Abstract
Background
Toxoplasma gondii is a zoonotic protozoan parasite infecting warm-blooded animals. Infection in people can occur through ingestion of oocysts passed in the faeces of the definitive hosts; ingestion of bradyzoites in the tissue of infected intermediate hosts; or exposure to tachyzoites in raw milk and eggs. Slaughterhouse workers are considered a high-risk group for T. gondii exposure because of their contact with raw meat, although a positive relationship between handling raw meat and T. gondii seropositivity has not been demonstrated in all studies. This study aimed to determine the seroprevalence of antibodies to T. gondii in slaughterhouse workers in Kenya and identify risk factors associated with seropositivity.
Methods
A survey of slaughterhouse workers was conducted in 142 slaughter facilities in the study area. Information regarding demographics, contact with livestock, meat consumption, and practices in the slaughterhouse was collected using structured questionnaires. Commercial ELISAs were used to detect IgM and IgG antibodies against T. gondii and a multi-level logistic regression model was used to identify potential risk factors for seropositivity in slaughterhouse workers.
Results
The apparent prevalence of antibodies to T. gondii was 84.0% (95% Confidence Interval (CI) 81.2–86.5%) for IgG and 2.2% (95% CI 1.3–3.5%) for IgM antibodies. All IgM positive individuals were IgG positive. Risk factors for exposure to T. gondii were: increasing age (Odds Ratio (OR) 1.03; 95% CI 1.01–1.05); owning poultry (OR 2.00; 95% CI 1.11–3.62); and consuming animal blood (OR 1.92; 95% CI 1.21–3.03).
Conclusions
The seroprevalence of antibodies to T. gondii was very high in this population and considerably higher than published values in the general population. Risk factors included age, owning poultry and drinking animal blood which were consistent with previous reports but none were specifically associated with working in the slaughterhouse. In this instance slaughterhouse workers may represent a useful sentinel for the general population where the level of exposure is also likely to be high and may signify an unidentified public health risk to vulnerable groups such as pregnant women. A detailed understanding of the epidemiology of infection is required, which should include an assessment of incidence, mortality, and burden since T. gondii infection is likely to have life-long sequelae.
Keywords: Slaughterhouse, Abattoir, Kenya, Toxoplasma, Zoonoses, Occupational health
Background
Toxoplasma gondii is a zoonotic protozoan parasite found worldwide. It infects a wide variety of warm-blooded animals including mammals and birds. Sexual reproduction of the parasite occurs in the definitive host (cats) and asexual reproduction occurs in the intermediate hosts (all warm blooded animals) [1]. Infection in people can occur through ingestion of oocysts passed in the faeces of the definitive hosts; ingestion of bradyzoites in the tissue of infected intermediate hosts which is the potential route of transmission to meat handlers including slaughterhouse workers; or exposure to tachyzoites in raw milk and eggs [2]. Vertical transmission is also possible across the placenta [1]. Sporulated oocysts are very robust and can survive in moist soils for more than 12 months [1]. Previously identified risk factors for toxoplasmosis in humans include contact with soil, water or unwashed raw vegetables that might be contaminated with cat faeces, and eating raw meat [2–5].
Toxoplasmosis is generally asymptomatic in immunocompetent individuals but might result in retinitis later in life [6]. Research has also demonstrated a relationship between latent T. gondii infection and some mental health disorders [7]. In immunocompromised individuals infection can result in encephalitis with neurological disturbances including seizures and loss of consciousness [8]. Infection in women during early pregnancy can result in foetal death or severe damage to the newborn including, retinochoroiditis, hydrocephalus, seizures and intracerebral calcification [8].
Previously the “gold standard” for definitive diagnosis of T. gondii infection was the Sabin Feldman dye test (SFDT), however ELISAs are now used for routine screening in the majority of laboratories [1]. Although the detection of IgG antibodies using ELISA is likely to be later in the course of infection there is relatively good agreement with the SFDT [1, 9, 10]. IgM antibodies may indicate a more recent exposure, but they can persist for several months to years, which means the presence of IgM antibodies does not indicate acute infection nor the time of exposure [11, 12].
There are few reports of exposure to T. gondii in Kenya. A study of blood donors in several regions of the country showed the seroprevalence to be 54% [13] and a more recent study of samples collected from women during antenatal clinics in Nairobi, Kisumu and Mombasa reported the prevalence to be 32% [14]. A more targeted study of school children indicated an increase in seroprevalence between preschool and primary school that might be the result of poor sanitation [15]. Recently a study in slaughterhouses demonstrated the prevalence of T. gondii by PCR in ruminant slaughterhouse workers to be 34.6% and 100% in chicken slaughterhouse workers [16].
Slaughterhouse workers are considered a high-risk group for T. gondii exposure because of their regular contact with raw meat. Although a positive relationship between handling raw meat and T. gondii seropositivity has not been demonstrated in all studies [17, 18]. The present study aimed to determine the seroprevalence of antibodies to T, gondii in slaughterhouse workers in Kenya and identify risk factors associated with seropositivity.
Methods
Study site and population
The study area, in western Kenya on the border with Uganda, is a predominantly rural region characterised by a smallholder mixed crop and livestock system that is broadly representative of the Lake Victoria Basin [19].
A census of all slaughterhouses was attempted with 156 slaughterhouses recruited in the study area – 88 ruminant and 68 porcine. Inclusion criteria were all workers aged over 18 years and present at the slaughterhouse on the day of sampling. Participants were excluded for third trimester pregnancy, severe inebriation, and being aged over 85 years.
Ethical approval
Ethical approval for this study was granted by the Kenya Medical Research Institute Ethical Review Committee (SCC Protocol 2086). The study was conducted in accordance with the Declaration of Helsinki and each participant gave signed informed consent.
Data collection and sampling
Data collection was conducted between February and November 2012. Data were collected regarding demographic characteristics of the participants, animal contacts, food consumption practices, and time and role in the slaughterhouse. Questionnaire data were recorded on a Palm operating system (Palm OS) Personal digital assistant (PDA) using Pendragon Forms 5.1 (Pendragon Software Corporation, Libertyville, IL). A clinical officer collected blood from each participant using a 21G or 23G BD Vacutainer® Safetylok™ blood collection set into 10 ml plain BD Vacutainers®.
The locations of slaughterhouses were georeferenced using a handheld GPS device (Garmin eTrex®) and mapped using ArcGIS™ version 10.2.2 (ESRI, Redlands, California, USA).
Laboratory analysis
Serum samples were screened with the Vir-ELISA Anti-Toxo-IgG (Viro-Immun, Oberursel, Germany) and the Vir-ELISA Anti-Toxo-IgM (Immunocaptureassay; Viro-Immun, Oberusel, Germany) following manufacturer’s instructions. Briefly, the steps for the IgG and IgM ELISAs were: 10 μL of sera were diluted in 1 ml of provided diluent and 100 μL added to each well of the provided precoated plate. For IgG 100 μL of negative control and 4 calibrators were added to the plate and for IgM 100 μL of positive and negative control were added. Plates were incubated at 37 °C for 60 min in a humid environment. Plates were washed four times and 100 μL of peroxidase conjugate added to each well and then incubated at 37 °C for 30 min in a humid environment. Plates were washed four times and then 100 μL of TMB substrate added to each well and incubated at room temperature (21–25 °C) for 15 min. Finally, 100 μL of 0.95 N sulphuric acid was added to each well and the plates read at 450 nm (Synergy HT, Biotek, USA).
Statistical analysis
Statistical analysis was performed in R (http://CRAN.R-project.org/). The apparent prevalence estimates and the true prevalence estimates were calculated using the truePrev function in the prevalence package [20] of R accounting for the test sensitivity and specificity reported by the manufacturer of 98.9% and 100.0% for the IgG ELISA and 94.3% and 98.0% for the IgM ELISA respectively. Design-based adjustment was done with the svydesign procedure in the Survey [21] package in R using sampling weights for each slaughterhouse calculated by dividing the number of expected workers by the number sampled.
Multivariable mixed effects (multi-level) logistic regression models were used to identify risk factors and quantify their association with T. gondii seropositivity in slaughterhouse workers. Univariable logistic regression was used to screen variables against T. gondii exposure at the individual level. The variables used were those previously reported to be associated with seropositivity to T. gondii and included age, gender, contact with livestock, drinking milk, eating meat, role in the slaughterhouse and types of animals slaughtered. All variables are listed in Table 1.
Table 1.
Results of univariable analysis for risk factors for seropositivity to IgG antibodies to Toxoplasma gondii in slaughterhouse workers from western Kenya
| Variable | Number (%) n = 737 | Number positive (%) n = 619 | OR (95%CI) | p–value |
|---|---|---|---|---|
| Individual variables | ||||
| Gender | ||||
| Female | 26 (3.5) | 18 (69.2) | 1 | Ref. |
| Male | 711 (96.5) | 601 (84.5) | 2.57 (0.98–6.72) | 0.055 |
| Age groups | ||||
| 18–27 | 167 (22.7) | 130 (77.8) | 1 | Ref. |
| 28–37 | 227 (30.8) | 186 (81.9) | 1.42 (0.82–2.46) | 0.217 |
| 38–47 | 147 (19.9) | 127 (86.4) | 1.95 (1.02–3.74) | 0.043 |
| 48 + | 196 (26.6) | 176 (89.8) | 2.79 (1.46–5.36) | 0.002 |
| Age (linear) | 1.03 (1.01–1.05) | 0.001 | ||
| Own cattle | ||||
| No | 252 (34.2) | 206 (81.7) | ||
| Yes | 485 (65.8) | 413 (85.2) | 1.34 (0.86–2.10) | 0.196 |
| Own sheep | ||||
| No | 624 (84.7) | 524 (84.0) | ||
| Yes | 113 (15.3) | 95 (84.1) | 1.02 (0.56–1.85) | 0.947 |
| Own goats | ||||
| No | 469 (63.6) | 391 (83.4) | ||
| Yes | 268 (36.4) | 228 (85.1) | 1.14 (0.73–1.80) | 0.558 |
| Own pigs | ||||
| No | 517 (70.1) | 429 (83.0) | ||
| Yes | 220 (29.9) | 190 (86.4) | 1.44 (0.87–2.36) | 0.156 |
| Own poultry | ||||
| No | 104 (14.1) | 80 (76.9) | ||
| Yes | 633 (85.9) | 539 (85.2) | 1.97 (1.11–3.48) | 0.020 |
| Drink animal blood | ||||
| No | 339 (46.0) | 271 (79.9) | ||
| Yes | 398 (54.0) | 348 (87.4) | 1.96 (1.26–3.06) | 0.003 |
| Drink cow’s milk | ||||
| No | 35 (4.7) | 32 (91.4) | ||
| Yes | 702 (95.3) | 587 (83.6) | 0.43 (0.12–1.52) | 0.191 |
| Drink goat’s milk | ||||
| No | 716 (97.2) | 601 (83.9) | ||
| Yes | 21 (2.8) | 18 (85.7) | 1.02 (0.27–3.84) | 0.980 |
| Eat beef | ||||
| No | 22 (3.0) | 20 (90.9) | ||
| Yes | 715 (97.0) | 599 (83.8) | 0.58 (0.13–2.70) | 0.491 |
| Eat pork | ||||
| No | 230 (31.2) | 191 (83.0) | ||
| Yes | 507 (68.8) | 428 (84.4) | 1.18 (0.74–1.89) | 0.478 |
| Eat at slaughterhouse | ||||
| No | 593 (80.5) | 494 (83.3) | ||
| Yes | 144 (19.5) | 125 (86.8) | 1.18 (0.64–2.17) | 0.600 |
| HIV positive | ||||
| No | 648 (87.9) | 540 (83.3) | ||
| Yes | 89 (12.1) | 79 (88.8) | 1.65 (0.79–3.42) | 0.179 |
| Job in slaughterhouse | ||||
| Slaughterman/foreman | 79 (10.7) | 65 (82.3) | 1 | Ref. |
| Flayer | 579 (78.6) | 487 (84.1) | 1.13 (0.58–2.18) | 0.727 |
| Cleans intestines | 43 (5.8) | 37 (86.0) | 1.09 (0.36–3.32) | 0.880 |
| Cleans slaughterhouse | 36 (4.9) | 30 (83.3) | 0.99 (0.32–3.05) | 0.989 |
| Other job | ||||
| No job | 137 (18.6) | 115 (83.9) | 1 | Ref. |
| Butcher | 299 (40.6) | 248 (82.9) | 0.92 (0.51–1.67) | 0.784 |
| Farmer | 213 (28.9) | 182 (85.4) | 1.04 (0.55–1.97) | 0.896 |
| Other | 88 (11.9) | 74 (84.1) | 0.96 (0.44–2.12) | 0.927 |
| Time as worker (linear) | 1.04 (1.01–1.07) | 0.019 | ||
| Slaughterhouse level variables | ||||
| Animal type | ||||
| Cattle, sheep, goats | 274 (37.2) | 231 (84.3) | 1 | Ref. |
| Cattle only | 292 (39.6) | 249 (85.3) | 1.18 (0.63–2.21) | 0.614 |
| Pigs only | 171 (23.2) | 139 (81.3) | 0.83 (0.43–1.60) | 0.574 |
A multivariable mixed effects logistic regression model was developed using variables with a p-value < 0.1 in the univariable analysis. The model was developed using the glmer function in the lme4 package [22] with slaughterhouse included as a random effect to account for clustering of the workers. Model selection was conducted using a backwards stepwise approach starting with a full model containing all predictors. Variables with the highest p-value were removed in a stepwise fashion until the model with the lowest Akaike information criterion (AIC) was identified. Variance Inflation Factors (VIF) were calculated from the final model to check for collinearity and variables with VIF > 4 were excluded.
Results
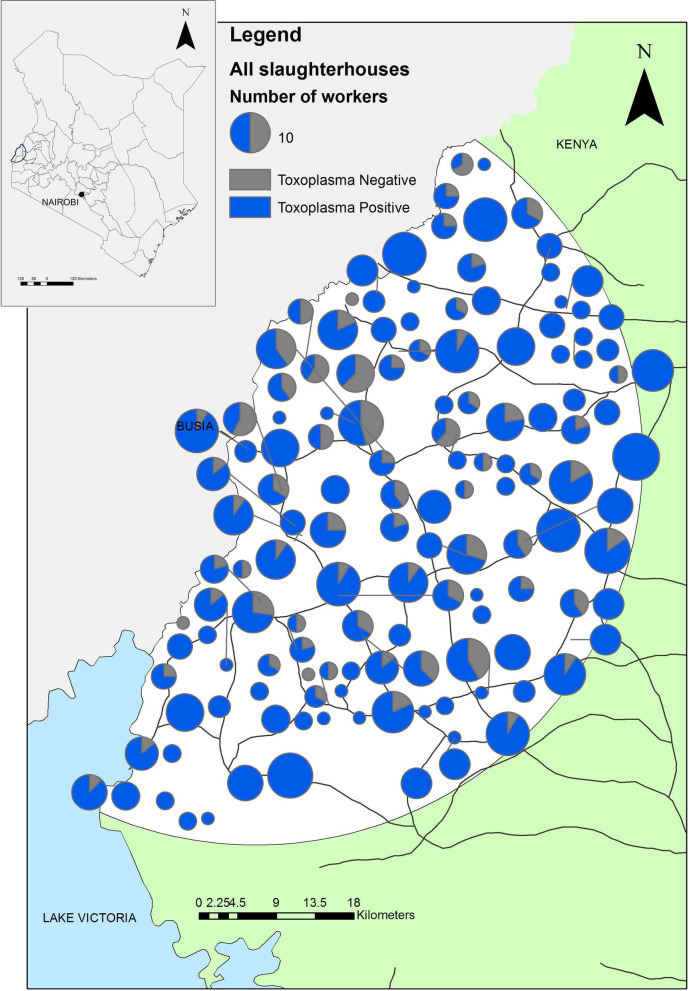
The study recruited 738 slaughterhouse workers from 142 ruminant slaughterhouses. Four ruminant slaughterhouses and 10 pig slaughterhouses refused to participate. Serum samples were available from 737 workers; 619 workers were seropositive for IgG antibodies to T. gondii with an apparent prevalence of 84.0% (95% CI 81.2–86.5%). The adjusted prevalence estimate accounting for the study design was 84.3% (95% CI 81.2–87.4%). The true prevalence was 84.8% (95% CI 82.1–87.5%) after adjustment for the sensitivity and specificity of the test. Sixteen (2.2%; 95% CI 1.3–3.5%) workers were positive for IgM antibodies. The adjusted prevalence estimate accounting for the study design was 2.4% (95% CI 1.2–3.7%). The true prevalence was 0.6% (95% CI 0–1.8%) after adjustment for the sensitivity and specificity of the test. All IgM positive individuals were also IgG positive. The distribution of T. gondii seropositive workers through the study area is demonstrated in Fig. 1.
Fig. 1.
Map of the distribution of Toxoplasma gondii seropositive and seronegative slaughterhouses. The size of the charts is proportional to the number of workers in each slaughterhouse. This is an original figure created using ArcGIS
The complete univariable analysis for risk factors for T. gondii IgG seropositivity in slaughterhouse workers included previously reported exposure variables (Table 1). The variables from the univariable analysis significantly associated with T. gondii seropositivity in slaughterhouse workers were: age (OR 1.03, 95% CI 1.01–1.05); owning poultry (OR 1.97, 95% CI 1.11–3.48); drinking animal blood (OR 1.96 95% CI 1.26–3.06); and length of time as a slaughterhouse worker (OR 1.04, (95% CI 1.01–1.07). There was no difference in T. gondii seropositivity between slaughterhouse types (Table 1): with seropositivity of 84.2% (230/273) in cattle, sheep and goat slaughterhouses; 85.3% (249/292) in cattle only slaughterhouses; and 81.3% (39/171) in pig only slaughterhouses. The univariable analysis was not conducted for IgM seropositivity as the numbers of positive workers was too small.
The final multivariable model for T. gondii IgG seropositivity in individual slaughterhouse workers included the variables gender, age, owning poultry and drinking animal blood. The results are shown in Table 2. Risk factors for exposure to T. gondii were: increasing age in years (OR 1.03; 95% CI 1.01–1.05); owning poultry (OR 2.00; 95% CI 1.11–3.62); and consuming animal blood (OR 1.92; 95% CI 1.21–3.03).
Table 2.
Results of the multivariable analysis for IgG antibodies to Toxoplasma gondii in slaughterhouse workers in western Kenya
| Variables | OR (95% CI) | p–value | VIF |
|---|---|---|---|
| Individual variables | |||
| Male gender | 2.56 (0.94–7.00) | 0.066 | 1.019 |
| Age | 1.03 (1.01–1.05) | 0.001 | 1.015 |
| Owning poultry | 2.00 (1.11–3.62) | 0.022 | 1.023 |
| Drinking animal blood | 1.92 (1.21–3.03) | 0.005 | 1.003 |
Discussion
The apparent seroprevalence of IgG antibodies to T. gondii in slaughterhouse workers in western Kenya was 84.0% (95% CI 81.2 – 86.5%). This is consistent with reports in slaughterhouse workers in countries from Latin America and Africa, 72% in Brazil [23], 72% in Mexico [24] and 55.8% in Nigeria [25] where the prevalence is reportedly high (> 50%) [1]. This contrasts to Europe where there is low-moderate seroprevalence (10—50%) with reports of T. gondii seropositivity in slaughterhouse workers of 45% [26].
We do not have an estimate of the seroprevalence in the general population in this study area to make a direct comparison, however, the prevalence in slaughterhouse workers is higher than that reported in the community (54%) in an earlier study conducted in Kenya [13] and a more recent report from western Kenya of 28.2% [27]. This may indicate that slaughterhouse workers have a higher seroprevalence for antibodies to T. gondii but it we cannot rule out that the difference may also be the result of a different test (ELISA versus haemagglutination and microsphere-based immunoassay), different environments, or another occupational exposure.
A previous study in Kenya demonstrated active infections (PCR positivity) in 34.6% of ruminant abattoir workers [16]. In our study it was not possible to determine if infections were active since seropositivity for IgM and IgG does not indicate or distinguish between active and chronic infections [11, 12]. The proportion of seropositive workers increased with increasing age as previously reported [1], however, the highest prevalence may be seen in childhood, particularly in areas with poor hygiene, as demonstrated elsewhere in Kenya [15]. Initial exposure to T. gondii occurs in childhood but it is believed that repeated exposure is required to sustain antibody levels [28], which appears to be occurring regularly among slaughterhouse workers in this setting.
There was no relationship between T. gondii seropositivity and the role in the slaughterhouse which has previously been identified as a risk factor [23], together with handling meat [23, 29]. A difference in risk between roles in the slaughterhouse might have been expected since the slaughtermen, foremen and cleaners do not handle meat compared to flayers. However, the lack of differential risk between workers might be explained by the method of batch slaughtering conducted in western Kenya where all processes (skinning, evisceration and cutting) are performed in the same spot without a clear demarcation of activities [30]. Workers may also be involved with slaughtering animals at home, with 15% of homesteads in the study area reporting home slaughter which may be an additional route of exposure [19]. Further research is required to compare the prevalence and risk factors in the community to determine if handling or consuming meat is a risk factor for slaughterhouse workers.
In our study seropositivity to T. gondii was associated with owning poultry. Poultry are known to be infected with T. gondii [31] with the prevalence in backyard chickens reaching 100% in some settings [32]. In western Kenya poultry are free ranging during the day and are kept in the house or kitchen at night [33], and 87.2% of households own poultry [19], which is an intensifying industry in the region [34]. Poultry slaughterhouse workers have been shown to have higher risk of being exposed to T. gondii [25, 35]. In this setting poultry are slaughtered at home which may indicate a potential point of exposure for poultry keepers. From the information available it is not possible to determine the potential role of chickens in the epidemiology of T. gondii in this setting but these findings suggest they may be important source of infection and further research is required.
Drinking animal blood was a risk factor for T. gondii seropositivity in slaughterhouse workers (OR 1.92; 95% CI 1.21–3.03). This is consistent with reports that consuming undercooked animal products is a risk factor for exposure to T. gondii [29]. Many workers in this study consume animal blood (54%) and education regarding the preparation of blood before consumption may reduce the risk of exposure.
Infection with T. gondii has previously been associated with severe disease in immunocompromised individuals [36]. The prevalence of HIV in the study area is the highest in Kenya [37]. A significant relationship between T. gondii infection and HIV was not identified in this study, and the lack of association may indicate a healthy worker effect [38] with affected individuals unable to work.
Conclusion
The results of this study indicate a high seroprevalence of antibodies to T. gondii in a population of slaughterhouse workers in western Kenya, which suggests repeated exposure. None of the risk factors identified here are specifically associated with working in the slaughterhouse. The high infection pressure identified in this study may signify an unidentified public health risk in this region which is a hazard to vulnerable groups such as people living with HIV or pregnant women. There are some gaps in the information which are required to understand the epidemiology of T. gondii in this setting such as information regarding raw meat consumption and contact with cats. There is currently no data on the disease incidence or mortality from toxoplasmosis in Kenya. In neighbouring Tanzania a 10 year hospital based study reported the mortality from toxoplasmosis to be 0.08% (188/247,976) of the total deaths recorded [39]. A detailed understanding of the epidemiology of infection is required, which needs to include an assessment of incidence, mortality, and burden since congenital T. gondii infection is likely to have life-long sequelae [40]. Future research agendas should consider investing in understanding zoonotic disease risks in sub-Saharan African settings such as this where there is intense animal contact and potential long-term issues.
Acknowledgements
We thank all of the team on the ‘PAZ’ project for their hard work and diligence We are grateful to all the members of the PAZ field and laboratory teams in Busia in particular Fred Amanya, James Akoko, Lorren Alumasa, Omoto Lazarus, Maseno Cleophas, Daniel Cheruiyot, George Omondi, John Mwaniki, Hannah Kariuki and Lilian Achola. We are grateful to all the participating slaughterhouse workers for their willingness to be involved in the research.
Abbreviations
- AIC
Akaike information criteria
- CI
Confidence interval
- ELISA
Enzyme-linked immunosorbent assay
- GPS
Global positioning system
- Ig
Immunoglobulin
- OR
Odds ratio
- PCR
Polymerase chain reaction
- PDA
Personal digital assistant
- SCC
Scientific Steering Committee
- SFDT
Sabin Feldman dye test
- VIF
Variance Inflation Factors
Authors' contributions
EAJC designed and conducted the data collection and interpretation. NG conducted laboratory analysis and interpretation. EK, EMF, LFT, WAdG and SK assisted with study design and conceptualization. All authors made contributions to conception, design, and revision of the manuscript. All authors read and approved the final manuscript.
Funding
We thank the Wellcome Trust (085308) for supporting EMF and the People, Animals and their Zoonoses project. Support was also received from the CGIAR Research Program on Agriculture for Nutrition and Health (A4NH), led by the International Food Policy Research Institute (IFPRI). We acknowledge the CGIAR Fund Donors (https://www.cgiar.org/funders/). This work was part-funded by the Global Challenges Research Fund (GCRF) One Health Regional Network for the Horn of Africa (HORN) Project, from UK Research and Innovation (UKRI) and Biotechnology and Biological Sciences Research Council (BBSRC) (project number BB/P027954/1).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the Kenya Medical Research Institute Ethical Review Committee (SCC Protocol 2086). All methods were performed in accordance with the Declaration of Helsinki and each participant gave signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests..
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elizabeth Anne Jessie Cook and Nduhiu Gitahi contributed equally to this work
References
- 1.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012 doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: From animals to humans. Int J Parasitol. 2000;30(12–13):1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook A, Gilbert R, Buffolano W, Zufferey J, Petersen E, Jenum P, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European Research Network on Congenital Toxoplasmosis. BMJ. 2000;321(7254):142–147. doi: 10.1136/bmj.321.7254.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapperud G, Jenum P, Sray-Pedersen B, Melby K, Esklin A, Eng J. Risk factors for Toxoplasma gondii infection in pregnancy. Results of a prospective case-control study in Norway. Am J Epidemiol. 1996;144(4):405–412. doi: 10.1093/oxfordjournals.aje.a008942. [DOI] [PubMed] [Google Scholar]
- 5.Baril L, Ancelle T, Goulet V, Thulliez P, Tirard-Fluery V, Carme B. Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis. 1999;31(3):305–309. doi: 10.1080/00365549950163626. [DOI] [PubMed] [Google Scholar]
- 6.Delair E, Monnet D, Grabar S, Dupouy-Camet J, Yera H, Brézin AP. Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am J Ophthalmol. 2008;146(6):851–855. doi: 10.1016/j.ajo.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Torrey EF, Yolken RH. Toxoplasma gondii and Schizophrenia. Emerg Infect Dis. 2003;9(11):1375–1380. doi: 10.3201/eid0911.030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill D, Dubey JP. Toxoplasma gondii: Transmission, diagnosis, and prevention. Clin Microbiol Infect. 2002;8(10):634–640. doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 9.Mondesire R, Charlton D, Tizard I. A standardized enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Toxoplasma gondii. J Immunoassay. 1981;2(1):45–57. doi: 10.1080/01971528108062991. [DOI] [PubMed] [Google Scholar]
- 10.Ybañez RHD, Ybañez AP, Nishikawa Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Front Cell Infect Microbiol. 2020;10(May):1–18. doi: 10.3389/fcimb.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gras L, Gilbert RE, Wallon M, Peyron F, Cortina-Borja M. Duration of the IgM response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and cross-sectional incidence studies. Epidemiol Infect. 2004;132(3):541–548. doi: 10.1017/S0950268803001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Q, Wang ZD, Huang SY, Zhu XQ. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors. 2015 doi: 10.1186/s13071-015-0902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin L, Williams KAB. Serological and parasitological survey of blood donors in Kenya for toxoplasmosis. Trans R Soc Trop Med Hyg. 1983;77(6):763–766. doi: 10.1016/0035-9203(83)90283-3. [DOI] [PubMed] [Google Scholar]
- 14.Nisbet AI, Omuse G, Revathi G, Adam RD. Seroprevalence data at a private teaching hospital in Kenya: an examination of Toxoplasma gondii, cytomegalovirus, rubella, hepatitis A, and Entamoeba histolytica. PLoS ONE. 2018;13(10):1–11. doi: 10.1371/journal.pone.0204867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowry T, Camargo M, Kinyanjui M. Sero-epidemiology of Toxoplasma gondii infection in young children in Nairobi, Kenya. Trans R Soc Trop Med Hyg. 1986;80:439–441. doi: 10.1016/0035-9203(86)90336-6. [DOI] [PubMed] [Google Scholar]
- 16.Thiongo SK, Ichagichu JM, Ngotho M, Aboge GO, Kagira JM, Karanja SM, et al. Use of the nested polymerase chain reaction for detection of Toxoplasma gondii in slaughterhouse workers in Thika District, Kenya. S Afr Med J. 2016;106(4):417–419. doi: 10.7196/SAMJ.2016.v106i4.8777. [DOI] [Google Scholar]
- 17.Gonçalves DD, Teles PS, Dos Reis CR, Lopes FMR, Freire RL, Navarro IT, et al. Seroepidemiology and occupational and environmental variables for leptospirosis, brucellosis and toxoplasmosis in slaughterhouse workers in the Paraná State, Brazil. Rev Inst Med Trop Sao Paulo. 2006;48(3):135–140. doi: 10.1590/S0036-46652006000300004. [DOI] [PubMed] [Google Scholar]
- 18.Alvarado-Esquivel C, Liesenfeld O, Estrada-Martínez S, Félix-Huerta J. Toxoplasma gondii infection in workers occupationally exposed to raw meat. Occup Med. 2011;61(4):265–269. doi: 10.1093/occmed/kqr032. [DOI] [PubMed] [Google Scholar]
- 19.Fèvre EM, de Glanville WA, Thomas LF, Cook EAJ, Kariuki S, Wamae CN. An integrated study of human and animal infectious disease in the Lake Victoria crescent small-holder crop-livestock production system Kenya. BMC Infect Dis. 2017 doi: 10.1186/s12879-017-2559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speybroeck N, Devleesschauwer B, Joseph L, Berkvens D. Misclassification errors in prevalence estimation: Bayesian handling with care. Int J Public Health. 2013;58(5):791–95. 10.1007/s00038-012-0439-9. [DOI] [PubMed]
- 21.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9(1):1–19. R package verson 2.2.
- 22.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48. 10.18637/jss.v067.i01.
- 23.Riemann HP, Brant PC, Behymer DE, Franti CE. Toxoplasma gondii and Coxiella burneti antibodies among Brazilian slaughterhouse employees. Am J Epidemiol. 1975;102(5):386–393. doi: 10.1093/oxfordjournals.aje.a112177. [DOI] [PubMed] [Google Scholar]
- 24.Galván-Ramírez M, Flores Orozco C, Soto MJ. Seroepidemiology of toxoplasma gondii in workers of slaughterhouse in Zapopan, Jalisco. Int J Infect Dis. 2008 doi: 10.1016/j.ijid.2008.05.1014. [DOI] [Google Scholar]
- 25.Ekanem U, Moses A, Abraham E, Motilewa O, Umo A, Itina E. Seroprevalence of anti-toxoplasma gondii igg antibody and risk factors among abattoir workers in Uyo, Southern Nigeria. Niger J Clin Pract. 2018;21:1662–1669. doi: 10.4103/njcp.njcp_44_18. [DOI] [PubMed] [Google Scholar]
- 26.Lings S, Lander F, Lebech M. Antimicrobial antibodies in Danish slaughterhouse workers and greenhouse workers. Int Arch Occup Environ Health. 1994;65(6):405–409. doi: 10.1007/BF00383252. [DOI] [PubMed] [Google Scholar]
- 27.Fujii Y, Kaneko S, Nzou SM, Mwau M, Njenga SM, Tanigawa C, et al. Serological surveillance development for tropical infectious diseases using simultaneous microsphere-based multiplex assays and finite mixture models. PLoS Negl Trop Dis. 2014 doi: 10.1371/journal.pntd.0003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rougier S, Montoya JG, Peyron F. Lifelong persistence of toxoplasma cysts: a questionable Dogma? Trends Parasitol. 2017;33(2):93–101. doi: 10.1016/j.pt.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. 2009;49(6):878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 30.Cook EAJ, De Glanville WA, Thomas LF, Kariuki S, de Bronsvoort BMC, Fèvre EM. Working conditions and public health risks in slaughterhouses in western Kenya. BMC Public Health. 2017;17(1):1–12. doi: 10.1186/s12889-016-3923-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mose JM, Kagira JM, Karanja SM, Ngotho M, Kamau DM, Njuguna AN, et al. Detection of natural Toxoplasma gondii Infection in chicken in Thika Region of Kenya using nested polymerase chain reaction. Biomed Res Int. 2016;2016:1–5. doi: 10.1155/2016/7589278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubey JP. Toxoplasma gondii infections in chickens (Gallus domesticus): prevalence, clinical disease, diagnosis and public health significance. Zoonoses Public Health. 2010;57(1):60–73. doi: 10.1111/j.1863-2378.2009.01274.x. [DOI] [PubMed] [Google Scholar]
- 33.Justus O, Owuor G, Bebe BO. Management practices and challenges in smallholder indigenous chicken production in western Kenya. J Agric Rural Dev Trop Subtrop. 2013;114(1):51–58. [Google Scholar]
- 34.Chaiban C, Robinson TP, Fèvre EM, Ogola J, Akoko J, Gilbert M, et al. Early intensification of backyard poultry systems in the tropics: a case study. Animal. 2020;14(11):2387–2396. doi: 10.1017/S175173112000110X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Setouhy M, Hussein H, Abdel Malek M. Toxoplasmosis among married female workers in poultiy slaughter houses in Cairo. A cross sectional study. Egypt J Community Med. 1997;15(2):21–26. [Google Scholar]
- 36.Basavaraju A. Toxoplasmosis in HIV infection: an overview. Trop Parasitol. 2016;6(2):129–135. doi: 10.4103/2229-5070.190817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NASCOP. Preliminary KENPHIA 2018 Report [Internet]. Nairobi; 2020 https://phia.icap.columbia.edu/wp-content/uploads/2020/04/KENPHIA-2018_Preliminary-Report_final-web.pdf. Accessed 18 May 2021.
- 38.Li CY, Sung FC. A review of the healthy worker effect in occupational epidemiology. Occup Med. 1999;49(4):225–229. doi: 10.1093/occmed/49.4.225. [DOI] [PubMed] [Google Scholar]
- 39.Mboera LEG, Kishamawe C, Kimario E, Rumisha SF. Mortality patterns of toxoplasmosis and its comorbidities in Tanzania: a 10-year retrospective hospital-based survey. Front Public Health. 2019;7:1–7. doi: 10.3389/fpubh.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nissen J, Jokelainen P, Stensvold CR, Trevisan C, Fuchs J, Burgdorf KS, et al. The disease burden of congenital toxoplasmosis in Denmark, 2014. PLoS ONE. 2017;12(5):1–12. doi: 10.1371/journal.pone.0178282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.