Abstract
Context:
By providing timely services at all steps along the continuum of the early hearing detection and intervention (EHDI) process, providers may be able to lessen potential adverse effects of late identification of hearing loss on children’s language development.
Objective:
To examine the timeliness of key events in the EHDI process from birth through diagnosis of hearing loss among different populations.
Design:
Retrospective, cross-sectional.
Setting:
Data pooled from 9 states’ EHDI information systems were used to determine the extent to which timely screening and diagnosis were achieved by 754 613 infants born in calendar year 2017. Enrollment into early intervention for children diagnosed is not examined here due to incomplete data.
Participants:
Nine state EHDI programs were selected to participate in this study for their successful experience in using EHDI-IS to collect detailed child-level data.
Main Outcome Measures:
Age of service, rate of service receipt.
Results:
Median age of newborn hearing screening was 1 day, and median age of hearing loss diagnosis was 68 days. Early completion of newborn hearing screening was associated with maternal education, maternal race/ethnicity, and admission into a neonatal intensive care unit (NICU). Receiving and completing follow-up diagnostic services were associated with maternal education, maternal race/ethnicity, age of screening, and enrollment into the Women, Infants, and Children program.
Conclusions:
Timely completion of the newborn hearing screening is achieved by most of the population among the participating states. Increased efforts may be considered by state EHDI programs to provide additional follow-up and education to underrepresented racial/ethnic groups, mothers with less education, and NICU infants and their families as these groups appear to be at an increased risk for delayed diagnostic testing for hearing loss.
Keywords: diagnostic evaluation, EHDI, hearing loss, hearing screening, socioeconomic disparities
Congenital hearing loss affects approximately 2 infants per 1000 live births and, if undetected, can delay speech, language, and cognitive development.1–3 All US states now have early hearing detection and intervention (EHDI) programs that work to ensure that all infants who are deaf or hard of hearing receive timely diagnosis and early intervention (EI). The cornerstone of EHDI programs is universal newborn hearing screening. Most states have enacted legislation to ensure that all babies undergo hearing screening, typically before hospital discharge.
Evidence of positive effects of universal newborn hearing screening on early hearing loss identification and entry into EI is well documented,4,5 which have both been linked with improved language outcomes.6–8 These studies have laid the foundation for establishing meaningful benchmarks to assess the effectiveness of EHDI programs. The EHDI 1–3-6 benchmarks (screening by 1 month, diagnosis by 3 months, and enrollment into EI by 6 months of age) were initially introduced by the Joint Committee on Infant Hearing (JCIH) in 20009 and re-endorsed in 2007.10 In the recent JCIH 2019 position statement,11 it is recommended that EHDI programs already meeting the 1–3-6 benchmarks consider setting a new target of 1–2-3 (screening by 1 month, diagnosis by 2 months, and EI by 3 months of age).
In the United States, every state EHDI program has implemented its own tracking and surveillance information system, or EHDI-IS. These population-based systems collect and consolidate data at different stages of the EHDI process to support effective tracking and follow-up of hearing services for all infants. Each year, states submit data from their EHDI-IS to the Centers for Disease Control and Prevention through the Hearing Screening and Follow-up Survey (HSFS). The HSFS provides data aggregated at the state level to assess the nation’s progress toward the EHDI 1–3-6 benchmarks.12,13 However, such aggregate data cannot be used to identify the determinants of failing to meet the benchmarks. Even with an overall national screening rate well above 90%, several recent studies showed that geographic, racial, and socioeconomic disparities exist in the provision of newborn hearing screening and diagnostic services.14–17 In addition, the HSFS can provide only binary measures to assess the receipt and timeliness of EHDI services. For example, to measure progress toward meeting the 1–3-6 benchmarks, the result was recorded simply as “Yes” or “No.” This does not provide the range of ages for services across the continuum of care.
In this study, we used standardized, child-level data obtained from 9 states’ EHDI-IS to evaluate the extent to which the national EHDI benchmarks for screening and diagnosis were met for babies born in 2017. We were able to look beyond binary results, to assess timeliness of services across the entire continuum of care, and thus to provide evidence about feasibility of benchmark updating. We also discuss several clinical, socioeconomic, and demographic contributors to disparities in order to identify areas for future program improvements.
Methods
Study population
The patient cohort for this study comprised 754 613 infants who were born January 1, 2017, through December 31, 2017, as documented in 9 participating states’ EHDI-IS. The 9 states were selected for their successful experience in using EHDI-IS to collect detailed child-level data.
Data collection
Child-level demographic, newborn hearing screening, follow-up screening, diagnostic evaluation, EI enrollment, and other clinical care and service data items used in this study (see Supplemental Data Content Appendix A, available at http://links.lww.com/JPHMP/A691) were extracted by each state program from its EHDI-IS and exported into a comma-separated vector (csv) file. The privacy of the patients’ records was maintained by removing personally identifiable information, such as names and street addresses, from each record before submission. The state project staff ensured records were deduplicated and coded consistently according to a standard specification using a validation software tool designed for this study. Once validated, the data files were transmitted via a secure data transport service available to each participating state.
Measures
Screening:
Newborn hearing screening is the first step in the EHDI process. The result of a hearing screening could be either pass or refer. Babies who do not pass the initial screening at birth are typically referred for rescreening or further testing.
Final screening is defined as the most recent hearing screening documented in the state EHDI-IS at the time of data submission. If a child received more than 1 screening, the outcome (pass or refer) is determined by the result of the final screening.
Age of screening:
For children who had more than 1 screening and had passing results, the age of screening is defined as the earliest age (in days) of the child when he or she received the screening. For all other cases, age of screening is defined as the age of the final screening in days.
Diagnostic evaluation is also known as a diagnostic test. It refers to a series of diagnostic procedures used to determine the type, degree, and configuration of hearing loss and is typically performed by an audiologist. The result of a diagnostic evaluation could be one of the following: (1) a confirmed diagnosis that the patient has normal hearing; (2) a confirmed diagnosis that the patient has permanent hearing loss; (3) the patient has transient hearing loss and should be evaluated again; or (4) the patient’s condition cannot be determined and should be evaluated again.
Diagnosis is defined as either normal hearing or permanent hearing loss. Transient hearing loss cases and undermined results of diagnostic evaluation were considered as no diagnosis.
Rate of diagnosis is defined as the percentage of children who have received a diagnosis among all children in the pooled data set who were referred to diagnostic evaluation on their final hearing screening.
Age of diagnosis is the age of the child, in days, when a diagnosis was first made.
Socioeconomic status (SES):
Since family income and parent’s occupational data were not requested from the participating states in this study, we used maternal education level as the primary indicator of the family’s SES. In addition, we used whether the family participated in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) as a secondary SES indicator, as there is an income limit for WIC eligibility.
Concentration curve and concentration index (CI) as measures of health inequalities:
Wagstaff et al18 argued that CI is the most appropriate measure of health inequality. As shown in the Figure, the CI is defined as twice the area between the concentration curve and the diagonal, ranging from −1 to +1. The sign of CI indicates the direction of any relationship between the health variable and the position in the SES distribution, and its magnitude, reflects both the strength of the relationship and the degree of variability in the health variable. The larger the absolute value of CI, the greater the inequality. CI equals zero when the concentration curve coincides with the diagonal, indicating there is no socioeconomic inequality in the health outcome. In this study, we define 2 health variables: screening by 30 days of age, and diagnosis by 90 days of age. If the curve lies above the diagonal (CI < 0), this indicates that meeting the screening by 30 days or diagnosis by 90 days benchmark is more concentrated among low-SES groups. If the concentration curve lies below the diagonal line (CI > 0), indicating that meeting either benchmark is more concentrated in high-SES groups.
FIGURE.
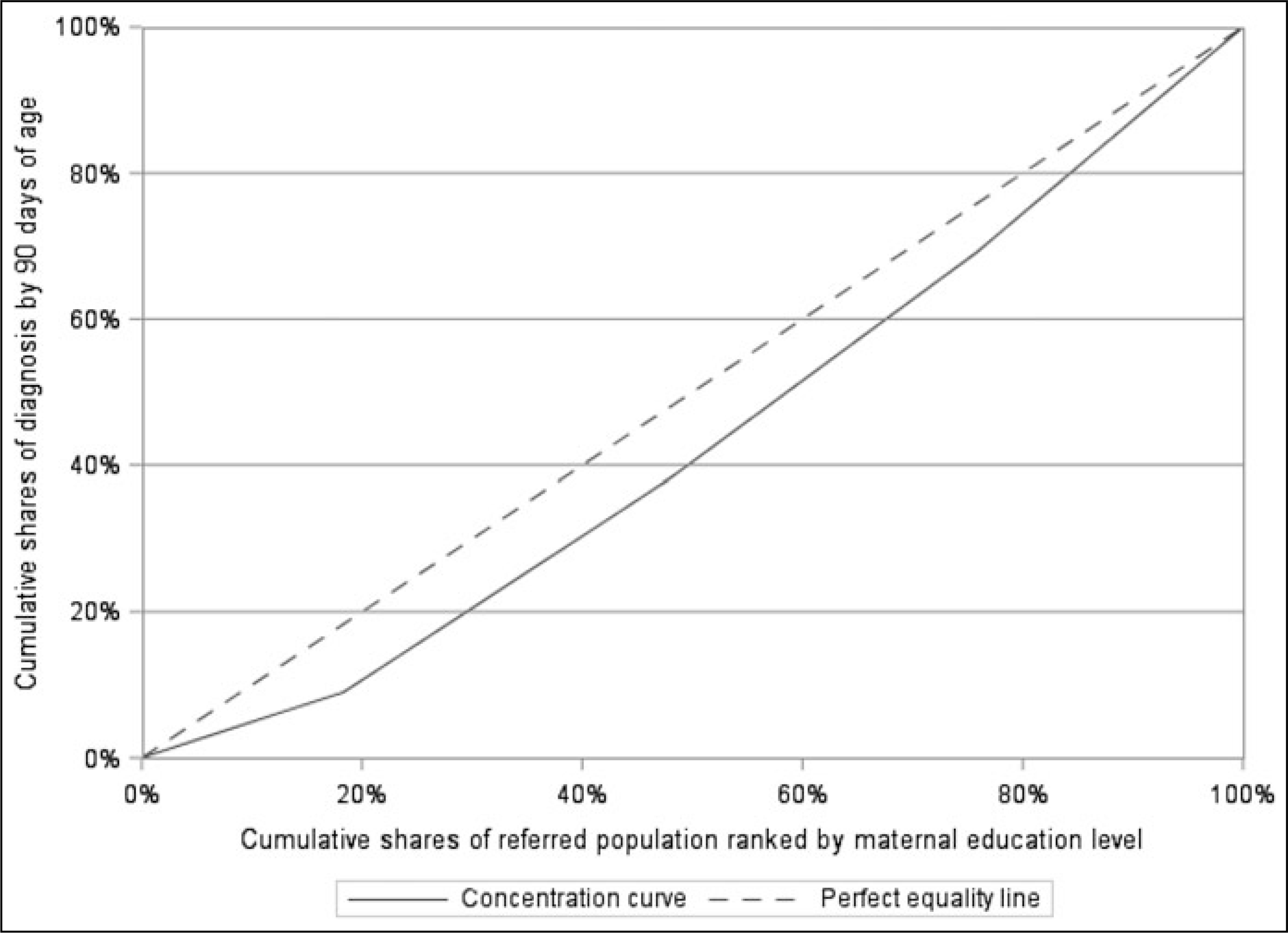
Concentration Curve of Audiological Diagnosis by 90 Days of Age, 9 States, 2017a
Abbreviation: SES, socioeconomic status.
aThe dotted diagonal was defined as the “equality line,” where achieving the desired health outcome (diagnosis by 90 days of age) or the burden of not achieving the desired health outcome is equally distributed across SES levels indicated by maternal education. The solid curve below the diagonal represents a progressive concentrative curve. It indicates that achieving the desired health outcome is concentrated more heavily among the higher-SES group.
Analysis
Data were combined across 9 states for analysis. We compared the screening and diagnostic evaluation processes based on birth admission (neonatal intensive care unit [NICU] vs well-baby nursery), demographic (maternal race/ethnicity), and socioeconomic (WIC, maternal education) status. Descriptive analysis consists of group frequencies for discrete variables and means, standard deviations, and intermediate percentiles for continuous variables. Inferential analysis consists of a series of univariate analyses including χ2 tests, logistic regression, and linear regression. Using maternal education level for SES, we calculated overall CI and group-specific CIs, stratified by maternal race/ethnicity using the method described by the World Bank.19 The t tests were conducted to determine whether these indices were significantly different from zero and to test the difference among group-specific CIs. SAS statistical software (version 9.4; SAS Institute) was used for the analyses. If more than 5% of data on a specific variable were missing or unknown, the missing data were listed as a separate category. Otherwise, the missing data were excluded from the corresponding analysis.
Human participant compliance statement
The information cited in this article is based on data reported by state programs and does not involve medical records or human tissues.
Results
In this sample, more than 97% of newborns met the timely screening benchmark of screening by 30 days of age (Table 1). However, this high proportion was heavily influenced by passes: almost all met the benchmark, with 95% screened within 1 week of birth. On the contrary, less than 90% of babies who did not pass newborn hearing screening met the timely screening benchmark and approximately 5% did not receive their final screening until over 2 months after birth.
TABLE 1.
Screening and Diagnosis Benchmarks and Age of Service Completion Among Infants Born in 2017, Overall and by NICU Status, 9 States
| Screening |
Diagnosis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Totala | Total Adjustedb | % Meeting Benchmark, Adjustedb,c | Age-of-Screening Percentiles, d |
Totala | % Evaluated | % Diagnosed | % Meeting Benchmarkc | Age-of-Diagnosis Percentiles, d |
||||||
| 50th | 75th | 95th | 50th | 75th | 95th | |||||||||
|
| ||||||||||||||
| Resultd | 754 613 | 733 685 | 97.1 | 1.0 | 2.0 | 7.0 | 8 829 | 66.8 | 55.8 | 41.6 | 49.0 | 92.6 | 291.0 | |
| + | 8 829 | 8 606 | 89.7 | 2.0 | 3.0 | 66.0 | 1 201 | n/a | n/a | 62.6 | 68.0 | 134.0 | 353.0 | |
| − | 733 303 | 712 598 | 98.9 | 1.0 | 2.0 | 7.0 | 3 727 | n/a | n/a | 78.4 | 44.0 | 81.0 | 263.0 | |
| NICU statuse | ||||||||||||||
| Yes | 70 017 | 66 654 | 82.1 | 5.0 | 14.0 | 57.0 | 1 801 | 73.9 | 56.2 | 31.5 | 79.0 | 151.0 | 356.0 | |
| No | 684 226 | 666 675 | 98.6 | 1.0 | 2.0 | 3.0 | 7 019 | 65.0 | 55.7 | 44.2 | 44.0 | 78.0 | 257.0 | |
Abbreviations: n/a, not applicable: NICU, neonatal intensive care unit.
Total births for screening, including 12 481 without screening results: total referred for diagnostic evaluation, including 3901 without diagnostic results.
Adjusted by excluding cases where the baby was screened but age of screening was missing.
Screening by 30 days of age: diagnosis by 90 days of age.
–: result for screening is pass: +: result for screening is refer; −: result for diagnosis is normal hearing: +: result for diagnosis is permanent hearing loss.
Status unknown: 370 for screening, 9 for diagnostics.
About two-thirds of the babies who did not pass screening underwent diagnostic evaluation, a little over half (55.8%) received a diagnosis of normal hearing or permanent hearing loss, and even fewer (41.6%) achieved the timely diagnosis benchmark of diagnosis by 90 days of age. Among all babies diagnosed with permanent hearing loss, less than two-thirds (62.6%) met the timely diagnosis benchmark. The median age of diagnosis for babies with normal hearing was 44 days and 68 days for those diagnosed with permanent hearing loss.
Approximately 80% of babies who have spent time in NICU nurseries met the timely screening benchmark as compared with more than 98% for infants in the well-baby nurseries. NICU babies also lag babies in the well-child nurseries in meeting the timely diagnosis benchmark. However, regardless of the timeliness of the service, NICU babies were more likely to receive a diagnostic evaluation than babies in the well-child nursery (χ21,8820 = 51.30, P < .01). Ultimately, there was no significant difference in the eventual rate of diagnosis between the 2 groups (χ21,8820 = 0.17, P = 0.68).
Among all listed racial/ethnic groups, infants born to non-Hispanic Black mothers had the lowest rate of meeting the screening benchmark (Table 2). Non-Hispanic Black and American Indian/Alaska Natives were the 2 groups that had the lowest rates of receiving diagnostic evaluation and meeting the timely diagnosis benchmark and the highest median age of diagnosis. A little over half (54.4%) of babies born to non-Hispanic Black mothers underwent diagnostic evaluation after not passing their final hearing screening, and less than one-third of these babies (30.5%) met the timely diagnosis benchmark compared with babies born to non-Hispanic White mothers where nearly three-fourths (73.1%) of the babies were evaluated and half of them (48.9%) were diagnosed before 90 days of age. The median age of diagnosis with permanent hearing loss for babies born to American Indian/Alaska Native mothers was 115 days, nearly 2 months later than babies born to non-Hispanic White mothers (62 days).
TABLE 2.
Screening and Diagnosis Benchmarks and Age of Diagnosis Among Infants Born in 2017, by WIC Status, Maternal Education, and Maternal Race/Ethnicity, 9 States
| Diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening |
Median Age of Diagnosis, d |
||||||||
| Total Births | Total Births, Adjusteda | % Meeting Benchmark, Adjusteda,b | Total Not Passing Final Screening | % Evaluated | % Diagnosed | % Meeting Benchmarkb | +/− Resultsc | + Resultc Only | |
|
| |||||||||
| WIC status | |||||||||
| Yes | 212 682 | 206 624 | 97.2 | 2 834 | 63.2 | 53.0 | 37.8 | 52.0 | 74.0 |
| No | 387 030 | 378 743 | 97.1 | 3 676 | 66.1 | 57.1 | 44.8 | 44.0 | 60.0 |
| Unknown | 154 834 | 148 318 | 90.9 | 2 319 | 72.4 | 57.2 | 42.3 | 52.0 | 76.0 |
| Maternal education | |||||||||
| Below high school | 87 214 | 84 341 | 95.0 | 1 523 | 56.7 | 44.9 | 29.0 | 63.0 | 87.0 |
| High school diploma | 177 408 | 172 361 | 96.9 | 2 495 | 60.6 | 49.5 | 35.6 | 52.0 | 83.0 |
| Some college/Associate degree | 202 488 | 196 872 | 97.1 | 2 285 | 68.5 | 58.3 | 43.8 | 49.0 | 62.0 |
| Bachelor’s degree and beyond | 252 187 | 247 005 | 97.8 | 2 013 | 80.1 | 69.8 | 57.1 | 41.0 | 54.0 |
| Unknown | 35 316 | 33 106 | 96.2 | 513 | 67.8 | 52.8 | 37.8 | 56.0 | 74.5 |
| Maternal race/ethnicity | |||||||||
| Non-Hispanic White | 405 637 | 396 176 | 97.4 | 4 025 | 73.1 | 61.9 | 48.9 | 44.0 | 62.0 |
| Non-Hispanic Black | 136 204 | 132 179 | 95.5 | 2 171 | 54.4 | 44.9 | 30.5 | 56.0 | 90.0 |
| Asian/Pacific Islander | 45 084 | 44 021 | 98.4 | 435 | 75.4 | 63.9 | 48.5 | 49.0 | 55.0 |
| American Indian/Alaska Natives | 4 173 | 4 006 | 97.1 | 77 | 58.4 | 46.9 | 27.3 | 74.0 | 115.0 |
| Hispanic | 106 753 | 103 360 | 97.8 | 1 391 | 67.1 | 55.6 | 39.6 | 54.0 | 69.0 |
| Undeterminedd | 56 762 | 53 943 | 96.8 | 730 | 64.2 | 51.2 | 35.3 | 63.0 | 71.0 |
Abbreviation: WIC, the Special Supplemental Nutrition Program for Women, Infants, and Children.
Adjusted by excluding cases where the baby was screened but age of screening was missing.
Screening by 30 days of age: diagnosis by 90 days of age.
−: result for diagnosis is normal hearing: +: result for diagnosis is permanent hearing loss.
Undetermined includes multirace, other races, and cases with missing or incomplete race/ethnicity information.
The rate of meeting the timely diagnosis benchmark was lower among babies who were enrolled in the WIC program than in those babies not enrolled in the WIC program. The rates of both the timely screening and diagnosis increased with higher maternal education level. Less than 30% of babies born to mothers with less than high school education met the timely diagnosis benchmark compared with nearly 60% (57.1%) for babies born to mothers who have completed college. We computed the CI to further quantify relative inequality in meeting these benchmarks among children of mothers with different education levels. For screening, the result (CI = 0.004, SE = 0.001, P < .01) indicated that timely screening was slightly concentrated among higher-SES populations. On the contrary, a CI of 0.13 (SE = 0.007, P < .01) confirmed that meeting the timely diagnosis benchmark was more heavily concentrated among economically better-off families.
We also compared the CIs across different racial/ethnic groups (see Supplemental Digital Content Appendix B, available at http://links.lww.com/JPHMP/A692). The CIs for children with non-Hispanic Black and non-Hispanic White mothers were positive (P < .01), indicating that meeting the timely diagnosis benchmark was more concentrated in the higher-SES non-Hispanic families. The CI for children with Hispanic mothers was also positive but smaller than that of the other 2 groups (P = .15), indicating that among the Hispanic population there was no strong socioeconomic inequality in relation to receiving diagnosis by 90 days. There was also no significant difference between CIs for the non-Hispanic Black and White groups (P = .87).
Among babies who did not pass newborn hearing screening, the increased likelihood of being diagnosed with normal hearing or permanent hearing loss was independently associated with maternal education and WIC receipt (Table 3). The odds of receiving a diagnosis were 40% higher for children who received WIC services than those who were not in the program. The odds of being diagnosed for children in the highest-SES group (maternal education was bachelor’s degree or higher) were nearly 4 times (odds ratio = 3.58) than those in the lowest-SES group (mother had not completed high school). Children in the highest-SES group were diagnosed more than 3 weeks earlier than those in the lowest-SES group. Babies born to Black or Hispanic mothers were diagnosed approximately 2 weeks later than their White peers. Finally, with all other factors being equal, age of screening was associated with both the receipt and timeliness of diagnosis: for every 7-day increase in age of final screening, there was a 2% increase in the odds of being diagnosed and nearly 5-day increase in age of diagnosis.
TABLE 3.
Results of Multiple Logistic and Linear Regression Predicting Receipt and Age of Diagnosis for Infants Who Were Referred to Diagnostic Audiological Evaluation, 2017, Across 9 States
| Diagnosis Receipta |
Age at Diagnosis, wkb |
||||
|---|---|---|---|---|---|
| Multivariatec | Univariate | b | SE | P | |
|
| |||||
| Age at screening, wk | 1.02 (1.01–1.03) | 1.02 (1.01–1.03) | 0.7 | 0.04 | <.01 |
| NICU status | |||||
| Yes | 0.93 (0.81–1.08) | 0.99 (0.89–1.11) | 3.0 | 0.56 | <.01 |
| No | Reference | ||||
| WIC status | |||||
| Yes | 1.40 (1.24–1.57) | 0.85 (0.77–0.94) | 0.4 | 0.51 | .45 |
| No | Reference | ||||
| Maternal education | |||||
| Bachelor’s and beyond | 3.58 (2.98–4.29) | 2.88 (2.51–3.32) | −3.4 | 0.78 | <.01 |
| Some college/associate degree | 2.12 (1.80–2.50) | 1.78 (1.56–2.04) | −2.0 | 0.74 | .01 |
| High school diploma | 1.29 (1.11–1.51) | 1.22 (1.07–1.39) | −1.5 | 0.72 | .05 |
| Below high school | Reference | ||||
| Maternal race/ethnicity | |||||
| Non-Hispanic Black | 0.58 (0.51–0.66) | 0.50 (0.45–0.56) | 1.9 | 0.57 | <.01 |
| Asian/Pacific Islander | 1.16 (0.90–1.50) | 1.08 (0.88–1.32) | 0.8 | 0.93 | .40 |
| Native American/Alaska Natives | 0.81 (0.47–1.42) | 0.54 (0.34–0.85) | 1.2 | 2.4 | .61 |
| Hispanics | 1.07 (0.92–1.24) | 0.77 (0.68–0.87) | 2.1 | 0.62 | <.01 |
| Non-Hispanic White | Reference | ||||
Abbreviations: NICU, neonatal intensive care unit; SE, standard error; WIC, the Special Supplemental Nutrition Program for Women, Infants, and Children.
The values are odds ratio (95% confidence interval) generated using logistic regression. Number of observations used = 7856 (diagnosed: yes = 4416; no = 3440).
The values are estimates and standard error generated using linear regression. Number of observations used = 3465.
Simultaneously controlled for all listed variables.
Discussion
This study revealed that more than 98% of reported newborns were screened, with more than 97% screened before 30 days of age, exceeding the initial JCIH benchmark of 95%. Diagnostic data were less encouraging: less than half of the at-risk babies were documented as meeting the timely diagnosis benchmark. However, around two-thirds of babies who did not pass their final screening received a diagnostic evaluation. Among those who completed their diagnostic process, more than half of them were diagnosed before 2 months of age and nearly 75% by 3 months. This suggests that the timely diagnosis benchmark is achievable for the majority of babies who are able to start the diagnostic process right after screening. For these children, setting the timely diagnosis benchmark to 2 months of age may be feasible.
On the contrary, these established national benchmarks for screening and diagnosis may not be realistic for certain populations such as NICU babies. NICU babies are at high risk of having congenital hearing loss20; yet, they are most likely to be delayed in the EHDI process. Their period of hospitalization may extend beyond the timely screening benchmark, and the delayed screening timeline subsequently affects the timeliness of diagnosis. In addition, given that many NICU babies were born prematurely, some of the differences in their meeting the timely service benchmarks can likely be attributed to the differences in gestational age compared with term babies. Gestational age was not reported in this study, and its impact on EHDI service timeliness and/or results is a subject for future investigation. Despite the differences in the timeliness of services, there was no difference when comparing the rate of diagnosis between the babies in the NICU and those in well-baby settings. If we examine the rate of receiving an initial diagnostic evaluation rather than a completed diagnosis, those in NICU had higher odds of receiving an initial test than well babies. This suggests that more babies in the NICU never received a confirmatory diagnosis after starting their diagnostic process. This could be due to other health problems these babies may have, which might impede the completion of the hearing-related diagnostic tests.
The associations we found between meeting the EHDI benchmarks and maternal education and race/ethnicity highlight a need to improve screening and diagnosis of hearing loss for children of non-Hispanic Black mothers and mothers without college degrees. This may help minimize delay in the receipt of critical diagnostic and EI services. Using CI, we were able to quantify the degree to which the EHDI benchmarks were disproportionately achieved among higher-SES groups. Future research on health inequality issues related to EHDI could compare this measure across different states and over time.
Past research has shown that WIC participation among children was associated with an increased use of preventive care and increased diagnosis and treatment of common childhood illnesses.21,22 In this study, analysis of association between WIC status and receipt of hearing loss diagnosis yielded additional findings of interest: when WIC status was examined alone, patients not enrolled in WIC were more likely to receive a diagnosis than patients enrolled in the WIC program. However, controlling for other child and family factors, the opposite pattern was found: compared with children not enrolled in WIC, children enrolled in the WIC program had 40% higher odds of receiving a diagnosis. Since both WIC and maternal education level are indicators of SES, these results suggest that the WIC program may aid in reducing health inequality by helping economically disadvantaged families obtain recommended diagnostic services.
Analysis of screening and diagnosis data showed that among those children who did not pass their final screening and subsequently received a diagnosis, early screening predicts early diagnosis. However, many who finished screening with a referral to diagnostic evaluation never had a documented diagnosis and those who completed their screening later were more likely to receive a diagnosis.
The point in time when an infant is considered to have completed the screening stage and moves into the diagnostic stage could be affected by the screening protocol used. Many US hospitals employ a 2-stage protocol that requires infants not passing their initial newborn hearing screening to receive a follow-up outpatient screening before being referred for a diagnostic evaluation. The 2-stage protocol can reduce unnecessary diagnostic tests due to false-positives (ie, a child did not pass the screening but had normal hearing). However, for true-positive cases (ie, a child did not pass hearing screening and had hearing loss), this may delay the diagnostic process. The 2-stage protocol also creates another possible point where a child may become lost to follow-up: either after the initial screening or after the outpatient screening. This may occur for many reasons, such as a program being unable to contact the family. Results from this study suggest that a child was more likely to be lost in the earlier stage of the screening-diagnostic process. Whether and how the use of a 2-stage screening protocol contributes to this effect is an area for further study.
In this study, we were not able to provide an accurate assessment of possible determinants of receipt and timeliness of EI enrollment, nor establish a connection between early diagnosis and early entry into EI. A recent survey (C. A. Mason, PhD, written/electronic communication, November 14, 2019) found considerable variability among states in the frequency with which they collect EI data, with some programs collecting data at least once a month, whereas others only obtain this information once per year. Consequently, these data are not always available for all children enrolled in EI at the time of reporting. In this study, more than 40% of patients diagnosed with permanent hearing loss did not have EI enrollment dates. It was unknown whether these children were not enrolled (ie, lost to follow up) or whether this information was not available to their state’s EHDI program at the time of data submission (ie, lost to documentation). In addition, children with hearing loss can have other disabilities and/or medical conditions that made them eligible for EI,23,24 which allows them to start EI services for non–hearing loss-related conditions.25 Some of these children may have been enrolled into EI before a hearing loss diagnosis was made. As a result, connections between diagnosis date and entry into EI can be difficult to establish.
There are at least 3 limitations of this study: First, data used in this study came from the participating states’ EHDI-IS and some data were not available at the time of data submission. WIC status and maternal demographic data were missing in more than 5% of records. Loss to documentation also contributed to an unknown portion of missing data in diagnostic information. All missing diagnostic data were treated as negative responses (not diagnosed) and therefore the result is a lower-end estimate of the true diagnosis rate. Second, all analyses were conducted on the entire data set consisting of 9 participating states. Each state has its own EHDI-IS, uses different tracking and surveillance methods, and has different population characteristics. The findings from this analysis reflect an aggregated effect rather than the distinct trends of any individual state. Third, only 1 year of newborn data were available for this study, with the results providing a one-time snapshot of the status of EHDI for the included population in 2017. Additional years of data and continued study are needed to validate the findings and provide a complete assessment of the trends and gaps in the EHDI follow-up and surveillance processes.
Supplementary Material
Implications for Policy & Practice.
Use of child-level data available from the state EHDI-IS allows for understanding, at the national level, the hearing health of different populations and makes it possible to identify both common challenges and issues unique to each state.
To encourage continued improvements for states that have consistently met or exceeded the current JCIH hearing screening benchmark (95% of newborns screened by 1 month of age), a higher benchmark may be considered.
For states where most babies can start the diagnostic process right after failed screening, moving up the timely diagnosis benchmark from current 3 to 2 months of age may be feasible.
State EHDI programs may consider providing additional follow-up and education to underrepresented racial/ethnic groups and mothers with less education. Pediatric audiologists and EHDI programs may also consider developing or updating best practice guidelines specifically for NICU infants and their families.
A new data collection and reporting mechanism may be needed in some states to obtain more complete EI information necessary to analyze trends and gaps in this third stage of the EHDI process.
Acknowledgments
The data set used in this study was provided by the following state EHDI programs: Georgia, Kentucky, Louisiana, Massachusetts, Minnesota, New Jersey, North Carolina, Washington, and Wisconsin. These programs are funded under the CDC-RFA-DD17–1701 cooperative agreement. The authors thank the EHDI coordinators from these states for their comments when reviewing the manuscript.
The opinions expressed in this article are the authors’ own and do not reflect the view of the Centers for Disease Control and Prevention, the Department of Health and Human Services, or the US government.
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://www.JPHMP.com).
Contributor Information
Xidong Deng, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia.
Suhana Ema, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia.
Craig Mason, College of Education and Human Development, University of Maine, Orono, Maine.
Ashley Nash, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia; Task Force for Global Health, Decatur, Georgia.
Eric Carbone, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia.
Marcus Gaffney, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- 1.Vohr B Overview: infants and children with hearing loss—part I. Ment Retard Dev Disabil Res Rev. 2003;9(2):62–64. [DOI] [PubMed] [Google Scholar]
- 2.Morton CC, Nance WE. Newborn hearing screening—a silent revolution. N Engl J Med. 2006;354(20):2151–2164. [DOI] [PubMed] [Google Scholar]
- 3.2017 CDC EHDI Hearing screening and follow-up survey—summary of diagnostics among infants not passing hearing screening. https://www.cdc.gov/ncbddd/hearingloss/2017-data/06-diagnostics.html. Published December 2019. Accessed March 4, 2020.
- 4.Halpin KS, Smith KY, Widen JE, Chertoff ME. Effects of universal newborn hearing screening on an early intervention program for children with hearing loss, birth to 3 yr of age. J Am Acad Audiol. 2010;21(3):169–175. [DOI] [PubMed] [Google Scholar]
- 5.Sininger YS, Martinez A, Eisenberg L, Christensen E, Grimes A, Hu J. Newborn hearing screening speeds diagnosis and access to intervention by 20–25 months. J Am Acad Audiol. 2009;20(1):49–57. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher A, Purcell AA, Baker E, Munro N. Listen up: children with early identified hearing loss achieve age-appropriate speech/language outcomes by 3 years-of-age. Int J Pediatr Otorhinolaryngol. 2012;76(12):1785–1794. [DOI] [PubMed] [Google Scholar]
- 7.Stika CJ, Eisenberg LS, Johnson KC, et al. Developmental outcomes of early-identified children who are hard of hearing at 12 to 18 months of age. Early Hum Dev. 2015;91(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshinaga-Itano C, Sedey AL, Wiggin M, Chung W. Early hearing detection and vocabulary of children with hearing loss. Pediatrics. 2017;140(2):e20162964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joint Committee on Infant Hearing; American Academy of Audiology; American Academy of Pediatrics; American Speech-Language-Hearing Association; Directors of Speech and Hearing Programs in State Health and Welfare Agencies. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2000;106(4):798–817. [DOI] [PubMed] [Google Scholar]
- 10.American Academy of Pediatrics, Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. [DOI] [PubMed] [Google Scholar]
- 11.Joint Committee on Infant Hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv. 2019;4(2):1–44. [Google Scholar]
- 12.Gaffney M, Green DR, Gaffney C. Newborn hearing screening and follow-up: are children receiving recommended services? Public Health Rep. 2010;125(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TR, Alam S, Gaffney M. Progress in identifying infants with hearing loss—United States, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64(13):351–356. [PMC free article] [PubMed] [Google Scholar]
- 14.Deng X, Finitzo T, Aryal S. Measuring early hearing detection and intervention (EHDI) quality across the continuum of care. EGEMS (Wash DC). 2018;6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantos PM, Maradiaga-Panayotti G, Barber X, et al. Geographic and racial disparities in infant hearing loss. Otolaryngol Head Neck Surg. 2018;9:194599818803305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bush ML, Bianchi K, Lester C, et al. Delays in diagnosis of congenital hearing loss in rural children. J Pediatr. 2014;164(2):393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham M, Thomson V, McKiever E, Dickinson LM, Furniss A, Allison MA. Infant, maternal, and hospital factors’ role in loss to follow-up after failed newborn hearing screening. Acad Pediatr. 2018;18(2):188–195. [DOI] [PubMed] [Google Scholar]
- 18.Wagstaff A, Paci P, van Doorslaer E. On the measurement of inequalities in health. Soc Sci Med. 1991;33(5):545–557. [DOI] [PubMed] [Google Scholar]
- 19.The concentration index. Quantitative techniques for health equity analysis—technical note #7. http://siteresources.worldbank.org/EXTEDSTATS/Resources/3232763-1171296378756/concentration.pdf. Accessed March 4, 2020.
- 20.Stadio AD, Molini E, Gambacorta V, et al. Sensorineural hearing loss in newborns hospitalized in neonatal intensive care unit: an observational study. Int Tinnitus J. 2019;23(1):31–36. [DOI] [PubMed] [Google Scholar]
- 21.Thomas TN, Kolasa MS, Zhang F, Shefer AM. Assessing immunization interventions in the Women, Infants, and Children (WIC) program. Am J Prev Med. 2014;47(5):624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buescher PA, Horton SJ, Devaney BL, et al. Child participation in WIC: medicaid costs and use of health care services. Am J Public Health. 2003;93(1):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nightenale E, Yoon P, Wolter-Warmerdam K, Daniels D, Hickey F. Understanding hearing and hearing loss in children with Down syndrome. Am J Audiol. 2017;26(3):301–308. [DOI] [PubMed] [Google Scholar]
- 24.Häkli S, Luotonen M, Bloiqu R, Majamaa K, Sorri M. Childhood hearing impairment in northern Finland, etiology and additional disabilities. Int J Pediatr Otorhinolaryngol. 2014;78(11):1852–1856. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast SG, Lartz MN, Fiedler BC. Ages of diagnosis, amplification, and early intervention of infants and young children with hearing loss: findings from parent interviews. Am Ann Deaf. 2002; 147(1):24–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


