Abstract
Patient: Male, 70-year-old
Final Diagnosis: Drug induced polycythemia
Symptoms: Sural pain
Medication: —
Clinical Procedure: Endovascular treatment
Specialty: Oncology • Pulmonology
Objective:
Unusual or unexpected effect of treatment
Background:
Osimertinib is an oral third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) approved as first-line therapy for advanced non-small cell lung cancer (NSCLC) with positive EGFR mutation. Rashes, nail toxicity, and diarrhea are common adverse events. Hematological adverse effects, including anemia, thrombocytopenia, and lymphocytopenia, have been reported. However, erythrocytosis has not been reported as an adverse event. To the best of our knowledge, we report the first case of acute lower extremity thrombosis presumably caused by osimertinib-induced erythrocytosis.
Case Report:
A 70-year-old man with epidermal EGFR-mutant advanced NSCLC presented with acute left sural pain. The patient’s left foot was cold, and peripheral arterial Doppler signals were absent. He had developed erythrocytosis of unknown etiology during osimertinib therapy. Hemoglobin (Hb) and hematocrit were 22.6 g/dL and 62.5%, respectively. Contrast-enhanced computed tomography showed thrombotic occlusion of the popliteal artery. Other than erythrocytosis, there was no possible cause of arterial thrombosis. Osimertinib was discontinued immediately because the NSCLC started to resist treatment and was presumed to be the cause of erythrocytosis. He received endovascular treatment (EVT). Following serial EVT and debridement, his fourth toe was amputated for necrosis. Erythrocytosis persisted 8 months during osimertinib therapy. Hb levels decreased to 15.4 mg/dL due to blood loss complicated with catheter thrombectomy and remained normal for 20 months after osimertinib discontinuation. The patient died of cancer progression.
Conclusions:
This case suggests the erythrocytosis was possibly caused by osimertinib. We may need to monitor Hb levels during osimertinib therapy and be alert to thrombosis once Hb starts to rise.
Keywords: Antineoplastic Agents; Carcinoma, Non-Small-Cell Lung; Embolism and Thrombosis; Long Term Adverse Effects; Molecular Targeted Therapy; Polycythemia
Background
The recent development of tyrosine kinase inhibitors (TKIs) has dramatically improved the prognosis of patients with advanced non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) gene mutations [1–4]. Osimertinib is an oral third-generation EGFR-TKI specifically designed to inhibit EGFR-sensitizing mutations and acquired T790M mutations [5]. It is an approved first-line therapy for EGFR-mutant advanced NSCLC [6]. Rashes, nail toxicity, and diarrhea are common adverse events of the drug [1,7]. Hematological adverse events such as anemia, thrombocytopenia, and lymphocytopenia have also been reported [7]. However, erythrocytosis has not been previously reported as an adverse event of osimertinib. To the best of our knowledge, we report the first case of acute lower extremity thrombosis caused by osimertinib-induced erythrocytosis.
Case Report
A 70-year-old man with a history of hypertension and hyper-uricemia was diagnosed with EGFR-mutant advanced NSCLC and underwent treatment with afatinib. Twelve months later, disease progression was observed, and a second biopsy of the lung tumor was taken to investigate the resistant mutation in EGFR. A T790M mutation was identified and we began osimertinib therapy. A partial response was observed. Four months later, erythrocytosis developed. The patient’s hemoglobin (Hb) levels increased to 18 g/dL over 5 months (Figure 1). He did not report vomiting or diarrhea, and there were no other clinical signs of volume contraction, such as reduced skin turgor or tachycardia. His bone marrow smear, pathological examination, and serum erythropoietin levels were normal and JAK2V617F was not mutated, ruling out polycythemia vera. The patient had quit smoking 10 years prior and did not have a history of sleep apnea syndrome; polysomnography also showed no evidence of sleep apnea syndrome. The tumor continued to respond to osimertinib therapy. Low-dose aspirin was administered to prevent thrombosis. Since the cause of erythrocytosis was unclear, we continued osimertinib therapy. Eight months later, the patient presented with acute left sural pain. His Hb level and hematocrit were 22.6 g/dL and 62.5%, respectively, with a mean corpuscular volume of 105.0 fL and mean corpuscular Hb of 38.0 pg.
Figure 1.
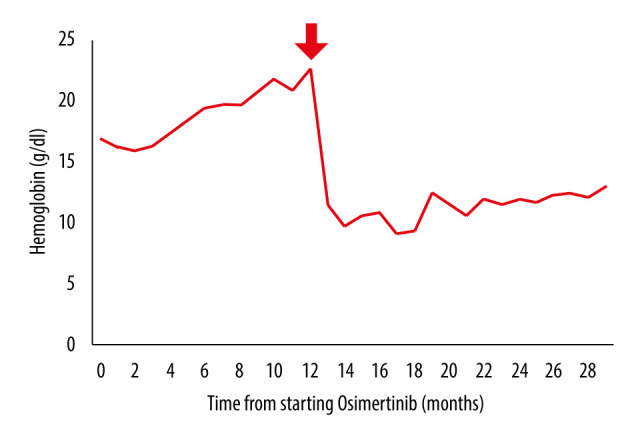
The trend of hemoglobin (Hb) level. The trend of Hb level from the initiation of osimertinib therapy is shown. The arrow indicates the timepoint at which acute arterial thrombosis developed and osimertinib was discontinued.
The patient’s vital signs on admission were as follows: body temperature, 36.7°C; blood pressure, 164/96 mm Hg; heart rate, 113 beats/min (regular); respiratory rate, 15 breaths/min; and oxygen saturation of 98% in ambient air. On examination, the patient’s left foot was cold, and the peripheral arterial Doppler signals were absent. The remaining complete blood cell count results revealed a white blood cell count of 3500/μL and a platelet count of 110×103/μL. The blood chemistry and coagulation panel results were as follows: blood urea nitrogen, 16.9 mg/dL; serum creatinine, 0.79 mg/dL; aspartate aminotransaminase, 39 U/mL; alanine aminotransferase, 27 U/mL; activated partial thromboplastin time, 40.5 s; and prothrombin time-international normalized ratio, 1.24. His lipid profile and HbA1c were within the reference ranges. Contrast-enhanced computed tomography (CT) and urgent angiography revealed thrombotic occlusion of the left popliteal artery. Progression of the tumor was also identified during CT. There was no evidence of severe atherosclerosis, aneurysm, or shunt in the pulmonary circulation. An electrocardiogram on presentation showed sinus tachycardia. The systolic function was normal, and cardiac defect or intracardiac thrombus were not noted on the transthoracic echocardiogram. Other than erythrocytosis, there was no possible cause of arterial thrombosis. Endovascular treatment (EVT) was successful, and the blood flow to the toe improved. Osimertinib was discontinued immediately because the lung cancer started to resist treatment and was presumed to be the cause of erythrocytosis.
The patient’s Hb levels decreased to 15.4 mg/dL due to blood loss complicated with catheter thrombectomy. Following serial EVT and debridement, his fourth toe was amputated because of necrosis. Anticoagulation therapy and wound care were continued.
On admission day 10, the patient was discharged. We initiated platinum-based combination chemotherapy in an outpatient setting. After the discontinuation of osimertinib, there was no recurrence of erythrocytosis or thrombosis (Figure 1). The cancer progressed, and the patient died 20 months after the thrombotic event.
Discussion
We reported the first case of arterial thrombosis associated with erythrocytosis caused by osimertinib therapy. Erythrocytosis increases the red blood cell (RBC) mass, leading to hyperviscosity and increased risk of both arterial and venous thrombosis [8]. In the present case, erythrocytosis was considered the most probable cause of acute lower extremity thrombosis because other possible causes of arterial thrombosis, such as atrial fibrillation, myocardial ischemia, severe arteriosclerosis, or paradoxical embolism through intracardiac or intrapulmonary shunt, were not identified. However, it could not be excluded that the cancer also led to the development of arterial thrombosis, in view of its progression [9]. Erythrocytosis is classified as either absolute or relative erythrocytosis [10]. The former is due to increased RBC production and the latter is due to decreased plasma volume [11]. Based on the patient’s medical history, physical examination, and normal blood urea nitrogen/creatinine levels, relative erythrocytosis (eg, dehydration) was excluded. Absolute erythrocytosis is further categorized into primary and secondary erythrocytosis [12]. The former includes polycythemia vera and other myeloproliferative neoplasms. According to the 2016 WHO classification, the patient did not meet the diagnostic criteria of polycythemia vera and other myeloproliferative neoplasms owing to the absence of hypercellularity of bone marrow and JAK2 gene mutation [13]. Secondary erythrocytosis occurs when RBC production is induced by increased erythropoietin [14]. The differential diagnosis of secondary erythrocytosis includes hypoxia-associated erythrocytosis and tumor-associated erythrocytosis. Chronic hypoxia could result from tobacco use, cyanotic heart disease, chronic obstructive pulmonary disease, carbon monoxide exposure, and sleep apnea syndrome [14]. These factors were not identified in the present case. Autonomous production of erythropoietin by various tumors, such as renal cell carcinoma, hepatocellular carcinoma, and hemangioblastoma, can cause secondary erythrocytosis [14]. They were also ruled out based on imaging studies, and the tumor continued to respond while the patient was receiving osimertinib therapy. Furthermore, he did not have any history of using anabolic steroids or exogenous erythropoietin that could be a pharmacological cause for erythrocytosis [15].
Erythrocytosis persisted for 8 months during osimertinib therapy. The patient’s Hb levels continued to be normal for 20 months after discontinuing osimertinib, even though the tumor progressed. This suggested that erythrocytosis was probably caused by osimertinib and was not a paraneoplastic effect. Previous clinical trials have not reported that erythrocytosis is associated with osimertinib therapy [1,7]. Vascular endothelial growth factor (VEGF)-TKIs, such as sunitinib, sorafenib, and pazopanib, have been reported to cause secondary erythrocytosis [16–19]. VEGF tyrosine kinases cause erythrocytosis, without elevating serum erythropoietin. Although the precise mechanism remains unclear, it is hypothesized that VEGF inhibition may sensitize erythropoietin or modulate the erythropoietic pathway [16]. Osimertinib is an EGFR-TKI and does not block the VEGF signaling pathway; therefore, osimertinib may cause erythrocytosis through a different mechanism. Further studies are required to investigate the association and mechanism of osimertinib-induced erythrocytosis.
Conclusions
We reported a case of arterial thrombosis which was probably due to osimertinib-induced erythrocytosis. We may need to consider monitoring Hb during osimertinib therapy and remain alert for thrombosis once Hb level starts to rise.
Footnotes
Conflicts of Interest
None declared.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 2.Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. Ann Oncol. 2015;26:1877. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 4.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemo-therapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 5.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network. (NCCN) Clinical practice guide-lines in oncology: NCCN guidelines for non-small cell lung cancer V.2. Fort Washington, PA: National Comprehensive Cancer Network; 2021. Available from: https://www.nccn.org. [Google Scholar]
- 7.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–40. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood. 2017;130:1795–99. doi: 10.1182/blood-2017-03-745349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Li A, Laureano M, et al. Frequency of arterial thromboembolism in populations with malignancies: A systematic review. Thromb Res. 2019;184:16–23. doi: 10.1016/j.thromres.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Silverstein MN. Relative and absolute polycythemia. How to tell them apart. Postgrad Med. 1987;81:285–88. doi: 10.1080/00325481.1987.11699802. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb NJ, Shih CF. Spurious polycythemia. Semin Hematol. 1975;12:397–407. [PubMed] [Google Scholar]
- 12.McMullin MF, Harrison CN, Ali S, et al. A guideline for the diagnosis and management of polycythaemia vera. A British Society for Haematology Guideline. Br J Haematol. 2019;184:176–91. doi: 10.1111/bjh.15648. [DOI] [PubMed] [Google Scholar]
- 13.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 14.Mithoowani S, Laureano M, Crowther MA, Hillis CM. Investigation and management of erythrocytosis. CMAJ. 2020;192:E913–18. doi: 10.1503/cmaj.191587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintzer DM, Billet SN, Chmielewski L. Drug-induced hematologic syndromes. Adv Hematol. 2009;2009:495863. doi: 10.1155/2009/495863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexandrescu DT, McClure R, Farzanmehr H, Dasanu CA. Secondary erythrocytosis produced by the tyrosine kinase inhibitors sunitinib and sorafenib. J Clin Oncol. 2008;26:4047–48. doi: 10.1200/JCO.2008.18.3525. [DOI] [PubMed] [Google Scholar]
- 17.Bukhari N, Winquist E. Secondary polycythemia due to pazopanib in patients with metastatic renal cell carcinoma. Can Urol Assoc J. 2017;11:E449–50. doi: 10.5489/cuaj.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harshman LC, Kuo CJ, Wong BY, Vogelzang NJ, Srinivas S. Increased hemoglobin associated with VEGF inhibitors in advanced renal cell carcinoma. Cancer Invest. 2009;27:851–56. doi: 10.1080/07357900902744528. [DOI] [PubMed] [Google Scholar]
- 19.Richard S, Croisille L, Yvart J, et al. Paradoxical secondary polycythemia in von Hippel-Lindau patients treated with anti-vascular endothelial growth factor receptor therapy. Blood. 2002;99:3851–53. doi: 10.1182/blood.v99.10.3851. [DOI] [PubMed] [Google Scholar]


