Abstract
Purpose:
We sought to evaluate whether provider volume or other factors are associated with chemotherapy guideline compliance in elderly patients with epithelial ovarian cancer (EOC).
Methods:
We queried the SEER-Medicare database for patients ≥66 years, diagnosed with FIGO stage II-IV EOC from 2004–2013 who underwent surgery and received chemotherapy within 7 months of diagnosis. We compared NCCN guideline compliance (6 cycles of platinum-based doublet) and chemotherapy-related toxicities across provider volume tertiles. Factors associated with guideline compliance and chemotherapy-related toxicities were assessed using logistic regression. Overall survival (OS) was compared across volume tertiles and Cox proportional-hazards model was created to adjust for case-mix.
Results:
1,924 patients met inclusion criteria. The overall rate of guideline compliance was 70.3% with a significant association between provider volume and compliance (64.5% for low-volume, 72.2% for medium-volume, 71.7% for high-volume, p=0.02). In the multivariate model, treatment by low-volume providers and patient age ≥80 years were independently associated with worse chemotherapy-guideline compliance. In the survival analysis, there was a significant difference in median OS across provider volume tertiles with median survival of 32.8 months (95%CI 29.6, 36.4) low-volume, 41.9 months (95%CI 37.5, 46.7) medium-volume, 42.1 months (95%CI 38.8, 44.2) high-volume providers, respectively (p<0.01). After adjusting for case-mix, low-volume providers were independently associated with higher rates of mortality (aHR 1.25, 95%CI: 1.08, 1.43).
Conclusions:
In a modern cohort of elderly Medicare patients with advanced EOC, we found higher rates of non-compliant care and worse survival associated with treatment by low-volume Medicare providers. Urgent efforts are needed to address this volume-outcomes disparity.
Keywords: Ovarian cancer, Treatment outcomes, Chemotherapy, Guideline compliance, Provider volume
INTRODUCTION
For the approximately 22,000 cases of epithelial ovarian cancer (EOC) diagnosed each year, the National Comprehensive Cancer Network (NCCN) recommends treatment with a combination of surgical debulking and 6–8 cycles of a platinum-containing chemotherapy regimen, most commonly carboplatin and paclitaxel. With guideline-compliant surgery and chemotherapy, approximately 35% of patients will be alive after 5 years [1].
Several recent drug trials involving bevacizumab and poly ADP-ribose polymerase (PARP) inhibitors have shown benefit when these agents are added to standard chemotherapy in the front-line treatment of patients with advanced EOC [2–4]. Recent FDA approvals of drugs including niraparib alone and olaparib plus bevacizumab are expected to shift the front-line treatment paradigm from universal platinum-doublet administration to nuanced clinical- and molecular genetics-guided treatment. While the addition of bevacizumab has resulted in a modest overall survival (OS) benefit in high-risk subgroups, PARP inhibitor maintenance therapy trials show an unprecedented progression-free survival (PFS) benefit in patients with germline or somatic BRCA mutations, making adoption and guideline compliance more important now than ever before.
Many studies have demonstrated that patients with ovarian cancer who receive care from high-volume providers or at high-volume centers are more likely to receive guideline-recommended care and have improved outcomes [1, 5–11]. However, most of these studies focus on surgical quality, and either omit or incompletely report chemotherapy-related process measures.
Given recent advances in the front-line treatment of advanced EOC and the absence of modern literature focusing specifically on chemotherapy guideline compliance, we sought to examine the association between provider volume and guideline-based care in a modern cohort of elderly Medicare patients.
METHODS
Approval to conduct this research was obtained from the Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB-A X16–044).
Data source and patient selection
We utilized the Surveillance, Epidemiology, and End Results (SEER) registry linked to Medicare claims to identify primary cases of ovarian cancer that were diagnosed between 2004 and 2013. The SEER cancer registry includes incident cancer cases representing almost 30% of the U.S. population. This registry collects information pertaining to site and extent of disease, sociodemographic characteristics, and survival. Patients aged 65 and older who are living in SEER registry regions have linked Medicare billing claims, which provides additional data regarding healthcare utilization such as medical provider visit information, patient comorbidities, chemotherapies administered, surgical procedures performed, and chemotherapy-associated toxicities. International Classification of Diseases, 9th revision (ICD-9), Current Procedural Terminology (CPT), and Healthcare Common Procedure Coding System (HCPCS) codes were used to extract data from the Medicare inpatient, outpatient, and physician claims files [12].
Of the 27,866 patients with ovarian cancer (ICD-O-3 site code C56.9), we included those who were diagnosed at age 66 or older to allow for a 1-year look-back period for comorbidities (n=8,982 excluded). We excluded patients with unknown date of diagnosis (n=173), non-epithelial histology (n=6,260), less than stage II disease (8,127), low-grade disease (n=13,376), or a diagnosis made on death certificate or after death (n=1,154).
Of the 6,401 patients who met the initial inclusion criteria, we included only those whose surgical management did not deviate from standard of care, so that chemotherapy-specific outcomes could be compared. Accordingly, those who never underwent an oophorectomy with omentectomy, lymph node dissection, or debulking surgery (n=2,788), or who underwent a interval or primary debulking surgery more than 7 months following diagnosis (n=97), were excluded (Supplementary Table 1).
Patients with incomplete Medicare fee-for-service coverage from 1 year pre-diagnosis through 8 months post-surgery, or who were enrolled in an HMO during this time, were excluded (n=1,102). Additional exclusions were patients with no chemotherapy claims through 7 months following surgery (n=351), patients whose plurality chemotherapy provider was not identifiable (n=74), and patients who went to a provider with Medicare patient volume exceeding 8,000 patients annually (which is thought likely to represent a coding error in the provider identification number) (n=65).
The final cohort included 1,924 patients with primary EOC diagnosed between 2004–2013 who underwent primary or interval debulking surgery within 7 months of diagnosis, and received chemotherapy within 7 months of surgery. For the 1,924 patients included in the analysis, SEER registry files were used to obtain demographic and clinical data including age (66–69, 70–74, 75–79, 80–84, ≥80 years), stage of disease (II, III, IV), grade (2 or 3), histology (serous, mucinous, endometrioid, clear cell, or other adenocarcinoma), marital status (married, not married/unknown), race/ethnicity (white, black, other), geographic region (Northeast, Midwest, South, West), and census tract median income (unknown, Quartiles 1, 2, 3 and 4). Stage of disease was determined based on American Joint Committee on Cancer (AJCC) criteria, which was consistent with International Federation of Gynecology and Obstetrics (FIGO) 2009 classifications. Residence was classified as metropolitan (metro), non-metropolitan (non-metro), or unknown. Comorbidity status was described using the Charlson comorbidity score (0, 1, ≥2) [13].
Defining primary chemotherapy provider
The provider administering a plurality of a patient’s chemotherapy was defined as the provider who administered the largest percentage of chemotherapy in a physician office or outpatient setting from the time of diagnosis through 7 months following surgery. The Unique Physician Identification Number (UPIN)-National Provider Identifier (NPI) crosswalk for Medicare data was utilized to uniquely identify providers across the two physician identification systems [14].
Defining provider volume
Provider volume was defined as the average annual number of patients with ovarian cancer who were seen between 2004 and 2014 by a given provider, only including years in which at least 1 patient was seen. For the volume calculation, all patients diagnosed with ovarian cancer in the database were included regardless of age, stage, grade, histology, duration of Medicare enrollment, and all other previously applied exclusion criteria. Provider volume was then calculated as the average annual volume of Medicare patients with EOC seen between 2004 and 2014, including only years when at least 1 Medicare patient was seen. Providers were categorized into volume-based tertiles and classified as low-, medium-, or high-volume based on tertile distributions. Low volume was defined as an average annual volume of 1–4, medium as a volume of 4–7, and high as a volume of 7–64 patients per year, respectively.
Statistical Analysis
We sought to examine the association between provider volume and NCCN guideline compliance, chemotherapy-related toxicities, and OS. The primary process measure evaluated was chemotherapy-specific NCCN guideline compliance. NCCN guideline compliance was defined as receiving 6 or more cycles of a platinum-containing doublet, which could consist of carboplatin or cisplatin in combination with paclitaxel, docetaxel, cyclophosphamide, gemcitabine, topotecan or doxorubicin. Cycles were defined by unique claim dates for a given chemotherapy. Patients who died within 2 months of receiving less than 6 cycles of chemotherapy were excluded, as they were ineligible to be guideline-compliant (n=61).
The association between provider volume tertile and guideline compliance was first assessed using a Chi-square test. A logistic regression model of guideline compliance adjusting for FIGO stage (II, III, IV), grade (2, 3), age at diagnosis (66–69, 70–74, 75–79, 80–84, 85+ years), marital status (married, not married/unknown), race (white, black, other/unknown), residence (metro, non-metro/unknown), geographic region (Northeast, Midwest, South, West), census tract median income (quartiles), Charlson comorbidity index in the year prior to diagnosis (0, 1, ≥2). Volume grouped in tertiles was created. A sensitivity analyses was performed using volume as a continuous variable. Odds ratios (OR) and 95% confidence intervals (CI) are reported. A sensitivity analysis was conducted including the 61 patients who were ineligible to achieve NCCN guideline compliance (six cycles of a platinum-based doublet), assuming they were not compliant.
The secondary outcome of interest was the occurrence of chemotherapy-related toxicity, requiring a higher level of care (inpatient hospitalization or emergency department visit). Chemotherapy-related toxicities were defined based on previously published reports as an inpatient hospitalization or emergency department visit within 30 days of receiving any chemotherapy for one or more of the following diagnoses: anemia, neutropenia, thrombocytopenia, mucositis, dehydration, nausea, or neuropathy [15]. Previous studies have evaluated chemotherapy-related toxicity as simply having a diagnosis code associated with an outpatient clinic visit. We chose to require an associated inpatient hospitalization or emergency department visit with chemotherapy-related toxicity as the primary discharge diagnosis, or as the secondary discharge diagnosis if the primary diagnosis was cancer.
The association between provider volume tertile and chemotherapy-related toxicity was first assessed using a Chi-square test. A logistic regression model with the same covariates as were used in the chemotherapy compliance model was applied. However, histology was excluded due to sparse cells and lack of clinical relevance.
OS was defined as time from date of diagnosis through death or the end of follow-up. The date of last follow-up was 12/31/2014 in the dataset. OS was estimated using Kaplan-Meier method and compared across volume tertiles using the Log-rank test, first for all patients and then stratified by stage. The median survival in years with a 95% CI is presented. For 2-, 3-, and 5-year survival, the percentage of patients surviving with 95% CI are reported. A Cox Proportional-Hazards model was created to control for possible confounders, and included the same variables used in the logistic regression model for chemotherapy compliance. In the Cox Proportional-Hazards model, histology was collapsed into serous versus non-serous due to sparse cells. A sensitivity analysis was performed, including guideline compliance in the model to determine if there was an independent relationship between volume and survival beyond that driven by chemotherapy-specific guideline compliance. Hazards ratios (HR) and 95% CI are presented. All analyses were computed in SAS v9.4 (Cary, NC).
RESULTS
We identified 1,924 women treated by 1,057 providers, including 411 (21.4%) by low-volume (1–4 average annual patients), 522 (27.1%) by medium-volume (4–7 average annual patients), and 991 (52.0%) by high-volume (7–64 average annual patients) providers, respectively. The majority of patients had Stage III disease (63.3%), serous histology (80.3%), were white (90.1%), lived in metropolitan areas (90.0%), and had a Charlson comorbidity index of 0 (72.2%). When we compared demographic and clinical characteristics across provider volume tertiles, provider volume was only associated with geographic region and Census tract median income (Table 1). Patients in the Midwest were most likely to receive chemotherapy from a high-volume provider (57.7%, n=112), compared to 48.1% in the West (n=435), 52.8% in the Northeast (n=187), and 54.6% in the South (n=257) (p<0.01). Additionally, patients in higher quartiles of Census tract median income were increasingly likely to receive care from high-volume providers (Quartile 1: 47.6% vs. Quartile 4: 56.6%, p=0.05).
Table 1.
Characteristics of patients in cohort by volume of provider of plurality of chemotherapy (n=1,924)
| Volume Categories | ||||
|---|---|---|---|---|
| Characteristics | Low volume n (col %) | Medium volume n (col %) | High volume n (col %) | Chi-square p-value |
| Volume category; Min, Max | 1, 4 | 4, 7 | 7, 64 | |
| Number of patients | 411 | 522 | 991 | |
| Number of providers | 354 | 351 | 352 | |
| Stage | 0.14 | |||
| Stage II | 27 (6.6%) | 39 (7.5%) | 89 (9.0%) | |
| Stage III | 251 (61.1%) | 328 (62.8%) | 640 (64.6%) | |
| Stage IV | 133 (32.4%) | 155 (29.7%) | 262 (26.4%) | |
| Grade | 0.90 | |||
| 2 | 51 (12.4%) | 68 (13.0%) | 132 (13.3%) | |
| 3 | 360 (87.6%) | 454 (87.0%) | 859 (86.7%) | |
| Histology | 0.25 | |||
| Other | 84 (20.4%) | 90 (17.2%) | 205 (20.7%) | |
| Serous | 327 (79.6%) | 432 (82.8%) | 786 (79.3%) | |
| Age at diagnosis (years) | 0.73 | |||
| 66–69 | 119 (29.0%) | 131 (25.1%) | 280 (28.3%) | |
| 70–74 | 129 (31.4%) | 164 (31.4%) | 311 (31.4%) | |
| 75–79 | 98 (23.8%) | 143 (27.4%) | 238 (24.0%) | |
| 80–84 | 53 (12.9%) | 65 (12.5%) | 119 (12.0%) | |
| 85+ | 12 (2.9%) | 19 (3.6%) | 43 (4.3%) | |
| Marital status | 0.45 | |||
| Not married/Unknown | 196 (47.7%) | 266 (51.0%) | 473 (47.7%) | |
| Married | 215 (52.3%) | 256 (49.0%) | 518 (52.3%) | |
| Race | 0.40 | |||
| White | 360 (87.6%) | 470 (90.0%) | 903 (91.1%) | |
| Black | 26 (6.3%) | 27 (5.2%) | 46 (4.6%) | |
| Other | 25 (6.1%) | 25 (4.8%) | 42 (4.2%) | |
| Residence | 0.53 | |||
| Non-metro | 47 (11.4%) | 48 (9.2%) | 102 (10.3%) | |
| Metro | 364 (88.6%) | 474 (90.8%) | 889 (89.7%) | |
| Geographic region | <.01 | |||
| West | 195 (47.4%) | 275 (52.7%) | 435 (43.9%) | |
| Northeast | 76 (18.5%) | 91 (17.4%) | 187 (18.9%) | |
| Midwest | 49 (11.9%) | 33 (6.3%) | 112 (11.3%) | |
| South | 91 (22.1%) | 123 (23.6%) | 257 (25.9%) | |
| Census tract median income quartile | 0.05 | |||
| Quartile 1 | 109 (26.5%) | 136 (26.1%) | 223 (22.5%) | |
| Quartile 2 | 86 (20.9%) | 128 (24.5%) | 239 (24.1%) | |
| Quartile 3 | 107 (26.0%) | 128 (24.5%) | 246 (24.8%) | |
| Quartile 4 | 87 (21.2%) | 110 (21.1%) | 257 (25.9%) | |
| Unknown | 22 (5.4%) | 20 (3.8%) | 26 (2.6%) | |
| Charlson comorbidity index | 0.39 | |||
| 0 | 291 (70.8%) | 367 (70.3%) | 731 (73.8%) | |
| 1 | 74 (18.0%) | 101 (19.3%) | 177 (17.9%) | |
| 2+ | 46 (11.2%) | 54 (10.3%) | 83 (8.4%) | |
| Chemotherapy compliance with NCCN guidelines 1 | 0.02 | |||
| Unknown | 14 | 15 | 32 | |
| Non-compliant | 141 (35.5%) | 141 (27.8%) | 271 (28.3%) | |
| Compliant | 256 (64.5%) | 366 (72.2%) | 688 (71.7%) | |
Excludes 61 patients who died < two months of last chemotherapy claim and prior to 6 cycles being complete
Chemotherapy compliance
Chemotherapy guideline compliance was assessed in 1,863 patients. The overall rate of compliance was 70.3%. There was a significant association between volume and compliance in the univariable analyses. Rates of chemotherapy guideline compliance were different based on provider volume: 64.5% compliance with low-volume providers; 72.1% with medium-volume providers; 71.2% with high-volume providers (P=0.02) (Table 1).
After adjusting for case-mix in the multivariate model, chemotherapy guideline compliance remained significantly associated with volume (Table 2). With high-volume providers as a reference, low-volume providers were significantly less likely to be compliant (aOR 0.71, 95% CI: 0.54–0.94). In the adjusted model, age ≥80 years was also independently associated with chemotherapy guideline compliance (80–84 years: aOR 0.67, 95% CI: 0.47, 0.95; ≥85 years: aOR 0.41, 95% CI: 0.24, 0.71). A sensitivity analysis was performed including the 61 excluded patients, and results were consistent with the primary analyses (Table 2).
Table 2.
Adjusted odds ratios and 95% confidence intervals (CI) for compliance with NCCN guidelines
| Characteristics | Adjusted odds ratio (95% CI) | Sensitivity Analysis |
|---|---|---|
| Provider volume tertile (across years patients were seen) | ||
| High | 1.00 (Reference) | 1.00 (Reference) |
| Low | 0.71 (0.54, 0.94)* | 0.72 (0.55, 0.94)* |
| Medium | 1.02 (0.78, 1.34) | 1.03 (0.79, 1.34) |
| Stage | ||
| Stage II | 1.00 (Reference) | 1.00 (Reference) |
| Stage III | 1.10 (0.75, 1.63) | 1.03 (0.70, 1.52) |
| Stage IV | 1.09 (0.72, 1.66) | 0.92 (0.61, 1.39) |
| Grade | ||
| 2 | 1.00 (Reference) | 1.00 (Reference) |
| 3 | 0.97 (0.71, 1.34) | 0.93 (0.68, 1.28) |
| Histology | ||
| Clear-cell carcinoma | 1.00 (Reference) | 1.00 (Reference) |
| Endometrioid carcinoma | 0.83 (0.33, 2.05) | 0.84 (0.35, 2.01) |
| Mucinous carcinoma | 0.57 (0.15, 2.21) | 0.50 (0.14, 1.80) |
| Other adenocarcinoma | 1.14 (0.49, 2.66) | 1.22 (0.54, 2.73) |
| Serous carcinoma | 1.34 (0.60, 2.96) | 1.42 (0.66, 3.04) |
| Age at diagnosis (years) | ||
| 66–69 | 1.00 (Reference) | 1.00 (Reference) |
| 70–74 | 1.22 (0.92, 1.63) | 1.20 (0.91, 1.58) |
| 75–79 | 0.88 (0.65, 1.18) | 0.85 (0.64, 1.12) |
| 80–84 | 0.67 (0.47, 0.95)* | 0.63 (0.44, 0.88)* |
| ≥85 | 0.41 (0.24, 0.71)* | 0.38 (0.23, 0.65)* |
| Marital status | ||
| Married | 1.00 (Reference) | 1.00 (Reference) |
| Not married/Unk | 0.81 (0.65, 1.01) | 0.78 (0.63, 0.97)* |
| Race | ||
| Black | 1.00 (Reference) | 1.00 (Reference) |
| Other | 1.31 (0.66, 2.60) | 1.28 (0.66, 2.50) |
| White | 1.34 (0.82, 2.20) | 1.24 (0.77, 2.01) |
| Residence | ||
| Metro | 1.00 (Reference) | 1.00 (Reference) |
| Non-metro | 0.76 (0.52, 1.12) | 0.70 (0.49, 1.02) |
| Geographic region | ||
| Midwest | 1.00 (Reference) | 1.00 (Reference) |
| Northeast | 1.07 (0.68, 1.68) | 1.01 (0.65, 1.58) |
| South | 1.36 (0.89, 2.09) | 1.23 (0.81, 1.88) |
| West | 1.16 (0.77, 1.73) | 1.07 (0.72, 1.59) |
| Census tract median income quartile | ||
| Quartile 1 | 1.00 (Reference) | 1.00 (Reference) |
| Quartile 2 | 0.90 (0.65, 1.24) | 0.92 (0.67, 1.25) |
| Quartile 3 | 0.88 (0.63, 1.23) | 0.93 (0.67, 1.28) |
| Quartile 4 | 0.96 (0.67, 1.36) | 1.04 (0.74, 1.47) |
| Unknown | 0.70 (0.38, 1.30) | 0.74 (0.41, 1.35) |
| Charlson comorbidity index | ||
| 0 | 1.00 (Reference) | 1.00 (Reference) |
| 1 | 0.86 (0.65, 1.13) | 0.84 (0.64, 1.10) |
| ≥2 | 0.81 (0.56, 1.17) | 0.75 (0.53, 1.07) |
p-value <0.05
An additional sensitivity analysis was performed to examine the association between continuous average annual provider volume and chemotherapy guideline compliance. This analysis showed that directionally, the trend towards improved guideline compliance with increasing average provider volume was also present. For example, the adjusted OR for an increase of 5 patients per year on average was 1.05 (95% CI: 1.00, 1.11) (Supplementary Table S2).
Chemotherapy-related toxicities
We then examined the relationship between provider volume and chemotherapy-related toxicities requiring an emergency room visit or inpatient stay. The unadjusted rate of chemotherapy-related toxicity in the cohort was 11.8%. The rates of chemotherapy-related toxicities were 11.9%, 11.7%, and 11.8% for low-, medium-, and high-volume providers, respectively (p-value >0.95). Similarly, in the adjusted logistic regression model there was no significant association between provider volume and chemotherapy-related toxicities (Table 3). Unsurprisingly, we did find that a higher Charlson comorbidity index was independently associated with chemotherapy-related toxicity (Index ≥ 2: aOR 1.73, 95% CI: 1.11, 2.170; Index=1: aOR 1.44, 95% CI: 1.01, 2.05). Black race was also independently associated with chemotherapy-associated toxicity compared to white race (aOR 1.75, 95% CI: 1.01, 3.03).
Table 3.
Adjusted odds ratios and 95% confidence intervals (CI) for chemotherapy-related toxicity
| Characteristics | Adjusted Odds Ratio (95% CI) |
|---|---|
| Volume tertile (across years patients were seen) | |
| High | 1.00 (Reference) |
| Low | 1.00 (0.69, 1.44) |
| Medium | 0.96 (0.68, 1.35) |
| Stage | |
| Stage II | 1.00 (Reference) |
| Stage III | 1.56 (0.85, 2.87) |
| Stage IV | 1.78 (0.94, 3.37) |
| Grade | |
| 2 | 1.00 (Reference) |
| 3 | 0.87 (0.58, 1.31) |
| Age at diagnosis (years) | |
| 66–69 | 1.00 (Reference) |
| 70–74 | 0.95 (0.65, 1.38) |
| 75–79 | 1.27 (0.86, 1.85) |
| 80–84 | 0.64 (0.37, 1.11) |
| ≥85 | 1.11 (0.51, 2.40) |
| Marital status | |
| Married | 1.00 (Reference) |
| Not married/Unk | 1.10 (0.82, 1.47) |
| Race | |
| White | 1.00 (Reference) |
| Black | 1.75 (1.01, 3.03)* |
| Other | 0.58 (0.25, 1.38) |
| Residence | |
| Metro | 1.00 (Reference) |
| Non-metro | 1.49 (0.93, 2.39) |
| Geographic region | |
| Midwest | 1.00 (Reference) |
| Northeast | 1.49 (0.82, 2.72) |
| South | 1.41 (0.80, 2.46) |
| West | 1.13 (0.66, 1.96) |
| Census tract median income quartile | |
| Quartile 1 | 1.00 (Reference) |
| Quartile 2 | 1.10 (0.73, 1.67) |
| Quartile 3 | 0.90 (0.58, 1.40) |
| Quartile 4 | 1.02 (0.64, 1.63) |
| Unknown | 0.71 (0.26, 1.92) |
| Charlson comorbidity index | |
| 0 | 1.00 (Reference) |
| 1 | 1.44 (1.01, 2.05)* |
| ≥2 | 1.73 (1.11, 2.70)* |
p-value <0.05
Overall survival
In the survival analysis there was a significant difference in OS by provider volume tertile. In the overall cohort, median OS was 39.8 months (95% CI 37.0, 42.0). There was a significant difference in median OS across provider volume tertiles, with a median survival of 32.8 months (95% CI 29.6, 36.4) with low-volume providers compared to 41.9 months (95% CI 37.5, 46.7) with medium-volume providers and 42.1 months (95% CI 38.8, 44.2) with high-volume providers (p<0.01) (Figure 1, Table 4). In the Cox Proportional-Hazards model, after controlling for case-mix and stage of disease, low-volume providers were independently associated with higher rates of mortality, with an adjusted HR of 1.25 (95% CI: 1.08, 1.43), compared with high-volume providers (Table 5). When we added chemotherapy guideline compliance as a variable to the Cox Proportional-Hazards model, we still observed a statistically significant relationship between provider volume tertiles and OS (Table 5).
Figure 1.
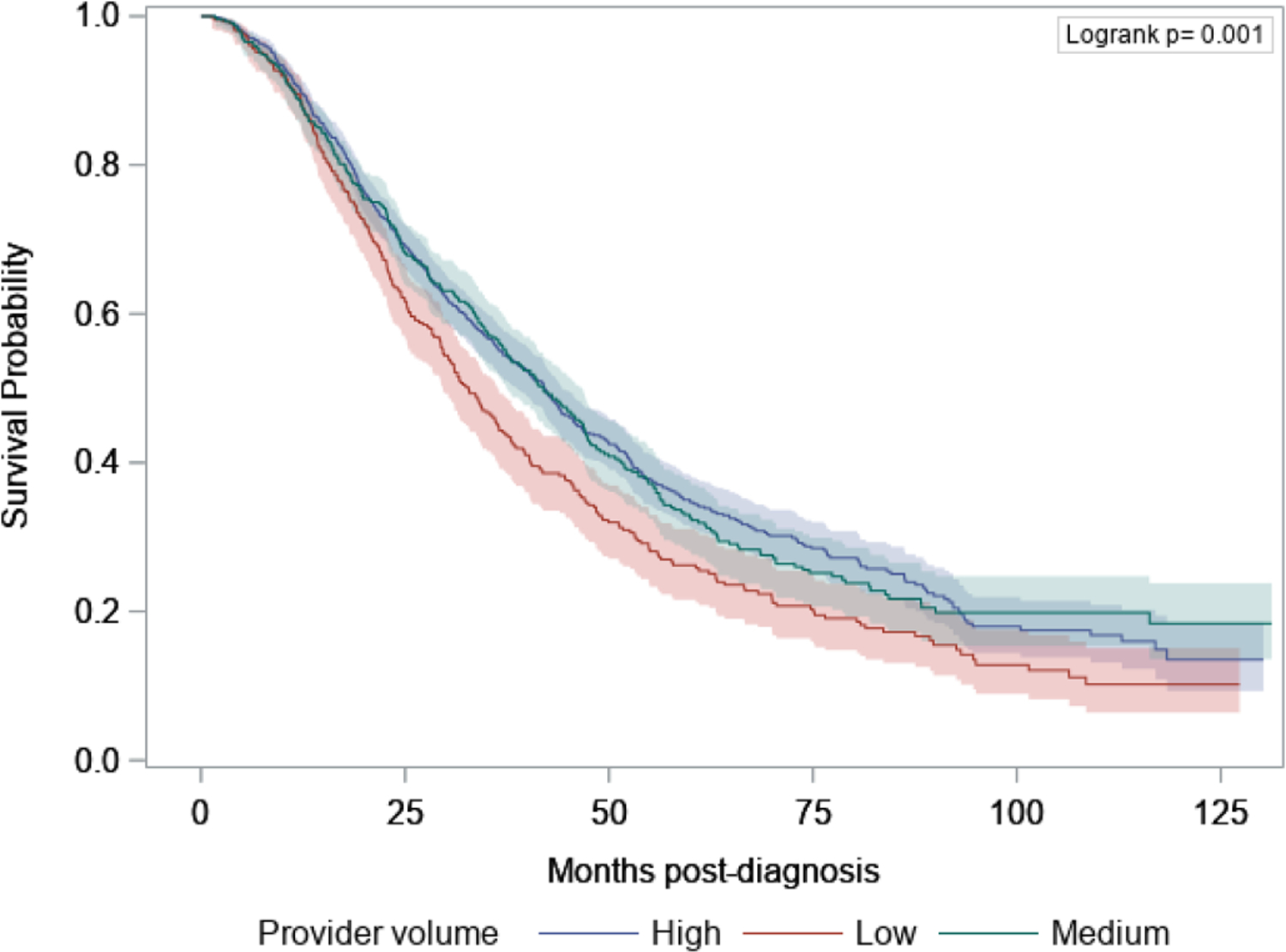
Kaplan-Meier survival plot comparing survival by tertile of provider volume
Table 4.
Comparison of process measures and outcomes across tertiles of volume
| Characteristics | Overall | Low | Medium | High | P-value |
|---|---|---|---|---|---|
| Guideline Compliance | 0.02 | ||||
| Compliant* | 70.3% | 64.5% | 72.1% | 71.2% | |
| Chemotherapy Associated Toxicity | >0.95 | ||||
| Yes | 11.8% | 11.9% | 11.7% | 11.8% | |
| Overall survival, months | <0.01 | ||||
| Median (95% CI) | 39.8 (37.0, 42.0) | 32.8 (29.6, 36.4) | 41.9 (37.5, 46.7) | 42.1 (38.8, 44.2) | |
| 2-year survival, % (95% CI) | 69.1% (67.0%, 71.2%) | 63.5% (58.5%, 68.0%) | 70.7% (66.5%, 74.5%) | 70.6% (67.6%, 73.4%) | |
| 3-year survival, % (95% CI) | 53.9% (51.5%, 56.2%) | 45.8% (40.7%, 50.8%) | 56.8% (52.1%, 61.1%) | 55.8% (52.5%, 59.0%) | |
| 5-year survival, % (95% CI) | 32.3% (29.8%, 34.7%) | 26.2% (21.6%, 31.1%) | 32.6% (27.9%, 37.4%) | 34.6% (31.2%, 38.1%) |
Excludes 61 patients who died < two months of last chemotherapy claim and prior to 6 cycles being complete
Table 5:
Multivariable Cox Proportional-Hazards model of mortality controlling for tertiles of volume
| Characteristics | Adjusted Hazards Ratio (95% CI) | Sensitivity Analysis |
|---|---|---|
| Provider volume tertile (across years patients were seen) | ||
| High | 1.00 (Reference) | 1.00 (Reference) |
| Low | 1.25 (1.08, 1.43)* | 1.23 (1.06, 1.42)* |
| Medium | 0.99 (0.86, 1.13) | 1.00 (0.87, 1.16) |
| Chemotherapy Compliance | ||
| No | - | 1.39 (1.23, 1.57)* |
| Yes | - | 1.00 (Reference) |
| Stage | ||
| Stage II | 1.00 (Reference) | 1.00 (Reference) |
| Stage III | 2.20 (1.71, 2.84)* | 2.23 (1.72, 2.88)* |
| Stage IV | 2.88 (2.21, 3.76)* | 2.85 (2.17, 3.73)* |
| Grade | ||
| 2 | 1.00 (Reference) | 1.00 (Reference) |
| 3 | 0.82 (0.70, 0.97)* | 0.81 (0.69, 0.96)* |
| Histology | ||
| Other | 0.90 (0.78, 1.05) | 0.87 (0.75, 1.01) |
| Serous carcinoma | 1.00 (Reference) | 1.00 (Reference) |
| Age at diagnosis (years) | ||
| 66–69 | 1.00 (Reference) | 1.00 (Reference) |
| 70–74 | 1.03 (0.89, 1.20) | 1.05 (0.90, 1.22) |
| 75–79 | 1.25 (1.07, 1.47)* | 1.24 (1.05, 1.45)* |
| 80–84 | 1.46 (1.21, 1.77)* | 1.38 (1.13, 1.68)* |
| ≥85 | 1.33 (0.98, 1.80) | 1.17 (0.85, 1.61) |
| Marital status | ||
| Married | 1.00 (Reference) | 1.00 (Reference) |
| Not married/Unk | 1.09 (0.97, 1.23) | 1.05 (0.94, 1.19) |
| Race | ||
| Black | 0.99 (0.76, 1.28) | 1.00 (0.77, 1.30) |
| Other | 0.99 (0.75, 1.31) | 1.02 (0.77, 1.36) |
| White | 1.00 (Reference) | 1.00 (Reference) |
| Residence | ||
| Metro | 1.00 (Reference) | 1.00 (Reference) |
| Non-metro | 0.89 (0.72, 1.10) | 0.82 (0.66, 1.02) |
| Geographic region | ||
| Midwest | 1.00 (0.79, 1.26) | 1.04 (0.82, 1.32) |
| Northeast | 1.00 (Reference) | 1.00 (Reference) |
| South | 1.07 (0.89, 1.29) | 1.07 (0.89, 1.30) |
| West | 0.89 (0.76, 1.04) | 0.88 (0.74, 1.03) |
| Census tract median income quartile | ||
| Quartile 1 | 1.00 (Reference) | 1.00 (Reference) |
| Quartile 2 | 1.01 (0.86, 1.19) | 1.01 (0.85, 1.20) |
| Quartile 3 | 0.98 (0.83, 1.17) | 1.00 (0.84, 1.19) |
| Quartile 4 | 0.83 (0.69, 1.00) | 0.86 (0.71, 1.03) |
| Unknown | 1.37 (1.03, 1.84)* | 1.41 (1.04, 1.89)* |
| Charlson comorbidity index | ||
| 0 | 1.00 (Reference) | 1.00 (Reference) |
| 1 | 0.98 (0.85, 1.14) | 0.95 (0.82, 1.11) |
| ≥2 | 1.32 (1.09, 1.60)* | 1.28 (1.05, 1.56)* |
p-value <0.05
DISCUSSION
In this study of Medicare patients with advanced EOC, we found an independent association between provider volume and receipt of guideline-based chemotherapy in the front-line setting. Despite no volume-based differences in chemotherapy-related toxicities, we found a significant and independent association between provider volume and survival outcomes.
Prior studies report rates of guideline-compliant ovarian cancer treatment in the U.S. that range from 30% to 68% [1,5,6,11]. In our cohort of Medicare patients who all received guideline-compliant surgery, the rate of chemotherapy guideline compliance was consistent with these estimates, at 70%. Numerous prior studies demonstrate an independent association between hospital or provider volume and NCCN guideline compliance [1,5,6,10]. Several have also demonstrated an independent association between hospital or provider volume and survival outcomes [1,5,6,7,8,16]. The results of these studies have led many to advocate for centralization of care to improve survival outcomes through improving surgical outcomes [17]. However, most of these studies focus on surgical quality, and either omit or incompletely report chemotherapy-related process measures. A California Cancer Registry study of 13,321 EOC patients treated from 1999–2006 explored the impact of hospital and surgeon volume on adherence to NCCN guidelines and disease-specific survival (DSS) [1]. The authors found that high-volume hospitals were independently associated with improved surgical and chemotherapy guideline compliance. However, in the adjusted analysis, while the association with guideline-compliant surgery persisted, surgeon volume was not independently associated with chemotherapy-specific guideline compliance [1]. Nevertheless, the author’s analysis showed that low-volume hospitals and surgeons were independently associated with decreased DSS [1]. A SEER-Medicare database study of 2,952 patients who received treatment for advanced EOC between 1992–1999 examined the impact of hospital and surgeon volume on survival after surgery and any post-operative chemotherapy [5]. The authors found that patients treated at high-volume hospitals or by high-volume providers were more likely to receive post-operative chemotherapy, but that volume was not a strong predictor of survival outcomes following surgery [5]. Lastly, a National Cancer Database study evaluating 96,802 EOC patients treated between 1998–2007 showed that higher hospital volume was independently associated with guideline-compliant care, and independently predicted OS [6]. However, due to limitations associated with the database, with respect to chemotherapy-specific guideline compliance the authors assessed only whether multiagent chemotherapy was given on at least one occasion, i.e., the evaluation was not based on completing 6 cycles of chemotherapy.
While surgical outcomes remain important in the treatment of advanced EOC, recent advances in medical management have increased the stakes of chemotherapy guideline compliance in the front-line setting. A major strength of our analysis is its focus on front-line chemotherapy guideline compliance and determining if volume or others factors might contribute to disparities in care. Based on our findings, both lower provider volume and patient age greater than 80 were independently associated with lower rates of compliance.
Previous studies have found an association between Black race, lower socioeconomic status (SES), and lower rates of guideline-based care, showing that Black race and lower SES are predictors of not receiving chemotherapy, or receiving only single-agent chemotherapy, and a lower likelihood of undergoing debulking surgery [6,10]. In our analysis, neither SES nor race were associated with chemotherapy guideline compliance or survival outcomes. However, both factors were independently associated with higher rates of chemotherapy-associated toxicities requiring emergency room visits or inpatient hospitalization. This might be explained by failure of providers to address complications in a timely manner, lack of access to walk-in outpatient clinics, lack of advanced practice provider support, or patient-specific barriers to report symptoms before they become severe enough to require admission. It might also be the case, particularly for patients of lower SES, that there is less social support to help prevent escalation of care to the hospital setting.
Our analysis showed that provider volume was associated with OS even after accounting for case mix. When we included chemotherapy-specific guideline compliance in the survival model, the association between provider volume and OS persisted. This suggests that other factors beyond chemotherapy-specific guideline compliance are also driving the survival benefit associated with high-volume providers. We believe that this is likely due to differences in surgical quality, as demonstrated in several previously published studies [5–11, 16]. Other possible explanations might include access to nursing support, clinical trials, or maintenance therapies.
This study has several limitations. First, though we attempted to minimize the influence of surgical quality on volume-based outcomes by ensuring that all patients received what would be considered a guideline-compliant surgery, in the absence of information regarding resection status, we recognize that our ability to do so is limited. There are notable differences in our cohort selection compared to previous studies that attempt to tailor it to the study of front-line chemotherapy. These include the exclusion of patients who did not receive a guideline-compliant surgery or whose surgery occurred greater than 7 months after diagnosis. In the absence of information regarding the completeness of the surgical resection, we believe this allowed for an assessment of chemotherapy-associated outcomes while minimizing surgical bias. Second, survival outcomes are undoubtedly affected by patients receiving later lines of chemotherapy. While we report differences in survival outcomes across volume settings, we recognize that the patient’s provider-volume status was assigned based on the provider who administered a plurality of front-line chemotherapy, and this does not account for potential patient migration to a new provider for later lines of chemotherapy. Third, there are limitations inherent in any large national database study. We specifically chose to query SEER-Medicare because it is well-suited for the study of EOC where the median age of diagnosis is between 60–65 years, and is a reliable database for studying chemotherapy administered over the course of months or even years. One limitation specific to using SEER-Medicare for a study of provider volume is that volume approximations were made using only a subset of patients seen by providers. By only capturing Medicare patient volume, there is a risk of misclassifying the relative volume of patients that a provider treats for ovarian cancer each year. A recently published study supports that fee-for-service Medicare volume is representative of all-payer volume; however, in that analysis, estimates of all-payer volume were less accurate for low-volume hospitals [18]. Finally, the categorization of volume into tertiles may not lead to characterization of truly high- or low-volume providers. To account for this potential source of bias, we performed sensitivity analyses using volume as a continuous variable throughout, and found that the modest association between tertile-based volume and guideline compliance was no longer present when volume was evaluated as a continuous variable. This is not surprising as the differences may not, in fact, be linear; or more substantial differences may be necessary in order to achieve significance when volume is considered as a continuous variable. Despite these limitations, we believe our findings have important implications for patients, health systems, and health policy makers.
With the approval of new life-extending therapies that increase the stakes of non-compliance, there should be a heightened focus on improving guideline compliant in front line chemotherapy for patients with ovarian cancer. First it is important to note that there are both provider and patient factors that may contribute to chemotherapy non-compliance. From a patient perspective, interventions that have shown to be effective in improving compliance include reducing out-of-pocket costs, employment of case managers, and providing enhanced patient education resources [19,20]. From a provider perspective, based on our analysis, low volume Medicare ovarian cancer providers are at increased risk of non-compliance and could be targeted for an intervention. Tumor boards, where treatment plans are discussed with a multidisciplinary team, have been shown to improve guideline compliance [21]. If it is the case that low volume provides are less likely to have access to or participate in tumor boards, making these available or mandatory may improve guideline adherence. Clinical pathways have been strongly advocated by payers and incorporated in certain alternative payment models due to their promise of standardizing care, decreasing costs, and improving outcomes [22]. Despite these reported advantages, pathway programs have received mixed reviews from clinicians who often find them to be overly burdensome to incorporate into clinical practice. Certain alternative payment models, such as bundled payments and Oncology Care Model have established incentives for providing guideline-based care and some early results show improvement in certain areas of guideline compliance with their use [22]. In these settings, timely updates to guidelines used to monitor compliance should occur, in order to incentivize early adoption by providers at participating practices.
In conclusion, with recent approval of life-extending drugs for front-line treatment of patients with EOC, the stakes of non-compliance are higher than ever. Our analysis of risk factors for guideline non-compliance suggest that low-volume Medicare providers and Medicare patients 80 years or older are at increased risk of receiving non-guideline-compliant chemotherapy for newly diagnosed EOC. Importantly, treatment by low-volume Medicare providers was modestly associated with worse survival outcomes. Given the increasing complexity anticipated in modern management of EOC in the front-line setting, urgent efforts are needed to address this volume-outcomes disparity.
Supplementary Material
Supplementary Table S1. ICD-9 procedure codes and HCPCS codes used to identify surgery and chemotherapy
Supplementary Table S2. Adjusted odds ratios and 95% confidence intervals for NCCN guideline compliance with volume as a continuous variable
Highlights:
Front-line chemotherapy in ovarian cancer is complex; greater survival benefits are associated with guideline-compliant care
Low provider volume was associated with lower rates of chemotherapy guideline compliance in Medicare patients
Low-volume providers were independently associated with higher mortality rates in elderly Medicare patients
FUNDING:
This work was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTERESTS: None declared
DISCLOSURES:
Dr. Lavery reports salary support for Project Genomics Evidence Neoplasia Information Exchange (GENIE) through the American Association for Cancer Research.
Dr. Long Roche reports travel expenses (airfare) from Intuitive Surgical Inc. (to a survivorship conference where she spoke), outside the submitted work.
Dr. Green reports personal fees from Clinical Congress Consultants/MJH Life Sciences, outside the submitted work.
Dr. Aghajanian reports personal fees from Tesaro; personal fees from Immunogen; grants and personal fees from Clovis; personal fees from Mateon Therapeutics; personal fees from Cerulean Pharma; grants from Genentech; grants from AbbVie; grants from Astra Zeneca, outside the submitted work.
Dr. O’Cearbhaill reports personal fees from Clovis; personal fees from Tesaro, outside the submitted work.
Dr. Jewell reports grant from Summit Biomedical, outside the submitted work.
Dr. Leitao is a consultant for Intuitive Surgical Inc., outside the submitted work.
Dr. Abu-Rustum reports grant from Stryker/Novadaq (paid to institution); grant from Olympus (paid to institution); grant from GRAIL (paid to institution), outside the submitted work.
Dr. Bach reports personal fees and non-financial support from American Society for Health-System Pharmacists; personal fees and non-financial support from Gilead Pharmaceuticals; personal fees from WebMD; personal fees from Goldman Sachs; personal fees from Defined Health; personal fees and non-financial support from Vizient; personal fees and non-financial support from Hematology Oncology Pharmacy Association; personal fees from JMP Securities; personal fees from Mercer; personal fees and non-financial support from United Rheumatology; personal fees from Foundation Medicine; personal fees from GRAIL; personal fees from Morgan Stanley; personal fees from NYS Rheumatology Society; personal fees and non-financial support from Oppenheimer & Co.; personal fees from Cello Health; personal fees and non-financial support from Oncology Analytics; personal fees from Anthem; personal fees from Magellan Health; personal fees and non-financial support from Kaiser Permanente Institute for Health Policy; personal fees and non-financial support from Congressional Budget Office; personal fees and non-financial support from America’s Health Insurance Plans; grants from Kaiser Permanente; grants from Arnold Ventures; personal fees and non-financial support from Geisinger; personal fees from EQRx, outside the submitted work.
REFERENCES
- [1].Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol 2013;121:1226–1234. doi: 10.1097/AOG.0b013e3182922a17 [DOI] [PubMed] [Google Scholar]
- [2].Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 2015;16:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 2018;379:2495–2505. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Eng J Med 2019;381:2391–2402. [DOI] [PubMed] [Google Scholar]
- [5].Schrag D, Earle C, Xu F, Panageas KS, Yarbroff KR, Bristow RE, Trimble EL, Warren JL. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. J Natl Cancer Inst 2006;98:163–171. DOI: 10.1093/jnci/djj018 [DOI] [PubMed] [Google Scholar]
- [6].Cliby WA, Powell MA, Al-Hammadi N, Chen L, Philip Miller J, Roland PY, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol 2015;136:11–17. doi: 10.1016/j.ygyno.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bristow RE, Zahurak ML, Diaz-Montes TP, Giuntoli RL, Armstrong DK. Impact of surgeon and hospital ovarian cancer surgical case volume on in-hospital mortality and related short-term outcomes. Gynecol Oncol 2009;115:334–338. doi: 10.1016/j.ygyno.2009.08.025 [DOI] [PubMed] [Google Scholar]
- [8].Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol 2014;132:403–410. doi: 10.1016/j.ygyno.2013.12.017 [DOI] [PubMed] [Google Scholar]
- [9].Liu FW, et al. Racial disparities and patterns of ovarian cancer surgical care in California. Gynecol Oncol 2014;132:221–226. DOI: 10.1016/j.ygyno.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Long B, Chang J, Ziogas A, Tewari KS, Anton-Culver H, Bristow RE. Impact of race, socioeconomic status, and the health care system on the treatment of advanced-stage ovarian cancer in California. Am J Obstet Gynecol 2015;212:e1–9. doi: 10.1016/j.ajog.2014.10.1104 [DOI] [PubMed] [Google Scholar]
- [11].Goff BA, Matthews BJ, Larson EH, Andrilla CHA, Wynn M, Lishner DM, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007;109:2031–2042. doi: 10.1002/cncr.22604 [DOI] [PubMed] [Google Scholar]
- [12].Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:Iv-3–18. [DOI] [PubMed] [Google Scholar]
- [13].Klabunde CN, Potosky AL, Legler JM, et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267. [DOI] [PubMed] [Google Scholar]
- [14].Parsons HM, Enewold LR, Banks R, Barrett MJ, Warren JL. Creating a National Provider Identifier (NPI) to Unique Physician Identification Number (UPIN) Crosswalk for Medicare Data. Med Care 2017;55:e113–e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Admissions and Emergency Department Visits for Patients Receiving Outpatient Chemotherapy Measure Technical Report March2016. Prepared by: Mathematica Policy Research. Prepared for: Centers for Medicare & Medicaid Services [Google Scholar]
- [16].Wright JD, Herzog TJ, Siddiq Z, et al. Failure to Rescue As a Source of Variation in Hospital Mortality for Ovarian Cancer. J Clin Oncol 2012;30:3976–3982. [DOI] [PubMed] [Google Scholar]
- [17].Cowan RA, O’Cearbhaill RE, Gardner GJ, et al. Is it time to centralize ovarian cancer care in the United States? Ann Surg Oncol 2016;23:989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li DG, Lavery JA, Panageas KS, et al. Estimating hospitals’ all-payer volume of cancer surgeries from Fee-for-Service Medicare claims. J Hosp Manag Health Policy [in press]. [Google Scholar]
- [19].Zullig L, Peterson E, Bosworth H. Ingredients of successful interventions to improve medication adherence. J Am Med Assoc 2013;310(24):2611–2612. [DOI] [PubMed] [Google Scholar]
- [20].Viswanathan M, Golin C, Jones C, et al. Interventions to improve adherence ot self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med 2012;157(11):785–795. [DOI] [PubMed] [Google Scholar]
- [21].Basta YL, Bolle S, Fockens P, Tytgat KMAJ. The value of multidisciplinary team meetings for patients with gastrointestinal malignancies: a systematic review. Ann Surg Oncol 2017September;24(9):2669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Loy BA, Shkedy CI, Powell AC, et al. : Do Case Rates Affect Physicians’ Clinical Practice in Radiation Oncology?: An Observational Study. PLoS One 2016;11(2):e0149449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. ICD-9 procedure codes and HCPCS codes used to identify surgery and chemotherapy
Supplementary Table S2. Adjusted odds ratios and 95% confidence intervals for NCCN guideline compliance with volume as a continuous variable


