Abstract
The mechanism of alopecia areata (AA) is not well-elucidated, and hair follicle melanogenesis pathways are implicated as possible sources for autoantigens. After a retrospective medical record review at a single tertiary medical center, the hair color of 112 AA patients were identified and compared to a control group of 104 androgenetic alopecia patients. There were no statistically significant differences in the natural hair color prevalence between the 2 groups (p = 0.164), and hair color was not a predictor of the alopecia type. Our results suggest hair pigmentation, determined by the eumelanin-to-pheomelanin ratio, is not a positive risk factor for AA development. We hope that our study will encourage multiple large-scale, collaborative, retrospective medical reviews to determine if our results are reproducible in diverse patient populations.
Keywords: Alopecia areata, Vitiligo, Autoimmune, Alopecia, Hair loss, Melanogenesis
Alopecia areata (AA) results from an autoimmune hair follicle attack; however, the autoantigen is not identified. Hair follicle melanogenesis pathways are an implicated AA target. Clinically, AA affects pigmented hair, and newly regrown hair is often unpigmented [1]. Mouse models implicate hair follicle melanocyte-incubated T cells; on histologic section, T cells are found surrounding peribulbar melanocytes [2]. Hair pigmentation depends on the ratio of eumelanin and pheomelanin synthesized by follicle melanocytes.
We performed a retrospective medical record review at a single, academic, tertiary center to determine if hair pigmentation is a risk factor for AA development. Chart review with documentation of patients' natural hair color and evaluation of clinical photographs from the dermatology electronic medical records was conducted from July 2016 to July 2018. Data were analyzed using the t test for continuous variables and the χ2 test for categorical variables. Logistic regression was used to assess if hair color could predict the disease type.
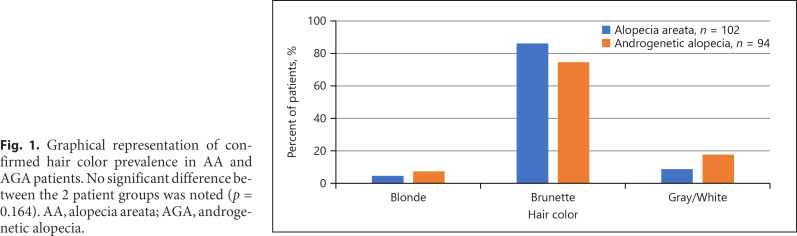
One hundred twelve patients were identified with AA. As controls, 104 androgenetic alopecia (AGA) patients were identified as they too suffer from hair loss, but without an autoimmune or inflammatory component (Table 1). Racial breakdown between the 2 groups was similar; however, the AGA group was older and had more women (p < 0.001). On average, AA patients presented 7.58 ± 11.87 years after initial disease onset, while AGA patients presented after 5.29 ± 6.56 years to our tertiary center. Of the initial cohort, 102 AA and 94 AGA patients had photographic confirmation of hair color; those who appeared to have dyed hair were categorized according to the color of their roots or documentation of natural hair color as indicated in physician notes. There were 86.2% of AA patients with brown hair color (vs. 74.5% of AGA), 8.8% with gray/white hair (vs. 18.1%), and 5% with blonde (vs. 7.4%) (Fig. 1). Although these differences appear clinically significant, statistical analysis found no significant differences in hair color prevalence between the 2 groups (p = 0.164), and hair color was not a predictor of the disease type.
Table 1.
Summary of demographics of AA and AGA patients from a single tertiary academic center over the period of 2016–2018
| AA | AGA | |
|---|---|---|
| N | 111 | 104 |
| Age, years | 38.47±17.02 | 48.81±16.81 |
| Gender, %* | ||
| Female | 54.1 | 79.8 |
| Male | 45.9 | 20.2 |
| Race, % | ||
| Asian | 15.3 | 14.4 |
| Other/mixed | 20.7 | 24.0 |
| White | 59.5 | 53.8 |
| Unknown | 4.5 | 7.7 |
| Time until disease presentation, years | 7.58±11.87 | 5.29±6.56 |
AA, alopecia areata; AGA, androgenetic alopecia. * Denotes a significant difference between the 2 patients groups with p < 0.001.
Fig. 1.
Graphical representation of confirmed hair color prevalence in AA and AGA patients. No significant difference between the 2 patient groups was noted (p = 0.164). AA, alopecia areata; AGA, androgenetic alopecia.
The pathogenesis of AA eludes researchers as they try to identify the target autoantigen(s) associated with AA development. AA is associated with various autoimmune and inflammatory conditions, including vitiligo, a disease resulting from autoimmune attack of melanogenesis pathways, most notably tyrosinase [3]. Regrowing hair in AA patches is often white [1, 4, 5], raising important questions regarding the role of pigmented hair in AA − is pigmented hair the specific target of AA; does pigment induce autoreactive T cells, thus promoting autoimmune hair follicle attack; or is hair follicle pigment just lost during the inflammatory process as a result of T-cell-mediated attack? In addition, vitiligo and AA share an interferon-γ, CXC motif chemokine ligand 9/10, and T-helper (Th)-1-driven inflammatory response [3]. With these observations in mind, hypotheses have emerged that melanogenesis pathways may have autoantigens of significance in AA, with potential disease incidence increased in patients with dark hair.
Our results demonstrate that there is no significant difference in the hair color prevalence between AA and AGA patients, and support that hair pigmentation, as determined by eumelanin-to-pheomelanin ratio, is not a positive risk factor for AA development. We encourage collaboration among multiple academic centers to complete retrospective medical record reviews and determine if these results are reproducible on a large scale, within diverse patient populations.
Statements
These data have not been previously presented. This work has not been submitted to another journal. No reprints are requested.
Statement of Ethics
This retrospective chart review was approved by the Institutional Review Board of the University of California, Irvine.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
This article has no funding source.
Author Contributions
M.J.: substantial contributions including the conception of the idea, acquisition of data, interpretation of data, drafting and revising the work, and approving the final version of the work; R.Z.: substantial contributions including analysis of data, drafting and revising the work, and approving the final version of the work; and N.A.M.: substantial contributions including the conception of the idea, drafting and revising the work, and approving the final version of the work. All authors agreed to be accountable for all aspects of this work.
References
- 1.Jia WX, Mao QX, Xiao XM, Li ZL, Yu RX, Li CR. Patchy alopecia areata sparing gray hairs: a case series. Postepy Dermatol Alergol. 2014;31:113–6. doi: 10.5114/pdia.2014.40956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trautman S, Thompson M, Roberts J, Thompson CT. Melanocytes: a possible autoimmune target in alopecia areata. J Am Acad Dermatol. 2009;61:529–30. doi: 10.1016/j.jaad.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Rork JF, Rashighi M, Harris JE. Understanding autoimmunity of vitiligo and alopecia areata. Curr Opin Pediatr. 2016;28:463–9. doi: 10.1097/MOP.0000000000000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol. 2013;22:785–9. doi: 10.1111/exd.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Mittal J, Mahajan B. Colocalization of vitiligo and alopecia areata: coincidence or consequence? Int J Trichology. 2013;5:50–2. doi: 10.4103/0974-7753.114705. [DOI] [PMC free article] [PubMed] [Google Scholar]