Abstract
Patient: Female, 38-year-old
Final Diagnosis: Coagulopathy • splenic marginal zone lymphoma
Symptoms: Abdominal swelling • early satiety
Medication: —
Clinical Procedure: Splenectomy
Specialty: Hematology
Objective:
Rare disease
Background:
Here, we report the novel presentation of a factor VII inhibitor in association with a new diagnosis of splenic marginal zone lymphoma in a previously healthy 38-year-old woman. There are only 4 reported cases of factor VII inhibitors, none of which are secondary to a splenic marginal zone lymphoma.
Case Report:
Our patient, a 38-year-old woman, presented reporting increased abdominal swelling and early satiety. She was found to have pancytopenia, an elevated international normalized ratio (INR), normal partial thromboplastin time (PTT), and massive splenomegaly. Further investigation revealed a morphology and immunopheno-type most consistent with splenic marginal zone lymphoma. A mixing study was unable to bring the INR into normal range after 60 min, confirming a factor VII inhibition. Therefore, the final diagnosis was primary splenic marginal zone lymphoma and secondary factor VII inhibitors. Owing to the elevated INR, both chemotherapy and splenectomy were avoided and we began a 4-week course of weekly rituximab infusions. After a second course of 4 treatments, there was a resolution of both the coagulopathy and the splenomegaly. At this point, the splenectomy was safely performed. Maintenance rituximab continued for 2 years. Our patient has now been in remission 12 years.
Conclusions:
We successfully treated a rare factor VII inhibitor and its underlying splenic marginal zone lymphoma with rituximab immunotherapy. A complete response was documented by splenectomy. The patient’s 12-year remission of both the lymphoma and the inhibitor helps to support the causative relationship between the lymphoma and the factor VII inhibitor.
Keywords: Blood Coagulation Factor Inhibitors; Factor VII; Hematology; Lymphoma, B-Cell, Marginal Zone; Rituximab; Splenectomy
Background
Here, we report the novel presentation of a factor VII inhibitor in association with a new diagnosis of splenic marginal zone lymphoma in a previously healthy 38-year-old woman.
Acquired factor VII inhibitors are exceedingly rare. Conversely, factor VIII inhibitors, although still rare, are well recognized as a condition known as acquired hemophilia A, with an estimated incidence of 1.3 to 1.5 cases per million per year. Factor VIII inhibitors are well characterized in the hemophilia A population requiring factor VIII replacement [1,2]. A patient with factor VIII inhibitors is thought to produce IgG against different epitopes on the factor VIII molecule [3,4]. These inhibitors are associated with pregnancy, rheumatic disease, drugs, and malignancy, and significantly increase the risk of bleeding in patients with hemophilia A. Factor VII deficiency is another rare inheritable bleeding disorder (1 in 500 000). Factor VII activity levels and inhibitors of the tissue factor: factor VII pathway do not correlate well with symptoms of bleeding, making reliable management of these patients difficult [5,6].
A patient with a factor VII inhibitor would present with a prolonged prothrombin time (PT) and a normal activated partial thromboplastin time (aPTT). In a factor deficiency, the mixing study would correct the PT. This is not the case in a factor inhibition. A low level or absence of factor VII activity, especially after prolonged incubation (minimum 1 h), is necessary to confirm the diagnosis of factor VII inhibitors. With both factor VIII and factor VII inhibitors, patient management should focus on controlling bleeding, decreasing the inhibitor concentration, and reversing the underlying cause [2]. The inhibitor can be quantified using the classic Bethesda clot-based method, where increasing dilutions of the patient’s plasma is mixed with an equal part of normal pooled plasma. It is incubated for 2 h, allowing time for the inhibitor to neutralize the factor at 37°C. A high titre is defined as a titre of at least 5 BU/mL [7].
There are only 4 cases of acquired factor VII inhibitors reported in the literature to date [8–11]. Here, we present the fifth case of factor VII inhibitor. This case presented with a new diagnosis of splenic marginal zone lymphoma (SMZL) in a 38-year-old woman. SMZL, a rare small B-cell lymphoma, often presents with splenomegaly as well as with bone marrow and blood involvement. While often asymptomatic, advanced-stage SMZL can present with symptomatic splenomegaly. Ten to twenty percent of patients also exhibit an associated autoimmune disease such as hemolytic anemia, immune thrombocytopenia, and circulating anticoagulants [12–14]. Owing to a lack of SMZL clinical trials, there are no specific treatment guidelines; however, recommendations include splenectomy, rituximab, and/or chemotherapy. SMZL can also be associated with hepatitis C virus, in which case an antiviral treatment is recommended. Survival varies widely and is highly dependent on clinical stage at the time of diagnosis as well as the treatment course [12,13].
Case Report
Our patient, a 38-year-old woman, presented to her family physician with increased abdominal swelling and early satiety. She had been previously worked up for anemia and placed on vitamin B12 and iron supplements. Due to persistent abdominal discomfort, she was referred to the Emergency Department for further investigation.
Upon admission, she was found to have pancytopenia (hemoglobin 68 g/L, white blood cells 2.0×109/L, platelets 103×109/L, neutrophils 0.98×109/L, lymphocytes 1.3×109/L, monocytes 0.18×109/L, eosinophils 0.03×109/L, basophils 0.03×109/L), an elevated INR of 3.2 (range, 0.8–1.2), and a normal PTT of 26 s (range, 22–36). An ultrasound revealed significant splenomegaly with associated hepatomegaly (17.6 cm), which were later confirmed by computed tomography (CT) scans of the abdomen and pelvis, with the spleen measuring 33 cm in the coronal plane images.
There was no evidence of adenopathy on ultrasound, no significant retroperitoneal or mesenteric lymphadenopathy on the CT scans, and no palpable cervical, supraclavicular, axillary, or inguinal adenopathy. A hepatitis screen was negative. IgM was elevated at 16.2 g/L (range, 0.46–3.04), with a discrete peak in the gamma region of the serum protein electrophoresis. Immunofixation was positive for IgM kappa. On a bone marrow biopsy analysis, patchy nodular infiltrates of small-to-medium sized lymphocytes involved one-third of the marrow. Immunohistochemical stains showed that these nodular collections of lymphocytes were strongly positive for CD20. CD23 was negative. CD5 staining was weaker and corresponded to the CD3 staining. CD138 and DBA44 were negative. Cyclin D1 was negative. Flow cytometry identified a monoclonal infiltrate positive for CD19, CD20, FMC7, and CD23 and was kappa light chain-restricted.
The differential diagnosis included other rare B-cell malignancies, such as hairy cell leukemia and B-cell prolymphocytic leukemia; however, these entities were ruled out by morphology, tissue immunohistochemistry, and flow cytometry. Hairy cell leukemia markers (CD11c, CD25, CD103) were negative by flow cytometry, and DBA-44 was negative by tissue immunohistochemistry. Prolymphocytes were not conspicuous in peripheral blood and bone marrow smears. B-cell prolymphocytic leukemia usually presents with a marked leukocytosis, which was not seen in this case. We know that one-third of splenic marginal zone lymphomas are associated with an elevated IgM, as was the case with this patient. Although polar villi were not apparent on circulating or bone marrow lymphocytes (Figure 1), the findings that the lymphoid neoplasm was associated with an elevated IgM and morphologic and immunophenotypic features were not compatible with a different lymphoproliferative neoplasm, supporting the diagnosis of splenic marginal zone lymphoma.
Figure 1.
Morphological features of circulating lymphocytes. Polar villi were not apparent in this case.
The coagulopathy was of particular interest because of the unusual presentation of an elevated PT and normal PTT. A mixing study did not bring the INR within normal range after 60 min. Instead, it remained elevated, indicating factor inhibition. A further coagulation study revealed a normal fibrinogen level of 2.78 g/L (range, 2.00–4.00) and a negative lupus anticoagulant. There was a markedly low factor VII level of 0.09 U/mL (range, 0.50–2.00) along with less severely decreased levels of factor II at 0.40 U/mL (range, 0.50–2.00), factor V at 0.37 U/mL (range, 0.50–2.00), and factor X at 0.46 U/mL (range, 0.50–2.00). Factor VII inhibitor was elevated at 4.8 Bethesda units and demonstrated cross reactivity with factors II, V, and VII. Therefore, the final diagnosis was twofold: primary splenic marginal zone lymphoma and a secondary factor VII inhibitor.
Before we were aware of the inhibitor, initial treatment involved several units of fresh frozen plasma (2–8 units on 4 separate days) along with intravenous vitamin K (10 units, administered twice). These interventions were ineffective in correcting the patient’s PT. Once the inhibitor was identified, 5 days of plasmapheresis partially corrected the PT, bringing it down from a high of 3.8 to 2.5.
As we assumed that the inhibitor was a consequence of the patient’s underlying lymphoma, lymphoma-directed treatment options, namely chemotherapy or splenectomy, were considered. Concerns regarding thrombocytopenia combined with an elevated INR during chemotherapy and the knowledge that fatal bleeding risk was common with factor VII inhibitor dissuaded us from this treatment. Splenectomy was also avoided because of the severe coagulopathy and associated surgical risk. After consultation, a 4-week course of weekly 375 mg/m2 rituximab infusions was started. Severe thrombocytopenia (14 000 platelet count) on the evening of her first dose was concerning; however, within the 4 days following the treatment, the platelet count rose above 75 000. The 4 cycles of rituximab yielded results of the INR normalizing to 1.4 and her spleen shrinking to being palpable only at the splenic tip. We repeated a second course of 4 treatments of rituximab, resulting in complete resolution of the coagulopathy and splenomegaly (Table 1, Figure 2). A splenectomy was performed following rituximab treatments since the coagulopathy was resolved. Immunohistochemical stains following the surgery revealed rare scattered B lymphocytes (CD20 and CD79a), explained by the previous rituximab treatment, and numerous macrophages (CD68 positive) in the red pulp. The lack of malignancy indicated complete remission to treatment.
Table 1.
Therapy timeline. An overview of the therapies employed in the first 2 months of treatment along with the associated international normalized ratio, platelet, and factor VII levels. “xx” indicates days when plasmapheresis was conducted.
| Date | Plasma-pheresis | IVIG (g) | Solumedrol (mg) | FFP units | Rituximab (mg) | INR | Platelet count | FVII (0.62–1.46 U/mL) |
|---|---|---|---|---|---|---|---|---|
| Feb 19 | 3.2 | 99 | ||||||
| Feb 20 | 110 | |||||||
| Feb 22 | 3.1 | |||||||
| Feb 23 | 2 | 2.9 | 112 | |||||
| Feb 24 | 4 | 3.1 | 114 | |||||
| Feb 25 | 100 | 2 | 2.9 | 104 | ||||
| Feb 26 | 620 | 3.8 | 101 | |||||
| Feb 27 | 50 | 8 | 2.9 | 14 | ||||
| Feb 28 | 50 | 3.1 | 31 | |||||
| Feb 29 | 3.1 | 0.09 | ||||||
| Mar 1 | 50 | 50 | 3.3 | 43 | ||||
| Mar 2 | 40 | 3.2 | 67 | |||||
| Mar 3 | xx | 40 | 3.4 | 79 | ||||
| Mar 4 | xx | 3.4 | 100 | |||||
| Mar 5 | xx | 3.2 | 105 | |||||
| Mar 5 | xx | 3.2 | 96 | |||||
| Mar 6 | xx | 2.7 | ||||||
| Mar 7 | xx | 2.5 | ||||||
| Mar 8 | 1.2 | 0.5 | ||||||
| Mar 9 | 2.1 | |||||||
| Mar 11 | 2.2 | 111 | ||||||
| Mar 12 | 620 | 2.2 | 63 | |||||
| Mar 13 | 2.2 | 56 | 0.73 |
INR – international normalized ratio; FFP – fresh frozen plasma; IVIG – intravenous immune globulin; FVII – factor VII.
Figure 2.
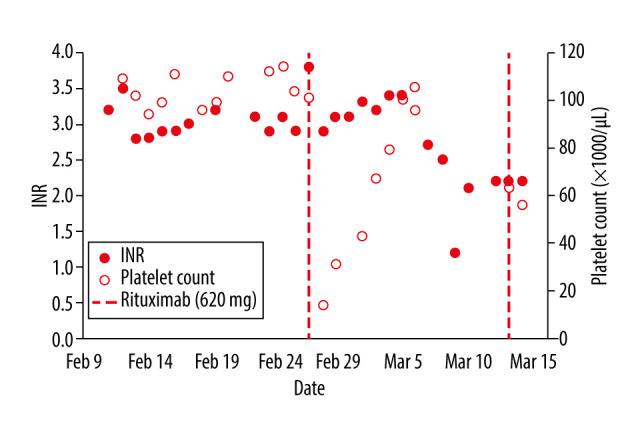
Plot of international normalized ratio (INR) and platelet counts. INR and platelet counts displayed over the first 2 months after initial presentation. The dashed lines indicated the first 2 rituximab treatments.
Maintenance rituximab, consisting of 1 dose every 3 months, began approximately 9.5 months following the patient’s initial presentation and continued for 2 years. She currently receives a quarterly examination of CBC and INR, annual abdominal ultrasound, and annual clinic visits. To date, she remains in ongoing remission 12 years after treatment, with a normal CBC, PT, and PTT and no signs or symptoms of recurrent disease. Treatment with only rituximab and splenectomy provided successful management of a factor VII inhibitor associated with primary splenic marginal zone lymphoma.
Discussion
We present a unique case of acquired factor VII inhibitor associated with splenic marginal zone lymphoma, successfully treated and in ongoing complete remission with 12 years of follow-up. The patient continues to have a completely normal INR. Three of the previously reported cases documented significant-to-life-threatening bleeding, which we were able to avoid with the use of single-agent rituximab [9–11]. The rituximab was chosen to control the underlying cause of the factor VII inhibitor, the splenic marginal zone lymphoma. The authors of a previous report used a combination of cytotoxic agents (cyclophosphamide) and steroids successfully [11]. To the best of our knowledge, this is the first factor VII inhibitor case to date that was related to lymphoma. Other cases of factor VII inhibitor related to malignancy included probable bronchogenic carcinoma [8] and breast cancer [11]. Although our patient experienced a dramatic decline in platelet count with the first rituximab infusion, she quickly recovered, and this did not recur with subsequent infusions. She experienced no bleeding episodes. We administered a total of 8 doses of rituximab to solidify the response of the lymphoma to the treatment. Once the INR was normal and the patient’s spleen was of normal size, we performed a splenectomy to consolidate the treatment.
There has been decreasing use of splenectomy for SMZL over the last decade, with the success of treatment with rituximab. Kalpadakis found that rituximab could replace splenectomy in SMZL [15,16], and it has since been identified that maintenance rituximab leads to better survival [17]. Our patient received both. However, because of the life-threatening presentation of this patient’s SMZL with secondary factor VII inhibitor, we may still consider proceeding with the splenectomy in the present case. This novel presentation required careful consideration of the underlying condition in light of the high risk of bleeding. Our successful treatment regimen relied heavily on immunotherapy, avoided cytotoxic chemotherapy, and delayed splenectomy. We are pleased to report a positive long-term follow-up of this patient, with no evidence of recurrence of either the lymphoma or the factor VII inhibitor.
Conclusions
We stabilized our patient with plasmapheresis, which decreased the inhibitor level reflected in a lower INR. Following this, we started immunotherapy with rituximab to treat both the inhibitor and what we felt was the underlying cause, the splenic marginal zone lymphoma. A complete response was documented by splenectomy, and the patient’s 12-year remission of the lymphoma and the inhibitor helps to support the causative relationship between the lymphoma and the factor VII inhibitor.
Footnotes
Conflicts of Interest
None declared.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Franchini M. Acquired hemophlia A: A concise review. Am J Hematol. 2005;80(1):55. doi: 10.1002/ajh.20390. [DOI] [PubMed] [Google Scholar]
- 2.Kruse-Jarres R, Kempton CL, Baudo F, et al. Acquired hemophilia A. Am J Hematol. 2017;92(7):962–64. doi: 10.1002/ajh.24777. [DOI] [PubMed] [Google Scholar]
- 3.Collins P, Macartney N, Davies R, et al. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124(1):86–90. doi: 10.1046/j.1365-2141.2003.04731.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins PW, Hirsch S, Baglin TP, et al. Acquired hemophilia A in the United Kingdom: A 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109(5):1870–77. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 5.Sevenet PO, Kaczor DA, Depasse F. Factor VII deficiency: From basics to clinical laboratory diagnosis and patient management. Clin Appl Thromb Hemost. 2017;23(7):703–10. doi: 10.1177/1076029616670257. [DOI] [PubMed] [Google Scholar]
- 6.Golino P. The inhibitors of the tissue factor: Factor VII pathway. Thromb Res. 2002;106(3):V257–65. doi: 10.1016/s0049-3848(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 7.Peerschke EI, Castellone DD, Ledford-Kraemer M, et al. NASCOLA Proficiency Testing Committee Laboratory assessment of factor VIII inhibitor titer: The North American Specialized Coagulation Laboratory Association experience. Am J Clin Pathol. 2009;131(4):552–58. doi: 10.1309/AJCPMKP94CODILWS. [DOI] [PubMed] [Google Scholar]
- 8.Campbell E, Sanal S, Mattson J, et al. Factor VII inhibitor. Am J Med. 1980;68(6):962–64. doi: 10.1016/0002-9343(80)90231-4. [DOI] [PubMed] [Google Scholar]
- 9.Delmer A, Horellou MH, Andreu G, et al. Life-threatening intracranial bleeding associated with the presence of an antifactor VII autoantibody. Blood. 1989;74(1):229–32. [PubMed] [Google Scholar]
- 10.Okajima K, Ishii M. Life-threatening bleeding in a case of autoantibody-induced factor VII deficiency. Int J Hematol. 1999;69(2):129–32. [PubMed] [Google Scholar]
- 11.Levitt M, Topillow A, Lerner W. A case of FVII inhibitor. Blood. 2010;116(21):3655. [Google Scholar]
- 12.Piris MA, Onaindia A, Mollejo M. Splenic marginal zone lymphoma. Best Pract Res Clin Haematol. 2017;30(1–2):56–64. doi: 10.1016/j.beha.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: From genetics to management. Blood. 2016;127(17):2072–81. doi: 10.1182/blood-2015-11-624312. [DOI] [PubMed] [Google Scholar]
- 14.Thieblemont C. Treatment of splenic marginal zone b cell lymphomas: An analyss of 81 patients. Clin Lymphoma. 2002;3(1):41–47. doi: 10.3816/clm.2002.n.010. [DOI] [PubMed] [Google Scholar]
- 15.Kalpadakis C, Pangalis GA, Dimopoulou MN, et al. Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol Oncol. 2007;25(3):127–31. doi: 10.1002/hon.820. [DOI] [PubMed] [Google Scholar]
- 16.Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. Treatment of splenic marginal zone lymphoma with rituximab monotherapy: Progress report and comparison with splenectomy. Oncologist. 2013;18(2):190–97. doi: 10.1634/theoncologist.2012-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalpadakis C, Pangalis GA, Sachanas S, et al. Rituximab monotherapy in splenic marginal zone lymphoma: Prolonged responses and potential benefit from maintenance. Blood. 2018;132(6):666–70. doi: 10.1182/blood-2018-02-833608. [DOI] [PubMed] [Google Scholar]


