Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease with neuronal cell inclusions composed of neurofilaments and other abnormal aggregative proteins as pathological hallmarks. Approximately 90% of patients have sporadic cases (sALS), and at least 4 genes, i.e. C9orf72, SOD1, FUS and TARDBP, have been identified as the main causative genes, while many others have been proposed as potential risk genes. However, these mutations could explain only ~ 10% of sALS cases. The neurofilament polypeptides encoded by NEFH, NEFM, and NEFL are promising protein biomarkers for ALS and other degenerative diseases. However, whether the genetic variants of these genes were associated with ALS remain ambiguous.
Methods
Here, we used PCR-Sanger to sequence the exons of these three genes in a cohort of 371 sALS patients and 711 healthy controls (Phase I) and validated the risk variant in another 300 sALS patients and 1076 controls (Phase II).
Results
A total of 92 variants were identified, including 36 rare heterozygous variants in NEFH, 27 in NEFM, and 16 in NEFL, and only rs568759161 (p.Ser787Arg) in NEFH reached nominal statistical power (P = 0.02 at Phase I, P = 0.009 at Phase II) in the case–control comparison. Together, the Phase I and II studies showed the significantly higher frequency of the variant in cases (9/1342, 0.67%) than in controls (2/3574, 0.07%) (OR 12.06; 95% CI 2.60–55.88; P = 0.0003). No variants passed multiple testing in the discovery cohort, but rs568759161 was associated with ALS in a replication cohort.
Conclusions
Our results confirmed that NEFH Ser787Arg is a novel sALS risk variant in Chinese subjects, but NEFM and NEFL were not associated with sALS. These data may have implications for genetic counselling and for understanding the pathogenesis of sALS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12920-021-01073-z.
Keywords: Sporadic amyotrophic lateral sclerosis, Neurofilament genes, Rare variant, Association
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by loss of motor neurons in the brain and spinal cord, resulting in muscle atrophy, swallowing disorders, and pyramidal tract signs. A known pathological hallmark of ALS is neuronal cell inclusions composed of neurofilaments and other abnormal aggregative proteins [1–3]. Epidemiological surveys show an incidence of 0.6–3.8 per 100,000 persons per year and a prevalence of 4.1–8.4 per 100,000 persons worldwide [4]. It has been reported that the yearly incidence is 0.8 (2010–2015) per 100,000 persons in China [5]. Approximately 10% of cases were familial, and 90% were sporadic cases. To date, the genetics of ALS are not fully understood. In 1993, SOD1 was discovered as the first ALS-causing gene. Since then, many other genes have been reported to be causative for (i.e. C9orf72, SOD1, FUS, TARDBP, etc.) or associated with the disease [6, 7]. Genetic studies have found that mutations in these genes were mainly identified in familial cases and could explain only approximately 10% of sporadic cases (sALS) [6]. With next-generation sequencing, novel genes and loci have been increasingly discovered [8], but the genetics of sALS are not fully understood.
Neurofilaments (NFs) are type IV intermediate filament heteropolymers composed of light (NEFL), medium (NEFM), and heavy (NEFH) subunits. The different NF subunits have the same conserved alpha-helical rod domain and differ in the head and tail domains. NFs function by determining axonal calibre, promoting axonal growth, and forming a 3-dimensional lattice that supports cytoplasmic organelle organization [9]. NFs have been considered to play an essential role in many neurodegenerative diseases, such as ALS, Charcot-Marie-Tooth disease, and Parkinson's disease [10]. Many studies have revealed the relationship between NFs and ALS. First, one of the important pathological features of ALS is the cytoplasmic inclusion bodies of NFs [11–14]. Second, motor function impairment is observed in NF-subunit-transgenic mice [15, 16]. Third, studies have found a decrease in NEFL mRNA expression in the spinal cord tissue of patients with ALS [17, 18]. Recently, NEFL and phosphorylated NEFH (pNEFH) were considered as promising novel biomarkers in the blood and cerebrospinal fluid of ALS patients during disease onset and progression [19–21]. In previous studies, NEFH variants were reported in approximately 1% sALS cases [22–26], and NEFH was considered a susceptibility gene for ALS. However, the conclusions from many different studies are contradictory [27–29]. The mechanism may involve abnormal protein modification, folding, clearance, and axonal transport [30]. However, NEFM and NEFL have not been linked to ALS, although upregulation of NEFM has been detected in spinal cord tissues in patients with ALS or ALS-like diseases [31, 32]. Thus far, systemic sequencing studies of NEFH/NEFM/NEFL with large samples have been rare, and most of the relevant studies have been restricted to the Caucasian population. Moreover, the distribution, burden, and significance of these genetic variations remain ambiguous, especially in the Chinese sALS cohort.
In this study, we sequenced the variants in the exons of NEFH, NEFM, and NEFL in a Chinese sALS cohort including 371 sALS patients and 711 healthy controls (Phase I) to identify the potentially associated variants and validate these variants in another 300 sALS patients and 1076 controls (Phase II). We found that rs568759161 (p.Ser787Arg) in NEFH was a novel risk variant associated with sALS, and the distribution of this genetic variant was different from that observed in previous studies. However, NEFM and NEFL were not definitively associated with sALS.
Materials and methods
Patients and controls
A total of 371 sALS patients and 711 healthy subjects of Han ethnicity were recruited from the Department of Neurology of three hospitals (Fujian Medical University Union Hospital, Sanming First Hospital Affiliated to Fujian Medical University, and Xuanwu Hospital of Capital Medical University) from Jan 2016 to Nov 2020 (Phase I). Another 1076 healthy elderly control subjects were recruited from communities in Beijing as a further validation control group, and 300 sALS patients were recruited from the aforementioned hospitals to confirm the risk variant (Phase II). The inclusion criteria of the control group were healthy elderly people without diseases history of motor neuron diseases, degenerative neurological disorders or malignancy.
All patients with ALS were diagnosed by at least two neuromuscular specialists in each hospital based on clinical and electrophysiological findings according to the revised El Escorial criteria [33]. We only recruited sporadic cases in the study, which were defined as the absence of a second patient within three generations of the family, and frontotemporal dementia (FTD) was excluded in each patient in this study. And all sALS patients fulfilled the criteria for probable, or definite ALS based on this criterion. Clinical data, including age at onset (AAO), initial site of impairment, core symptoms and signs, electromyography, and nerve conduction velocity assessment, were reviewed and analysed reciprocally by researchers and specialists from the hospitals. Family inquiry was performed to exclude the existence of kinship among samples within at least three generations.
NEFH/NEFM/NEFL genetic analysis
Three millilitres of blood were obtained from sALS patients and controls. Polymerase chain reaction (PCR) assays and extension primers for exons were designed using Oligo 6.0 software (Molecular Biology Insights, Inc., CO, USA). The primer sequences for amplifying the exons of NEFL, NEFM, and NEFH are listed in Additional file 1: Table S1. PCR products were purified and sequenced by an ABI 3730 DNA Analyzer (Applied Biosciences, Inc., CT, USA). Chromas 2.22 software was used for sequence reading. The variant position at the genomic level was based on GenBank accession number NC_000022.10/NC_000008.10/NC_000008.10, the transcript position was based on NM_021076.3/NM_005382.2/NM_006158.4, and the protein-level position was based on NP_066554.2/NP_005373.2/NP_006149.2, according to the hg19/GRCh37 reference sequence. The chromosomal position, frequencies, and other relevant information of the variants were annotated using the 1000 Genome Project database (http://www.1000genomes.org), dbSNP version 147 (http://www.ncbi.nlm.nih.gov/projects/SNP), and the Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/) database. Variants were classified into the following categories according to their minor allele frequencies (MAFs): MAF > 0.05, common variant; 0.01 ≤ MAF ≤ 0.05, low-frequency variant; and MAF < 0.01, rare variant. We paid close attention to the rare variant (MAF < 0.01). The ExAC_EAS (for the East Asian population) and GnomAD_exome_EAS databases were used as references. Novel variants were defined as those that were not indexed in any of the databases, irrespective of ethnic population. The functional effect of the variants was predicted by combined annotation-dependent depletion (CADD) (https://cadd.gs.washington.edu/snv).
Statistical analysis
Low-frequency and common variants located in the NEFH, NEFM, and NEFL coding regions in the control group were tested for deviations from Hardy–Weinberg equilibrium using the χ2 test. Allele frequencies of common and low-frequency variants in patients and controls were compared by χ2 statistics using SPSS 22 software. Nominal P values were corrected for the number of variants tested using Bonferroni correction. The burden test for rare coding variants across the full NEFH, NEFM, and NEFL coding sequences was performed by the sequence kernel association test (SKAT-O) using R software (version 4.0.0). Differences in AAO between patients carrying and not carrying rare variants were calculated using an unpaired nonparametric (Mann–Whitney) test. P < 0.05 was considered statistically significant.
Results
Demographic data of the cohort
The cohort consisted of two phases of cases and controls. Phase I included 371 patients with sALS and 711 healthy controls. The AAO of the patients was 53.42 ± 10.28 years. Phase II included another 300 sALS patients and 1076 controls. The AAO was 53.49 ± 9.56 years in the cases. It has been reported that ALS was more prevalent in men, and the mean AAO was 51 (IQR 43–59) years in China [34]. Therefore, we chose more female individuals in the controls, who were older (69.41 ± 8.42 and 69.83 ± 7.70 years) than the cases selected (Table 1).
Table 1.
Demographic data of the study subjects
| Clinical features | Phase I | Phase II | Combined | |||
|---|---|---|---|---|---|---|
| sALS (n = 371) | Control (n = 711) | sALS (n = 300) | Control (n = 1076) | sALS (n = 671) | Control_2 (n = 1787) | |
| Sex, M/F (ratio) | 227/144 (1.58:1) | 283/428 (0.66:1) | 183/117 (1.56:1) | 453/623 (0.73:1) | 410/261 (1.57:1) | 736/1051 (0.70:1) |
| Age (year, mean ± SD) | 55.13 ± 10.28 | 69.41 ± 8.42 | 55.16 ± 9.82 | 69.83 ± 7.70 | 55.14 ± 9.97 | 69.66 ± 7.91 |
| Age at onset (year, mean ± SD) | 53.42 ± 10.28 | – | 53.49 ± 9.56 | – | 53.45 ± 9.96 | – |
| Site of onset, bulbar (%) | 73/371 (19.68%) | – | 59/300 (19.61) | – | 132/671 (19.67%) | – |
sALS, sporadic amyotrophic lateral sclerosis; AAO, age at onset
Rare coding variants identified in the NEFH, NEFM, and NEFL genes
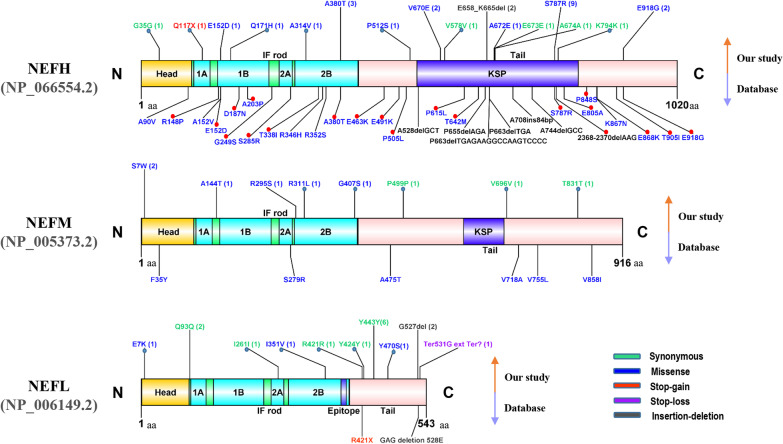
We screened the exons and their flanking sequences in the NEFH, NEFM, and NEFL genes by PCR and Sanger sequencing (Additional file 6: Fig. S1). We identified 92 variants, including 36 rare heterozygous variants in NEFH, 27 in NEFM, and 16 in NEFL. There were 16 rare coding variants of NEFH in 20 sALS cases, 8 of NEFM in 9 sALS cases, and 10 of NEFL in 17 sALS cases. The rare variants included 1 stop-gain, 1 stop-loss, 4 frameshift, 2 insertion/deletion, 44 missense, 1 intron-harboured, and 26 synonymous variants in 44 sALS cases (20 cases carried rare variants in NEFH, 9 in NEFM, and 17 in NEFL). Of the sALS patients who carried rare variants, 2 had variants in two genes. Of the rare nonsynonymous variants, 4 in NEFH, 2 in NEFM, and 3 in NEFL were identified only in cases, while 14 in NEFH, 13 in NEFM, and 5 in NEFL were identified only in control subjects. Seven variants in NEFH, 3 in NEFM, and 2 in NEFL were identified in both the cases and controls. Given these genes, 9 heterozygous missense mutations, 1 in-frame deletion, and 1 nonsense mutation in NEFH were distributed in 4 exons, especially in exons 4 and 1; 5 missense variants were distributed in exons 3 and 1 in NEFM; and 1 stop-loss, 1 in-frame deletion and 3 missense variants were distributed in the exons of NEFL (Fig. 1). Upon comparing the cases and controls, we found that only one variant, rs568759161 (c.2361C > G, p.Ser787Arg), in NEFH was nominally more frequent in cases than in controls (OR 9.64; 95% CI 1.12–82.67; P = 0.02) (Table 2 and Additional file 2: Table S2). However, no variants passed the Bonferroni multiple comparison test.
Fig. 1.
Comparison of the variants found in our research and previous literature reports. Numbers at the end of the variant name represent the number of patients. Variants detected in cases only are indicated by blue dots. Variants detected in next generation of sequencing (NGS) are indicated by red dots. 1A, 1B, 2A, 2B: The Coil 1A, Coil 1B, Coil 2A, Coil 2B regions, respectively; Rod: intermediate filament rod; KSP repeats, repeats of lysine-serine-proline; Epitope: recognized by an IF-specific monoclonal antibody; SubA, SubB: subdomain A, subdomain B (acidic). The amino acid position and functional domains are depicted according to the UniProt database (http://www.uniprot.org/uniprot/). The plot was created with DOG v. 2.0 software (http://dog.biocuckoo.org/). Abbreviations: ALS, amyotrophic lateral sclerosis; NEFH, neurofilament heavy polypeptide. NEFM, neurofilament medium polypeptide. NEFL neurofilament light polypeptide
Table 2.
Rare nonsynonymous coding variants identified in NEFH
| Group | Chr_Position | dbSNP | cDNA_change | AA _change | ExAC_EAS | gnomAD_exome_EAS | CADD | No. Carriers (n = 371) | No. Controls (n = 711) | P value | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case only | 22:29,876,600 | – | c.349C > T | p.Gln117Ter | – | 7.54E-05 | Damaging | 1 | 0 | 0.34 | Inf (Na-Inf) |
| 22:29,876,764 | – | c.513G > C | p.Gln171His | – | – | Tolerable | 1 | 0 | 0.34 | Inf (Na-Inf) | |
| 22:29,879,421 | rs539511579 | c.941C > T | p.Ala314Val | 2.32E-04 | 1.74E-04 | Damaging | 1 | 0 | 0.34 | Inf (Na-Inf) | |
| 22:29,885,163 | – | c.1534C > T | p.Pro512Ser | – | – | Damaging | 1 | 0 | 0.34 | Inf (Na-Inf) | |
| Control only | 22:29,876,270 | – | c.19G > A | p.Ala7Thr | – | – | Damaging | 0 | 1 | 1.00 | Na |
| 22:29,876,409 | rs772280985 | c.158C > T | p.Thr53Met | 0.00 | 0.00 | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,876,520 | rs61556467 | c.269C > T | p.Ala90Val | 0.00 | 0.00 | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,876,710 | rs763364083 | c.469_491del | p.Val157ArgfsTer115 | 0.00 | 1.29E-04 | – | 0 | 1 | 1.00 | Na | |
| 22:29,879,444 | rs778265423 | c.964C > T | p.Arg322Trp | 0.00 | 0.00 | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,884,842 | rs200464796 | c.1213C > A | p.Leu405Ile | 0.00 | 0.00 | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,885,182 | – | c.1553C > T | p.Ser518Leu | – | – | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,885,198 | rs138278265 | c.1569G > C | p.Glu523Asp | 0.00 | 0.00 | Tolerable | 0 | 1 | 1.00 | Na | |
| 22:29,885,215 | – | c.1586A > C | p.Glu529Ala) | – | – | Tolerable | 0 | 1 | 1.00 | Na | |
| 22:29,885,735 | – | c.2106_2110del | p.Lys703ProfsTer2 | – | – | – | 0 | 1 | 1.00 | Na | |
| 22:29,885,741 | – | c.2112_2232del | p.Pro705SerfsTer17 | – | – | – | 0 | 1 | 1.00 | Na | |
| 22:29,885,917 | – | c.2288C > T | p.Ser763Phe | – | – | Damaging | 0 | 1 | 1.00 | Na | |
| 22:29,886,118 | rs201757428 | c.2489C > T | p.Pro830Leu | 4.81E-04 | 2.91E-04 | Tolerable | 0 | 2 | 0.54 | Na | |
| 22:29,886,426 | rs777317391 | c.2797C > T | p.Pro933Ser | 9.53E-04 | 1.33E-03 | Tolerable | 0 | 1 | 1.00 | Na | |
| Both | 22:29,876,707 | rs774792100 | c.456G > C | p.Glu152Asp | 7.46E-03 | 4.57E-03 | Tolerable | 1 | 1 | 1.00 | 1.92 (0.12–30.70) |
| 22:29,881,766 | rs201416955 | c.1138G > A | p.Ala380Thr | 5.78E-03 | 5.33E-03 | Damaging | 3 | 8 | 0.76 | 0.72 (0.19–2.71) | |
| 22:29,885,581 | rs267607533 | c.1965_1988del | p.Glu658_Lys665del | 1.16E-04 | 8.37E-03 | – | 2 | 6 | 0.72 | 0.64 (0.13–3.17) | |
| 22:29,885,638 | rs190692435 | c.2009 T > A | p.Val670Glu | 1.17E-04 | 1.91E-03 | Tolerable | 2 | 5 | 1.00 | 0.77 (0.15–3.96) | |
| 22:29,885,644 | – | c.2015C > A | p.Ala672Glu | 1.17E-04 | 1.60E-03 | Tolerable | 1 | 5 | 0.67 | 0.38 (0.05–3.28) | |
| 22:29,885,990 | rs568759161 | c.2361C > G | p.Ser787Arg | 1.39E-03 | 2.38E-03 | Damaging | 5 | 1 | 0.02 | 9.64 (1.12–82.67) | |
| 22:29,886,382 | rs189881592 | c.2753A > G | p.Glu918Gly | 3.34E-03 | 3.53E-03 | Damaging | 2 | 2 | 0.61 | 1.92 (0.27–13.65) |
cDNA-level nomenclature was based on NM_021076.3. According to hg19/GRCh37, protein-level nomenclature was based on NP_066554.2; dbSNP, accession number of the variant in the Database of Single-Nucleotide Polymorphisms 147; MAF, minor allele frequency; ExAC_EAS and GnomAD_exome_EAS, MAFs of variants in the Exome Aggregation Consortium (ExAC) and GnomAD_exome databases for the East Asian population; In silico prediction by Combined Annotation Dependent Depletion (CADD); AA, amino acid; Ifn, infinity; 95% CI, 95% confidence interval; Na, not available,. A value of P < 0.05 was considered statistically significant (P < 0.0005 after Bonferroni correction)
Burden test for rare variants of NEFH and NEFL in ALS
To investigate the enrichment of rare coding variants in ALS, we performed the SKAT-O burden test for each gene. We chose the dominant inheritance (Dom) model for nonsynonymous coding variants and the non-benign and loss-of-function (LoF) variants. As shown in Additional file 3: Table S3, none of the genes showed significant enrichment of rare variants in cases.
Validation of the association of NEFH Ser787Arg variant with sALS
No variants passed multiple testing in the discovery cohort, in which the only rare variant, rs568759161 (p.Ser787Arg), in NEFH, was nominally associated with ALS. This variant was found in 5 sALS patients and 1 control in Phase I (MAF: 5/742, 0.67% and 1/1422, 0.07%; P = 0.02; OR 9.64; 95% CI 1.12–82.67). Due to the lack of significance in the Bonferroni correction, we added 300 cases and 1076 controls for sequencing (Phase II validation). The variant was found in 4 sALS patients and 1 control in Phase II (MAF: 4/600, 0.67% and 1/2152, 0.05%; P = 0.009; OR 14.43; 95% CI 1.61–129.40). Upon combining the two phases, the variant was shown to be significantly more abundant in cases than in controls (OR 12.06; 95% CI 2.60–55.88; P = 0.0003) (Table 3).
Table 3.
Statistical outcome in variant (NEFH p.Ser787Arg) carriers
| Variables | Phase I | Phase II | Combined | |||
|---|---|---|---|---|---|---|
| sALS (n = 371) | Control (n = 711) | sALS (n = 300) | Control (n = 1076) | sALS (n = 671) | Control_2 (n = 1787) | |
| No. carriers | 5 | 1 | 4 | 1 | 9 | 2 |
| MAF | 6.73E-03 | 7.03 E-04 | 6.67E-03 | 4.65 E-04 | 6.71E-03 | 5.60 E-04 |
| P value | 0.02 | 0.009 | 0.0003 | |||
| OR (95% CI) | 9.64 (1.12–82.67) | 14.43 (1.61–129.40) | 12.06 (2.60–55.88) | |||
sALS, sporadic amyotrophic lateral sclerosis; MAF, minor allele frequency; P value, determined using Fisher's exact test; 95% CI, 95% confidence interval
In clinical aspects, one female and 8 male sALS patients shared the NEFH p.Ser787Arg variant with AAO at 53.44 ± 13.51 years, which was not different from other sALS cases. Most (7/9) of these patients initially presented with limb symptoms (Additional file 4: Table S4).
Low-frequency and common coding variants identified in the NEFH, NEFM, and NEFL genes
Among all the nonsynonymous variants, 5 low-frequency (0.01 ≤ MAF ≤ 0.05) were identified in NEFH, 1 was in NEFM and 1 was in NEFL. For common (MAF > 0.05) variants, 4 were revealed in NEFH, 1 was in NEFM and 1 was in NEFL (Additional file 5: Table S5). The results suggested that genotype frequencies of NEFH, NEFM and NEFL were Hardy–Weinberg equilibrium in the control group. However, there were no statistically significant differences in common or low-frequency variants between the case and control groups.
Discussion
In our study, it was found that many domains harboured rare variants in NEFH, NEFM, and NEFL in sALS patients, which is different from the results of previous studies [35] and the ALSod database (https://alsod.ac.uk/output/gene.php#variants). Previous ALS studies have indicated that the NEFH mutations are insertions and deletions and are mostly located at the tail [22, 26]. Our study demonstrated more point variants than insertion/deletion variants in NEFH (Fig. 1). Second, our data showed more carriers with rare nonsynonymous variants (17/371, 4.58%) in NEFH than previous reports showed in other populations [25, 29, 36–38]. Third, mutation of NEFL has traditionally been recognized as a cause of Charcot-Marie-Tooth disease [39], congenital myopathy in humans [40], and motor neuron disease in mice [32]. NEFM is linked to Parkinson's disease [41]. However, NEFL and NEFM were not associated with sALS in our study. Although we had shown variants in these genes in sALS, we did not find significant differences in clinical characteristics (sex, AAO, onset site) between cases carrying and not carrying the variants. The differences further confirmed the genetic heterogeneity in sALS among different ethnicities and highlight the association of NEFH, but not NEFL or NEFM, with ALS.
This study found that the p.Ser787Arg variant in NEFH was associated with sALS in Chinese subjects. Notably, rs568759161 is only found in only the East Asian population according to ExAC (MAF 0.14%) and gnomAD (MAF 0.24%), and their MAFs were slightly higher than those of our control group (0.07% in Phase I and 0.05% in Phase II) (Table 3). We assume that the difference in allele frequency between the two databases and our study might be due to the population differences. Moreover, we found that some variants reported by other studies were not associated with ALS. For example, A380T in NEFH was identified only in cases previously [29], but our study suggested it was identified in both case and control groups. So, we believed study of rare variants need large samples of controls. In our study, we recruited relatively large controls (n = 711 in Phase I and n = 1076 in Phase II) to decrease the chance of false positive or false negative.
The phosphorylation of NF subunits has been considered a critical process regulating the formation and function of NFs [10]. The variant p.Ser787Arg is located in the phosphorylated region in a conserved sequence. Proper phosphorylation/dephosphorylation of NEFH may be considered a protective mechanism under conditions of cellular stress [16, 42], indicating that the modification of NEFH plays a significant role in maintaining the normal function of neurons. We hypothesized that the NEFH-S787R variant changes the phosphorylation of the protein. However, because of the unavailability of an antibody against the site, we did not test the hypothesis in this study. In the future, we need to synthesize antibodies against the phosphorylated NEFH-Ser787 site to further explore the changes in phosphorylation levels.
Recently, next-generation sequencing technology have identified many genes, including NEFH [43], as causative for or associated with ALS. In NEFH, 20 variants have been reported in ALS cases (Fig. 1) [25, 36–38, 44, 45], but none was conclusively related to the disease. The p.Ser787Arg was only reported by Chen et al. [45], but its association with ALS was not confirmed. Our study provided the spectrum of NEFH variants and confirmed the association of p. Ser787Arg with Chinese sALS.
Conclusion
In this study, we analysed the mutational spectrum of NEFH, NEFM, and NEFL genes in an sALS Chinese cohort and identified the variant (rs568759161) locating in the phosphorylated site of the KSP domain of NEFH as a risk variant associated with sALS in Chinese. Functional studies will be necessary to assess its role in ALS pathogenesis.
Supplementary Information
Additional file 1. Primers for amplification of exons.
Additional file 2. Rare non-synonymous coding variants in NEFM and NEFL.
Additional file 3. Burden in all rare variants.
Additional file 4. Clinical features of NEFH (S787R) carriers.
Additional file 5. Low frequency and common variants identified in NEFH, NEFM and NEFL genes.
Additional file 6. Workflow of the study design.
Acknowledgements
We thank all the participants of this study. We’re also grateful for Dr. Xiuli Feng and Dr. Shu Xie for their technical assistance, including PRC and Sanger sequencing.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- NFs
Neurofilaments
- FTD
Frontotemporal dementia
- AAO
Age at onset
- PCR
Polymerase chain reaction
- ExAC
Exome aggregation consortium
- MAFs
Minor allele frequencies
- CADD
Combined annotation-dependent depletion
- SKAT-O
Sequence kernel association test
- LoF
Loss-of-function
Authors' contributions
FL and HH initiated the project and wrote the manuscript. FL, WL, and CZ designed the experiments. FL, CZ, JL, and XL performed the experiments. FL, ZW, and CW analysed the data. FL, XL, JZ and JL constructed the figures. FL, XL, JZ and XL created the tables. All authors read and approved the final manuscript.
Funding
This study was supported by funds from the Innovation of Science and Technology, Fujian Province (Grant Number 2017Y9058), to Prof. Huapin Huang; the Fujian Provincial Health Technology Project (Grant Number 2019-ZQN-38), to Dr. Wanhui Lin; the Fujian Sanming Science and Technology Plan Project (Grant Number 2019-S-3) and Fujian Provincial Science and Technology Project (Grant Number 2020J011271), to Dr. Feng Lin; and the Ministry of Science and Technology (Grant Number 2016YFC1306000), Special Fund from Key Laboratory of Neurodegenerative Diseases, Ministry of Education of China (PXM2019_026283_000002), and National Natural Science Foundation (Grant Number 81771212) to Prof. Chaodong Wang.
Availability of data and materials
All genetic polymorphisms identified in this study is available at Table 2, Additional file 2: Table S2 and Additional file 5: Table S5. The primer sequences are shown in Additional file 1: Table S1. The original sequencing and clinical datasets generated during the current study are not publicly available due to maintaining patient confidentiality but are available from the corresponding author (hh-p@163.com) on reasonable request.
Declarations
Ethics approval and consent to participate
All research participants or their legal representatives signed informed consent forms for participation in clinical and genetic research. The medical ethics committee approved the protocol and provided informed consent (2021KY016) of the whole study at Fujian Medical University Union Hospital, Fuzhou, China. In this study, all methods were performed in accordance with the relevant guidelines and regulations in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
None of the authors declared conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leung CL, He CZ, Kaufmann P, Chin SS, Naini A, Liem RK, et al. A pathogenic peripherin gene mutation in a patient with amyotrophic lateral sclerosis. Brain Pathol. 2004;14:290–296. doi: 10.1111/j.1750-3639.2004.tb00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlary L, Plotkin SS, Yerbury JJ, Cashman NR. Prion-like propagation of protein misfolding and aggregation in amyotrophic lateral sclerosis. Front Mol Neurosci. 2019;12:262. doi: 10.3389/fnmol.2019.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin H, Schlaepfer WW. Role of neurofilament aggregation in motor neuron disease. Ann Neurol. 2006;60:399–406. doi: 10.1002/ana.20965. [DOI] [PubMed] [Google Scholar]
- 4.Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol. 2019;32:771–776. doi: 10.1097/WCO.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou S, Zhou Y, Qian S, Chang W, Wang L, Fan D. Amyotrophic lateral sclerosis in Beijing: epidemiologic features and prognosis from 2010 to 2015. Brain Behav. 2018;8:e01131. doi: 10.1002/brb3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor JP, Brown RH, Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17085. doi: 10.1038/nrdp.2017.85. [DOI] [PubMed] [Google Scholar]
- 8.Pampalakis G, Mitropoulos K, Xiromerisiou G, Dardiotis E, Deretzi G, Anagnostouli M, Katsila T, Rentzos M, Patrinos GP. New molecular diagnostic trends and biomarkers for amyotrophic lateral sclerosis. Hum Mutat. 2019;40(4):361–373. doi: 10.1002/humu.23697. [DOI] [PubMed] [Google Scholar]
- 9.Gentil BJ, Tibshirani M, Durham HD. Neurofilament dynamics and involvement in neurological disorders. Cell Tissue Res. 2015;360:609–620. doi: 10.1007/s00441-014-2082-7. [DOI] [PubMed] [Google Scholar]
- 10.Al-Chalabi A, Miller CC. Neurofilaments and neurological disease. BioEssays. 2003;25:346–355. doi: 10.1002/bies.10251. [DOI] [PubMed] [Google Scholar]
- 11.Kobayakawa Y, Sakumi K, Kajitani K, Kadoya T, Horie H, Kira J, et al. Galectin-1 deficiency improves axonal swelling of motor neurones in SOD1(G93A) transgenic mice. Neuropathol Appl Neurobiol. 2015;41:227–244. doi: 10.1111/nan.12123. [DOI] [PubMed] [Google Scholar]
- 12.Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, Maruyama S, Yamane K, Sakuma H, Takeishi M. Swellings of proximal axons in a case of motor neuron disease. Ann Neurol. 1989;25:520–522. doi: 10.1002/ana.410250520. [DOI] [PubMed] [Google Scholar]
- 15.Elder GA, Friedrich VL, Jr, Pereira D, Tu PH, Zhang B, et al. Mice with disrupted midsized and heavy neurofilament genes lack axonal neurofilaments but have unaltered numbers of axonal microtubules. J Neurosci Res. 1999;57:23–32. doi: 10.1002/(SICI)1097-4547(19990701)57:1<23::AID-JNR3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Xie F, Siedlak SL, Nunomura A, Honda K, Moreira PI, et al. Neurofilament proteins in neurodegenerative diseases. Cell Mol Life Sci. 2004;61:3057–3075. doi: 10.1007/s00018-004-4268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge WW, Leystra-Lantz C, Wen W, Strong MJ. Selective loss of trans-acting instability determinants of neurofilament mRNA in amyotrophic lateral sclerosis spinal cord. J Biol Chem. 2003;278:26558–26563. doi: 10.1074/jbc.M302886200. [DOI] [PubMed] [Google Scholar]
- 18.Bergeron C, Beric-Maskarel K, Muntasser S, Weyer L, Somerville MJ, Percy ME. Neurofilament light and polyadenylated mRNA levels are decreased in amyotrophic lateral sclerosis motor neurons. J Neuropathol Exp Neurol. 1994;53:221–230. doi: 10.1097/00005072-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Poesen K, Van Damme P. Diagnostic and prognostic performance of neurofilaments in ALS. Front Neurol. 2018;9:1167. doi: 10.3389/fneur.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 21.Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R, et al. Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:404–413. doi: 10.3109/21678421.2016.1167913. [DOI] [PubMed] [Google Scholar]
- 22.Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 23.Skvortsova V, Shadrina M, Slominsky P, Levitsky G, Kondratieva E, Zherebtsova A, et al. Analysis of heavy neurofilament subunit gene polymorphism in Russian patients with sporadic motor neuron disease (MND) Eur J Hum Genet. 2004;12:241–244. doi: 10.1038/sj.ejhg.5201144. [DOI] [PubMed] [Google Scholar]
- 24.Al-Chalabi A, Andersen PM, Nilsson P, Chioza B, Andersson JL, Russ C, et al. Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet. 1999;8:157–164. doi: 10.1093/hmg/8.2.157. [DOI] [PubMed] [Google Scholar]
- 25.Tripolszki K, Gampawar P, Schmidt H, Nagy ZF, Nagy D, Klivényi P, et al. Comprehensive genetic analysis of a Hungarian amyotrophic lateral sclerosis cohort. Front Genet. 2019;10:732. doi: 10.3389/fgene.2019.00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomkins J, Usher P, Slade JY, Ince PG, Curtis A, Bushby K, et al. Novel insertion in the KSP region of the neurofilament heavy gene in amyotrophic lateral sclerosis (ALS) NeuroReport. 1998;9:3967–3970. doi: 10.1097/00001756-199812010-00036. [DOI] [PubMed] [Google Scholar]
- 27.Vechio JD, Bruijn LI, Xu Z, Brown RH, Jr, Cleveland DW. Sequence variants in human neurofilament proteins: absence of linkage to familial amyotrophic lateral sclerosis. Ann Neurol. 1996;40:603–610. doi: 10.1002/ana.410400410. [DOI] [PubMed] [Google Scholar]
- 28.Rooke K, Figlewicz DA, Han FY, Rouleau GA. Analysis of the KSP repeat of the neurofilament heavy subunit in familiar amyotrophic lateral sclerosis. Neurology. 1996;46:789–790. doi: 10.1212/WNL.46.3.789. [DOI] [PubMed] [Google Scholar]
- 29.Garcia ML, Singleton AB, Hernandez D, Ward CM, Evey C, Sapp PA, et al. Mutations in neurofilament genes are not a significant primary cause of non-SOD1-mediated amyotrophic lateral sclerosis. Neurobiol Dis. 2006;21:102–109. doi: 10.1016/j.nbd.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Julien JP. Amyotrophic lateral sclerosis. Unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/S0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 31.Wu YY, Kuo HC. Functional roles and networks of non-coding RNAs in the pathogenesis of neurodegenerative diseases. J Biomed Sci. 2020;27:49. doi: 10.1186/s12929-020-00636-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bomont P, Cavalier L, Blondeau F, Ben Hamida C, Belal S, Tazir M, et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet. 2000;26:370–374. doi: 10.1038/81701. [DOI] [PubMed] [Google Scholar]
- 33.Brooks BR, Miller RG, Swash M, Munsat TL. World federation of neurology research group on motor neuron diseases: El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 34.Dorst J, Chen L, Rosenbohm A, Dreyhaupt J, Hübers A, Schuster J, et al. Prognostic factors in ALS: a comparison between Germany and China. J Neurol. 2019;266:1516–1525. doi: 10.1007/s00415-019-09290-4. [DOI] [PubMed] [Google Scholar]
- 35.Yuan A, Rao MV, Veeranna Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9:a018309. doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura R, Sone J, Atsuta N, Tohnai G, Watanabe H, Yokoi D, et al. Next-generation sequencing of 28 ALS-related genes in a Japanese ALS cohort. Neurobiol Aging. 2016;39(219):e1–8. doi: 10.1016/j.neurobiolaging.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Nishiyama A, Niihori T, Warita H, Izumi R, Akiyama T, Kato M, et al. Comprehensive targeted next-generation sequencing in Japanese familial amyotrophic lateral sclerosis. Neurobiol Aging. 2017;53:194.e1–194.e8. doi: 10.1016/j.neurobiolaging.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Morgan S, Shatunov A, Sproviero W, Jones AR, Shoai M, Hughes D, et al. A comprehensive analysis of rare genetic variation in amyotrophic lateral sclerosis in the UK. Brain. 2017;140:1611–1618. doi: 10.1093/brain/awx082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maciel R, Correa R, Bosso Taniguchi J, Prufer Araujo I, Saporta MA. Human tridimensional neuronal cultures for phenotypic drug screening in inherited peripheral neuropathies. Clin Pharmacol Ther. 2020;107:1231–1239. doi: 10.1002/cpt.1718. [DOI] [PubMed] [Google Scholar]
- 40.Agrawal PB, Joshi M, Marinakis NS, Schmitz-Abe K, Ciarlini PD, Sargent JC, et al. Expanding the phenotype associated with the NEFL mutation: neuromuscular disease in a family with overlapping myopathic and neurogenic findings. JAMA Neurol. 2014;71:1413–1420. doi: 10.1001/jamaneurol.2014.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavedan C, Buchholtz S, Nussbaum RL, Albin RL, Polymeropoulos MH. A mutation in the human neurofilament M gene in Parkinson's disease that suggests a role for the cytoskeleton in neuronal degeneration. Neurosci Lett. 2002;322:57–61. doi: 10.1016/S0304-3940(01)02513-7. [DOI] [PubMed] [Google Scholar]
- 42.Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PL, Shimohama S, et al. High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem. 2002;277:4644–4648. doi: 10.1074/jbc.M110913200. [DOI] [PubMed] [Google Scholar]
- 43.Pecoraro V, Mandrioli J, Carone C, Chiò A, Traynor BJ, Trenti T. The NGS technology for the identification of genes associated with the ALS. A systematic review. Eur J Clin Invest. 2020;50(5):e13228. doi: 10.1111/eci.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu ZJ, Lin HX, Wei Q, Zhang QJ, Chen CX, Tao QQ, Liu GL, Ni W, Gitler AD, Li HF, Wu ZY. Genetic spectrum and variability in Chinese patients with amyotrophic lateral sclerosis. Aging Dis. 2019;10(6):1199–1206. doi: 10.14336/AD.2019.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen W, Xie Y, Zheng M, Lin J, Huang P, Pei Z, Yao X. Clinical and genetic features of patients with amyotrophic lateral sclerosis in southern China. Eur J Neurol. 2020;27(6):1017–1022. doi: 10.1111/ene.14213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Primers for amplification of exons.
Additional file 2. Rare non-synonymous coding variants in NEFM and NEFL.
Additional file 3. Burden in all rare variants.
Additional file 4. Clinical features of NEFH (S787R) carriers.
Additional file 5. Low frequency and common variants identified in NEFH, NEFM and NEFL genes.
Additional file 6. Workflow of the study design.
Data Availability Statement
All genetic polymorphisms identified in this study is available at Table 2, Additional file 2: Table S2 and Additional file 5: Table S5. The primer sequences are shown in Additional file 1: Table S1. The original sequencing and clinical datasets generated during the current study are not publicly available due to maintaining patient confidentiality but are available from the corresponding author (hh-p@163.com) on reasonable request.