Abstract
Background
Idiopathic granulomatous mastitis (IGM) is a rare, recurrent and progressive breast disease with an unknown etiology. Patients with IGM will probably face stressful, time-consuming treatment procedures with side effects due to medications. There are different treatment modalities in clinical use including medical and surgical interventions.
Objective
The aim of this study was to present the results of using the combination therapy of low-dose methotrexate (MTX) and steroid in IGM.
Methods
Seventeen patients diagnosed with IGM and treated with MTX were included into the study. Low-dose MTX at 5 mg/week and 8 mg/day prednisone were given for 2–3 months.
Results
After 2–3 months of treatment, 10 patients exhibited (58.5%) complete, 3 patients (17.6%) partial recovery, and no response to the treatment process was observed in 4 patients (23.5%). No side effects of MTX and recurrent events were noted in any of the patients.
Conclusion
Low-dose MTX and prednisone treatment for IGM patients, who did not respond to steroids alone, should be considered as an alternative treatment method instead of surgical intervention.
Keywords: Idiopathic granulomatous mastitis, Methotrexate, Treatment
Introduction
Idiopathic granulomatous mastitis (IGM) is a breast disease which progresses with limited inflammation and granulation in breast lobules. It was first defined by Kessler and Wolloch [1]. It usually affects parous women and its incidence among benign breast diseases is about 1.8% [2]. Although the exact etiology is unknown, it is believed that autoimmune reactions, infectious diseases and hormonal disorders trigger this disease [3, 4]. Initial symptoms and examination findings are usually confused with breast cancer. Patients generally present with a painful mass in the breast. Moreover, symptoms such as abscess, deformity, nipple retraction, and orange peel views can be seen. Its differential diagnosis from other diseases associated with granulosis in breast and breast cancer should be made [5]. Such diseases are tuberculosis, syphilis, corynebacterium, parasitic and fungal infections as well as sarcoidosis, Wegener's granulomatosis, giant cell arthritis and poly-arthritis nodosa. Fat necrosis and foreign body reactions should also be excluded. In differential diagnosis, inflammatory breast cancer should always be kept in mind [6]. IGM diagnosis can be made neither clinically nor radiologically. Definitive diagnosis is established histologically. Tissue biopsies are often collected with Tru-Cut or incisional/excisional biopsy methods. In histopathological examination, non-caseating granulomas and chronic inflammatory cells, such as histiocytes and multinucleated giant cells, are seen.
IGM treatment includes quite different modalities. Although there are clinicians who recommend only follow-up, steroid-based medical treatments are still valid. Methotrexate (MTX) is used alone or in combination with steroids. Besides, bromocriptine and cabergoline are also used [7, 8, 9]. There are studies recommending surgical approach alone [10]. Nowadays, medical approach is the first-line treatment in IGM management, and in case of remaining lesions, surgical interventions are used for complete cure of IGM [11, 12, 13, 14, 15]. Methotrexate, which is a folic acid analogue and immunosuppressive agent, was used alone or in addition to current therapy in patients with inadequate response to steroid treatment [8]. In this study, the result of using the combination therapy of low-dose steroid and MTX is discussed.
Materials and Methods
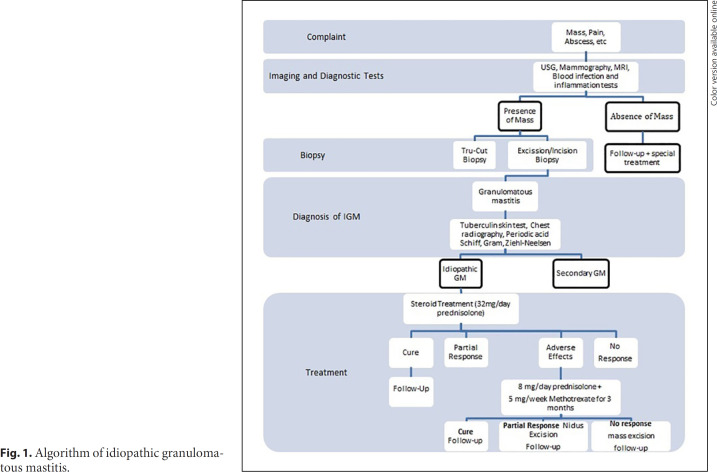
This study was conducted in Dicle University School of Medicine Department of General Surgery between January 2010 and December 2016. The study was approved by Dicle University Human Ethics Committee (No.: 26.11.2020–357), Diyarbakır, Turkey. Seventeen patients diagnosed with IGM and treated with MTX were included into the study. Their medical records were retrieved and analyzed retrospectively. Our approach to management of IGM is given as an algorithm in Figure 1. Histopathological confirmation of IGM diagnosis and treatment are as follows.
Fig. 1.
Algorithm of idiopathic granulomatous mastitis.
Histopathological and Serological Evaluation
Sampling of the mass for histopathological was determination performed by Tru-Cut needle biopsy or excisional biopsy accompanied with abscess drainage (in the presence of larger abscess) where appropriate. Examination of haematoxylin and eosin staining of pathological slides showed lobulocentric non-necrotizing granulomas with infiltrating multinucleated giant cells, neutrophils, lymphocytes, plasma cells and eosinophils. Inflammation sources causing granulomatous mastitis were excluded by Ziehl-Neelsen (acid-fast bacilli), Gram (microorganisms), and periodic acid-Schiff (fungal infections) stainings. Tuberculin test was performed in order to exclude tuberculosis mastitis.
Treatment
Patients diagnosed with IGM were treated with systemic steroids (32 mg/day prednisone for 3 months). Methotrexate treatment (5 mg/week) was added to the treatment and prednisone dose was reduced to 8 mg/day when we had partial or no response (the regression of the mass volume does not exceed 50%) and in the case of adverse effects.
Treatment Response Criteria for Both Steroids and Methotrexate
Listed conditions should be maintained at least for 3 weeks. (I) Reduction of pain; (II) reduction of abscess; (III) loss of indurations; (IV) loss of sensitivity; (V) infection and inflammation markers within normal range; and (VI) shrinkage of mass.
Patients whose diagnosis of IGM was confirmed by the methods mentioned above and who received low-dose steroid therapy were included in the study. Patients with suspected IGM diagnosis and those treated with other treatment methods were excluded from the study.
Statistical Analysis
IBM SPSS 21.0 (Statistical Package for the Social Sciences version 21, SPSS Inc., Chicago, IL, USA) for windows statistical package program was used for the statistical evaluation of our research data. Measuring variables were presented as mean ± SD, and categorical variables were presented as numbers and percentages. χ2 test was used to compare qualitative variables. Hypotheses were considered two-way, p ≤ 0.05 was accepted as a statistically significant result.
Results
All women enrolled into the study were in childbearing age (40.4 ± 5.3), none of whom were pregnant nor breastfeeding their child at the time of presentation. Patients' demographic variables including duration of symptoms, parity, breastfeeding duration, tobacco consumption, and geographic distribution are given in Table 1.
Table 1.
Demographic variables of the study population
| N (%; n = 17) | |
|---|---|
| Geographic distribution | |
| Urban | 14 (82.4) |
| Rural | 3 (17.6) |
| Complaint duration, months | |
| 1–6 | 11 (64.7) |
| 7–12 | 5 (29.4) |
| ≥13 | 1 (5.9) |
| Tobacco consumption | |
| No | 12 (70.6) |
| Yes | 5 (29.4) |
| Parity | |
| 1–3 | 10 (58.8) |
| 4–6 | 7 (41.2) |
| Breastfeeding, years | |
| 1–3 | 9 (52.9) |
| 4–6 | 7 (41.2) |
| ≥7 | 1 (5.9) |
Patients were admitted to General Surgery Clinic with complaints such as mass (29.4%), pain (23.5%), and abscess or combinations (47.1%). After physical examination, lesions palpated in all patients were one-sided, 11 patients had lesions on the left side and 6 on the right. Three patients had complications, one had fistula and 2 of them had erythema. Ultrasonography (USG) was applied to all patients. The determined mass was generally located on the upper outer quadrant (47.1%). Axillary lymphadenopathy was present in 9 patients (52.9%). Advanced imaging technics such as mammography (MG; applied to patients ≥40 year old) and magnetic resonance imaging (MRI; applied to patients <40 year old) were used for detailed imaging of the mass. According to BIRADS classification of the mass category, 3, 4 and 5 were observed. For sampling of the mass, excisional biopsy from mass wall accompanied with abscess drainage (4 patients) and Tru-Cut biopsy (13 patients) procedure was performed. Clinical presentations of the patients are given in Table 2.
Table 2.
Clinical presentation of the patients
| N (%; n = 17) | |
|---|---|
| Affected side | |
| Left | 11 (64.7) |
| Right | 6 (35.3) |
| Localization | |
| Upper outer | 8 (47.1) |
| Upper inner | 1 (5.9) |
| Lower outer | 0 (0) |
| Lower inner | 4 (23.5) |
| Retroareolar space | 4 (23.5) |
| Axillary LAP | |
| Negative | 8 (47.1) |
| Positive | 9 (52.9) |
| Additional disease | |
| Negative | 14 (82.4) |
| Positive | 3 (17.6) |
| Initial complaint | |
| Pain | 2 (11.8) |
| Mass | 4 (23.5) |
| Pain + abscess | 3 (17.6) |
| Pain + mass | 4 (23.5) |
| Mass + abscess | 1 (5.9) |
| Pain + mass + abscess | 3(17.6) |
| Complication | |
| No complication | 14 (82.4) |
| Erythema | 2 (11.8) |
| Fistula | 1 (5.9) |
| Imaging | |
| USG | 8 (47.1) |
| USG + MG | 5 (29.4) |
| USG + MG + MRI | 4 (23.5) |
| BIRADS | |
| 1 | 0 (0) |
| 2 | 0 (0) |
| 3 | 9 (52.9) |
| 4 | 7 (41.2) |
| 5 | 1 (5.9) |
| Biopsy | |
| Tru-Cut | 13 (76.5) |
| Drainage + incisional biopsy | 4 (23.5) |
USG, ultrasonography; MG, mammography; MRI, magnetic resonance imaging.
Prednisone was given 32 mg/day as a first-line treatment. In 2 diabetic patients, glucose levels increased as a side effect of prednisone. Four patients did not respond to prednisone treatment. In 11 patients, the expected adequate regression of the mass was lacking. Due to these issues, the prednisone dose was decreased to 8 mg/day, and 5 mg/week methotrexate was added to the treatment. Complete remission was seen in 10 patients after MTX treatment. Partial remission was observed in 3 patients where the remaining mass was removed with surrounding normal tissues by surgical intervention. There was no response to methotrexate treatment in 4 patients. In this case, the mass with surrounding normal tissue was surgically removed. Treatment procedures and results were seen in Table 3. All patients were invited to the General Surgery outpatient clinic for routine follow-up procedures every 2 weeks during treatment. Signs and symptoms such as, mouth sores, nausea, leukopenia, adverse effects on the liver or kidney, allergic reaction-like findings (itching, rash, etc.), signs of infection (fever, cough, sputum, burning while urinating), cough, shortness of breath, diarrhea, and so on, were questioned and screened.
Table 3.
Treatment procedures and results
| N (%; n = 17) | |
|---|---|
| Steroid treatment duration, months | |
| 1–3 | 10 (58.8) |
| 4–6 | 6 (35.3) |
| ≥7 | 1 (5.9) |
| Steroid response | |
| No response | 4 (23.5) |
| Partial response | 11 (64.7) |
| Adverse effect | 2 (11.8) |
| Methotrexate treatment duration, months | |
| 2 | 4 (23.5) |
| 3 | 13 (76.5) |
| Methotrexate response | |
| No response | 4 (23.5) |
| Partial response | 3 (17.6) |
| Complete response | 10 (58.8) |
| Surgery approach | |
| No surgery | 10 (58.8) |
| Remaining mass excision after partial remission | 3 (17.6) |
| Whole mass excision | 4 (23.5) |
| Follow-up period, months | |
| 4–6 | 7 (41.2) |
| 7–12 | 6 (35.3) |
| ≥13 | 4 (23.5) |
Discussion
IGM is a rare, recurrent and progressive inflammatory disease of the breast. It is suggested that successful treatment with immunosuppressive therapy supports autoimmune etiology [8, 16]. However, the reasons or causes that trigger epithelioid damage, which creates histopathological features of the disease, are still unknown [1, 17]. Patients are generally at childbearing age, but there are reports regarding women affected at post-menopausal age [4, 11]. IGM tends to occur in younger, parous women at an average age of 35 years, and rarely occurs in men. All of the women in this study were parous and nursed their child at least for 1 year. Most of the patients describe a slow-onset and gradually growing mass with or without pain, which was the most significant symptom [5, 7, 17, 18, 19, 20].
Signs of both acute mastitis (abscess, erythema, etc.) and chronic mastitis (fistula, nipple retraction, orange peel appearance, etc.) are complaints that are usually reported on admission [6, 21, 22]. There is no specific imaging method; USG, MG and MRI are applied to appropriate patients. Observed masses are generally located on one side, bilateral location is rarely observed, all of our patients had mass on one side, mostly on the left side (64%) [11, 12, 23]. There is no specific location for the mass on the breast, whereas Boufettal et al. [11] reported that upper outer quadrant is the most frequent location of the mass similar to our data (47%). Axillary nodes may accompany the mass on the same side. Gautier et al. [21] observed axillary node frequency as 61%, it was 18% in the study by Aghajanzadeh et al. [7]. Axillary node frequency in our study was 52.9%, all occurred on the same side. All cases were subjected to the Breast Imaging Reporting and Data System (BIRADS) classification on mammography or ultrasonography. BIRADS categories 3, 4 and in one case BIRADS 5 were observed in our study.
Multidisciplinary approach is needed during diagnosis. Diagnosis can only be made with histopathological examination via excluding other causes of granulomatous breast diseases. Tru-Cut and excisional/incisional biopsies are used for sampling. In the presence of abscess, incisional biopsy during drainage due to abscess is a good option that we carried out in 4 patients [5, 7]. Other cases were subjected to Tru-Cut biopsying. All other granulomatous mastitis causes were excluded with Ziehl-Neelsen (acid-fast bacilli), Gram (microorganisms), and periodic acid-Schiff (fungal infections) stainings of the pathology slides.
In IGM, the treatment process is difficult and reduces the quality of life considerably. The reasons that decrease the quality of life are as follows; breast abscesses, fistulas, ulcers with various sizes and localizations, recurrence and chronicity [6]. Clinics and radiological images mimic primary breast cancer which increases the psychological stress of the treatment process [24, 25]. Therefore, early diagnosis and effective treatment are very important. Although many algorithms have been described up to now, there is no consensus on the treatment of IGM [3, 10]. In addition to only follow-up without any medication and medical treatments, such as antibiotics, prolactin inhibitors, immunosuppressive agents and wide surgical excision, are still in use [7, 15, 26, 27, 28]. Although cultures for IGM are generally sterile (abscess content and tissue cultures), antibiotics were reported to be effective in some studies. While some authors recommended surgical excision due to faster healing and definitive cure, there are studies suggesting fistula formation, local recurrence and poor wound healing due to surgical interventions [6, 10, 12, 23]. Due to high success rates of immunosuppressive based treatments, they were preferred instead of surgical treatment approaches as a first-line treatment [3, 16, 26, 27]. In 1980, DeHertogh et al. [3] used high dose 60 mg/day for the first time in 2 IGM patients and reported a definite cure within 3 weeks of treatment and follow-up of 9 months. Akahane et al. [19] treated 10 IGM patients with a median dose of 30 mg/day prednisone for 5 months (1–12 months) and got complete response without any surgical intervention. Altintoprak et al. [29] tried topical steroid for 15 weeks in treatment, reporting a success ratio of more than 90% after 37 months of follow-up. There are studies suggesting surgical excision after shrinkage of mass, and disappearance of abscess formations with steroid treatment is the best way to treat IGM [5, 11, 30]. Adverse effects, such as hyperglycemia, moon face, and acne, occurred during systemic steroid treatment in some patients. In these cases, the authors reduced the dose of steroids and/or performed directly to surgical excision of the mass [5, 10]. In the medical treatment of IGM, prolactin inhibitors were also used, and results were not successful enough to become a widespread treatment procedure [7]. In 2009, Schmajuk et al. [8] treated 2 IGM patients with MTX monotherapy. In this study, 2 patients were steroid resistant and were given 15 mg/week MTX in combination with folic acid for 6 weeks, then the dose was increased to 20 mg/week, and within 12 months, there were no palpable mass, erythema and fistula. Kim et al. [16] were the first to use MTX to cure IGM in patients who were resistant to steroids or had a side effect, obtaining promising results. In this case report, 15 mg/week MTX in combination with 25 mg/day prednisone was applied to 4 cases for at least 3–6 months, 2 of whom recovered well without any recurrence within 5 years of follow-up; the other 2 cases were subjected to surgical intervention. Joseph et al. [25] pointed out that they had success over 80% through the steroid and MTX combination. Similarly, Aghajanzadeh et al. [7] tried steroid+ MTX combination, and their success rate of treatment was reported as 71.4%. Akbulut et al. [31] investigated a case series consisting of 12 patients from different clinics in a literature review and reported that the recovery ratio with a steroid + MTX combination therapy was 83%. Seventeen patients enrolled in this study were given a low-dose 5 mg/week MTX in combination with 8 mg/day prednisone for 3 months due to steroid resistance, partial cure and side effects to prednisone. Complete remission was seen in 10 patients (58.8%) after combination therapy. Partial remission was observed in 3 patients (17.6%), and the remaining mass was removed with surrounding normal tissues by surgical intervention. There was no response to MTX treatment in 4 patients (23.5%). In this case, the mass with surrounding normal tissue was surgically removed. None of the patients after medical and surgical treatment had recurrence with a median follow-up period of 8–9 months. A total success rate of 76.5% had been achieved in accordance with the literature on MTX [7, 25, 30, 31, 32, 33]. Joseph et al. [25] reported side effects, such as hair loss and elevated liver function tests, due to MTX administration in 2 patients out of 7. No side effects were observed due to the prednisone + MTX combination in our study.
Conclusion
An approach with low-dose MTX and prednisone to IGM patients not responding to steroids alone should be considered as an alternative treatment method instead of surgical intervention.
Statement of Ethics
The study was in line with the principles set out in the Declaration of Helsinki. All patients signed informed consent for their data to be used for research purposes after clear and complete explanation, and their consent was recorded in the patients' medical records. The Institutional Review Board of Dicle University, Diyarbakır, Turkey, approved this study (No.: 26.11.2020–357).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare that this study has received no financial support.
Author Contributions
M.T.K. and S.G. concept; M.T.K. and M.V.B. design; M.T.K. supervision; M.V.B. and S.G. findings; M.V.B. and S.G. materials; M.T.K., M.V.B., and S.G. data collection and/or processing; M.T.K. analysis and/or interpretation; M.T.K. literature review; M.T.K. and M.V.B. writing; M.T.K. and S.G. critical review.
References
- 1.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972 Dec;58((6)):642–6. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 2.Baslaim MM, Khayat HA, Al-Amoudi SA. Idiopathic granulomatous mastitis: a heterogeneous disease with variable clinical presentation. World J Surg. 2007 Aug;31((8)):1677–81. doi: 10.1007/s00268-007-9116-1. [DOI] [PubMed] [Google Scholar]
- 3.DeHertogh DA, Rossof AH, Harris AA, Economou SG. Prednisone management of granulomatous mastitis. N Engl J Med. 1980 Oct;303((14)):799–800. doi: 10.1056/NEJM198010023031406. [DOI] [PubMed] [Google Scholar]
- 4.Erhan Y, Veral A, Kara E, Ozdemir N, Kapkac M, Ozdedeli E, et al. A clinicopthologic study of a rare clinical entity mimicking breast carcinoma: idiopathic granulomatous mastitis. Breast. 2000 Feb;9((1)):52–6. doi: 10.1054/brst.1999.0072. [DOI] [PubMed] [Google Scholar]
- 5.Gurleyik G, Aktekin A, Aker F, Karagulle H, Saglamc A. Medical and surgical treatment of idiopathic granulomatous lobular mastitis: a benign inflammatory disease mimicking invasive carcinoma. J Breast Cancer. 2012 Mar;15((1)):119–23. doi: 10.4048/jbc.2012.15.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira FA, Mudgil AV, Macias ES, Karsif K. Idiopathic granulomatous lobular mastitis. Int J Dermatol. 2012 Feb;51((2)):142–51. doi: 10.1111/j.1365-4632.2011.05168.x. [DOI] [PubMed] [Google Scholar]
- 7.Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, Alavi A, Hemmati H, Esmaeili Delshad MS, et al. Granulomatous mastitis: Presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast. 2015 Aug;24((4)):456–60. doi: 10.1016/j.breast.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Schmajuk G, Genovese MC. First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy. J Rheumatol. 2009 Jul;36((7)):1559–60. doi: 10.3899/jrheum.090091. [DOI] [PubMed] [Google Scholar]
- 9.Bouton ME, Winton LM, Gandhi SG, Jayaram L, Patel PN, O' Neill PJ, et al. Temporal resolution of idiopathic granulomatous mastitis with resumption of bromocriptine therapy for prolactinoma. Int J Surg Case Rep. 2015;10:8–11. doi: 10.1016/j.ijscr.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hur SM, Cho DH, Lee SK, Choi MY, Bae SY, Koo MY, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc. 2013 Jul;85((1)):1–6. doi: 10.4174/jkss.2013.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boufettal H, Essodegui F, Noun M, Hermas S, Samouh N. Idiopathic granulomatous mastitis: a report of twenty cases. Diagn Interv Imaging. 2012 Jul;93((7-8)):586–96. doi: 10.1016/j.diii.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Salehi M, Salehi H, Moafi M, Taleban R, Tabatabaei SA, Salehi M, et al. Comparison of the effect of surgical and medical therapy for the treatment of idiopathic granulomatous mastitis. J Res Med Sci. 2014 Mar;19(Suppl 1):S5–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Postolova A, Troxell ML, Wapnir IL, Genovese MC. Methotrexate in the Treatment of Idiopathic Granulomatous Mastitis. J Rheumatol. 2020 Jun;47((6)):924–7. doi: 10.3899/jrheum.181205. [DOI] [PubMed] [Google Scholar]
- 14.Akcan A, Oz AB, Dogan S, Akgün H, Akyüz M, Ok E, et al. Idiopathic Granulomatous Mastitis: Comparison of Wide Local Excision with or without Corticosteroid Therapy. Breast Care (Basel) 2014 May;9((2)):111–5. doi: 10.1159/000360926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey TS, Mackinnon JC, Bressler L, Millar A, Marcus EE, Ganschow PS. Idiopathic granulomatous mastitis—a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014 May-Jun;20((3)):258–66. doi: 10.1111/tbj.12263. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Tymms KE, Buckingham JM. Methotrexate in the management of granulomatous mastitis. ANZ J Surg. 2003 Apr;73((4)):247–9. doi: 10.1046/j.1445-1433.2002.02564.x. [DOI] [PubMed] [Google Scholar]
- 17.Tse GM, Poon CS, Ramachandram K, Ma TK, Pang LM, Law BK, et al. Granulomatous mastitis: a clinicopathological review of 26 cases. Pathology. 2004 Jun;36((3)):254–7. doi: 10.1080/00313020410001692602. [DOI] [PubMed] [Google Scholar]
- 18.Kehribar DY, Duran TI, Polat AK, Ozgen M. Effectiveness of Methotrexate in Idiopathic Granulomatous Mastitis Treatment. Am J Med Sci. 2020 Nov;360((5)):560–5. doi: 10.1016/j.amjms.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Akahane K, Tsunoda N, Kato M, Noda S, Shimoyama Y, Ishigakis S, et al. Therapeutic strategy for granulomatous lobular mastitis: a clinicopathological study of 12 patients. Nagoya J Med Sci. 2013 Aug;75((3-4)):193–200. [PMC free article] [PubMed] [Google Scholar]
- 20.Skandarajah A, Marley L. Idiopathic granulomatous mastitis: a medical or surgical disease of the breast? ANZ J Surg. 2015 Dec;85((12)):979–82. doi: 10.1111/ans.12929. [DOI] [PubMed] [Google Scholar]
- 21.Gautier N, Lalonde L, Tran-Thanh D, El Khoury M, David J, Labelle M, et al. Chronic granulomatous mastitis: Imaging, pathology and management. Eur J Radiol. 2013 Apr;82((4)):e165–75. doi: 10.1016/j.ejrad.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Sheybani F, Sarvghad M, Naderi HR, Gharib M. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015 Apr;125((4)):801–7. doi: 10.1097/AOG.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 23.Kiyak G, Dumlu EG, Kilinc I, Tokaç M, Akbaba S, Gurer A, et al. Management of idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. BMC Surg. 2014 Sep;14((1)):66. doi: 10.1186/1471-2482-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman CM, Xia BT, Wilson GC, Lewis JD, Khan S, Lee SJ, et al. Idiopathic granulomatous mastitis: A diagnostic and therapeutic challenge. Am J Surg. 2017 Oct;214((4)):701–6. doi: 10.1016/j.amjsurg.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Joseph KA, Luu X, Mor A. Granulomatous mastitis: a New York public hospital experience. Ann Surg Oncol. 2014 Dec;21((13)):4159–63. doi: 10.1245/s10434-014-3895-z. [DOI] [PubMed] [Google Scholar]
- 26.Haddad M, Sheybani F, Arian M, Gharib M. Methotrexate-based regimen as initial treatment of patients with idiopathic granulomatous mastitis. Breast J. 2020 Feb;26((2)):325–7. doi: 10.1111/tbj.13590. [DOI] [PubMed] [Google Scholar]
- 27.Tekgöz E, Çolak S, Çinar M, Yilmaz S. Treatment of idiopathic granulomatous mastitis and factors related with disease recurrence. Turk J Med Sci. 2020 Aug;50((5)):1380–6. doi: 10.3906/sag-2003-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haitz K, Ly A, Smith G. Idiopathic granulomatous mastitis. Cutis. 2019 Jan;103((1)):38–42. [PubMed] [Google Scholar]
- 29.Altintoprak F, Kivilcim T, Yalkin O, Uzunoglu Y, Kahyaoglu Z, Dilek ON. Topical Steroids Are Effective in the Treatment of Idiopathic Granulomatous Mastitis. World J Surg. 2015 Nov;39((11)):2718–23. doi: 10.1007/s00268-015-3147-9. [DOI] [PubMed] [Google Scholar]
- 30.Maione C, Palumbo VD, Maffongelli A, Damiano G, Buscemi S, Spinelli G, et al. Diagnostic techniques and multidisciplinary approach in idiopathic granulomatous mastitis: a revision of the literature. Acta Biomed. 2019 Jan;90((1)):11–5. doi: 10.23750/abm.v90i1.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akbulut S, Arikanoglu Z, Senol A, Sogutcu N, Basbug M, Yeniaras E, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011 Nov;284((5)):1189–95. doi: 10.1007/s00404-010-1825-2. [DOI] [PubMed] [Google Scholar]
- 32.Konan A, Kalyoncu U, Dogan I, Kiliç YA, Karakoç D, Akdogan A, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care (Basel) 2012 Aug;7((4)):297–301. doi: 10.1159/000341388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan ME, Morgan M, Heilat GB, Kanesalingam K. Granulomatous lobular mastitis: clinical update and case study. Aust J Gen Pract. 2020 Jan-Feb;49((1-2)):44–7. doi: 10.31128/AJGP-08-19-5042. [DOI] [PubMed] [Google Scholar]