Abstract
Adult-onset Still’s disease is a rare inflammatory disorder usually affecting young adults. Elderly-onset Still’s disease (EOSD) is reported in some cases, commonly in Japan, the USA and Europe. One of the most commonly used criteria for diagnosing EOSD is Yamaguci criteria. In elderly patients more severe course of the disease and more complications may be expected than in the younger group of patients with Still’s disease. The lungs involvement is rather rare manifestation of this disease. In our article we discuss the problem of both the development of Still’s disease in the elderly and interstitial lung changes in the course of the disease, based on available literature and own cases from one centre.
Keywords: Still’s disease, lung, aged, adult-onset
Introduction
Adult-onset Still’s disease (AOSD) is a rare inflammatory disorder of unknown etiology with an estimated prevalence of 1 to 10 cases per million [1, 2]. It usually affects young patients between 16 and 35 years old, but some cases of elderly patients have been reported [3–7]. AOSD clinical presentation includes four main symptoms: spiking fever, arthralgia or arthritis, skin rash and leukocytosis ≥ 10,000 cells/µl with neutrophils ≥ 80%.
An elevation in the total and glycosylated ferritin levels is suggestive of AOSD but is not pathognomonic [2]. Many other manifestations are possible, making diagnosis challenging, especially in the elderly [3, 4]. Several features have been found to be associated with this category of patients with a higher frequency of complications and poor survival [3, 4]. However, the number of elderly-onset Still’s disease (EOSD) cases remains insufficient to explore their clinical features.
We aim through this article to highlight the fact that the elderly can develop typical AOSD with some characteristic features and discuss these characteristics for this age group by reviewing the literature.
Material and methods
The aims of this case-based review were to report three cases of EOSD (among 21 patients with AOSD) and to perform a literature review of EOSD case reports and series.
Searches were conducted in PubMed, Scopus (ScienceDirect) and Google Scholar, for the period between January 1983 and May 2021. Search terms included: “adult-onset Still’s disease”, “elderly-onset Still’s disease”, “elderly” and “aged”.
Inclusion criteria included case reports or series reporting specific characteristics of EOSD (age ≥ 60 years old) fulfilling the diagnostic criteria proposed by Yamaguchi [2]. Only English reports (full texts or abstracts) were considered. The references of the studies obtained were also examined to identify additional reports.
Among 74 articles resulting from the search, 43 were finally selected for the review (41 case reports [3, 5–44] and 2 case series [4, 45]) (Fig. 1). Suda et al. [3] and Mollaeian et al. [42] reported 1 case of EOSD and reviewed, from the literature, 24 and 38 cases, respectively.
Fig. 1.
Literature review flow chart of elderlyonset Still’s disease case reports and series.
Results
Case 1
A 61-year-old woman, with no pathological history, was admitted for fever, evanescent maculopapular rash on the limbs, polyarthralgia of small and large joints and sore throat for 1 month. Laboratory tests showed a biologic inflammatory syndrome.
The C-reactive protein (CRP) was 354 mg/l and the erythrocyte sedimentation rate (ESR) was 104 mm/hour. The white blood cell count (WBC) was elevated to 14 500 cells/µl.There were hepatic cytolysis and cholestasis. Serum protein electrophoresis showed polyclonal hypergammaglobulin at 16.7 g/l.
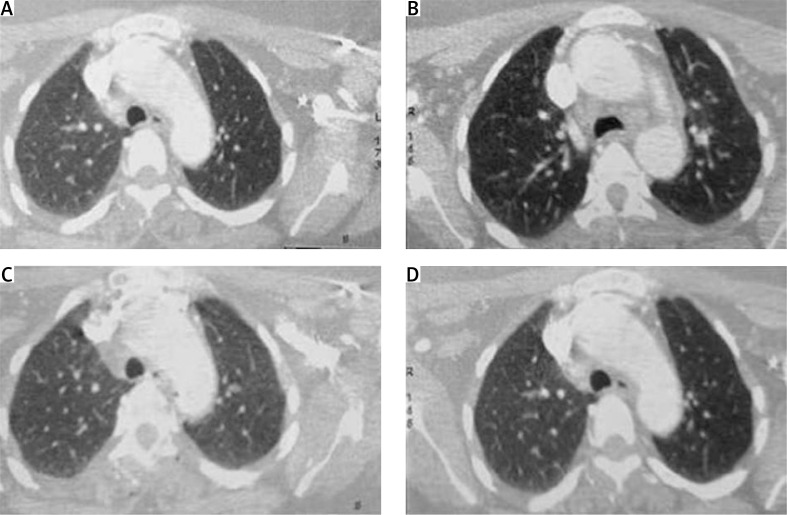
There was no biological evidence of infections. The serum ferritin was higher than 2000 ng/ml. Rheumatoid factor (RF), anti-citrullinated peptide antibodies (ACPA) and anti-nuclear antibodies (ANA) were not present. Cardiac ultrasound was normal. Thoraco-abdominopelvic computed tomography (CT) showed diffuse pulmonary nodules (Fig. 2), deep lymph nodes, splenomegaly and hepatomegaly.
Fig. 2.
Computed tomography: diffuse pulmonary nodules.
Histopathology of the liver biopsy found periportal non-specific inflammation. Joint radiography was normal. We diagnosed EOSD according to the Yamaguchi criteria. Initially, the patient was treated with glucocorticoids (GCs) 1 mg/kg/day, obtaining remission and stopping treatment after 14 months.
The patient relapsed 3 and 4 years later. The treatment with GCs 0.5–1 mg/kg/day has been applied. The complication of GCs treatment was weight gain and developement of arteruial hypertension. A control CT of the lungs showed diffuse pulmonary nodules at the upper and middle right lobe of the lung. Methotrexate (MTX) at the initial dose 12.5 mg/week was initiated 5 years later with a good outcome over a 3-year follow-up.
Case 2
A 68-year-old woman, with no pathological history, presented for 6 weeks deterioration of the general state, fever, evanescent maculopapular rash on the trunk, sore throat and tenosynovitis of the hands.
The CRP was 230 mg/l and the ESR was 80 mm/hour. The WBC was elevated to 14 600 cells/µl. Normochromic normocytic anemia at 10.8 g/dl was found. Serum protein electrophoresis showed polyclonal hypergammaglobulinemia. The serum ferritin was 11.420 ng/ml.
There was no biological evidence of infections. RF, ACPA and ANA antibodies were not present. Cardiac ultrasound was normal. Thoraco-abdominopelvic CT showed diffuse interstitial lung infiltrates. Joint radio-graphy was normal.
We diagnosed EOSD according to the Yamaguchi criteria. The patient was treated with indomethacin without clinical improvement. Corticosteroid therapy at 0.5 mg/kg/day was initiated with a good outcome over a 2-year follow-up.
Case 3
A 70-year-old man was admitted with fever, deterioration of the general condition, weight loss and myalgia. He had hypertension treated with irbesartan hydrochlorothiazide, diabetes mellitus treated with oral antidiabetics and a history of colonic adenocarcinoma treated a year earlier and in remission.
The described patient had high elevated inflammatory parameters including CRP of 183 mg/l and ESR of 130 mm/hour. The procalcitonin level was 0.148 ng/ml. The WBC was 17 800 cells/µl with neutrophils ≥ 80%. Serum protein electrophoresis did not indicate monoclonal gammopathy or hypogammaglobulinemia. The microbiological assessment did not confirm any infections. The serum ferritin was 1000 ng/ml. There was no presence of RF, ACPA, ANA antibodies or neutrophilic anticytoplasmic (cANCA) in the patient’s serum.
The cardiac ultrasound was normal. Thoracic-abdominopelvic CT showed reticulation and honeycombing involving mainly the lung periphery, without other abnormalities. The endoscopic examination showed antro-fundal gastritis and no malignancy was identified in the biopsies. The colonoscopy was normal. Doppler ultrasound and temporal artery biopsy were normal. The bone marrow biopsy was normal. The glucocorticosteroid therapy at dose a 1 mg/kg/day was prescribed as an initial dose with subsequent dose reduction and good outcome over a 3-year follow-up.
Discussion
Usually, the onset of AOSD is in early adulthood, but in our 3 cases the onset was at an advanced age. EOSD is reported in some cases [3–45], and its frequency is still increasing because of the aging of society.
According to a Japanese study in 2016, about 23% developed EOSD at ≥ 65 years old and about 16% at ≥ 75 years old among AOSD cases [53]. According to a review of the literature, EOSD has been described more often in Japan (27/43 articles), the USA (7/43 articles) and Europe (2/38 articles) (Table I) [3–45].
Table I.
Summary of case reports and series of elderly onset Still’s disease available in the literature
| Authors, year | Country | No. of cases | Age, years | Gender | Clinical features | Complications | Medications | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Suda et al., 2019 [3] | Japan | 1 | 74 | Female | Fever, rash and sore throat | MAS, cytomegalovirus (CMV) infection | Corticosteroids, cyclosporine | Remission, 2 years |
| Maruyama et al., 2020 [4] | Japan | 47 | 71 ±7.3 | 39 females, 8 males | Rash (21.3%), sore throat (55.3%) | Pleurisy (27.7%), DIC (19.1%) and MAS (17%) | Cyclosporine: 61.7% Plasma exchange: 8.5% |
Death: 14.9% Remission: 14.7% |
| Stoica et al., 2002 [5] | USA | 1 | 74 | Female | Severe respiratory failure and multiorgan dysfunction Chest computed tomography = non-specific pulmonary fibrosis Histology = extensive diffuse interstitial fibrosis with organizing pneumonitis |
Corticosteroids and IV gammaglobulin | Death | |
| Kurasawa et al., 2007 [6] | Japan | 1 | 83 | Female | Fever, sore throat, polyarthralgia, rash, appetite loss, leukocytosis, elevated ferritin and liver dysfunction | Pneumocystis carinii, pneumonia | Corticosteroids, methotrexate | Favorable, 2 months |
| Apostolova et al., 2011 [7] | USA | 1 | 72 | Male | Generalized weakness, weight loss, fever and arthralgia, leukocytosis, elevated ESR, CRP, ferritin and liver enzymes | Pulmonary hypertension and bilateral pleurisy | Corticosteroids | Favorable |
| Jiao et al., 2020 [8] | China | 1 | 77 | Female | Bilateral total knee arthroplasty 6 years ago Fever, right knee arthritis, rash and sore throat |
– | Corticosteroids | Favorable |
| Halpern et al., 2019 [9] | – | 1 | 68 | Female | Fever, rash, polyarthritis, sore throat, diarrhea, leukocytosis, elevated ferritin and liver enzymes | – | Corticosteroids | Favorable |
| Rubenstein et al., 2004 [10] | USA | 1 | 75 | Male | Fever, arthritis, rash, sore throat, leukocytosis, and elevated ferritin | – | Corticosteroids, IV immunoglobulin, methotrexate | Remission |
| Hartman et al., 2013 [11] | USA | 1 | 75 | Male | Fever, arthralgia, rash, leukocytosis and hyperferritinemia | – | Corticosteroids | Polycyclic, 2 years |
| Tamura et al., 1994 [12] | Japan | 1 | 74 | Female | Fever, sore throat, eruption, arthralgia, and lymphadenopathy | – | NSAID, corticosteroids | Favorable |
| Vilá et al., 2007 [13] | Puerto Rico | 1 | 76 | Female | Fever, arthritis, rash, pleurisy, abdominal pain, lymphadenopathy, chronic anemia and thrombocytosis | – | Corticosteroids and methotrexate | Favorable |
| Steffe et al., 1983 [14] | USA | 1 | 70 | Female | Fever, rash, polyarthritis, pleurisy | – | Aspirin | Remission, 2 years |
| Koga et al., 1992 [15] | Japan | 1 | 72 | Female | Fever, rash, polyarthritis, sore throat, leukocytosis, elevated ferritin and liver enzymes | – | NSAID, corticosteroids | Favorable |
| Ichiki et al., 1992 [16] | Japan | 2 | 61, 83 | Female | Fever, arthralgia, sore throat, rash and leukocytosis | – | Corticosteroids | Favorable |
| Takami et al., 1995 [17] | Japan | 1 | 74 | Female | Fever, rash, polyarthralgia, lymphadenopathy, leukocytosis and elevated ferritin | Submassive hepatic necrosis | Corticosteroids | Favorable |
| Yokoyama et al., 1995 [18] | Japan | 1 | 71 | Male | Fever, sore throat, myalgia and rash and elevated ferritin | Respiratory distress syndrome, DIC | Corticosteroids, nafamostat mesylate | Favorable |
| Kurabayashi et al., 1996 [19] | Japan | 1 | 75 | Female | Fever, rash and polyarthritis | Cerebral hemorrhage | NSAID, corticosteroids, cyclophosphamide, mizoribine and auranofin | Favorable |
| Sanada et al., 1997 [20] | Japan | 1 | 82 | Female | Fever, rash, swollen axillary lymph nodes, leukocytosis, and abnormal liver function tests | Subdural hematoma, DIC | – | – |
| Schifter et al., 1998 [21] | Israel | 1 | 66 | Female | Fever, rash and polyarthritis; adenocarcinoma 16 years ago | – | NSAID, corticosteroids, methotrexate | Polycyclic, 27 months |
| Limsukon et al., 2009 [22] | Japan | 1 | 60 | Male | Fever, myalgia, fatigue, pleuropericarditis, leukocytosis, elevated ESR and ferritin | Focal pulmonary capillaritis with alveolar hemorrhage, coma, tamponade | Corticosteroids, etanercept and anakinra | Remission, 6 months |
| Sumida et al., 2010 [23] | Japan | 1 | 69 | Female | Fever, polyarthralgia, sore throat, rash, leukocytosis, elevated ESR, ferritin and liver dysfunction, chest CT = bilateral pleurisy and diffuse alveolar densities | Thrombotic thrombocytopenic purpura and multiorgan dysfunction (respiratory distress, coma and microangiopathic cerebral infarction) | Corticosteroids, cyclosporine A, plasma exchange, etanercept, tocilizumab | Favorable |
| Yoshioka et al., 2011 [24] | Japan | 1 | 61 | Female | Fever, sore throat, polyarthralgia, rash, leukocytosis, elevated ESR, CRP, ferritin and liver enzymes | – | Corticosteroids | Favorable |
| Ertugrul et al., 2012 [25] | Turkey | 1 | 83 | Female | Fever, rash, arthromyalgia and hepatomegaly | – | Corticosteroids | Favorable |
| Kato et al., 2012 [26] | Japan | 1 | 78 | Male | Fever, rash, arthralgia, myalgia, episcleritis, leukocytosis, elevated ESR, CRP, ferritin and liver enzymes | Bacterial pneumonia (tocilizumab); reaction of injection site, angioedema (etanercept) | Corticosteroids, methotrexate, cyclosporine, tacrolimus, tocilizumab, etanercept | Polycyclic |
| Naniwa et al., 2013 [27] | Japan | 1 | 64 | Female | Fever, rash, arthritis, leukocytosis and liver dysfunction | DIC | Corticosteroids, cyclosporine, tacrolimus, immunoglobulin IV, tocilizumab | Remission, 1 year |
| Kiyonaga et al., 2014 [28] | Japan | 1 | 84 | Male | Fever, arthralgia, leukocytosis, elevated ferritin and liver dysfunction | – | Corticosteroids, cyclosporine methotrexate, etanercept | Polycyclic (1 year), remission (1 year) |
| Kumano et al., 2014 [29] | Japan | 1 | 80 | Female | Fever, rash and lichenoid plaques, sore throat, arthralgia, leukocytosis, elevated ferritin and liver dysfunction | – | Corticosteroids and NSAID | Polycyclic, 2 years |
| Umeda et al., 2014 [30] | Japan | 1 | 71 | Female | Fever, myalgia, throat pain, arthralgia, rash, lymph node swelling, hepatosplenomegaly | MAS and inflammatory myopathy | Corticosteroids | Favorable |
| Watanabe et al., 2016 [31] | Japan | 1 | 71 | Female | Fever, fatigue, rash, sore throat, loss of appetite, lymphadenopathy | DIC, MAS, CMV infection, Pneumocystis jirovecii pneumonia | Corticosteroids, tocilizumab, tacrolimus | Remission, 18 months |
| Yamashita et al., 2017 [32] | Japan | 1 | 88 | Female | Fever, rash, splenomegaly and pleurisy | DIC, MAS | Corticosteroids, cyclosporine | Favorable |
| Usuda et al., 2018 [33] | Japan | 1 | 66 | Female | Fever, arthralgia and rash | – | Corticosteroids, cyclosporine | Favorable |
| Ito et al., 2019 [34] | Japan | 1 | 63 | Male | Rash, arthralgia, lymphadenopathy and splenomegaly, leukocytosis, elevated ferritin and liver enzymes | Aseptic meningitis, acute renal insufficiency | Corticosteroids | Favorable |
| Kato et al., 2020 [35] | Japan | 1 | 69 | Male | Fever, arthralgia, leukocytosis, elevated ferritin and liver enzymes | Thrombotic thrombocytopenic purpura, septic shock and multiple organ failure | Plasma exchange, corticosteroids, cyclophosphamide and cyclosporine | Death |
| Bhamra et al., 2020 [36] | USA | 1 | 66 | Female | Shortness of breath, rash, myalgia, arthralgia. | Segmental pulmonary embolism | Corticosteroids and methotrexate | Favorable |
| Borg et al., 2020 [37] | Malta | 1 | 73 | Male | Fever, sore throat, pleurisy, rash, neutrophilia and hyperferritinaemia | – | Corticosteroids | Monocyclic, 3 months |
| Ohmura et al., 2020 [38] | Japan | 1 | 73 | Female | Fevers, rash, sore throat, oligoarthritis and splenomegaly | Severe refractory MAS | Corticosteroids, cyclosporine, methotrexate, etoposide, tocilizumab | Remission, 17 months |
| Mok et al., 1998 [39] | China | 1 | 80 | Female | Fever, arthritis, sore throat, hepatosplenomegaly, leukocytosis, anemia and abnormal liver function tests | – | NSAID | Remission, 11 months |
| Kamada et al., 2020 [40] | Japan | 1 | 88 | Female | Fever, arthritis, sore throat, leukocytosis, elevated ferritin and liver enzymes | Pulmonary tuberculosis | Corticosteroids | Remission, 2 years |
| Jeong et al., 2021 [41] | Korea | 1 | 77 | Female | Fever, myalgia, arthralgia, and sore throat , lymphadenopathy, splenomegaly and pleurisy, leukocytosis, elevated ferritin and liver enzymes | – | Corticosteroids | Favorable |
| Mollaeian et al., 2021 [42] | USA | 1 | 73 | Female | Fever, rash, arthralgia, leukocytosis, lymphadenopathy on CT scan | – | Corticosteroids, anakinra | Favorable |
| Koizumi et al., 2000 [43] | Japan | 1 | 74 | Female | Fever, sore throat, rash, arthralgia, leukocytosis and elevated ferritin | – | NSAID, corticosteroids | Favorable |
| Goh et al., 2020 [44] | India | 1 | 78 | Male | Fever, myalgia, arthralgia, fatigue and weight loss | Acute ischemic cerebral infarcts | Corticosteroids | Favorable |
| Kikuschi et al., 2014 [45] | Japan | 4 | 77 ±6.6 | Females | Fever, arthralgia, rash (100%), lymphadenopathy (75%), fatigue (25%), leukocytosis, elevated ferritin and liver dysfunction (100%) | Pericardial and pleural effusions on CT scan (25%), MAS (50%) | Corticosteroids | Favorable |
NSAID – non-steroidal anti-inflammatory drug, ESR – erythrocyte sedimentation rate, IV – intravenous, CT – computed tomography, DIC – disseminated intravascular coagulation, MAS – macrophage activation syndrome.
One case was reported from North Africa (Tunisia) [51] and it was associated with squamous cell carcinoma. The mean age of onset was 73.5 ±7 years and 79.5% were female.
A study compared the clinical features between 25 EOSD cases (age ≥ 70 years) and a group of 166 AOSD cases [3]. In this literature review, the overall AOSD group included less than 10–20% EOSD cases because reports of AOSD included all ages [3]. The mean age of onset was 76.6 ±4.9 and 24–46 years in the EOSD and AOSD groups, respectively [3]. Seventy-five percent of the patients were female [3]. Elderly-onset Still’s disease showed similar clinical signs and laboratory findings to overall AOSD [3]. Indeed, patients often presented with fever (higher than 39°C), evanescent rash, lymphadenopathy, sore throat, arthralgia/arthritis, myalgia, and serositis [7] (Table I). Another recent Japanese study demonstrated that EOSD (> 60 years old) patients less often had typical skin rashes and sore throat and more often had pleuritis than AOSD [4].
In case 3, the patient presented deterioration of the general condition and weight loss, which is rarely reported in the literature [7, 44]. Neoplasia, infections and systemic diseases need to be ruled out before the diagnosis is established.
Parenchymal lung involvement (PLI) was found in the 3 patients. In the literature, two cases were reported in EOSD with severe respiratory failure [5, 22] and coma [22]. However, two other patients in the 7th decade showed pleuritis and pneumonitis according to an abstract not included in the literature review (lack of information) [54]. Gerfaud-Valentin et al. [55] reported that PLI occurred in nearly 5% of AOSD cases. In the parenchymal lung involvement group, the patients were younger at AOSD onset compared with the non-PLI group. The distribution of PLI was equivalent between men and women [55]. The chest X-ray and CT revealed unilateral or bilateral interstitial hyperdensities in 72% and alveolar hyperdensities in 50% with an air bronchogram in 33% [55].
Rare cases of bilateral pulmonary nodules have been reported [56, 57]. Parenchymal lung involvement was classified into 2 groups: predominant airway involvement (bronchiolitis and bronchitis) and predominant interstitial lung disease (nonspecific interstitial pneumonia, organizing pneumonia, or unclassifiable interstitial pneumonia) [55].
Concerning the laboratory findings, leukocytosis (≥ 10,000 cells/µl) and liver abnormalities have been described in 84% of EOSD cases [3]. Likewise, leukocytosis, elevated ferritin level and liver enzymes were frequent in our study (Table I). In the Japanese study, aspartate aminotransferase and ferritin (12.700 ng/ml vs. 2.526 ng/ml, respectively; p < 0.0001) were higher compared with the younger patients [7].
EOSD complications occurred in 54.3% (n = 25/46) of the reviewed case reports (one case series excluded [4]): disseminated intravascular coagulation (10.8%, n = 5/46) and infections (13%) were the most common (Table I). In agreement, disseminated intravascular coagulation was found to occur more frequently in the EOSD group than in the AOSD group [3, 4].
However, the frequency of macrophage activation syndrome (MAS) was similar between the AOSD and EOSD groups [3, 4]. The Yamaguchi criteria have been used to aid in the diagnosis with high sensitivity (above 93%) and specificity (92.1%) [7]. So, as elderly patients can develop typical AOSD, physicians should not exclude EOSD from the differential diagnosis.
As for the medications, corticosteroids at 0.5–1 mg/kg/day were given for our 3 patients, and methotrexate was useful for controlling the disease and for corticosteroid-sparing treatment in our first case. According to our literature review and that of Suda et al. [3], corticosteroids were commonly used in EOSD (95.1% in the reviewed case reports), and methylprednisolone therapy was used in about one-third of cases. Methotrexate and biologics were prescribed in 17.7% of cases (Table I).
In the review cited above, immunosuppressants (methotrexate and cyclosporin) and biologics were used less often in cases of EOSD than AOSD (24% vs. 80.7% and 8% vs. 19.9%, respectively). Methotrexate was often used in overall AOSD (41%) [3].
Another Japanese study found that corticosteroids (including methylprednisolone), methotrexate, and biological agents were not different between the two groups. However, cyclosporine and plasma exchange was more frequently used in the elderly-onset group due to the higher prevalence of complications [4].
Biologics such as tocilizumab, etanercept and anakinra have demonstrated good outcomes, especially in refractory EOSD or in case of complications [22, 23, 28, 31, 35, 38, 58, 59]. These treatments need to be prescribed carefully in EOSD because of serious adverse events including opportunistic infections, anaphylactic reactions or biologic-associated macrophage activation syndrome [26, 31].
Regarding the evolution, it was polycyclic in our first case and monocyclic in our two subsequent cases. In the literature, the comparison of rates of remission, relapse and death between AOSD and EOSD is controversial. It was similar for Suda et al. [3], but the observation period was longer in the overall AOSD group.
According to Maruyama et al. [4], the EOSD-related mortality rate was higher, at 10.6% (p = 0.0023), and infection was the cause of death. Fewer elderly-onset patients achieved drug-free remission (remission rate at 5 years: 14.7% vs. 45.6%, p = 0.003) [4].
Conclusions
Adult Still’s disease should not be overlooked in elderly patients. However, it remains a diagnosis of elimination, particularly in the presence of fever or deterioration of the general condition. Parenchymal pulmonary involvement, found in the three described patients, is seldom encountered in adult and elderly Still’s disease.
Unlike our cases, the clinical features of the literature review were characterized by a higher frequency of complications that need immunosuppressive drugs. However, these treatments should be used with extreme caution in elderly-onset patients because of the risk of serious adverse events.
Footnotes
The authors declare no conflict of interest.
References
- 1.Evensen KJ, Nossent HC. Epidemiology and outcome of adult-onset Still’s disease in Northern Norway. Scand J Rheumatol. 2006;35:48–51. doi: 10.1080/03009740510026616. [DOI] [PubMed] [Google Scholar]
- 2.Fautrel B. Adult-onset Still’s disease. Best Pract Res Clin Rheumatol. 2008;22:773–792. doi: 10.1016/j.berh.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Suda T, Zoshima T, Takeji A, et al. Elderly-onset Still’s disease complicated by macrophage activation syndrome: a case report and review of the literature. Intern Med. 2020;59:721–728. doi: 10.2169/internalmedicine.3727-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama A, Kokuzawa A, Yamauchi Y, et al. Clinical features of elderly-onset adult-onset Still’s disease. Mod Rheumatol. 2020:1–7. doi: 10.1080/14397595.2020.1829340. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Stoica GS, Cohen RI, Rossoff LJ. Adult Still’s disease and respiratory failure in a 74 year old woman. Postgrad Med J. 2002;78:97–98. doi: 10.1136/pmj.78.916.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurasawa M, Kotani K, Kurasawa G, et al. Adult-onset Still’s disease in a patient over 80 years old successfully treated with low-dose methotrexate therapy. Age Ageing. 2007;36:104–106. doi: 10.1093/ageing/afl128. [DOI] [PubMed] [Google Scholar]
- 7.Apostolova M, Shoib M, Glynn M. A rare presentation of adult-onset Still’s disease in an elderly patient. Rheumatology Reports. 2011;3:e10–e10. doi: 10.4081/rr.2011.e10. [DOI] [Google Scholar]
- 8.Jiao X, Li Z, An S, et al. An elderly female with adult-onset Still’s disease initially misdiagnosed as prosthetic joint infection after total knee arthroplasty: lessons in differential diagnosis and treatment. BMC Geriatr. 2020;20:512. doi: 10.1186/s12877-020-01925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern D, Fagan M, Duh E. Adult-onset Still’s disease in an elderly patient. Pediatrics. 2019;144 10.1542/peds.144.2_MeetingAbstract.589. [Google Scholar]
- 10.Rubenstein EJ, Arkfeld DG. Adult Still’s disease in a 75-year-old patient. J Am Geriatr Soc. 2004;52:2144–2145. doi: 10.1111/j.1532-5415.2004.52579_5.x. [DOI] [PubMed] [Google Scholar]
- 11.Hartman Adult-onset Still’s disease in a geriatric patient: Updates and pearls in diagnosis. JAAD. 2013;68(Suppl 1):AB8. doi: 10.1016/j.jaad.2012.12.035. [DOI] [Google Scholar]
- 12.Tamura K, Kubota K, Kurabayashi H, et al. Elderly onset of adult Still’s disease: report of a case. Clin Rheumatol. 1994;13:117–118. doi: 10.1007/BF02229879. [DOI] [PubMed] [Google Scholar]
- 13.Vilá LM, Molina MJ. Chronic anemia and thrombocytosis as the initial presentation of Still’s disease in an elderly patient. Gerontology. 2007;53:289–292. doi: 10.1159/000102949. [DOI] [PubMed] [Google Scholar]
- 14.Steffe LA, Cooke CL. Still’s disease in a 70-year-old woman. JAMA. 1983;249:2062–2063. doi: 10.1001/jama.1983.03330390066034. [DOI] [PubMed] [Google Scholar]
- 15.Koga T, Tokunaga N, Ichikawa Y, Oizumi K. A 72-year-old female with adult Still’s disease. Intern Med. 1992;31:1356–1358. doi: 10.2169/internalmedicine.31.1356. [DOI] [PubMed] [Google Scholar]
- 16.Ichiki H, Shishido M, Nishiyama S. Two cases of adult onset of Still’s disease in the elderly. Nihon Ronen Igakkai Zasshi. 1992;29:960–964. doi: 10.3143/geriatrics.29.960. [Article in Japanese] [DOI] [PubMed] [Google Scholar]
- 17.Takami A, Nakao S, Miyamori H, et al. Adult-onset Still’s disease with submassive hepatic necrosis. Intern Med. 1995;34:89–91. doi: 10.2169/internalmedicine.34.89. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama M, Suwa A, Shinozawa T, et al. A case of adult onset Still’s disease complicated with adult respiratory distress syndrome and disseminated intravascular coagulation. Nihon Rinsho Meneki Gakkai Kaishi. 1995;18:207–214. doi: 10.2177/jsci.18.207. [Article in Japanese] [DOI] [PubMed] [Google Scholar]
- 19.Kurabayashi H, Kubota K, Tamura K, Shirakura T. Cerebral haemorrhage complicating adult-onset Still’s disease: a case report. J Int Med Res. 1996;24:492–494. doi: 10.1177/030006059602400608. [DOI] [PubMed] [Google Scholar]
- 20.Sanada I, Kawano F, Tsukamoto A, et al. Disseminated intravascular coagulation in a case of adult-onset Still’s disease. Rinsho Ketsueki. 1997;38:1194–1198. [PubMed] [Google Scholar]
- 21.Schifter T, Lewinski UH. Adult-onset Still’s disease associated with Epstein-Barr virus infection in a 66-year-old woman. Scand J Rheumatol. 1998;27:458–460. doi: 10.1080/030097498442307. [DOI] [PubMed] [Google Scholar]
- 22.Limsukon A, Jones HD, Feinstein J. A 60-year-old Japanese man with fevers, myalgias, pharyngitis, and right knee pain. Chest. 2009;136:1428–1431. doi: 10.1378/chest.09-0722. [DOI] [PubMed] [Google Scholar]
- 23.Sumida K, Ubara Y, Hoshino J, et al. Etanercept-refractory adult-onset Still’s disease with thrombotic thrombocytopenic purpura successfully treated with tocilizumab. Clin Rheumatol. 2010;29:1191–1194. doi: 10.1007/s10067-010-1418-2. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka K, Fujimoto S, Oba H, et al. Onset of adult-onset Still’s disease following influenza vaccination. Mod Rheumatol. 2011;21:432–435. doi: 10.1007/s10165-011-0418-7. [DOI] [PubMed] [Google Scholar]
- 25.Ertugrul BM, Gencer P, Ozturk B, et al. Adult-onset Still’s disease in an 83-year-old. J Am Geriatr Soc. 2012;60:162–164. doi: 10.1111/j.1532-5415.2011.03735.x. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Noguchi K, Uehara M, et al. Angioedema of the periorbital region that developed during treatment with etanercept in a case of refractory adult-onset Still’s disease. Intern Med. 2012;51:2801–2804. doi: 10.2169/internalmedicine.51.8243. [DOI] [PubMed] [Google Scholar]
- 27.Naniwa T, Ito R, Watanabe M, et al. Case report: successful use of short-term add-on tocilizumab for multirefractory systemic flare of adult-onset Still’s disease. Clin Rheumatol. 2013;32:103–106. doi: 10.1007/s10067-010-1562-8. [DOI] [PubMed] [Google Scholar]
- 28.Kiyonaga Y, Maeshima K, Imada C, et al. Steroid-sparing effects of etanercept in a patient with steroid-dependent adult-onset Still’s disease. Intern Med. 2014;53:1209–1213. doi: 10.2169/internalmedicine.53.1488. [DOI] [PubMed] [Google Scholar]
- 29.Kumano T, Ishibashi H, Kubota Y, et al. Adult-onset Still’s disease in an elderly woman with atypical presentation. Case Reports in Internal Medicine. 2014;1:158. doi: 10.5430/crim.v1n2p158. [DOI] [Google Scholar]
- 30.Umeda M, Origuchi T, Fujikawa K, et al. Hemophagocytic syndrome and inflammatory myopathy with abundant macrophages in a patient with adult-onset Still’s disease. Intern Med. 2014;53:2385–2389. doi: 10.2169/internalmedicine.53.1081. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe E, Sugawara H, Yamashita T, et al. Successful tocilizumab therapy for macrophage activation syndrome associated with adult-onset Still’s disease: a case-based review. Case Rep Med. 2016:2016. doi: 10.1155/2016/5656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita S, Furukawa NE, Matsunaga T, et al. Extremely high serum ferritin: an instrumental marker of masquerading adult-onset Still’s Disease with hemophagocytic syndrome. Am J Case Rep. 2017;18:1296–1301. doi: 10.12659/ajcr.905684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Usuda D, Furumura Y, Takeshima K, et al. Interleukin-18 as a diagnostic marker of adult-onset Still’s disease in older patients: a case report and review of the literature. J Med Case Rep. 2018;12:198. doi: 10.1186/s13256-018-1735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito N, Takahashi M, Miwa Y, et al. Adult-onset Still’s disease presenting with aseptic meningitis as the first symptom in an elderly patient. eNeurologicalSci. 2019;16:100202. doi: 10.1016/j.ensci.2019.100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato R, Ikeuchi T, Tomita K, Yamasaki A. Adult-onset Still’s disease with concurrent thrombotic thrombocytopenic purpura: case report and literature review. BMJ Case Rep. 2020;13:e235786. doi: 10.1136/bcr-2020-235786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhamra M, Amarnani A, Ozeri D. Unprovoked pulmonary embolism identified on initial presentation of adult-onset Still’s disease in an elderly patient with no malignancy. J Clin Rheumatol. 2020;26:e40–e42. doi: 10.1097/RHU.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 37.Borg J, Camilleri ML, Cassar PJ. Adult-onset Still’s disease in a 73-year-old Maltese man. BMJ Case Rep. 2020;13:e234752. doi: 10.1136/bcr-2020-234752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohmura SI, Uehara K, Yamabe T, et al. Successful use of short-term add-on tocilizumab for refractory adult-onset Still’s disease with macrophage activation syndrome despite treatment with high-dose glucocorticoids, cyclosporine, and etoposide. Modern Rheumatology Case Rep. 2020;4:202–207. doi: 10.1080/24725625.2020.1741073. [DOI] [PubMed] [Google Scholar]
- 39.Mok CC, Lau CS, Wong RW. Still’s disease in an 80-year-old woman. Age and Ageing. 1998;27:407–409. doi: 10.1093/ageing/27.3.407. [DOI] [Google Scholar]
- 40.Kamada M, Kenzaka T. Effectiveness of profiling serum IL-18 and neopterin in diagnosis of adult-onset Still’s Disease complicated by pulmonary tuberculosis: a case report. Tohoku J Exp Med. 2020;250:201–206. doi: 10.1620/tjem.250.201. [DOI] [PubMed] [Google Scholar]
- 41.Jeong WS, Kim J, Heo ST, et al. Atypical cutaneous features in a patient with adult-onset Still’s disease. Korean J Intern Med. 2021;36:742–743. doi: 10.3904/kjim.2019.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mollaeian A, Chen J, Chan NN, et al. Adult-onset Still’s disease in the elderly: a case-based literature review. BMC Rheumatology. 2021;5:12. doi: 10.1186/s41927-021-00183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koizumi R, Tsukada Y, Ideura H, et al. Treatment of adult Still’s disease with dexamethasone, an alternative to prednisolone. Scand J Rheumatol. 2000;29:396–398. doi: 10.1080/030097400447624. [DOI] [PubMed] [Google Scholar]
- 44.Goh Y, Wong VY, Tan WL, et al. An unusual cause of acute ischemic stroke: adult-onset Still’s disease. J Thromb Thrombolysis. 2020;49:141–144. doi: 10.1007/s11239-019-02006-5. [DOI] [PubMed] [Google Scholar]
- 45.Kikuchi N, Satoh M, Ohtsuka M, Yamamoto T. Persistent pruritic papules and plaques associated with adult-onset Still’s disease: report of six cases. J Dermatol. 2014;41:407–410. doi: 10.1111/1346-8138.12426. [DOI] [PubMed] [Google Scholar]
- 46.Yoshioka K, Fukushima H, Ishii N, et al. A case of chronic active Epstein-Barr virus infection mimicking adult-onset Still’s disease. Mod Rheumatol. 2013;23:162–166. doi: 10.1007/s10165-012-0620-2. [DOI] [PubMed] [Google Scholar]
- 47.Rossio R, Colombo G, Piconi S, Peyvandi F. Adult onset Still’s disease after human Herpesvirus 6 Infection in an elderly patient: a case rep. J Clin Rheumatol. 2019 doi: 10.1097/RHU.0000000000001225. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Nielly H, Jacquier C, Cournac JM, et al. Maladie de Still du sujet âgé: une revue systématique. Rev Med Interne. 2015;36:A160. doi: 10.1016/j.revmed.2015.03.184. [Article in French] [DOI] [Google Scholar]
- 49.Michels V, Delwaide J, Vermeulen P, et al. Clinical case of the month. Adult onset Still’s disease: a rare cause of acute febrile hepatitis. Rev Med Liege. 2003;58:729–733. [Article in French] [PubMed] [Google Scholar]
- 50.Bonnet F, Algayres J, Coutant G, et al. Still disease in the elderly. Rev Med Interne. 1997;18:170–171. doi: 10.1016/S0248-8663(97)84685-1. [Article in French] [DOI] [PubMed] [Google Scholar]
- 51.Marzouk S, Frikha O, Guermazi M, et al. Adult Still’s disease and squamous cell carcinoma in a 69-year-old woman. Pan Afr Med J. 2019;34:17. doi: 10.11604/pamj.2019.34.17.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lozano Morillo F, de la Cámara Fernández I, Enríquez Me-rayo E. Adult-onset Still’s disease associated with gastric cancer. Rev Colomb Reumatol. 2019;26:220–222. doi: 10.1016/j.rcreue.2018.09.007. [DOI] [Google Scholar]
- 53.Sakata N, Shimizu S, Hirano F, Fushimi K. Epidemiological study of adult-onset Still’s disease using a Japanese administrative database. Rheumatol Int. 2016;36:1399–1405. doi: 10.1007/s00296-016-3546-8. [DOI] [PubMed] [Google Scholar]
- 54.Wouters JM, van Rijswijk MH, van de Putte LB. Adult-onset Still’s disease in the elderly: a report of two cases. J Rheumatol. 1985;12:791–793. [PubMed] [Google Scholar]
- 55.Gerfaud-Valentin M, Cottin V, Jamilloux Y, et al. Parenchymal lung involvement in adult-onset Still’s disease. Medicine (Baltimore) 2016;95:e4258. doi: 10.1097/MD.0000000000004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perry R, Christidis D, Nicholson AG, et al. A case report of adult-onset Still’s disease presenting with acute fibrinous and organizing pneumonia. JRSM Open. 2020;11:0954406220913584. doi: 10.1177/2054270419894834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi H, Yin C, Xiao H, Duan T. A rare case of diffuse pulmonary nodules in a patient with adult-onset Still’s disease. Intern Med. 2014;53:1869–1872. doi: 10.2169/internalmedicine.53.1868. [DOI] [PubMed] [Google Scholar]
- 58.Kaneko Y, Kameda H, Ikeda K, et al. Tocilizumab in patients with adult-onset Still’s disease refractory to glucocorticoid treatment: a randomised, double-blind, placebo-controlled phase III trial. Ann Rheum Dis. 2018;77:1720–1729. doi: 10.1136/annrheumdis-2018-213920. [DOI] [PubMed] [Google Scholar]
- 59.Suematsu R, Ohta A, Matsuura E, et al. Therapeutic response of patients with adult Still’s disease to biologic agents: multicenter results in Japan. Mod Rheumatol. 2012;22:712–719. doi: 10.1007/s10165-011-0569-6. [DOI] [PubMed] [Google Scholar]