Abstract
Background
In older adults, elevated gait variability when walking has been associated with both cognitive impairment and future falls. This study leveraged 3 existing data sets to determine relationships between gait variability and the strength of functional connectivity within and between large-scale brain networks in healthy older adults, those with mild-to-moderate functional impairment, and those with Parkinson’s disease (PD).
Method
Gait and resting-state functional magnetic resonance imaging data were extracted from existing data sets on: (i) 12 older adults without overt disease yet with slow gait and mild executive dysfunction; (ii) 12 older adults with intact cognitive-motor function and age- and sex-matched to the first cohort; and (iii) 15 individuals with PD. Gait variability (%, coefficient of variation of stride time) during preferred walking speed was measured and correlated with the degree of functional connectivity within and between 7 established large-scale functional brain networks.
Results
Regression models adjusted for age and sex revealed that in each cohort, those with less gait variability exhibited greater negative correlation between fluctuations in resting-state brain activity between the default network and the dorsal attention network (functionally limited older: β = 4.38, p = .027; healthy older: β = 1.66, p = .032; PD: β = 1.65, p = .005). No other within- or between-network connectivity outcomes were consistently related to gait variability across all 3 cohorts.
Conclusion
These results provide strong evidence that gait variability is uniquely related to functional connectivity between the default network and the dorsal attention network, and that this relationship may be independent of both functional status and underlying brain disease.
Keywords: Brain networks, Falls, Functional magnetic resonance imaging, Resting state, Unsteady gait
The ability to walk safely is essential to maintaining functional independence in older adults. Walking is a continuous task that requires the repetition of gait cycles, defined as the body movements that occur in between 2 consecutive heel strikes of the same foot. Similar to heart beat dynamics, no two gait cycles are exactly the same, and the degree to which they vary provides important insight into health. Specifically, elevated stride time variability is a sensitive predictor of future falls (1), frailty (2), and cognitive decline (3–6). It is also particularly elevated in those suffering from progressive neurological diseases including advanced Parkinson’s disease (PD) (7,8), Huntington’s disease (7), multiple sclerosis (6,9,10), and cerebellar ataxia (11).
Walking requires activation of brain networks to attend to task-relevant aspects of the body and external environment, and at the same time avoid task-irrelevant distractors, in order to make proactive and reactive corrections to body movements over time. In a previous small study of older adults without overt disease, yet who presented with slow gait and mild-to-moderate executive dysfunction, we observed a significant correlation between gait variability and the strength of resting-state functional connectivity between 2 large-scale cortical networks in the brain; namely, the dorsal attention network and the default network (12). In those who walked with less gait variability, fluctuations in resting state activity within these two networks exhibited stronger negative correlation with one another. In other words, when one network exhibited an increase in activity, the other tended to decrease activity, and vice versa (13–15).
The relationship between the default network and the dorsal attention network is critical to cognitive function, and in particular, appears to subserve the ability to engage in a cognitive task and sustain performance in that cognitive task over time (16–18). We therefore contend that this important aspect of brain function is also critical to one’s ability to maintain consistent walking patterns over time, as measured by gait variability. As the previously published cohort (12) consisted only of a small sample of older adults with clinically significant functional limitations, the purpose of this study was to determine if the observed relationship between gait variability and resting-state brain activity of the dorsal attention and default networks was also present in (i) healthy older adults matched to the original cohort for both age and sex, and (ii) individuals with early-stage PD. We examined PD in particular because this disease is often associated with marked movement disorder including elevated gait variability.
Method
Standard Protocol Approvals, Registrations, and Patient Consents
We conducted a secondary analysis of existing data from a cohort of functionally limited older adults, healthy older adults and a separate cohort of individuals with PD. Each data set included baseline brain magnetic resonance imaging (MRI) scan and a gait analysis, conducted similarly to that previously published (12). All participants provided written informed consent as approved by the Institutional Review Board of either Hebrew SeniorLife (functionally limited and healthy older adult cohorts) or the Brigham and Women’s Hospital (PD cohort).
Participants
Functionally limited older adults
This previously reported cohort was recruited into a double-blinded, pilot randomized controlled trial testing the effects of noninvasive brain stimulation on cognitive-motor function (NC02436915) (19). Participants included men and women aged 65 years who presented with slow preferred walking speed (4-m over-ground preferred walking speed <1.0 m/s) and mild-to-moderate cognitive “executive” dysfunction (Trail-Making Test [TMT] B time within the bottom 25th percentile of age- and education-based norms). Twelve participants also completed baseline structural and functional brain imaging and were included in this secondary analysis (76.2 ± 9.5 years; 4 males and 8 females). Full details of this cohort subset have been published elsewhere (12). Briefly, exclusion criteria for the parent trial included the inability to walk independently, severe arthritis, pain, peripheral neuropathy, or other symptoms that may have affected walking performance, a Mini-Mental State Examination score <24, a clinical history of stroke, PD, or other physician-diagnosed neurological disorders, self-report of physician-diagnosed schizophrenia, bipolar disorder or other psychiatric illness, or depressive symptoms as indicated by a Geriatric Depression Scale >9. Those who completed baseline MRIs also presented without any contraindication to this imaging.
Healthy older adults
We then recruited 12 participants matched to the functionally limited cohort for both sex and age (74.7 ± 7.7 years old, 4 males and 8 females). Specifically, we first divided 12 cases in the functionally limited older adults cohort into 2 sex (ie, male, female) and 3 age (ie, 65–74, 75–84, 85, and above) categories. We then recruited “healthy” older adults to ensure equal numbers of healthy and functionally limited participants in each of the 6 possible sex–age categories. All healthy participants, however, were required to have a 4-m preferred walking speed ≥1.0 m/s and normal executive function defined by TMT B time ≥25th percentile of age- and education-based norms.
PD cohort
This cohort was originally recruited into a pilot study of the long-term impact of a 6-month Tai Chi training program in individuals with PD (NCT02418780). Briefly, participants included men and women aged 40–75 years who were diagnosed with idiopathic PD within 10 years and had limited disease progression (ie, Hoehn and Yahr stages 1–2.5). Exclusion criteria included diagnosis of atypical parkinsonism, history of stroke, head trauma, brain tumor, brain injury, seizures or other central nervous system conditions, orthopedic impairments, or other diseases that may contribute to gait disturbance or parkinsonism, history of severe hypertension or diabetes not currently controlled by medication, family history of seizures or unexplained loss of consciousness, current history of dementia or severe psychiatric illness that predated or was deemed unrelated to PD, DSM-V criteria for alcohol or substance abuse within the past 6 months or 10+ years of heavy alcohol use, acute illness requiring hospitalization within the past 3 months, history of deep brain stimulation or other brain surgery, regular use of walking aid, lack of English fluency, use of medications that affect cerebral vascular tone, or any contraindications to MRI. Fifteen participants (63.2 ± 6.5 years old, 8 males 7 females) with both neuroimaging and gait data were included for the secondary analysis.
Data Acquisition
All participants completed baseline MRIs and gait assessments as follows:
Resting-state functional MRI data acquisition
Functionally limited older adults and healthy older adults cohorts
MRIs were acquired with a GE Signa HDxt 3 Tesla system with an 8-channel head coil for the functionally limited older adults cohort and a GE Discovery 3T MR750 system with a 32-channel head coil for the healthy older adults cohort. Both scanners were located within the Center for Advanced MR Imaging at the Beth Israel Deaconess Medical Center. Although the scanners were different, the setting, sequence parameters, and MRI technician were the same. Standard structural imaging was first acquired (MDEFT sequence acquired axially with: 1.000 × 0.9375 × 0.9375 mm3 resolution; 6.616 ms repetition time (TR), 2.84 ms echo time (TE); 15 deg flip angle; 1100 ms inversion time) followed by 3 6-minute runs of resting-state functional magnetic resonance imaging (rs-fMRI) blood-oxygen-level-dependent (BOLD) sequences (3 × 3. 75 × 3.75 mm3, 3200 ms TR, 30 ms TE, 90 deg flip angle, 52 axial slices). Participants visually fixated on a cross within the MR bore and were asked to not think of anything specific for the entire period of the resting runs.
PD cohort
This cohort completed MRIs in a Siemens 3.0 Skyra MRI scanner with a 20-channel head coil at the Brigham and Women’s MRI Research Center (BWMRC). Structural imaging was first acquired (MPRAGE sequence: 0.94 × 0. 9 × 0.94 mm3 resolution; 1170 ms TR; 2.84 ms TE; 9 deg flip angle; field of view = 240 mm; 224 coronal slices with thickness = contiguous 0.9 mm), followed by 2 to 4 7-minute runs of rs-fMRI BOLD sequences, depending on time constraints and patient compliance (3000 ms TR, 30 ms TE, 3 mm slices). Participants were asked to close their eyes and were asked to let their mind freely wander without focusing on any particular thoughts for the entire period of the resting runs.
Gait assessment
Functionally limited older adults and healthy older adults cohorts
Gait assessments were acquired using the same protocol for both cohorts. Participants performed one practice and 5 official trials of over-ground walking at self-selected comfortable speed on a 60-foot oval indoor track with a 16-foot GAITRite mat placed along one side (CIR systems, Inc., Franklin, NJ, 100 Hz sampling frequency). Participants walked approximately 1.25 times around the track such that they passed over the mat twice per trial. Participant instructions were as follows: “When I say go, walk across the mat and then continue walking until I tell you to stop. Walk at your normal speed, as if you were walking down the street to go to the store.” Participants were encouraged to rest as much as needed in between walking trials. All participants completed 4–5 walking trials.
PD cohort
Participants completed 2, 90-second trials of continuous over-ground walking at self-selected comfortable speed along a long corridor (~35 m). Gait performance was captured through the Shimmer wearable sensor system (Shimmer Research, Dublin, Ireland) comprised of instrumented shoe insoles and inertial sensors placed on the waist and both wrists, thighs, and ankles.
Timed Up and Go test
In addition to gait data, all participants in each cohort also completed the Timed Up and Go (TUG) test of mobility. In this test, participants began in a seated position, stood up from a chair, walked for a 3-m distance at a comfortable pace, turned around, walked back to the chair, and sat down. Participants performed the TUG test twice and the averaged time needed to complete the trials was calculated and used for analysis.
Data Analysis
We applied the same methods to process and analyze both resting state functional connectivity and gait across all 3 cohorts.
Resting-state fMRI data analysis
Resting-state BOLD signals were processed with custom software combining a mix of FSL, SPM, and 4dfp routines, which has been previously employed in prior projects (20–22). This pipeline performed commonly accepted standard preprocessing steps (23) including spatial normalization to the MNI template, slice-time correction, motion-correction, and bandpass filtered for low-frequency data (0.08–2 Hz) spatial smoothing (7 mm FWHM). Ventricles, white matter, and the global signal nuisance signals were regressed from the time series.
After preprocessing, 7 networks were identified based on a previously defined and highly replicated parcellation from 1000 brains (20) including the visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal control, and default networks. Due to our main hypothesis, we focused only on the functional connectivity between the dorsal attention network and the default network. Time series were extracted from each of the abovementioned 7 network masks to calculate the strength of within- and between-network functional connectivity. The strength of the within-network functional connectivity was determined by the magnitude of the z-transferred Pearson correlation coefficients of the averaged time series of each voxel’s time series within each network mask. The strength of the between-network functional connectivity was determined by the magnitude of the z-transformed Pearson correlation coefficients between averaged time series from each of the network masks.
Gait data analysis
Gait variability and gait speed were derived from each trial based upon concatenated footfalls from passes over the instrumented mat (ie, functionally limited older adults and healthy older adults) or the wearable sensor system (ie, PD). Gait variability, stride-to-stride time variability, was defined as the coefficient of variation about the mean stride times of the right foot. Each gait metric was averaged across all available trials for each participant. Both the GAITRite and Shimmer systems have demonstrated high concurrent validity and test–retest reliability (24,25).
Statistical Analysis
Descriptive statistics were used to summarize participant characteristics including age, sex, BMI, TUG time, TMT A and B scores, gait speed, gait variability and the strength of the dorsal attention network, and default network functional connectivity. Linear regression analyses were used to determine the correlation between gait variability and functional connectivity outcomes, in each cohort separately. Models were completed with and without adjusting for age and sex. The level of statistical significance was set at 0.05. All analyses were performed using the JMP software version 14 (SAS Institute, Cary, NC).
Data Availability
The study data are available and will be shared at the request of other investigators for purposes of replicating results.
Results
Characteristics of the 3 cohorts have been summarized in Table 1. As expected, the PD cohort was significantly younger than both the healthy and functionally limited older adult cohorts. The functionally limited older adult cohort exhibited worse performance in mobility (ie, TUG, gait speed, gait variability) and cognition (ie, TMT A and B) as compared to the other two cohorts. The healthy older adult and PD cohorts did not differ significantly in gait speed, gait variability, TUG, TMT A, or TMT B scores.
Table 1.
Basic Characteristics (mean ± SD) of Study Participants
| Healthy Older Adults (N = 12) |
Functionally Limited Older Adults (N = 12) | Individuals With Parkinson’s Disease (N = 15) | |
|---|---|---|---|
| Age (y/o) | 74.7 ± 7.7 | 76.2 ± 9.5 | 63.2 ± 6.2* |
| Sex | 4M8F | 4M8F | 8M7F |
| BMI (kg/m2) | 28.8 ± 5.7 | 30.2 ± 5.8 | 25.9 ± 5.2 |
| TUG (sec) | 9.3 ± 2.1 | 16.0 ± 5.3* | 8.1 ± 1.8 |
| Gait speed (m/s) | 1.2 ± 0.2 | 0.7 ± 0.2* | 1.3 ± 0.2 |
| Gait variability (%) | 2.1 ± 0.8 | 4.7 ± 1.6* | 2.4 ± 0.6 |
| TMT A (sec) | 28.4 ± 6.3 | 66.0 ± 32.0* | 41.1 ± 19.9 |
| TMT B (sec) | 67.0 ± 27.3 | 247.5 ± 118.6* | 83.8 ± 31.3 |
| Hoehn & Yahr stage | NA | NA | 2.1 ± 0.2 |
Notes: BMI = body mass index; TMT = Trail Making Test; TUG = Timed Up and Go test.
* indicates the variable within this cohort is significantly different from the other two cohorts.
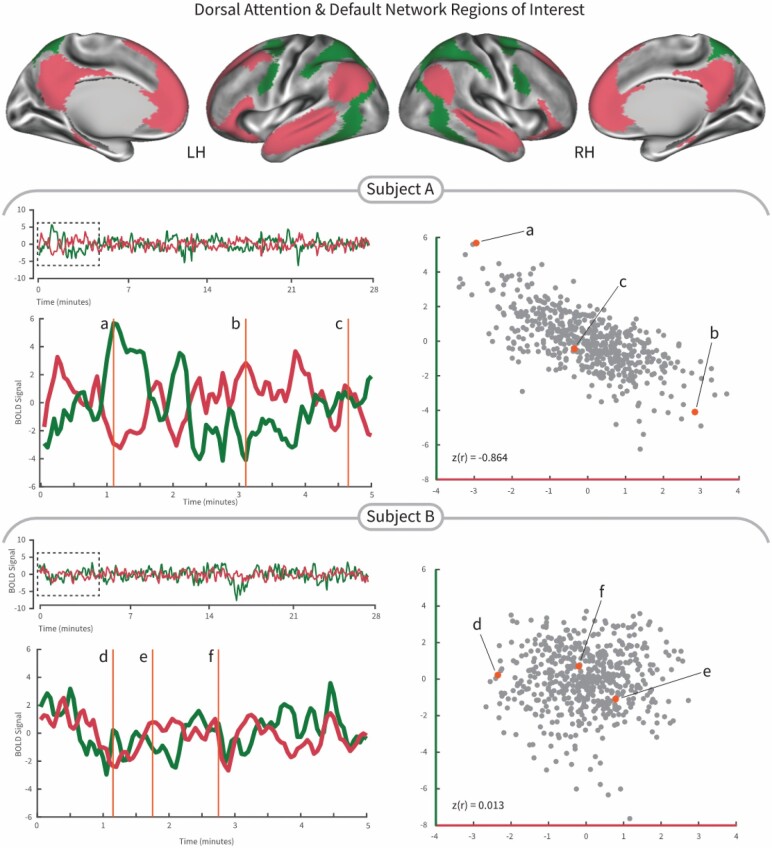
Figure 1 illustrates the negative correlation between the dorsal attention network and the default network in two representative participants (Figure 1). Subject A represents a strong negative correlation between these two networks, whereas subject B represents a weak negative correlation.
Figure 1.
Demonstration of the interaction between the dorsal attention network (green color) and the default network (crimson color) from two subjects in the Parkinson’s cohort. Subject A exhibited relatively strong negative correlation between the dorsal attention network and the default network, that is, the correlation value between 2 networks was −0.864 and the stride time variability was low (1.98%). We selected 3 time points in the fluctuation of the BOLD signal time series to highlight time points with high dorsal attention network and low default network (a), equal dorsal attention and default networks (b), and low dorsal attention network and high default network (c) activity illustrating the connectivity correlation. Subject B exhibited relatively weak correlation strength between activity of the dorsal attention network and the default network, that is, the correlation value between two networks was 0.013 and the stride time variability was high (3.87%). We selected 3 time points (d–f) in the fluctuation of the BOLD signal time series to highlight how similar time epochs do not differentiate the default and dorsal attention networks activity. LH = left hemisphere; RH = right hemisphere.
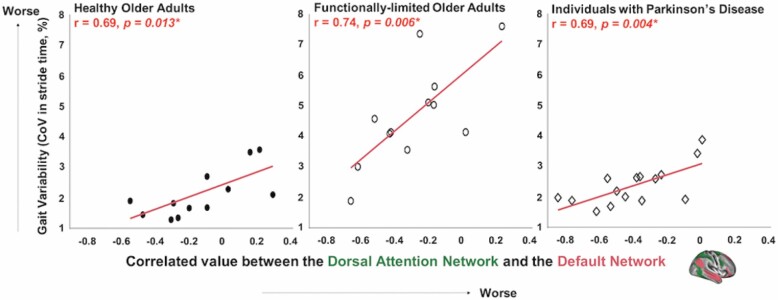
All 3 cohorts exhibited significant correlation between the magnitude of gait variability and the strength of negative correlation between the dorsal attention and default networks. In each cohort, those with lower (better) gait variability exhibited greater strength of negative correlation between the dorsal attention network and the default network; that is, more consistent anti-phase fluctuations in network activity (Figure 2). After adjusting for age and sex, the relationship between gait variability and the strength of this functional connectivity remained significant within each cohort (Healthy older adults: B = 1.66, p = .032, Functionally limited older adults: B = 4.38, p = .027, and Individuals with PD: B = 1.65, p = .005).
Figure 2.
Gait variability is associated with the negative correlation between the dorsal attention and default networks in the 3 cohorts. For the 3 cohorts—healthy older adults, functionally limited older adults, and individuals with Parkinson’s disease, those with less gait variability (ie, greater coefficient of variation in stride time) exhibited stronger negative correlation between the dorsal attention network and the default network. The correlation remains significant after adjusting for age and sex. The functionally limited cohort scatterplot was adapted from the original publication (12).
No other within- or between-network connectivity outcomes were consistently related to gait variability across all 3 cohorts. Several between-network connectivity outcomes, especially those related to the default network, were also correlated with gait variability in one or more of the cohorts. Specifically, lower gait variability was associated with (i) stronger negative correlation between the default network and the somatomotor network in the healthy older cohort (B = 1.47, p = .017) and the PD cohort (B = 1.31, p = .017), (ii) stronger negative correlation between the default network and the ventral attention network for the healthy older adult cohort (B = 1.70, p = .006), (iii) stronger negative correlation between the default network and the frontoparietal control network for the healthy older adult cohort (B = 1.65, p = .010), and (iv) stronger negative correlation between the default network and the limbic network in the PD cohort (B = 1.31, p = .034). No other significant correlation between gait variability and resting state functional connectivity was found to be stronger than the above-mentioned relationships.
Discussion
Despite its sensitivity to aging, functional status, and adverse health outcomes, the neural substrate of gait variability remains poorly understood. Our results suggest that gait variability is linked to the degree to which resting-state activity fluctuations in the dorsal attention and default networks are negatively correlated with one another. Across 3 heterogeneous cohorts—healthy older adults, older adults with mild-to-moderate cognitive-motor limitations, and individuals with PD—those who walked with less gait variability exhibited greater negative correlation between these two networks.
The dorsal attention network has been implicated in a variety of externally directed processes, including orienting one’s attention toward external targets and goal-oriented tasks. The default network is involved in a variety of internally directed processes, including mind-wandering, self-reflection, and conceptual processing. Negative correlation between the dorsal attention network and the default network is thus believed to represent an intrinsic and dynamic aspect of functional brain organization reflecting one’s ability to switch between external, attention-demanding cognitive functions and internal, self-related thinking processes (13,26–28).
Stronger negative correlation between the dorsal attention network and default network has been linked to greater ability to sustain one’s attention to a given task over time, an ability fundamental to higher-level cognitive functions such as learning and memory (17,18,29). Sustained attention, or sustained performance, refers to moment-to-moment fluctuations in cognitive behavioral performance over time and is most often quantified by the degree of intertrial variability in performance during a continuous cognitive task, such as the gradual-onset continuous performance task (gradCPT) (18) or the Eriksen flanker task (17). In the same light, gait variability captures the degree of temporospatial fluctuation in (one aspect of) system output during the continuous task of walking. Our results suggest that those with lower gait variability, that is, more consistent stride timing over time, also exhibit stronger negative correlation between the dorsal attention network and the default network. Intriguingly, both gait variability and sustained attention have been linked to falls (1,30), frailty (2,31), and Alzheimer’s disease (32–34). We thus contend that the magnitude of gait variability over a bout of walking similarly reflects one’s ability to consistently monitor task-relevant information, and avoid “mind-wandering” and task irrelevant stimuli, and is thus dependent upon the functionality of the same neural substrates that give rise to sustained attention—the reciprocal-like interaction between the dorsal attention and default networks.
There is mounting evidence that gait variability is correlated with multiple cortical, subcortical, and white matter elements of the brain (35). In particular, elevated stride time variability has been linked to atrophy of the right parietal cortex (36) and thalamus (37), smaller global white matter volume (38), and reduced integrity of thalamo-cortical white matter tracts (37). These brain regions and tracts are in fact well-aligned with the anatomical regions of and connections between the dorsal attention network and the default network. Both of these networks include various parietal regions: the dorsal attention network includes the superior parietal lobule and the inferior parietal sulcus; and the default network includes the angular gyrus of the parietal lobe. Furthermore, both the dorsal attention network and the default network project to different sub-nuclei in the thalamus (39). Results of the current study are thus corroborated by these known anatomic links to gait variability and provide a potential functional pathway through which such structural characteristics may disrupt locomotor control in older adults and in those with progressive neurologic disease.
Our results suggest that the interplay between the dorsal attention network and default network plays an essential role in regulating gait variability beyond older adults with cognitive and motor limitations. The observed correlation in healthy older adults suggests that functional interactions between these two networks may underlie gait variability even in the absence of clinically significant cognitive-motor decline. The same observation in the cohort with PD suggests that the communication between these two networks may contribute to gait variability even in the presence of a progressive neurological disease that, at least initially, spares these two cortical networks (40–42). Indeed, the present PD cohort consisted of patients at younger age and a relatively early disease stage as signified by comparatively similar gait variability and other aspects of functional performance to the healthy cohort. Future studies are therefore needed to examine if the observed relationship holds in later disease stages, where the progressive neurologic damage of PD may uniquely disrupt cortical network function.
Although large-scale data collections such as the Human Connectome Aging data sets exist to establish the relationship between the dorsal attention network and default network, these data sets do not include gait variability. Thus, while our present analyses are limited in sample size, this concern is mitigated by the fact that this study reproduces a prior finding (12). Testing the reproducibility of the relationship between the dorsal attention-default network and gait viability was the primary aim of this study. Secondary post-hoc analyses of other network relationships confirmed this primary measure as the strongest relationship observed. Future studies with higher spatial and temporal resolution imaging protocols and individualized network parcellations are needed to identify if these cross-subject relationships are the product of large scale dysconnectivity throughout the networks or changes in topographical organization as gait variability increases.
Recent evidence now suggests that the interactions between the dorsal attention and default networks are more sophisticated than previously believed. We now know that these networks are not strictly antagonistic and can vary across default subsystems, time, and cognitive states. For example, interactions between these two networks can be modulated by other brain networks, such as the frontoparietal control network, under various cognitive states (43,44). Our current results suggest that the complex and dynamic interactions between the dorsal attention and default networks at least partially play a role in regulating walking under relatively simple task conditions (ie, walking at preferred speed with no additional cognitive effort). Future studies are therefore warranted to delineate how interactions between the dorsal attention and default networks relate to gait in more complicated conditions (eg, cognitive “dual” tasking), as well as how other brain networks including the frontoparietal control network might influence dorsal attention and default network interactions as they relate to locomotion control.
Imaging and gait data from each cohort included in this study were obtained on different MR scanners, methods, and via different gait assessment tools. These differences limited our ability to combine data sets or compare functional connectivity values across cohorts. On the other hand, such differences suggest that the consistent, observed link between gait variability and brain connectivity was in fact independent of the methods used for measurement and analysis. The same global signal regression was used for preprocessing resting-state data in all 3 cohorts. One limitation for using global signal regression is this method may result in shifting a centered value from a slightly positive value (eg, ~ 0.2) to around zero (eg, ~ 0) (45–48), potentially leading a more negative correlation between the dorsal attention and default networks and reducing the sensitivity of detecting true correlations. However, direct comparisons between global and nonglobal preprocessing methods on resting-state data indicate that these methodological differences do not significantly impact observed correlations between the dorsal attention and default networks (45). Lastly, the current work utilized cross-sectional data sets and gait variability values obtained from walking at each participant’s preferred speed. Larger-scale confirmatory studies are thus needed to investigate whether changes in the functional connectivity between the dorsal attention and default networks are linked to changes in gait variability over time, and whether this aspect of functional connectivity predicts gait variability in habitual and/or more challenging walking conditions.
Author Contributions
O.-Y. L., M.A.H., and B.M. contributed the design and conceptualization of the study. O.-Y. L., M.A.H., and K.J.D. contributed the analysis and interpretation of the data. O.-Y. L. drafted the manuscript. M.A.H., K.J.D., P.M.W., L.A.L., and B.M. provided critical revisions of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (T32-AG023480, KL2-TR002542, R01-AG041785, R21-AG064575, K24-AT009282, R01-MH111868, R01-MH117063), the Interventional Studies in Aging Center (ISAC) pilot grant, the Dr. Ralph and Marian Falk Medical Research Trust, the Boston Claude F. Pepper Older Americans Independence Center (P30-AG013679), and a grant from the Osher Center for Integrative Medicine. L.A.L. holds the Irvin and Edyth S. Usen Chair in Geriatric Medicine at Hebrew SeniorLife.
Conflict of Interest
All authors report no conflicts of interest relevant to the manuscript. P.M.W. is the founder and sole owner of the Tree of Life Tai Chi Center. His interests were reviewed and managed by the Brigham and Women’s Hospital and Partner’s Health- Care in accordance with their conflict of interest policies.
References
- 1.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893 [DOI] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Muir SW, Hall M, et al. Gait variability is associated with frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:568–576. doi: 10.1093/gerona/glr007 [DOI] [PubMed] [Google Scholar]
- 3.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ijmker T, Lamoth CJ. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012;35:126–130. doi: 10.1016/j.gaitpost.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 5.Byun S, Han JW, Kim TH, et al. Gait variability can predict the risk of cognitive decline in cognitively normal older people. Dement Geriatr Cogn Disord. 2018;45:251–261. doi: 10.1159/000489927 [DOI] [PubMed] [Google Scholar]
- 6.Kalron A, Achiron A, Menascu S. Gait variability, not walking speed, is related to cognition in adolescents with multiple sclerosis. J Child Neurol. 2019;34:27–32. doi: 10.1177/0883073818808034 [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13:428–437. doi: 10.1002/mds.870130310 [DOI] [PubMed] [Google Scholar]
- 8.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16:53–58. doi: 10.1177/0891988702250580 [DOI] [PubMed] [Google Scholar]
- 9.Allali G, Laidet M, Herrmann FR, et al. Gait variability in multiple sclerosis: a better falls predictor than EDSS in patients with low disability. J Neural Transm (Vienna). 2016;123:447–450. doi: 10.1007/s00702-016-1511-z [DOI] [PubMed] [Google Scholar]
- 10.Hsieh KL, Sun R, Sosnoff JJ. Cognition is associated with gait variability in individuals with multiple sclerosis. J Neural Transm (Vienna). 2017;124:1503–1508. doi: 10.1007/s00702-017-1801-0 [DOI] [PubMed] [Google Scholar]
- 11.Schniepp R, Wuehr M, Schlick C, et al. Increased gait variability is associated with the history of falls in patients with cerebellar ataxia. J Neurol. 2014;261:213–223. doi: 10.1007/s00415-013-7189-3 [DOI] [PubMed] [Google Scholar]
- 12.Lo OY, Halko MA, Zhou J, Harrison R, Lipsitz LA, Manor B. Gait speed and gait variability are associated with different functional brain networks. Front Aging Neurosci. 2017;9:390. doi: 10.3389/fnagi.2017.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- 14.Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648 [DOI] [PubMed] [Google Scholar]
- 15.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Liu J, Wang Z, Sun P, Li K, Liang P. Dysfunctional interactions between the default mode network and the dorsal attention network in subtypes of amnestic mild cognitive impairment. Aging (Albany NY). 2019;11:9147–9166. doi: 10.18632/aging.102380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 18.Esterman M, Noonan SK, Rosenberg M, Degutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex. 2013;23:2712–2723. doi: 10.1093/cercor/bhs261 [DOI] [PubMed] [Google Scholar]
- 19.Manor B, Zhou J, Harrison R, et al. Transcranial direct current stimulation may improve cognitive-motor function in functionally limited older adults. Neurorehabil Neural Repair. 2018;32:788–798. doi: 10.1177/1545968318792616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 2014;34:12049–12056. doi: 10.1523/JNEUROSCI.1776-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006 [DOI] [PubMed] [Google Scholar]
- 24.McDonough AL, Batavia M, Chen FC, Kwon S, Ziai J. The validity and reliability of the GAITRite system’s measurements: a preliminary evaluation. Arch Phys Med Rehabil. 2001;82:419–425. doi: 10.1053/apmr.2001.19778 [DOI] [PubMed] [Google Scholar]
- 25.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x [DOI] [PubMed] [Google Scholar]
- 26.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 27.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortenbaugh FC, Rothlein D, McGlinchey R, DeGutis J, Esterman M. Tracking behavioral and neural fluctuations during sustained attention: a robust replication and extension. Neuroimage. 2018;171:148–164. doi: 10.1016/j.neuroimage.2018.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Halloran AM, Pénard N, Galli A, Fan CW, Robertson IH, Kenny RA. Falls and falls efficacy: the role of sustained attention in older adults. BMC Geriatr. 2011;11:85. doi: 10.1186/1471-2318-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Halloran AM, Finucane C, Savva GM, Robertson IH, Kenny RA. Sustained attention and frailty in the older adult population. J Gerontol B Psychol Sci Soc Sci. 2014;69:147–156. doi: 10.1093/geronb/gbt009 [DOI] [PubMed] [Google Scholar]
- 32.Webster KE, Merory JR, Wittwer JE. Gait variability in community dwelling adults with Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:37–40. doi: 10.1097/01.wad.0000201849.75578.de [DOI] [PubMed] [Google Scholar]
- 33.Berardi AM, Parasuraman R, Haxby JV. Sustained attention in mild Alzheimer’s disease. Dev Neuropsychol. 2005;28:507–537. doi: 10.1207/s15326942dn2801_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huntley JD, Hampshire A, Bor D, Owen AM, Howard RJ. The importance of sustained attention in early Alzheimer’s disease. Int J Geriatr Psychiatry. 2017;32:860–867. doi: 10.1002/gps.4537 [DOI] [PubMed] [Google Scholar]
- 35.Tian Q, Resnick S, Bair W, Ferrucci L, Studenski SA. Gait variability and longitudinal cognitive change in aging. Innovation in Aging. 2017;1:1206–1207. doi: 10.1016/j.neubiorev.2017.01.020 [DOI] [Google Scholar]
- 36.Beauchet O, Annweiler C, Celle S, Bartha R, Barthélémy JC, Roche F. Higher gait variability is associated with decreased parietal gray matter volume among healthy older adults. Brain Topogr. 2014;27:293–295. doi: 10.1007/s10548-013-0293-y [DOI] [PubMed] [Google Scholar]
- 37.Verlinden VJ, de Groot M, Cremers LG, et al. Tract-specific white matter microstructure and gait in humans. Neurobiol Aging. 2016;43:164–173. doi: 10.1016/j.neurobiolaging.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 38.Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40:175–180. doi: 10.1161/STROKEAHA.108.524355 [DOI] [PubMed] [Google Scholar]
- 39.Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37:5594–5607. doi: 10.1523/JNEUROSCI.0067-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg-Katz K, Herman T, Jacob Y, et al. Fall risk is associated with amplified functional connectivity of the central executive network in patients with Parkinson’s disease. J Neurol. 2015;262:2448–2456. doi: 10.1007/s00415-015-7865-6 [DOI] [PubMed] [Google Scholar]
- 41.Jiang S, Wang M, Zhang L, et al. Regional homogeneity alterations differentiate between tremor dominant and postural instability gait difficulty subtypes of Parkinson’s disease. J Neural Transm (Vienna). 2016;123:219–229. doi: 10.1007/s00702-015-1490-5 [DOI] [PubMed] [Google Scholar]
- 42.Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2019;63:77–82. doi: 10.1016/j.parkreldis.2019.02.036 [DOI] [PubMed] [Google Scholar]
- 43.Dixon ML, De La Vega A, Mills C, et al. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc Natl Acad Sci USA. 2018;115:E1598–E1607. doi: 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dixon ML, Andrews-Hanna JR, Spreng RN, et al. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage. 2017;147:632–649. doi: 10.1016/j.neuroimage.2016.12.073 [DOI] [PubMed] [Google Scholar]
- 45.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data are available and will be shared at the request of other investigators for purposes of replicating results.