Abstract
Background
Sodium cromoglycate has been recommended as maintenance treatment for childhood asthma for many years. Its use has decreased since 1990, when inhaled corticosteroids became popular, but it is still used in many countries.
Objectives
To determine the efficacy of sodium cromoglycate compared to placebo in the prophylactic treatment of children with asthma.
Search methods
We searched the Cochrane Airways Group Trials Register (October 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 3, 2009), MEDLINE (January 1966 to October 2009), EMBASE (January 1985 to October 2009) and reference lists of articles. We also contacted the pharmaceutical company manufacturing sodium cromoglycate.
Selection criteria
All double‐blind, placebo‐controlled randomised trials, which addressed the effectiveness of inhaled sodium cromoglycate as maintenance therapy, studying children aged 0 up to 18 years with asthma.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We pooled study results.
Main results
Of 3500 titles retrieved from the literature, 24 papers reporting on 23 studies could be included in the review. The studies were published between 1970 and 1997 and together included 1026 participants. Most were cross‐over studies. Few studies provided sufficient information to judge the concealment of allocation. Four studies provided results for the percentage of symptom‐free days. Pooling the results did not reveal a statistically significant difference between sodium cromoglycate and placebo. For the other pooled outcomes, most of the symptom‐related outcomes and bronchodilator use showed statistically significant results, but treatment effects were small. Considering the confidence intervals of the outcome measures, a clinically relevant effect of sodium cromoglycate cannot be excluded. The funnel plot showed an under‐representation of small studies with negative results, suggesting publication bias.
Authors' conclusions
There is insufficient evidence to be sure about the efficacy of sodium cromoglycate over placebo. Publication bias is likely to have overestimated the beneficial effects of sodium cromoglycate as maintenance therapy in childhood asthma.
Plain language summary
The effects of sodium cromoglycate compared with placebo for chronic asthma in children
In this review we aimed to determine whether there is evidence for the effectiveness of inhaled sodium cromoglycate as maintenance treatment in children with chronic asthma. Most of the studies were carried out in small groups of patients. Furthermore, we suspect that not all studies undertaken have been published. The results show that there is insufficient evidence to be sure about the beneficial effect of sodium cromoglycate compared to placebo. However, for several outcome measures the results favoured sodium cromoglycate.
Background
Since the late 1960s, disodium cromoglycate (DSCG) has been used as maintenance treatment for children with moderate asthma, although the precise mechanism of action is still not fully understood. No serious side effects have been reported in trials, but cases of dysuria, urticaria, bronchospasm, angio‐oedema and anaphylaxis have been ascribed to the use of DSCG, once with death as a result (Lester 1997; Leynadier 1985).
In the early 1990s, many guidelines recommended use of DSCG. Gradually, corticosteroids have come to the fore as first choice maintenance therapy (BAG 1997; Ernst 1996), or were recommended alongside DSCG for mild persistent asthma (NIH 1997). Other guidelines continued to recommend DSCG as first choice in young children (Sly 1997). The most recent revisions of the GINA and NIH guidelines (GINA 2005; NIH 2002) consider the role of DSCG in children to be limited. Inhaled glucocorticosteroids are the first choice; DSCG is only recommended as one of the alternative treatment options for children with mild persistent asthma. Canadian guidelines no longer recommend DSCG as maintenance therapy for children, nor do British guidelines for children aged 5 to 12 years (Becker 2005; BTS 2003, page i20).
The long‐term side effects of asthma treatment with inhaled steroids in early childhood are not clear. Nevertheless, there is concern that treating very mild cases of asthma with inhaled steroids may have an adverse effect on the balance between risk and benefit. A Cochrane review has shown an effect of inhaled beclomethasone on linear growth in children (Sharek 1999). Therefore, physicians involved in the treatment of asthma in children may still prefer sodium cromoglycate as first choice maintenance treatment.
The use of DSCG has decreased since 1990, while the use of inhaled corticosteroids is increasing. The discrepancy between guidelines and the debate on the role of DSCG, which led to its recent withdrawal as first line maintenance treatment in young children in some countries, was the rationale to review the efficacy of inhaled DSCG as maintenance treatment for chronic childhood asthma.
Objectives
To determine whether there is evidence for the efficacy of inhaled sodium cromoglycate as maintenance treatment in children with asthma.
Methods
Criteria for considering studies for this review
Types of studies
All double‐blind, placebo‐controlled, randomised clinical trials, which addressed the effectiveness of DSCG as maintenance therapy.
Types of participants
Children aged 0 up to 18 years with asthma in all settings (general practice, emergency departments, outpatient departments, hospitalised). We only included studies including both children and adults when results for children were presented separately. When the number of children in these studies was less than five, we did not include the study.
Types of interventions
Inhaled sodium cromoglycate, delivered via any device: nebulised, by Spinhaler or by metered dose inhaler, with or without holding chamber. We only included trials that compared DSCG with placebo. No co‐interventions were permitted other than rescue medication as needed.
Types of outcome measures
Primary outcomes
The primary outcome measure was the difference in percentage of days without asthma symptoms, between placebo and cromoglycate treatment.
Secondary outcomes
Symptom scores (day cough, day wheeze, daytime asthma score, day activity, night cough, night wheeze, night‐time asthma score, sleep disturbance, overall symptom/severity score)
Auscultation score
Preference of patients/parents and clinicians
Overall success rate
Bronchodilator use, use of oral steroids, hospital admission
Side effects
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details).
Additonal searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 3, 2009), MEDLINE (January 1966 to October 2009) and EMBASE (1979 to October 2009) were also conducted. For MEDLINE and EMBASE we used the Cochrane sensitive search strategy to select all RCTs (Dickersin 1994) and in all databases, we used the following search terms:
cromolyn* OR dscg OR cromoglycate* or cromoglicate* OR cromone* or intal*
Searches are current to October 2009.
Searching other resources
We contacted the pharmaceutical company manufacturing DSCG (Rhone‐Poulenc‐Rorer, formerly Fisons plc, Loughborough, UK), checked bibliographies of retrieved trials and contacted primary authors of trials published after 1990 for any additional trials.
Data collection and analysis
Data extraction and management
Two authors extracted data. When using symptom scores, most studies used a scale of 0 to 3 points; where a different scale was used we transformed the mean and standard deviation for our purposes. We calculated confidence intervals for the treatment effect (difference in symptom score) for individual studies assuming a t‐distribution.
Assessment of risk of bias in included studies
Two authors independently scored the methodological quality of all trials using three sets of criteria: Chalmers (Chalmers 1981), Jadad (Jadad 1996) and the Cochrane criteria for concealment. A third author determined the final decision if there was lack of consensus. Trials in which one of the authors was involved were also scored by an impartial author. We did not contact authors of the trials for confirmation of methodology and data extraction, because most of the studies were performed many years ago and we considered it unlikely that this would provide further useful information. When updating the review in 2007, a 'Risk of Bias' table was added (Figure 1).
Dealing with missing data
If no standard error of the treatment effect of a particular outcome measure was available, and could not be calculated, we imputed it from a study with a similar design (cross‐over or parallel) (cf Follman 1999). If more than one study was available for imputation, we selected the largest study, unless this choice would lead to inconsistencies with the results in the original study (e.g. when the authors reported no significant difference, but the imputed data would change this). In that case the second largest study was taken.
Data synthesis
We computed pooled estimates of the treatment effect and the pooled 95% confidence intervals (CI), combining parallel and cross‐over studies (Elbourne 2002). For cross‐over studies we used the results of paired analyses, extracting treatment effect, standard error and within patient correlation between DSCG and placebo period (rho) from papers. When rho was not given, we imputed this in the same way as stated above for missing standard errors. We tested for homogeneity (Dersimonian 1986). When heterogeneity was found (P < 0.05), we did not use the fixed‐effect model to compute a pooled estimate and confidence interval, but only used the random‐effects model (Dersimonian 1986). To investigate causes for heterogeneity, we evaluated the influence of study characteristics (year of publication, mean age of children, method of delivery, asthma severity of the study population, methodological quality, doses per day and duration). Assessment of asthma severity was based on the description of the study population in the papers (see 'Characteristics of included studies' table). As there was no single outcome measure available for all studies, we selected those outcomes for which at least 10 studies were available. To include as many studies as possible in the funnel plot (see below) and the meta‐regression analysis, we combined various outcome measures that used a similar scale, taking the first available from overall symptom score, day wheeze, day cough, day activity and daytime asthma score.
For all study characteristics except asthma severity, we used univariate and multivariate meta‐regression analysis (Fleiss 1993), weighing observations by the reciprocal of the square of the standard error of the mean difference between placebo and DSCG. Thus, all pooled outcomes are presented as weighted mean differences (WMD). Study characteristics were either entered as categorical (design, type of delivery) or as continuous (publication year, quality score, etc.). For asthma severity, we used the asthma score in the placebo group (or period) as study characteristic. Because this measurement is subject to measurement error as much as the outcome variables are, ordinary regression analysis is inappropriate, as this technique only assumes measurement error in the outcome variable. Therefore, we used an analysis technique called functional relationships (Nagelkerke 1992) to evaluate the influence of asthma severity of the study population on the outcome for cough, wheeze and overall symptom score.
We performed all analyses using SPSS version 10 for the initial review.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses when outcome data were available from at least 10 studies, using the following characteristics for subgroup identification: asthma severity (moderate versus severe), health care settings (hospitalised/institutionalised versus other settings), type of delivery (nebulised versus other), age (using a mean age of five years as the cut‐off point), duration of follow up (using three months follow up as the cut‐off point) and methodological quality (for Jadad's scoring system three points or higher versus lower; for Chalmers' summated items the 13 best studies versus the remaining studies).
To explore heterogeneity further and visualise possible publication bias, we constructed a funnel plot of the effect estimate (delta) against the precision (Egger 1995), using the same combination of outcome measures as for meta‐regression analysis. The precision of a trial was defined as one divided by the standard error. The symmetry of the funnel plot was tested using a significance level of 0.10 (Egger 1997).
Results
Description of studies
Results of the search
Searching the literature databases resulted in retrieval of over 3500 titles (MEDLINE: 1500; EMBASE: 1400; Cochrane Airways Group Trials Register: 850 titles). We read about 200 papers in full; 65 of these were evaluated by two authors according to a structured inclusion criteria form. The final set consisted of 24 papers, reporting on 23 studies. For one study, two papers were published reporting on different outcome measures (Yuksel 1992). Update searches were conducted in November 2006 and October 2007. These identified 181 titles, which were screened, and 10 were obtained as full papers for further assessment. None of these fulfilled our inclusion criteria, but several were added to the list of excluded studies. An updated search in October 2008 did not identify any new studies for consideration in the review. The latest search was in July 2010 and it did not return any eligible studies although two studies were added to Characteristics of excluded studies.
Included studies
Most of the included studies were European (13 studies, nine of which were from the UK) or North American. Two were from Israel, three from Japan and one from Thailand. All but three papers were written in English. One study was in Norwegian (Dalene 1977), the other two in Japanese (Kobayashi 1970; Mikawa 1986).
The studies were published between 1970 and 1997. Twelve studies were published in the 1970s, eight studies in the 1980s, and four in the 1990s. Detailed information on each study is given in the table of 'Characteristics of included studies'.
The age range of the children in the included studies varied considerably. Eleven studies included children not older than four years of age. In one study (Easton 1973) the age of the children was not specified. Before 1977, none of the studies included children below the age of four.
Most of the studies had a cross‐over design. Four were parallel group studies. The cross‐over studies typically were divided into two periods of three or four weeks treatment, with sometimes a washout period in between. In some of the cross‐over studies, the first two weeks of each period were ignored in the analysis.
In nine studies the study drugs (DSCG or placebo) were nebulised. Nine studies used dry powder in capsules, most often with the Spinhaler as device, but sometimes without a device being mentioned. In five studies the drugs were administered as aerosols with a spacer and sometimes a facemask.
In several papers it was not clear whether and what concurrent medication was permitted during the trial. We included these studies. Compliance with the therapy regimen was only discussed in a minority of papers.
Most of the studies were carried out in a hospital setting, usually with outpatients. For several studies, no information about the setting could be found. Based on the authors' affiliations, we assumed that these were hospital outpatients. In these cases we have added a question mark after 'hospital outpatients' in the table of study characteristics. Only one study recruited patients in general practice (Tasche 1997).
Regarding asthma severity, most of the studies included children that would be classified as having moderate or severe asthma by current standards (e.g. GINA 2005). Many children had one or more hospital admissions for asthma in the past. The three studies with probably the largest proportion of mild asthmatic children were Edmunds 1980; Furfaro 1994 and Tasche 1997.
The size of the trials varied between 10 and 232 participants. Only two trials included more than 100 children (Mikawa 1986; Tasche 1997). As can be expected, the parallel‐group trials had larger patient groups than the cross‐over trials (parallel group trials had on average 131 participants versus 26 for cross‐over studies). Altogether, the 23 studies included 1026 participants.
The length of the period during which the children used either active medication or placebo in the trials varied from three weeks to 26 weeks. For 15 studies (of which 14 had a cross‐over design), this was three or four weeks, while only two studies had a duration of over 10 weeks (Cogswell 1985; Tasche 1997).
Several study characteristics were strongly correlated. Dose (corrected for type of delivery), method of delivery, year of publication, age of children and length of treatment period showed Pearson correlations up to 0.75.
The variety of outcome measures on which data were reported was large. Likewise, for most outcome measures only few studies reported comparable data. The outcome measures that were reported most often were asthma scores (10 studies), daytime wheeze scores (10 studies), daytime cough scores (nine studies) and bronchodilator use (10 studies). Several studies reporting on hospital admittance and steroid use provided insufficient information to be included in the pooled results.
Excluded studies
Excluded studies were either not blinded, not randomised, not placebo‐controlled, did not concern the appropriate age group, or investigated the effectiveness of DSCG in exercise induced asthma. One study (Kraemer 1993) was misclassified and hence erroneously included in the first version of the review: this trial was removed from this update (see 'Characteristics of excluded studies').
Risk of bias in included studies
See: table 'Characteristics of included studies'.
The methodological quality, as assessed by two scoring methods, varied considerably (see Table 1; Table 2). Only one study attained the maximum score of five points on the Jadad list (Mikawa 1986). The proportion of items fulfilled on Chalmers's list varied between 24 and 79% (mean 44% (SD 11.9)). Of the papers reporting cross‐over studies, only few stated explicitly that the sequence of both treatments had been randomised. In the analysis, we assumed they were.
1. Methodological quality scores according to Chalmers.
| Study | Selection & reject log | Randomisation & concealment | Blinding | Therap regimens | Withdrawals | Compliance | Numbers & statistics | Timing | Total score (%) |
| Maximum score study | 6 | 13 | 23 | 6 | 7 | 6 | 24 | 10 | 95 (100%) |
| Bertelsen 1986 | 1 | 3 | 12 | 3 | 3 | 0 | 6 | 3 | 31/95 = 33% |
| Cogswell 1985 | 1 | 10 | 13 | 4 | 3 | 1/3 | 9/22 | 2 | 43/91 = 47% |
| Collins 1971 | 2 | 7 | 18 | 6 | 0 | 0 | 3 | 3/9 | 39/94 = 41% |
| Dalene 1977 | 2 | 2 | 16 | 4 | 3 | 1 | 3/20 | 2 | 32/91 = 35% |
| Easton 1973 | 1 | 4 | 17 | 5 | 1 | 0 | 4/16 | 3 | 35/87 = 40% |
| Edmunds 1980 | 0 | 4 | 14 | 0 | 1 | 2 | 6/20 | 2 | 29/91 = 33% |
| Furfaro 1994 | 3 | 13 | 13 | 5 | 4 | 3 | 11 | 4 | 56/95 = 59% |
| Geller 1982 | 1 | 4 | 16 | 2 | 3 | 0 | 6/20 | 2 | 34/91 = 37% |
| Geller 1983 | 2 | 6 | 18 | 3 | 4 | 1 | 5/19 | 2 | 41/90 = 46% |
| Glass 1981 | 0 | 4 | 14 | 2 | 0 | 1 | 6 | 2/9 | 29/94 = 31% |
| Henry 1984 | 0 | 0 | 16 | 3 | 0 | 0 | 2 | 2/9 | 23/94 = 24% |
| Hiller 1975 | 2 | 10 | 18 | 4 | 3 | 0 | 5/18 | 2/9 | 44/87 = 51% |
| Hiller 1977 | 0 | 10 | 14 | 4 | 3 | 0 | 2/18 | 1/9 | 34/87 = 39% |
| Hyde 1970 | 1 | 6 | 13 | 4 | 3 | 0 | 6/18 | 5/9 | 38/87 = 44% |
| Kobayashi 1970 | 2 | 4 | 16 | 5 | 3 | 0 | 13/19 | 2 | 45/90 = 50% |
| Limburg 1971 | 1 | 4 | 13 | 5 | 3 | 0 | 11/23 | 8 | 45/94 = 48% |
| Matthew 1977 | 0 | 4 | 13 | 4 | 3 | 0 | 3/16 | 2 | 29/87 = 33% |
| Mikawa 1986 | 3 | 4 | 16 | 5 | 2 | 0 | 721 | 6 | 43/92 = 47% |
| Shioda 1970 | 3 | 6 | 18 | 4 | 2 | 0 | 5 | 4 | 42/95 = 44% |
| Smith 1970 | 2 | 4 | 16 | 6 | 3 | 0 | 4/21 | 8 | 43/92 = 47% |
| Tasche 1997 | 6 | 11 | 20 | 3 | 6 | 3 | 21 | 5 | 75/95 = 79% |
| Tuchinda 1974 | 1 | 5 | 19 | 6 | 3 | 0 | 11/15 | 4 | 49/86 = 57% |
| Yuksel 1992 | 3 | 4 | 16 | 5 | 3 | 0 | 5/21 | 3/9 | 39/91 = 43% |
"/" means denominator adapted because items non‐applicable.
2. Methodological quality scores according to Jadad's criteria.
| Study | Randomisation | Randomisation detail | Double‐blind | Blinding details | Withdrawals | Total |
| Bertelsen 1986 | 1 | 0 | 1 | 0 | 1 | 3 |
| Cogswell 1985 | 1 | 0 | 1 | 1 | 1 | 4 |
| Collins 1971 | 0 | 0 | 1 | 1 | 0 | 2 |
| Dalene 1977 | 1 | 0 | 1 | 0 | 1 | 3 |
| Easton 1973 | 1 | 0 | 1 | 0 | 0 | 2 |
| Edmunds 1980 | 1 | 0 | 1 | 0 | 0 | 2 |
| Furfaro 1994 | 1 | 1 | 1 | 0 | 1 | 4 |
| Geller 1982 | 1 | 0 | 1 | 1 | 1 | 4 |
| Geller 1983 | 1 | 0 | 1 | 1 | 1 | 4 |
| Glass 1981 | 1 | 0 | 1 | 0 | 0 | 2 |
| Henry 1984 | 0 | 0 | 1 | 0 | 1 | 2 |
| Hiller 1975 | 1 | 0 | 1 | 0 | 1 | 3 |
| Hiller 1977 | 1 | 0 | 1 | 1 | 1 | 4 |
| Hyde 1970 | 0 | 0 | 1 | 1 | 1 | 3 |
| Kobayashi 1970 | 1 | 0 | 1 | 0 | 1 | 3 |
| Limburg 1971 | 0 | 0 | 1 | 1 | 1 | 3 |
| Matthew 1977 | 1 | 0 | 1 | 1 | 0 | 3 |
| Mikawa 1986 | 1 | 1 | 1 | 1 | 1 | 5 |
| Shioda 1970 | 1 | 0 | 1 | 0 | 1 | 3 |
| Smith 1970 | 1 | 1 | 1 | 0 | 1 | 4 |
| Tasche 1970 | 1 | 0 | 1 | 0 | 1 | 3 |
| Tuchinda 1974 | 0 | 1 | 1 | 1 | 1 | 4 |
| Yuksel 1992 | 1 | 0 | 1 | 0 | 0 | 2 |
| Yuksel 1993 | 1 | 0 | 1 | 0 | 1 | 3 |
When updating the review in 2007, 'Risk of Bias' tables were produced, and a summary table was added to this review (Figure 1). For further explanation of this table, see the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8 (Higgins 2008).
1.
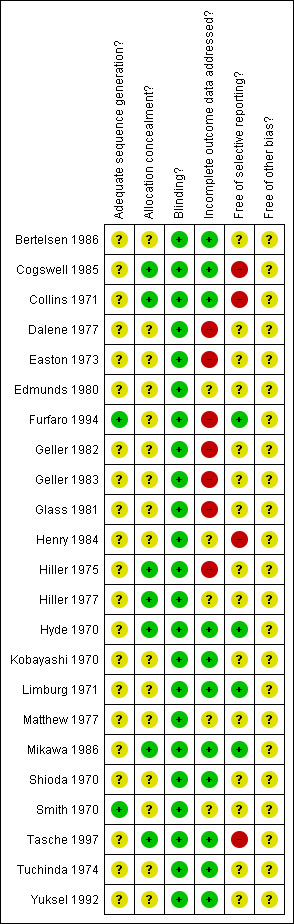
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
For several items (sequence generation, allocation concealment, selective reporting and other sources of bias), only few studies provided a clear answer. The proportion of question marks (for which the study reports do not provide enough information) is high. Blinding was considered to be adequate for all studies, which is no surprise, as this was an inclusion criterion. Several studies inadequately reported on incomplete outcome data or reported selectively. None of the studies attained the maximum score for 'withdrawals', and 20 of the 23 studies scored less than 50% on this item (Table 1).
Effects of interventions
Study outcomes have been gathered into Additional tables 3 to 19 and summarised in Table 20. The tables give pooled point estimates for the difference between DSCG and placebo (i.e. DSCG minus placebo), and confidence intervals, assuming homogeneity (fixed‐effect) and heterogeneity (random‐effects). Below we report the results for the outcome measures for which a considerable number of studies were available. These study outcomes are now also shown as forest plots for the primary outcome measure and all secondary outcomes with more than five contributing studies.
Symptoms
Only four studies provided results for the percentage of symptom‐free days: our primary outcome measure (Figure 2; Table 3). In all but one of the studies (Cogswell 1985), the confidence interval included the point of no difference. Pooling the results revealed no significant difference between DSCG and placebo (WMD 6.76% favouring DSCG, 95% CI ‐2.18 to 15.70), random‐effects model.
2.
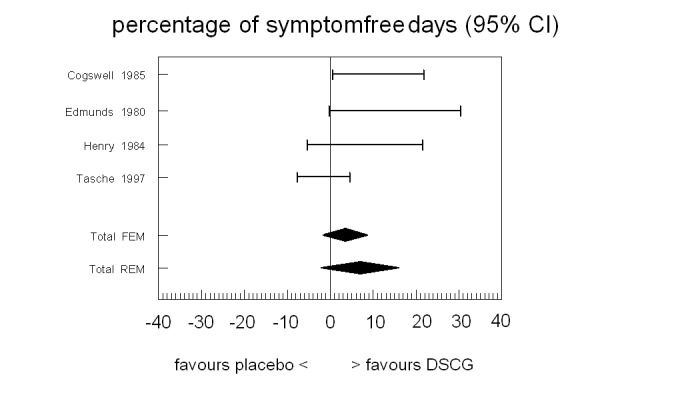
Forest plot of the primary outcome of the review: mean difference in % symptom‐free days between sodium cromoglycate and placebo
3. Primary outcome: percentage of symptom‐free days.
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Cogswell 1985 | 24 | 0.34 | 0.34 | 11.10 | 5.10 | 5.10 | 0.50 | 21.70 | — |
| Edmunds 1980 | 30 | 0.25 | 0.25 | 15.00 | 7.50 | 7.50 | ‐0.32 | 30.32 | — |
| Henry 1984 | 20 | NA | NA | 8.00 | NA | 9.89 | ‐5.50 | 21.50 | Edmunds 1980 |
| Tasche 1997 | 218 | 0 | 0 | ‐1.60 | 3.10 | 3.10 | ‐7.70 | 4.60 | — |
| Homogeneity test | Chi2 = 7.48, P = 0.06 | ||||||||
| Pooled results mean (95% CI) | Fixed‐effect model | 3.57 (‐1.18 to 8.32) | |||||||
| Random‐effects model | 6.76 (‐2.18 to 15.70) | ||||||||
Rho = correlation between DSCG and placebo period (cross‐over studies). NA = not available in paper.
A variety of other symptom and hindrance scores was found. In tables 4 to 19 we present the results for outcome measures for which at least two studies provided data. Here we describe the results for the symptom scores with the largest number of studies: day cough score (nine studies), day wheeze score (10 studies), and overall symptom/severity score (10 studies).
For daytime cough, the difference between placebo and DSCG favoured DSCG in all but one study (Bertelsen 1986) (Figure 3; Table 4). The confidence intervals included the point of no difference for seven out of the nine studies. Pooling the results (random‐effects model because of heterogeneity) did result in a statistically significant difference between placebo and DSCG favouring DSCG (WMD ‐0.18, 95% CI ‐0.32 to ‐0.04).
3.
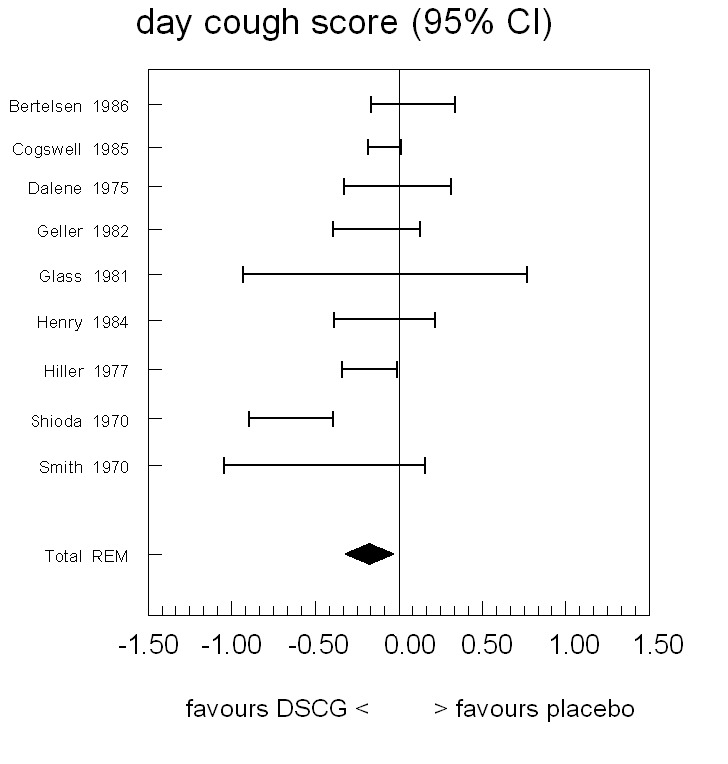
Forest plot of mean difference in symptom scores for day cough between sodium cromoglycate and placebo
4. Day cough score.
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Bertelsen 1986 | 58 | 0 | 0 | 0.08 | 0.13 | 0.13 | ‐0.17 | 0.33 | — |
| Cogswell 1985 | 24 | NA | NA | ‐0.09 | 0.05 | 0.05 | ‐0.19 | 0.01 | — |
| Dalene 1975 | 18 | NA | NA | ‐0.12 | NA | 0.16 | ‐0.44 | 0.20 | Shioda 1970 |
| Geller 1982 | 44 | NA | 0.63 | ‐0.14 | NA | 0.13 | ‐0.40 | 0.12 | Shioda 1970 |
| Glass 1981 | 14 | NA | NA | ‐0.09 | 0.39 | 0.39 | ‐0.93 | 0.76 | — |
| Henry 1984 | 20 | NA | NA | ‐0.09 | NA | 0.15 | ‐0.39 | 0.21 | Shioda 1970 |
| Hiller 1977 | 17 | NA | NA | ‐0.18 | 0.08 | 0.08 | ‐0.34 | ‐0.01 | — |
| Shioda 1970 | 33 | 0.63 | 0.63 | ‐0.65 | 0.12 | 0.12 | ‐0.89 | ‐0.40 | — |
| Smith 1970 | 18 | 0 | 0 | ‐0.45 | 0.28 | 0.28 | ‐1.05 | 0.14 | — |
| Homogeneity test | Chi2 = 23.44, P < 0.001 | ||||||||
| Pooled results: mean (95% CI) | Random‐effects model | ‐0.18 (‐0.32 to ‐0.04) | |||||||
NA = not available in paper.
For daytime wheeze the pooled results show a small but significant difference favouring DSCG: a difference of ‐0.11 (WMD) on a scale of 0 to 3 (95% CI ‐0.19 to ‐0.03; random‐effects model) (Figure 4; Table 5).
4.
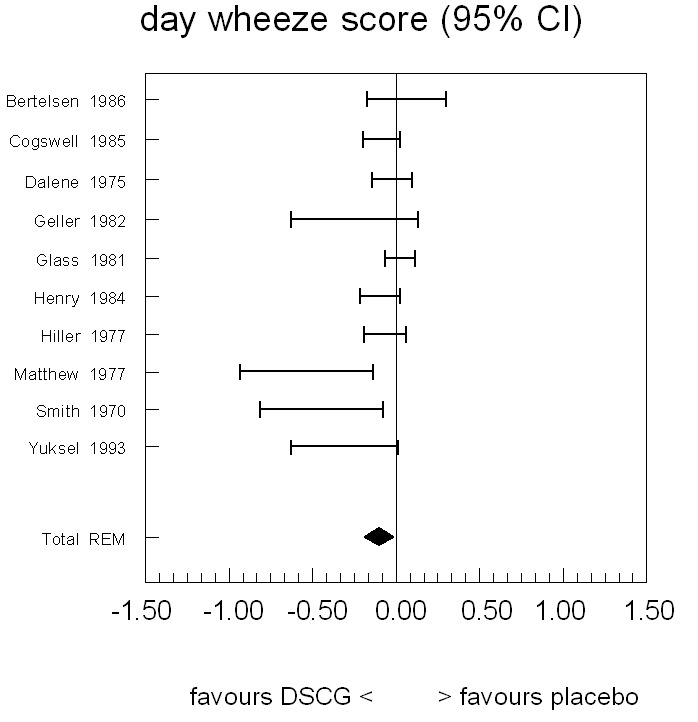
Forest plot of mean difference in symptom scores for day wheeze between sodium cromoglycate and placebo
5. Day wheeze score.
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Bertelsen 1986 | 58 | 0 | 0 | 0.06 | 0.12 | 0.12 | ‐0.17 | 0.30 | |
| Cogswell 1985 | 24 | NA | NA | ‐0.09 | 0.05 | 0.05 | ‐0.21 | 0.01 | |
| Dalene 1975 | 18 | NA | NA | ‐0.03 | NA | 0.06 | ‐0.15 | 0.09 | Cogswell 1985 |
| Geller 1982 | 44 | NA | 0.34 | ‐0.25 | NA | 0.19 | ‐0.63 | 0.13 | Matthew 1977 |
| Glass 1981 | 14 | NA | NA | 0.02 | 0.04 | 0.04 | ‐0.07 | 0.11 | |
| Henry 1984 | 20 | NA | NA | ‐0.10 | NA | 0.06 | ‐0.22 | 0.02 | Cogswell 1985 |
| Hiller 1977 | 17 | NA | NA | ‐0.07 | NA | 0.06 | ‐0.19 | 0.05 | Cogswell 1985 |
| Matthew 1977 | 8 | 0.26 | 0.26 | ‐0.54 | 0.18 | 0.18 | ‐0.94 | ‐0.14 | |
| Smith 1970 | 18 | 0 | 0 | ‐0.45 | 0.16 | 0.16 | ‐0.82 | ‐0.08 | |
| Yuksel 1993 | 16 | 0.34 | 0.34 | ‐0.31 | 0.15 | 0.15 | ‐0.63 | 0.01 | |
| Homogeneity test | Chi2 = 23.47, P = 0.01 | ||||||||
| Pooled results: mean (95% CI) | Random‐effects model | ‐0.11 (‐0.19 to ‐0.03) | |||||||
NA = not available in paper.
Mean overall symptom scores favoured DSCG in direction in six out of ten studies (Figure 5; Table 6). The 95% confidence intervals of four of the studies included the point of no difference. Pooling the results (test of homogeneity rejected, hence random‐effects model) showed an overall mean difference of ‐0.22 symptom score points (WMD), favouring the DSCG group (95%CI ‐0.34 to ‐0.09), hence statistically significant.
5.
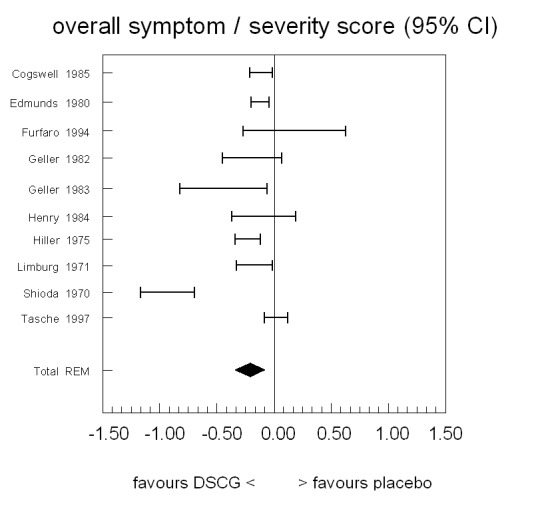
Forest plot of mean difference in overall symptoms between sodium cromoglycate and placebo
6. Overall symptom/severity score.
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Cogswell 1985 | 24 | NA | NA | ‐0.12 | 0.05 | 0.05 | ‐0.22 | ‐0.02 | — |
| Edmunds 1980 | 30 | NA | NA | ‐0.13 | 0.04 | 0.04 | ‐0.21 | ‐0.05 | — |
| Furfaro 1994 | 31 | 0 | 0 | 0.17 | 0.22 | 0.22 | ‐0.28 | 0.62 | — |
| Geller 1982 | 44 | NA | 0.70 | ‐0.20 | NA | 0.13 | ‐0.46 | 0.06 | Hyde 1970 |
| Geller 1983 | 46 | NA | NA | ‐0.45 | 0.19 | 0.19 | ‐0.83 | ‐0.07 | — |
| Henry 1984 | 20 | NA | NA | ‐0.10 | NA | 0.14 | ‐0.38 | 0.18 | Shioda 1970 |
| Hiller 1975 | 9 | NA | NA | ‐0.24 | 0.05 | 0.05 | ‐0.35 | ‐0.13 | — |
| Limburg 1971 | 27 | 0.67 | 0.67 | ‐0.18 | 0.07 | 0.07 | ‐0.34 | ‐0.03 | — |
| Shioda 1970 | 33 | 0.70 | 0.70 | ‐0.94 | 0.11 | 0.11 | ‐1.17 | ‐0.70 | — |
| Tasche 1997 | 218 | 0 | 0 | 0.01 | 0.05 | 0.05 | ‐0.09 | 0.11 | — |
| Homogeneity test | Chi2 = 70.76, P < 0.001 | ||||||||
| Pooled results: mean (95% CI) | Random‐effects model | ‐0.22 (‐0.34 to ‐0.09) | |||||||
NA = not available in paper.
Use of other medication
The use of bronchodilators was reported in ten studies (Figure 6; Table 7). Seven of these reported a difference in favour of DSCG. Five of the studies had confidence intervals excluding the point of no difference. Pooling the data (null hypothesis of homogeneity rejected) resulted in an overall estimated difference of ‐0.24 daily doses (WMD) favouring the DSCG group (95% CI ‐0.07 to ‐0.42, random‐effects model), which is statistically significant.
6.
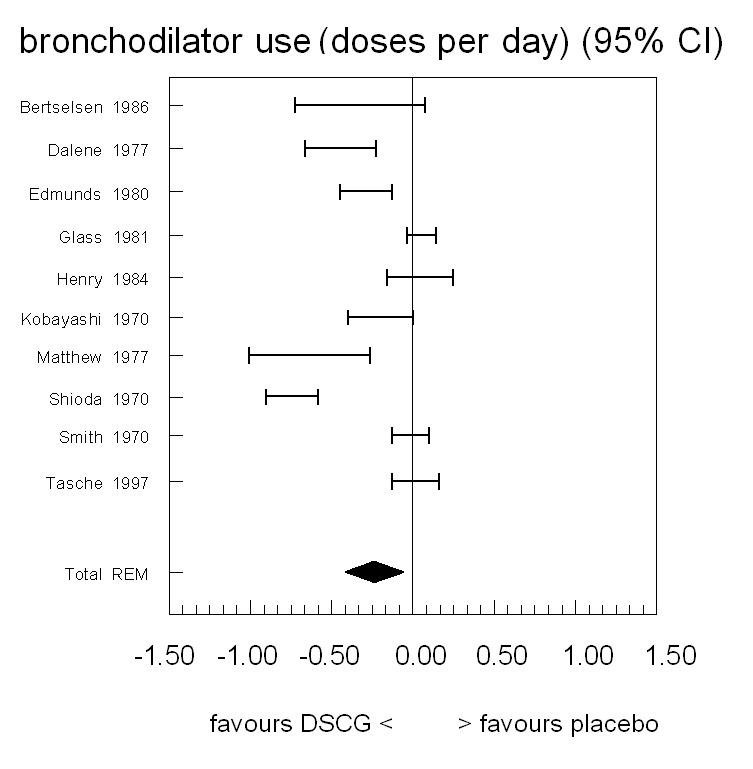
Forest plot of mean difference in bronchodilator use between sodium cromoglycate and placebo
7. Bronchodilator use (number of doses per day).
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Bertselsen 1986 | 58 | 0 | 0 | ‐0.33 | 0.20 | 0.20 | ‐0.73 | 0.07 | — |
| Dalene 1977 | 18 | NA | NA | ‐0.45 | NA | 0.11 | ‐0.67 | ‐0.23 | Shioda 1970 |
| Edmunds 1980 | 30 | 0.50 | 0.50 | ‐0.29 | 0.08 | 0.08 | ‐0.45 | ‐0.13 | — |
| Glass 1981 | 14 | NA | NA | 0.05 | 0.04 | 0.04 | ‐0.04 | 0.14 | — |
| Henry 1984 | 20 | NA | NA | 0.04 | NA | 0.10 | ‐0.16 | 0.24 | Shioda 1970 |
| Kobayashi 1970 | 25 | NA | NA | ‐0.20 | 0.09 | 0.09 | ‐0.40 | 0.00 | — |
| Matthew 1977 | 25 | 0.92 | 0.92 | ‐0.64 | 0.16 | 0.16 | ‐1.01 | ‐0.27 | — |
| Shioda 1970 | 33 | 0.79 | 0.79 | ‐0.75 | 0.08 | 0.08 | ‐0.91 | ‐0.59 | — |
| Smith 1970 | 15 | 0 | 0 | ‐0.02 | 0.05 | 0.05 | ‐0.13 | 0.10 | — |
| Tasche 1997 | 218 | 0 | 0 | 0.01 | 0.07 | 0.07 | ‐0.13 | 0.16 | — |
| Homogeneity test | Chi2 = 116.06, P < 0.001 | ||||||||
| Pooled results: mean (95% CI) | Random‐effects model | ‐0.24 (‐0.42 to ‐0.07) | |||||||
NA = not available in paper.
Steroid use in case of exacerbations was also addressed as an outcome measure: systemic or inhaled, or sometimes unspecified. Seven studies provided these data (Table 8). Only one study (Shioda 1970) found a significant difference. The pooled results did not show a significant difference (OR 0.76, 95% CI 0.34 to 1.72).
8. Steroid use (ln (OR steroid) DCSG/placebo).
| Study | n | Rho from paper | Rho paper or imputed | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Glass 1981 | 14 | NA | NA | ‐4.96 | 8.14 | 8.14 | ‐20.91 | 11.00 | — |
| Hyde 1970 | 42 | NA | NA | ‐0.69 | 0.88 | 0.88 | ‐2.41 | 1.03 | — |
| Kobayashi 1970 | 25 | NA | NA | ‐3.91 | 6.63 | 6.63 | ‐16.90 | 9.08 | — |
| Limburg 1971 | 27 | NA | NA | ‐3.61 | 5.66 | 5.66 | ‐14.70 | 7.48 | — |
| Shioda 1970 | 33 | NA | NA | ‐1.95 | 0.87 | 0.87 | ‐3.66 | ‐0.24 | — |
| Smith 1970 | 15 | 0 | 0 | ‐5.40 | 12.92 | 12.92 | ‐30.72 | 19.92 | — |
| Tasche 1997 | 218 | 0 | 0 | ‐0.17 | 0.39 | 0.39 | ‐0.93 | 0.59 | — |
| Combining parallel studies (Smith and Tasche) | |||||||||
| Homogeneity test | Chi2 = 0.57, P = 0.45 | ||||||||
| Pooled results: mean (95% CI) (ln (OR)) | Fixed‐effect model | ‐0.27 (‐1.09 to 0.54) | |||||||
| (0 = no difference) | Random‐effects model | ‐0.27 (‐1.09 to 0.54) | |||||||
| Pooled results: mean (95% CI) OR | All models | 0.76 (0.34 to 1.72) | |||||||
| (1 = no difference) | |||||||||
NA = not available in paper.
Hospital admission
Hospital admission was reported in three studies (Table 9). None of these found a significant difference between DSCG and placebo. Pooling the results of the parallel studies did not result in a significant difference (OR 0.93, 95% CI 0.40 to 2.56).
9. Hospital admittance (ln (OR hospital) DSCG/placebo).
| Study | n | Rho from paper | Rho imputed or paper | Diff (c‐p) | SE from paper | SE imputed or paper | 95% CI left | 95% CI right | Imputations from |
| Bertelsen 1986 | 58 | 0 | 0 | ‐0.07 | 0.55 | 0.55 | ‐1.17 | 1.03 | — |
| Furfaro 1994 | 31 | 0 | 0 | ‐0.07 | 1.46 | 1.46 | ‐2.97 | 2.85 | — |
| Glass 1981 | 14 | NA | NA | NA | NA | 8.14 | ‐20.91 | 11.00 | — |
| Pooling parallel studies (Bertelsen and Furfaro) | |||||||||
| Homogeneity test | Chi2 = 0, P = 1.0 | ||||||||
| Pooled results: mean (95% CI) (ln (OR)) | Fixed‐effect model | ‐0.07 (‐1.08 to 0.94) | |||||||
| (0 = no difference) | Random‐effects model | ‐0.07 (‐1.08 to 0.94) | |||||||
| Pooled results: mean (95% CI) OR | All models | 0.93 (0.40 to 2.56) | |||||||
| (1 = no difference) | |||||||||
NA = not available in paper.
Lung function parameters
Thirteen studies assessed lung function parameters. Eight of these reported no statistically significant difference between DSCG and placebo groups/periods, sometimes without providing exact figures. The variety of parameters, methods used, time of day tests were performed and the way they were presented made it impossible to pool data. Five of the 13 studies reported differences to be statistically significant for one or more lung function parameters (Geller 1983; Hiller 1975; Limburg 1971; Matthew 1977; Yuksel 1992).
Side effects
Twelve studies did not report on side effects (Table 10). The reported side effects of DSCG and placebo in the other 11 studies were mild and of short duration (minutes to a few days). Overall, differences between DCSG and placebo were small.
10. Side effects reported in included studies.
| Study ID | Side effects DCSG | Side effects placebo |
| Bertelsen 1986 | Eczema oral (1) Cough (1) | Cough (3) |
| Cogswell 1985 | Not mentioned | Not mentioned |
| Collins 1971 | Bitter taste (20) Cough (11) Dry mouth (4) Dizziness (2) Nausea (2) Sore throat (0) Headache (2) | Bitter taste (13) Cough (1) Dry mouth (2) Dizziness (0) Nausea (0) Sore throat (1) Headache (0) |
| Dalene 1977 | Not registered | Not registered |
| Easton 1973 | Not mentioned | Not mentioned |
| Edmunds 1980 | Nausea, vomiting, abdominal pain, headache 5% | Nausea, vomiting, abdominal pain, headache 5% |
| Furfaro 1994 | Not mentioned | Not mentioned |
| Geller 1982 | Not mentioned | Not mentioned |
| Geller 1983 | None | Throat irritation (1) |
| Glass 1981 | Well‐tolerated | Well‐tolerated |
| Henry 1984 | Not mentioned | Not mentioned |
| Hiller 1975 | Not mentioned | Not mentioned |
| Hiller 1977 | Not mentioned | Not mentioned |
| Hyde 1970 | Duration mild side effect less than 5 minutes Throat irritation (4) Headache (1) Brief coughing (4) Wheezing (2) | Cough (1) Wheezing (1) Headache (1) |
| Kobayashi 1970 | No side effects | No side effects |
| Limburg 1971 | Cough (2) | Cough (1) |
| Matthew 1977 | Not mentioned | Not mentioned |
| Mikawa 1986 | Mild nausea (1) | Mild nausea (1) Mild sore throat (1) |
| Shioda 1970 | Mild Perioral dermatitis (3) Headache (1) | None |
| Smith 1970 | Not mentioned | Not mentioned |
| Tasche 1997 | Mild side effects (40) Eczema mask (5) Cough after inhalation (9) | Mild side effects (33) Eczema (0) Cough after inhalation (1) |
| Tuchinda 1974 | No side effect experienced | No side effect experienced |
| Yuksel 1992 | Not mentioned | Not mentioned |
| Yuksel 1993 | Not mentioned | Not mentioned |
Subgroup analysis
Subgroup analyses were performed for four outcome measures: day time wheeze (10 studies), overall asthma symptom/severity score (10 studies), bronchodilator use (10 studies) and a combination of outcome measures using the same scale (19 studies, see 'Data collection and analysis'). For day time wheeze and for bronchodilator use, the differences between subgroups were either not significant or one of the groups contained only one or two studies. For the asthma symptom/severity score, the age of the children and duration of follow up showed statistically significant differences. Studies that included children with a mean age lower than five showed less effect than studies that (also) included older children (estimate of difference between DSCG and placebo ‐0.06 (95% CI ‐0.15 to 0.02) versus ‐0.30 (95% CI ‐0.49 to ‐0.11), favouring DSCG, P = 0.03). The three studies that had at least three months follow up showed less effect than the eight shorter studies (0.04 versus 0.27, favouring DSCG, P = 0.01).
The combined outcome measure showed subgroup differences for way of administration of the drug, hospitalisation, age and duration of follow up. Studies that applied nebulised DSCG showed less effect than studies that used other methods of administration (0.08 versus 0.32 on a 0 to 3 point symptom scale, P = 0.01). Studies in hospitalised patients showed less effect than studies in other patients (0.08 versus 0.34, P = 0.01). Subgroup analyses for age and for duration of follow up both showed differences of the same magnitude and in the same direction for the combined outcome measure as reported above for asthma symptom/severity score.
The subgroup analyses for the above mentioned four outcome measures were also performed separately for studies with higher methodological quality (see 'Data collection and analysis' for cut‐offs). Comparing the results of this subgroup of studies to the overall results revealed only minor differences, in the same direction as in our primary analysis, sometimes more in favour of the subgroup of better quality studies.
The same analyses were done excluding cross‐over studies that did not take account of period effects (or did not report they did). For the asthma symptom score (five studies) the pooled difference was ‐0.06, with 95% CI (‐0.16 to 0.03) (random‐effects model). For bronchodilator use (four studies) the pooled difference was ‐0.05 doses (‐0.12 to 0.02) (fixed‐effect), the random‐effects model gave ‐0.08 (‐0.19 to 0.04), all not statistically significant. Both these outcomes are smaller than found for the whole group of studies (see Tables). For the combined outcome measures (see 'Data collection and analysis') nine studies provided data. The mean difference was ‐0.20, with random‐effects, 95% CI ‐0.49 to 0.09.
Funnel plot
For the funnel plot, showing the mean difference in effect between DSCG and placebo treatment against precision of the study, we could include 19 studies. The symmetry test gave a value of ‐1.95 for the constant (SE 1.12, P = 0.09), which means that the hypothesis of symmetry was rejected. Especially imprecise (small or heterogeneous) studies with results favouring placebo were under‐represented (Figure 7).
7.
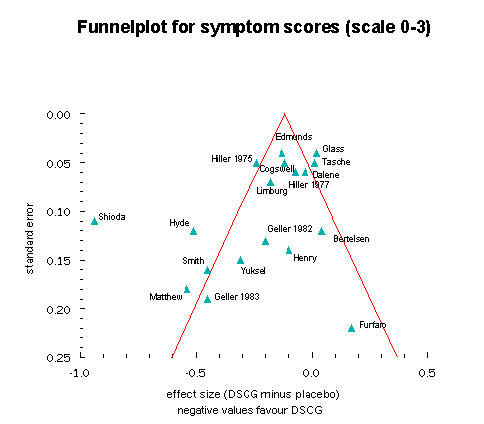
Meta‐regression analysis and functional relationships
Seven study characteristics showed relationships with the (combined) outcome variable (P < 0.25). As only 19 studies provided data for this combined outcome measure, the power of a meta‐regression analysis would be very low. Furthermore, several study characteristics were strongly related to each other (e.g. age, publication year, and method of administration of DSCG). Hence, we decided to refrain from the planned analysis.
There was no influence of placebo symptom level on study outcomes (day cough, day wheeze, overall severity score and bronchodilator use), assessed by means of functional relationships.
Discussion
Summary of main results
This systematic review, involving 1026 children in 23 trials performed between 1970 and 1997, provides conflicting evidence regarding the superiority of DSCG over placebo in children with asthma. There is no evidence to support the superiority of DSCG over placebo in the percentage of symptom‐free days, the main outcome of this review, although this is limited by the small number of trials reporting on this outcome.
For several secondary outcomes, especially symptom scores and bronchodilator use, we found significant group differences between DSCG and placebo, favouring DSCG. The overall treatment effect for these outcomes appears to be quite small, with a mean difference of 0.2 to 0.3 symptom score on a scale from 0 to 3 and less than ¼ puff per day for bronchodilator use. However, considering the confidence intervals of the outcome measures, a clinically relevant effect of sodium cromoglycate cannot be excluded.
For mild persistent asthma, evidence is only available for children below the age of four. For this subgroup, we can rule out important benefit in terms of symptom scales but not in terms of symptom‐free days. We cannot rule out the possibility that DSCG is of benefit in children above the age of four.
Overall completeness and applicability of evidence
Although DSCG has been advocated as maintenance treatment for mild to moderate asthma, and nowadays only for mild persistent asthma (GINA 2005), nearly all trials were hospital based, and included children with moderate to severe asthma. Three studies appear to have included a considerable proportion of children with mild asthma (Edmunds 1980; Furfaro 1994; Tasche 1997). The study by Edmunds showed positive outcomes on four outcome measures but was methodologically weak. The two other studies had negative conclusions, i.e. DSCG was not more effective than placebo. Both studies were carried out in young children (below the age of four). Studies in children above the age of five found more favourable effects than studies in children below that age.
In nine studies, the drug was administered with a nebuliser. Spinhalers were used in eight studies. Metered dose inhalers with spacer devices, nowadays the preferred method of administration for young children, were used in only two studies (Tasche 1997; Yuksel 1992). The method of administration, a critical factor in delivery of drugs to the lungs, was a predictor of outcome (combined outcome measure): studies that used nebulisation showed less effect than studies that used other methods.
The year of publication of the study and the age of the children turned out to be strongly related. In multivariate analysis, results proved to be instable, sometimes favouring age, sometimes publication year. It is impossible to disentangle these two factors: in the early days of DSCG, studies were carried out in older children and only after 1977 did studies start to include children below the age of four.
Quality of the evidence
Heterogeneity of study results is apparent for several outcome measures. The methodological quality of the studies, especially regarding sequence generation and concealment of allocation, was often impossible to assess (see 'Risk of Bias' table Figure 1), and varied considerably for other aspects.
The absence of small trials favouring placebo, as shown in the funnel plot, indicates possible publication bias. This bias is likely to result in an overestimation of the efficacy of DSCG, especially because when applying a random‐effects model the small positive studies we included received a relatively large weight.
Potential biases in the review process
It has been questioned whether the (difference in) percentage of symptom‐free days should be the primary outcome measure, given the fact that only a minority of studies included this (see Feedback (Edwards et al)). However, we believe that the choice of primary outcome measure should not be driven by availability, but by clinical relevance. We feel supported by national and international guidelines, where the aim of the treatment of asthma focuses on leading a normal life with few or no complaints.
Lung function parameters could not be aggregated due to incomplete reporting of data.
Agreements and disagreements with other studies or reviews
The effects of treatment with DSCG have been reviewed previously. As early as 1974 a narrative review was published in JAMA (Dykes 1974), based on data provided by the manufacturers, but giving no references to published data. Edwards 1994 examined the evidence for the anti‐inflammatory action of DSCG in adults and children in a large number of controlled and uncontrolled studies but it is unclear how these were selected. Hoag 1991 summarised studies on the effect of DSCG on bronchial hyperreactivity in adults and children. Schweitzer 1994 discussed the role of DSCG in children below two years of age and concluded that evidence was lacking; this conclusion was shared by Carlsen 1996. Holgate 1996 reviewed recent trials with metered dose inhalers in children and adults and discussed challenge studies, therapeutic studies, and the long‐term effects of DSCG. Other reviews were Berman 1983; Carlsen 1996; Church 1985; Kuzemko 1989; Shapiro 1985; Storms 2005. None of the reviews mentioned above were systematic, assessed the methodological quality of studies or tried to quantify treatment effects. With the exception of Schweitzer 1994, all of these reviews came to conclusions in favour of DSCG.
Our group published a systematic review of inhaled DSCG as maintenance therapy in children in 2000 (Tasche 2000). The current review differs from the previous one in several respects. Seven studies that were included in the previous review were excluded in this one, either because of different exclusion criteria, especially regarding continuous use of steroids (Crisp 1974; Fox 1972; Hyde 1973; Miraglia 1982; Sly 1970), or because we initially overlooked the fact that the placebo drug contained isoprenaline and hence was not a true placebo (Silverman 1972; Smith 1968). The current review included six studies that were not included in the previous one, because of more thorough searching and the withdrawal of language restrictions (Easton 1973; Dalene 1977; Kobayashi 1970; Mikawa 1986; Smith 1970; Tuchinda 1974). Another important difference is that the previous review only considered symptom scores for cough and wheeze as outcome measures. The overall results of the previous and the first version of the current review are similar. For the 2007 update, changing our focus for the pooled results from the tolerance interval to the random‐effects model interval has slightly affected the interpretation of our results in favour of DSCG. For this update, we excluded Kraemer 1993, for reasons mentioned above.
The funnel plot was similar to the one published in our earlier review (for 'wheeze'), although a different outcome was used in order to include as many studies as possible (Figure 7). As we have put forward before, when discussing our previous review (Tasche 2000; Tasche 2001), publication bias may be an explanation for the asymmetry. More specifically we think it is possible that small studies that did not find a beneficial effect for DSCG may not have been submitted to journals, or may not have been published.
In order to appreciate the results of this review in the context of other relevant treatments for childhood asthma, we refer to several recently published Cochrane reviews (Adams 1999; Arnold 2008; Guevara 2006; Gøtzsche 2008; Manning 2008; Seddon 2006; Sridhar 2006).
The possibility of publication bias could be further explored by trying to obtain information about studies that have been performed but were never published. However, since most studies we traced were published more than 20 years ago, and expecting unpublished studies to be at least as old, this does not appear to be a very promising endeavour.
This review only addressed DSCG as maintenance therapy in childhood asthma. Other studies have investigated the role of DSCG in attenuating exercise‐induced bronchoconstriction, but we are unaware of a systematic review comparing DSCG to placebo for this condition. Indirect evidence from two systematic reviews in this area suggests that DSCG may be beneficial in both adults and children (Kelly 2003; Spooner 2003).
Authors' conclusions
Implications for practice.
A considerable number of trials has been performed. Together, they show heterogeneous effects for DSCG compared to placebo as maintenance therapy for childhood asthma. Given the strong indication of publication bias, the small overall treatment effect, and the pooled confidence intervals including zero for our primary outcome measure and several others, we conclude that it is not justified to recommend DSCG as first line maintenance therapy in childhood asthma. This recommendation is further supported by the availability of alternatives with proven effectiveness, i.e. inhaled corticosteroids. For mild persistent asthma evidence is only available for children below the age of four. For this subgroup, there is no good evidence that DSCG is much more effective than placebo. We cannot rule out the possibility that DSCG is of benefit in older children.
Implications for research.
Given the place of DSCG in current guidelines, the lack of studies in children from age four onwards with mild persistent asthma is surprising. A large parallel study in this group, of high methodological quality and extended follow up (at least six months), could fill this gap. Preferably, such a study should not only compare DSCG to placebo, but also contain a study arm with low dose inhaled steroids. As the primary outcome measure we would recommend symptoms, either as a symptom score or as a percentage of symptom‐free days. Given ongoing concern about the side effects of inhaled steroids, such a study should also address secondary outcomes like growth, adrenal function and bone density. Leukotriene‐modifying drugs would be another class of drugs that could be compared to DSCG.
Feedback
Criticism of conclusions and methods
Summary
1. The primary outcome measure, symptom free days is directionally in favour of SCG in 3 of 4 studies. The results are dominated by one study in which we doubt whether the dosage was adequate. 2. Of the 16 secondary outcome measures, 8 were statistically significant in favour of SCG. None were in favour of placebo. 3. Of the 17 outcome measures, 11 are to be found in less than 5 studies. Of the 6 outcome measures that included 5 or more studies, 4 are statistically significantly in favour of SCG. None are in favour of placebo. 4. The presentation of the results is misleading. 5. Three methods of drug delivery are included with a dose range of 1 mg 3 times daily to 40 mg 3 times daily. No account is taken of the consequences of efficacy on this dose range. There is ample evidence that 2 of the delivery systems, pMDI and nebulization may not provide an adequate dose particularly in children below the age of 5 years. 6. The diagnosis of asthma is difficult to make with confidence in children below the age of 5 years. At least half the included studies are in children in this age group. Drug delivery also presents problems in this age group. 7. A number of relevant studies have been excluded. We have identified 16 studies that should have been included. Five were excluded as they apparently included subjects over the age of 17 years. Our examination of the papers shows this either not to be the case or results for subjects below 18 years were presented separately. These studies should have been included. The exclusion of studies due to some children being on regular steroid therapy is not justified in those studies in which the steroid dose was kept fixed. If this exclusion was consistent, 2 further studies should have been excluded. It is doubtful if the exclusion of studies in which a fixed dose of bronchodilator was added to both SCG and placebo treatment arms is justified if this review is considered to be representative of SCG in childhood asthma.
Reply
We have replied to most of these criticisms before in response to letters by Edwards et al (2002), commenting on a previous version of our review, published in Thorax. Our conclusions are based on both the fact that the confidence interval that we a priori chose to be our guidance (the tolerance interval) does include 'no effect' for most of the outcome measures as well as the strong suspicion of publication bias, as reflected in the funnel plot.
The fact that the diagnosis of asthma is difficult in young children and drug delivery may pose problems, does not mean that doctors should not treat these children, nor does it mean that investigators should not assess the effectiveness of therapeutic options in this group of children. The protocol of our review was clear in excluding studies that included patients over the age of 17 years. In this update, we excluded the study by Kraemer et al for the reasons suggested by the authors of the criticism.
Contributors
JBL Howell, MT Stevens, AM Edwards, N Åberg, B Callaghan, S Godfrey, ST Holgate, P König, A Morikawa, D Reinhardt, B Stenius‐Aarniala, JO Warner, Weinberg
Criticism of updated review, 7 July 2010
Summary
The current version of the review addresses many concerns which we submitted as a comment previously. We thank the authors for addressing our comments and producing a clearer presentation of the results of their work.
Our criticisms of this review, the previous review and earlier papers on which they were based relate to the statistical methods used, the presentation of the data the interpretation of the data and the conclusions drawn. A full account of our criticisms can be found in paper by three of us [1]. This was accompanied by two commentaries by statisticians in the same journal [2,3].
The first Cochrane review concluded that 'The evidence of the efficacy of sodium cromoglicate (DSCG) over placebo is not proven'. In the latest review, the conclusion is 'There is insufficient evidence to be sure about the efficacy of (DSCG) over placebo'. This is a justified shift in stance, but still understates the case for DSCG. Although there was no statistically significant difference between DSCG and placebo on the primary outcome (symptom‐free days), seven secondary outcome variables for which data were available in four or more studies were, according to the authors, all in favour of DSCG, and six were statistically significant. Rather than providing 'conflicting evidence regarding the superiority of DSCG over placebo' we believe the review provides overwhelming evidence for the efficacy of DSCG compared to placebo. We do not believe that the authors have fully justified their choice of the primary outcome, given the low power of the of this outcome in that only four studies were included, and one study dominated these results. In one of the commentaries to our paper the author states 'it seems inappropriate to put major emphasis on the meta‐analysis of a primary outcome that is reported in very few of the trials' [3] They have also not provided an evidence‐based response to the criticism that the dose used in this study was probably inadequate.
The authors claim that there may be publication bias, yet this is only weakly supported by their funnel plots which are potentially subject to criticism as they include different outcome variables.
The size of the overall treatment effect is claimed to be small but should be viewed in the light of the mild symptoms experienced by the children. On‐treatment mean symptom scores, where given, were less than one (on a 0‐3 scale) in both DSCG and placebo treatment groups. Given the relatively low margin for improvement, the treatment effects seen are indeed relevant.
Based on the above, it is surprising that the authors conclude that 'it is not justified to recommend DSCG as first line therapy in childhood asthma' (the objective was in any case to assess maintenance therapy). The drug has established evidence of safety and efficacy in a wide number of indications, and has a role in both first line and maintenance therapy.
The authors conclude that 'a clinically relevant effect of DSCG cannot be excluded'. We suggest that this review provides strong support for the beneficial effect of DSCG over placebo in childhood asthma, particularly those over four years of age.
1. Stevens MT, Edwards AM, Howell JBL. Sodium cromoglicate: an ineffective drug or meta‐analysis misused? Pharmaceut Statist 2007; 6: 123‐137.
2. Lewis S, Deeks J. Re Sodium Cromoglicate: An Ineffective Drug or Meta‐analysis Misused? Pharmaceut. Statist. 2007; 6: 139–140
3. Lewis JA. Comment on sodium cromoglicate: an ineffective drug or meta‐analysis misused? by Stevens et al.; Pharmaceut. Statist. 2007; 6: 141–143
Reply
We thank Dr Edwards and colleagues for their continued interest in our review. The points raised in their comment are not new, and we have carefully considered these when updating our review. As we have already clarified in previous replies, changing the primary endpoint of our review due to its infrequent availability relative to other measurements in the studies would violate elementary methodological principles.
Contributors
A M Edwards, M T Stevens, S T Holgate, SD Anderson, JBL Howell.
Declaration of interest: AME was employed by the originators or sodium cromoglicate, Fisons Pharmaceuticals from 1974 to 1995. MTS was employed by Fisons Pharmaceuticals from 1968 to 1996. STH, SDA and JBHL have all conducted clinical trials with inhaled sodium cromoglicate in the past. None have any financial interest or connection with the current manufacturers.
What's new
| Date | Event | Description |
|---|---|---|
| 17 November 2010 | Feedback has been incorporated | Feedback has been received and appended to the review. The authors have responded to the feedback, but there have been no changes made to the review. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 28 July 2010 | New search has been performed | Literature search re‐run, no new included studies found. Two new excluded studies found. |
| 7 October 2009 | New search has been performed | Litertaure search re‐run; no new studies found. |
| 27 February 2009 | Amended | Risk of bias tables completed, copy edited table of included study |
| 25 October 2008 | New search has been performed | In response to external peer review: Modified overall description of outcomes, not excluding a clinically relevant benefit. Added forest plots based on values provided in Additional tables 3‐19, we could not use the forest plots provided in RevMan, as we assumed a t‐distribution when calculating confidence intervals for individual studies. Yuksel 1992 and 1993 combined, as these papers refer to the same study. |
| 30 May 2008 | Amended | Converted to new review format. |
| 19 December 2007 | New citation required and conclusions have changed | In response to comments by Edwards et al, one study was excluded (Kraemer (1993)). Searches performed for years 2003‐2007 did not reveal any new studies, but did lead to new 'excluded studies'. Paragraph and table added on side effects as reported in included studies. Paragraph 'other reviews' in Discussion was extended. Tolerance intervals for pooled results removed. Risk of Bias tables added. Discussion rewritten. |
| 1 November 2007 | New search has been performed | Literature search re‐run in November 2007 |
Acknowledgements
We are grateful to the following people for:
searching literature databases: Louis Volkers (Erasmus MC), Karen Blackhall, Liz Arnold (Cochrane Airways Group);
tracing papers: Philippa Mills (Cochrane Airways Group);
providing studies: Rhone‐Poulenc‐Rorer, formerly Fisons plc, Loughborough, UK (Ivo Knottnerus), Alan Edwards;
their help in translating papers from foreign languages: Toby Lasserson (Cochrane Airways Group, coordination), Keiji Hayashi and Meg Meguro (Japanese), Helena Varonen (Finnish), Diego Martínez de la Concha (Spanish), Dan Peretianu (Rumanian), Luca Richeldi (Italian), Vasily Vlassov (Russian), Charlotta Pisinger (Czech), Translingua Rotterdam;
scoring the methodological quality of our own trial: Sita Bierma‐Zeinstra;
statistical advice: Theo Stijnen, Nico Nagelkerke;
providing feedback on the synopsis: Alison Whitton.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertelsen 1986.
| Methods | DESIGN Parallel‐group METHODOLOGICAL QUALITY Chalmers score 31/95; Jadad score 3 WITHDRAWALS/ DROPOUTS 5 | |
| Participants | SETTING Hospital outpatients? AGE 1 to 4 years INCLUSION CRITERIA Recurrent wheezy bronchitis demanding treatment at least once a month during preceding winter or later N = 59 | |
| Interventions | 4 to 8 weeks baseline 10 weeks treatment 3 dd 20 mg Nebulised | |
| Outcomes | Day wheezing Day cough Sleep disturbance Bronchodilator use Hospital admissions | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Low risk | 10 weeks: 5/59 missing, reasons provided, well balanced across groups |
| Free of selective reporting? | Unclear risk | Protocol not available |
| Free of other bias? | Unclear risk | Unclear |
Cogswell 1985.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 43/91; Jadad score 4 WITHDRAWALS/ DROPOUTS 3 | |
| Participants | SETTING Hospital outpatients? AGE 1 to 4 years INCLUSION CRITERIA Regular attacks of asthma that required at least one admission to hospital N = 27 | |
| Interventions | 4 weeks baseline, 2 x 26 weeks cross‐over treatment 4 dd 20 mg nebulised | |
| Outcomes | % Symptom‐free days Day cough Day wheeze Day activity Night cough Overall asthma severity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'packaged in identical ampoules' |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and 'identical ampoules' |
| Incomplete outcome data addressed? All outcomes | Low risk | 24/27 analysed, reasons for withdrawal provided, seems unbiased |
| Free of selective reporting? | High risk | Protocol not available, choice of one outcome (symptom free days) seems post‐hoc (see Discussion) |
| Free of other bias? | Unclear risk | unclear |
Collins 1971.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 39/94; Jadad score 2 WITHDRAWALS/ DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 7 to 17 years INCLUSION CRITERIA Severe allergic asthma, wheeze at least once a week N = 14 | |
| Interventions | 2 weeks baseline 2 x 4 weeks cross‐over treatment 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores Clinical assessment Lung function | |
| Notes | Nr. of patients differs from previous version of review, due to patients on steroids Study provided no data for meta‐analysis due to incomplete reporting |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'identical' and 'coded by manufacturer' |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' 'physicians nor parents' |
| Incomplete outcome data addressed? All outcomes | Low risk | Apparently no missing data |
| Free of selective reporting? | High risk | No results of hemograms, BUN and SGOT |
| Free of other bias? | Unclear risk | Unclear |
Dalene 1977.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 32/91; Jadad score 3 WITHDRAWALS/ DROPOUTS 2 | |
| Participants | SETTING Hospital outpatients? AGE 1 to 4 years INCLUSION CRITERIA Repeated episodes of virus induced asthma N = 20 | |
| Interventions | 2 x 10 weeks Cross‐over treatment 4 dd 2 ml 1% solution Nebulised | |
| Outcomes | Day cough Day wheeze Auscultation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | High risk | 2/20 did not complete, unclear in which group |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Easton 1973.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 35/87 = 40%; Jadad score 2 WITHDRAWALS/ DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE Children of unspecified age INCLUSION CRITERIA Daily extrinsic asthma, stable symptoms, total blood eosinophil counts > 500 cells/cu mm N = 25 | |
| Interventions | Baseline period unspecified 2 x 3 weeks cross‐over treatment 4 dd 20 mg capsule | |
| Outcomes | Total eosinophil count | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote 'double‐blind' and 'identical placebo' |
| Incomplete outcome data addressed? All outcomes | High risk | One patient seems missing from figure. Not mentioned in text |
| Free of selective reporting? | Unclear risk | Protocol not available |
| Free of other bias? | Unclear risk | Unclear |
Edmunds 1980.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 29/91; Jadad score 2 WITHDRAWALS/ DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 5 to 15 years INCLUSION CRITERIA Perennial asthma N = 30 | |
| Interventions | 3 x 4 weeks (incl. additional treatment) Cross‐over 4 dd 1 capsule | |
| Outcomes | Symptom score % Symptom‐free days | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Unclear risk | N of results is unclear |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Furfaro 1994.
| Methods | DESIGN Parallel‐group METHODOLOGICAL QUALITY Chalmers score 56/95; Jadad score 4 WITHDRAWALS/ DROPOUTS 6 | |
| Participants | SETTING Outpatients referred to pulmonary clinic AGE 0 to 1 years INCLUSION CRITERIA Chronic pulmonary symptoms for at least one month and wheezing documented by a physician + symptoms in baseline period N = 37 | |
| Interventions | 3 weeks baseline, 6 weeks treatment 3 dd 40 mg nebulised | |
| Outcomes | Symptom score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Table of random numbers |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | High risk | 3 parental withdrawn, 3 poor compliance, group assignment of these children unclear |
| Free of selective reporting? | Low risk | Comprehensive listing of outcome measures |
| Free of other bias? | Unclear risk | No details |
Geller 1982.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 34/91 = 37%; Jadad score 4 WITHDRAWALS/ DROPOUTS 5 | |
| Participants | SETTING Hospital outpatients? AGE 0 to 2 years INCLUSION CRITERIA Frequent troublesome wheezy bronchitis despite regular bronchodilator therapy + symptoms in baseline period N = 49 | |
| Interventions | 2 weeks baseline 2 x 4 weeks cross‐over treatment 4 dd 2 ml nebulised | |
| Outcomes | Symptom score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind', 'matching placebo' |
| Incomplete outcome data addressed? All outcomes | High risk | 44/49 analysed, 'one withdrawn while failing to improve during placebo period' |
| Free of selective reporting? | Unclear risk | Protocol not available |
| Free of other bias? | Unclear risk | Unclear |
Geller 1983.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 41/90; Jadad score 4 WITHDRAWALS/ DROPOUTS 5 | |
| Participants | SETTING Hospital outpatients? AGE 4 to 13 years INCLUSION CRITERIA Moderately severe or severe extrinsic asthma for at least 12 months, not taken DSCG or steroids for at least 6 months before trial N = 48 | |
| Interventions | 2 weeks baseline 2 x 6 weeks treatment 4 dd 2 mg aerosol | |
| Outcomes | Symptom score Asthma severity score Lung function Patients', parents' and physicians' preferences | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | High risk | 43/48, 5 withdrawn, 'one failed to improve on placebo' |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Glass 1981.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 29/94; Jadad score 2 WITHDRAWALS/ DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 1 to 4 years INCLUSION CRITERIA Poor control of asthma under routine treatment N = 16 | |
| Interventions | 4 weeks baseline, 3 x 8 weeks cross‐over treatment incl. additional study arm 4 dd 20 mg nebulised | |
| Outcomes | Cough Wheeze Activity Sleep disturbance Additional treatment, Hospital admission Parental preference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | High risk | Tables seem to be based on fewer than 16 children (first sentence Results) |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Henry 1984.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 23/94; Jadad score 2 WITHDRAWALS/ DROPOUTS 3 | |
| Participants | SETTING Hospital outpatients? AGE 0 to 1 years INCLUSION CRITERIA Suffered from recurrent attacks of wheezing, asthma considered troublesome by paediatricians and parents N = 23 | |
| Interventions | 2 weeks baseline, 3 x 8 weeks cross‐over treatment incl. additional study arm 3 dd 20 mg nebulised | |
| Outcomes | Wheeze Cough % Symptom‐free days | |
| Notes | Number of withdrawals probably higher | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 20/23 analysed, unclear whether withdrawal was related to outcome |
| Free of selective reporting? | High risk | Biased description of favourable results in some individual patients (p. 56) |
| Free of other bias? | Unclear risk | Unclear |
Hiller 1975.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 44/87; Jadad score 4 WITHDRAWALS/ DROPOUTS 2 | |
| Participants | SETTING Hospital outpatients? AGE 9 to 13 years INCLUSION CRITERIA Chronic perennial asthma, symptoms inadequately controlled by DSCG and bronchodilators N = 11 | |
| Interventions | 4 x 1 month cross‐over treatment, including 2 additional treatment arms 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores Clinical assessment Additional medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'packed and coded by manufacturers' |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | High risk | 2/23 withdrawn because of severe symptoms |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Hiller 1977.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 34/87; Jadad score 3 WITHDRAWALS/DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 2 to 4 years INCLUSION CRITERIA Frequent troublesome asthma N = 17 | |
| Interventions | 1 week baseline 2 x 8 weeks cross‐over treatment 3 dd 20 mg nebulised | |
| Outcomes | Daily symptoms Clinical assessment Lung function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'coded by manufacturers' |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Unclear risk | All children completed the trial, for 3 children no peak flow |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | unclear |
Hyde 1970.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 38/87; Jadad score 3 WITHDRAWALS/ DROPOUTS 3 | |
| Participants | SETTING Hospital outpatients? AGE 6 to 16 years INCLUSION CRITERIA Duration of asthma > 1 year, definite symptoms before inclusion N = 60 | |
| Interventions | 2 x 3 weeks Cross‐over treatment 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores Clinical assessment Lung function Additional treatment Eosinophil level | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'Labels code' (p. 450‐1) |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and taste |
| Incomplete outcome data addressed? All outcomes | Low risk | 57/60 completed, withdrawals due to failure to cooperate or keep adequate records |
| Free of selective reporting? | Low risk | All outcomes seem to have been addressed |
| Free of other bias? | Unclear risk | unclear |
Kobayashi 1970.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 45/90; Jadad score 3 WITHDRAWALS/DROPOUTS 7 | |
| Participants | SETTING Hospital outpatients AGE 6 to 15 years INCLUSION CRITERIA Moderate to severe asthma N = 37 | |
| Interventions | 1 to 2 weeks baseline 2 x 4 weeks cross‐over treatment 3 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom score Physician's and patient's impression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and 'code remained unbroken' |
| Incomplete outcome data addressed? All outcomes | Low risk | 7/37 withdrawn, seemingly unrelated to outcome |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | Unclear |
Limburg 1971.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 45/94; Jadad score 3 WITHDRAWALS/ DROPOUTS 1 | |
| Participants | SETTING Asthma centre, inpatients AGE 6 to 16 years INCLUSION CRITERIA Regular asthma symptoms N = 30 | |
| Interventions | 2 x 4 weeks 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores Lung function Additional treatment Eosinophilia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and 'matching placebo' |
| Incomplete outcome data addressed? All outcomes | Low risk | 1/30 withdrawn nothing to do with this therapy (p. 368) |
| Free of selective reporting? | Low risk | All outcomes seem to have been reported |
| Free of other bias? | Unclear risk | unclear |
Matthew 1977.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 29/87; Jadad score 3 WITHDRAWALS/ DROPOUTS 1 | |
| Participants | SETTING Hospital outpatients AGE 3 to 6 years INCLUSION CRITERIA Severe chronic perennial asthma + symptoms in baseline period N = 10 | |
| Interventions | 8 weeks baseline, 2 x 4 weeks cross‐over treatment 4 dd 20 mg nebulised | |
| Outcomes | Daily symptom scores Clinical assessment Lung function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | 'double‐blind' and 'placebo identical' |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Initial number of patients unclear and probably greater than 9 for which data are reported (Nine children completed? p. 36) |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | unclear |
Mikawa 1986.
| Methods | DESIGN Parallel‐group METHODOLOGICAL QUALITY Chalmers score 43/92; Jadad score 5 WITHDRAWALS/DROPOUTS 49 | |
| Participants | SETTING Hospital outpatients? AGE 6 to 15 years INCLUSION CRITERIA Mild to moderate asthma N = 196 | |
| Interventions | 2 weeks baseline 4 weeks treatment 4 dd 20 mg aerosol | |
| Outcomes | Symptom scores Side effects Patients' and parents' assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'randomly allocated six in a group, key codes sealed and kept by controller' |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and 'matching placebo' |
| Incomplete outcome data addressed? All outcomes | Low risk | 145/196 completed. Reasons for exclusion well described, these seem unrelated to outcome (table 1) |
| Free of selective reporting? | Low risk | All outcomes seem to be reported |
| Free of other bias? | Unclear risk | Unclear |
Shioda 1970.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 42/95; Jadad score 3 WITHDRAWALS/ DROPOUTS 1 | |
| Participants | SETTING Hospital, inpatients and outpatients AGE 6 to 15 years INCLUSION CRITERIA Perennial asthma N = 34 | |
| Interventions | 2 x 4 weeks cross‐over treatment 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores Lung function Clinical assessment Additional medication School absence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' and 'neither patients, parents nor clinicians were aware' |
| Incomplete outcome data addressed? All outcomes | Low risk | 1/34 withdrawn, unrelated to outcomes (although probably a side‐effect) |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | unclear |
Smith 1970.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 43/92; Jadad score 4 WITHDRAWALS/DROPOUTS 3 | |
| Participants | SETTING Hospital outpatients? AGE 7 to 16 years INCLUSION CRITERIA Hay fever and pollen asthma confirmed by skin prick tests N = 18 | |
| Interventions | 4 weeks 4 dd 20 mg Spinhaler | |
| Outcomes | Daily symptom scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | List of random numbers |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 15/18 completed (368), unclear in which group |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | unclear |
Tasche 1997.
| Methods | DESIGN Parallel‐group METHODOLOGICAL QUALITY Chalmers score 75/95; Jadad score 3 WITHDRAWALS/ DROPOUTS 14 | |
| Participants | SETTING General practice AGE 1 to 4 years INCLUSION CRITERIA Previously been prescribed asthma medication and meeting criteria for moderate asthma N = 232 | |
| Interventions | 4 weeks baseline 22 weeks treatment 3 dd 10 mg aerosol + spacer (Aerochamber) + face mask | |
| Outcomes | % Symptom‐free days Daily symptom scores Additional medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Low risk | Quote: 'treatment allocation was concealed from parents, patients, GPs, research physician and nurses' (p. 1061) |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind' |
| Incomplete outcome data addressed? All outcomes | Low risk | 167/218 completed study. Equally divided and not related to primary outcome (Fig 1) |
| Free of selective reporting? | High risk | All outcome measures seem to be reported |
| Free of other bias? | Unclear risk | unclear |
Tuchinda 1974.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 49/86; Jadad score 4 WITHDRAWALS/ DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 7 to 12 years INCLUSION CRITERIA Chronic asthma N = 17 | |
| Interventions | 2 x 4 weeks cross‐over treatment 4 dd 20 mg Spinhaler | |
| Outcomes | Perceived improvement Medication score Lung function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | Quote: 'double‐blind', 'identical in taste and color' |
| Incomplete outcome data addressed? All outcomes | Low risk | All patients completed the study |
| Free of selective reporting? | Unclear risk | No results on symptoms |
| Free of other bias? | Unclear risk | Unclear |
Yuksel 1992.
| Methods | DESIGN Cross‐over METHODOLOGICAL QUALITY Chalmers score 32/91; Jadad score 2 WITHDRAWALS/DROPOUTS 0 | |
| Participants | SETTING Hospital outpatients? AGE 0 to 2 years INCLUSION CRITERIA Preterm born, wheeze and/or cough 3 to 4 days/week for previous 4 weeks + symptoms for at least 3 days following respiratory infections N = 16 | |
| Interventions | 2 to 3 weeks treatment 4 dd 5 mg Aerosol + face mask (coffee cup) | |
| Outcomes | Daily symptom scores Additional treatment Lung function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not mentioned |
| Allocation concealment? | Unclear risk | Not mentioned |
| Blinding? All outcomes | Low risk | 'double‐blind' and 'similarly shaped and sized cannister' (Yuksel 1993) |
| Incomplete outcome data addressed? All outcomes | Low risk | All 16 patients completed the study |
| Free of selective reporting? | Unclear risk | Protocol not available, planned outcome measures unknown |
| Free of other bias? | Unclear risk | unclear |
Assessment of concealment of allocation for cross‐over studies applies only to initial allocation. Adequacy of washout period not taken into account because of incomplete reports. Possible unblinding due to perceived differences were not taken into account. Setting = ? where not clearly stated, but deducted from authors' affiliations. dd: doses per day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agbayani 1984 | Double dummy, no placebo arm |
| Anastasatu 1979 | Not an RCT |
| Anonymous 1969 | Included adults as well, but no age specific results |
| Arndt 1975 | Not placebo‐controlled |
| Avital 1991 | Theophylline versus placebo (cross‐over, double dummy), no placebo arm |
| Berman 1975 | Partly > 18 years, no separate results for children |
| Bernstein 1972 | Results for children not presented separately (except patient preference) Continuous use of steroid was allowed |
| Blumenthal 1988 | Results for children not presented separately |
| Bonifazi 1985 | Age 10 to 50, results for children not presented separately |
| Booij‐Noord 1971 | Results for children not presented separately DSCG not as maintenance therapy |
| Bruderman 1990 | Not an RCT |
| Carrasco 1989 | Results for children not presented separately |
| Carrà 2001 | No placebo arm |
| Chai 1973 | All children on steroids continuously |
| Chan‐Yeung 1971 | Only two children |
| Chyrek‐Borowska 1975 | Results for children not presented separately |
| Ciszek 1974 | Results for children not presented separately |
| Crawford 1974 | Incomplete report |
| Crawford 1974b | Most children on steroids continuously (281 doses of 5 mg prescribed in 30 children for 4 weeks) |
| Crimi 1988 | Not maintenance therapy (adenosine‐induced bronchoconstriction) |
| Crisp 1974 | Steroid use was one of the selection criteria, no separate results for non‐users |
| Croce 1995 | Patients 6 to 24 years Results for children not presented separately |
| De Baets 1998 | No placebo arm |
| Dickson 1969 | Open study |
| Droszcz 1973 | Ages 15 to 63 years Results for children not presented separately |
| Edmunds 1994 | No placebo arm |
| Engström 1975 | No placebo for asthma treatment Same data as Engström 1977 |
| Engström 1977 | No placebo arm |
| Exline 1972 | Results for children not presented separately |
| Forster 1998 | Abstract only |
| Fox 1972 | 9 patients were on regular steroids, no separate results for non‐users |
| Friday 1973 | No information on age, included patients above 18 years, no age‐specific results |
| Fuleihan 1973 | Includes both adults and children, no separate results for children |
| Furukawa 1999 | Results for children not presented separately |
| Garcio Velloso 1984 | No information on age |
| Gaur 1997 | No information on blinding (possibly not blinded), no placebo Results on children not presented separately |
| Geller‐Bernstein 198 | No placebo arm |
| Gemicioglu 1993 | Ages 15 to 46, results for children not presented separately |
| Glazer 1971 | No information on age |
| Godfrey 1975 | Study conducted in exercise‐induced bronchoconstriction |
| Gomez‐Orozco 1976 | Not an RCT |
| Graber 1998 | Not original, refers to Tasche 1997 |
| Grifoni 1971 | Age 5 to 63 years Results for children not presented separately |
| Gulyas 1984 | Combined therapy, not DSCG alone |
| Guminski 1976 | No placebo arm |
| Haber 1989 | Patients 16 to 41 years Results for children not presented separately |
| Herjavecz 1982 | No placebo arm Results for children not presented separately |
| Hermance 1973 | Steroid use was continued |
| Hobday 1970 | No placebo arm (isoprenaline) |
| Hyde 1971 | Not an RCT |
| Hyde 1973 | 15 of 57 children were on daily steroids Results for non‐users not presented separately |
| Inoue 1970 | No placebo arm |
| Irani 1972 | Results for children not presented separately |
| Ito 1971 | only one child below age of 18 |
| Jenssen 1973 | Results for children not presented separately |
| Johannessen 1975 | Results for children not presented separately |
| Jones 1970 | Single‐blind |
| Kehnscherper 1993 | No placebo arm |
| Kennedy 1969 | Primary reference gives no information on age Secondary reference gives 10 patients in age range 11 to 20, no specific data |
| Khurana 1977 | Open study |
| Kidner 1968 | Results for children not presented separately |
| Kimmel 1974 | Open study |
| Klein 1980 | No placebo arm |
| Klein 1981 | No placebo arm |
| Knezevic 1997 | (Abstract only) No placebo arm No information on randomisation |
| Kotaniemi 2005 | Not blinded |
| Kraemer 1986 | DSCG single‐blind |
| Kraemer 1987 | Single‐blind with respect to SCG |
| Kraemer 1993 | Daily use of bronchodilator in both arms, irrespective of symptoms |
| Kuzemko 1974 | Study compares 2 active treatments Placebo period not double‐blind |
| Kuzemko 1977 | No placebo |
| König 1973 | Not double‐blind No placebo period |
| Lahoz 1973 | Adults |
| Lecks 1974 | Included children > 17 years of age, probably above 18 years |
| Lenney 1978 | Exercise induced asthma |
| Linehan 1970 | Single‐blind |
| Löwhagen 1985 | Parallel‐group trial with only 1 child |
| Macdonald 1979 | Cromoglycate not compared to placebo |
| Mahashur 1981 | Not double‐blind, not randomised, no separate results for children |
| Marks 1974 | Incomplete results: only patients who had benefit reported |
| Marshall 1969 | Placebo‐controlled study included both children and adults |
| Masood 1978 | Results for children not presented separately |
| Matsumoto 1994 | Not randomised |
| Mattoli 1986 | Allergen‐induced challenge |
| McLean 1973 | Included children > 18 yearrs |
| Mellon 1982 | No placebo arm |
| Menardo 1998 | No placebo arm |
| Miraglia 1981 | Not an RCT |
| Miraglia 1982 | 10 of 31 children use steroids continuously, no separate results for non‐users |
| Mitchell 1976 | No placebo arm |
| Moeller 2009 | No placebo arm |
| Molema 1989 | Results for children not reported separately |
| Moran 1968 | Results for children not presented separately |
| Muittari 1969 | Not randomised |
| Munro Ford 1969 | No information on age, probably adults |
| Naganathan 1975 | 37 patients age 11 to 53 years, 6 patients 11 to 20 years, no age‐specific results |
| Ng 1977 | Study compares 2 active treatments Placebo period not double‐blind |
| Orefice 1990 | Not randomised |
| Pesic 1975 | No placebo arm |
| Petersen 1996 | No placebo arm |
| Price 1995 | Not blinded, no placebo |
| Rafinski 1977 | Not an RCT |
| Rauber 1983 | No placebo arm |
| Reid 1988 | Results for children not presented separately |
| Robertson 1969 | Only 1 child |
| Romano 1970 | No information on ages Description of methods very incomplete No useful results |
| Sarlet 1973 | Not double‐blind No placebo arm |
| Schmidt 1973 | No placebo arm |
| Selcow 1983 | Not an RCT |
| Selcow 1989 | Age 8 to 20 years Results for children not presented separately |
| Sellars 1975 | Age of children > 17 years, above 18? |
| Shioda 1973 | Overlapping data with Shioda 1970, but no data on RCT |
| Shiota 1984 | Adults only |
| Shore 1971 | Not an RCT |
| Sienra Monge 1990 | Not an RCT |
| Silverman 1972 | Not placebo‐controlled (isoprenaline) |
| Sly 1970 | 2 of 21 children used prednisone continuously, results for non‐users not presented separately |
| Smith 1968 | Not placebo‐controlled (isoprenaline) |
| Smith 1980 | Not an RCT |
| So 1981 | Aerosol compared to powder No placebo Ages 12 to 31 |
| Streumer 1970 | Incomplete description of methods, probably not a RCT |
| Thompson 1974 | Age 7 to 28 years Results for children not presented separately |
| Toshner 1974 | 16 patients were on continuous steroids |
| Turpeinen 2010 | not randomised, no placebo arm |
| Varsano 1983 | Upper respiratory tract infections, not asthma |
| Viscardi 1997 | Not asthma |
| Watanabe 1992 | Not an RCT |
| Weinbren 1969 | Isoprenaline is not placebo |
| Wells 1979 | Not maintenance therapy Cat fur challenge |
| Wheatley 1981 | No placebo arm |
| Zarkovic 1991 | Open study No placebo |
Contributions of authors
JCvdW drafted text of protocol and review. MJAT, JHJMU and JCvdW searched papers, assessed inclusion criteria and methodological quality. RMDB and JCvdW extracted data. RMDB performed statistical analysis. JHJMU and JCvdW drafted the 2008 update. All authors commented on versions of the protocol and review.
Sources of support
Internal sources
Department of General Practice, Erasmus MC ‐ University Medical Center Rotterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
The authors were involved in a placebo‐controlled trial (Tasche 1997) and in an earlier systematic review comparing DSCG and placebo (Tasche 2000). Both studies had negative conclusions.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Bertelsen 1986 {published data only}
- Bertelsen A, Andersen JB, Busch P, Daugbjerg P, Friis B, Hansen L, et al. Nebulised sodium cromoglycate in the treatment of wheezy bronchitis. Allergy 1986;41(4):266‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cogswell 1985 {published data only}
- Cogswell JJ, Simpkiss MJ. Nebulised sodium cromoglycate in recurrently wheezing preschool children. Archives of Disease in Childhood 1985;60(8):736‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1971 {published data only}
- Collins‐Williams C, Chiu AW, Lamenza C, Lewis‐McKinley CA, Williams H, Levison H. Treatment of bronchial asthma with disodium cromoglycate (Intal) in children. Annals of Allergy 1971;29(12):613‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Dalene 1977 {published data only}
- Dalene S. Sodium cromoglycate (DCG) "Lomudal" nebuliser solution in asthmatic infants [Dinatriumkromoglykat (DCG) "Lomudal" nebulisatoropploesning hos smaabarn met astma]. Inhal Treat Prophyl Asthma 1977:48‐51. [MEDLINE: ] [Google Scholar]
Easton 1973 {published data only}
- Easton JG. Effect of cromolyn sodium (disodium) cromoglycate on the peripheral eosinophilia of asthmatic children. Annals of Allergy 1973;31(3):134‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Edmunds 1980 {published data only}
- Edmunds AT, Carswel F, Robinson P, Hughes AO. Controlled trial of cromoglycate and slow‐release aminophylline in perennial childhood asthma. British Medical Journal 1980;281(6244):842. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Furfaro 1994 {published data only}
- Furfaro S, Spier S, Drblik SP, Turgeon JP, Robert M. Efficacy of cromoglycate in persistently wheezing infants. Archives of Disease in Childhood 1994;71(4):331‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geller 1982 {published data only}
- Geller‐Bernstein C, Levin S. Nebulised sodium cromoglycate in the treatment of wheezy bronchitis in infants and young children. Respiration 1982;43(4):294‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geller 1983 {published data only}
- Geller‐Bernstein C, Levin S. Sodium cromoglycate pressurised aerosol in childhood asthma. Current Therapeutic Research 1983;34(2):345‐9. [MEDLINE: ] [Google Scholar]
Glass 1981 {published data only}
- Glass J, Archer LNJ, Adams W, Simpson H. Nebulised cromoglycate, theophylline, and placebo in preschool asthmatic children. Archives of Disease in Childhood 1981;56(8):648‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 1984 {published data only}
- Henry RL, Hiller EJ, Milner AD, Hodges IGC, Stokes GM. Nebulised ipratropium bromide and sodium cromoglycate in the first two years of life. Archives of Disease in Childhood 1984;59(1):54‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiller 1975 {published data only}
- Hiller EJ, Milner AD. Betamethasone 17 valerate aerosol and disodium chromoglycate in severe childhood asthma. British Journal of Diseases of the Chest 1975;69(2):103‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiller 1977 {published data only}
- Hiller EJ, Milner AD, Lenney W. Nebulized sodium cromoglycate in young asthmatic children. Double‐blind trial. Archives of Disease in Childhood 1977;52(11):875‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyde 1970 {published data only}
- Hyde JS, Buranakul B, Vithayasai V. Effect of cromolyn sodium on childhood asthma. Annals of Allergy 1970;28(10):449‐58. [MEDLINE: ] [PubMed] [Google Scholar]
Kobayashi 1970 {published data only}
- Kobayashi N, Takashima H, Kono M, Haruna H, Hayakawa H, Juji A, et al. [Double‐blind cross‐over evaluation of effectiveness of disodium cromoglycate in children with special reference to the application of a score method for the symptoms and treatments]. [Japanese]. Arerugi ‐ Japanese Journal of Allergology 1970;19(1):31‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Limburg 1971 {published data only}
- Limburg M. Treatment of children in an asthma centre with disodium cromoglycate. Double‐blind crossover trial. Acta Allergologica 1971;26(5):367‐82. [MEDLINE: ] [PubMed] [Google Scholar]
Matthew 1977 {published data only}
- Matthew DJ. The use of nebulised sodium cromoglycate in children. Acta Allergologica 1977;suppl 13:34‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Mikawa 1986 {published data only}
- Mikawa H, Baba M, Mishima T. [Clinical effect of disodium cromoglycate pressurised aerosol on bronchial asthma in children]. [Japanese]. Japanese Journal of Pediatrics 1986;39:2529‐38. [MEDLINE: ] [Google Scholar]
Shioda 1970 {published data only}
- Shioda H, Murano J, Mishima K, Iikura Y, Tanaka F, Izeki M. Disodium cromoglycate (Intal) in the treatment of bronchial asthma in children. Acta Allergologica 1970;25:221‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1970 {published data only}
- Smith JM. Disodium cromoglycate in the treatment of asthma in children. Disodium Cromoglycate. Papers presented at the 7th International Congress of Allergology. Rome: CEPI, 1970:109‐13. [Google Scholar]
- Smith JM, Mills P. Disodium cromoglycate in pollen asthma. Acta Allergologica 1970;25(5):365‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tasche 1997 {published data only}
- Tasche MJA, Wouden JC, Uijen JHJM, Ponsioen BP, Bernsen RMD, Suijlekom‐Smit LWA, et al. Randomised placebo‐controlled trial of inhaled sodium cromoglycate in 1‐4‐year‐old children with moderate asthma. Lancet 1997;350(9084):1060‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tuchinda 1974 {published data only}
- Tuchinda M, Habanananda S, Pongpipat D. A "double‐blind" trial of disodium cromoglycate in Thai asthmatic children. Journal of the Medical Association of Thailand 1974;57(6):289‐93. [MEDLINE: ] [PubMed] [Google Scholar]
Yuksel 1992 {published data only}
- Yuksel B, Greenough A. Inhaled sodium cromoglycate for pre‐term children with respiratory symptoms at follow‐up. Respiratory Medicine 1992;86:131‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yuksel B, Greenough A. The effect of sodium cromoglycate on upper and lower respiratory symptoms in children born prematurely. European Journal of Pediatrics 1993;152:615‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agbayani 1984 {published data only}
- Agbayani BF, Nantes WJ. A double‐blind comparative trial of sodium cromoglycate using pressurized aerosol vs. spinhaler in the treatment of asthma. Clinical trial. Philippine Journal of Internal Medicine 1984;22:42‐50. [Google Scholar]
Anastasatu 1979 {published data only}
- Anastasatu C, Kercea V, Dutu S, Algeorge G. [Disodium cromoglycate treatment of bronchial asthma and spastic bronchitis (preliminary results)] [Tratamentul cu cromoglicat disodic in astmul bronsic si in bronsita spastica (rezultate preliminare)]. Revista de Igienă, Bacteriologie, Virusologie, Parazitologie, Epidemiologie, Pneumoftiziologie. Pneumoftiziologia 1979;28:17‐24. [PubMed] [Google Scholar]
Anonymous 1969 {published data only}
- Anonymous. General practitioner clinical trials. Disodium cromoglycate in asthma. The Practitioner 1969;203:220‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Arndt 1975 {published data only}
- Arndt M. Cutaneous allergen test in children: frequency of conversion of prick‐test reactions, IgE concentrations in serum and course of the disease in young asthmatics during or without treatment with "Dinatrium cromoglicicum" inhalation [Cutaner Allergentest bei Kindern: Konversionshäufigkeit von Pricktestreaktionen, IgE‐Konzentration im Serum und Krankheitsverlauf bei jugendlichen Asthmatikern während und ohne Therapie met Ninatrium cromoglicicum]. Klinische Pädiatrie 1975;187:496‐512. [MEDLINE: ] [PubMed] [Google Scholar]
Avital 1991 {published data only}
- Avital A, Steljes DG, Pasterkamp H, Kryger M, Sanchez I, Chernick V. Sleep quality in children with asthma treated with theophylline or cromolyn sodium. Journal of Pediatrics 1991;119(6):979‐84. [DOI] [PubMed] [Google Scholar]
Berman 1975 {published data only}
- Berman BA, Fenton MM, Girsh LS, Haddad ZH, Sellars WA, Strem EL, et al. Cromolyn sodium in the treatment of children with severe, perennial asthma. Pediatrics 1975;55(5):621‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Bernstein 1972 {published data only}
- Bernstein IL, Siegel SC, Brandon ML, Brown EB, Evans RR, Feinberg AR, et al. A controlled study of cromolyn sodium sponsored by the drug committee of the American Academy of Allergy. Journal of Allergy and Clinical Immunology 1972;50(4):235‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blumenthal 1988 {published data only}
- Blumenthal MN, Selcow J, Spector S, Zeiger RS, Mellon M. A multicenter evaluation of the clinical benefits of cromolyn sodium aerosol by metered‐dose inhaler in the treatment of asthma. Journal of Allergy and Clinical Immunology 1988;81(4):681‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bonifazi 1985 {published data only}
- Bonifazi F, Antonicelli L, Pieretti C, Piunti E. Metered aerosol of disodium cromoglycate in the treatment of seasonal allergic asthma: a trial controlled and aerobiologic monitoring [Il DSCG in aerosol dosato nell trattamento dell'asma allergico stagionale: studio controllato e monitoraggio aerobiologico]. Giornale Italiano Malattia del Torace 1985;39(1):35‐8. [Google Scholar]
Booij‐Noord 1971 {published data only}
- Booij‐Noord H, Orie NGM, Vries K. Immediate and late bronchial obstructive reactions to inhalation of house dust and protective effects of disodium cromoglycate and prednisolone. Journal of Allergy and Clinical Immunology 1971;48(6):344‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruderman 1990 {published data only}
- Bruderman I, Cohen R, Schachter J. Bronchial response to methacholine in "healthy" children of asthmatic parents. Effect of treatment with cromoly sodium. Chest 1990;97(2):285‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carrasco 1989 {published data only}
- Carrasco E, Sepulveda R. Comparison of 1 mg and 5 mg sodium cromoglycate metered dose inhalers in the treatment of asthma: a 12‐week double‐blind, parallel group trial. Current Medical Research and Opinion 1989;11(6):341‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carrà 2001 {published data only}
- Carrà S, Gagliardi L, Zanconato S, Scollo M, Azzolin N, Zacchello F, et al. Budesonide but not nedocromil sodium reduces exhaled nitric oxide levels in asthmatic children. Respiratory Medicine 2001;95:734‐9. [DOI] [PubMed] [Google Scholar]
Chai 1973 {published data only}
- Chai H. The long‐term efficacy and safety of Intal therapy in asthmatic children. In: Pepys J, Yamamura Y editor(s). Intal in Bronchial Asthma. Papers presented at the 8th International Congress of Allergology. Loughborough: Fisons, 1973:55‐64. [Google Scholar]
Chan‐Yeung 1971 {published data only}
- Chan‐Yeung M, Morton J, Grzybowski S. A double‐blind trial of disodium cromoglycate (Intal) in the treatment of bronchial asthma. Canadian Medical Association Journal 1971;105:827‐31. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Chyrek‐Borowska 1975 {published data only}
- Chyrek‐Borowska S, Obrzut D, Kowal E, Hofman J, Gruszecka M, Zietkowski B. Clinical evaluation of Intal in the treatment of asthma [Ocena kliniczna stosowania intalu u chorych na dychawice oskrzelowa]. Polski Tygodnik Lekarski 1975;30(5):213‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Ciszek 1974 {published data only}
- Ciszek J, Kazimierczak A, Walczak J. Clinical studies on Intal in the treatment of patients with bronchial asthma [Badania kliniczne nad Intalem u chorych z astma oskrzelowa]. Gruzlica 1974;42(8):779‐87. [MEDLINE: ] [PubMed] [Google Scholar]
Crawford 1974 {published data only}
- Crawford LV, Lecks HI, Marks MB, Mascia AV. Cromolyn sodium in the management of the child with asthma: a symposium. Preliminary data from a collaborative group study. Clinical Pediatrics (Philadelphia) 1973;12(9):518‐24. [MEDLINE: ] [PubMed] [Google Scholar]
Crawford 1974b {published data only}
- Crawford LV. Cromolyn sodium in childhood asthma. Southern Medical Journal 1974;67(11):1285‐7. [DOI] [PubMed] [Google Scholar]
Crimi 1988 {published data only}
- Crimi N, Palermo F, Vancheri C, Oliveri R, Distefano SM, Polosa R, et al. Effect of sodium cromoglycate and nifedipine on adenosine‐induced bronchoconstriction. Respiration 1988;53:74‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crisp 1974 {published data only}
- Crisp J, Ostrander C, Giannini A, Stroup G, Deamer WC. Cromolyn sodium therapy for chronic perennial asthma. Journal of the American Medical Association 1974;229(7):787‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Croce 1995 {published data only}
- Croce J, Negreiros EB, Mazzei JAM, Isturiz G. A double‐blind placebo‐controlled comparison of sodium cromoglycate and ketotifen in the treatment of childhood asthma. Allergy 1995;50:524‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Baets 1998 {published data only}
- Baets F, Daele S, Frankx H, Vinaimont F. Inhaled steroids compared with disodium cromoglycate in preschool children with episodic viral wheeze. Pediatric Pulmonology 1998;25:361‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dickson 1969 {published data only}
- Dickson W. A one year's trial of Intal compound in 24 children with severe asthma. In: Pepys J, Frankland AW editor(s). Disodium Cromoglycate in Allergic Airway Disease. London: Butterworths, 1969:105‐19. [Google Scholar]
Droszcz 1973 {published data only}
- Droszcz W, Madalinska M. Clinical evaluation of Intal in the treatment of atopic bronchial asthma (double‐blind study) [Kliniczna ocena intalu w leczeniu atopowej astmy oskrzelowej (podwojna slepa proba]. Polskie Archiwum Medycyny Wewnętrznej 1973;50:603‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Edmunds 1994 {published data only}
- Edmunds AT, Goldberg RS, Duper B, Devichand P, Follow RMA. A comparison of budesonide 800 mug and 400 mug daily via Turbuhaler with disodium cromoglycate via Spinhaler for asthma prophylaxis in children. British Journal of Clinical Research 1994;5:11‐23. [Google Scholar]
Engström 1975 {published data only}
- Engström I, Oberger E. Experience with sodium cromoglycate treatment of pollen‐triggered asthma in children and adolescents [Erfarenheter av natriumkromoglikat‐behandling vid pollenutlost astma hos barn och ungdom]. Läkartidningen 1978;75:141‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Engström 1977 {published data only}
- Engström I. Evaluation of Lomudal treatment in children. Scandinavian Journal of Respiratory Diseases 1977;101 suppl:49‐56. [PubMed] [Google Scholar]
Exline 1972 {published data only}
- Exline AL. Bronchial asthma treated with cromolyn sodium: a double‐blind crossover study. Journal of Asthma Research 1972;9:121‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Forster 1998 {published data only}
- Forster TJ, Uryniak T, Simpson B, McGuire K, Casty FE, et al. Intal 1mg CFC‐free is an effective therapy for both adolescent and adult asthmatics. American Journal of Respiratory and Critical Care Medicine 1998;157(Suppl 3):A637. [Google Scholar]
Fox 1972 {published data only}
- Fox ZR, Brickman HF, Beaudry PH, Liddell FDK, Eisen AH. Response to disodium cromoglycate in children with chronic asthma. Canadian Medical Association Journal 1972;106(9):975‐9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Friday 1973 {published data only}
- Friday GA, Facktor MA, Bernstein RA, Fireman P. Cromolyn therapy for severe asthma in children. Journal of Pediatrics 1973;83(2):299‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fuleihan 1973 {published data only}
- Fuleihan FJD, Kiblawi S, O'Brien MA, Haamari SM, Feisal KA. Clinical response to disodium cromoglycate. A double blind study. Lebanon Medical Journal 1973;26(2):95‐108. [PubMed] [Google Scholar]
Furukawa 1999 {published data only}
- Furukawa C, Atkinson D, Forster TJ, Nazzario K, Simpson B, Uryniak T, et al. Controlled trial of two formulations of cromolyn sodium in the treatment of asthmatic patients > or = 12 years of age. Intal Study Group. Chest 1999;116(1):65‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garcio Velloso 1984 {published data only}
- Garcia Velloso MA, Maresca OD, Marzorati EH, Perez DL, Pellegrini HMM, Vit E, et al. Controlled trial with cromoglycate in the bronchial asthma [Ensayo controlado del cromoglicato en el asma bronquial]. Prensa Medica Argentina 1984;71:42‐6. [EMBASE: 84197455] [Google Scholar]
Gaur 1997 {published data only}
- Gaur SN, Agarwal G, Gupta SK. Use of LPC antagonist, choline, in the management of bronchial asthma. Indian Journal of Chest Diseases and Allied Sciences 1997;39:107‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Geller‐Bernstein 198 {published data only}
- Geller‐Bernstein C, Sheh N. The management of bronchial asthma in children under the age of 3 1/2 years using Intal (sodium cromoglycate) administered by Spinhaler. Clinical Allergy 1980;10 Suppl:503‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gemicioglu 1993 {published data only}
- Gemicioglu B, Gürsoy M, Umut S, Yildirim H, Özüner Z. Effects of beclomethasone dipropionate, disodium cromoglycate and terfenadine on blood histamine levels [Brons Hiperreaktivitesi Olgularinda Beklometazon Diproppiunot, Disodyum Kromoglikat ve Terfenadinin Kan Histamin Düzeyine Etkileri]. Klinik Gelisim 1993;6:2426‐9. [Google Scholar]
Glazer 1971 {published data only}
- Glazer I, Racz I, Molho M. Double blind single crossover clinical evaluation of disodium cromoglicate in bronchial asthma. International Archives of Allergy and Applied Immunology 1971;41(1):161‐2. [DOI] [PubMed] [Google Scholar]
Godfrey 1975 {published data only}
- Godfrey S, Balfour‐Lynn L, König P. The place of cromolyn sodium in the long‐term management of childhood asthma based on 3‐ to 5‐year follow‐up. Journal of Pediatrics 1975;87(3):465‐73. [DOI] [PubMed] [Google Scholar]
Gomez‐Orozco 1976 {published data only}
- Gomez‐Orozco. Use of disodium cromoglycate in the treatment of asthma in children [Utilidad del cromoglicato disodico en el tratamiento del asma en el niño]. Alergia 1976;23(2):37‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Graber 1998 {published data only}
- Graber MA. Cromolyn use in children with asthma. Journal of Family Practice 1998;46(2):113‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Grifoni 1971 {published data only}
- Grifoni V, Giacco GS, Manconi PE. Disodium cromoglycate in the treatment of bronchial asthma: a controlled clinical trial. Folia Allergologica (Roma) 1971;18:145‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Gulyas 1984 {published data only}
- Gulyas A. Double‐blind study between reproterol and a fixed combination of reproterol and disodium cromoglycate in children and adolescents [Doppelblindstudie Zur Therapeutischen Wirksamkeit Von Reproterolhydrochlorid Allein Und Einer Kombination Aus Cromoglicinsaure, Dinatriumsalz Und Reproterolhydrochlorid]. Pharmakotherapie 1984;7:51‐9. [EMBASE: 85054923] [Google Scholar]
Guminski 1976 {published data only}
- Guminski T, Rokicki W, Szumilas Z. Intal in in‐patient and out‐patient treatment of children with asthma [Stosowanie preparatu Intal w leczeniu klinicznym i ambulatoryjnym dzieci chorych na astme oskrzelowa]. Polski Tygodnik Lekarski 1976;31(24):1043‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Haber 1989 {published data only}
- Haber P, Geyer K, Burghuber OC. Long‐term treatment with disodium cromoglycate does not alter bronchial hyperreactivity in patients with perennial bronchial asthma. Respiration 1989;55:44‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herjavecz 1982 {published data only}
- Herjavecz I, Böszörmény‐Nagy Gy. Drug prevention of allergic bronchial asthma. Comparison of the protective effects of ketotifen and cromoglycic acid [Medikamentose Prophylaxe bei allergischem Asthma bronchiale. Vergleich der protektiven Wirkung von Ketotifen und Cromoglicinsaure]. Zeitschrift für Allgemeinmedizin 1982;58:1427‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Hermance 1973 {published data only}
- Hermance WE, Brown EB. Cromolyn sodium (disodium cromoglycate) in treatment of asthma. New York State Journal of Medicine 1973;73:430‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Hobday 1970 {published data only}
- Hobday JD. The effect of disodium cromoglycate on asthma in children. Australian Paediatric Journal 1970;6:14‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hyde 1971 {published data only}
- Hyde JS, Swarts C. Long term prophylaxis of childhood asthma using cromolyn sodium. Annals of Allergy 1971;29:483‐91. [MEDLINE: ] [PubMed] [Google Scholar]
Hyde 1973 {published data only}
- Hyde JS, Isenberg PD, Floro LD. Short‐ and long‐term prophylaxis with cromolyn sodium in chronic asthma. Chest 1973;63(6):875‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inoue 1970 {published data only}
- Inoue S. Effects of disodium cromoglycate on airway histamine hypersensitivity and daily pulmonary function in asthmatic children. Disodium Cromoglycate. Papers presented at the 7th International Congress of Allergology. Rome: CEPI, 1970:133‐9. [Google Scholar]
Irani 1972 {published data only}
- Irani FA, Jones NJ, Gent M, Newhouse MT. Evaluation of disodium cromoglycate in intrinsic and extrinsic asthma. American Review of Respiratory Disease 1972;106(2):179‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ito 1971 {published data only}
- Ito K, Hattori J, Mizutani N, Iwakura M. [Clinical trial of disodium cromoglycate in bronchial asthma ‐ evaluation by double‐blind crossover test]. [Japanese]. Arerugi ‐ Japanese Journal of Allergology 1971;20:779‐89. [PubMed] [Google Scholar]
Jenssen 1973 {published data only}
- Jenssen AO. Disodium cromoglycate (Lomudal) as a prophylactic agent in allergen‐induced asthma [Dinatriumkromoglykat (Lomudal) som profylaktikum ved allergenprovosert astma]. Tidsskrift for den Norske Laegeforening 1973;93:17‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Johannessen 1975 {published data only}
- Johannessen H. The efficiency of hyposensitization and disodium cromoglycate (Lomudal) in bronchial asthma [Effekten av hyposensibilisering og dinatrium kromoglykat (lomudal) ved asthma bronchiale]. Tidsskrift for den Norske Laegeforening 1975;95:595‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Jones 1970 {published data only}
- Jones RS, Blackhall MI. Role of disodium cromoglycate ('Intal') in treatment of childhood asthma. Archives of Diseases in Childhood 1970;45:49‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehnscherper 1993 {published data only}
- Kehnscherper M. DNCG (acecromol) and ketotifen (Zatofug) in treatment of children with bronchial asthma [DNCG (acecromol) und Ketotifen (Zatofug) in der Behandlung von Kindern mit Asthma bronchiale]. Kinderärztliche Praxis 1993;61:276‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kennedy 1969 {published data only}
- Kennedy MCS. Disodium cromoglycate in the control of asthma. British Journal of Diseases of the Chest 1969;63:96‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kennedy MCS. Long‐term therapy and effect of disodium cromoglycate in adolescents and adults. In: Pepys J, Frankland AW editor(s). Disodium Cromoglycate in Allergic Airway Disease. London: Butterworths, 1969:121‐58. [Google Scholar]
Khurana 1977 {published data only}
- Khurana S, Hyde JS. Cromolyn sodium, five to six years later. Annals of Allergy 1977;39:94‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Kidner 1968 {published data only}
- Kidner PH, Meisner P, Pride NB, Bruce Pearson RS. Disodium cromoglycate in the treatment of bronchial asthma. Lancet 1968;2(7569):655‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kimmel 1974 {published data only}
- Kimmel Z, Kachel S, Skowronek S, Kokurewizc H. Treatment of asthma with Intal (disodium cromoglycate) [Wyniki leczenia dychawicy oskrzelowej intalem (disodium cromoglycicum)]. Polskie archiwum medycyny wewnętrznej 1974;52:79‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Klein 1980 {published data only}
- Klein G, Urbanek R, Matthys H, Hamm M. Protective effect of ketotifen and disodium cromoglycate in bronchial challenge tests with allergens [Schutzwirkung von Ketotifen und Dinatrium cromoglicicum auf die inhalativ provozierte allergogene Bronchialobstruktion]. Deutsche Medizinische Wochenschrift 1980;105(38):1313‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klein 1981 {published data only}
- Klein G, Urbanek R, Matthys H. Long‐term study of the protective effect of ketotifen in children with allergic bronchial asthma. The value of a provocation test in assessment of treatment. Respiration 1981;41:128‐32. [DOI] [PubMed] [Google Scholar]
Knezevic 1997 {published data only}
- Knezevic J, Stojcic V. Influence of therapy with antiinflammatory drugs on changing the baseline FEV1. Journal of Investigational Allergology and Clinical Immunology 1997;7(5):522. [Google Scholar]
Kotaniemi 2005 {published data only}
- Kotaniemi‐Sierjänen A, Reijonen TM, Korhonen K, Korppi M. Sodium cromoglycate therapy in wheezing infants: preliminary evidence of beneficial outcome at early school age. Pediatrics International 2005;47:627‐34. [DOI] [PubMed] [Google Scholar]
Kraemer 1986 {published data only}
- Kraemer R, Sennhauser F. Inhaled beclomethasone and cromoglycate on bronchial hyperreactivity in asthmatic children [Einfluss der topischen Anwendung von Beclometason und Cromoglykat auf die bronchiale Hyperirritabilität bei Kindern mit Asthma bronchiale]. Atemwege und Lungkrankheiten 1986;12(3):110‐3. [Google Scholar]
Kraemer 1987 {published data only}
- Kraemer R, Sennhauser F, Reinhardt M. Effects of regular inhalation of beclamethasone diproprionate and sodium cromoglycate on bronchial hypereactivity in asthmatic children. Acta Paediatrica Scandinavica 1987;76(1):119‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kraemer 1993 {published data only}
- Kraemer R, Modelska K, Casaulta Aebischer C, Schöni MH. Comparison of different inhalation schedules to control childhood asthma. Agents and Actions 1993;40(Suppl):211‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuzemko 1974 {published data only}
- Kuzemko JA, Bedford S, Wilson L, Walker SR. A comparison of betamethasone valerate aerosol and sodium cromoglycate in children with reversible airways obstruction. Postgraduate Medical Journal 1974;50(suppl 4):53‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Kuzemko 1977 {published data only}
- Kuzemko JA. Long‐term experiences in the use of sodium cromoglycate (SCG) in young children with asthma. Acta Allergologica 1977;Suppl 13:28‐33. [MEDLINE: ] [PubMed] [Google Scholar]
König 1973 {published data only}
- König P, Godfrey S. The effect of frequent administration of sodium cromoglycate to asthmatic children who previously respondend poorly. Clinical Allergy 1973;3:395‐402. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lahoz 1973 {published data only}
- Lahoz F, Bensabat Z, Marín F, Pérez Guerrero J, Sastre A. Disodium cromoglycate and bronchial asthma (double‐blind study) [Cromoglicato disódico y asma bronquial (estudio en doble ciego).]. Boletín de la Fundación Jiménez Díaz 1973;5:449‐52. [Google Scholar]
Lecks 1974 {published data only}
- Lecks HI, Kravis LP, Wood DW. Clinical experiences with the use of cromolyn sodium in asthmatic children. Clinical Pediatrics 1974;13:420‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lenney 1978 {published data only}
- Lenney W, Milner AD. Nebulised sodium cromoglycate in the preschool wheezy child. Archives of Diseases in Childhood 1978;53:474‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linehan 1970 {published data only}
- Linehan WD. A clinical trial of disodium cromoglycate in asthma. Journal of the Irish Medical Association 1970;63:265‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Löwhagen 1985 {published data only}
- Löwhagen O, Rak S. Modification of bronchial hyperreactivity after treatment with sodium cromoglycate during pollen season. Journal of Allergy and Clinical Immunology 1985;75(4):460‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Macdonald 1979 {published data only}
- Macdonald TH, McWilliam R. Monitoring response to bronchodilator therapy in asthma in childhood. Journal of International Medical Research 1979;7(suppl 1):87‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Mahashur 1981 {published data only}
- Mahashur AA, Chandrasekharan M, Kamat SR. Disodium cromoglycate in bronchial asthma. Indian Journal of Chest Diseases and Allied Sciences 1981;23:4‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Marks 1974 {published data only}
- Marks MB. Therapeutic efficacy of cromolyn in childhood asthma. American Journal of Diseases of Children 1974;128(3):301‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marshall 1969 {published data only}
- Marshall JS, Birtwhistle IH. Clinical trial of disodium cromoglycate in general practice. In: Pepys J, Frankland AW editor(s). Disodium Cromoglycate in Allergic Airway Disease. A symposium held at the Royal Society of Medicine, London, on 5th March 1969. London: Butterworths, 1969:171‐5. [Google Scholar]
Masood 1978 {published data only}
- Masood G, Hasan NW. Disodium cromoglycate in allergic bronchial asthma: a report on twentyone patients. Journal of the Pakistan Medical Association 1978;28(10):142‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Matsumoto 1994 {published data only}
- Matsumoto T, Miike T. Effect of sodium cromoglycate on the peak expiratory flow rate in children with bronchial asthma. Pediatric Asthma, Allergy and Immunology 1994;8(2):105‐10. [Google Scholar]
Mattoli 1986 {published data only}
- Mattoli S, Foresi A, Corbo GM, Polidori G, Ciappi G. Protective effect of disodium cromoglycate on allergen‐induced bronchoconstriction and increased hyperresponsiveness: a double‐blind placebo‐controlled study. Annals of Allergy 1986;57:295‐300. [MEDLINE: ] [PubMed] [Google Scholar]
McLean 1973 {published data only}
- McLean WL, Lozano J, Hannaway P, Sakowitz S, Mueller HL. Cromolyn treatment of asthmatic children. American Journal of Diseases of Children 1973;125(3):332‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mellon 1982 {published data only}
- Mellon MH, Harden K, Zeiger RS. The effectiveness and safety of nebulizer cromolyn solution in the young childhood asthmatic. Immunology and Allergy Practice 1982;IV(5):36‐40. [Google Scholar]
Menardo 1998 {published data only}
- Menardo J‐L, Wessel F, Cougnard J, Czarlewski W. Propylactic treatment with loratidine versus cromolyn sodium in children with mild‐to‐moderate perennial alergic asthma. Current Therapeutic Research 1998;59(8):567‐78. [Google Scholar]
Miraglia 1981 {published data only}
- Miraglia del Giudice M, Capristo A, Maiello N. Preventive treatment of asthma due to exertion in children: comparative study of 4 drugs [Ricerche sulla terapia preventiva dell'asma da sforzo in età pediatrica: rilieve comparativi fra 4 farmaci]. Pediatria (Napoli) 1981;89:679‐92. [PubMed] [Google Scholar]
Miraglia 1982 {published data only}
- Miraglia del Giudice M, Capristo A, Maiello N, Apuzzo G. Nebulized sodium cromoglycate for the treatment of asthma in children under five years of age. Modern Problems in Paediatrics 1982;21:122‐7. [Google Scholar]
Mitchell 1976 {published data only}
- Mitchell I, Paterson IC, Cameron SJ, Grant IWB. Treatment of childhood asthma with sodium cromoglycate and beclomethasone dipropionate aerosol singly and in combination. British Medical Journal 1976;2(6033):457‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moeller 2009 {published data only}
- Moeller A, Spescha H, Knauer N, Inci D, Wildhaber JH. Efficacy of an isotonic small droplet size nebulized DSCG on asthma control in children. American Journal of Respiratory and Critical Care Medicine 2009;179:A4802. [Google Scholar]
Molema 1989 {published data only}
- Molema J, Herwaarden CLA, Folgering HT. Effects of long‐term treatment with inhaled cromoglycate and budesonide on bronchial hyperresponsiveness in patients with allergic asthma. European Respiratory Journal 1989;2(4):308‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Moran 1968 {published data only}
- Moran F, Bankier JDH, Boyd G. Disodium cromoglycate in the treatment of allergic bronchial asthma. Lancet 1968;2(7560):137‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Muittari 1969 {published data only}
- Muittari A. Prevention of the bronchial obstruction induced by inhalation allergy with disodium cromoglycate [Natriumkromoglykaatti inhalaatioallergeenin auheuttaman bronkusobstruktion ehkäisemissä]. Duodecim 1969;85:1493‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Munro Ford 1969 {published data only}
- Munro Ford R. Disodium cromoglycate in the treatment of seasonal and perennial asthma. Medical Journal of Australia 1969;2(11):537‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naganathan 1975 {published data only}
- Naganathan N, Cheah PS, Feng PH. Evaluation of sodium cromoglycate BP (Intal) in the prophylaxis of bronchial asthma in asians. Singapore Medical Journal 1975;16:204‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Ng 1977 {published data only}
- Ng SH, Dash CH, Savage SJ. Betamethasone valerate compared with sodium cromoglycate in asthmatic children. Postgraduate Medical Journal 1977;53:315‐20. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orefice 1990 {published data only}
- Orefice U, Struzzo P, Ferrazzano PL, Pitzalis G, Dorigo R. Ketotifen and disodium cromoglycate in the treatment of allergic bronchial asthma in children [Terapia con Ketotifen e Disodiocromoglicato dell'asma bronchiale allergico nei bambini]. Lotta contro la TBC e Malattie Polmonari 1990;60:238‐44. [Google Scholar]
Pesic 1975 {published data only}
- Pesic V, Tesic R, Ojkic B, Armacki Z, Djordjevic M, Maksimovic V. Long‐term use of disodium chromoglycate (Intal) in the treatment of asthma in children [Dugotrajna primena dinatrijum hromoglikata (Intal) u lecenju decje astme]. Plucne Bolesti Tuberk 1975;27(1‐2):87‐97. [MEDLINE: ] [PubMed] [Google Scholar]
Petersen 1996 {published data only}
- Petersen W, Karup‐Pedersen F, Friis B, Howitz P, Nielsen F, Strömquist L‐H. Sodium cromoglycate as a replacement for inhaled corticosteroids in mild‐to‐moderate childhood asthma. Allergy 1996;51:870‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Price 1995 {published data only}
- Price JF, Weller PH. Comparison of fluticasone propionate and sodium cromoglycate for the treatment of childhood asthma (an open parallel group study). Respiratory Medicine 1995;89:363‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rafinski 1977 {published data only}
- Rafinski T, Chobot‐Maciejewska H, Mrozikiewicz D. Late results of combined treatment of asthma in children [Odlegle wyniki skojarzonego leczenia astmy oskrzelowej u dzieci]. Polski Tygodnik Lekarski 1977;32(30):1157‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Rauber 1983 {published data only}
- Rauber G, Stauder J, Steurich F. Therapy for bronchial asthma ‐ DSCG and ketotifen in a double‐blind trial [Zur Therapie des Asthma bronchiale ‐ Dinatrium cromoglicicum and Ketotifen im Doppelblindversuch]. Atemwegs Lungenkrankrankheiten 1983;9(11):465‐71. [Google Scholar]
Reid 1988 {published data only}
- Reid JJ. Evaluation of the addition of sodium cromoglycate to therapy in the long‐term management of asthma in New Zealand. New Zealand Family Physician 1988;15:86‐9. [CENTRAL: CN‐00269870] [Google Scholar]
Robertson 1969 {published data only}
- Robertson DG, Epstein SW, Warrell DA. Trial of disodium cromoglycate in bronchial asthma. British Medical Journal 1969;643:552‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 1970 {published data only}
- Romano C. Clinical study of disodium cromoglycate in the treatment of chronic allergic asthma in children [Studio clinico del cromoglicato disodico nel trattamento dell'asma cronica allergica del bambino]. Disodium cromoglycate, 7th International Congress Allerg. Spec. Sect. Meetings, Florence. Rome: CEPI, 1970:115‐23. [Paper provided by Rhone‐Poulenc‐Rhorer]
Sarlet 1973 {published data only}
- Sarlet E. Clinical evaluation of the effect of DSCG on allergic dyspnea in children [Etude experimentale clinique sur l'emploi de cromoglycate disodique dans les dyspnees allergiques chez l'enfant]. Acta Paediatrica Belgica 1973;27:390‐405. [MEDLINE: ] [PubMed] [Google Scholar]
Schmidt 1973 {published data only}
- Schmidt E, Pfingsten S. Treatment of bronchial asthma in childhood using disodium cromoglycate [Zur Behandlung des kindlichen Asthma bronchiale mit Dinatrium cromoglicicum]. Zeitschrift für Allgemeinmedizin 1973;14:686‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Selcow 1983 {published data only}
- Selcow JE. Cromolyn therapy in children. Journal of Asthma 1983;20:361‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Selcow 1989 {published data only}
- Selcow JE, Mendelson LM, Rosen JP. Clinical benefits of cromolyn sodium aerosol (MDI) in the treatment of asthma in children. Annals of Allergy 1989;62:195‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Sellars 1975 {published data only}
- Sellars WA, Pflanzer J. Cromolyn sodium in the treatment of asthma: its effectiveness and use. Southern Medical Journal 1975;68(8):970‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shioda 1973 {published data only}
- Shioda H. Clinical aspects of disodium cromoglycate in childhood asthma. In: Pepys J, Yamamura Y editor(s). Intal in Bronchial Asthma. Papers presented at the 8th International Congress of Allergology. Loughborough: Fisons, 1973:65‐73. [Google Scholar]
Shiota 1984 {published data only}
- Shiota K, Hamada A, Shida T, Nagano H, Nakajima S, et al. [Clinical evaluation of Travanax Sodium (Y‐12141), a new oral anti‐allergic compound, in bronchial asthma. Multi‐center double‐blind study in comparison with DSCG and placebo]. [Japanese]. Clinical Evaluation 1984;12:475‐555. [Google Scholar]
Shore 1971 {published data only}
- Shore SC. A clinical trial of disodium cromoglycate (Lomudal) in asthmatic children. South‐African Medical Journal 1971;45:141‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Sienra Monge 1990 {published data only}
- Sienra Monge JJL, Baeza Bacab MA, Casillas Miranda R, Cavazos Galvan M, Real Sanchez H. Use of sodium cromoglycate in nebulizations for asthmatic children less than 7 years of age [Uso de cromoglicato de sodio por nebulizaciones en ninos asmaticos menores de siete anos]. Revista Alergia Mexico 1990;37(1):7‐12. [MEDLINE: ] [PubMed] [Google Scholar]
Silverman 1972 {published data only}
- Silverman M, Connolly NM, Balfour‐Lynn L, Godfrey S. Long‐term trial of disodium cromoglycate and isoprenaline in children with asthma. British Medical Journal 1972;823(3):378‐81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sly 1970 {published data only}
- Sly RM. Evaluation of disodium cromoglycate in asthmatic children. Annals of Allergy 1970;28:299‐306. [PubMed] [Google Scholar]
Smith 1968 {published data only}
- Smith JM. Long‐term results with disodium cromoglycate in the treatment of children with asthma. In: Pepys J, Frankland AW editor(s). Disodium Cromoglycate in Allergic Airway Disease. A symposium held at the Royal Society of Medicine, London, on 5th March 1969. London: Butterworths, 1970. [Google Scholar]
- Smith JM, Devey GF. Clinical trial of disodium cromoglycate in treatment of asthma in children. British Medical Journal 1968;601:340‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1980 {published data only}
- Smith JM. Prolonged use of disodium cromoglycate in children and young persons ‐ ten years experience. Schweizerische Medizinische Wochenschrift. Journal Suisse de Medecine 1980;110(6):183‐4. [PubMed] [Google Scholar]
So 1981 {published data only}
- So SY, Yu DYC. Sodium cromoglycate delivered by pressurized aerosol in the treatment of asthma. Clinical Allergy 1981;11:479‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Streumer 1970 {published data only}
- Streumer J. Treatment of asthma with DSCG in juveniles during and after hospitalization. Respiration 1970;27(Suppl):363‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Thompson 1974 {published data only}
- Thompson HC, Cochran HD. Use of cromolyn sodium in childhood asthma. Arizona Medicine 1974;31:501‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Toshner 1974 {published data only}
- Toshner D, Frank M. A double‐blind crossover trial of disodium cromoglycate in asthmatic patients living under environmental control. Israel Journal of Medical Sciences 1974;10(5):509‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Turpeinen 2010 {published data only}
- Turpeinen M, Raitio H, Pelkonen AS, Nikander K, Korva S, Selroos O, Juntunen‐Backman K, Haahtela T. Skin thickness in children treated with daily or periodical inhaled budesonide for mild persistant asthma. Pediatric Research 2010;67:221‐25. [DOI] [PubMed] [Google Scholar]
Varsano 1983 {published data only}
- Varsano I, Mukamel M, Shuper A, Volovitz B, Shrem M, Jaber L. The efficiency of nebulizer treatment with water compared to sodium cromoglycate in reducing upper respiratory infections in children. Helvetica Paediatrica Acta 1983;38:335‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Viscardi 1997 {published data only}
- Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbel AB, Palmer TW. Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 1997;156(5):1523‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watanabe 1992 {published data only}
- Watanabe H. The effect of disodium cromoglycate against bronchial hyperresponsiveness in asthmatic children. Journal of Asthma 1992;29:117‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weinbren 1969 {published data only}
- Weinbren I, Bound J, Capper L. A double‐blind trial of disodium cromoglycate in bronchial asthma with assessment by four observers. British Journal of Diseases of the Chest 1969;63:155‐64. [DOI] [PubMed] [Google Scholar]
Wells 1979 {published data only}
- Wells A, Taylor B. A placebo‐controlled trial of ketotifen (HC 20‐511, Sandoz) in allergen induced asthma and comparison with disodium cromoglycate. Clinical Allergy 1979;9:237‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wheatley 1981 {published data only}
- Wheatley D. Pelletised sodium cromoglycate. Current Therapeutic Research 1981;30(5):655‐60. [Google Scholar]
Zarkovic 1991 {published data only}
- Zarkovic J, Angermayr R, Covi B, Danhorn H, Eber E, Emhofer J, et al. Effectiveness and tolerance of aerosol disodium cromoglycate in children with bronchial asthma [Wirksamkeit und Vertraglichkeit von vernebeltem Dinatrium Cromoglykat bei Kindern mit Asthma bronchiale]. Pädiatrie und Pädologie 1991;26:107‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Adams 1999
- Adams N, Bestall J, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [Art. No.: CD003274 DOI: 10.1002/14651858.CD003274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arnold 2008
- Arnold E, Clark CE, Lasserson TJ, Wu T. Herbal interventions for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 1. [Art. No.: CD005989. DOI: 10.1002/14651858.CD005989.pub2] [DOI] [PubMed] [Google Scholar]
BAG 1997
- British Asthma Guidelines Coordinating Committee. British guidelines on asthma management: 1995 review and position statement commenting on the guidelines in the light of recent evidence. Thorax 1997;52 (suppl):1‐21. [Google Scholar]
Becker 2005
- Becker A, Lemière C, Bérubé D, Boulet L‐P, Ducharme FM, FitzGerald M, Kovesi T, on behalf of the Asthma Guidelines Working Group of the Canadian Network for Asthma Care and the Canadian Thoracic Society. Summary of recommendations from the Canadian Asthma Consensus Guidelines, 2003. Canadian Medical Association Journal 2005;173(6 suppl):S1‐S56. [PMC free article] [PubMed] [Google Scholar]
Berman 1983
- Berman BA, Ross RN. Cromolyn. Clinical Reviews in Allergy 1983;1(1):105‐21. [DOI] [PubMed] [Google Scholar]
BTS 2003
- British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax 2003;58(Suppl I):i1‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calpin 1997
- Calpin C, Macarthur C, Stephens D, Feldman W, Parkin PC. Effectiveness of prophylactic inhaled steroids in childhood asthma: a systematic review of the literature. Journal of Allergy and Clinical Immunology 1997;100:452‐7. [DOI] [PubMed] [Google Scholar]
Carlsen 1996
- Carlsen K‐H, Larsson K. The efficacy of inhaled disodium cromoglycate and glucocorticoids. Clinical and Experimental Allergy 1996;20(Suppl 4):8‐17. [DOI] [PubMed] [Google Scholar]
Chalmers 1981
- Chalmers TC, Smith H, Blackburn B, Silverman B, Schroeder B, Reitman D, et al. A method for assessing the quality of a randomized control trial. Controlled Clinical Trials 1981;2:31‐49. [DOI] [PubMed] [Google Scholar]
Church 1985
- Church MK, Warner JO. Sodium cromoglycate and related drugs. Clinical Allergy 1985;15:311‐20. [DOI] [PubMed] [Google Scholar]
Dersimonian 1986
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dykes 1974
- Dykes MH. Evaluation of an asthmatic agent cromolyn sodium (Aarane, Intal). Journal of the American Medical Association 1974;227:1061‐2. [PubMed] [Google Scholar]
Edwards 1994
- Edwards AM. Sodium cromoglycate (Intal) as an anti‐inflammatory agent for the treatment of chronic asthma. Clinical and Experimental Allergy 1994;24:612‐23. [DOI] [PubMed] [Google Scholar]
Egger 1995
- Egger M, Davey Smith G. Misleading meta‐analysis. British Medical Journal 1995;310:752‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
Ernst 1996
- Ernst P, Fitzgerald JM, Spier S. Canadian Asthma‐consensus Conference ‐ Summary of recommendations. Canadian Respiratory Journal 1996;3:89‐100. [Google Scholar]
Fleiss 1993
- Fleiss JL. The statistical basis of meta‐analysis. Statistical Methods in Medical Research 1993;2:121‐45. [DOI] [PubMed] [Google Scholar]
Follman 1999
- Follman D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epdeimiology 1999;45:769‐73. [DOI] [PubMed] [Google Scholar]
GINA 2005
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Vol. NIH‐Publication No 02‐3659. Issued January 1996. Updated 2002‐2005, Bethesda, Maryland, USA: National Institutes of Health, 2002 (revised). [Google Scholar]
Greenwood 1999
- Greenwood CM, Midgley JP, Matthew AG, Logan AG. Statistical issues in a metaregression analysis of randomized trials: impact on the dietary sodium intake and blood pressure relationship. Biometrics 1999;55:630‐6. [DOI] [PubMed] [Google Scholar]
Guevara 2006
- Guevara JP, Ducharme FM, Keren R, Nihtianova S, Zorc J. Inhaled corticosteroids versus sodium cromoglycate in children and adults with asthma. Cochrane Database of Systematic Reviews 2006, Issue 2. [Art. No.: CD003558. DOI: 10.1002/14651858.CD003558.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gøtzsche 2008
- Gøtzsche PC, Johansen HK. House dust mite control measures for asthma. Cochrane Database of Systematic Reviews 2008, Issue 2. [Art. No.: CD001187. DOI: 10.1002/14651858.CD001187.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG. Assessing risk of bias in included studies. Green S, Higgins JPT (eds). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008]. The Cochrane Collaboration (available from www.cochrane‐handbook.org) 2008.
Hoag 1991
- Hoag JE, McFadden ER. Long‐term effect of cromolyn sodium on non‐specific bronchial hyperresponsiveness: a review. Annals of Allergy 1991;66:53‐63. [PubMed] [Google Scholar]
Holgate 1996
- Holgate ST. Inhaled sodium cromoglycate. Respiratory Medicine 1996;90(7):387‐90. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moor A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Kelly 2003
- Kelly K, Spooner CH, Rowe BH. Nedocromil sodium versus sodium cromoglycate for preventing exercise‐induced bronchoconstriction in asthmatics. Cochrane Database of Systematic Reviews 2000, Issue 3. [Art. No.: CD002731. DOI: 10.1002/14651858.CD002731] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuzemko 1989
- Kuzemko JA. Twenty years of sodium cromoglycate treatment: a short review. Respiratory Medicine 1989;83(suppl):11‐16. [DOI] [PubMed] [Google Scholar]
Lester 1997
- Lester ML, Bratton DL. Adverse reactions to cromolyn sodium: patient report and review of the literature. Clinical Pediatrics 1997;36:707‐10. [DOI] [PubMed] [Google Scholar]
Leynadier 1985
- Leynadier F, Pujade‐Lauraine MD, Cornaille G, Dry J. Death after cromoglycate. Allergy 1985;40:540‐1. [DOI] [PubMed] [Google Scholar]
Manning 2008
- Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus other inhaled steroids for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 2008, Issue 2. [Art. No.: CD007031. DOI: 10.1002/14651858.CD007031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagelkerke 1992
- Nagelkerke NJD. Maximum likelihood estimation of functional relationships. New York: Springer, 1992. [Google Scholar]
NIH 1997
- National Heart, Lung and Blood Institute. Guidelines for the diagnosis and management of asthma. Bethesda: National Institutes of Health, 1997:97‐4051. [PubMed] [Google Scholar]
NIH 2002
- National Institutes of Health. National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma ‐ Update on selected topics 2002. Journal of Allergy and Clinical Immunology 2002;110(5 pt 2):S141‐S219. [PubMed] [Google Scholar]
Schweitzer 1994
- Schweitzer M, Brossier Ballano K. Cromolyn use in young children. Annals of Pharmacotherapy 1994;28(7‐8):886‐7. [PubMed] [Google Scholar]
Seddon 2006
- Seddon P, Bara A, Ducharme FM, Lasserson TJ. Oral xanthines as maintenance treatment for asthma in children. Cochrane Database of Systematic Reviews 2006, Issue 1. [Art. No.: CD002885. DOI: 10.1002/14651858.CD002885.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapiro 1985
- Shapiro GG, König P. Cromolyn sodium: a review. Pharmacotherapy 1985;5:156‐70. [DOI] [PubMed] [Google Scholar]
Sharek 1999
- Sharek PJ, Bergman DA, Ducharme F. Beclomethasone for asthma in children: effects on linear growth. Cochrane Database of Systematic Reviews 1999, Issue 3. [Art. No.: CD001282. DOI: 10.1002/14651858.CD001282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sly 1997
- Sly RM. New guidelines for diagnosis and management of asthma. Annals of Allergy, Asthma, and Immunology 1997;78:427‐37. [DOI] [PubMed] [Google Scholar]
Spooner 2003
- Spooner CH, Saunders LD, Rowe BH. Nedocromil sodium for preventing exercise‐induced bronchoconstriction. Cochrane Database of Systematic Reviews 2002, Issue 1. [Art. No.: CD001183. DOI: 10.1002/14651858.CD001183.] [DOI] [PubMed] [Google Scholar]
Sridhar 2006
- Sridhar AV, McKean M. Nedocromil sodium for chronic asthma in children. Cochrane Database of Systematic Reviews 2006, Issue 3. [Art. No.: CD004108. DOI: 10.1002/14651858.CD004108.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Storms 2005
- Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. Journal of Asthma 2005;42:47‐89. [PubMed] [Google Scholar]
Tasche 2001
- Tasche MJA, Uijen JHJM, Bernsen RMD, Jongste JC, Wouden JC. Re: Sodium cromoglycate in childhood asthma (Authors' reply). Thorax 2001;56:331‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Houwelingen 1995
- Houwelingen HC. Meta‐analysis; methods, limitations and applications. Biocybernetics and Biomedical Engineering 1995;15:53‐61. [Google Scholar]
References to other published versions of this review
Tasche 2000
- Tasche MJA, Uijen JHJM, Bernsen RMD, Jongste JC, Wouden JC. Inhaled disodium cromoglycate (DSCG) as maintenance therapy in children with asthma: a systematic review. Thorax 2000;55:913‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Wouden 2003
- Wouden JC, Tasche MJA, Bernsen RMD, Uijen JHJM, Jongste JC, Ducharme FM. Sodium cromoglycate for asthma in children. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD002173. DOI: 10.1002/14651858.CD002173] [DOI] [PubMed] [Google Scholar]


