2-2 | Lung cancer cells | TGF-β-mediated exosomal lnc-MMP2-2 might regulate the migration and invasion of lung cancer cells into the vasculature by promoting MMP2 expression | Regulate migration | [44] | |
| Lung cancer | Cell culture fluid | TGF-β; IL-10 | Lung cancer cells NCI-H1688 | Exosomes derived from cancer cells regulate the cellular migration of tumor cells through TGF-β and IL-10 | Regulate migration | [45] | |
| Lung cancer (NSCLC) | Cell culture fluid | PEDF; THBS1 | Lung cancer cells | Exosomes from PEDF-treated cells contain THBS1, which inhibit cytoskeletal remodeling and exosome-induced lung cancer cell motility, migration, and invasion | Inhibit invasion and migration | [46] | |
Abbreviations: ALDH3A1 Aldehyde dehydrogenase 3A1, ALDOA Aldolase A, ASMA Alpha-smooth muscle actin, BALF Bronchoalveolar lavage fluid, BCL2L1 B-cell lymphoma-2, CHO Chinese hamster ovary, COX-2 Cyclooxygenase-2, CysLT1 Cysteinyl leukotriene 1, FECR FLI1 exonic circular RNAs, FSCN1 Fascin homolog 1, actin-bundling protein 1, GGT-1 γ-glutamyl transpeptidase 1, HBMECs Human brain microvascular endothelial cells, HIF-1α Hypoxia inducible factor-1, IL Interleukin, iNOS Inducible nitric-oxide synthase, KLF9 Kriippel-like factor9, LMO7 LIM-domain only protein 7, LT Leukotriene, MALAT-1 Metastasis-associated lung adenocarcinoma transcript 1, MMP Matrix metalloproteinase, NSCLC Non-small cell lung cancer, ROCK1 Rho Associated Coiled-Coil Containing Protein Kinase 1 gene, PANC-1 Human pancreatic cancer cell line, PEDF Pigment epithelium-derived factor, PI3Kγ PI-3 kinase gamma, PTEN Phosphatase and tensin homolog deleted on chromosome ten, SCLC Small cell lung cancer, TGF Transforming growth factor, THBS1 Thrombospondin 1, TNF Tumor necrosis factor
mRNA transcripts of GGT-1, LTC4, FECR1, FECR2, miR-106b, ALDOA, ALDH3A1, and other genes in exosomes derived from lung cancer cells act as important regulators by playing an enabling role in promoting the invasion and migration of lung cancer cells. Tumor-derived exosomes derived from lung cancer cells promote the invasion and migration of lung cancer cells by regulating the expression levels of specific genes, including those encoding the matrix metalloproteinases MMP-2, MMP-9, PTEN, E-cadherin, and ROCK1 [33–38]. Nontumor exosomes derived from adipocytes increase the activity of MMP9 by delivering MMP3 to lung cancer cells, thereby promoting the invasion and migration of lung cancer cells [39].
Lung cancer cell-derived exosomes also promote lung cancer metastasis by inhibiting apoptosis. These exosomes contribute to lung cancer progression by delivering transcripts of ASMA, S100A16, and the long noncoding RNA (lncRNA) MALAT-1, all of which promote tumor growth and migration and prevent apoptosis [40–42]. In contrast, macrophage cell-derived exosomes contain let-7a-5p, which promotes autophagic cell death of lung cancer cells through the BCL2L1-mediated PI3Kγ signaling pathway [43]. Thus, exosomal let-7a-5p plays a significant role in inhibiting tumor invasion and metastasis.
In terms of the simple regulation of lung cancer migration, studies have confirmed that transcripts of TGF-β, lnc-MMP2-2, IL-10, as well as those of other genes in exosomes derived from lung cancer cells have regulatory functions. They play a key role in regulating the migration ability of lung cancer cells by targeting and regulating related genes, thus participating in the biological process of lung cancer metastasis [44, 45]. Pigment epithelium-derived factor (PEDF)-treated exosomes from lung cancer cells inhibit cytoskeletal remodeling by secreting THBS1, which reduces the vitality of lung cancer cells and inhibits cell invasion and migration [46].
These findings have demonstrated that tumor-derived exosomes participate in the biological process of lung cancer metastasis by regulating cell apoptosis, cell proliferation, and cell migration. It can be predicted that blocking the biological functions of these key genes in tumor-derived exosomes to inhibit apoptosis, promote proliferation and migration will provide a new approach for the treatment of lung cancer metastasis induced by tumor exosomes. However, additional in-depth studies are needed to drive clinical transformation research and new clinical practice in lung cancer metastasis.
Tumor exosomes influence lung cancer metastasis by regulating immunity and angiogenesis
Many studies have shown that exosomes from lung cancer or other tumor sources can suppress immunity leading to immune escape and promote metastasis of lung cancer cells (Table 3). For instance, exosomes from lung cancer cells express PD-L1 and promote tumor growth by reducing T cell activity to induce immune escape. Exosomal PD-L1 inhibits the secretion of interferon-γ (IFN-γ) by Jurkat T cells. Exosomes impair immune function and promote lung cancer metastasis by reducing cytokine production and inducing apoptosis of CD8+ T cells [47]. Exosomes from Lewis lung carcinoma cells block the differentiation of bone marrow progenitor cells into CD11c+ dendritic cells (DCs) and induce apoptosis. However, after tumor exosome PD-L1 was blocked, the immunosuppressive ability of DCs was partially restored [48].
Table 3.
Exosomes related with lung cancer metastasis by the regulation of immune and angiogenesis
| Cancer type | Exosomes source | Related genes or pathway | Tissues and/or cells | Experimental data | Function | Refs |
|---|---|---|---|---|---|---|
| Immune regulation | ||||||
| Lung cancer (NSCLC) | Cell culture fluid | PD-L1 | Lung cancer cells | Reduce T cell activity resulting in immune escape, and promote tumor growth | Immune inhibition; immune escape | [47] |
| Lung cancer | Lung cancer cells (LLC Lewis) | PD-L1 | LLC Lewis lung carcinomacells | Tumor exosome treatment can inhibit the maturation and migration of DCs and promote DCs immunosuppression | Immune inhibition | [48] |
| Lung cancer | Tumors secreting | miR-21; miR-29a; TLR7; TLR8 | Lung cancer cells (A549) | miR-21 and miR-29a bind to TLR7 and TLR8, triggering a TLR-mediated prometastatic inflammatory response that leads to tumor growth and metastasis | Immune inhibition | [49] |
| Lung cancer | TD-MVs | TGF-β1; miR-23a | Lung cancer cells | TD-MVS transferred TGF-β1 into NK cells and inhibited NK cell function under hypoxia | Immune inhibition | [50] |
| Lung cancer | Patients serum | EGFR | Lung cancer patients | Induction of immune tolerance in dendritic cells by inhibiting tumor antigen-specific CD8 + T cells | Immune inhibition | [51] |
| Lung cancer | MDA-MB-231 cell culture fluid | miR-126; PTEN/PI3K/ Akt signaling pathway | Lung cancer cells (A549) | MDA-MB-231 cell-derived exosomes can recognize A549 cells in the blood and effectively escape from in vitro immune monitoring systems; miR-126–231-Exo strongly inhibits the proliferation and migration of A549 cells by blocking the PTEN/PI3K/ Akt signaling pathway | Immune escape | [52] |
| Angiogenesis regulation | ||||||
| Lung cancer | Cell culture fluid | miR-23a; PHD1; PHD2; ZO-1 | Lung cancer cells | miR-23a up-regulated in exosomes of lung cancer under hypoxic conditions. miR-23a inhibits PHD1 and 2, leading to HIF-1 accumulation to enhance angiogenesis and, in addition, inhibits ZO-1, increases vascular permeability and cancer transepithelial migration | Enhance angiogenesis | [53] |
| Lung cancer | Cell culture fluid (malignant transformation of HBE cells) | exosomal miR-21; STAT3; VEGF | HBE cells | miR-21 in exosomes activates STAT3, increases VEGF levels in recipient cells, and promotes angiogenesis and malignant transformation of HBE cells | Enhance angiogenesis | [54] |
| Lung cancer | Serum from patients with NSCLC | exosomal miR-126 | HBE cells | miR-126 in exosomes induces angiogenesis and malignant transformation of HBE cells. | Enhance angiogenesis | [55] |
| Lung cancer | Lung cancer cells secreting (Hypoxic conditions) | HIF-1α; COX-2; miR-135b; miR-210 | Lung cancer cells (A549) |
Hypoxia-induced exosomes can promote the proliferation, migration and angiogenesis of A549 cells. Aspirin attenudes this stimulative effect by inhibiting the proliferation of hypoxic A549 cells, reducing exosome secretion and changing exosome composition. |
Enhance angiogenesis | [29] |
| Lung cancer (SCLC) | NCI-H69 SCLC cells secreting | sFlt-1 | HUVECs | Exosomes of SCLC cell lines contain very low level of sFlt-1, which significantly increases the migration of HUVECs and weakens the inhibitory effect of NCI-H69-Exo on angiogenesis. | Enhance angiogenesis | [56] |
| Lung cancer | TECs (derived from ADC and SCC) | CDH2; MAPK/ERK and MAPK/JNK signaling pathways | Lung cancer patients | CDH2 significantly promoted angiogenesis in vivo and in vitro. | Enhance angiogenesis | [57] |
Abbreviations: ADC Adenocarcinoma, CDH2 Cadherin-2, DCs Dendritic cells, HBEs Human bronchial epithelial cells, HUVEC Human umbilical vein endothelial cells, PHD Prolyl hydroxylase, TD-MVs Tumor-derived microvesicles, TECs Tumor-derived endothelial cells, TLR Toll-like receptor, NSCLC Non-small cell lung cancer, SCC Squamous cell carcinoma, SCLC Small cell lung cancer, sFlt-1 Soluble fms-like tyrosine kinase-1, VEGF Vascular endothelial growth factor
As miRNAs are involved in tumor–immune system communication and play an important role in tumor growth and progression, they are a promising target of cancer therapy. MiR-21 and miR-29a bind to TLR7 and TLR8, thereby stimulating a TLR-mediated prometastatic inflammatory response that leads to tumor growth and metastasis [49]. Tumor-derived microvesicles (TD-MVs) transfer TGF-β1 to natural killer (NK) cells and decrease the expression of the activated receptor NKG2D on the cell surface, while high levels of miR-210 and miR-23a in hypoxic TD-MVs act as additional immunosuppressors that directly affect the expression of CD107a in NK cells and reduce the antitumor immune response of NK cells [50].
Epidermal growth factor receptor (EGFR) is closely related to the occurrence and development of lung cancer. The presence of EGFR in lung cancer exosomes induces tolerant DCs. Tolerant DCs and Th0 cells were co-cultured to produce tumor antigen-specific regulatory T cells (Tregs), which inhibit tumor antigen-specific CD8+ T cells and cause immune tolerance in patients with lung cancer [51]. Additionally, MDA-MB-231 cell-derived exosomes recognize A549 cells in blood, thereby promoting immune escape and distant metastasis of lung cancer cells [52].
A number of studies have reported that lung cancer exosomes also promote lung cancer cell metastasis by increasing angiogenesis (summarized in Table 3). Lung cancer cell-derived exosomal miR-23a directly inhibits the prolyl hydroxylases PHD1 and PHD2, leading to the accumulation of hypoxia-inducible factor-1 (HIF-1) in endothelial cells to enhance angiogenesis. MiR-23a also inhibits the tight junction protein ZO-1, thereby increasing vascular permeability and cancer trans epithelial migration [53]. Further, miR-21 in cigarette smoke extract-transformed HBEC exosomes induces STAT3 activation, which increases VEGF levels in recipient cells and promotes angiogenesis and malignant transformation of HBECs [54]. MiR126 is mainly present in the exosomes of patients with NSCLC. Exosomal miR126 induces angiogenesis and malignant transformation of HBECs [55]. Hypoxia-induced lung cancer stem cell-derived exosomes promote proliferation, migration, and angiogenesis of A549 cells, whereas aspirin attenuates this effect by reducing exosome secretion and altering exosome components (i.e., upregulating HIF-1α/COX-2 and downregulating exosomal miR-135b and miR-210) [29]. Exosomes derived from cells of the SCLC cell line NCI-H69 contain a low level of sFlt-1, which can significantly increase the migration ability of human umbilical vein vascular endothelial cells (HUVECs) and weaken the inhibitory effect of NCI-H69-derived exosomes on angiogenesis. Exosomes rich in sFlt-1 inhibit HUVEC migration induced by NCI-H69-derived exosomes, and thus, may be an effective therapeutic agent for inhibiting SCLC metastasis [56]. Cadherin-2 (CDH2) expression was significantly elevated in tumor-derived endothelial cells derived from both ADC and squamous cell carcinoma (SCC). CDH2 significantly promoted angiogenesis and increased sensitivity to antagonists in vivo and in vitro. The MAPK/ERK and MAPK/JNK signaling pathways may play an important role in CDH2-induced HIF-1α/VEGF-mediated angiogenesis [57].
These studies demonstrate the involvement of tumor-derived exosomes in lung cancer metastasis by regulating immune function and angiogenesis and also provide clues for further research directions toward improving diagnosis and treatment of lung cancer metastasis. By targeting specific genes in tumor-derived exosomes associated with immunosuppression and tumor angiogenesis, blocking or reversing the biological functions of these two aspects, it is expected to have a certain therapeutic effect on the metastasis of lung cancer.
Other mechanisms
Tumor-derived exosomes also promote tumor progression through other mechanisms, such as via inflammation-related pathways, induction of the EGFR pathway in preosteoclasts, or interactions with adjacent cells. Additionally, exosomes from the serum of patients with lung cancer are closely related to tumor stage and metastatic progression (summarized in Table 4).
Table 4.
Exosomes related with lung cancer metastasis by other mechanisms
| Cancer type | Exosomes source | Related genes or pathway | Tissues and/or cells | Experimental data | Function | Refs |
|---|---|---|---|---|---|---|
| Lung cancer | Lung cancer cells | TRIM59; NLRP3;, IL-1β | Lung cancer cells; macrophages | Lung cancer cells-derived exosomal TRIM59 converts macrophages via regulating ABHD5 proteasomal degradation, to activate NLRP3 inflammasome signaling pathway to promote lung cancer progression by IL-1β secretion | Promote lung cancer progression | [58] |
| Lung cancer (NSCLC) | Lung cancer cells | AREG; EGFR; RANKL | Lung cancer cells | NSCLC-exosomes, containing AREG, induce EGFR pathway activation in pre-osteoclasts that in turn causes an increased expression of RANKL | Promote bone metastasis | [59] |
| Lung cancer | Cell culture fluid | COX-2 | Lung cancer cells; THP-1 | COX-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes | Involve in the interaction with neighbor cells | [60] |
| Lung cancer | Lung cancer cells | lncRNA | Lung cancer cells | Lung cancer exosomes initiate global long non-coding RNA changes in mesenchymal stem cells to inhibit MSCs osteogenic and adipogenic differentiation | Tumor exosomes contribute to interactions between MSCs and tumor cells | [61] |
| Lung cancer (NSCLC) | Serum | miR-222-3p; SOCS3 | Gemcitabine- resistant A549 cells | Exosomic miR-222-3p enhances the proliferation, gemcitabine resistance, migration, invasion, and anti-anoikis of parental sensitive cells by directly targeting the promoter of SOCS3 | Enhance proliferation and metastasis | [62] |
| Lung cancer | Serum | Exosomal miR-21 and miR-155 | Nude mouse model with lung cancer | Exosomal miRNAs, miR-21 and miR-155, were significantly upregulated in recurrent tumors compared to primary tumors | Exosomal miRNAs as biomarkers of recurrent lung cancer | [63] |
| Lung cancer | Plasma; Lung cancer cells | Lung cancer cells and lung cancer patients | Including stage I and II cancer patients, plasma exosomes of 90.7% patients had higher similarity to lung cancer cell exosomes than the average of the healthy controls. Such similarity was proportional to the progression of cancer | Similarity between plasma and lung cancer cell relates to progression of cancer | [64] | |
| Lung cancer (NSCLC) | Plasma | Lung cancer patients | Plasma exosome level correlates with tumor stage and may serve as a prognostic factor for NSCLC | Plasma exosome correlate with tumor stage | [65] | |
| Lung cancer (NSCLC) | Plasma | Exosomal Tim-3 and Galectin-9 | Lung cancer patients | High levels of Exo-Tim-3 and Exo-Galectin-9 were all positively correlated with larger tumor size, advanced stages, and more distant metastasis | Correlate with metastasis | [66] |
| Lung cancer (NSCLC) | Serum | Exo-GAS5 | NSCLC patients | Patients with NSCLC with larger tumor size and advanced TNM classification showed lower Exo-GAS5 expression | Exo-GAS5 expression is associated with advanced TNM classification | [67] |
Abbreviations: ABHD5 Abhydrolase domain containing 5, AREG Amphiregulin, COX-2 Cyclooxygenase-2, EGFR Epidermal growth factor receptor, GASS Growth arrest-specific transcript 5, IL Interleukin, MSCs Mesenchymal stem cells, NLRP3 NOD-LRR-and pyrin domain-containing protein 3, NSCLC Non-small cell lung cancer, RANKL Receptor Activator for Nuclear Factor-κ B Ligand, SERS Surface-enhanced Raman scattering, SOCS3 Suppressors-of-cytokine-signaling 3, THP-1 Human myeloid leukemia mononuclear cells, TNM T: extent of the primary tumor; N: lymph node involvement; M: metastatic disease, TRIM59 Tripartite motif-containing 59
Exosomes derived from lung cancer cells secrete TRIM59 and transform macrophages into tumor-promoting macrophages by regulating ABHD5 proteasome degradation, which activates the NLRP3 inflammasome signaling pathway and promotes the progression of lung cancer by secreting IL-1 [58]. NSCLC-derived exosomes containing amphiregulin (AREG) induce activation of the EGFR pathway in preosteoclast cells, which in turn leads to increased RANKL expression and induced expression of proteolytic enzymes that are known as markers of osteoclast formation, triggering a vicious cycle of osteolytic bone metastasis [59].
Lung cancer cells communicate with neighboring cells via exosomes. For instance, exosomes of lung cancer cells contain COX-2. Celecoxib induces COX-2 expression in lung cancer cells, and high expression of COX-2 in exosomes can be transferred to other cells, thereby participating in interactions with neighboring cells [60]. Previous studies have shown that exosomes derived from lung cancer cells inhibit osteogenic and adipogenic differentiation of MSCs, and lncRNAs in exosomes are involved in the regulation of MSC characteristics [61]. These findings bring new insights into the mechanisms of interaction between tumor cell exosomes and environmental components of MSCs. Exosomes from gemcitabine-resistant cells of the NSCLC cell line A549 contain miR-222-3p, which targets SOCS3 as a major regulator of gemcitabine resistance and malignancy characteristics. Exosomal miR-222-3p promotes tumor progression by directly targeting the SOCS3 promoter to enhance proliferation, gemcitabine resistance, migration, and invasion of parental sensitive cells [62]. Thus, exosomal miR-222-3p in serum may be a potential prognostic biomarker for gemcitabine sensitivity in patients with NSCLC.
In a related in vivo study using lung cancer H1299 cells, it was found that levels of miR-21 and miR-155 were significantly higher in relapsed tumors than in primary tumors of nude mouse models of subcutaneous xenotransplantation of primary and recurrent lung cancers. In fact, exosomal miRNA signatures may reflect the true pathological features of lung cancer [63]. Therefore, exosomal miRNAs may be used as biomarkers for noninvasive diagnosis of this disease. In a study of plasma-derived exosomes of 43 patients with stage I and II lung cancer evaluated by surface-enhanced Raman spectroscopy and exploration of the characteristics of exosomes of normal and lung cancer cell lines using deep learning, it was found that 90.7% of patient plasma-derived exosomes had higher similarity with those of lung cancer cells. Furthermore, this similarity was in direct proportion to cancer progression [64]. These results show the considerable potential of combining exosome analysis with deep learning as a liquid biopsy method for early and advanced lung cancer diagnosis and monitoring.
In an association study to determine whether there was a relationship between plasma exosome level and clinical characteristics and prognosis in 208 patients with NSCLC, it was found that exosome levels were significantly associated with tumor stage. Cox proportional risk analysis showed that higher exosome levels were independently associated with poorer overall survival [65]. High levels of exosomal Tim-3 and galectin 9 (also known as LGALS9) in plasma exosomes from patients with lung cancer were positively correlated with tumor size, advanced stage, lymph node metastasis, and distant metastasis. Further, plasma levels of exosomal Tim-3 and LGALS9 are higher in patients with lung SCC than in patients with lung ADC [66]. Additionally, exosomal GAS5 expression was downregulated in the serum of patients with NSCLC, and those patients with large tumors and advanced TNM stage showed lower levels of exosomal GAS5 expression [67]. Thus, exosomal Tim-3, LGALS9, and GAS5 may be ideal noninvasive serum markers to identify patients with early and advanced lung cancer, or even therapeutic targets.
Discussion
As an important information carrier between cells, exosomes participate in many physiologically important processes of the body. However, they also have a role in pathological conditions. In addition to physiological functions such as cell communication and signal transmission, exosomes also play a role in the regulation of local microenvironment by tumor cells. Tumor metastasis is a complex multi-step process involving cancer cell invasion, vascular survival, attachment and host organ colonization. Study confirmed that tumor-derived exosomes promote tumor metastasis by promoting EMT of tumor cells, inducing angiogenesis, the establishment of a premetastatic microenvironment, immune escape, the formation of a metastatic niche, and organ propensity to guide tumor metastasis [68]. Exosomes affect every step of the tumor metastasis cascade and can be targeted by tumor therapy. Thus, exosomes provide a new direction for researchers to study the mechanism of tumor metastasis and clinical translational research.
Tumor-derived exosomes is the main mechanism of intracellular communication between tumor cells and host cells, enabling tumor cells to adapt to their surroundings to favor an optimal microenvironment for tumor initiation and progression. And tumor-derived exosomes contains a large number of different immune-activating and immunosuppressive factors to support the cellular programming of host cells. Tumor-derived exosomes can also drive metastasis by creating a pre-metastatic niche and directing tumor cells to future metastatic sites [69]. Although exosomes have an important role in the occurrence and development of lung cancer and much exploratory research has been performed on exosomes as an important part of the tumor microenvironment in the context of early diagnosis and treatment of lung cancer, the specific mechanisms of exosomes in tumor evolution remain unclear [70]. There are still many questions of the mechanisms about tumor-derived exosomes and cancer metastasis need to be clarified. Further, the sensitivity and specificity of exosomes in the diagnosis and treatment of lung cancer remain to be improved. Therefore, greater attention to the mechanisms underlying exosomes in tumor progression and efforts in translational medicine research will provide a richer basis and support for a clinical role of exosomes in the early diagnosis and treatment of lung cancer [71].
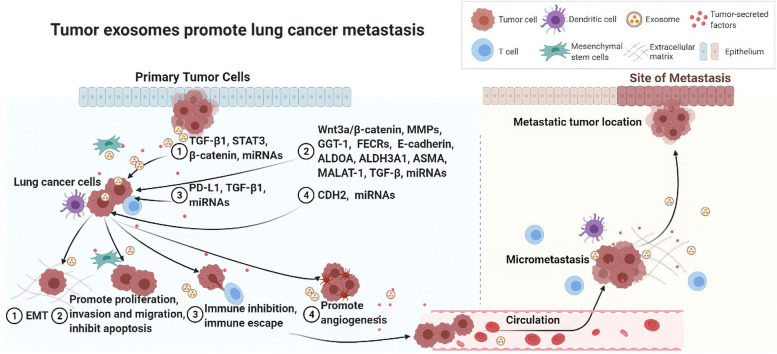
This review provides a systematic summary of the current understanding of tumor-derived exosomes, especially lung cancer exosomes in lung cancer metastasis. The studies in this article describe the influence of lung cancer-associated exosomes that range from promoting cellular EMT and exerting an effect on lung cancer metastasis through regulating cell proliferation, apoptosis, and migration to regulating immune function and angiogenesis; activating inflammation-related pathways or inducing tumor metastasis signaling pathways; and interactions with neighboring cells to promote tumor progression (mainly summarized in Fig. 2). These studies are mainly the results of in vitro studies on the interactions between cells through exosomes, but they also include a small number of in vivo studies using animal models and analyses of clinical cases. Importantly, these studies have identified a number of genes of high research significance toward exosomes and lung cancer development, such as ZEB1, TGF-β1, KLF9, LMO7, ASMA, S100A16, MALAT-1, PD-L1, EGFR, sFlt-1, Tim-3, and LGALS9. Additionally, other important genes that are regulated by exosomes and participate in invasion and metastasis of lung cancer were identified, including those encoding MMPs such as MMP-1 and MMP-9, N-cadherin, and Vimentin.
Fig. 2.
Tumor-derived exosomes promote lung cancer metastasis. Tumor-derived exosomes, especially lung cancer exosomes participate in lung cancer metastasis by promoting cellular epithelial–mesenchymal transformation (EMT); by regulating cell proliferation, apoptosis, and migration; by regulating immune function and angiogenesis; by activating inflammation-related pathways or inducing tumor metastasis signaling pathways; and by interacting with neighboring cells to promote lung cancer progression
Among the transcripts of regulatory genes contained in these exosomes, exosomal miRNAs are key regulatory factors of gene expression that participate in cell–cell communication in the tumor microenvironment, mediate immune escape, regulate drug resistance, and promote tumor cell metastasis and angiogenesis, thereby affecting the occurrence and development of lung cancer [72]. Targeting tumor-specific exosomal miRNAs provides a new strategy for the clinical treatment of lung cancer metastases [73]. Additionally, as potential noninvasive biomarkers, exosomal miRNAs would be of clinical value in the early diagnosis of lung cancer metastasis [74]. In fact, the involvement of exosomal miRNAs in the occurrence and progression of tumors brings new opportunities for clinical diagnosis and treatment of lung cancer [75]. However, because of the early stage of research in this field, the clinical application of exosomal miRNAs in lung cancer is still in the preliminary stage of exploration. The exosomal miRNAs described in this review as key regulatory genes for lung cancer metastasis include miR-193a-3p, miR-210-3p, miR-5100, miR-326, miR-135b, miR-210, miR-660-5p, miR-96, miR-23a, miR-21, miR126, and miR-135b. Despite the potential of exosomal miRNAs as diagnostic markers of lung cancer metastasis and potential new targets for treatment, because of their wide variety, the potential regulatory mechanisms in the occurrence and development of lung cancer warrant further research.
Exosomes are enriched in circulating body fluids. The continuous development of liquid biopsy technology accelerates the clinical application of exosomes, and exosome contents are expected to become the next generation of emerging biomarkers [76]. Although research on lung cancer exosomes has made a breakthrough, controversies and challenges remain. For instance, exosomes can inhibit tumor metastasis in lung cancer [77]. Thus, they may play a contradictory dual role that needs to be better understood by further studies. Additionally, exosomes are involved in multiple steps and links in the process of lung cancer metastasis, but the most critical and core links need to be determined. Further extensive systematic and in-depth studies are also needed to determine whether differential expression of exosome contents can be monitored and followed up, leading to effective clinical intervention before tumor metastasis occurs.
Conclusions
Collectively, exosomes exist as a key pathway of information transmission in tumor cells. At present, positive results have been achieved in studies toward understanding the mechanisms of exosomes in lung cancer metastasis; however, in-depth and systematic studies are still lacking. There are still many questions to be clarified regarding the mechanism of tumor-derived exosomes and lung cancer metastasis. For example, how tumor-derived exosomes and lung cancer cells or host cells recognize each other and how vesicle contents are selected to play a role in regulating tumor metastasis. Which is more influential in metastatic tumors than gene signals from exosomes or gene signals expressed by metastatic tumor cells, etc. Additional exploration is needed to broaden the existing limited information known regarding exosomes and lung, and continued efforts in basic and clinical translational research on exosomes and lung cancer metastasis should be intensified. These future studies will provide stronger guidance for the diagnosis and treatment of lung cancer in the future, especially the diagnosis and clinical treatment of lung cancer metastatic progression, and ensure more positive breakthroughs.
Acknowledgements
None.
Authors’ contributions
C.Y.J. conceived and designed this review writing. C.Y.J. and N.Z. mainly presented all tables. C.Y.J. presented the figure. All the authors participated writing the paper. All authors reviewed and agreed the manuscript.
Funding
This work was supported by the Natural Science Foundation of Tianjin-Municipal Science and Technology Commission (CN) (Grant Number: 20JCYBJC01030) and Social Development Science and Technology Project of TaiZhou City (Grant Number: 21ywb99).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors are approved the manuscript and consent for publication.
Competing interests
There are no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunyang Jiang, Email: chunyangjiang@126.com.
Hongyan Wang, Email: tiger_wt@sina.com.
References
- 1.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 2.Yu Z, Zhao S, Ren L, Wang L, Chen Z, Hoffman RM, Zhou J. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017;8(38):63461–63483. doi: 10.18632/oncotarget.18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Lv T, Zhang Q, Zhu Q, Zhan P, Zhu S, Zhang J, Song Y. The biology, function and clinical implications of exosomes in lung cancer. Cancer Lett. 2017;407:84–92. doi: 10.1016/j.canlet.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Aghabozorgi AS, Ahangari N, Eftekhaari TE, Torbati PN, Bahiraee A, Ebrahimi R, Pasdar A. Circulating exosomal miRNAs in cardiovascular disease pathogenesis: new emerging hopes. J Cell Physiol. 2019;234(12):21796–21809. doi: 10.1002/jcp.28942. [DOI] [PubMed] [Google Scholar]
- 5.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91(4):431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoda BB, Ajit SK. Modulation of immune responses by exosomes derived from antigen-presenting cells. Clin Med Insights Pathol. 2016;9(Suppl 1):1–8. doi: 10.4137/CPath.S39925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu M, Wang G, Hu W, Yao Y, Yu XF. Emerging roles and therapeutic value of exosomes in cancer metastasis. Mol Cancer. 2019;18(1):53. doi: 10.1186/s12943-019-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta. 2019;1871(2):455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar A, Deep G. Exosomes in hypoxia-induced remodeling of the tumor microenvironment - ScienceDirect. Cancer Lett. 2020;488:1–8. doi: 10.1016/j.canlet.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. doi: 10.1016/j.actbio.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Lee S, Shin E, Seong KM, Jin YW, Youn H, Youn B. The emerging roles of exosomes as EMT regulators in cancer. Cells. 2020;9(4):861. doi: 10.3390/cells9040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L. Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):1–14. doi: 10.1038/s41467-017-02088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Li D, Shen L, Hu D, Tang B, Guo W, Wang Z, Zhang Z, Wei G, He D. Exosomes derived from Piwil2induced cancer stem cells transform fibroblasts into cancerassociated fibroblasts. Oncol Rep. 2020;43(4):1125–1132. doi: 10.3892/or.2020.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olejarz W, Dominiak A, Żołnierzak A, Kubiak-Tomaszewska G, Lorenc T. Tumor-derived exosomes in immunosuppression and immunotherapy. J Immunol Res. 2020;2020(1):6272498. doi: 10.1155/2020/6272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobb RJ, Lima LG, Möller A. Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol. 2017;67:3–10. doi: 10.1016/j.semcdb.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, Song X, Liu L, Niu L, Wang X, Song X, Xie L. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. 2018;109(5):1701–1709. doi: 10.1111/cas.13581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan T, Sun N, He J. Exosome-derived LncRNAs in lung cancer. Front Oncol. 2020;10:1728. doi: 10.3389/fonc.2020.01728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C, Meiners S, Lukas C, Stathopoulos GT, Chen J. Role of exosomal microRNAs in lung cancer biology and clinical applications. Cell Prolif. 2020;53(6):e12828. doi: 10.1111/cpr.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You J, Li M, Cao LM, Gu QH, Deng PB, Tan Y, Hu CP. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM. 2019;112(8):581–590. doi: 10.1093/qjmed/hcz093. [DOI] [PubMed] [Google Scholar]
- 20.Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Möller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. 2017;141(3):614–620. doi: 10.1002/ijc.30752. [DOI] [PubMed] [Google Scholar]
- 21.Kim E, Kim W, Lee S, Chun J, Kang J, Park G, Han I, Yang HJ, Youn H, Youn B. TRAF4 promotes lung cancer aggressiveness by modulating tumor microenvironment in normal fibroblasts. Sci Rep. 2017;7(1):8923. doi: 10.1038/s41598-017-09447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Wu X, Qian M, Song Y, Wu D, Zhang W. Knockdown of TGF-β1 expression in human umbilical cord mesenchymal stem cells reverts their exosome-mediated EMT promoting effect on lung cancer cells. Cancer Lett. 2018;428:34–44. doi: 10.1016/j.canlet.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Tang YT, Huang YY, Li JH, Qin SH, Xu Y, An TX, Liu CC, Wang Q, Zheng L. Alterations in exosomal miRNA profile upon epithelial-mesenchymal transition in human lung cancer cell lines. BMC Genomics. 2018;19(1):802. doi: 10.1186/s12864-018-5143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, Li G, Tang J, Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18(1):40. doi: 10.1186/s12943-019-0959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, He J, Hu H, Tu L, Sun Z, Liu Y, Luo F. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell Mol Med. 2020;24(11):6324–6339. doi: 10.1111/jcmm.15274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman MA, Barger JF, Lovat F, Gao M, Otterson GA, Nana-Sinkam P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget. 2016;7(34):54852–54866. doi: 10.18632/oncotarget.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Jiao X, Wu Y, Li S, Cao L, Dong L. Exosomes derived from PM2.5-treated lung cancer cells promote the growth of lung cancer via the Wnt3a/β-catenin pathway. Oncol Rep. 2019;41(2):1180–1188. doi: 10.3892/or.2018.6862. [DOI] [PubMed] [Google Scholar]
- 28.Zhang N, Nan A, Chen L, Li X, Jia Y, Qiu M, Dai X, Zhou H, Zhu J, Zhang H, Jiang Y. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol Cancer. 2020;19(1):101. doi: 10.1186/s12943-020-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Xu R, Xia J, Huang J, Su B, Wang S. Aspirin inhibits hypoxia-mediated lung cancer cell stemness and exosome function. Pathol Res Pract. 2019;215(6):152379. doi: 10.1016/j.prp.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Qi Y, Zha W, Zhang W. Exosomal miR-660-5p promotes tumor growth and metastasis in non-small cell lung cancer. J BUON. 2019;24(2):599–607. [PubMed] [Google Scholar]
- 31.Wu H, Zhou J, Mei S, Wu D, Mu Z, Chen B, Xie Y, Ye Y, Liu J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J Cell Mol Med. 2017;21(6):1228–1236. doi: 10.1111/jcmm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108(1):42–52. doi: 10.1111/cas.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Yang Y, Huang C, Cao P, Wu Q, Chen S, Chen F. Human THP-1 macrophages activated by exosomes derived from lung adenocarcinoma cells promote lung cancer cell invasion. Chin J Cell Mol Immunol. 2019;35(11):967–972. [PubMed] [Google Scholar]
- 34.Lukic A, Wahlund CJE, Gómez C, Brodin D, Samuelsson B, Wheelock CE, Gabrielsson S, Rådmark O. Exosomes and cells from lung cancer pleural exudates transform LTC4 to LTD4, promoting cell migration and survival via CysLT1. Cancer Lett. 2019;444:1–8. doi: 10.1016/j.canlet.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu Z, Li S, Wang M, Dai D, Jing H, Liu L. Upregulation of E-cadherin in bronchoalveolar lavage fluid-derived exosomes in patients with lung cancer. Thorac Cancer. 2020;11(1):41–47. doi: 10.1111/1759-7714.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Li W, Chen N, Zhao H, Xu G, Zhao Y, Pan X, Zhang X, Zhou L, Yu D, Li A, Hu JF, Cui J. FLI1 exonic circular RNAs as a novel oncogenic driver to promote tumor metastasis in small cell lung cancer. Clin Cancer Res. 2019;25(4):1302–1317. doi: 10.1158/1078-0432.CCR-18-1447. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Chen H, Xu C, Zhang Y, Zhang Q, Chen L, Ding Q, Deng Z. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020;244:117297. doi: 10.1016/j.lfs.2020.117297. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Xu J, Yuan D, Bai Y, Pan Y, Zhang J, Shao C. Exosomes carrying ALDOA and ALDH3A1 from irradiated lung cancer cells enhance migration and invasion of recipients by accelerating glycolysis. Mol Cell Biochem. 2020;469(1–2):77–87. doi: 10.1007/s11010-020-03729-3. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Wu Y, Guo J, Fei X, Yu L, Ma S. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget. 2017;8(47):81880–81891. doi: 10.18632/oncotarget.18737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Ding Z, Luo Q, Xu W. Cancer cell-derived exosomes promote cell proliferation and inhibit cell apoptosis of both normal lung fibroblasts and non-small cell lung cancer cell through delivering alpha-smooth muscle actin. Am J Transl Res. 2019;11(3):1711–1723. [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang R, Xia Y, Wang Z, Zheng J, Chen Y, Li X, Wang Y, Ming H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem Biophys Res Commun. 2017;490(2):406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 42.Xu ZH, Miao ZW, Jiang QZ, Gan DX, Wei XG, Xue XZ, Li JQ, Zheng F, Qin XX, Fang WG, Chen YH, Li B. Brain microvascular endothelial cell exosome-mediated S100A16 up-regulation confers small-cell lung cancer cell survival in brain. FASEB J. 2019;33(2):1742–1757. doi: 10.1096/fj.201800428R. [DOI] [PubMed] [Google Scholar]
- 43.Duan S, Yu S, Yuan T, Yao S, Zhang L. Exogenous let-7a-5p induces A549 lung cancer cell death through BCL2L1-mediated PI3Kγ signaling pathway. Front Oncol. 2019;9:808. doi: 10.3389/fonc.2019.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu DM, Deng SH, Liu T, Han R, Zhang T, Xu Y. TGF-β-mediated exosomal lnc-MMP2-2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Med. 2018;7(10):5118–5129. doi: 10.1002/cam4.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Yi J, Chen X, Zhang Y, Xu M, Yang Z. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-β and IL-10. Oncol Lett. 2016;11(2):1527–1530. doi: 10.3892/ol.2015.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang WT, Chong IW, Chen HL, Li CY, Hsieh CC, Kuo HF, Chang CY, Chen YH, Liu YP, Lu CY, Liu YR, Liu PL. Pigment epithelium-derived factor inhibits lung cancer migration and invasion by upregulating exosomal thrombospondin 1. Cancer Lett. 2019;442:287–298. doi: 10.1016/j.canlet.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 47.Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, Sung YH, Pack CG, Jung MK, Han B, Kim K, Kim WS, Nam SJ, Choi CM, Yun M, Lee JC, Rho JK. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51(8):1–13. doi: 10.1038/s12276-019-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y, Pan J, Qi C. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36–43. doi: 10.1016/j.imlet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109(31):E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, Chouaib S. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. Oncoimmunology. 2015;5(4):e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest. 2013;31(5):330–335. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 52.Nie H, Xie X, Zhang D, Zhou Y, Li B, Li F, Li F, Cheng Y, Mei H, Meng H, Jia L. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale. 2020;12(2):877–887. doi: 10.1039/C9NR09011H. [DOI] [PubMed] [Google Scholar]
- 53.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH, Wu CY, Kuo PL. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36(34):4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Luo F, Wang B, Li H, Xu Y, Liu X, Shi L, Lu X, Xu W, Lu L, Qin Y, Xiang Q, Liu Q. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370(1):125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Grimolizzi F, Monaco F, Leoni F, Bracci M, Staffolani S, Bersaglieri C, Gaetani S, Valentino M, Amati M, Rubini C, Saccucci F, Neuzil J, Tomasetti M, Santarelli L. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci Rep. 2017;7(1):15277. doi: 10.1038/s41598-017-15475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao D, Li Y, Zhao G, Zhang M. Soluble fms-like tyrosine kinase-1-enriched exosomes suppress the growth of small cell lung cancer by inhibiting endothelial cell migration. Thorac Cancer. 2019;10(10):1962–1972. doi: 10.1111/1759-7714.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhuo H, Zhao Y, Cheng X, Xu M, Wang L, Lin L, Lyu Z, Hong X, Cai J. Tumor endothelial cell-derived cadherin-2 promotes angiogenesis and has prognostic significance for lung adenocarcinoma. Mol Cancer. 2019;18(1):34. doi: 10.1186/s12943-019-0987-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang M, Chen X, Wang L, Qin L, Wang H, Sun Z, Zhao W, Geng B. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. 2020;39(1):176. doi: 10.1186/s13046-020-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taverna S, Pucci M, Giallombardo M, Di Bella MA, Santarpia M, Reclusa P, Gil-Bazo I, Rolfo C, Alessandro R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep. 2017;7(1):3170. doi: 10.1038/s41598-017-03460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J, Hong SW, Kim S, Kim D, Hur DY, Jin DH, Kim B, Kim YS. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int J Oncol. 2018;52(2):613–620. doi: 10.3892/ijo.2017.4227. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Li X, Zhu R, Han Q, Zhao RC. Lung cancer exosomes initiate global long non-coding RNA changes in mesenchymal stem cells. Int J Oncol. 2016;48(2):681–689. doi: 10.3892/ijo.2015.3272. [DOI] [PubMed] [Google Scholar]
- 62.Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, Ding L, Zhang Y, Zhang L, Li N, Li Y, Liu Y. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017;16(1):132. doi: 10.1186/s12943-017-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munagala R, Aqil F, Gupta RC. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumor Biology. 2016;37(8):10703–10714. doi: 10.1007/s13277-016-4939-8. [DOI] [PubMed] [Google Scholar]
- 64.Shin H, Oh S, Hong S, Kang M, Kang D, Ji YG, Choi BH, Kang KW, Jeong H, Park Y, Hong S, Kim HK, Choi Y. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. 2020;14(5):5435–5444. doi: 10.1021/acsnano.9b09119. [DOI] [PubMed] [Google Scholar]
- 65.Liu Q, Xiang Y, Yuan S, Xie W, Li C, Hu Z, Wu N, Wu L, Yu Z, Bai L, Li Y. Plasma exosome levels in non-small-cell lung cancer: correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018;22(2):267–274. doi: 10.3233/CBM-170955. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Qiu X, Li X, Fan H, Zhang F, Lv T, Song Y. Expression profiles and clinical value of plasma exosomal Tim-3 and Galectin-9 in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;498(3):409–415. doi: 10.1016/j.bbrc.2018.02.114. [DOI] [PubMed] [Google Scholar]
- 67.Li C, Lv Y, Shao C, Chen C, Zhang T, Wei Y, Fan H, Lv T, Liu H, Song Y. Tumor-derived exosomal lncRNA GAS5 as a biomarker for early-stage non-small-cell lung cancer diagnosis. J Cell Physiol. 2019;234(11):20721–20727. doi: 10.1002/jcp.28678. [DOI] [PubMed] [Google Scholar]
- 68.Weidle UH, Birzele F, Kollmorgen G, Rüger R. The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics. 2017;14(1):1–15. doi: 10.21873/cgp.20015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alipoor SD, Mortaz E, Varahram M, Movassaghi M, Kraneveld AD, Garssen J, Adcock IM. The potential biomarkers and immunological effects of tumor-derived exosomes in lung cancer. Front Immunol. 2018;9:819. doi: 10.3389/fimmu.2018.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu K, Zhang C, Du T, Gabriel ANA, Wang X, Li X, Sun L, Wang N, Jiang X, Zhang Y. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134:111111. doi: 10.1016/j.biopha.2020.111111. [DOI] [PubMed] [Google Scholar]
- 71.Masaoutis C, Mihailidou C, Tsourouflis G, Theocharis S. Exosomes in lung cancer diagnosis and treatment. From the translating research into future clinical practice. Biochimie. 2018;151:27–36. doi: 10.1016/j.biochi.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Yin Z, Fan J, Zhang S, Yang W. The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;13(4):47. doi: 10.1038/s41392-019-0080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanni I, Alama A, Grossi F, Dal Bello MG, Coco S. Exosomes: a new horizon in lung cancer. Drug Discov Today. 2017;22(6):927-36. [DOI] [PubMed]
- 74.Chen R, Xu X, Qian Z, Zhang C, Niu Y, Wang Z, Sun J, Zhang X, Yu Y. The biological functions and clinical applications of exosomes in lung cancer. Cell Mol Life Sci. 2019;76(23):4613-33. [DOI] [PMC free article] [PubMed]
- 75.Amiri A, Pourhanifeh MH, Mirzaei HR, Nahand JS, Moghoofei M, Sahebnasagh R, Mirzaei H, Hamblin MR. Exosomes and lung cancer: roles in pathophysiology, diagnosis and therapeutic applications. Curr Med Chem. 2021;28(2):308–328. doi: 10.2174/0929867327666200204141952. [DOI] [PubMed] [Google Scholar]
- 76.Fortunato O, Gasparini P, Boeri M, Sozzi G. Exo-miRNAs as a new tool for liquid biopsy in lung cancer. Cancers. 2019;11(6):888. doi: 10.3390/cancers11060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu S, Zheng L, Kang L, Xu H, Gao L. microRNA-let-7e in serum-derived exosomes inhibits the metastasis of non-small-cell lung cancer in a SUV39H2/LSD1/CDH1-dependent manner. Cancer Gene Ther. 2021;28(3-4):250-64. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.