Abstract
Introduction
The aim of this study was to evaluate the accuracy and reliability of the Magseed magnetic marker in breast cancer surgery.
Methods
Thirty-nine patients with 41 implanted Magseeds undergoing surgical treatment in 3 surgical oncology departments were included in the retrospective trial to study pilot use of the Magseed magnetic marker in the Czech Republic for localisation of breast tumours or pathological axillary nodes in breast cancer patients.
Results
Thirty-four breast cancer and 7 pathological lymph node localisations were performed by Magseed implantation. No placement failures, or perioperative detection failures of Magseeds were observed (0/41, 0.0%), but one case of Magseed migration was present (1/41, 2.4%). All magnetic seeds were successfully retrieved (41/41, 100.0%). Negative margins were achieved in 29 of 34 (85.3%) breast tumour localisations by Magseed.
Conclusion
Magseed is a reliable marker for breast tumour and pathological axillary node localisation in breast cancer patients. Magseed is comparable to conventional localisation methods in terms of oncosurgical radicality and safety.
Keywords: Magseed, Magnetic marker, Breast cancer surgery, Tumour localisation, Axillary node localisation
Introduction
The development of mammography screening programmes in recent decades has led to an increasing number of impalpable breast tumours with a requirement for precise preoperative localisation when breast-conserving surgery is performed [1].
Various methods have been presented from traditional widely used wire localisation to modern localisation techniques such as radioactive seed localisation using radioactive iodine seeds (125I) [2], SAVI-SCOUT based on infrared light with radar technology [3], or radiofrequency identification tags (RFIDs) using radio wave transmission [4]. Apart from techniques with specific detection systems, there is a well-known and low-cost localisation method using carbon injections.
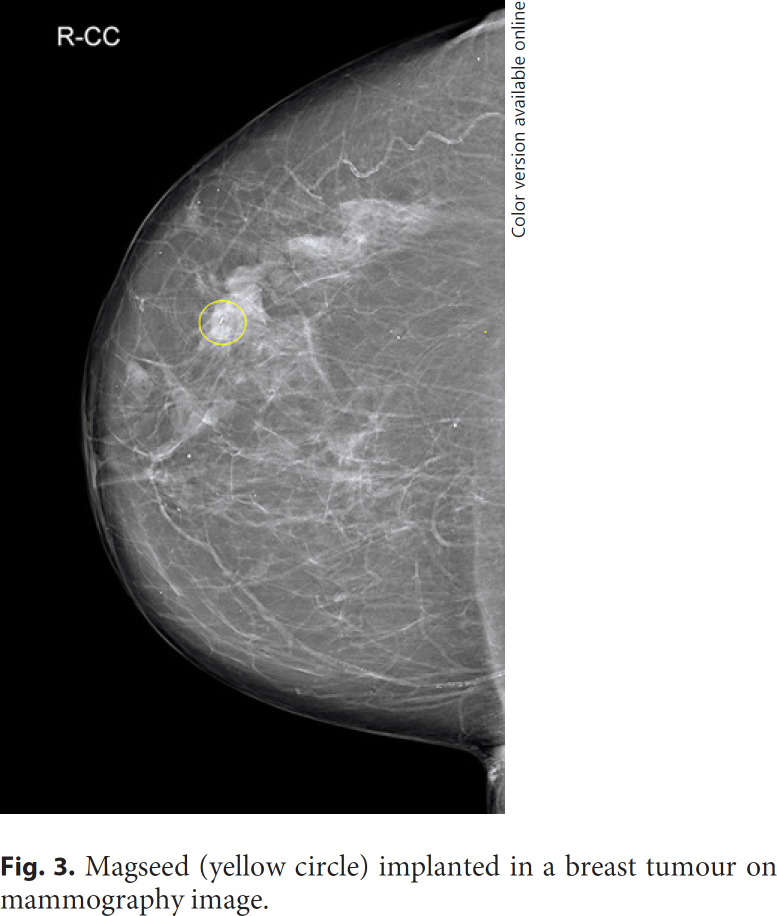
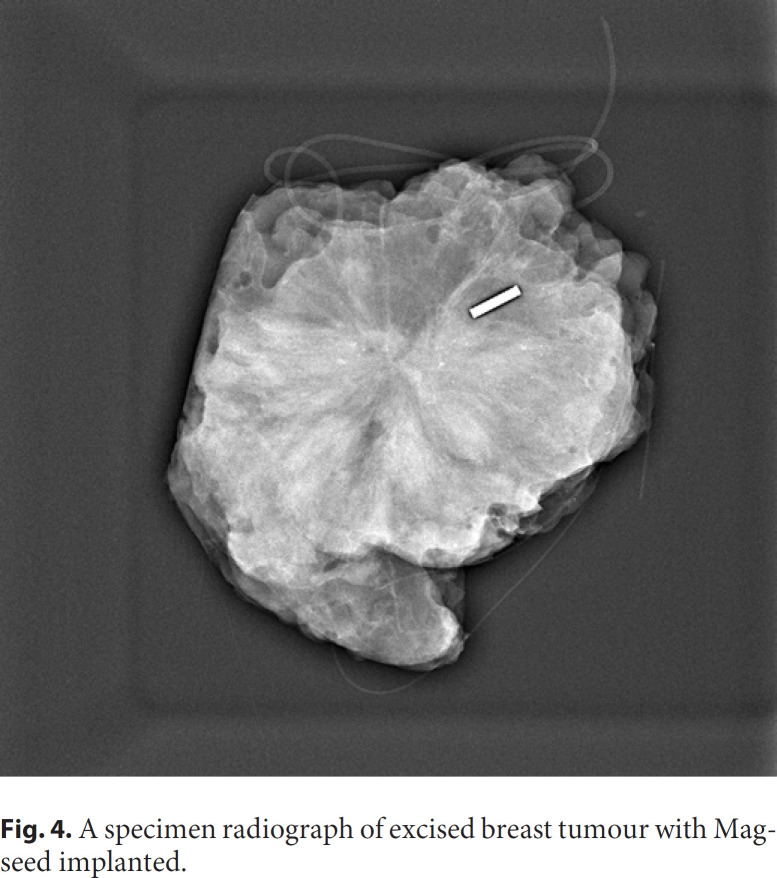
In 2016 the first Magseed magnetic marker (Fig. 1) with the SentiMag localisation probe (Endomagnetics, Inc.; Fig. 2) was approved by the Food and Drug Administration (FDA) [5]. The Magseed is a 1 × 5 mm stainless steel magnetic seed deployed in an 18-G sterile needle introducer [6]. Magseed implantation into a breast tumour (Fig. 3, 4) or pathological lymph node is performed under ultrasound or mammography guidance. The possibility of long-term Magseed deployment with no restrictions enables tumour and lymph node marking in patients with breast carcinoma undergoing neoadjuvant chemotherapy (NAC) [7]. Magseed is detected before and during surgery by the SentiMag handheld probe using a phenomenon called magnetic susceptibility. The recommended depth for Magseed implantation and detection is up to 30 mm, but deeper Magseeds can be detected by probe palpation [7].
Fig. 1.
Magseed − magnetic marker.
Fig. 2.
Sentimag − magnetic marker detection probe.
Fig. 3.
Magseed (yellow circle) implanted in a breast tumour on mammography image.
Fig. 4.
A specimen radiograph of excised breast tumour with Magseed implanted.
An innovative Magseed use is pathologic lymph node marking in breast cancer patients with NAC. After NAC, sentinel lymph node biopsy by various tracers and excision with histological evaluation of marked formerly pathological lymph node can be performed according to NCCN guidelines [8]. This procedure, known as a targeted axillary dissection, has a lower false negativity rate compared to sentinel lymph node biopsy only and less postoperative complications than axillary dissection of level I and II, but it is dependent on a reliable marker [9].
The aim of this study was to evaluate the accuracy and reliability of the Magseed magnetic marker in breast cancer surgery.
Materials and Methods
The study was designed as a clinical retrospective multicentre trial evaluating pilot use of the Magseed magnetic marker in the Czech Republic. Three surgical oncology departments specialising in breast cancer surgery were included in the study − the Department of Surgery, University Hospital Ostrava, the Department of Surgical Oncology, Masaryk Memorial Cancer Institute, Brno, and the Oncogynecology Centre and The Institute for the Care of Mother and Child, Prague. The study was approved by the Ethics Committee of the Faculty of Medicine, University of Ostrava and by local Ethics Committees.
Patients with breast cancer confirmed by biopsy and preoperative localisation of tumour or pathological metastatic involved axillary node by Magseed following surgical treatment in one of three surgical oncology departments were included in the trial. The exclusion criterium was previous disagreement with participation in any study.
The Magseed was inserted into the tumour or the axillary lymph node by the radiologist with ultrasonography or mammography guidance. Patients were operated on in the period from February 21, 2018 to February 1, 2020, breast-conserving surgery or mastectomy and sentinel lymph node biopsy, targeted axillary dissection, or axillary dissection of level I and II were performed according to the recommendation of a multidisciplinary team. The Magseed was perioperatively detected by the SentiMag probe, after resection of the specimen with the seed, a specimen radiograph was performed. Surgical resection specimens were histologically examined in the appropriate histopathology department.
Data were retrospectively collected from the medical records. Observed patients' parameters were age, side and breast quadrant, duration and type of surgery, tumour type, tumour size, TNM classification, tumour grading, Magseed placement complications, Magseed migration, depth of Magseed placement, perioperative complications with Magseed detection, time from localisation to surgery, tumour positive margins according to histological finding, use of a neoadjuvant therapy, and the final histological finding of lymph nodes.
The primary outcome was to evaluate the accuracy and reliability of the Magseed magnetic marker used for localising the tumour and pathological axillary lymph nodes in breast cancer patients. Mean values, percentages, and ranges were calculated.
Results
Thirty-nine patients with 41 implanted Magseeds were included in the study meeting inclusion criteria; individual surgical oncology departments contributed with 5, 15, and 19 patients. Thirty-four Magseeds were used for localization of impalpable breast lesions and 7 Magseeds were used for the localization of the initially pathological axillary lymph node that was marked with a HydroMARK® before the neoadjuvant treatment. Two patients had 2 Magseeds implanted each. One patient had 1 Magseed for breast tumour localisation and another localised pathological node. Another patient had multicentric breast tumour and 2 Magseeds were implanted into medial and lateral tumour centres in the lower breast quadrants. The mean age of patients was 61.6 years, median 66 years, with a minimum of 40 and a maximum of 78 years.
All patients suffered from invasive or preinvasive breast carcinoma (39/39, 100.0%). Thirty-one patients (79.5%) underwent surgical therapy first, 8 patients (20.5%) were operated after neoadjuvant systemic treatment. The most common tumour type was invasive carcinoma NST in 32 out of 39 patients (82.1%). Further details about histological tumour characteristics are listed in Table 1. The most common tumour size according to TNM classification was T1b in 15 patients (38.5%) and T1c in 12 patients (30.8%). Lymph node involvement was present in 11 patients (28.2%) and distant metastasis was not proved in any patient (Table 2).
Table 1.
Number of patients in subgroups according to tumour type and grade
| Grade 1 | Grade 2 | Grade 3 | Total (% of 39 patients) | |
|---|---|---|---|---|
| Invasive carcinoma | 12 | 16 | 4 | 32 (82.1) |
| NST | ||||
| Lobular carcinoma | 2 | 3 | − | 5 (12.8) |
| Mucinous carcinoma | − | − | 1 | 1 (2.6) |
| DCIS | 3 | 1 | − | 4 (10.3) |
Table 2.
Number of patients in subgroups according to TNM classification
| TNM classification | Patients, n (%) |
|---|---|
| T1a | 2 (5.1) |
| T1b | 15 (38.5) |
| T1c | 12 (30.8) |
| T2 | 9 (23.1) |
| Tis | 1 (2.6) |
| N0 | 28 (71.8) |
| N1 | 11 (28.2) |
| M0 | 39 (100.0) |
In 38 cases, breast-conserving surgery was performed including 3 oncoplastic surgeries. One patient had a bilateral mastectomy. Magseed was used for pathological axillary lymph node localisation in this patient. Seven targeted axillary dissections by Magseed (7/39, 18.0%) and 32 sentinel lymph node biopsies by various tracers (32/39, 82.1%) were performed. In 6 patients (6/39, 15.4%), axillary dissection of level I and II was further indicated due to the recommendation of a multidisciplinary team. The mean operation time was 45.5 min, minimum 20 and maximum 105 min, depending on surgery type − longer operation times were observed in oncoplastic breast surgeries.
No complications were observed during Magseed implantation into tumour or lymph node, so the placement failure rate was 0/41 seeds (0.0%). One inadvertent premature deployment of the seed occurred during manipulation with needle delivery system before implantation by the radiologist, another seed was used for tumour localisation. The depth of Magseed deployment was recorded in 33/42 seeds; the mean depth was 15.4 mm with minimum 3 mm and maximum 50 mm.
The mean time from Magseed placement to surgical retrieval was 9.5 days, with a range from 0 (same day) to a maximum of 34 days. Magseed migration affecting perioperative tumour localisation was present in one case (1/41 seeds, 2.4%), but no case of perioperative detection failure was reported (0/41 seeds, 0.0%). All magnetic seeds were successfully retrieved (41/41, 100.0%).
Negative margins were achieved in 29 of 34 (85.3%) breast tumour localisations by Magseed. In 4 patients (11.8%) a positive resection margin was found in final histological report and in 1 patient (2.9%) the tumour was missed completely due to perioperative Magseed migration, so re-operation was necessary in 5 patients (14.7%). The baseline characteristics and results summarizing Magseed use in our trial are listed in Table 3.
Table 3.
Baseline characteristics and results
| Characteristics | |
| Number of implanted seeds | 41 |
| Number of patients | 39 |
| Number of patients with TAD | 7 |
| Placement failure, n (%) | 0/41 (0.0) |
| Seed migration, n (%) | 1/41 (2.4) |
| Detection failure, n (%) | 0/41 (0.0) |
| Range of depth of marker deployment, mm | 3–50 |
| Negative margins, n (%) | 29/34 (85.3) |
| Range of time to operation, days | 0–34 |
| Mean time to operation, days | 9.5 |
| Malignant tumours, n (%) | 39/39 (100.0) |
| Patients after NAC, n (%) | 8/39 (20.5) |
| Complications rate, n (%) | 4/39 (10.3) |
Seven patients underwent pathological axillary lymph node localisation by hydroMARK clip before NAC as a standard procedure. With a range of 0–29 days (mean time 12.7 days) before surgery, the Magseed was introduced into the lymph node according to hydroMARK placement. Targeted axillary dissection and successful retrieval of originally pathological lymph nodes was performed in 6/7 patients (85.7%). In one patient only an adipose tissue marked by hydroMARK clip and Magseed was found in a histological sample, possibly due to placement failure or migration of the hydroMARK clip. Axillary dissection was performed in 3 patients (42.9%) in this subgroup.
Wound complications after surgical treatment were present in 4 patients (10.3%). Two patients had a wound infection (Clavien Dindo Grade I), 1 patient had a wound haematoma (CD I), and 1 patient had a haematoma with a wound necrosis requiring excision and re-suture under local anaesthesia (CD IIIa).
Discussion
According to the literature review, our study is the second trial reporting Magseed use for preoperative localisation of pathological axillary lymph nodes worldwide. Greenwood et al. [10] performed a retrospective analysis of 35 patients with 38 Magseeds implanted into axillary lymph nodes proving its safety and feasibility. Greenwood et al. [10] reported no complications during Magseed placement and successful retrieval of 37/38 Magseeds (97%) due to one seed lost possibly during local suction. Our limited results proving retrieval of 7/7 (100%) Magseeds and successful targeted axillary dissection in 6/7 patients (85.7%), in one patient, previous hydroMARK placement failure or migration led to a finding of no lymph node in histological analysis and axillary dissection was further indicated.
In comparison, Hartmann et al. [11] used a clip for lymph node localisation before NAC. After primary systemic therapy, wire-guided localisation was performed according to clip placement and a clip identification rate on specimen radiograph after excision was possible in 17/24 cases (70.8%) [11]. Kim et al. [12] presented an identification rate and successful retrieval of 23/24 clips (95.8%) inserted into pathological lymph node before NAC. Several studies investigated lymph node localisation with a radioactive iodine-125 seed with promising results of 97–100% successful lymph node retrieval [13]. Khallaf et al. [14] used carbon for lymph node localisation in 20 patients after NAC with 95% identification rate. According to the results mentioned above, the Magseed lymph node localisation seems to be comparable to other localisation methods, even if up-to-date evidence is scarce and further studies are needed. Additionally, primary localisation of the lymph node by the magnetic seed without previous clip localisation should affect the marker migration rate, also it reduces the overall number of the invasive procedures the patient undergoes through the treatment process.
Breast tumour localisation by Magseed has been reported by several authors [6, 15, 16, 17, 18, 19]. Harvey et al. [6] demonstrated as a first the feasibility and safety of magnetic seeds by a prospectively maintained study with 28 patients and 29 Magseeds implanted into breast tumour and a negative margin rate of 29/29 (100%). Other authors reported a negative margin rate in a range from 78.1 to 100% with a quantity of patients from 10 to 137 [15, 16, 17, 18, 19]. Our results with negative margins in 29/34 patients (85.3%) confirmed previous data. In comparison with other localisation methods, the negative margin rate in wire-guided localisation is 70–88% [20], in radioactive iodine seed localisation 73.5–96.7% [3], in SAVI-SCOUT 85.1–92.6% [3], in carbon marking 81% [4], and in clip markers 90–92% [21]. Magseed seems to be comparable to the breast cancer localisation methods mentioned above in terms of oncosurgical safety [7].
To our knowledge, only one prospective comparative study between Magseed and other breast localisation methods has been published. Zacharioudakis et al. [22] arranged a study comparing WGL and Magseed with 100 patients by each method and reported the effectiveness of Magseed localisation to be the same as WGL in terms of lesion identification and negative margin rate.
The main advantages of magnetic marker localisation are no need for radiation safety policy, no operating theatre delays due to the possibility of arranging localisation to any day before the operation, no marker migration, and intuitive detection [7]. Several authors reported no seed migration affecting perioperative tumour localisation [6, 16, 17, 19]. To our knowledge no study has published data about Magseed migration yet, but we report a Magseed migration in one seed (1/41, 2.4%) probably during breast tumour excision, so we conclude a Magseed migration could occur. Also, we observed one case of Magseed displacement from the breast specimen during excision of the targeted lesion before specimen radiography. This parameter was not observed in our study, so we cannot evaluate the rate, but in comparison with iodine seeds, McGhan et al. [23] published a displacement in 2.6%.
Breast surgeons from all surgical oncology departments included in our study were well experienced in breast cancer surgery and became familiar with the Magseed system in a short time. The magnetic marker detection was easy to learn for surgeons, probably because there is an analogy with sentinel lymph node biopsy by gamma probe, which is routinely used in breast cancer surgery.
The maximum time from Magseed deployment to retrieval in our study was 34 days with no observed complication or marker migration. Price et al. [15] published a maximum time of Magseed deployment in breast tumour of 40 days, which is to our knowledge the longest reported time. According to these findings and FDA approval, we suggest Magseed seems to be usable for long-time deployment especially in patients before NAC for breast tumour and pathological lymph node localisation.
However, the main Magseed drawbacks should be mentioned. The depth of marker implantation is limited to up to 30 mm according to the manufacturer's recommendation [6]. Deeper implantation could result in problematic detection [6], but 2 patients included in our study had a depth of marker implantation over 30 mm (33 and 50 mm) with no complications during detection. A probe palpation, meaning pressing with the probe on the breast to achieve a shorter distance between the probe and the marker, should be beneficial in more deeply implanted Magseeds.
One of the biggest disadvantages of magnetic marker localisation system use is a frequent need for probe recalibration [7]. The Sentimag probe interferes with paramagnetic surgical instruments and with electrocautery, so the recalibration process could prolong operation time [5, 20, 24].
Another drawback is the high cost of the Sentimag probe and each magnetic seed in comparison to other localisation methods, for example, WGL or carbon marking [7]. Several authors suggested a cost-effectiveness analysis to review all factors participating in the final price of the surgery [6, 22], but none have been published to date.
Compatibility with magnetic resonance (MRI) is another important factor, especially in patients after NAC, where MRI is used for restaging. Magseed is compatible with MRI but has a bloom effect up to 6 cm affecting the final scan [4]. In comparison, radioactive seed localisation is compatible with MRI with a minimum artefact [25] and SAVI-SCOUT can be used under MRI without any artefact [3]. Unfortunately, none of these three new localisation techniques can be implanted under MRI, because its needle delivery system is not compatible [26], but radioactive seed localisation has already a published protocol for MRI localisation using a titanium delivery needle [25].
The limitation of our study is in its retrospective arrangement; further trials are needed because of the paucity of evidence about Magseed localisation in breast cancer surgery. A prospective comparative study between Magseed and different localisation methods should be beneficial.
Conclusion
The Magseed is a reliable marker for breast tumours and pathological axillary node localisation in breast cancer patients. The Magseed is comparable to conventional localisation methods in terms of oncosurgical radicality and safety, but further trials are needed.
Statement of Ethics
The research was approved by the Ethics Committee of Faculty of Medicine, University of Ostrava (EK 22/2019). Patients have given their written informed consent.
Conflict of Interest Statement
Magseeds were provided by Sysmex CZ, Inc. without any charge as part of the Magseed pilot use in the Czech Republic project. The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by grant from the Ministry of Health of the Czech Republic − Conceptual Development of a Research Organization (MMCI 00209805).
Author Contributions
J.Ž.: literature search, manuscript writing; O.K.: topic selection, manuscript revisions; O.C.: manuscript revisions, data preparation; M.K.: data preparation; A.F.: manuscript revisions, data preparation; K.R.: data preparation; M.L.: literature search; M.P.: manuscript writing; R.B.: final revisions.
References
- 1.Wyld L, Markopoulos C, Leidenius M, Senkus-Konefka E, editors. Springer International Publishing; 2018. Breast Cancer Management for Surgeons: A Euro¬pean Multidisciplinary Textbook. [Google Scholar]
- 2.Dauway EL, Saunders R, Friedland J. Innovative diagnostics for breast cancer new frontiers for the new millennium using radioactive seed localization. Surgical forum: 85th annual American college of surgeons clinic congress. Chicago, IL: American College of Surgeons. 1999;Vol. 50 [Google Scholar]
- 3.Cheang E, Ha R, Thornton CM, Mango VL. Innovations in image-guided preoperative breast lesion localization. Br J Radiol. 2018 May;91((1085)):20170740. doi: 10.1259/bjr.20170740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayes MK. Update on preoperative breast localization. Radiol Clin North Am. 2017 May;55((3)):591–603. doi: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Jeffries DO, Dossett LA, Jorns JM. Localization for breast surgery: the next generation. Arch Pathol Lab Med. 2017 Oct;141((10)):1324–9. doi: 10.5858/arpa.2017-0214-RA. [DOI] [PubMed] [Google Scholar]
- 6.Harvey JR, Lim Y, Murphy J, Howe M, Morris J, Goyal A, et al. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Cancer Res Treat. 2018 Jun;169((3)):531–6. doi: 10.1007/s10549-018-4709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Žatecký J, Kubala O, Jelínek P, et al. Magnetic marker localisation in breast cancer surgery. Arch Med Sci. 2020;••• doi: 10.5114/aoms.2020.93673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Breast Cancer (Version 3. 2020). https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf. [Google Scholar]
- 9.Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016 Apr;34((10)):1072–8. doi: 10.1200/JCO.2015.64.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood HI, Wong JM, Mukhtar RA, Alvarado MD, Price ER. Feasibility of Magnetic Seeds for Preoperative Localization of Axillary Lymph Nodes in Breast Cancer Treatment. AJR Am J Roentgenol. 2019 Oct;213((4)):953–7. doi: 10.2214/AJR.19.21378. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018 Sep;44((9)):1307–11. doi: 10.1016/j.ejso.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Kim EY, Byon WS, Lee KH, Yun JS, Park YL, Park CH, et al. Feasibility of Preoperative Axillary Lymph Node Marking with a Clip in Breast Cancer Patients Before Neoadjuvant Chemotherapy: A Preliminary Study. World J Surg. 2018 Feb;42((2)):582–9. doi: 10.1007/s00268-017-4171-8. [DOI] [PubMed] [Google Scholar]
- 13.Woods RW, Camp MS, Durr NJ, Harvey SC. A Review of Options for Localization of Axillary Lymph Nodes in the Treatment of Invasive Breast Cancer. Acad Radiol. 2019 Jun;26((6)):805–19. doi: 10.1016/j.acra.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Khallaf E, Wessam R, Abdoon M. Targeted axillary dissection of carbon-tattooed metastatic lymph nodes in combination with post-neo-adjuvant sentinel lymph node biopsy using 1% methylene blue in breast cancer patients. Breast J. 2020 May;26((5)):1061–3. doi: 10.1111/tbj.13736. [DOI] [PubMed] [Google Scholar]
- 15.Price ER, Khoury AL, Esserman LJ, Joe BN, Alvarado MD. Initial Clinical Experience With an Inducible Magnetic Seed System for Preoperative Breast Lesion Localization. AJR Am J Roentgenol. 2018 Apr;210((4)):913–7. doi: 10.2214/AJR.17.18345. [DOI] [PubMed] [Google Scholar]
- 16.Hersi AF, Eriksson S, Ramos J, Abdsaleh S, Wärnberg F, Karakatsanis A. A combined, totally magnetic technique with a magnetic marker for non-palpable tumour localization and superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in breast cancer surgery. Eur J Surg Oncol. 2019 Apr;45((4)):544–9. doi: 10.1016/j.ejso.2018.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Pohlodek K, Foltín M, Mečiarová I, Ondriaš F. Simultaneous use of magnetic method in localization of impalpable breast cancer and sentinel lymph nodes detection: initial experience. Nanomedicine (Lond) 2018 Dec;13((24)):3075–81. doi: 10.2217/nnm-2018-0220. [DOI] [PubMed] [Google Scholar]
- 18.Lamb LR, Bahl M, Specht MC, D'Alessandro HA, Lehman CD. Evaluation of a Nonradioactive Magnetic Marker Wireless Localization Program. AJR Am J Roentgenol. 2018 Oct;211((4)):940–5. doi: 10.2214/AJR.18.19637. [DOI] [PubMed] [Google Scholar]
- 19.Thekkinkattil D, Kaushik M, Hoosein MM, Al-Attar M, Pilgrim S, Gvaramadze A, et al. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin Radiol. 2019 Dec;74((12)):974.e7–11. doi: 10.1016/j.crad.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Green M, Vidya R. Techniques Used to Localize Occult Breast Lesions: an Update. Clin Breast Cancer. 2018 Jun;18((3)):e281–3. doi: 10.1016/j.clbc.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Corsi F, Sorrentino L, Bossi D, Sartani A, Foschi D. Preoperative localization and surgical margins in conservative breast surgery. Int J Surg Oncol. 2013;2013:793819. doi: 10.1155/2013/793819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharioudakis K, Down S, Bholah Z, Lee S, Khan T, Maxwell AJ, et al. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol. 2019 Nov;45((11)):2016–21. doi: 10.1016/j.ejso.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 23.McGhan LJ, McKeever SC, Pockaj BA, Wasif N, Giurescu ME, Walton HA, et al. Radioactive seed localization for nonpalpable breast lesions: review of 1,000 consecutive procedures at a single institution. Ann Surg Oncol. 2011 Oct;18((11)):3096–101. doi: 10.1245/s10434-011-1910-1. [DOI] [PubMed] [Google Scholar]
- 24.Chacko SM, Marshall HN. Implementation of Preoperative Magnetic Seed Localization for Breast and Axillary Lesions: An Alternative to Wires and Radioactive Seeds. J Radiol Nurs. 2018 Sep;37((3)):154–7. [Google Scholar]
- 25.Lee C, Bhatt A, Felmlee JP, Trester P, Lanners D, Paulsen A, et al. How to Safely Perform Magnetic Resonance Imaging-guided Radioactive Seed Localizations in the Breast. JCIS. Scientific Scholar; 2020 Apr 9;10:19. doi: 10.25259/JCIS_11_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwood HI, Dodelzon K, Katzen JT. Impact of Advancing Technology on Diagnosis and Treatment of Breast Cancer. Surg Clin North Am. 2018 Aug;98((4)):703–24. doi: 10.1016/j.suc.2018.03.006. [DOI] [PubMed] [Google Scholar]