Abstract
Sudden cardiac death (SCD) of an athlete is a rare but tragic event and sport activity might play a trigger role in athletes with underlying structural or electrical heart diseases. Preparticipation screenings (PPs) have been conceived for the potential to prevent SCD in young athletes by early identification of cardiac diseases. The European Society of Cardiology protocol for PPs includes history collection, physical examination and baseline electrocardiogram, while further examinations are reserved to individuals with abnormalities at first-line evaluation. Nevertheless, transthoracic echocardiography has been hypothesized to have a primary role in the PPs. This review aims to describe how to approach an athlete-focused echocardiogram, highlighting what is crucial to focus on for the different diseases (cardiomyopathies, valvulopathies, congenital heart disease, myocarditis and pericarditis) and when is needed to pay attention to overlap diagnostic zone (“grey zone”) with the athlete's heart. Once properly tested, focused echocardiography by sports medicine physicians may become standard practice in larger screening practices, potentially available during first-line evaluation.
Keywords: Echocardiogram, Athletes, Sport eligibility, Pre-participation screening, Sudden cardiac death, Sport cardiology
Core Tip: Echocardiography is a helping tool in detecting several cardiovascular (CV) conditions afflicting athletes. As physicians become more experienced with sonography, focused echocardiography by sports medicine physicians may become standard practice in larger screening practices. This technique could help to detect some hidden CV condition and to distinguish between physiological and pathological adaptation to physical activity, assisting in sport eligibility process. In this review we aimed at describing athlete-focused echocardiogram that could be an effective time and cost-saving first line evaluation in granting sport eligibility to athletes.
INTRODUCTION
The advantages of sport practicing in improving cardiovascular (CV) health are evident, but a concomitant raise in CV events has been seen during exercise[1]. Physical activity therefore is a double-edge sword, with both benefits and risks mostly in predisposed subjects, even if not all people are conscious about it[2].
Sudden cardiac death (SCD) of an athlete is as uncommon as shocking event[3]. Sport practice might be a trigger event of cardiac arrest in athletes with underlying heart diseases, with syncope as a frequent first manifestation[4]. Indeed, these diseases are often clinically silent and unsuspecting[5]. Athletes have an increased risk of SCD compared with their non-active counterpart, related to pathologies theoretically identifiable through pre-participation screening (PPS)[6]. Therefore, several PPSs have been developed with the aim to uncover possible hidden and fatal CV condition in athletes. There is a significative debate about the efficacy and feasibility of different kind of PPSs proposed by national and international society worldwide. While the American Heart Association has never recommended a history collection and a physical examination, opposing the routine use of electrocardiogram (ECG)[7], the European Society of Cardiology (ESC) protocol focuses on history collection, physical examination, and ECG[8] and it has been adopted by different sport committees and international federations[9]. Controversies arise regarding the sensitivity and specificity of individual tests as well as the availability of expertise and cost effectiveness[10-13].
Nevertheless, some cardiac structural diseases can be difficult to detect on physical examination and ECG, but they may definitely be recognized with further cardiac investigations. About 10% of SCD cases have been related to cardiac diseases showing structural but no electrical manifestations[14]. While in Italy an exercise stress test is mandatory before engaging in competitive sports, transthoracic echocardiography has also been hypothesized to have a role in PPS: it might be a useful, convenient and noninvasive tool to increase diagnostic power of screening evaluation[15]. Even if echocardiography is frequently adopted as a first-line screening tool for athletes[16], also by major sporting bodies such a Federation Internationale de Football Association (FIFA) or Union of European Football Association (UEFA), and mainly as a valuable second line investigation to the diagnose malignant cardiac conditions[17,18], it has never been recommended as a first-line screening tool in athletes[19,20], nor included in recent ESC 2020 guidelines on sport cardiology[3]. Diagnostic necessity, time constrains and cost-effectiveness are probably unfavorable reasons for its use[21], even if there are only few data[22] regarding the cost-effectiveness of including an echocardiography in the PPS.
However, the use of echocardiography is highly increasing among non-cardiologist physicians and it could represent a powerful tool in the sport eligibility process by sport medicine physicians.
Therefore, the aim of the present paper is to review athlete-related echocardiographic features in order to develop a first-line screening athlete-focused echocardiogram. How should an echocardiography of the athlete be performed? What should a sport physician know about echocardiography? What would an athlete-focused echocardiogram be like?
EPIDEMIOLOGY AND ETIOLOGY OF SCD
SCD could literally be defined as “natural death due to cardiac causes, heralded by abrupt loss of consciousness within one hour after the onset of symptoms”[23]. Current epidemiology of the incidence of SCD in professional athletes varies from almost 1/1000000 to 1/5000 subjects per year[24,25]. Indeed, the definition of the precise frequency of SCD is made difficult by heterogeneous study methodologies. In literature, the incidence of SCD in young athletes is fluctuating[14,26-28], with a prevalence of CV predisposing conditions of about 0.2%–0.7%[29].
Several causes of SCD in athletes are related to the age of onset. In patients from birth to adolescence, the primary cause of SCD is a congenital abnormality[30]. The great majority of young victims (age ≤ 35 years) present a concealed structural heart disease[31]. Coronary artery disease is the most frequent cause of SCD in aged athletes (35 years or older)[32]. Main causes of SCD are summarized in Table 1.
Table 1.
Common cardiovascular diseases associated with sudden cardiac deaths in athletes (adapted from[15,21])
|
Type of pathology
|
Pathology
|
| Cardiomyopathies | HCM |
| DCM | |
| LVNC | |
| ARVC | |
| CAD | |
| Myocarditis | |
| Congenital defects | AOCA |
| BAV | |
| Valvulopathies | MVP |
| Aortic diseases | Aortic dissection |
| Aortic rupture | |
| Aortic aneurism | |
| Kawasaki disease | |
| Idiopathic scarring | |
| Conduction defects | WPW |
| Channelopathies | LQTs |
| Brugada syndrome | |
| CPVT | |
HCM: Hypertrophic cardiomyopathy; DCM: Dilated cardiomyopathy; LVNC: Left ventricle non compaction; ARVC: Arrhythmogenic right ventricle cardiomyopathy; CAD: Coronary artery disease; AOCA: Anomalous origin of coronary arteries; BAV: Bicuspid aortic valve; MVP: Mitral valve prolapse; WPW: Wolff-Parkinson-White syndrome; LQTs: Long QT syndrome; CPVT: Catecholaminergic polymorphic ventricular tachycardia.
ECHOCARDIOGRAPHY IN ATHLETE’S HEART
Even if a minimum dataset is recommended for all echocardiograms[33] (Table 2), previous knowledge of some demographics data, patient’s personal history and physical examination, and ECG findings will help to focus the examination and the interpretation of findings: sex, age, body mass index, ethnicity, ECG changes, symptoms, training volume, type of sport and level, and family history of unexplained cardiac death < 40 years[34]. A focused echocardiogram with real-time interpretation can considerably cut the cost and time spent performing the study, as previously demonstrated[35]. Indeed, Niederseer et al[21] proposed to include a double screening echocardiography in the athlete: in adolescence to rule out structural heart disease, and over the age of 30 to evaluate pathological cardiac remodeling to exercise, cardiomyopathies and wall motion anomalies. Often coronary artery disease is missed by classing screening method, so in a master athlete (> 35 years) echocardiography detection of atherosclerotic plaque[36], could represent a valid example of a focused exam.
Table 2.
Minimum dataset for transthoracic echocardiography (adapted from[33])
|
Echo views
|
Structure
|
Measure
|
| PLAX | LV | IVS |
| End diastolic diameter | ||
| Posterior wall | ||
| Wall motion | ||
| Mitral Valve | Leaflets and annulus | |
| Color | ||
| Aortic Valve | Anulus | |
| Valsalva Sinus | ||
| STJ | ||
| Color | ||
| Ascending aorta | Size | |
| RV | RVOT | |
| LA | Size | |
| PSAX–aortic valve | Aortic Valve | Morphology |
| OCA | ||
| RV | RVOT | |
| Pulmonary valve | Color | |
| PW | ||
| PSAX-base | Mitral Valve | Leaflets and annulus |
| PSAX–mid/apex | LV | Wall motion |
| A4C | LV | EF |
| Wall motion | ||
| Wall thickness | ||
| End diastolic area/volume | ||
| VSD | ||
| ASD | ||
| LA | LAVI | |
| Mitral valve | Color | |
| PW | ||
| TDI | ||
| RV | RVD1 | |
| RVD2 | ||
| RVD3 | ||
| Wall motion | ||
| TAPSE | ||
| TV | Color | |
| CW | ||
| Pulmonary veins | PW | |
| A5C | Aortic valve | Color |
| CW | ||
| A2C | LV | Wall motion |
| Subcostal | ASD | |
| Inferior vena cava | Size | |
| Breath collapsibility | ||
| Pericardium | Pericardial effusion | |
| Abdominal aorta | Size | |
| Suprasternal | Aortic arch | Size |
| Color | ||
| CW |
PLAX: Parasternal long axis view; PSAX: Parasternal short axis view; A4C: Apical 4 chambers view; A5C: Apical 5 chambers view; A2C: Apical 2 chambers view; LV: Left ventricle; BSA: Body surface area; EF: Ejection fraction; RVOT: Right ventricle outflow tract; RV: Right ventricle; FAC: Fractional area charge; LAVI: Left atrium ventricle index; LA: Left atrium; IVS: Inter ventricular septum; PW: Power doppler wave; CW: Continuous doppler wave; RA: Right atrium; STJ: Sinotubular junction; OCA: Origin of coronary arteries; PDA: Patent ductus arteriosus; COA: Aortic coarctation; ASD: Atria septum defect; VSD: Ventricular septal defect; RVD: Right ventricle diameter; TAPSE: Tricuspid annular plane excursion.
Moreover, prolonged physical activity causes structural, functional, and electrical heart modifications that represent the physiological responses (“adaptation”) of the heart during physical effort: this series of remodeling is named as “athlete's heart”[37] and it can be appreciated both in ECG[38] and in echocardiograms. These adaptations, involving all the heart chambers[39] and strictly dependent upon on the duration, type and intensity of training, are often benign and physiological but, sometimes, may predispose to pathological conditions[40,41]. The challenges posed by athlete’s heart require detailed assessment in order to distinguish between physiological adaptation and life-threatening cardiomyopathies[16,42-44], that often coexist in the so called “grey zones”.
Also, novel advanced echocardiographic techniques play an emerging role in the echocardiographic investigation of athlete[41]: Exercise stress echocardiography in the differentiation between athlete’s heart and dilated cardiomyopathy, coronary artery disease and pulmonary hypertension; speckle tracking [with left ventricular (LV) global longitudinal strain] in the differentiation between athlete’s heart and dilated cardiomyopathy and hypertrophic cardiomyopathy, other than the characterization of wall motion abnormalities and right ventricle description; 3D echocardiography in estimation of cardiac mass.
Therefore, echocardiography has a major role in sports cardiology, and it can help physicians not only to investigate structural diseases invisible both at anamnesis and ECG, but also to rule out these physio-pathological modifications.
ATHLETE-FOCUSED ECHOCARDIOGRAM
The concept of point-of-care ultrasound is increasingly emerging in literature[45], in different body systems[46,47], including heart. Even if it is crucial to consider the ultrasound scanner and the degree of competence and knowledge of the sonographer[48], a focused protocol for point-of-care echocardiography could be developed for physicians to help screen out major CV diseases afflicting athletes in granting sport eligibility. Such protocols may gain much more attention by the fact that nowadays, portable and handheld ultrasound devices are spreading and have proven to be equally reliable and accurate in evaluating cardiac features[49,50].
Several of athlete-focused echocardiography protocols have been proposed in literature[19,51,52] by different authors[53-55], and some are reported in Table 3. Some of them are direct to rule out specific diseases, while others are designed for global viewing but only last a few minutes. Moreover, several sportive federations, such as FIFA, have their own precompetition medical assessment that includes echocardiographic examination.
Table 3.
Athlete-focused echo protocols
|
Ref.
|
Echo view (parameters assessed)
|
Estimated exam time
|
| Feinstein et al[51] | PLAX (LV IVS, LV posterior wall, LVOT) | 1-2 min |
| Wyman et al[53] | PLAX (aortic arch size, aortic valve characteristics, mitral valve characteristics, LV wall motion, LV mass); PSAX (aortic valve characteristics, aortic valve morphology, origin of coronary arteries, CW pulmonary valve, LV wall motion, LV wall thickness); A4C (tricuspid valve characteristics, mitral valve characteristics, RV size, RV wall motion, LV size); A5C (CW aortic valve) | Not specified |
| Weiner et al[52] | PLAX (aortic valve characteristics, mitral valve characteristics, CW tricuspid valve); PSAX (aortic valve characteristics, pulmonary valve characteristics, LV wall motion); A4C (RV size, RV wall motion, LV size, LV wall motion, PW mitral valve, TDI mitral valve, tricuspid valve characteristics); A5C (CW aortic valve); A2C (LV wall motion) | 13 min |
| Yim et al[55] | PLAX (IVS, LV end diastolic diameter, PW, aortic arch size) | Not specified |
| Fishman et al[54] | LV IVS, LV posterior wall, LV end diastolic diameter, PW, EF, AVR, MVR aortic valve regurgitation, mitral valve regurgitation, aortic valve morphology, aortic root dimension | 1 min |
PLAX: Parasternal long axis view; PSAX: Parasternal short axis view; A4C: Apical 4 chambers view; A5C: Apical 5 chambers view; A2C: Apical 2 chambers view; IVS: Inter ventricular septum; LVOT: Left ventricle outflow tract; LV: Left ventricle; CW: Continuous wave; RV: Right ventricle; PW: Power doppler wave; TDI: Tissue doppler imaging; EF: Ejection fraction; AVR: Aortic valve regurgitation; MVR: Mitral valve regurgitation.
We want to propose our version of first-line athlete-focused echocardiography, as seen in Table 4 and Figures 1 and 2. Some measurements are intentionally missed: visually estimated left ventricular ejection fraction and wall motion has been found to be closely related to formal quantitative methods[56,57]; valvular heart diseases can be firstly screened by color doppler[58]. We also consider a visual assessment of cardiac chambers’ dimensions. All these first-line measurements, if pathological, will be eventually followed by other measurements (“second-line”), as seen in Table 5.
Table 4.
Proposed echocardiographic protocol for athletes
|
Echo views
|
Structure
|
Measure
|
| PLAX | LV | IVS |
| End diastolic diameter | ||
| Posterior wall | ||
| Mitral valve | Leaflets | |
| Color | ||
| Aortic valve | Valsalva sinus | |
| Color | ||
| Ascending aorta | Size | |
| PSAX–aortic valve | Aortic valve | Morphology |
| OCA | ||
| RV | RVOT | |
| PDA | ||
| A4C | LV | Trabeculations |
| Wall motion | ||
| VSD | ||
| ASD | ||
| Mitral valve | Color | |
| PW | ||
| TV | Color | |
| CW | ||
| A5C | Aortic valve | Color |
| CW | ||
| Subcostal | ASD | |
| Inferior vena cava | Size | |
| Breath collapsibility | ||
| Pericardium | Pericardial effusion | |
| Suprasternal | Aortic arch | Size |
| COA |
PLAX: Parasternal long axis view; PSAX: Parasternal short axis view; A4C: Apical 4 chambers view; A5C: Apical 5 chambers view; A2C: Apical 2 chambers view; LV: Left ventricle; BSA: Body surface area; EF: Ejection fraction; RVOT: Right ventricle outflow tract; RV: Right ventricle; FAC: Fractional area charge; LAVI: Left atrium ventricle index; LA: Left atrium; IVS: Inter ventricular septum; PW: Power doppler wave; CW: Continuous doppler wave; RA: Right atrium; STJ: Sinotubular junction; OCA: Origin of coronary arteries; PDA: Patent ductus arteriosus; COA: Aortic coarctation; ASD: Atria septum defect; VSD: Ventricular septal defect; RVD: Right ventricle diameter; TAPSE: Tricuspid annular plane excursion.
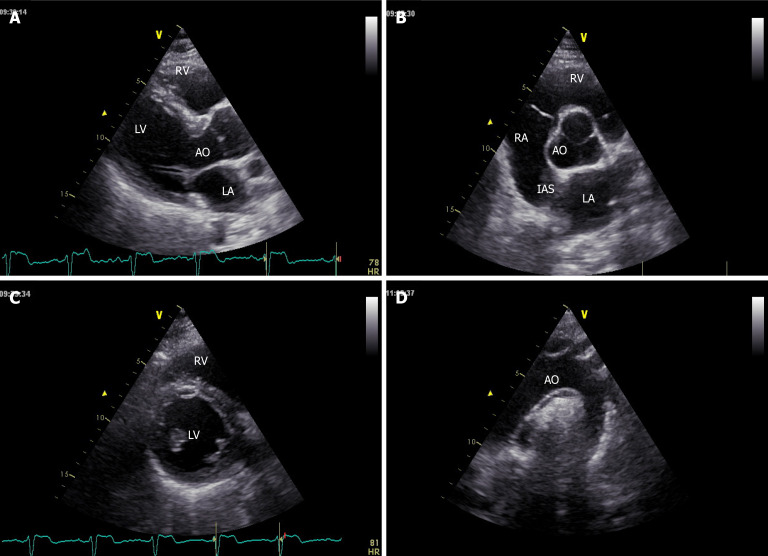
Figure 1.
Parasternal long-axis, short axis and suprasternal windows showing left and right chambers and aortic valve and arch. A: Long-axis; B and C: Short axis; D: Suprasternal windows. LV: Left ventricle; LA: Left atrium: RV: Right ventricle; RA: Right atrium; AO: Aorta; IAS: Interatrial septum.
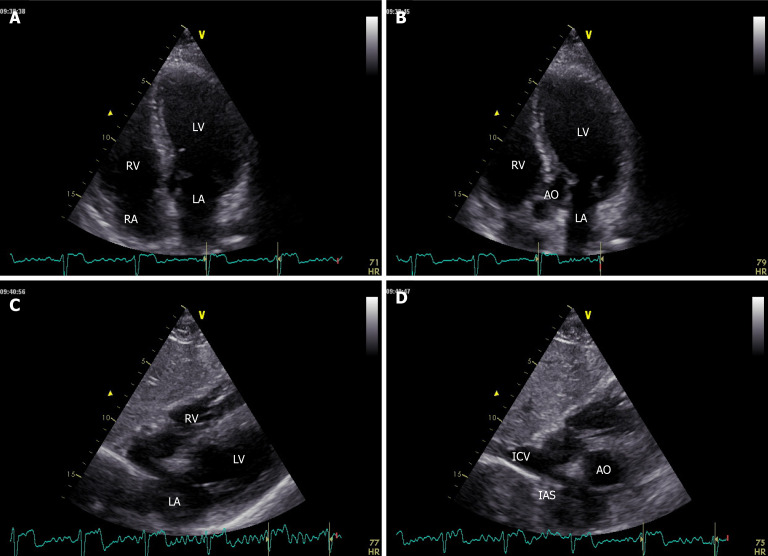
Figure 2.
Apical 4-chamber, 5-chamber and subcostal windows showing left and right chambers, inferior cava vein and pericardium. A: 4-chamber; B: 5-chamber; C and D: Subcostal windows. LV: Left ventricle; LA: Left atrium: RV: Right ventricle; RA: Right atrium; AO: Aorta; IAS: Interatrial septum; ICV: Inferior cava vein.
Table 5.
Main echo findings of cardiovascular pathologies in athletes
|
Pathology
|
What to assess? (echo view)
|
Cut-off mm (mean mm)
|
If pathological, what to assess? (echo view)
|
Cut-off mm (mean mm)
|
| HCM | LV Max end-diastolic wall thickness | M white 15 (10); M Afro-American 16 (11.5); F white 11; F Afro-American 13 (9.5); M/F adolescent 16 (12) | LV wall thickness distribution | Asymmetric (HCM) |
| LV end diastolic diameter (A4C) | M 70 (55); F 66 (49); Adolescent 60 (51) | |||
| LV mass/BSA | M 117 mm/m2 (83 mm/m2); F 143 mm/m2 (101 mm/m2) | |||
| LVOT obstruction | ||||
| E/A (A4C) | 1.3 (1.93) | |||
| DCM | LV end diastolic diameter (PLAX) | M 70 (50); F 66 (49); Adolescent 60 (51) | EF (A4C) | 55% (64%) |
| LVNC | LV trabeculation | NC/C layer ratio > 2.0 in systole | EF (A4C) | 55% (64%) |
| Thickness of compact layer in systole | 8 | |||
| E/A (A4C) | 1.3 (1.93) | |||
| ARVC | RVOT/BSA (PSAX) | > 21 mm/m2 | RV inflow (A4C)/ LV end diastolic diameter (PLAX) | > 0.9 |
| RVOVT/BSA (PLAX) | > 19 mm/m2 | RV wall motion abnormalities | ||
| RV FAC | 33% | |||
| Aortic dilatation | Aortic valve max dimension (PLAX) | M 40 (32); F 34 (28) | Other congenital defects (BAV) | |
| Ascending aorta dimension (PLAX) | Aortic regurgitation | |||
| Mitral prolapse | Mitral prolapse (PLAX) | Abnormal systolic bulging of leaflets > 2 mm toward LA | Mitral regurgitation | |
| PAPS (A4C) | 40 mmHg (24 mmHg) | |||
| Pulmonary veins flow | Reverse | |||
| EF (A4C) | 55% (64%) | |||
| LV mass/BSA | M 117 mm/m2 (83 mm/m2); F 143 mm/m2 (101 mm/m2) | |||
| LAVI | M 36 mm/m2 (28 mm/m2); F 33 mm/m2 (26.5 mm/m2) | |||
| AOCA | Coronary arteries origin (PSAX) | |||
| BAV | Aortic morphology (PSAX) | Aortic stenosis | ||
| Aortic regurgitation | ||||
| Aortic root max dimension (PLAX) | M 40 (32); F 34 (28) | |||
| Other congenital defects (coarctation of the aorta, interrupted aortic arch, patent ductus arteriosus, coronary anomaly or hypoplastic left heart, as well as Williams or Turner syndrome) | ||||
| ASD | ASD | RV dimension (A4C) | Basal RV: M 55 (43.5), F 49 (39); Medial RV: M 47 (34), F 43 (32); Longitudinal RV: M 109 (89), F 100 (82) | |
| RA area/BSA (A4C) | M 28 mm/m2 (19.5 mm/m2); F 24 mm/m2 (15.5 mm/m2) | |||
| PAPS (A4C) | 40 mmHg (24 mmHg) | |||
| VSD | VSD | LV mass/BSA (PLAX) | M 117 mm/m2 (83 mm/m2); F 143 mm/m2 (101 mm/m2) | |
| PAPS (A4C) | 40 mmHg (24 mmHg) | |||
| Aortic regurgitation | ||||
| Other congenital defects (aneurysm of Valsalva sinus, ToF, TGA, DCRV) | ||||
| PDA | PDA (PSAX) | LA/Aortic root ratio | ≥ 1.4 | |
| LV mass/BSA (PLAX) | M 117 mm/m2 (83 mm/m2); F 143 mm/m2 (101 mm/m2) | |||
| PAPS (A4C) | 40 mmHg (24 mmHg) | |||
| Pulmonary artery size (PSAX) | ||||
| RV dimension (A4C) | Basal RV: M 55 (43.5), F 49 (39); Medial RV: M 47 (34), F 43 (32); Longitudinal RV: M 109 (89), F 100 (82) | |||
| RA area/BSA (A4C) | M 28 mm/m2 (19.5 mm/m2); F 24 mm/m2 (15.5 mm/m2) | |||
| Other congenital defects (COA, pulmonary atresia) | ||||
| COA | COA (PSAX) | Aortic stenosis | ||
| Mitral stenosis | ||||
| LV mass/BSA (PSAX) | M 117 mm/m2 (83 mm/m2); F 143 mm/m2 (101 mm/m2) | |||
| EF (A4C) | 55% (64%) | |||
| Other congenital defects (BAV, ascending aortic aneurysm) | ||||
| Myocarditis | EF (A4C) | 55% (64%) | ||
| LV wall motion abnormalities | ||||
| Pericardial effusion | ||||
| Increased LV wall thickness | ||||
| Pericarditis | Pericardial effusion | |||
| Kawasaki disease | Coronary artery abnormalities | |||
| EF (A4C) | 55% (64%) | |||
| LV wall motion abnormalities | ||||
| Mitral regurgitation | ||||
| Aortic regurgitation | ||||
| Pericardial effusion | ||||
PLAX: Parasternal long axis view; PSAX: Parasternal short axis view; A4C: Apical 4 chambers view; HCM: Hypertrophic cardiomyopathy; DCM: Dilated cardiomyopathy; LVNC: Left ventricle non compaction; ARVC: Arrhythmogenic right ventricular cardiomyopathy; BAV: Bicuspid aortic valve; AOCA: Anomalous origin of coronary arteries; ASD: Atrial septum defects; VSD: Ventricular septal defects; PDA: Patent ductus arteriosus; CoA: Aortic coarctation; M: Male; F: Female; LV: Left ventricle; BSA: Body surface area; EF: Ejection fraction; LVOT: Left ventricle outflow tract; NC/C: Non-compact/compact; RVOT: Right ventricle outflow tract; RV: Right ventricle; FAC: Fractional area charge; PAPS: Pulmonary artery systolic pressure; LAVI: Left atrium ventricle index; RA: Right atrium; ToF: Tetralogy of fallot; TgA: Transposition of great arteries; DCRV: Double-chambered right ventricle.
Below we will describe main echo findings and grey zone in the more frequent CV conditions in athletes (Table 5), with cut off based on latest recommendations[42].
CARDIOMYOPATHIES
The diagnosis of these cardiomyopathies and their differentiation with athlete’s heart still represent one of the biggest challenges of sport cardiology
HYPERTROPHIC CARDIOMYOPATHY
Hypertrophic cardiomyopathy (HCM) is one of the most common etiologies of SCD in athletes and represents one of the most frequent reason for denied sport eligibility according to both American and European guidelines[59-61].
Echocardiography is crucial for the diagnosis and monitoring of HCM. In the absence of another reason of hypertrophy, it can be diagnosed with a maximal end-diastolic wall thickness of ≥ 15 mm in any zone of the left ventricle; less marked hypertrophy (13–14 mm) can be diagnostic when present in relatives of a patient with HCM or along with a positive genetic test. For children, a threshold of z > 2.5 may be useful to identify early HCM in asymptomatic subject with no family history, while for children with a positive family history or a positive genetic test, a threshold of z > 2 could be sufficient for early diagnosis.
Measurements of LV wall thickness should be executed at end diastole, in parasternal long axis (PLAX) or in parasternal short axis (PSAX) views[15].
Grey zone: The diagnosis of HCM in competitive athletes may be difficult when LV wall thickness ranges from 13 to 16 mm[62-64]. The overlap between athlete’s heart and HCM is challenging in Afro-American individuals[65]. Indeed, the extreme value of LV hypertrophy in athletes is found in male (15 mm in white and 16 mm in Afro-American athletes[66]), while lower values are found in female (11 mm in whites and 13 mm in Afro-Americans[67]). The type and the intensity of sport practiced are also important aspects to consider.
Differential diagnosis features between HCM and athlete’s heart in the gray-zone is seen in Table 6. The most practical morphologic criterion is the assessment of LV cavity size and geometry, enlarged (end-diastolic diameter > 54 mm) and with an eccentric pattern only in athlete’s heart[62,68]. On the other side, an asymmetric and heterogeneous pattern of LV hypertrophy represents features of HCM[62]. Furthermore, athletes usually show a homogeneous distribution of wall thickness and a normal diastolic function[69], besides a reduction of wall thickness after a detraining period.
Table 6.
Differential diagnosis between hypertrophic cardiomyopathy and athlete’s heart, in the grey-zone (adapted from[42])
|
HCM
|
Findings
|
Athlete’s heart
|
| Normal, reduced | LV cavity size | Enlarged, eccentric pattern |
| Asymmetric and heterogeneous | LV hypertrophy | Symmetric and homogeneous |
| Present | LVOT obstruction | Absent |
| Abnormal | LV diastolic function | Normal |
| Unchanged | LV wall thickness after detraining | Reduced |
HCM: Hypertrophic cardiomyopathy; LV: Left ventricle; LVOT: Left ventricle outflow tract.
What to assess? LV wall thickness. If pathological, what to assess? LV end diastolic diameter + LV mass + LV outflow tract (LVOT) obstruction + LV wall thickness distribution + LV diastolic function (E/A).
DILATED CARDIOMYOPATHY
Dilated cardiomyopathy (DCM) is a not-so rare cause of SCD in athletes[70,71]. It is defined as (left) ventricular systolic dysfunction and dilatation not explained by other loading conditions. Systolic dysfunction is measured by abnormal LV ejection fraction (EF) (through biplane Simpson’s rule). LV dilatation is defined by LV end-diastolic diameters > 2 SD from normal, indexed by body surface area (BSA), age and gender. LV shape can provide additional information (“sphericity index”)[72].
Grey zones: Left ventricular cavity increasing in athletes should be interpreted in the context of sport played and it is usually associated with increased wall thickness[73,74]. A significant dilation is often seen in endurance athletes. Almost 40% of athletes showed an increase of LV end diastolic diameter (> 55 mm), and almost 10% even bigger cavity (> 60 mm, with 99th percentile corresponding to 65 mm). LV dilation in athletes is frequently presented with EF > 55%. Moreover, indices of diastolic function are within normal values[75]. In case of mildly reduced ejection fraction, it may be useful to perform a stress test echocardiography, along with the measure of stroke volume.
Differential diagnosis features between DCM and athlete’s heart in the grey zone is showed in Table 7.
Table 7.
Differential diagnosis between dilated cardiomyopathy and athlete’s heart (adapted from[42])
|
DCM
|
Findings
|
Athlete’s heart
|
| > 60 mm | LV end diastolic diameter | < 60 mm |
| Reduced | EF | Normal |
| Abnormal | Diastolic function | Normal |
DCM: Dilated cardiomyopathy; LV: Left ventricle; EF: Ejection fraction.
What to assess? LV end diastolic diameter. If pathological, what to assess? EF.
LEFT VENTRICLE NON COMPACTION
Left ventricle non-compaction (LVNC) is a cardiomyopathy identified by increased myocardial trabeculations and inter-trabecular recesses[76,77], ranging clinically from asymptomatic to SCD outcomes. Diagnosis is based on three proposed echocardiographic criteria, showing high non-compacted to compacted ratio within the LV myocardium[78,79].
Grey zones: About 1 in 5 athletes has LV hypertrabeculation, with about 10% complying LVNC diagnostic criteria[80,81]. This may be due to cardiac adaptation to load training. ESC guidelines on cardiovascular imaging[42] proposed the threshold for defining an LV trabeculations on echocardiography: a non-compacted to compacted (NC/C) layer ratio > 2.0 in systole[82].
In athletes achieving criteria for LVNC, an history of cardiac symptoms or family SCD, are suggestive of pathology. Additional echocardiography features of LVNC include low EF not improving with exercise, altered diastolic function and a thickness of compacted layer in systole < 8 mm.
Differential diagnosis features between LVNC and athlete’s heart in the gray-zone is showed in Table 8.
Table 8.
Differential diagnosis between left ventricle non compaction and athlete’s heart, in the grey zone (adapted from[42])
|
LVNC
|
Findings
|
Athlete’s heart
|
| Reduced | LV systolic function | Normal |
| Reduced | Thickness of compact layer | Normal |
| Abnormal | Diastolic function | Normal |
LVNC: Left ventricle non compaction; LV: Left ventricle.
What to assess? LV trabeculation. If pathological, what to assess? FE (Simpson) + thickness of compact layer in systole + LV diastolic function (E/A).
ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a cardiomyopathy characterized by progressive waste of myocytes that are substituted by fibrofatty tissue. It typically (but not only) involves the right ventricle (RV).
Diagnostic imaging criteria have been defined in 2010[83]: Disproportionate enlargement of the right ventricle outflow tract (RVOT) and RV motion abnormalities are essential characteristics of this disease[84].
Grey zones: Long-term physical activity could promote the expression of ARVC: exercise remodeling includes RV (basal) enlargement and mild reduction of global RV peak systolic longitudinal strain[15]. ESC guidelines on cardiovascular imaging[42] propose that only major dimensional criteria, indexed by BSA, should be used to define RV enlargement in athletes: specifically, RVOT > 19 mm/m2 in PLAX, and/or > 21 mm/m2 in PSAX. Other useful measures include the ratio of RV inflow dimension (in apical 4 chambers view (A4C))/LV end-diastolic dimension (PLAX) > 0.9[85], the RV fractional area change (FAC) < 33%[86], and a subclinical RV dysfunction with reduced RV global longitudinal strain[87]. About 50% of ARVC subjects could have regional akinesia, dyskinesia, or aneurysmal deformation.
Differential diagnosis features between ARVC and athlete’s heart is seen in Table 9.
Table 9.
Differential diagnosis between arrhythmogenic right ventricular cardiomyopathy and athlete’s heart, in the grey-zone (adapted from[42])
|
ARVC
|
Findings
|
Athlete’s heart
|
| Exceeding major criteria for ARVC | RV size | Not exceeding major criteria for ARVC |
| Abnormal | Regional RV wall motion | Normal |
| Abnormal | Global RV function | Normal |
ARVC: Arrhythmogenic right ventricular cardiomyopathy; RV: Right ventricle.
What to assess? RVOT PSAX + RVOT PLAX. If pathological, what to assess? RV inflow apical/LV end diastolic PLAX + RV regional wall abnormalities + RV FAC.
AORTIC DISEASES
Aortic pathologies, such as aortic dissection or aortic aneurism, are important etiologies of SCD in athletes[88,89] and could be related to Marfan disease. Practically, aortic root diameter measurements should be performed from the PLAX view using the leading edge-to-leading edge convention (except for the measurement of the annulus, in which it is used inner edge to inner edge convention) and preferring 2D measurements[90,91]. Otherwise, in children and in subjects ≤ 25 years, aortic root dimensions should be carried out using the inner-wall-to-inner-wall technique during systole[92]. Measurements should embrace the aortic valve annulus, the sinuses of Valsalva, the sinotubular junction and the proximal ascending aorta.
Grey zone: Remodeling of the aortic root in athletes is strictly related to the overload training[93-95]. Large increases in aortic size are rare in athletes and often related to an underlying potentially fatal aortic disease[96]. The American College of Cardiology and the American Heart Association have proposed the use of z-scores to identify subjects with significant aortic root dilation[97]. If dilated aortic root dimensions are found, it is recommended to exclude the presence of associated conditions such as Marfan Syndrome of bicuspid aortic valve (BAV)[98,99]. In athletes with dilated aortic root, a periodical diagnostic assessment to monitor dimension is recommended.
What to assess? aortic root dimension + ascending aortic diameter. If pathological, what to assess? BAV + aortic valve regurgitation.
VALVULOPATHIES
Mitral valve prolapse
Mitral valve prolapse (MVP) is a common valve abnormality in population that, in some case, has been associated to SCD especially in young individuals[100]. Echocardiography, ideally in PLAX view, is essential for MVP diagnosis[40]: It is literally defined by “abnormal systolic bulging of one or both leaflets toward the left atrium (LA) with displacement of coaptation point into the LA (> 2 mm beyond a line connecting the annular hinge point)”. Leaflets are usually elongated and thickened: “classic” MVP is characterized by a leaflet thickness ≥ 5 mm, while “non-classic” MVP is defined by leaflet thickness < 5 mm. Furthermore, mitral annulus is usually enlarged.
Grey zone: MVP is one of the most frequent cause of mitral valve (MV) regurgitation in athletes[101]. If rare, cases of malignant arrhythmic prolapse have been reported and therefore this is a must be ruled-out condition[102-104]. Athletes usually has mild mitral regurgitation (MR), even if severe MR must be excluded (Table 10). Also pulmonary artery pressures (PAPS) and reverse flow in the pulmonary veins assessment should be performed. LV and LA dimensions must be evaluated to exclude cardiac chamber dilatation associated.
Table 10.
Echocardiographic criteria for the definition of severe valve regurgitation (adapted from[165])
|
|
AVR
|
MVR
|
TVR
|
| Vena contracta width (mm) | > 6 | ≥ 7 | ≥ 7 |
| Other | Pressure half-time < 200 ms | TVI mitral/TVI aortic > 1.4 | PISA radius > 9 mm |
| EROA (mm2) | ≥ 30 | ≥ 40 | ≥ 40 |
| Regurgitant volume (mL/beat) | ≥ 60 | ≥ 60 | ≥ 45 |
AVR: Aortic valve regurgitation; EROA: Effective regurgitant orifice area; MVR: Mitral valve regurgitation; PISA: Proximal isovelocity surface area; TVR: Tricuspid valve regurgitation; TVI: Time velocity integral.
What to assess? MV prolapse. If pathological, what to assess? MR (vena contracta, CW jet area, PISA, MR flow, CW doppler, E wave) + PAPS + pulmonary vein flow + LV mass + LV function + LA dimension.
Other valvulopathies
Valvular heart disease is frequently related to degenerative processes and usually age-related[14], but may also involve younger individuals. Moreover, specific recommendations exist for athletes with valvulopathies[105]. Congenital defects are common cause of valvular heart disease in young individuals. Although cardiac magnetic resonance offers a better quality of imaging[106], these are conditions to be considered when performing an echocardiogram in athletes. Right-sided valvular pathologies and regurgitant lesions are better tolerated[107].
Valvular Stenosis
Aortic valve stenosis (AVS) counts for 5% of congenital heart defects, and is frequently caused by BAV[108]. Even if mostly asymptomatic, these subjects have a small but significant risk of sudden death[109]. Echocardiography is the gold standard diagnostic tool[110] (Table 11).
Table 11.
Echocardiographic parameters indicative of the degree of severity of different valve stenosis (adapted from[111,165])
| AVS | MVS | TVS | PVS | |||||||
|
Low
|
Moderate
|
Severe
|
Low
|
Moderate
|
Severe
|
Clinically significant
|
Low
|
Moderate
|
Severe
|
|
| V max (m/s) | 2.6-2.9 | 3.0-3.4 | ≥ 4.0 | < 3 | 3-4 | ≥ 4 | ||||
| DP mean (mmHg) | < 30 | 30-40 | ≥ 40 | < 5 | 5-10 | > 10 | > 5 | < 30 | 30-50 | > 50 |
| Valve orifice area (cm2) | > 1.5 | 1.0-1.5 | < 1.0 | > 1.5 | 1.0-1.5 | < 1.0 | ||||
AVS: Aortic valve stenosis; MVS: Mitral valve stenosis; TVS: Tricuspid valve stenosis; PVS: Pulmonary valve stenosis; PAP: Pulmonary artery pressure.
Mitral valve stenosis (MVS) in young individuals can be of rheumatic origin. It results in elevated LA pressure and eventually pulmonary hypertension. An exercise-related cardiac output increase can lead to acute pulmonary edema[111]. The degree of MVS can be categorized through echocardiography (Table 8).
Tricuspid valve stenosis (TVS) can be caused by rheumatic fever and is frequently associated with MVS, rather than alone[111].
Pulmonary valve stenosis accounts for about 10% of the congenital heart syndrome[112]. It is commonly present in tetralogy of Fallot, Nooan syndrome and maternal rubella syndrome[113]. Most of these patients are asymptomatic, but rarely SCD. Evaluation of pulmonary stenosis is best evaluated with echocardiography[114,115].
Valvular regurgitation
Valvular regurgitation is a common and often harmless condition in athletes. In particular, mitral and tricuspid regurgitation are widely diffused in young subjects[116].
The more common causes of aortic valve regurgitation (AVR) include BAV, rheumatic fever, endocarditis, Marfan syndrome and aortic dissection. AVR causes LV dilatation with subsequent LV pressure and volume loading. LV dilatation should be evaluated by echocardiography. Often LV cavity dimension can be raised in healthy athletes[111]. Quantification of AVR is seen in Table 9.
There are many causes of mitral valve regurgitation (MVR), including MVP, endocarditis, rheumatic heart disease, coronary artery disease, and dilated cardiomyopathy[117]. In athletes, mitral regurgitation is mostly related to MVP. Quantification of MVR is showed in Table 9.
Tricuspid valve regurgitation (TVR) is often the result of RV dilatation. Primary TVR leads to RV volume overload and increased venous pressure. The severity of TVR can be determined by echocardiography (Table 10)[111].
CONGENITAL HEART DISEASES
A once-in-a-lifetime echocardiography allows to identify congenital heart disease in order to plan an individualized follow-up and exercise recommendation, other than screening of the athlete’s family.
ANOMALOUS ORIGIN OF CORONARY ARTERIES
Anomalous origin of coronary arteries (AOCA) is a rare but recognized cause of sports related SCD[108], often missed to ECG. Identification of AOCA is not easy because these are often asymptomatic individuals and do not necessarily exhibit distinctive features on ECG stress testing. Even if echocardiography is not the gold standard for its diagnosis, this examination is the first line test recommended when there is such a suspicion also because it is the only noninvasive tool to visualize the ostia and first tracts of coronary arteries[118-120]. Each coronary artery (CA) usually originates from its respective sinus above the aortic valve leaflets, with the right CA (RCA) arising from the right sinus of Valsalva and the left main CA (LCA) arising from the left sinus of Valsalva[121]. Not all AOCA have the same prognostic impact: the most at-risk anomalies include an anomalous origin from a wrong aortic sinus [aortic anomalous origin of coronary arteries (AAOCA)[15]].
About the AAOCA, the ostium and proximal course of the left main and right coronary arteries can be visualized by echocardiography respectively in over 98% and 80% of subjects[122], especially from a PSAX view. The left main CA origin is approximately 4 o'clock and the RCA origin at 11 o'clock. Additional color flow mapping can help in the identification process[123]. Occasionally, clockwise rotation of the transducer in the PSAX view frequently allows the identification of the left main CA as it bifurcates into the left anterior descending branch and the left circumflex branch.
In infants and small children with good subcostal windows, the LCA origin can be seen from a coronal plane; the RCA origin is frequently best seen from a PLAX view.
What to assess? Origin of coronary arteries (PSAX). if pathological, what to assess? Additional color flow mapping of the inter-arterial space.
BICUSPID AORTIC VALVE
BAV is one of the most common congenital cardiac abnormalities[124]. Subjects with BAV may consequently have larger dimensions of the aortic sinus, ascending aorta, and aortic arch[15,89], beside possible progression to subsequent valvulopathies. The most common fusion pattern involves the right and left cusps[125]. Moreover, BAV may be associated with other diseases such as coarctation of the aorta, interrupted aortic arch, patent ductus arteriosus, coronary anomaly, as well as Williams or Turner syndrome[126,127]. The leaflets number and fusion, and the presence of a ‘raphe’ can be easily evaluated in the PSAX view, while systolic doming is better seen in PLAX view. Doppler echocardiography permits assessment of valve dysfunction[110,128]
What to assess? Aortic valve morphology (PSAX). If pathological, what to assess? Aortic stenosis + aortic regurgitation + dimension of aortic root (PLAX) + other congenital defects (coarctation of the aorta, interrupted aortic arch, patent ductus arteriosus, coronary anomaly or hypoplastic left heart, as well as Williams or Turner syndrome).
ATRIAL SEPTAL DEFECTS
Atrial septal defect (ASD), the failure to close the communication between RA and LA, afflicts 1 in 4 children[129]. There are five subtypes of atrial septal defects: patent foramen ovalis, ostium secundum defect, ostium primum defect, sinus venosus defect, and coronary sinus defect[89,130]. The atrial septum can be assessed from subcostal, apical and PSAX[131,132] views, even if subcostal is the preferred one. The four-chamber view allows hemodynamic assessment of the ASD (RA and RV dilatation) RV pressure estimation (TVR jet velocity). Associated lesions include anomalous pulmonary venous connection, pulmonary valve stenosis, and mitral valve prolapse. This condition is frequent in Ebstein anomaly[89].
What to assess? ASD (subcostal). If pathological, what to assess? ASD (A4C/PSAX) + RA dimension + RV dimension + PAPS.
VENTRICULAR SEPTAL DEFECTS
Ventricular septal defects (VSD) are the most common congenital cardiac abnormality in young individuals and the second one in adults, following only BAV[133]. Four locations of the defect within the interventricular septum are possible. Multiple windows can be used to assess the ventricular septum[131], such as PSAX and A4C views. VSD is also a common component of complex anomalies, such as Tetralogy of Fallot (ToF) and transposition of the great arteries (TGA)[89]. Key findings to provide are location, number, and size of defects, severity of LV volume overload, and estimated PAPS. Double chambered right ventricle (DCRV) and sinus of Valsalva aneurysm must be ruled out[89].
What to assess? VSD (A4C). If pathological, what to assess? LV mass + PAPS + aortic regurgitation + other congenital defects (aneurysm of Valsalva sinus, ToF, TGA, DCRV).
PATENT DUCTUS ARTERIOSUS
Patent ductus arteriosus (PDA) is the lasting communication between the proximal left pulmonary artery (PA) and the descending aorta, just distal to the left subclavian artery[89]. Echocardiography is choice diagnostic tool131]. PDA is best seen from the PSAX and suprasternal windows, where the duct can be visualized along the left border of the pulmonary artery: there is bidirectional flow across the duct, confirmed on pulsed wave doppler (PW); moreover, LA/Aortic root ratio ≥ 1.4 is suggestive of shunt[134]. A complete echocardiographic assessment should be performed to exclude pulmonary atresia or coarctation of the aorta. Furthermore, it must be assessed the degree of LV volume overload, PAPS, PA size, and right heart change.
What to assess? PDA (PSAX). If pathological, what to assess? PWPDA (PSAX) + LA/Aortic root ratio ≥ 1.4 + LV mass + PAPS + PA size + RV dimension + RA dimension.
AORTIC COARCTATION
Aortic coarctation (CoA) is considered a part of a more general arterial disease[89]: It occurs as a stenosis or as a hypoplastic aortic (arch) segment. Associated lesions include BAV ascending aortic aneurysm, aortic stenosis, mitral stenosis or complex congenital heart defects. Echocardiography provides information regarding site, structure, and extent of CoA, LV function and LVH, and aortic vessel diameters[89].
What to assess? Aortic coarctation (PSAX). If pathological, what to assess? Aortic stenosis + mitral stenosis + FE + LV mass + other congenital defects (BAV, ascending aortic aneurysm).
OTHER CONGENITAL DISEASES
Improvements in modern medicine has led to an increase in the number of adults with congenital heart disease engaging in sports activities[89,135]. Therefore, an echocardiography assessment of others various congenital heart diseases is crucial for sport eligibility.
ToF is a congenital cardiac malformation made by VSD, RVOT obstruction, override of the ventricular septum by the aortic root, and RV hypertrophy[136]. The initial presentation of ToF varies depending on the severity lung blood flow obstruction. Echocardiography has become the standard modality in its diagnosis[137]. The ventricular septal defect, the aortic override, and RV hypertrophy can be seen in PLAX view, while the pulmonary outflow tract obstruction in PSAX view[138].
Transposition of the great vessels is a group of congenital heart defects involving an abnormal spatial arrangement of any of the great vessels[139]. Congenital heart diseases involving only the primary arteries (pulmonary artery and aorta) belong to a sub-group called TGA: the aorta aligns with the RV and the pulmonary artery aligns with the LV[140]. It is the fourth most common type of major cardiac defect[141] and the second most common cyanotic lesion after ToF[140]; if not treated, it is the leading cause of SCD in neonates and infants[142]. Important echocardiographic assessments in case of TGA are position of the great arteries in PLAX or subcostal views, presence of ASD and VSD, presence of LVOT obstruction, eventual anomalies of coronary arteries[140].
Ebstein's anomaly (EA) is a malformation of the tricuspid valve (TV) with myopathy of the RV[143]. Initial presentation of EA in adulthood is common, and natural history demonstrates decreased survival with biventricular failure[144]. Echocardiography is the imaging standard in EA and provides a platform for TV leaflet, determination of right-heart size and function and dynamic evaluation of intracardiac shunts and defects, through color doppler[143,145].
Complete atrioventricular canal, also referred to as complete atrioventricular septal defect, is characterized by an ostium primum atrial septal defect, a common atrioventricular valve and a variable deficiency of the ventricular septum inflow[146]. It is a rare congenital heart condition. Echocardiography is the key tool for its diagnosis and anatomic classification[147].
OTHER PATHOLOGIES
Even if these are acute conditions that usually manifest with other symptoms, these pathologies are often cause of sudden cardiac death in athletes and therefore need to be screened.
MYOCARDITIS
Myocarditis accounts for almost 15% of SCDs in younger populations, especially in athletes[148]. The clinical presentation varies from chest pain, exertional dyspnea and fatigue, to cardiogenic shock[148]. Some patients heal completely while other may evolve in DCM[149]. An higher incidence of sport related SCD in case of myocarditis is documented[148], for the higher rate of life-threatening ventricular arrhythmias[150]. In acute form, echocardiography may show global LV dysfunction[151], wall motion abnormalities and pericardial effusions[15,152,153], an diffuse myocardial edema (increased wall thickness)[154-157]. In healed form, echocardiographic LV systolic dysfunction is found rarely because there is often small myocardial fibrosis, with undetectable scar at echocardiography[158]: Its evaluation still represent one of the bigger challenges of sport cardiology.
What to assess? EF; LV wall motion abnormalities; pericardial effusion; increased LV wall thickness.
PERICARDITIS
In athletes presenting with chest pain, the diagnosis of pericarditis should always be taken in consideration[159], particularly in younger ones[160]. Pericarditis and myocarditis may coexist in 20%–30% of patients[135]. Specific sport recommendations exist for patients with this disease[135]. Echocardiography is the diagnostic test of choice. The presence of pericardial fluid is recorded as an echo-free space between the posterior pericardium and left ventricular epicardium (in case of small effusion) or anterior right ventricular epicardium (in case of larger effusion)[159]. It can be assessed mostly in subcostal view.
What to assess? Pericardial effusion.
KAWASAKI DISEASE
Kawasaki disease (KD) is an acute vasculitis affecting young children, resulting in coronary artery abnormalities in case of delayed diagnosis[161,162].
Echocardiography is the imaging modality of choice for its detection: LV function and wall motion, of valvar regurgitation (particularly the mitral and aortic valves), and pericardial effusion should be assessed[163,164].
What to assess? Coronary artery abnormalities (PSAX/PLAX); EF; LV wall motion abnormalities; mitral regurgitation; aortic regurgitation; pericardial effusion.
CONCLUSION
Echocardiography is a valuable tool helping in detecting several CV conditions afflicting athletes. As physicians become more experienced with sonography, focused echocardiography by sports medicine physicians may become standard practice in larger screening practices. This technique could help to detect some hidden CV condition and to distinguish between physiological and pathological adaptation to physical activity, assisting in sport eligibility process. Even if we admit that a good manual skill, a training period and echocardiography experience should be required to perform it, our athlete-focused echocardiography could be an effective time-saving first line evaluation tool in sport eligibility process, eventually followed by a carefully exam. As such, a focused cardiac ultrasound examination may optimize cost-effectiveness: early detection of asymptomatic structural heart conditions could have important prognostic implications, with a relative less expensive cost than a complete echocardiogram. Nevertheless, further clinical trials should investigate its efficacy, accuracy, and applicability.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 25, 2021
First decision: April 26, 2021
Article in press: July 14, 2021
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reddy RS, Sempokuya T S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Stefano Palermi, Public Health Department, University of Naples Federico II, Naples 80131, Italy.
Alessandro Serio, Public Health Department, University of Naples Federico II, Naples 80131, Italy.
Marco Vecchiato, Sport and Exercise Medicine Division, Department of Medicine, University Hospital of Padova, Padova 35128, Italy.
Felice Sirico, Public Health Department, University of Naples Federico II, Naples 80131, Italy.
Francesco Gambardella, Public Health Department, University of Naples Federico II, Naples 80131, Italy.
Fabrizio Ricci, Department of Neuroscience, Imaging and Clinical Sciences, “G. d’Annunzio” University of Chieti-Pescara, Chieti 66100, Italy; Department of Clinical Sciences, Faculty of Medicine, Lund University, Clinical Research Center, Malmö 21428, Sweden; Casa di Cura Villa Serena, Pescara 65013, Italy.
Franco Iodice, Unit of Cardiology, Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy.
Juri Radmilovic, Unit of Cardiology and Intensive Coronary Care, “Umberto I” Hospital, Nocera Inferiore 84014, Italy.
Vincenzo Russo, Unit of Cardiology, Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy.
Antonello D'Andrea, Unit of Cardiology, Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Monaldi Hospital, Naples 80131, Italy. antonellodandrea@libero.it; Unit of Cardiology and Intensive Coronary Care, “Umberto I” Hospital, Nocera Inferiore 84014, Italy.
References
- 1.Manolis AS, Manolis AA. Exercise and Arrhythmias: A Double-Edged Sword. Pacing Clin Electrophysiol. 2016;39:748–762. doi: 10.1111/pace.12879. [DOI] [PubMed] [Google Scholar]
- 2.Palermi S, Sacco AM, Belviso I, Romano V, Montesano P, Corrado B, Sirico F. Guidelines for Physical Activity-A Cross-Sectional Study to Assess Their Application in the General Population. Have We Achieved Our Goal? Int J Environ Res Public Health. 2020;17:3980. doi: 10.3390/ijerph17113980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17–96. doi: 10.1093/eurheartj/ehaa605. [DOI] [PubMed] [Google Scholar]
- 4.Ricci F, Sutton R, Palermi S, Tana C, Renda G, Gallina S, Melander O, De Caterina R, Fedorowski A. Prognostic significance of noncardiac syncope in the general population: A systematic review and meta-analysis. J Cardiovasc Electrophysiol. 2018;29:1641–1647. doi: 10.1111/jce.13715. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med. 2010;152:276–286. doi: 10.1059/0003-4819-152-5-201003020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malhotra A, Dhutia H, Finocchiaro G, Gati S, Beasley I, Clift P, Cowie C, Kenny A, Mayet J, Oxborough D, Patel K, Pieles G, Rakhit D, Ramsdale D, Shapiro L, Somauroo J, Stuart G, Varnava A, Walsh J, Yousef Z, Tome M, Papadakis M, Sharma S. Outcomes of Cardiac Screening in Adolescent Soccer Players. N Engl J Med . 2018;379:524–534. doi: 10.1056/NEJMoa1714719. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Friedman RA, Kligfield P, Levine BD, Viskin S, Chaitman BR, Okin PM, Saul JP, Salberg L, Van Hare GF, Soliman EZ, Chen J, Matherne GP, Bolling SF, Mitten MJ, Caplan A, Balady GJ, Thompson PD American Heart Association Council on Clinical Cardiology; Advocacy Coordinating Committee; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Surgery and Anesthesia; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; Council on Quality of Care and Outcomes Research. and American College of Cardiology. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people (12-25 years of age): a scientific statement from the American Heart Association and the American College of Cardiology. J Am Coll Cardiol. 2014;64:1479–1514. doi: 10.1016/j.jacc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Pelliccia A, Bjørnstad HH, Vanhees L, Biffi A, Borjesson M, Panhuyzen-Goedkoop N, Deligiannis A, Solberg E, Dugmore D, Mellwig KP, Assanelli D, Delise P, van-Buuren F, Anastasakis A, Heidbuchel H, Hoffmann E, Fagard R, Priori SG, Basso C, Arbustini E, Blomstrom-Lundqvist C, McKenna WJ, Thiene G Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:516–524. doi: 10.1093/eurheartj/ehi108. [DOI] [PubMed] [Google Scholar]
- 9.Ljungqvist A, Jenoure P, Engebretsen L, Alonso JM, Bahr R, Clough A, De Bondt G, Dvorak J, Maloley R, Matheson G, Meeuwisse W, Meijboom E, Mountjoy M, Pelliccia A, Schwellnus M, Sprumont D, Schamasch P, Gauthier JB, Dubi C, Stupp H, Thill C. The International Olympic Committee (IOC) Consensus Statement on periodic health evaluation of elite athletes March 2009. Br J Sports Med. 2009;43:631–643. doi: 10.1136/bjsm.2009.064394. [DOI] [PubMed] [Google Scholar]
- 10.Myerburg RJ, Vetter VL. Electrocardiograms should be included in preparticipation screening of athletes. Circulation. 2007;116:2616–26; discussion 2626. doi: 10.1161/CIRCULATIONAHA.107.733519. [DOI] [PubMed] [Google Scholar]
- 11.Asif IM, Drezner JA. Sudden cardiac death and preparticipation screening: the debate continues-in support of electrocardiogram-inclusive preparticipation screening. Prog Cardiovasc Dis. 2012;54:445–450. doi: 10.1016/j.pcad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Williams EA, Pelto HF, Toresdahl BG, Prutkin JM, Owens DS, Salerno JC, Harmon KG, Drezner JA. Performance of the American Heart Association ( AHA ) 14-Point Evaluation Versus Electrocardiography for the Cardiovascular Screening of High School Athletes: A Prospective Study. J Am Heart Assoc. 2019;8:e012235. doi: 10.1161/JAHA.119.012235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampert R. ECG screening in athletes: differing views from two sides of the Atlantic. Heart. 2018;104:1037–1043. doi: 10.1136/heartjnl-2016-309448. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 15.Radmilovic J, D'Andrea A, D'Amato A, Tagliamonte E, Sperlongano S, Riegler L, Scarafile R, Forni A, Muscogiuri G, Pontone G, Galderisi M, Russo MG. Echocardiography in Athletes in Primary Prevention of Sudden Death. J Cardiovasc Echogr. 2019;29:139–148. doi: 10.4103/jcecho.jcecho_26_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Ascenzi F, Anselmi F, Mondillo S, Finocchiaro G, Caselli S, Garza MS, Schmied C, Adami PE, Galderisi M, Adler Y, Pantazis A, Niebauer J, Heidbuchel H, Papadakis M, Dendale P. The use of cardiac imaging in the evaluation of athletes in the clinical practice: A survey by the Sports Cardiology and Exercise Section of the European Association of Preventive Cardiology and University of Siena, in collaboration with the European Association of Cardiovascular Imaging, the European Heart Rhythm Association and the ESC Working Group on Myocardial and Pericardial Diseases. Eur J Prev Cardiol. 2020:2047487320932018. doi: 10.1177/2047487320932018. [DOI] [PubMed] [Google Scholar]
- 17.D'Ascenzi F, Fiorentini C, Anselmi F, Mondillo S. Left ventricular hypertrophy in athletes: How to differentiate between hypertensive heart disease and athlete's heart. Eur J Prev Cardiol. 2020:2047487320911850. doi: 10.1177/2047487320911850. [DOI] [PubMed] [Google Scholar]
- 18.D'Ascenzi F, Solari M, Anselmi F, Maffei S, Focardi M, Bonifazi M, Mondillo S, Henein M. Atrial chamber remodelling in healthy pre-adolescent athletes engaged in endurance sports: A study with a longitudinal design. The CHILD study. Int J Cardiol. 2016;223:325–330. doi: 10.1016/j.ijcard.2016.08.231. [DOI] [PubMed] [Google Scholar]
- 19.Magalski A, McCoy M, Zabel M, Magee LM, Goeke J, Main ML, Bunten L, Reid KJ, Ramza BM. Cardiovascular screening with electrocardiography and echocardiography in collegiate athletes. Am J Med. 2011;124:511–518. doi: 10.1016/j.amjmed.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Grazioli G, Merino B, Montserrat S, Vidal B, Azqueta M, Pare C, Sarquella-Brugada G, Yangüas X, Pi R, Til L, Escoda J, Brugada J, Sitges M. Usefulness of echocardiography in preparticipation screening of competitive athletes. Rev Esp Cardiol (Engl Ed) 2014;67:701–705. doi: 10.1016/j.rec.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Brugada Terradellas J, Carré F, Guasch E, Heidbuchel H, La Gerche A, Lampert R, McKenna W, Papadakis M, Priori SG, Scanavacca M, Thompson P, Sticherling C, Viskin S, Wilson M, Corrado D Reviewers. Lip GY, Gorenek B, Blomström Lundqvist C, Merkely B, Hindricks G, Hernández-Madrid A, Lane D, Boriani G, Narasimhan C, Marquez MF, Haines D, Mackall J, Manuel Marques-Vidal P, Corra U, Halle M, Tiberi M, Niebauer J, Piepoli M. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Eur J Prev Cardiol. 2017;24:41–69. doi: 10.1177/2047487316676042. [DOI] [PubMed] [Google Scholar]
- 22.Koch S, Cassel M, Linné K, Mayer F, Scharhag J. ECG and echocardiographic findings in 10-15-year-old elite athletes. Eur J Prev Cardiol. 2014;21:774–781. doi: 10.1177/2047487312462147. [DOI] [PubMed] [Google Scholar]
- 23.Evéquoz D, Zuber M, Erne P. [Sudden cardiac death: definition, mechanisms and risk factors] Praxis (Bern 1994) 1996;85:188–196. [PubMed] [Google Scholar]
- 24.Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, Zigman ML, Ellenbogen R, Rao AL, Ackerman MJ, Drezner JA. Incidence, Cause, and Comparative Frequency of Sudden Cardiac Death in National Collegiate Athletic Association Athletes: A Decade in Review. Circulation. 2015;132:10–19. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts WO, Stovitz SD. Incidence of sudden cardiac death in Minnesota high school athletes 1993-2012 screened with a standardized pre-participation evaluation. J Am Coll Cardiol. 2013;62:1298–1301. doi: 10.1016/j.jacc.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 26.Van Camp SP, Bloor CM, Mueller FO, Cantu RC, Olson HG. Nontraumatic sports death in high school and college athletes. Med Sci Sports Exerc. 1995;27:641–647. [PubMed] [Google Scholar]
- 27.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123:1594–1600. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]
- 28.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Corrado D, Schmied C, Basso C, Borjesson M, Schiavon M, Pelliccia A, Vanhees L, Thiene G. Risk of sports: do we need a pre-participation screening for competitive and leisure athletes? Eur Heart J. 2011;32:934–944. doi: 10.1093/eurheartj/ehq482. [DOI] [PubMed] [Google Scholar]
- 30.Yow AG, Rajasurya V, Sharma S. Sudden Cardiac Death. 2021 May 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 31.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, Lloyd G, Masani N, Mathew T, Oxborough D, Rana B, Sandoval J, Wheeler R, O'Gallagher K, Sharma V. A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2015;2:G9–G24. doi: 10.1530/ERP-14-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxborough D, Augustine D, Gati S, George K, Harkness A, Mathew T, Papadakis M, Ring L, Robinson S, Sandoval J, Sarwar R, Sharma S, Sharma V, Sheikh N, Somauroo J, Stout M, Willis J, Zaidi A. A guideline update for the practice of echocardiography in the cardiac screening of sports participants: a joint policy statement from the British Society of Echocardiography and Cardiac Risk in the Young. Echo Res Pract. 2018;5:G1–G10. doi: 10.1530/ERP-17-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modaff DS, Hegde SM, Wyman RA, Rahko PS. Usefulness of Focused Screening Echocardiography for Collegiate Athletes. Am J Cardiol. 2019;123:169–174. doi: 10.1016/j.amjcard.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Desai MY, Cremer PC, Schoenhagen P. Thoracic Aortic Calcification: Diagnostic, Prognostic, and Management Considerations. JACC Cardiovasc Imaging. 2018;11:1012–1026. doi: 10.1016/j.jcmg.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Fagard R. Athlete's heart. Heart (British Cardiac Society) 2003;89:1455–1461. doi: 10.1136/heart.89.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, Asif IM, Owens DS, Chung EH, Emery MS, Froelicher VF, Heidbuchel H, Adamuz C, Asplund CA, Cohen G, Harmon KG, Marek JC, Molossi S, Niebauer J, Pelto HF, Perez MV, Riding NR, Saarel T, Schmied CM, Shipon DM, Stein R, Vetter VL, Pelliccia A, Corrado D. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39:1466–1480. doi: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 39.D'Andrea A, Mele D, Palermi S, Rizzo M, Campana M, Di Giannuario G, Gimelli A, Khoury G, Moreo A a nome dell’Area Cardioimaging dell’Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) [Grey zones in cardiovascular adaptations to physical exercise: how to navigate in the echocardiographic evaluation of the athlete's heart] G Ital Cardiol (Rome) 2020;21:457–468. doi: 10.1714/3359.33330. [DOI] [PubMed] [Google Scholar]
- 40.Carbone A, D'Andrea A, Riegler L, Scarafile R, Pezzullo E, Martone F, America R, Liccardo B, Galderisi M, Bossone E, Calabrò R. Cardiac damage in athlete's heart: When the "supernormal" heart fails! World J Cardiol. 2017;9:470–480. doi: 10.4330/wjc.v9.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niederseer D, Rossi VA, Kissel C, Scherr J, Caselli S, Tanner FC, Bohm P, Schmied C. Role of echocardiography in screening and evaluation of athletes. Heart. 2020 doi: 10.1136/heartjnl-2020-317996. [DOI] [PubMed] [Google Scholar]
- 42.Pelliccia A, Caselli S, Sharma S, Basso C, Bax JJ, Corrado D, D'Andrea A, D'Ascenzi F, Di Paolo FM, Edvardsen T, Gati S, Galderisi M, Heidbuchel H, Nchimi A, Nieman K, Papadakis M, Pisicchio C, Schmied C, Popescu BA, Habib G, Grobbee D, Lancellotti P Internal reviewers for EAPC and EACVI. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. Eur Heart J. 2018;39:1949–1969. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 43.D'Ascenzi F, Solari M, Corrado D, Zorzi A, Mondillo S. Diagnostic Differentiation Between Arrhythmogenic Cardiomyopathy and Athlete's Heart by Using Imaging. JACC Cardiovasc Imaging. 2018;11:1327–1339. doi: 10.1016/j.jcmg.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Calò L, Martino A, Tranchita E, Sperandii F, Guerra E, Quaranta F, Parisi A, Nigro A, Sciarra L, Ruvo E, Casasco M, Pigozzi F. Electrocardiographic and echocardiographic evaluation of a large cohort of peri-pubertal soccer players during pre-participation screening. Eur J Prev Cardiol. 2019;26:1444–1455. doi: 10.1177/2047487319826312. [DOI] [PubMed] [Google Scholar]
- 45.Neskovic AN, Edvardsen T, Galderisi M, Garbi M, Gullace G, Jurcut R, Dalen H, Hagendorff A, Lancellotti P European Association of Cardiovascular Imaging Document Reviewers: Popescu BA, Sicari R, Stefanidis A. Focus cardiac ultrasound: the European Association of Cardiovascular Imaging viewpoint. Eur Heart J Cardiovasc Imaging. 2014;15:956–960. doi: 10.1093/ehjci/jeu081. [DOI] [PubMed] [Google Scholar]
- 46.Via G, Hussain A, Wells M, Reardon R, ElBarbary M, Noble VE, Tsung JW, Neskovic AN, Price S, Oren-Grinberg A, Liteplo A, Cordioli R, Naqvi N, Rola P, Poelaert J, Guliĉ TG, Sloth E, Labovitz A, Kimura B, Breitkreutz R, Masani N, Bowra J, Talmor D, Guarracino F, Goudie A, Xiaoting W, Chawla R, Galderisi M, Blaivas M, Petrovic T, Storti E, Neri L, Melniker L International Liaison Committee on Focused Cardiac UltraSound (ILC-FoCUS); International Conference on Focused Cardiac UltraSound (IC-FoCUS) International evidence-based recommendations for focused cardiac ultrasound. J Am Soc Echocardiogr 2014; 27: 683.e1-683. :e33. doi: 10.1016/j.echo.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, Flachskampf FA, Hassager C, Pasquet A, Gargani L, Galderisi M, Cardim N, Haugaa KH, Ancion A, Zamorano JL, Donal E, Bueno H, Habib G. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2015;4:3–5. doi: 10.1177/2048872614568073. [DOI] [PubMed] [Google Scholar]
- 48.Pinto A, Pinto F, Faggian A, Rubini G, Caranci F, Macarini L, Genovese EA, Brunese L. Sources of error in emergency ultrasonography. Crit Ultrasound J. 2013;5 Suppl 1:S1. doi: 10.1186/2036-7902-5-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coletta C, De Marchis E, Lenoli M, Rosato S, Renzi M, Sestili A, Romano P, Infusino T, Ricci R, Ceci V. Reliability of cardiac dimensions and valvular regurgitation assessment by sonographers using hand-carried ultrasound devices. Eur J Echocardiogr. 2006;7:275–283. doi: 10.1016/j.euje.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Yim ES, Gillis EF, Ojala K, MacDonald J, Basilico FC, Corrado GD. Focused transthoracic echocardiography by sports medicine physicians: measurements relevant to hypertrophic cardiomyopathy. J Ultrasound Med. 2013;32:333–338. doi: 10.7863/jum.2013.32.2.333. [DOI] [PubMed] [Google Scholar]
- 51.Feinstein RA, Colvin E, Kimoh M. Echocardiographic Screening as Part of a Preparticipation Examination. Clin J Sport Med . 1993;3 [Google Scholar]
- 52.Weiner RB, Wang F, Hutter AM Jr, Wood MJ, Berkstresser B, McClanahan C, Neary J, Marshall JE, Picard MH, Baggish AL. The feasibility, diagnostic yield, and learning curve of portable echocardiography for out-of-hospital cardiovascular disease screening. J Am Soc Echocardiogr . 2012;25:568–575. doi: 10.1016/j.echo.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Wyman RA, Chiu RY, Rahko PS. The 5-minute screening echocardiogram for athletes. J Am Soc Echocardiogr. 2008;21:786–788. doi: 10.1016/j.echo.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Fishman ML, Shea J, Choi BG, Solomon AJ. Sports and Exercise Medicine Feasibility of Focused Cardiac Ultrasound in Pre-participation Screening. Int J Sports Exerc Med. 2015;1:011. [Google Scholar]
- 55.Yim ES, Basilico F, Corrado G. Early screening for cardiovascular abnormalities with preparticipation echocardiography: utility of focused physician-operated echocardiography in preparticipation screening of athletes. J Ultrasound Med. 2014;33:307–313. doi: 10.7863/ultra.33.2.307. [DOI] [PubMed] [Google Scholar]
- 56.Gudmundsson P, Rydberg E, Winter R, Willenheimer R. Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiol. 2005;101:209–212. doi: 10.1016/j.ijcard.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 57.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Lee RT, Bhatia SJ, St John Sutton MG. Assessment of valvular heart disease with Doppler echocardiography. JAMA. 1989;262:2131–2135. [PubMed] [Google Scholar]
- 59.Pelliccia A, Fagard R, Bjørnstad HH, Anastassakis A, Arbustini E, Assanelli D, Biffi A, Borjesson M, Carrè F, Corrado D, Delise P, Dorwarth U, Hirth A, Heidbuchel H, Hoffmann E, Mellwig KP, Panhuyzen-Goedkoop N, Pisani A, Solberg EE, van-Buuren F, Vanhees L, Blomstrom-Lundqvist C, Deligiannis A, Dugmore D, Glikson M, Hoff PI, Hoffmann A, Horstkotte D, Nordrehaug JE, Oudhof J, McKenna WJ, Penco M, Priori S, Reybrouck T, Senden J, Spataro A, Thiene G Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology; Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26:1422–1445. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 60.Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd, Cooper LT Jr, Link MS, Maron MS American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology. Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 3: Hypertrophic Cardiomyopathy, Arrhythmogenic Right Ventricular Cardiomyopathy and Other Cardiomyopathies, and Myocarditis: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2015;132:e273–e280. doi: 10.1161/CIR.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 61.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff H V, Semsarian C, Sorajja P. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy. Circulation. 2020;142:e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 62.Caselli S, Maron MS, Urbano-Moral JA, Pandian NG, Maron BJ, Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2014;114:1383–1389. doi: 10.1016/j.amjcard.2014.07.070. [DOI] [PubMed] [Google Scholar]
- 63.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 64.Sheikh N, Papadakis M, Schnell F, Panoulas V, Malhotra A, Wilson M, Carré F, Sharma S. Clinical Profile of Athletes With Hypertrophic Cardiomyopathy. Circ Cardiovasc Imaging. 2015;8:e003454. doi: 10.1161/CIRCIMAGING.114.003454. [DOI] [PubMed] [Google Scholar]
- 65.Riding NR, Sharma S, Salah O, Khalil N, Carré F, George KP, Hamilton B, Chalabi H, Whyte GP, Wilson MG. Systematic echocardiography is not efficacious when screening an ethnically diverse cohort of athletes in West Asia. Eur J Prev Cardiol. 2015;22:263–270. doi: 10.1177/2047487313506549. [DOI] [PubMed] [Google Scholar]
- 66.Basavarajaiah S, Boraita A, Whyte G, Wilson M, Carby L, Shah A, Sharma S. Ethnic differences in left ventricular remodeling in highly-trained athletes relevance to differentiating physiologic left ventricular hypertrophy from hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;51:2256–2262. doi: 10.1016/j.jacc.2007.12.061. [DOI] [PubMed] [Google Scholar]
- 67.Pelliccia A, Maron BJ, Culasso F, Spataro A, Caselli G. Athlete's heart in women. Echocardiographic characterization of highly trained elite female athletes. JAMA. 1996;276:211–215. doi: 10.1001/jama.276.3.211. [DOI] [PubMed] [Google Scholar]
- 68.De Castro S, Caselli S, Maron M, Pelliccia A, Cavarretta E, Maddukuri P, Cartoni D, Di Angelantonio E, Kuvin JT, Patel AR, Pandian NG. Left ventricular remodelling index (LVRI) in various pathophysiological conditions: a real-time three-dimensional echocardiographic study. Heart. 2007;93:205–209. doi: 10.1136/hrt.2006.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lewis JF, Spirito P, Pelliccia A, Maron BJ. Usefulness of Doppler echocardiographic assessment of diastolic filling in distinguishing "athlete's heart" from hypertrophic cardiomyopathy. Br Heart J. 1992;68:296–300. doi: 10.1136/hrt.68.9.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, Mellor G, Merghani A, Malhotra A, Behr E, Sharma S, Sheppard MN. Etiology of Sudden Death in Sports: Insights From a United Kingdom Regional Registry. J Am Coll Cardiol. 2016;67:2108–2115. doi: 10.1016/j.jacc.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 71.Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 72.Faganello G, Doimo S, DI Nora C, DI Lenarda A. Cardiac imaging in patients with acute or chronic heart failure. Minerva Cardioangiol. 2017;65:589–600. doi: 10.23736/S0026-4725.17.04387-0. [DOI] [PubMed] [Google Scholar]
- 73.Douglas PS, O'Toole ML, Katz SE, Ginsburg GS, Hiller WD, Laird RH. Left ventricular hypertrophy in athletes. Am J Cardiol. 1997;80:1384–1388. doi: 10.1016/s0002-9149(97)00693-0. [DOI] [PubMed] [Google Scholar]
- 74.Finocchiaro G, Dhutia H, D'Silva A, Malhotra A, Steriotis A, Millar L, Prakash K, Narain R, Papadakis M, Sharma R, Sharma S. Effect of Sex and Sporting Discipline on LV Adaptation to Exercise. JACC Cardiovasc Imaging. 2017;10:965–972. doi: 10.1016/j.jcmg.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28:236–244. doi: 10.1016/j.echo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, Sharma S, Elliott PM. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29:89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 78.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82:507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 79.Stöllberger C, Winkler-Dworak M, Blazek G, Finsterer J. Prognosis of left ventricular hypertrabeculation/noncompaction is dependent on cardiac and neuromuscular comorbidity. Int J Cardiol. 2007;121:189–193. doi: 10.1016/j.ijcard.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Papadakis M, Carre F, Kervio G, Rawlins J, Panoulas VF, Chandra N, Basavarajaiah S, Carby L, Fonseca T, Sharma S. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J. 2011;32:2304–2313. doi: 10.1093/eurheartj/ehr140. [DOI] [PubMed] [Google Scholar]
- 81.de la Chica JA, Gómez-Talavera S, García-Ruiz JM, García-Lunar I, Oliva B, Fernández-Alvira JM, López-Melgar B, Sánchez-González J, de la Pompa JL, Mendiguren JM, Martínez de Vega V, Fernández-Ortiz A, Sanz J, Fernández-Friera L, Ibáñez B, Fuster V. Association Between Left Ventricular Noncompaction and Vigorous Physical Activity. J Am Coll Cardiol. 2020;76:1723–1733. doi: 10.1016/j.jacc.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 82.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH, Watkins H, Neubauer S. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46:101–105. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 83.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, van Tintelen JP, Wilde AAM, Zareba W. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Zaidi A, Sheikh N, Jongman JK, Gati S, Panoulas VF, Carr-White G, Papadakis M, Sharma R, Behr ER, Sharma S. Clinical Differentiation Between Physiological Remodeling and Arrhythmogenic Right Ventricular Cardiomyopathy in Athletes With Marked Electrocardiographic Repolarization Anomalies. J Am Coll Cardiol. 2015;65:2702–2711. doi: 10.1016/j.jacc.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 86.La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, Droogne W, Van Cleemput J, Claus P, Heidbuchel H. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36:1998–2010. doi: 10.1093/eurheartj/ehv202. [DOI] [PubMed] [Google Scholar]
- 87.Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith HJ, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail. 2014;16:1337–1344. doi: 10.1002/ejhf.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Corrado D, Basso C, Poletti A, Angelini A, Valente M, Thiene G. Sudden death in the young. Is acute coronary thrombosis the major precipitating factor? Circulation. 1994;90:2315–2323. doi: 10.1161/01.cir.90.5.2315. [DOI] [PubMed] [Google Scholar]
- 89.Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 90.Feigenbaum H. Echocardiographic Chamber Quantification in the Era of Multimodality Imaging: Beware of Unintended Consequences. J Am Soc Echocardiogr. 28:847–850. doi: 10.1016/j.echo.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Roman MJ, Devereux RB, Kramer-Fox R, O'Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507–512. doi: 10.1016/0002-9149(89)90430-x. [DOI] [PubMed] [Google Scholar]
- 92.Colan SD, McElhinney DB, Crawford EC, Keane JF, Lock JE. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J Am Coll Cardiol. 2006;47:1858–1865. doi: 10.1016/j.jacc.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 93.Yim ES. Aortic root disease in athletes: aortic root dilation, anomalous coronary artery, bicuspid aortic valve, and Marfan's syndrome. Sports Med. 2013;43:721–732. doi: 10.1007/s40279-013-0057-6. [DOI] [PubMed] [Google Scholar]
- 94.Kinoshita N, Mimura J, Obayashi C, Katsukawa F, Onishi S, Yamazaki H. Aortic root dilatation among young competitive athletes: echocardiographic screening of 1929 athletes between 15 and 34 years of age. Am Heart J. 2000;139:723–728. doi: 10.1016/s0002-8703(00)90055-3. [DOI] [PubMed] [Google Scholar]
- 95.Pelliccia A, Di Paolo FM, Quattrini FM. Aortic root dilatation in athletic population. Prog Cardiovasc Dis. 2012;54:432–437. doi: 10.1016/j.pcad.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 96.Churchill TW, Groezinger E, Kim JH, Loomer G, Guseh JS, Wasfy MM, Isselbacher EM, Lewis GD, Weiner RB, Schmied C, Baggish AL. Association of Ascending Aortic Dilatation and Long-term Endurance Exercise Among Older Masters-Level Athletes. JAMA Cardiol. 2020;5:522–531. doi: 10.1001/jamacardio.2020.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Braverman AC, Harris KM, Kovacs RJ, Maron BJ American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology. Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 7: Aortic Diseases, Including Marfan Syndrome: A Scientific Statement From the American Heart Association and American College of Cardiology. Circulation. 2015;132:e303– e309. doi: 10.1161/CIR.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 98.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009;53:2288–2295. doi: 10.1016/j.jacc.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yetman AT, Graham T. The dilated aorta in patients with congenital cardiac defects. J Am Coll Cardiol. 2009;53:461–467. doi: 10.1016/j.jacc.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 100.Spartalis M, Tzatzaki E, Spartalis E, Athanasiou A, Moris D, Damaskos C, Garmpis N, Voudris V. Mitral valve prolapse: an underestimated cause of sudden cardiac death-a current review of the literature. J Thorac Dis. 2017;9:5390–5398. doi: 10.21037/jtd.2017.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation. 2014;129:2158–2170. doi: 10.1161/CIRCULATIONAHA.113.006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cavarretta E, Peruzzi M, Versaci F, Frati G, Sciarra L. How to manage an athlete with mitral valve prolapse. Eur J Prev Cardiol. 2020:2047487320941646. doi: 10.1177/2047487320941646. [DOI] [PubMed] [Google Scholar]
- 103.Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, Rigato I, Migliore F, Pilichou K, Bertaglia E, Cacciavillani L, Bauce B, Corrado D, Thiene G, Iliceto S. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 104.Jeresaty RM. Mitral valve prolapse: definition and implications in athletes. J Am Coll Cardiol. 1986;7:231–236. doi: 10.1016/s0735-1097(86)80286-8. [DOI] [PubMed] [Google Scholar]
- 105.van Buuren F, Gati S, Sharma S, Papadakis M, Adami PE, Niebauer J, Pelliccia A, Rudolph V, Börjesson M, Carre F, Solberg E, Heidbuchel H, Caselli S, Corrado D, Serratosa L, Biffi A, Pressler A, Schmied C, Panhuyzen-Goedkoop NM, Rasmussen HK, La Gerche A, Faber L, Bogunovic N, D'Ascenzi F, Mellwig KP. Athletes with valvular heart disease and competitive sports: a position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2021 doi: 10.1093/eurjpc/zwab058. [DOI] [PubMed] [Google Scholar]
- 106.Ricci F, Aung N, Gallina S, Zemrak F, Fung K, Bisaccia G, Paiva JM, Khanji MY, Mantini C, Palermi S, Lee AM, Piechnik SK, Neubauer S, Petersen SE. Cardiovascular magnetic resonance reference values of mitral and tricuspid annular dimensions: the UK Biobank cohort. J Cardiovasc Magn Reson. 2020;23:5. doi: 10.1186/s12968-020-00688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gati S, Malhotra A, Sharma S. Exercise recommendations in patients with valvular heart disease. Heart. 2019;105:106–110. doi: 10.1136/heartjnl-2018-313372. [DOI] [PubMed] [Google Scholar]
- 108.Kastellanos S, Aznaouridis K, Vlachopoulos C, Tsiamis E, Oikonomou E, Tousoulis D. Overview of coronary artery variants, aberrations and anomalies. World J Cardiol. 2018;10:127–140. doi: 10.4330/wjc.v10.i10.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Singh GK. Congenital Aortic Valve Stenosis. Children (Basel) 2019;6 doi: 10.3390/children6050069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baumgartner H Chair, Hung J Co-Chair, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:254–275. doi: 10.1093/ehjci/jew335. [DOI] [PubMed] [Google Scholar]
- 111.van Buuren F, Mellwig KP. Specific Cardiovascular Diseases and Competitive Sports Participation: Valvular Heart Disease. In: Pressler A, Niebauer J. Textbook of Sports and Exercise Cardiology. Springer International Publishing; 2020: 291-302. [Google Scholar]
- 112.Stephensen SS, Sigfusson G, Eiriksson H, Sverrisson JT, Torfason B, Haraldsson A, Helgason H. Congenital cardiac malformations in Iceland from 1990 through 1999. Cardiol Young. 2004;14:396–401. doi: 10.1017/S1047951104004081. [DOI] [PubMed] [Google Scholar]
- 113.Balzer D. Pulmonary Valve Replacement for Tetralogy of Fallot. Methodist Debakey Cardiovasc J. 2019;15:122–132. doi: 10.14797/mdcj-15-2-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 115.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 116.Douglas PS, Berman GO, O'Toole ML, Hiller WD, Reichek N. Prevalence of multivalvular regurgitation in athletes. Am J Cardiol. 1989;64:209–212. doi: 10.1016/0002-9149(89)90459-1. [DOI] [PubMed] [Google Scholar]
- 117.Bonow RO, Cheitlin MD, Crawford MH, Douglas PS. Task Force 3: valvular heart disease. J Am Coll Cardiol. 2005;45:1334–1340. doi: 10.1016/j.jacc.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 118.Zeppilli P, dello Russo A, Santini C, Palmieri V, Natale L, Giordano A, Frustaci A. In vivo detection of coronary artery anomalies in asymptomatic athletes by echocardiographic screening. Chest. 1998;114:89–93. doi: 10.1378/chest.114.1.89. [DOI] [PubMed] [Google Scholar]
- 119.Pelliccia A, Spataro A, Maron BJ. Prospective echocardiographic screening for coronary artery anomalies in 1,360 elite competitive athletes. Am J Cardiol. 1993;72:978–979. doi: 10.1016/0002-9149(93)91120-7. [DOI] [PubMed] [Google Scholar]
- 120.Bianco F, Colaneri M, Bucciarelli V, Surace FC, Iezzi FV, Primavera M, Biasi A, Giusti G, Berton E, Baldoni M, Renda G, Baldinelli A, Gallina S, Pozzi M. Echocardiographic screening for the anomalous aortic origin of coronary arteries. Open Heart. 2021;8 doi: 10.1136/openhrt-2020-001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frommelt P, Lopez L, Dimas VV, Eidem B, Han BK, Ko HH, Lorber R, Nii M, Printz B, Srivastava S, Valente AM, Cohen MS. Recommendations for Multimodality Assessment of Congenital Coronary Anomalies: A Guide from the American Society of Echocardiography: Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2020;33:259–294. doi: 10.1016/j.echo.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 122.Freeman RV, Otto CM. Bicuspid aortic valve and aortopathy: see the first, then look at the second. JACC Cardiovasc Imaging. 2013;6:162–164. doi: 10.1016/j.jcmg.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 123.Frommelt PC, Frommelt MA, Tweddell JS, Jaquiss RD. Prospective echocardiographic diagnosis and surgical repair of anomalous origin of a coronary artery from the opposite sinus with an interarterial course. J Am Coll Cardiol. 2003;42:148–154. doi: 10.1016/s0735-1097(03)00503-5. [DOI] [PubMed] [Google Scholar]
- 124.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 125.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 126.Cripe L, Andelfinger G, Martin LJ, Shooner K, Benson DW. Bicuspid aortic valve is heritable. J Am Coll Cardiol. 2004;44:138–143. doi: 10.1016/j.jacc.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 127.Hallidie-Smith KA, Karas S. Cardiac anomalies in Williams-Beuren syndrome. Arch Dis Child. 1988;63:809–813. doi: 10.1136/adc.63.7.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 129.Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O'Fallon WM, Pascoe RD, Enriquez-Sarano M, Seward JB, Covalt JL, Sicks JD, Wiebers DO. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74:862–869. doi: 10.4065/74.9.862. [DOI] [PubMed] [Google Scholar]
- 130.Celermajer DS. Atrial septal defects: even simple congenital heart diseases can be complicated. Eur Heart J. 2018;39:999–1001. doi: 10.1093/eurheartj/ehx633. [DOI] [PubMed] [Google Scholar]
- 131.Deri A, English K. Educational series in congenital heart disease: Echocardiographic assessment of left to right shunts: atrial septal defect, ventricular septal defect, atrioventricular septal defect, patent arterial duct. Echo Res Pract. 2018;5:R1–R16. doi: 10.1530/ERP-17-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Silvestry FE, Cohen MS, Armsby LB, Burkule NJ, Fleishman CE, Hijazi ZM, Lang RM, Rome JJ, Wang Y American Society of Echocardiography; Society for Cardiac Angiography and Interventions. Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. 2015;28:910–958. doi: 10.1016/j.echo.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 133.Hopkins MK, Goldstein SA, Ward CC, Kuller JA. Evaluation and Management of Maternal Congenital Heart Disease: A Review. Obstet Gynecol Surv. 2018;73:116–124. doi: 10.1097/OGX.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 134.Johnson GL, Breart GL, Gewitz MH, Brenner JI, Lang P, Dooley KJ, Ellison RC. Echocardiographic characteristics of premature infants with patient ductus arteriosus. Pediatrics. 1983;72:864–871. [PubMed] [Google Scholar]
- 135.Budts W, Pieles GE, Roos-Hesselink JW, Sanz de la Garza M, D'Ascenzi F, Giannakoulas G, Müller J, Oberhoffer R, Ehringer-Schetitska D, Herceg-Cavrak V, Gabriel H, Corrado D, van Buuren F, Niebauer J, Börjesson M, Caselli S, Fritsch P, Pelliccia A, Heidbuchel H, Sharma S, Stuart AG, Papadakis M. Recommendations for participation in competitive sport in adolescent and adult athletes with Congenital Heart Disease (CHD): position statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), the European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease and the Sports Cardiology, Physical Activity and Prevention Working Group of the Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2020;41:4191–4199. doi: 10.1093/eurheartj/ehaa501. [DOI] [PubMed] [Google Scholar]
- 136.Bailliard F, Anderson RH. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4:2. doi: 10.1186/1750-1172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mertens LL, Cohen MS, Geva T, Lai WW. Echocardiography in Pediatric and Congenital Heart Disease. 2nd edition. Wiley-Blackwell. 2016: 928. [Google Scholar]
- 138.Silverman NH. The essential echocardiographic features of tetralogy of Fallot. Cardiol Young. 2013;23:871–882. doi: 10.1017/S1047951113001704. [DOI] [PubMed] [Google Scholar]
- 139.Squarcia U, Macchi C. Transposition of the great arteries. Curr Opin Pediatr. 2011;23:518–522. doi: 10.1097/MOP.0b013e32834aa551. [DOI] [PubMed] [Google Scholar]
- 140.Cohen MS, Mertens LL. Educational series in congenital heart disease: Echocardiographic assessment of transposition of the great arteries and congenitally corrected transposition of the great arteries. Echo Res Pract. 2019;6:R107–R119. doi: 10.1530/ERP-19-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Unolt M, Putotto C, Silvestri LM, Marino D, Scarabotti A, Valerio Massaccesi, Caiaro A, Versacci P, Marino B. Transposition of great arteries: new insights into the pathogenesis. Front Pediatr. 2013;1:11. doi: 10.3389/fped.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Samánek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young. 2000;10:179–185. doi: 10.1017/s1047951100009082. [DOI] [PubMed] [Google Scholar]
- 143.Holst KA, Connolly HM, Dearani JA. Ebstein's Anomaly. Methodist Debakey Cardiovasc J. 2019;15:138–144. doi: 10.14797/mdcj-15-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Attie F, Rosas M, Rijlaarsdam M, Buendia A, Zabal C, Kuri J, Granados N. The adult patient with Ebstein anomaly. Outcome in 72 unoperated patients. Medicine (Baltimore) 2000;79:27–36. doi: 10.1097/00005792-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 145.Booker OJ, Nanda NC. Echocardiographic assessment of Ebstein's anomaly. Echocardiography. 2015;32 Suppl 2:S177–S188. doi: 10.1111/echo.12486. [DOI] [PubMed] [Google Scholar]
- 146.Calabrò R, Limongelli G. Complete atrioventricular canal. Orphanet J Rare Dis. 2006;1:8. doi: 10.1186/1750-1172-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Meza JM, Devlin PJ, Overman DM, Gremmels D, Baffa G, Cohen MS, Quartermain MD, Caldarone CA, Pourmoghadam K, DeCampli WM, Fackoury CT, Mertens L. The Congenital Heart Surgeon's Society Complete Atrioventricular Septal Defect Cohort: Baseline, Preintervention Echocardiographic Characteristics. Semin Thorac Cardiovasc Surg. 2019;31:80–86. doi: 10.1053/j.semtcvs.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 148.Cooper LT Jr, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014;9:121–129. doi: 10.1016/j.gheart.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 149.Basso C, Carturan E, Corrado D, Thiene G. Myocarditis and dilated cardiomyopathy in athletes: diagnosis, management, and recommendations for sport activity. Cardiol Clin. 2007;25:423–429, vi. doi: 10.1016/j.ccl.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 150.Eichhorn C, Bière L, Schnell F, Schmied C, Wilhelm M, Kwong RY, Gräni C. Myocarditis in Athletes Is a Challenge: Diagnosis, Risk Stratification, and Uncertainties. JACC Cardiovasc Imaging. 2020;13:494–507. doi: 10.1016/j.jcmg.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 151.Vio R, Zorzi A, Corrado D. Myocarditis in the Athlete: Arrhythmogenic Substrates, Clinical Manifestations, Management, and Eligibility Decisions. J Cardiovasc Transl Res. 2020;13:284–295. doi: 10.1007/s12265-020-09996-1. [DOI] [PubMed] [Google Scholar]
- 152.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 153.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 154.Bière L, Piriou N, Ernande L, Rouzet F, Lairez O. Imaging of myocarditis and inflammatory cardiomyopathies. Arch Cardiovasc Dis. 2019;112:630–641. doi: 10.1016/j.acvd.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 155.Pinamonti B, Alberti E, Cigalotto A, Dreas L, Salvi A, Silvestri F, Camerini F. Echocardiographic findings in myocarditis. Am J Cardiol. 1988;62:285–291. doi: 10.1016/0002-9149(88)90226-3. [DOI] [PubMed] [Google Scholar]
- 156.Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6:833–839. doi: 10.1161/CIRCIMAGING.113.000416. [DOI] [PubMed] [Google Scholar]
- 157.Compagnucci P, Volpato G, Falanga U, Cipolletta L, Conti MA, Grifoni G, Ciliberti G, Stronati G, Fogante M, Bergonti M, Sommariva E, Guerra F, Giovagnoni A, Dello Russo A, Casella M. Myocardial Inflammation, Sports Practice, and Sudden Cardiac Death: 2021 Update. Medicina (Kaunas) 57:277. doi: 10.3390/medicina57030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seidenberg PH, Haynes J. Pericarditis: diagnosis, management, and return to play. Curr Sports Med Rep. 2006;5:74–79. doi: 10.1007/s11932-006-0034-z. [DOI] [PubMed] [Google Scholar]
- 160.Imazio M, Gaita F, LeWinter M. Evaluation and Treatment of Pericarditis: A Systematic Review. JAMA. 2015;314:1498–1506. doi: 10.1001/jama.2015.12763. [DOI] [PubMed] [Google Scholar]
- 161.McCrindle BW, Cifra B. The role of echocardiography in Kawasaki disease. Int J Rheum Dis. 2018;21:50–55. doi: 10.1111/1756-185X.13216. [DOI] [PubMed] [Google Scholar]
- 162.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E American Heart Association Rheumatic Fever; Endocarditis , and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 163.Marchesi A, Tarissi de Jacobis I, Rigante D, Rimini A, Malorni W, Corsello G, Bossi G, Buonuomo S, Cardinale F, Cortis E, De Benedetti F, De Zorzi A, Duse M, Del Principe D, Dellepiane RM, D'Isanto L, El Hachem M, Esposito S, Falcini F, Giordano U, Maggio MC, Mannarino S, Marseglia G, Martino S, Marucci G, Massaro R, Pescosolido C, Pietraforte D, Pietrogrande MC, Salice P, Secinaro A, Straface E, Villani A. Kawasaki disease: guidelines of the Italian Society of Pediatrics, part I - definition, epidemiology, etiopathogenesis, clinical expression and management of the acute phase. Ital J Pediatr . 2018;44:102. doi: 10.1186/s13052-018-0536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fuse S, Kobayashi T, Arakaki Y, Ogawa S, Katoh H, Sakamoto N, Hamaoka K, Saji T. Standard method for ultrasound imaging of coronary artery in children. Pediatr Int. 2010;52:876–882. doi: 10.1111/j.1442-200X.2010.03252.x. [DOI] [PubMed] [Google Scholar]
- 165.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, Rosenhek R, Sjögren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]