Abstract
Pathogenic and likely pathogenic variants in the ATM gene are associated both with Ataxia-telangiectasia disease or ATM syndrome and an increased cancer risk for heterozygous carriers. We identified a novel compound heterozygous mutation c.3955_3958dup (p.Asp1320delinsValTer) and c.5825C>T (p.Ala1942Val) in the ATM gene in a Peruvian patient with progressive ataxia combined with other movement disorders, mild conjunctival telangiectasia and increased alpha-fetoprotein, without history of recurrent infection or immunodeficiency. We also determined the carrier status of the family members, and we were able to detect gastric and breast cancer at an early stage during the cancer risk assessment in the mother (c.3955_3958dup). Here, we describe clinical evidence for the novel compound heterozygous mutation and c.3955_3958dup not previously reported.
Keywords: Ataxia-telangiectasia, ATM, c.3955_3958dup, c.5825C>T, rs1591646379, rs730881394
Introduction
Ataxia-telangiectasia (A-T; OMIM 208900) or ATM syndrome is a rare autosomal recessive disease (homozygous or compound heterozygous) characterized by ataxia combined with a variety of movement disorders, immunodeficiency, increased levels of alpha-fetoprotein (AFP) and cancer predisposition. This rare disease is associated with pathogenic variants within the ATM gene [Rothblum-Oviatt et al., 2016]. Heterozygous ATM carriers have a higher risk for cancer and must have appropriate genetic counseling [van Os et al., 2016].
The ATM gene (OMIM 607585), located in chromosome 11q22.3, encodes the serine-threonine kinase protein responsible for cell cycle checkpoint control, apoptosis, DNA damage response, oxidative stress, and mitochondrial metabolism [Verhagen et al., 2012]. Nowadays, approximately 1,184 variants of known pathogenicity in the ATM gene have been reported in ClinVar. Regarding genotype-phenotype correlations, biallelic truncating mutations are associated with a classic A-T phenotype and an important minority of missense mutations with a milder phenotype [Schon et al., 2019; van Os et al., 2019].
In Peru, there is still limited access to molecular diagnosis for most the genetic disorders including A-T. To the best of our knowledge, there are only clinical reports of A-T based on clinical criteria [Lazo Rivera et al., 2014; García Gomero et al., 2018].
Here, we describe a Peruvian girl with A-T and a novel compound heterozygous mutation in the ATM gene (c.3955_3958dup and c.5825C>T). Her mother, an obligate carrier, developed 2 different types of cancer.
Materials and Methods
Genetic Testing
DNA was obtained from peripheral blood (proband) and saliva (individuals I-2, II-3, and II-5, shown in Fig. 1). For the proband, clinical sequencing analysis and deletion/duplication testing was performed using Invitae Multi-Cancer Panel of 83 genes. Sanger sequencing was performed for confirmation of clinically relevant variants. For the mother's and sister's samples, analysis of the ATM gene looking for sequence changes and exonic deletions/duplications was performed by Sanger sequencing.
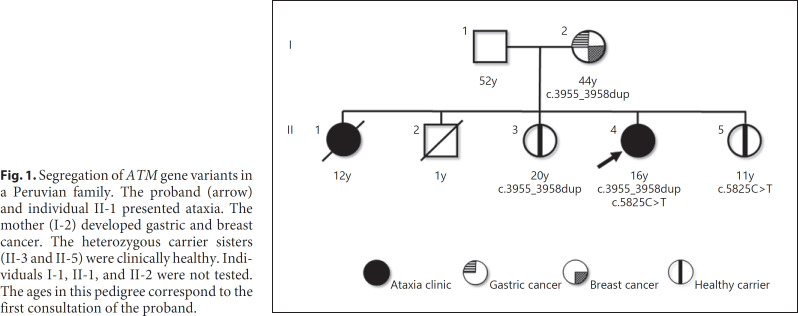
Fig. 1.
Segregation of ATM gene variants in a Peruvian family. The proband (arrow) and individual II-1 presented ataxia. The mother (I-2) developed gastric and breast cancer. The heterozygous carrier sisters (II-3 and II-5) were clinically healthy. Individuals I-1, II-1, and II-2 were not tested. The ages in this pedigree correspond to the first consultation of the proband.
Analysis and Interpretation of Genetic Variants
Pathogenicity of identified variants was analyzed following the recommendations of the ACMG [Richards et al., 2015]. We described the interest variants based on ATM transcript variant NM_000051.3 at GenBank. In addition, we reviewed the UniProt in https://www.uniprot.org/, ClinVar in https://www.ncbi.nlm.nih.gov/clinvar/, dbSNP in https://www.ncbi.nlm.nih.gov/snp/, gnomAD in https://gnomad.broadinstitute.org/,and in silico tools, MutationTaster [Schwarz et al., 2014], PolyPhen-2 [Adzhubei et al., 2010], and SIFT [Kumar et al., 2009].
Results
Clinical Vignette
A Peruvian family of nonconsanguineous parents with 5 children is shown in Figure 1. The proband (II-4) is a 16-year-old girl with slowly progressive gait instability, associated with recurrent falls since 2 years of age. At 6 years, a neuropsychological assessment found diminished attention, auditory memory difficulties, and moderate dysarthria. By the age of 10, she was unable to walk independently and complained about generalized pruritus. A year after, she was confined to a wheelchair. There is no history of any recurrent respiratory or digestive infectious disease.
Physical examination performed at age 16 revealed moderate bilateral dysarthria, dysmetria, dysdiadochokinesia and mild global bradykinesia, as well as the difficulty to stand without permanent support. Motor strength is preserved; however, there is generalized muscle atrophy with bilateral extensor plantar reflexes. Ocular examination revealed multidirectional nystagmus with oculomotor apraxia and mild conjunctival telangiectasias. She has generalized episodic jerk movements with predominance in the cephalic region and dystonic postures of the upper limbs. Rating scale examination for ataxia scored 26 for SARA and 15 for NESSCA.
Laboratory testing showed hematological and biochemistry battery in normal ranges. An increased level of AFP, 610 ng/mL (normal: 0–10 ng/mL). Neuroconduction study confirmed an axonal sensory neuropathy in the lower limbs. Brain MRI showed severe global cerebellar atrophy.
Her older sister (II-1) experienced gait disturbances since she was 8 years old. By the age of 11, she was diagnosed with non-Hodgkin lymphoma and died at 12 years affected by complicated varicella zoster during chemotherapy. Her mother (I-2), a healthy carrier at the age of 44, started cancer risk assessment; 1 month later she was diagnosed with gastric signet ring cell carcinoma T2N0M0 and 7 months later invasive ductal carcinoma T1bN0(i+)M0.
Clinical Molecular Testing
Targeted sequencing using a panel of 83 cancer predisposition genes was performed detecting 2 heterozygous variants in the ATM gene: NM_000051.3: c.3955_3958dup (p.Asp1320delinsValTer) and NM_000051.3: c.5825C>T (p.Ala1942Val). The search for carriers in first-degree relatives, clinically healthy, detected c.3955_3958dup in the mother at 44 years (I-2) and in the sister at 20 years (II-3). Likewise, the other variant c.5825C>T was detected in the 11-year-old sister (II-5).
Discussion and Conclusion
Here, we describe a Peruvian family with ATM-related conditions. The proband experienced progressive ataxia combined with hyperkinetic movements. Her mother, an obligate carrier, developed gastric and breast cancer.
Individuals with homozygous or compound heterozygous pathogenic variants in the ATM gene usually develop an ataxia plus syndrome, meaning ataxia plus other systemic symptoms. This case presents with early-onset ataxia, hyperkinetic movements, mild conjunctival telangiectasia, increased levels of AFP, and the absence of recurrent infections. The classical A-T phenotype combines progressive ataxia in more than 96%, oculocutaneous telangiectasia up to 97%, recurrent infections almost 80%, cancer predisposition 10–25%, and increased levels of AFP in more than 90% [Levy and Lang, 2018; Amirifar et al., 2019]. However, with the discovery of the ATM gene, an expanding phenotype involves other movement disorders in addition to ataxia such as myoclonus 92%, dystonia and choreoathetosis 89%, tremor 74% and parkinsonism 41%, even without ataxia or telangiectasia [Levy and Lang, 2018; Teive et al., 2018]. Cases genetically confirmed as A-T and without telangiectasia have been previously described; hence, there is a trend to recognize telangiectasia as a typical but not pathognomonic sign of A-T [Teive et al., 2015]. It is not clear what the origin is of the high serum AFP level in patients with A-T. However, it has been shown that AFP levels increase proportionally with age; thus, it is considered as a diagnostic marker [Stray-Pedersen et al., 2007]. The lack of a history of recurrent infections in patients with A-T is unusual, although it is postulated that this characteristic occurs in patients with a normal immunoglobulin profile [Amirifar et al., 2019].
We established a cancer risk assessment with an annual breast MRI based on the NCCN guideline [Daly et al., 2020] and a control endoscopy based on a personal history of burning epigastric pain. The obligate carrier mother developed gastric and breast cancer later on, as described in previous studies associated with pathogenic variants in the ATM gene [van Os et al., 2016]. Early clinical diagnosis of A-T should lead to genetic testing and multidisciplinary management due to the cancer risk of the patients and the carrier relatives. Although the proband came to us late, we deemed that we determine carrier status of the family members and detected 2 types of cancer at an early stage in the carrier mother.
Novel Compound Heterozygous Mutation Identified within in ATM Gene
The c.3955_3958dup is reported as pathogenic in ClinVar with ID 653063 (https://www.ncbi.nlm.nih.gov/clinvar/variation/653063), and it is part of the dbSNP collection with ID rs1591646379 (https://www.ncbi.nlm.nih.gov/snp/rs1591646379). According to available evidence, it meets ACMG criteria: PVS1 “pathogenic-very strong 1” − this change produces a premature stop signal at exon 26. PM2 “pathogenic-moderate 2” − not found in gnomAD. Consequently, this variant is classified as pathogenic according to the recommendations of the ACMG [Richards et al., 2015]. This nonsense variant has not been previously reported in individuals with ATM-related phenotype. Our case report provides clinical evidence for both A-T and associated cancers, individuals II-4 and I-2, respectively, shown in Figure 1.
The c.5825C>T variant is reported as conflicting interpretations of pathogenicity in ClinVar with ID 181999 (https://www.ncbi.nlm.nih.gov/clinvar/variation/181999/),and it is part of the dbSNP collection with ID rs730881394 (https://www.ncbi.nlm.nih.gov/snp/rs730881394). This missense variant has been observed in individuals with A-T [Carney et al., 2012; Davis et al., 2013]. According to available evidence, it meets ACMG5 criteria: PM1 “pathogenic-moderate 1” − located in the FAT domain with 230 coding variants (94.7% pathogenicity) in ATM HUMAN UniProt. PM2 “pathogenic-moderate 2” − allele frequency = 0.000004 in gnomAD Exome. PP3 “pathogenic-supporting 3” − multiple in silico tools (MutationTaster, PolyPhen-2 and SIFT) predict pathogenicity.
Here, both ATM variants of the proband were confirmed to be in compound heterozygous status by segregation. That is, the patient inherited the c.3955_3958dup pathogenic variant from the mother (an affected allele) and the c.5825C>T variant, most likely, from the father (the other affected allele) since her younger sister (II-5) harbors the c.5825C>T variant, and the oldest deceased sister had a history of a similar A-T phenotype, shown in Figure 1. Therefore, the variant meets the ACMG PM3 “pathogenic-moderate 3” criterion: for recessive disorders, detected in trans with a pathogenic variant [Richards et al., 2015]. In addition, c.5825C>T has been reported to result in absence of ATM activity by western blot [Carney et al., 2012]. However, there are no well-established functional studies supportive of a damaging effect on the gene or gene product, according to current recommendations for meeting the PS3 “pathogenic-strong 3” criterion [Richards et al., 2015; Gelman et al., 2019].
Consequently, the c.5825C>T variant is classified as likely pathogenic according to the recommendations of the ACMG [Richards et al., 2015].
In conclusion, we report clinical evidence of A-T for the novel compound heterozygous mutation (c.3955_3958dup and c.5825C>T) and 2 cancers in a c.3955_3958dup carrier. These clinical findings reinforced the evidence of pathogenicity for these variants and allow timely decisions to be made in risk assessment of ATM-associated conditions.
Statement of Ethics
The Institutional Ethics Committee at Instituto Nacional de Ciencias Neurologicas approved the study with IRB No. 622-2020-CIE-INCN. Written informed consent was obtained for the use of photographs, videos and research findings.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
A.R.-V. is partially supported by NIH training grants #D43TW009345 and #D43TW009137.
Author Contributions
All authors reviewed and approved the final manuscript. R.S.R., M.C.-O., and J.B.-M. prepared the manuscript. R.S.R., J.B.-M., M.T.-L., and Y.S.-A. performed the analysis and interpretation of the variants. M.C.-O., E.S.-C., and A.R.-V. performed the clinical assessment and follow-up. Y.S.-A. coordinated the genetic testing and performed the family cancer risk assessment.
Acknowledgements
We thank the patient and her family members for their participation in the study. The authors thank Miluska Loarte-Villarreal, Eng and Miguel Inca-Martinez, BS for the critical review of the manuscript.
References
- 1.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7((4)):248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amirifar P, Ranjouri MR, Yazdani R, Abolhassani H, Aghamohammadi A. Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr Allergy Immunol. 2019;30((3)):277–88. doi: 10.1111/pai.13020. [DOI] [PubMed] [Google Scholar]
- 3.Carney EF, Srinivasan V, Moss PA, Taylor AM. Classical ataxia telangiectasia patients have a congenitally aged immune system with high expression of CD95. J Immunol. 2012;189((1)):261–8. doi: 10.4049/jimmunol.1101909. [DOI] [PubMed] [Google Scholar]
- 4.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18((4)):380–91. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 5.Davis MY, Keene CD, Swanson PD, Sheehy C, Bird TD. Novel mutations in ataxia telangiectasia and AOA2 associated with prolonged survival. J Neurol Sci. 2013;335((1-2)):134–8. doi: 10.1016/j.jns.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Gomero D, Córdova-Calderón W, Aldave-Becerra J. Registro de pacientes con inmunodeficiencias primarias en los tres principales centros de referencia del Perú (in Spanish) Rev Peru Med Exp Salud Publica. 2018;35((3)):538–40. doi: 10.17843/rpmesp.2018.353.3317. [DOI] [PubMed] [Google Scholar]
- 7.Gelman H, Dines JN, Berg J, Berger AH, Brnich S, Hisama FM, et al. Recommendations for the collection and use of multiplexed functional data for clinical variant interpretation. Genome Med. 2019;11((1)):85. doi: 10.1186/s13073-019-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4((7)):1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 9.Lazo Rivera E, Pastor Vizcarra LF. Ataxia-Telangiectasia: Reporte de caso. Rev Neuropsiquiatr. 2014;77((4)):283–7. [Google Scholar]
- 10.Levy A, Lang AE. Ataxia-telangiectasia: A review of movement disorders, clinical features, and genotype correlations. Mov Disord. 2018;33((8)):1238–47. doi: 10.1002/mds.27319. [DOI] [PubMed] [Google Scholar]
- 11.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17((5)):405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis. 2016;11((1)):159. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schon K, van Os NJH, Oscroft N, Baxendale H, Scoffings D, Ray J, et al. Genotype, extrapyramidal features, and severity of variant ataxia-telangiectasia. Ann Neurol. 2019;85((2)):170–80. doi: 10.1002/ana.25394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11((4)):361–2. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 15.Stray-Pedersen A, Borresen-Dale AL, Paus E, Lindman CR, Burgers T, Abrahamsen TG. Alpha fetoprotein is increasing with age in ataxia-telangiectasia. Eur J Paediatr Neurol. 2007;11((6)):375–80. doi: 10.1016/j.ejpn.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Teive HAG, Moro A, Moscovich M, Arruda WO, Munhoz RP, Raskin S, et al. Ataxia-telangiectasia - A historical review and a proposal for a new designation: ATM syndrome. J Neurol Sci. 2015;355((1-2)):3–6. doi: 10.1016/j.jns.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teive HAG, Camargo CHF, Munhoz RP. More than ataxia - Movement disorders in ataxia-telangiectasia. Parkinsonism Relat Disord. 2018;46:3–8. doi: 10.1016/j.parkreldis.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 18.van Os NJ, Roeleveld N, Weemaes CM, Jongmans MC, Janssens GO, Taylor AM, et al. Health risks for ataxia-telangiectasia mutated heterozygotes: a systematic review, meta-analysis and evidence-based guideline. Clin Genet. 2016;90((2)):105–17. doi: 10.1111/cge.12710. [DOI] [PubMed] [Google Scholar]
- 19.van Os NJH, Chessa L, Weemaes CMR, van Deuren M, Fiévet A, van Gaalen J, et al. Genotype-phenotype correlations in ataxia telangiectasia patients with ATM c.3576G>A and c.8147T>C mutations. J Med Genet. 2019;56((5)):308–16. doi: 10.1136/jmedgenet-2018-105635. [DOI] [PubMed] [Google Scholar]
- 20.Verhagen MM, Last JI, Hogervorst FB, Smeets DF, Roeleveld N, Verheijen F, et al. Presence of ATM protein and residual kinase activity correlates with the phenotype in ataxia-telangiectasia: a genotype-phenotype study. Hum Mutat. 2012;33((3)):561–71. doi: 10.1002/humu.22016. [DOI] [PubMed] [Google Scholar]