Abstract
Background
Although hearing impairment (HI) is linked to poorer physical functioning, the longitudinal associations between HI and higher-level functional measures are unclear.
Method
Data are from the Baltimore Longitudinal Study of Aging (2012–2019). Using pure-tone audiometry, we categorized hearing into normal, mild, and moderate or greater HI. Physical function was assessed with the expanded Short Physical Performance Battery (eSPPB) and walking endurance with time to walk 400 m. Multivariable and mixed-effects linear models tested the hypotheses that participants with HI, at baseline, have poorer physical performance and walking endurance, and faster decline over time (up to 6 measurements). In a subset (n = 526), we further adjusted for vestibular function. Among participants with HI, we evaluated the differences in eSPPB scores and walking endurance between hearing aid users and nonusers.
Results
Of 831 participants, 26% had mild, and 17% moderate or greater HI. After adjustment for demographics and medical history, moderate or greater impairment versus normal hearing was associated with poorer function (0.17 [95% CI: 0.09, 0.26] lower eSPPB score, and 13.3 [95% CI: 3.31, 23.4] seconds slower 400-m walk time) and faster decline in these parameters over 6 years. Adjustment for vestibular function did not attenuate these associations. Hearing aid users walked 400 m 24 seconds faster than nonusers (p = .001).
Conclusion
Moderate or greater HI is associated with poorer initial and greater decline in higher-level physical performance. The observation that hearing aid users had better walking endurance suggests that screening for and treatment of HI may delay or slow progression of hearing-related functional decline.
Keywords: Fitness, Hearing loss, Physical function
Hearing impairment is highly prevalent among older adults, affecting nearly 2 out of 3 persons aged 70 and older (1), and is associated with poorer physical function, including lower walking endurance (2,3). However, several previous studies investigating the association between hearing impairment and physical functioning used self-reported measures of hearing (4,5), which are more prone to measurement error than audiometry (6), or were cross-sectional (7–9). Moreover, only a few studies investigated the association between hearing impairment and walking endurance (2,3,5,10)—a predictor of reduced functional capacity and disability risk (11) that captures decrements in performance earlier in the functional spectrum, when interventions may be more effective—and have longitudinal data on objectively measured endurance.
Several mechanisms have been hypothesized to explain associations between hearing and physical outcomes, including a pathway mediated by social isolation, cognitive impairment, and restricted physical activity. All of which may arise from reduced auditory environmental cues important for movement as well as common or shared causes of hearing and physical impairment including neurodegeneration and cardiovascular disease (12). Additionally, this association may be confounded by concomitant vestibular and hearing impairments due to shared causes (eg, ototoxic medications) (12) and the anatomical proximity of the hearing and vestibular systems. Both vestibular and auditory inputs go through the VIII cranial nerve (vestibulocochlear) and the vestibular system plays a critical role in self-motion perception and balance (13,14). The observed association between hearing postural control and falls (15,16), and the correlation between hearing and vestibular measures seen in previous epidemiological studies (17–19) further support this perspective.
Previous studies that assessed the role of vestibular function as a potential confounder in the association between hearing impairment and falls had conflicting results (20,21). Whether the hearing impairment–physical function association is independent of vestibular function is relevant since current treatments for hearing impairment do not target the vestibular system. Evaluating the potential contribution of vestibular function may clarify the independent and synergistic effects of the auditory and vestibular systems in conditioning physical functional decline in old age and identify appropriate and most effective interventions.
We investigated the cross-sectional and longitudinal associations of hearing impairment with physical function and walking endurance in a cohort of well-functioning middle-aged and older adults in the United States. We explored the role of vestibular function as a potential confounder of these associations. Further, although randomized clinical trials are best suited to study whether hearing aid users have better physical function than nonusers, some observational studies have found no differences in physical performance scores or incidence of disability between hearing aid users and nonusers (8). Thus, we also assessed whether hearing aid users had better physical function measures compared to nonusers.
We hypothesized that, in a dose–response fashion, hearing impairment contributes to poorer physical performance, slower walking endurance, and a faster decline in these capacities over time and that vestibular function partially explains these associations.
Method
Study Population
This study uses data from the Baltimore Longitudinal Study of Aging (BLSA), a cohort study of aging conducted by the Intramural Research Program of the National Institute on Aging. General descriptions of the participants, and enrollment procedures and criteria have been previously published (22). Briefly, BLSA participants consist of community-dwelling volunteers who, at enrollment, are free of all major chronic conditions (except for controlled hypertension) and cognitive or physical impairments. Participants are followed for life, every 1–4 years depending on age: <60 every 4 years, 60–79 every 2 years, and ≥80 every year. At each visit, participants undergo extensive medical interviews, examinations, and physical and cognitive testing. The Internal Review Board of the Intramural Research Program of the National Institutes of Health approved the study protocol, and all participants provided written informed consent.
Our analyses included 831 participants aged 40 years or older, free of stroke or heart failure who completed the 400-m walk, and had 1 or more in-clinic visits between October 2012 and December 2019. We defined the baseline visit as the first visit for each participant in which hearing, physical performance, and walking endurance were measured. In a subsample of 526 participants who underwent vestibular testing (between February 2013 and December 2018), we evaluated the potential confounding role of vestibular function with the same functional measures. Finally, 532 participants had multiple visits, with a maximum of 6 (mean follow-up time was 2.7 years [SD = 1.2, range: 0.9–6]) years allowing for longitudinal analyses.
Hearing Assessment
Pure-tone audiometric testing was performed at baseline in a sound-attenuating booth using an Interacoustics AD629 audiometer (Interacoustics A/S, Assens, Denmark) with ER-3A insert earphones. Participants wearing hearing aids were asked to remove them for testing. Audiometric thresholds were obtained in decibels hearing level (dB HL) at standard octaves from 0.5 to 8 kHz using best-practice procedures (23). For each ear, we calculated a 4-frequency (0.5, 1, 2, and 4 kHz) pure-tone average and used the better-hearing ear's pure-tone average (BPTA) for analyses. Using the World Health Organization thresholds (commonly used in hearing impairment literature), hearing status was categorized into normal hearing (BPTA ≤ 25 dB HL), mild hearing impairment (BPTA 26–40 dB HL), and moderate or greater hearing impairment (BPTA > 40 dB HL; moderate and severe [>60 dB HL] hearing impairments were combined into one group due to a small sample size [14 participants with severe hearing impairment]) (24).
Assessment of the Outcomes
Physical performance
Lower extremity physical performance was assessed using the expanded Short Physical Performance Battery (eSPPB) which is particularly useful for distinguishing higher levels of function (25). The test has 4 components: balance (ability to hold 3 progressively more difficult standing positions for 30 seconds each: semi-tandem, full tandem, and single-legged stances), a 6-m walk at usual gait speed, a usual-paced 6-m narrow walk (20-cm wide, the fastest of 3 attempts is used for the score), and pace (stands/second) of 5 chair stands from an armless chair (25). Each test is scored on a scale from 0 to 1, where the participant’s observed performance is divided by a previously established maximal performance for each test: 90 seconds for the standing balance test, 2.0 m/s for the standard and narrow walks, and 1.0 stand/second for the chair stands (25). If a participant’s performance is better than the maximum, a score of 1 is assigned. The final eSPPB score is the sum of the 4 tests’ score; hence, ranging from 0 to 4, where higher scores represent better physical performance. We used the continuous eSPPB score as an outcome.
Walking endurance
Walking endurance was assessed using the long-distance corridor walk (LDCW), a fast-paced endurance walking test, and a validated measure of cardiorespiratory fitness (26,27). Walking aids (ie, cane, walker) were permitted. The test was performed on a 20-m course in an uncarpeted, unobstructed corridor, marked by traffic cones at each end. In the first phase, participants walk for 2.5 minutes at their usual pace. In the second phase, immediately after the first phase, participants are instructed to complete 10 laps (400 m) “as quickly as possible, without running at a pace that can be maintained.” Standard encouragement and the number of remaining laps are given. The total time to complete the LDCW was recorded in seconds and used as a continuous outcome.
Assessment of Vestibular-Evoked Myogenic Potentials
To measure vestibular function, we used a commercial electromyographic (EMG) system (Carefusion Synergy, software version 14.1, Dublin, OH). Adhesive electrodes (GN Otometrics, Schaumburg, IL) were used to record EMG signals. The signals were amplified (2 500×) and filtered (28).
Ocular vestibular-evoked myogenic potential (oVEMP) testing was performed with participants in a supine position with the upper body supported at a 30° angle from the horizontal plane with the participants’ eyes fixed at a specified level (looking upward). Four self-adhesive electrodes were placed on the cheeks (2 on each cheek [noninverting and inverting]) directly below the eyes and with 2 cm of separation, and a ground electrode was placed on the sternum. The electrode placement was tested for symmetry by asking the participant to perform vertical movements. Vibration stimuli were given via head taps using a reflex hammer with an inertial microswitch trigger at a point on the head known as the Fz (midline at the hairline, at 1/3 of the distance between the inion and nasion) (29).
Cervical vestibular-evoked myogenic potential (cVEMP) testing was performed in the same position as oVEMP. Four electrodes were placed on the sternocleidomastoids (SCMs) muscles, 2 at each SCM; one (noninvertive) at the midpoint of the SCM, and one (inverting) on the sternoclavicular junction; a ground electrode was placed on the sternum. Before delivering the stimulus, a background SCM activity sample was obtained by asking participants to lift their heads. Audible stimuli were delivered through Audiocups noise-canceling headphones from Amplivox (Eden Prairie, MN). The stimulus was a 500 Hz, 125 dB Sound Pressure Level tone burst, with a repetition rate of 5 Hz, a 1-ms rise/fall time, and a 2-ms plateau. For a cVEMP tracing to be valid, the background EMG signal was required to reach at least 30 mV over the 10 ms prior to the applied stimulus (30).
Participants were categorized into 2 groups based on the presence of VEMP responses, for cVEMP and oVEMP, respectively: individuals with any VEMP (ie, bilateral or unilateral VEMP response) and individuals with bilaterally absent VEMP responses (vestibular dysfunction). We also used the mean amplitude of the cVEMP as a continuous variable among participants who had any cVEMP response (n = 438).
Assessment of Other Covariates
We assessed covariates which were identified in our literature review as potential confounders in the hearing–physical function association. Demographic characteristics including age, sex, race (White, African American, or other), highest education attained (≤high school, some college, college, and post-college), and history of smoking (ever vs never) were self-reported. History of diabetes and hypertension was obtained by asking participants if a health professional had ever told them, they had that condition. Participants were defined as hearing aid users if they self-reported using hearing aids in either ear. Finally, height (meters) and weight (kilograms) were measured following standard clinical procedures.
Statistical Analysis
The demographic and medical characteristics of participants were summarized and compared across hearing categories. For continuous variables, we used mean ± SD and analysis of variance tests. We described categorical variables using frequencies and percentages and tested for differences in percentages using chi-squared tests.
Cross-sectional analyses
We used linear regression models to estimate the association of hearing categories with physical function and walking endurance separately. We built 2 models: model 1: adjusted for demographics (age, sex, race, education), and model 2: adjusted for demographics, anthropometric, and medical characteristics (model 1 + height, weight, diabetes, hypertension, and smoking). In addition, to estimate the role of vestibular dysfunction as a potential confounder of the association between hearing, physical function and walking endurance, in the subsample who received vestibular testing (n = 526), we built additional models in which we further adjusted for vestibular function: model 2 + cVEMP and model 2 + oVEMP. Moreover, we adjusted for the continuous cVEMP mean amplitude among participants with cVEMP present (model 2 + cVEMP mean amplitude, n = 438).
Longitudinal analyses
We used 2 separate linear mixed-effects models with unstructured covariance matrix to estimate the rate of change in physical function and walking endurance over time (measured in years from the first hearing assessment from October 2012 to December 2019) across hearing categories. We allowed for between-subject heterogeneity over time in our outcomes with random effects for intercept and slope. We included interaction terms between hearing categories and time to estimate mean differences in annual rate of change in physical outcomes across hearing categories. We evaluated model assumptions with residual plots and present adjusted (model 2) longitudinal models.
Secondary analyses
Among 355 participants who had any degree of hearing impairment (mild or worse), and hearing aid use data available, we used the aforementioned models to estimate cross-sectional differences in physical function, and walking endurance comparing hearing aid users and nonusers adjusting for variables in model 2 + degree of hearing impairment. We also examined differences in the rate of change in physical outcomes by hearing aid status adjusting for covariates in model 2 + degree of hearing impairment using mixed-effect models.
Sensitivity analyses
We performed an analysis restricted to participants who did not use walking aids in the 400-m test. Also, in adjusted models (variables from model 2), we tested for interactions between hearing categories and age, and hearing categories and sex to assess whether these factors modified the association between hearing and physical function measures.
We performed analyses using Stata version 15 (Statacorp, College Station, TX). Two-sided p values <.05 were considered statistically significant.
Results
At baseline, 474 (57%) participants had normal hearing, 212 (26%) had mild hearing impairment, and 145 (17%) had moderate or greater hearing impairment. In descriptive unadjusted comparisons, participants with hearing impairment were older, more likely to be male, White, have hypertension, and lower weight than participants with normal hearing. Mean eSPPB score and 400-m walk time were 2.7 points (SD = 0.5), and 274 seconds (SD = 57), respectively. Participants with hearing impairment had poorer physical function and walking endurance (Table 1). Among the 526 participants with vestibular testing, 17% and 10% exhibited vestibular dysfunction according to cVEMP and oVEMP, respectively. The proportion of participants with vestibular dysfunction was greater among those with hearing impairments (p < .001 for both cVEMP and oVEMP).
Table 1.
Demographic Characteristics and Medical History by Hearing Categories at the First Hearing Assessment
| Total | Normal Hearing (BPTA ≤ 25 dB HL) | Mild Hearing Impairment (BPTA 26–40 dB HL) | Moderate or Greater Hearing Impairment (BPTA > 40 dB HL) | P Value | |
|---|---|---|---|---|---|
| N = 831 | n = 474 | n = 212 | n = 145 | ||
| Age [range] | 69.2 (12.0) [40–92] | 63.4 (11.1) [40–88] | 74.8 (8.5) [41–92] | 79.9 (7.0) [60–92] | <.001 |
| Sex, male | 371 (45%) | 172 (36%) | 106 (50%) | 93 (64%) | <.001 |
| Race | <.001 | ||||
| White | 528 (64%) | 247 (52%) | 165 (78%) | 116 (80%) | |
| Black | 216 (26%) | 165 (35%) | 32 (15%) | 19 (13%) | |
| Other | 87 (10%) | 62 (13%) | 15 (7%) | 10 (7%) | |
| Education | .20 | ||||
| ≤High school | 32 (4%) | 18 (4%) | 6 (3%) | 8 (6%) | |
| Some college | 91 (11%) | 43 (9%) | 31 (15%) | 17 (12%) | |
| College completed | 180 (22%) | 113 (24%) | 38 (18%) | 29 (20%) | |
| Post-college | 528 (64%) | 300 (63%) | 137 (65%) | 91 (63%) | |
| Diabetes | 129 (16%) | 75 (16%) | 34 (16%) | 20 (14%) | .82 |
| Hypertension | 358 (43%) | 187 (39%) | 89 (42%) | 82 (57%) | .001 |
| Weight (kg) | 76.7 (16.1) | 78.0 (16.5) | 75.4 (15.9) | 74.3 (14.9) | .023 |
| Height (cm) | 167.9 (9.0) | 167.8 (8.8) | 167.9 (9.2) | 168.1 (9.2) | .91 |
| Ever smoked | 275 (33%) | 143 (30%) | 74 (35%) | 58 (40%) | .072 |
| LDCW, time to walk 400 m, s | 274.0 (57.3) | 261.2 (48.6) | 282.2 (56.7) | 304.0 (70.0) | <.001 |
| eSPPB, points | 2.67 (0.50) | 2.82 (0.42) | 2.55 (0.53) | 2.36 (0.53) | <.001 |
| n = 526 | n = 321 | n = 119 | n = 86 | P Value | |
| Ocular VEMP bilateral absent | 51 (10%) | 21 (7%) | 14 (12%) | 16 (19%) | <.001 |
| Cervical VEMP bilateral absent | 88 (17%) | 39 (12%) | 21 (18%) | 28 (33 %) | <.001 |
Notes: BPTA = better-hearing ear pure-tone average; eSPPB = expanded Short Physical Performance Battery; HL = hearing level; LDCW = long-distance corridor walk; VEMP = vestibular-evoked myogenic potentials. Data are means (SDs) or frequencies (proportions).
Cross-Sectional Findings
Physical performance
Compared to normal hearing, moderate or greater hearing impairment was associated with poorer physical performance in both models. The eSPPB scores were on average 0.16 (95% CI: 0.07, 0.25), and 0.17 (95% CI: 0.09, 0.26) points lower in models 1–2, respectively (Table 2).
Table 2.
Associations of Hearing With Physical Function and Walking Endurance (N = 831)
| Normal Hearing (BPTA ≤ 25 dB HL) |
Mild Hearing Impairment (BPTA 26–40 dB HL) |
Moderate or Greater Hearing Impairment (BPTA > 40 dB HL) |
P Trend | |
|---|---|---|---|---|
| n = 474 | n= 212 | n = 145 | ||
| Physical function outcome, β-coefficient (95% CI) | ||||
| eSPPB, score | ||||
| Model 1 | Reference | −0.06 (−0.14, 0.01) | −0.16 (−0.25, −0.07) | .002 |
| Model 2 | Reference | −0.07 (−0.14, 0.01) | −0.17 (−0.26, −0.09) | <.001 |
| LDCW, time to walk 400 m, s | ||||
| Model 1 | Reference | −1.35 (−10.20, 7.50) | 10.60 (−0.18, 21.39) | .093 |
| Model 2 | Reference | −0.03 (−8.26, 8.19) | 13.34 (3.31, 23.37) | .019 |
Notes: BPTA = better-hearing ear pure-tone average; eSPPB = expanded Short Physical Performance Battery; HL = hearing level; LDCW = long-distance corridor walk. Model 1: adjusted for age, sex, race, and education. Model 2: adjusted for age, sex, race, education, height, weight, diabetes, hypertension, and smoking. Bold indicates statistically significant differences compared to normal hearing. β coefficient represent the mean difference in eSPPB scores and LDCW compared to participants with normal hearing.
Further adjustment for vestibular function did not substantively attenuate the association between hearing and physical performance among participants with vestibular testing (Table 3). In this subsample, the difference between participants with moderate or greater hearing impairment and those with normal hearing was −0.18 (95% CI: −0.29, −0.07) points in model 2; −0.18 (95% CI: −0.29, −0.07) points adjusting for variables in model 2 + oVEMP; and −0.17 (95% CI: −0.28, −0.06) in the model adjusted for variables in model 2 + cVEMP.
Table 3.
Associations Between Hearing and Physical Function and Walking Endurance Adjusted for Vestibular Function (n = 526)
| Model 2 (adjusted except vestibular function) | Model 2 (adjusted) + oVEMP | Model 2 (adjusted) + cVEMP | |
|---|---|---|---|
| Physical Function Outcome, β Coefficient (95% CI) | |||
| eSPPB, score | |||
| Normal hearing, n = 321 | Reference | Reference | Reference |
| Mild hearing impairment, n = 119 | −0.13 (−0.22, −0.04) | −0.13 (−0.22, −0.04) | −0.13 (−0.22, −0.04) |
| Moderate + hearing impairment, n = 86 | −0.18 (−0.29, −0.07) | −0.18 (−0.29, −0.07) | −0.17 (−0.28, −0.06) |
| oVEMP | — | −0.03 (−0.15, 0.09) | — |
| cVEMP | — | — | −0.08 (−0.18,0.01) |
| LDCW, time to walk 400 m, s | |||
| Mild hearing impairment | 1.87 (−9.35, 13.08) | 1.83 (−9.38, 13.04) | 2.11 (−9.08, 13.31) |
| Moderate + hearing impairment | 20.27 (6.97, 33.56) | 19.68 (6.35, 33.01) | 19.17 (5.84, 32.50) |
| oVEMP | — | 8.13 (−5.95, 22.20) | — |
| cVEMP | — | — | 9.98 (−1.29, 21.25) |
Notes: cVEMP = cervical vestibular-evoked myogenic potential; eSPPB = expanded Physical Performance Battery; LDCW = long-distance corridor walk; oVEMP = ocular vestibular-evoked myogenic potential. Model 2: adjusted for age, sex, race, education, height, weight, diabetes, hypertension, and smoking. Estimates differ from those in Table 2, because of differences in sample size (N = 831 for Table 2 and N = 526 for Table 3 [subsample with vestibular tests]). Bold indicates statistically significant differences compared to normal hearing. β coefficient represent the mean difference in eSPPB scores and LDCW compared to participants with normal hearing.
Walking endurance
Compared to participants with normal hearing, participants with moderate or greater hearing impairment walked 400 m, on average, 10.6 (95% CI: −0.2, 21.4) seconds slower in model 1, and 13.3 (95% CI: 3.31, 23.4) seconds slower in model 2. Similarly, further adjustment for vestibular dysfunction did not attenuate the difference in time to complete the 400-m walk between participants with moderate or greater hearing impairment and those with normal hearing (Table 3). The difference in time to complete the walk was 20.3 (95% CI: 7.0, 33.6) in model 2; 19.7 (95% CI: 6.4, 33.0) in model 2 + oVEMP; and 19.2 (95% CI: 5.8, 32.5) in model 2 + cVEMP. Also, among the 438 participants with cVEMP present, we built another model that included variables in model 2 + mean cVEMP amplitude as a continuous variable and the results remained almost identical for both physical performance and walking endurance (data not shown).
Longitudinal Findings
Physical performance
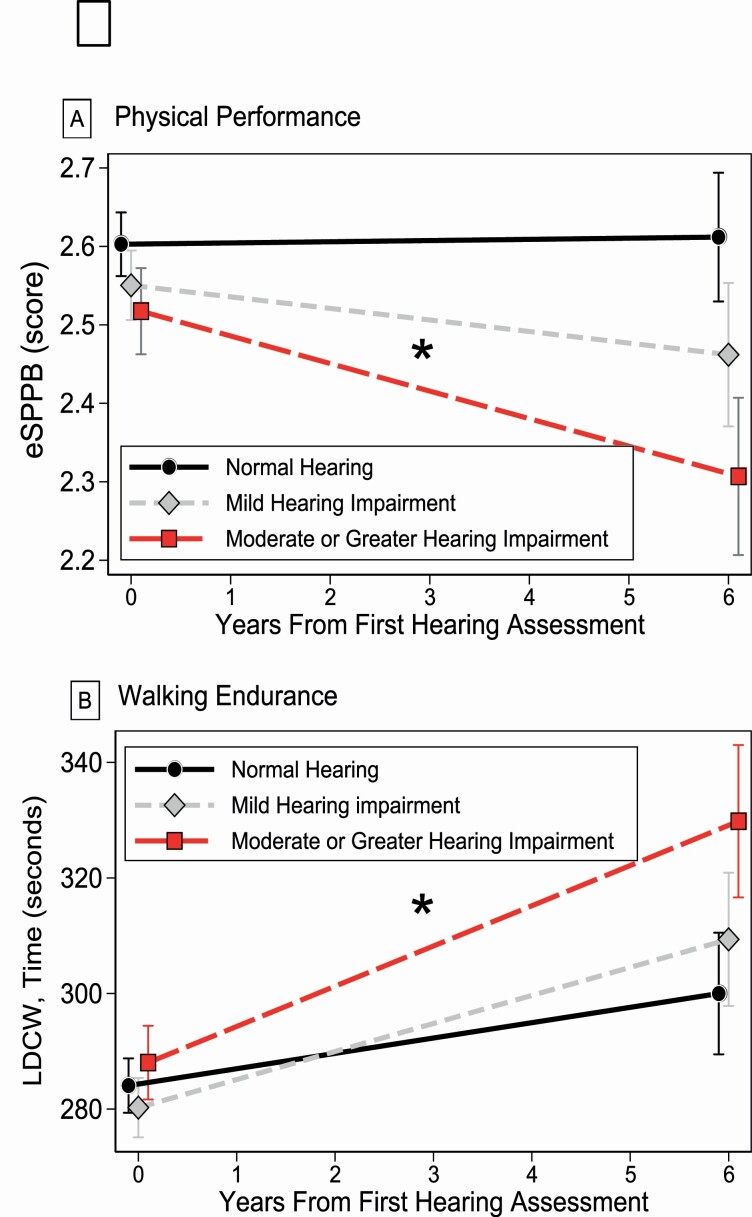
For the 831 participants, there were 1 701 observations (mean = 2, max = 6) of eSPPB scores over a 6.1-year period (mean follow-up time for participants with >1 visit [n = 513] = 2.8, SD = 1.2 years). The rate of change in physical performance for participants with normal hearing was 0.001 (95% CI: −0.012, 0.015) eSPPB points/year. Compared to participants with normal hearing, those with mild hearing impairment had a rate of change of −0.015 (95% CI: −0.031, 0.001; β for interaction [Time × Mild impairment] = −0.02 [95% CI: −0.04, 0.004]) points/year; participants with moderate or greater hearing impairment had a rate of −0.035 (95% CI: −0.052, −0.018; β for interaction [Time × Moderate or greater impairment] = −0.037 [95% CI: −0.058, −0.015], p for interaction < .001) points/year.
Walking endurance
There were 1 659 400-m walk observations (mean = 2, max = 6) over the 6.1-year period (mean follow-up time for participants with >1 visit [N = 491] = 2.8, SD = 1.2 years). On average, we observed that the time needed to complete the 400-m walk increased over time for all participants, but the increase rate varied by hearing loss status. Compared to those with normal hearing, whose mean time to complete the walked increased by 2.66 (95% CI: 0.89, 4.42) seconds/year; those with mild hearing impairment slowed down in the 400-m walk by 4.86 ([95% CI: 2.83, 6.88]; β for interaction [Time × Mild impairment] = 2.20 [95% CI: −0.6, 4.42]) seconds/year, but the difference compared to those with normal hearing was not significant. Participants with moderate or greater hearing impairment slowed down by 6.96 ([95% CI: 4.67, 9.26]; β for interaction [Time × Moderate or greater impairment] = 4.31 [95% CI: 1.47, 7.15], p for interaction = .003) seconds/year. We show our findings from longitudinal adjusted models in Figure 1.
Figure 1.
Longitudinal associations between hearing impairment, (A) eSPPB score, and (B) time to complete the 400-m walk. eSPPB = expanded Short Physical Performance Battery. *Statistically different rate of change from normal hearing. Adjusted for age, sex, race, education, height, weight, diabetes, hypertension, and smoking.
Secondary Analyses
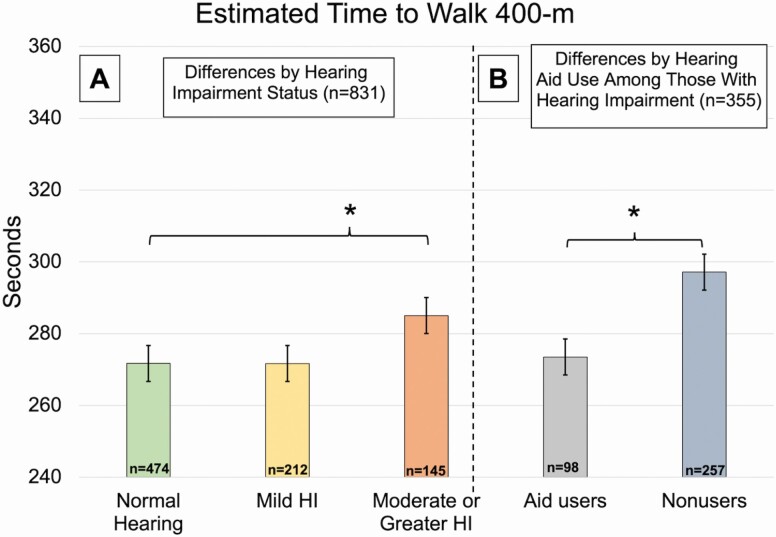
Out of 355 participants with at least mild hearing impairment (BPTA ≥ 25 dB), 98 (28%) reported hearing aid use in at least one ear (differences in demographic and medical characteristics between hearing aid users and nonusers are shown in the Supplementary Material). Adjusting for variables in model 2 + degree of hearing impairment, there were no statistically significant differences in eSPPB score comparing hearing aid users vs. nonusers (β for hearing aid use = 0.05, 95% CI: −0.07, 0.16 eSPPB points), but for walking endurance, we found that hearing aid users walked 400-m 24 seconds faster compared to nonusers (β for hearing aid use = −23.6, 95% CI: −38.1, −9.2 seconds [Figure 2]). We found no statistically significant differences in our longitudinal models between hearing aid users and nonusers.
Figure 2.
Cross-sectional differences in time to complete the 400-m walk by hearing impairment and aid use. HI = hearing impairment. *Statistically significant difference (p < .05). (A) Estimated mean time to walk 400-m from model 2, adjusted for age, sex, race, education, height, weight, diabetes, hypertension, and smoking. (B) Estimated mean time to walk 400-m from secondary analysis, adjusted for age, sex, race, education, height, weight, diabetes, hypertension, smoking, and degree of hearing impairment.
Sensitivity Analysis
There were 5 participants who used walking aids during the 400-m walk. Excluding them from our analyses did not change our findings. When we tested for interactions between hearing and age, we found that the association between hearing impairment and physical performance and walking endurance was magnified with increasing age (p value for age-hearing interaction terms <.01 for all). The interaction between sex and hearing was not statistically significant for physical performance. However, for walking endurance, we found that the association between mild hearing impairment and time to walk 400 m was stronger among women (Mild impairment × Sex interaction, p = .026), although not statistically significant (β for mild impairment vs normal hearing for women = 7.11 seconds slower, p = .208). The interaction between moderate or greater hearing impairment and sex was not statistically significant.
Discussion
In a community-dwelling population of well-functioning middle-aged and older adults in the United States, we found that participants with audiometrically assessed moderate or greater hearing impairment had poorer physical performance, slower walking endurance, and faster rates of decline of both over up to 6.1 years of follow-up. The participants with normal hearing or mild hearing impairment did not experience statistically significant deterioration in eSPPB scores over time in our study. Our findings were robust to adjustment for demographic, anthropometric, and comorbidity characteristics, including physiologically assessed vestibular function. Secondary analyses also showed that among participants with any degree of hearing loss, hearing aid users exhibited better walking endurance after adjusting for confounders including degree of hearing loss. Collectively, these results suggest that individuals with hearing impairment exhibit earlier and accelerated decrements in performance, independent of vestibular function, warranting regular screening and intervention as needed.
Using data from the Health Aging and Body Composition study, Chen et al. (31) described the association between audiometrically defined hearing impairment and physical performance using the SPPB. Their analyses found that, compared to participants with normal hearing, those with hearing impairments had faster rates of decline in physical performance (SPPB and gait speed, evaluated separately). We used a different tool to measure physical performance, the eSPPB, to help identify higher-level differences in physical performance among well-functioning individuals, such as BLSA participants. In agreement and extension of their results, we also found a faster decline in physical performance among those with hearing impairment, and no cross-sectional or longitudinal differences physical function scores between hearing aid users and nonusers. These findings indicate that, even among relatively healthy, well-functioning adults, those with hearing loss are at greater risk of mobility limitations than those with normal hearing.
The association between hearing and walking endurance has been previously described; Mikkola et al. (5) reported an association between self-reported hearing and perceived difficulty walking 2 km, but not 500 m; Viljanen et al. (3) found an association between audiometrically defined hearing and a performance-based assessment of walking endurance—the 6-minute walk. However, their findings were restricted to women and not significant after covariate adjustment. A previous study, from our group (2), found a graded association between hearing impairment and slower walking endurance during the 2-minute walk test in the Atherosclerosis Risk in Communities Study, but this finding was limited to a cross-sectional analysis. Our results are consistent with these previous findings and expand upon them by assessing the longitudinal association with walking endurance. To our knowledge, we are the first to describe the association between hearing and walking endurance over time using audiometrically defined hearing impairment and performance-based tests of walking endurance. Furthermore, in contrast to these findings, we found that cross-sectionally, hearing aid use was associated with better walking endurance.
To place our findings in context, because there are no minimal clinically meaningful differences for the eSPPB score, we compared our estimate of the association between moderate or greater hearing impairment (lower eSPPB score by 0.17 points) with the β coefficient for age in model 2. Having moderate or greater hearing loss was roughly equivalent to a 7-year age difference (β for 1-year difference = 0.024 points). For walking endurance, having moderate or greater hearing impairment (β for time to complete the 400-m walk = 13.3 seconds) was roughly equivalent to a 5-year difference in age (β for 1-year difference = 2.50 seconds).
Our finding that, over time, those with moderate or greater hearing impairment exhibit poorer walking endurance suggests that hearing impairment may contribute to detraining possibly due to hearing-related activity restriction, given that those who used a hearing aid did not show diminished exercise tolerance (26,32). Our finding that, among those with hearing impairment, hearing aid users were faster compared to nonusers should be interpreted with caution (magnitude of the association equivalent to a 5.8-year difference in age [β for 1-year difference = 4.05 seconds]). Hearing aid users are more likely to be White and have higher socioeconomic status, factors associated with better physical function (33,34), and even though our results were adjusted for race and education, residual confounding is possible. Besides, our assessment of hearing aid use was limited to a binary (yes/no) question, thus not fully characterizing hearing aid use patterns. However, these findings are suggestive of a potential beneficial effect of hearing aids on physical function.
Although the hearing and vestibular systems are distinct systems (30), previous literature posited that concomitant hearing and vestibular dysfunction may explain the association between hearing and physical function and that vestibular dysfunction may confound or underlie the association between hearing loss and physical function and endurance (12). Despite this speculation, previous studies investigating the association between hearing and physical outcomes did not adjust for vestibular function (7,9,31). Two previous studies examining hearing impairment and falls risk using measures of postural balance which is related to but not the same as vestibular function had slightly different findings. Viljanen et al. (15) found attenuation of fall risk after adjusting for postural balance; whereas Lin and Ferrucci (21) found that the association between hearing and falls remained significant after adjusting for a modified Romberg test performance. Using a clinical definition of vertigo as a proxy for vestibular function, Heitz et al. (20) found that adjusting for vertigo attenuated the association between hearing and falls.
To our knowledge, this is the first study to evaluate the association between hearing impairment and physical function after adjusting for physiologically assessed vestibular function measured by oVEMP and cVEMP. Although vestibular dysfunction modestly attenuated the association between hearing and physical outcomes, the magnitude of the associations was virtually unchanged with less than a 6% reduction of all β coefficients compared to models that did not include vestibular function. Also, the association between moderate or greater hearing impairment and higher-order physical function remained statistically significant (Table 3), suggesting that vestibular function does not underlie these associations. We note that although the coefficients for the association between VEMP and physical function were not statistically significant, there was a trend for absent oVEMP and cVEMP responses to be associated with poorer endurance walk performance. This finding merits further exploration in even larger studies.
Several other mechanisms could explain an association between hearing loss and physical function/endurance outcomes. First, there may be a causal relationship mediated by social isolation, cognitive impairment, and reduced physical activity (35–37). Second, auditory cues contribute to postural control and balance; thus, hearing impairment may directly interfere with these functions leading to poorer physical performance. Third, a common cause (ie, neurodegeneration, metabolic, microvascular damage (12)) contributing to both poor hearing and physical performance might underlie this association. More research is needed to understand the mechanisms, and temporality of these factors in the pathway between hearing impairment and functional deficits.
Our study has the following strengths: a longitudinal design, pure-tone audiometry to categorize hearing, and the ability to empirically evaluate the role of vestibular function as a confounder of the association between hearing and physical outcomes. This work also has limitations. Residual confounding by unmeasured factors such as living environment is a possibility in observational studies. We did not adjust for physical activity levels, cognition, or depression; however, we considered these factors mediators of the association we investigated. The decision not to adjust for them was made a priori. BLSA participants have better physical function, lower prevalence of chronic conditions, and higher education levels than the general U.S. adult population. These characteristics may limit the generalization of our findings. However, the associations we found would likely be stronger in a population in which the prevalence of other factors that impair physical performance is higher.
Conclusion
Hearing impairment, a highly prevalent and treatable condition, is associated with poorer higher-level physical performance and slower walking endurance independent of vestibular function. Moreover, these physical outcomes declined faster over time among those with moderate or greater hearing impairment, indicating these individuals are at greater risk of low fitness and mobility disability. The observation that hearing aid users had better walking endurance than nonusers supports the need for randomized studies to elucidate whether hearing rehabilitation strategies can help slow physical decline among individuals with hearing impairment.
Supplementary Material
Funding
This work was supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health. P.M.-A. is supported by the Cochlear Center for Hearing and Public Health. N.S.R. is supported by the National Institute on Aging grant K23AG065443. Y.A. and J.A.S are supported by the National Institute on Aging grant R01 AG061786. J.A.D. is supported by the National Institute on Aging grant K01AG054693.
Conflict of Interest
E.M.S, L.F, F.R.L., and J.A.S serve on the editorial board of Journal of Gerontology: Medical Sciences. N.S.R. is scientific advisory board member with no financial ties to Shoebox Inc. and Good Machine Studio. F.R.L. is consultant to frequency therapeutics, speaker honoraria from Caption Call, and director of a public health research center funded in part by a philanthropic gift from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. All other authors declare no conflicts of interest.
Author Contributions
Study concept and design: P.M.-A., P.-L.K., J.A.D., and J.A.S. Data acquisition: P.M.-A., P.-L.K., and J.A.S. Analysis of the data: P.M.-A, J.A.D., and J.A.S. Preparation of the manuscript, interpretation of results, and review: all authors contributed.
References
- 1.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66A(5):582–590. doi: 10.1093/gerona/glr002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Amezcua P, Powell D, Kuo P-L, et al. . Association of age-related hearing impairment with physical functioning among community-dwelling older adults in the US. JAMA Netw Open. 2021;4(6):e2113742–e2113742. doi: 10.1001/jamanetworkopen.2021.13742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viljanen A, Kaprio J, Pyykkö I, Sorri M, Koskenvuo M, Rantanen T. Hearing acuity as a predictor of walking difficulties in older women. J Am Geriatr Soc. 2009;57(12):2282–2286. doi: 10.1111/j.1532-5415.2009.02553.x [DOI] [PubMed] [Google Scholar]
- 4.Liljas AEM, Carvalho LA, Papachristou E, et al. . Self-reported hearing impairment and incident frailty in English community-dwelling older adults: a 4-year follow-up study. J Am Geriatr Soc. 2017;65(5):958–965. doi: 10.1111/jgs.14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikkola TM, Polku H, Portegijs E, Rantakokko M, Rantanen T, Viljanen A. Self-reported hearing status is associated with lower limb physical performance, perceived mobility, and activities of daily living in older community-dwelling men and women. J Am Geriatr Soc. 2015;63(6):1164–1169. doi: 10.1111/jgs.13381 [DOI] [PubMed] [Google Scholar]
- 6.Choi JS, Betz J, Deal J, et al. . A comparison of self-report and audiometric measures of hearing and their associations with functional outcomes in older adults. J Aging Health. 2016;28(5):890–910. doi: 10.1177/0898264315614006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Simonsick EM, Ferrucci L, Lin FR. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38(1):25–29. doi: 10.1016/j.gaitpost.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen DS, Genther DJ, Betz J, Lin FR. Association between hearing impairment and self-reported difficulty in physical functioning. J Am Geriatr Soc. 2014;62(5):850–856. doi: 10.1111/jgs.12800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deal JA, Richey Sharrett A, Bandeen-Roche K, et al. . Hearing impairment and physical function and falls in the atherosclerosis risk in communities hearing pilot study. J Am Geriatr Soc. 2016;64(4):906–908. doi: 10.1111/jgs.14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosiano MF, Jannat-Khah D, Lin FR, Goyal P, McKee M, Sterling MR. Hearing loss and physical functioning among adults with heart failure: data from NHANES. Clin Interv Aging. 2020;15:635–643. doi: 10.2147/CIA.S246662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonsick EM, Newman AB, Visser M, et al. ; Health, Aging and Body Composition Study . Mobility limitation in self-described well-functioning older adults: importance of endurance walk testing. J Gerontol A Biol Sci Med Sci. 2008;63(8):841–847. doi: 10.1093/gerona/63.8.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos J, Ramkhalawansingh R, Pichora-Fuller MK. Hearing, self-motion perception, mobility, and aging. Hear Res. 2018;369:42–55. doi: 10.1016/j.heares.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 13.Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35(3):185–196. doi: 10.1016/j.tins.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938. doi: 10.1001/archinternmed.2009.66 [DOI] [PubMed] [Google Scholar]
- 15.Viljanen A, Kaprio J, Pyykkö I, et al. . Hearing as a predictor of falls and postural balance in older female twins. J Gerontol A Biol Sci Med Sci. 2009;64(2):312–317. doi: 10.1093/gerona/gln015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiam NT, Li C, Agrawal Y. Hearing loss and falls: a systematic review and meta-analysis. Laryngoscope. 2016;126(11):2587–2596. doi: 10.1002/lary.25927 [DOI] [PubMed] [Google Scholar]
- 17.Zuniga MG, Dinkes RE, Davalos-Bichara M, et al. . Association between hearing loss and saccular dysfunction in older individuals. Otol Neurotol. 2012;33(9):1586–1592. doi: 10.1097/MAO.0b013e31826bedbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agmon M, Lavie L, Doumas M. The association between hearing loss, postural control, and mobility in older adults: a systematic review. J Am Acad Audiol. 2017;28(6):575–588. doi: 10.3766/jaaa.16044 [DOI] [PubMed] [Google Scholar]
- 19.Bang S-H, Jeon J-M, Lee J-G, Choi J, Song J-J, Chae S-W.. Association between hearing loss and postural instability in older Korean adults. JAMA Otolaryngol Head Neck Surg. 2020:146(6):530–534. doi: 10.1001/jamaoto.2020.0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitz ER, Gianattasio KZ, Prather C, Talegawkar SA, Power MC. Self-reported hearing loss and nonfatal fall-related injury in a nationally representative sample. J Am Geriatr Soc. 2019;67(7):1410–1416. doi: 10.1111/jgs.15849 [DOI] [PubMed] [Google Scholar]
- 21.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369–371. doi: 10.1001/archinternmed.2011.728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21(4):575–580. doi: 10.1093/geronj/21.4.575 [DOI] [PubMed] [Google Scholar]
- 23.Carhart R, Jerger JF. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord. 1959;24(4):330–345. doi: 10.1044/jshd.2404.330 [DOI] [Google Scholar]
- 24.World Health Organization (WHO). Grades of Hearing Impairment. http://www.who.int/deafness/hearing_impairment_grades/en/. Accessed September 30, 2019.
- 25.Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group . Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 26.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x [DOI] [PubMed] [Google Scholar]
- 27.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x [DOI] [PubMed] [Google Scholar]
- 28.Nguyen KD, Welgampola MS, Carey JP. Test-retest reliability and age-related characteristics of the ocular and cervical vestibular evoked myogenic potential tests. Otol Neurotol. 2010;31(5):793–802. doi: 10.1097/MAO.0b013e3181e3d60e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li C, Layman AJ, Carey JP, Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging. Clin Neurophysiol. 2015;126(11):2207–2215. doi: 10.1016/j.clinph.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gadkaree SK, Sun DQ, Li C, et al. . Does sensory function decline independently or concomitantly with age? Data from the Baltimore Longitudinal Study of Aging. J Aging Res. 2016;2016:1865038. doi: 10.1155/2016/1865038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen DS, Betz J, Yaffe K, et al. ; Health ABC Study . Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70(5):654–661. doi: 10.1093/gerona/glu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman AB, Simonsick EM, Naydeck BL, et al. . Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA J Am Med Assoc. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 33.Chiles Shaffer N, Simonsick EM, Thorpe RJ, Studenski SA. The roles of body composition and specific strength in the relationship between race and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 2020;75(4):784–791. doi: 10.1093/gerona/glz103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: the role of health and socioeconomic status over the life course. J Gerontol B Psychol Sci Soc Sci. 2012;67B(2):238–248. doi: 10.1093/geronb/gbr155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shukla A, Harper M, Pedersen E, et al. . Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngol Neck Surg. 2020;162(5):622–633. doi: 10.1177/0194599820910377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo PL, Di J, Ferrucci L, Lin FR. Analysis of hearing loss and physical activity among US adults aged 60–69 years. JAMA Netw Open. 2021;4(4):e215484. doi: 10.1001/jamanetworkopen.2021.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.