Abstract
In the 1990s and early 2000s, the common definition for sarcopenia was age-related loss of skeletal muscle, and low levels of muscle mass were central to sarcopenia diagnosis. In more recent consensus definitions, however, low muscle strength displaces low muscle mass as a defining feature of sarcopenia. The change stems from growing evidence that muscle weakness is a better predictor of adverse health outcomes (eg, mobility limitations) than muscle mass. This evidence accompanies an emerging recognition that central neural mechanisms are critical determinants of age-related changes in strength and mobility that can occur independently of variations in muscle mass. However, strikingly little practical attention is typically given to the potential role of the central nervous system in the etiology or remediation of sarcopenia (ie, low muscle function). In this article, we provide an overview of some mechanisms that mediate neural regulation of muscle contraction and control, and highlight the specific contributions of neural hypoexcitability, dopaminergic dysfunction, and degradation of functional and structural brain connectivity in relation to sarcopenia. We aim to enhance the lines of communication between the domains of sarcopenia and neuroscience. We believe that appreciation of the neural regulation of muscle contraction and control is fundamental to understanding sarcopenia and to developing targeted therapeutic strategies for its treatment.
Keywords: Aging, Dynapenia, Physical function, Strength, Weakness
The concept of sarcopenia arose in the late 1980s and early 1990s to describe age-related loss of muscle mass (1). Sarcopenia has since become recognized as an important geriatric condition and a key precursor to the development of frailty, prompting an exponential increase in scientific research into this condition. Such efforts supported an evolution in the definition and conceptualization of sarcopenia; current views more commonly consider sarcopenia as an age-related loss of muscle mass and muscle function (namely muscle strength) (2).
Between 2010 and 2014, 3 different working groups comprising international panels of geriatricians and scientists from academia and industry proposed a series of consensus definitions of sarcopenia (3–5). These definitions centered on the general premise that advancing age results in changes in body composition, particularly muscle wasting (ie, atrophy), which leads to muscle weakness that precipitates reduced physical function and mobility limitations. These early consensus definitions highlighted low levels of muscle mass as central to sarcopenia diagnosis. However, recent working groups called for substantial revisions to these definitions, with low muscle strength (ie, weakness) displacing low muscle mass as a principal defining feature of sarcopenia. This proposition stems from growing evidence that muscle weakness is a better predictor of adverse health outcomes (eg, mobility limitations) than is muscle mass (6–8).
The increased emphasis on muscle weakness in sarcopenia is, in our opinion, well-motivated. The premise that age-related loss of muscle mass was a direct cause of age-related decrease in muscle strength stemmed from associations that were ultimately not as strong as initially expected. For instance, both cross-sectional and longitudinal study data clearly indicate that age-related loss of strength far exceeds the loss of muscle mass (9,10). In fact, Delmonico et al reported that older adults who gained weight and increased their muscle mass (assessed via computed tomography imaging) over a 5-year period (n = 333) still exhibited substantial loss of strength (Figure 1A) (10). Recently, Cawthon et al demonstrated that measurement of muscle mass using the D3-creatine dilution technique resulted in stronger associations between muscle mass and lower extremity function than the more commonly employed dual-energy x-ray absorptiometry technique of measuring muscle mass (11). Thus, the role of muscle mass in measures of strength and mobility is liable to further revision. Nonetheless, it seems safe to conclude that age-related loss of strength is not due solely to muscle atrophy, and that nonmuscle mass-related factors may play critical roles in development of weakness.
Figure 1.
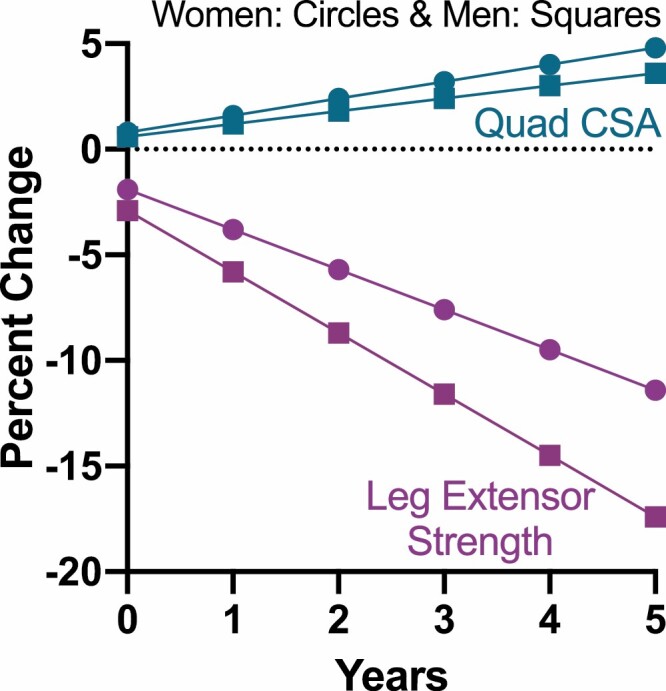
Longitudinal data over a 5-year period illustrating that older adults who gained weight and increased their quadriceps femoris cross-sectional area (Quad CSA) still exhibited a dramatic reduction in leg extensor muscle strength. Adapted from Delmonico and colleagues (10).
The most recent definitions of sarcopenia suggest that consensus has shifted from the historic muscle-centric view to a recognition that manifestation of strength is mediated by a host of neuromuscular mechanisms (12,13). However, the potential role of the central nervous system (CNS) in the etiology or remediation of sarcopenia remains poorly understood. In this article, we provide an overview of some mechanisms that mediate neural regulation of muscle contraction and control, and highlight the specific contributions of neural hypoexcitability, dopaminergic dysfunction, and degradation of functional and structural brain connectivity in relation to sarcopenia. By promoting awareness and understanding of the neural regulation of muscle contraction and control in the context of sarcopenia, we hope to facilitate development of alternative strategies to treat this condition.
Sarcopenia, Muscle Function, and the Nervous System
With respect to sarcopenia, the term “muscle function” is generally used to encompass muscle strength, muscle power (the product of the force and velocity of muscle contraction), and fatigability (2). Most work to date focused on the degree to which measures of muscle strength and power predict negative health outcomes in older adults. The results are incontrovertible. Weakness places older adults at greater risk of mobility limitations (14,15), falls (15), impairments in instrumental activities of daily living (16), and even premature death (16–18). Weakness is more prevalently associated with frailty than chronological age (19).
Muscle power declines earlier and more precipitously with advancing age than maximal muscle strength. Muscle power is of greater importance than muscle strength for many everyday tasks such as rising from a chair and climbing stairs and has emerged as an important predictor of functional limitations in older adults (20,21). This is not surprising; for strength to confer functional benefits, the rapidity with which muscle force is generated must be sufficient for the task at hand. For example, muscle force must be generated quickly to shift one’s base of support and restore an upright stance following a disturbance that would otherwise induce a fall. The speed of recruitment and maximal discharge rate of motor neurons largely determines the velocity at which muscles can generate force (22,23). Thus, nervous system integrity is critical.
The ability to selectively engage muscles in a coordinated context-sensitive manner is of commensurate importance to physical function. If a perturbation occurs during gait, the observed adaptive response extends across the whole body, involving not only coordinated lower limb muscle activity, but also goal-directed engagement of the torso and arm muscles (see (24) for review). The pattern of muscle activation manifested in such circumstances is often described with reference to the term synergy—defined as mechanisms used by the CNS to coordinate groups of motor units (motor neurons and the muscle fibers they innervate) into functional assemblies (eg, (22)). While it is perhaps obvious that measures of muscle strength depend upon the capability of the CNS to recruit motor units and discharge them at optimal rates, it is less widely appreciated that all assessments requiring contractions in humans necessarily also reflect the organization of muscle synergies. That measures of muscle performance are more strongly predictive of physical function and adverse health outcomes than measures of muscle mass is, in our opinion, due to dependence of muscle performance on the integrity of neural systems necessary for motor unit recruitment and coordination.
Impairments in Muscle Synergy Formation and Neural Activation Are Key Contributors to Weakness
Grip strength is a robust prospective predictor of negative health outcomes in older adults. The extent to which tests of human grip strength impose demands on the CNS (for review, see (23)) is less well recognized. These demands are dictated by the complexity and redundancy of the musculoskeletal apparatus of the human hand. Tests of maximum grip strength require that the brain modulates recruitment of 19 muscles situated entirely within the hand and another 20 muscles located in the forearm in a spatially and temporally differentiated pattern. The force measured during grip strength testing depends not only on activation of muscles that flex the phalanxes against the dynamometer, but also upon the ability of the CNS to engage the muscles that orient the fingers with respect to one another, and those that stabilize the position of the hand and wrist. The degree to which these various requirements can be balanced determines the reading that will be obtained.
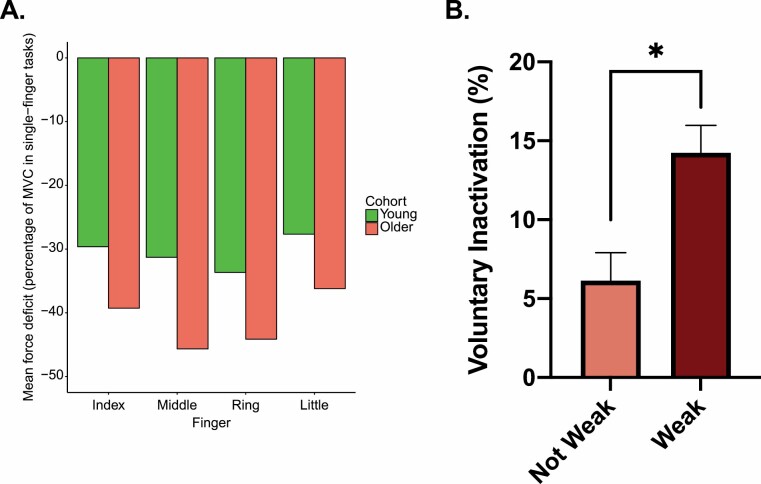
The significance of the constraints imposed on expressions of strength by the demands for muscle coordination can be illustrated by the “multi-finger force deficit” phenomenon. This is the decline in the flexion force that can be generated by an individual finger as the number of other fingers contributing to the grip is increased (25). When all 4 fingers are engaged together, the force applied by each finger is about half that which can be applied when the finger is used in isolation. The magnitude of this deficit is greater in older adults (26), consistent with evidence that deficiencies in muscle synergy formation contribute to the difficulties experienced by older adults in many movement tasks (27,28) (Figure 2A).
Figure 2.
(A) Multi-finger force deficit exhibited by a group of 6 male (29.3 ± 3.6 years) and 6 female (29.8 ± 4.6 years) young; and 6 male (87.2 ± 4.5 years) and 6 female (76.3 ± 4.0 years) older participants. Negative values indicate that the force generated by each digit in the 4-finger test is substantially lower than that produced when a digit is engaged in isolation. Diminution of the force applied by individual digits during 4-finger tasks is greater in older persons than in young persons. This original plot was generated using data extracted from Shinohara et al. (28). (B) Older adults with clinically meaningful leg extensor weakness exhibit significantly higher levels of leg extensor voluntary inactivation (ie, impairments in the nervous system to fully activate their quadriceps musculature) than older adults who are not weak. This original figure was generated using data extracted from Clark et al. (29).
Substantial improvements in performance can be achieved in the absence of changes in muscle mass, further emphasizing the critical role of the CNS in grip strength (30). Work toward the end of the 19th century demonstrated that grip strength is enhanced entirely as a consequence of training performed by the opposite limb (31). There have been many further demonstrations of this “cross-education” phenomenon (eg, (32)), in tasks that engage muscles of the upper or lower limbs. The most parsimonious interpretation is that improved control of muscle coordination and activation—instantiated by CNS adaptations—is the primary agent of change.
There is also evidence that age-related changes in the strength of other muscle groups are critically determined by central neural mechanisms. In addition to affecting muscle synergy, the health of the CNS is the key factor determining the sufficiency of neuromuscular activation. Numerous studies used the interpolated twitch method or a derivative thereof (33,36) to examine whether aging results in impairment of the CNS in fully activating skeletal muscle volitionally (see (35) for review). These reports yielded discrepant findings, likely due to variations in the muscle groups investigated, as well as the inherent heterogeneity of aging (36). However, we recently reported strong evidence that older adults with clinically meaningful leg extensor weakness exhibit significant deficits in the ability of their nervous system to fully activate their leg extensor muscles (Figure 2B) (29,37). Interestingly, stronger older adults did not exhibit these deficits. These findings indicate that the nervous system is a key culprit in clinically meaningful age-related weakness. This is arguably the most interesting phenotype from a clinical care and treatment perspective. Moreover, these findings suggest that aging is not inherently associated with impairments in neural activation (as stronger older adults exhibited minimal deficits). These outcomes also highlight the heterogeneity of aging and raise the possibility that reduced physical activity or other factors (opposed to age per se) lead to impaired neural regulation of muscle contraction and control.
Neural Control of Muscle Contraction and Control: A Primer
Voluntary engagement of a muscle is initiated by activity in brain networks (eg, the primary motor cortex), resulting in elevated firing of (descending) corticospinal neurons and consequential recruitment of spinal motor neurons, and hence muscle fibers. As descending drive increases, a greater number of spinal motor neurons are recruited, they discharge more rapidly, and additional contractile force is generated (38). When a motor neuron fires sufficiently fast, the muscle fibers it innervates produce a fused contraction. In this context, there are multiple influences on the state of spinal motor neurons (in addition to descending drive from the motor cortex and other supraspinal centers), such as those mediated by excitatory and inhibitory afferent projections, and alterations in motor neuron properties that may make them more or less responsive to synaptic input (39).
Although all actions are mediated by integrative interactions at multiple sites within the nervous system, ultimately the only available output channel is provided by motor neurons (40). The pyramidal tract, which originates in the deeper structures of the cerebral cortex, is the best-studied output pathway. Many axons in this tract intersect in the lower medulla and form the corticospinal tract that projects to the spinal cord. Several cortical areas, including the premotor and parietal areas as well as the primary motor cortex, contribute axons to the corticospinal tract, with many of these axons terminating on spinal interneurons in the intermediate region of the spinal cord. The terminals of some corticospinal axons also extend into the ventral horn of the spinal cord, where they branch out and contact monosynaptically the dendrites of spinal motor neurons (corticomotoneurons). A single corticomotoneuron axon often terminates on and excites spinal motor neurons for several different agonist muscles. Further, this neuron can influence the contractile activity of additional muscles through synapses on spinal interneurons that act indirectly to suppress the activity of antagonist muscles. Monosynaptic projections from the primary motor cortex onto spinal motor neurons are most profuse for the distal arm, hand, and finger muscles. This arrangement allows the primary motor cortex to regulate the activity of these muscles directly, in contrast to indirect muscle regulation through the reflex and pattern-generating functions of spinal circuits. The corticospinal tract is not, however, the only pathway for descending control signals to spinal motor circuits since the spinal cord also receives inputs from the rubrospinal, reticulospinal, and vestibulospinal tracts.
A muscle may be controlled (innervated) by several hundred alpha motor neurons; however, there is wide variability across muscles. Each alpha motor neuron will innervate anywhere from a few to several thousand muscle fibers (ie, the motor unit). The motor unit is the elementary constituent of motor control because it represents the final pathway transmitting the neural signal to skeletal muscle fibers. The number of muscle fibers innervated by a motor neuron (innervation number) varies primarily based on the function of a given muscle, with muscles responsible for the most precise control (eg, eye muscles, hand muscles) having a lower innervation number than those responsible for higher force and power production. The amount of force that can be produced by the activation of a motor unit is related to the number of muscle fibers innervated by the motor neuron.
Muscle force is modulated primarily by the recruitment and discharge rate of motor units, whereby force can be increased by activation of additional motor units or by discharging an active motor unit at a faster rate. For a motor unit to be activated, the alpha motor neuron must reach threshold. The recruitment threshold depends on the membrane resistance of the motor neuron, which results in small-diameter motor neurons being recruited first. Motor neuron discharge rates depend on the level of net excitatory input to the motor neuron, coupled with its intrinsic membrane properties, which ultimately determine the magnitude of neuronal depolarization. These properties can be dramatically altered by input from monoaminergic neurons in the brain stem. For instance, when the monoamines serotonin and norepinephrine activate L-type Ca2+ channels on the dendrites of the motor neurons, the resulting inward Ca2+ currents can enhance synaptic currents up to 10-fold. Thus, high monoaminergic drive can result in “self-sustained firing” of the motor neurons (ie, they continue to fire repetitively after being activated by a brief, excitatory synaptic input). Notably, motor units vary based on their contraction speed, maximal force, and ability to withstand fatigue.
There is compelling evidence that aging affects most of the neural processes described above (for review, see (23,35,41)). Undoubtedly, these changes contribute mechanistically to the declines in physical capability and muscle function commonly observed with advancing age. In the next few sections, we highlight our work and that of others demonstrating the specific contributions of neural hypoexcitability, dopaminergic dysfunction, and degradation of functional and structural brain connectivity to sarcopenia.
The Contribution of Neural Hypoexcitability
Neural excitability can be defined, depending on the level of detail, as the readiness of a nerve cell or neural circuit to respond to a stimulus (42–44). The response is typically in the form of an action potential, a transient change of electrical charge (polarization) of the neuronal membrane. The action potential can be measured either individually, at the level of an individual nerve cell, or as sum of action potentials in form of a compound action potential or an evoked potential, at the level of group of neurons or neural circuits (42,43). Neural hypoexcitability may be a key contributor to weakness (eg, a neuron with low excitability will, conceptually, have a lower maximal steady-state firing frequency (44)).
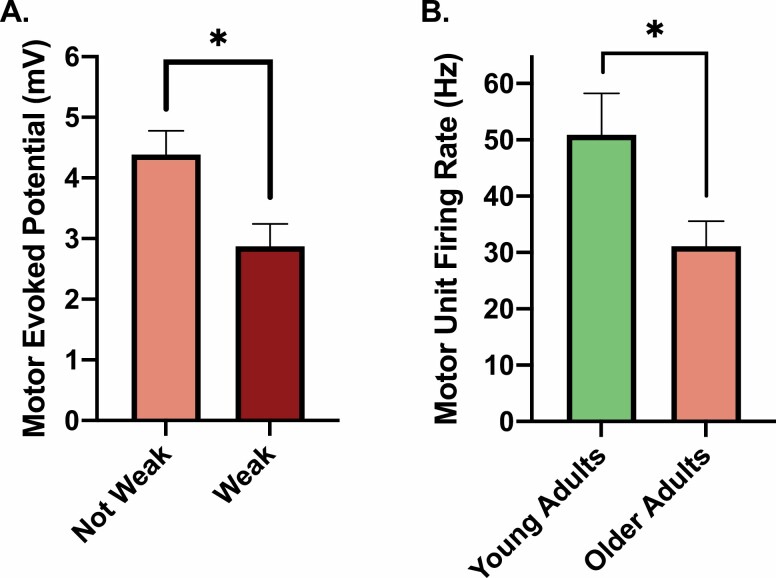
We and others previously suggested that aging-associated weakness, as well as a myriad of other disorders and conditions (eg, disuse, injury, and sepsis), may be due, in part, to neural hypoexcitability (29,45–47). Older adults with clinically meaningful leg extensor weakness exhibit corticospinal hypoexcitability (eg, they have motor evoked potentials approximately half that of the strong older adults and ~25% longer silent period durations) (Figure 3) (29). Moreover, these indices of excitability explain ~33% of between-subject variability in older adults’ leg extensor strength, which is slightly more than that explained by thigh lean mass (29).
Figure 3.
(A) Older adults with clinically meaningful leg extensor weakness exhibit indices of neural hypoexcitability (ie, significantly smaller motor evoked potentials) in comparison to nonweak older adults as assayed using transcranial magnetic stimulation. This original figure was generated using data extracted from Clark et al. (29). (B) Older adults (n = 7) exhibit significantly slower motor unit firing rates of the first dorsal interosseous (finger abductor) during a maximal voluntary contraction (n = 7). This finding suggests that reductions in muscle strength in older adults are partially due to an impaired ability to fully drive the surviving motor units. Figure created from data presented by Kamen et al. (48).
Recognizing the limitations of cross-sectional studies for drawing cause-and-effect conclusions (49), these findings nonetheless suggest that weakness is due, in part, to neural hypoexcitability. While these findings do not provide insight into whether the dysfunction is at the level of the cortical or spinal motor neurons, prior work indicates that aging is associated with hypoexcitability of both upper and lower motor neurons. For instance, paired-pulse brain stimulation paradigms that permit inferences in relation to intracortical excitability demonstrate that older adults have greater indices of cortical hypoexcitability than young adults (50,51). Human and animal studies also suggest that aging results in reduced α-motor neuron excitability (eg, greater and longer afterhyperpolarization potentials and lower minimal and maximal steady-state firing frequencies) (Figure 3B) (48,52–54). Findings of this nature raise the question of whether neurotherapeutic interventions targeting excitability could be a viable approach to increasing muscle strength to reduce the risk of physical impairments in later life.
Contribution of Dopaminergic Dysfunction
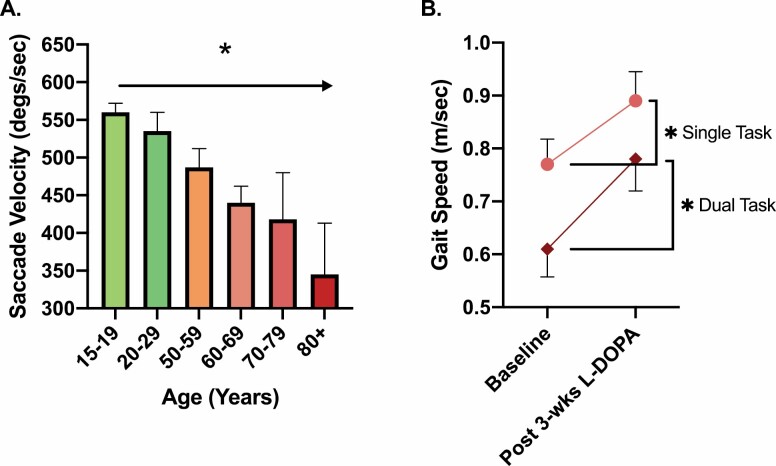
It has been proposed that dysregulation of the basal ganglia, a group of subcortical nuclei, contributes to age-related reductions in mobility by virtue of its dopaminergic function. Progressive degeneration of midbrain dopaminergic neurons is associated with deficits in initiation, speed, and fluidity of voluntary movement (55–57). Lower rates of voluntary force development presage an elevated risk of falls (58). The ability of the nervous system to rapidly drive muscle force production is also linked to indices of mobility (59,60). Moreover, peak horizontal saccade velocity—which, in principle, should not be constrained by musculoskeletal mechanisms—decreases with age. Older adults exhibit saccade velocities that are about half those of young adults (Figure 4A) (61).
Figure 4.
(A) Peak horizontal saccade velocity—which theoretically should not be affected by musculoskeletal mechanisms and processes—decreases with age. Figure created based on estimated values from Irving et al. (61). This article did not report specific pairwise comparisons across groups, but a group main effect was observed, denoted here with an asterisk. (B) Older adults with depression (n = 16) and slow gait speed were given a 3-week treatment with carbidopa/levodopa (L-DOPA), which resulted in significant improvement in single- and dual-task usual gait speed (37.5 mg carbidopa/150 mg levodopa once daily for the first week, twice a day for the second week, and 3 times a day for the third week). Figure created from data presented by Rutherford et al. (62).
Studies of the aging human brain show that regulation of dopamine (DA) action is significantly reduced in old age. This change occurs as a result of structural degradation, including neuronal loss, reduction of neuroreceptor sites, and a decline of transporter molecules (63). Age-dependent declines of brain DA levels in the basal ganglia were reported postmortem, specifically in the dorsal striatum (64). In vivo imaging studies later confirmed these findings (63). Moreover, after the age of 20, the availability of DA D1-like receptors declines in the human striatum at a rate of ~7% per decade (65,66), with the D2-like family demonstrating a similar decrease in receptor density (~5–10%/decade) (40,67) and receptor binding potential (~6–8%/decade) (39,68,69).
Several studies directly examined the relationship between striatal DA, age-related changes in gait, and other measures of motor function. For instance, Volkow et al reported that age-related decreases in brain DA activity in nonparkinsonian older adults, assessed using positron emission tomography with sensitivity to DA D2 receptors in the caudate and putamen, are associated with finger-tapping speed (70). Similar studies reported that lower striatal DA transporter activity explains ~23% and 35% of the between-subject variance in “comfortable-pace” gait speed and cadence, respectively (71). Moreover, several investigations reported a relationship between dopaminergic genotype (catechol-O-methyltransferase genotype) and mobility (59,72,73). These studies generally suggest that genotypes resulting in intermediate levels of tonic DA (ie, the Val158Met polymorphism) are associated with faster gait and movement speeds (59,72,73). This is likely due to an influence of these genotypes on balancing the roles of tonic and phasic DA (ie, tonic–phasic regulation of DA transmission) (74–76). Lastly, a recent pilot study that treated a small number of older adults (n = 16; mean age 72.5 years) who suffered from depression with L-DOPA for 3 weeks reported a 16% and 28% significant increase in single-task and dual-task usual gait speed, respectively (Figure 4B) (62). Collectively, findings of this nature support the premise that dopaminergic system status is linked directly to preservation of gait speed and mobility. Further work on this topic appears warranted.
Degradation of Brain Connectivity
There is a vast literature that documents age-related variations in functional and structural brain connectivity (77). A substantial volume of work deals specifically with the relationships between these variations and preservation of physical function (78). A general conclusion to emerge from this work is that the manner in which the brain is affected by neural degeneration is not “diffuse, random, or confluent” (79). Rather, neural degeneration proceeds in an orderly and sequential manner, affecting brain networks that regulate related functions. Notably, grip strength is a strong predictor of subsequent declines in mobility, as gauged by gait speed (80,81).
Here, we will focus on a subdomain of this literature: interhemispheric (corpus callosum [CC]) projections. A fundamental role of these CC pathways is to support contrast-enhancing and integrative functions by co-opting the computational resources of the 2 cerebral hemispheres (82). Thus, it is predicted that reductions in the structural integrity of the callosal fiber bundles connecting nodes of the cortical (motor) network will give rise to reductions in motor capability. The CC plays a key pathophysiological role in aging and in the early phases of neurodegenerative disease (83). In the following section, we highlight the literature on age-related changes in CC microstructure, focusing on studies that used diffusion-weighted magnetic resonance imaging at high angular resolution. Such methods permit more satisfactory biophysical modeling of the lateral cortical projections of the CC, which contain complex architectures such as crossing fibers (84–86).
The CC exhibits structural and functional segmentation. The local tissue microstructure of callosal streamlines linking anatomically defined regions in the 2 cerebral hemispheres can be inferred by tractography (eg, (87)). Alternatively, tissue microstructure may be estimated by looking at subdivisions of the CC delineated using histological methods (eg, (88)) or by interrogating the functional connectivity of white matter networks (eg, (89)). Using such methods, the subdivisions typically associated with (sensori-) motor functions are the body and the isthmus of the CC. A negative association between age and white matter structural integrity is observed in the body of the CC (90,91), which is particularly pronounced after 40 years of age (92). A similar pattern of age-related change is observed in CC fibers projecting to the precentral gyrus using an approach based on whole‐brain tractography and delineation of tracts of interest (93). Although, to the best of our knowledge, longitudinal assessments have thus far only been conducted using low-angular-resolution diffusion-weighted magnetic resonance imaging sequences, in a cohort of 215 individuals assessed twice over an interval ranging between 2.7 and 4.7 years, annual decreases in fractional anisotrophy (FA) and increases in radial diffusivity, axial diffusivity, and mean diffusivity—reflecting myelin disruption or degeneration—were detected in the body of the CC. The start of decline was placed in the fifth decade ((94), see also (95)). Therefore, it appears that structural integrity of the callosal fibers connecting nodes of the cortical motor network tends to diminish with age. Significantly, age-related variations in callosal integrity are associated with measures of strength and mobility.
Several studies suggested that gait velocity is related to the burden of white matter hyperintensities within the CC (41,96,97). These findings suggest that the parts of the CC that connect frontal, (pre)motor, and sensory areas exhibit the closest associations with gait abnormalities (eg, (98)). Additionally, in studies employing the diffusion tensor model, lower white matter integrity in the body of the CC accompanies higher temporal gait variability (99) and poorer performance on clinical mobility assessment scales (97). Zhou et al observed associations between FA values registered for the genu of the CC and walking speed in older adults (100).
Prospective studies also reveal that the integrity of the CC is indicative of future declines in mobility. Tian et al reported that the volume of the whole CC predicted subsequent changes in gait speed over a period of 4 years (101). In a prospective study spanning 3 years, Ryberg et al noted that the size of isthmus of the CC at baseline was inversely related to the prevalence of falls (102). In contrasting groups of individuals who had either “improved” or “not responded” to a home-based physical activity intervention over a period of 2 years, Venkatraman and colleagues observed that diffusion tensor modeled mean diffusivity of the (whole) CC at baseline was predictive of differences between the groups in performance of the Timed Up and Go test (103). Moreover, van der Holst et al observed that over a period of ~5.4 years, declines in stride length were associated with decreases in white matter FA and increases in mean diffusivity in the whole CC (104). In cross-sectional studies, the relationship between FA values in the body of the CC and both gait speed and variability of gait speed remains present after adjustment for the presence of white matter hyperintensities ((105), see also (106)).
On the basis of anatomical magnetic resonance images obtained from a large (~500) cohort of older adults (60–64 years), Anstey et al noted an association between grip strength and the area of the midbody of the CC (107). To date, no studies employed high-angular-resolution diffusion-weighted magnetic resonance imaging to directly assess the relationship between callosal white matter integrity and grip strength. Using a test that measures sensory function and upper limb strength, Cunningham et al reported an association between (diffusion tensor modeled) FA values derived for the body of the CC and test performance in a small group of adults (age ~50 years) (108). Similar associations are observed in patient cohorts (109). In a 2-year longitudinal study, changes in white matter hyperintensity burden in the body and splenium of the CC were associated with aging-related declines in the time taken to rise from chair to stand, a task that is heavily dependent on strength (110).
The effects of age-related degeneration on the callosal fiber bundles connecting nodes of the cortical motor network suggest that changes in central neurological processes mediate the relationship between life-span variations in grip strength and gait sufficiency (and cognitive function). More generally, it is possible to delineate specific changes in brain structure that precipitate weakness and limitations of mobility.
Conclusion
We used the evolving conceptualization of sarcopenia as the context in which to assess the proposition that central neural mechanisms are critical determinants of age-related changes in strength and mobility. In this vein, we provided an overview of the mechanisms that mediate neural regulation of muscle contraction and control, and highlighted the instrumental contributions to sarcopenia of neural hypoexcitability, dopaminergic dysfunction, and degradation of functional and structural brain connectivity. These examples of age-related changes in neural function are by no means exhaustive. They do, however, point to the influence of a broad range of neural factors in the expression of sarcopenia. Many of these factors have yet to be studied comprehensively. There is certainly still much to be learned about these factors and the manner in which the extent of their influence varies across different types of sarcopenia. However, a general point can be made: aging of the nervous system plays a critical role in precipitating loss of muscle strength and reduction in physical function.
In some circumstances, a lack of knowledge about the mechanistic basis of age-related declines in function may have little practical impact, particularly if the approach to remediation is concerned with the symptoms rather than the causes. In other instances, the consequences of deficits in basic knowledge are not so benign. Age-related changes in strength and mobility provide a case in point. Most nonexercise-based therapeutic interventions for sarcopenia currently being developed or deployed focus on increasing muscle mass. While these may succeed in their narrow intent (ie, gains in muscle mass), they do not provide a means of addressing deficits in neuromuscular control. It is therefore unlikely that this approach represents the most effective allocation of resources. More particularly, it is a concern that older adults are being denied CNS-focused intervention strategies that, we hypothesize, have the potential to yield more substantial gains in functional capacity than increases in muscle mass alone. While the lines of communication between the domains of sarcopenia and neuroscience have recently expanded, further dialogue is essential in order to enhance the breadth of understanding upon which the development of effective therapeutic strategies for the treatment of sarcopenia must be based.
Acknowledgments
The authors conceived of the work together. Both were deeply involved in the writing of the article and contributed equally.
Funding
This work was supported, in part, by the National Institutes of Health (NIA R01AG044424 to B.C.C.).
Conflict of Interest
In the past 5 years, B.C.C. has received research funding from NMD Pharma, Regeneron Pharmaceuticals, Astellas Pharma Global Development, Inc., and RTI Health Solutions for contracted studies that involved aging and neuromuscular-related research. In the past 5 years, B.C.C. has received consulting fees from Regeneron Pharmaceuticals, Zev industries, and the Gerson Lehrman Group for consultation specific to age-related muscle weakness. B.C.C. is a cofounder with equity of OsteoDx, Inc.
References
- 1.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5 [DOI] [PubMed] [Google Scholar]
- 2.Correa-de-Araujo R, Hadley E. Skeletal muscle function deficit: a new terminology to embrace the evolving concepts of sarcopenia and age-related muscle dysfunction. J Gerontol A Biol Sci Med Sci. 2014;69:591–594. doi: 10.1093/gerona/glt208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307 e302. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc. 2020;68:1410–1418. doi: 10.1111/jgs.16372 [DOI] [PubMed] [Google Scholar]
- 9.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 10.Delmonico MJ, Harris TB, Visser M, et al. ; Health, Aging, and Body . Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cawthon PM, Orwoll ES, Peters KE, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group . Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. doi: 10.1093/bmb/ldq008 [DOI] [PubMed] [Google Scholar]
- 13.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9:3–19. doi: 10.1002/jcsm.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x [DOI] [PubMed] [Google Scholar]
- 15.Cawthon PM, Manini T, Patel SM, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020;68:1429–1437. doi: 10.1111/jgs.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGrath R, Erlandson KM, Vincent BM, Hackney KJ, Herrmann SD, Clark BC. Decreased handgrip strength is associated with impairments in each autonomous living task for aging adults in the United States. J Frailty Aging. 2019;8:141–145. doi: 10.14283/jfa.2018.47 [DOI] [PubMed] [Google Scholar]
- 17.Duchowny K. Do nationally representative cutpoints for clinical muscle weakness predict mortality? Results from 9 years of follow-up in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2019;74:1070–1075. doi: 10.1093/gerona/gly169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leong DP, Teo KK, Rangarajan S, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 19.Syddall H, Cooper C, Martin F, Briggs R, Aihie Sayer A. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111 [DOI] [PubMed] [Google Scholar]
- 20.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192 [DOI] [PubMed] [Google Scholar]
- 21.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40:4–12. doi: 10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Windhort U, Burke R, Dieringer N, et al. What are the outputs of motor behavior and how are they controlled? In: Humphrey DR, Freund H-J, eds. Motor Control: Concepts and Issues. New York, NY: Wiley; 1991:101–119. [Google Scholar]
- 23.Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189–222. doi: 10.1016/j.neurobiolaging.2018.07.023 [DOI] [PubMed] [Google Scholar]
- 24.Marigold DS, Misiaszek JE. Whole-body responses: neural control and implications for rehabilitation and fall prevention. Neuroscientist. 2009;15:36–46. doi: 10.1177/1073858408322674 [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuki T. Inhibition of individual fingers during grip strength exertion. Ergonomics. 1981;24:21–36. doi: 10.1080/00140138108924827 [DOI] [PubMed] [Google Scholar]
- 26.Shinohara M, Li S, Kang N, Zatsiorsky VM, Latash ML. Effects of age and gender on finger coordination in MVC and submaximal force-matching tasks. J Appl Physiol (1985). 2003;94:259–270. doi: 10.1152/japplphysiol.00643.2002 [DOI] [PubMed] [Google Scholar]
- 27.Barry BK, Riek S, Carson RG. Muscle coordination during rapid force production by young and older adults. J Gerontol A Biol Sci Med Sci. 2005;60:232–240. doi: 10.1093/gerona/60.2.232 [DOI] [PubMed] [Google Scholar]
- 28.Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol (1985). 2003;95:1361–1369. doi: 10.1152/japplphysiol.00070.2003 [DOI] [PubMed] [Google Scholar]
- 29.Clark LA, Manini TM, Wages NP, Simon JE, Russ DW, Clark BC. Reduced neural excitability and activation contribute to clinically-meaningful weakness in older adults. J Gerontol A Biol Sci Med Sci. 2021;76:692–702. doi: 10.1093/gerona/glaa157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speed CA, Campbell R. Mechanisms of strength gain in a handgrip exercise programme in rheumatoid arthritis. Rheumatol Int. 2012;32:159–163. doi: 10.1007/s00296-010-1596-x [DOI] [PubMed] [Google Scholar]
- 31.Scripture E, Smith T, Brown E. On the education of muscular control and power. Stud Yale Psychol Lab. 1894;2:111e119. [Google Scholar]
- 32.Shields RK, Leo KC, Messaros AJ, Somers VK. Effects of repetitive handgrip training on endurance, specificity, and cross-education. Phys Ther. 1999;79:467–475. [PubMed] [Google Scholar]
- 33.Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090 [DOI] [PubMed] [Google Scholar]
- 34.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725 [DOI] [PubMed] [Google Scholar]
- 35.Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. 2011;4:192–199. doi: 10.2174/1874609811104030192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. doi: 10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark BC, Manini TM, Wages NP, Simon JE, Clark LA. Voluntary vs electrically stimulated activation in age-related muscle weakness. JAMA Netw Open. 2019;2:e1912052. doi: 10.1001/jamanetworkopen.2019.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashe J. Force and the motor cortex. Behav Brain Res. 1997;87:255–269. doi: 10.1016/s0166-4328(97)00752-3 [DOI] [PubMed] [Google Scholar]
- 39.Rinne JO, Hietala J, Ruotsalainen U, et al. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab. 1993;13:310–314. doi: 10.1038/jcbfm.1993.39 [DOI] [PubMed] [Google Scholar]
- 40.Liddell EGT, Sherrington CS. Recruitment and some factors of reflex inhibition. Proc R Soc Lond B Biol Sci. 1925;97:488–518. [Google Scholar]
- 41.Wilson J, Allcock L, Mc Ardle R, Taylor JP, Rochester L. The neural correlates of discrete gait characteristics in ageing: a structured review. Neurosci Biobehav Rev. 2019;100:344–369. doi: 10.1016/j.neubiorev.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Konstantinovic LM, Fliipovic SR. Effects of near-infrared low-level laser stimulation on neuronal excitability. In: Hamblin MR, Huang Y-Y, eds. Photobiomodulation in the Brain. Elsevier. 2019. [Google Scholar]
- 43.Kandel ER, Schwartz JH, Jessel TM, Siegelbaum SA, Hudspeth AJ.. Principles of Neural Science. 5th ed. New York, NY: McGraw Hill; 2013. [Google Scholar]
- 44.Schulz DJ, Baines RA, Hempel CM, Li L, Liss B, Misonou H. Cellular excitability and the regulation of functional neuronal identity: from gene expression to neuromodulation. J Neurosci. 2006;26:10362–10367. doi: 10.1523/JNEUROSCI.3194-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark BC, Mahato NK, Nakazawa M, Law TD, Thomas JS. The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J Neurophysiol. 2014;112:3219–3226. doi: 10.1152/jn.00386.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nardelli P, Vincent JA, Powers R, Cope TC, Rich MM. Reduced motor neuron excitability is an important contributor to weakness in a rat model of sepsis. Exp Neurol. 2016;282:1–8. doi: 10.1016/j.expneurol.2016.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefanelli L, Lockyer EJ, Collins BW, et al. Delayed-onset muscle soreness and topical analgesic alter Corticospinal excitability of the biceps brachii. Med Sci Sports Exerc. 2019;51:2344–2356. doi: 10.1249/MSS.0000000000002055 [DOI] [PubMed] [Google Scholar]
- 48.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol (1985). 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908 [DOI] [PubMed] [Google Scholar]
- 49.Caruana EJ, Roman M, Hernández-Sánchez J, Solli P. Longitudinal studies. J Thorac Dis. 2015;7:E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGinley M, Hoffman RL, Russ DW, Thomas JS, Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45:671–678. doi: 10.1016/j.exger.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark BC, Taylor JL, Hong SL, Law TD, Russ DW. Weaker seniors exhibit motor cortex hypoexcitability and impairments in voluntary activation. J Gerontol A Biol Sci Med Sci. 2015;70:1112–1119. doi: 10.1093/gerona/glv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalmar JM, Button DC, Gardiner K, Cahill F, Gardiner PF. Caloric restriction does not offset age-associated changes in the biophysical properties of motoneurons. J Neurophysiol. 2009;101:548–557. doi: 10.1152/jn.90617.2008 [DOI] [PubMed] [Google Scholar]
- 53.Christie A, Kamen G. Short-term training adaptations in maximal motor unit firing rates and afterhyperpolarization duration. Muscle Nerve. 2010;41:651–660. doi: 10.1002/mus.21539 [DOI] [PubMed] [Google Scholar]
- 54.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795. doi: 10.1152/jn.00685.2005 [DOI] [PubMed] [Google Scholar]
- 55.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(Pt 11):2131–2146. doi: 10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- 56.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764 [DOI] [PubMed] [Google Scholar]
- 57.Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamo T, Asahi R, Azami M, et al. Rate of torque development and the risk of falls among community dwelling older adults in Japan. Gait Posture. 2019;72:28–33. doi: 10.1016/j.gaitpost.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 59.Moskowitz S, Russ DW, Clark LA, et al. Is impaired dopaminergic function associated with mobility capacity in older adults? GeroScience. In press. doi: 10.1007/s11357-020-00303-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark DJ, Pojednic RM, Reid KF, et al. Longitudinal decline of neuromuscular activation and power in healthy older adults. J Gerontol A Biol Sci Med Sci. 2013;68:1419–1425. doi: 10.1093/gerona/glt036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Irving EL, Steinbach MJ, Lillakas L, Babu RJ, Hutchings N. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006;47:2478–2484. doi: 10.1167/iovs.05-1311 [DOI] [PubMed] [Google Scholar]
- 62.Rutherford BR, Choi J, Slifstein M, et al. Neuroanatomical predictors of L-DOPA response in older adults with psychomotor slowing and depression: A pilot study. J Affect Disord. 2020;265:439–444. doi: 10.1016/j.jad.2020.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Kaasinen V, Rinne JO. Functional imaging studies of dopamine system and cognition in normal aging and Parkinson’s disease. Neurosci Biobehav Rev. 2002;26:785–793. doi: 10.1016/s0149-7634(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 64.Carlsson A, Winblad B. Influence of age and time interval between death and autopsy on dopamine and 3-methoxytyramine levels in human basal ganglia. J Neural Transm. 1976;38:271–276. doi: 10.1007/BF01249444 [DOI] [PubMed] [Google Scholar]
- 65.Suhara T, Fukuda H, Inoue O, et al. Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology (Berl). 1991;103:41–45. doi: 10.1007/BF02244071 [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Chan GL, Holden JE, et al. Age-dependent decline of dopamine D1 receptors in human brain: a PET study. Synapse. 1998;30:56–61. doi: [DOI] [PubMed] [Google Scholar]
- 67.Wong DF, Young D, Wilson PD, Meltzer CC, Gjedde A. Quantification of neuroreceptors in the living human brain: III. D2-like dopamine receptors: theory, validation, and changes during normal aging. J Cereb Blood Flow Metab. 1997;17:316–330. doi: 10.1097/00004647-199703000-00009 [DOI] [PubMed] [Google Scholar]
- 68.Antonini A, Leenders KL. Dopamine D2 receptors in normal human brain: effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Ann N Y Acad Sci. 1993;695:81–85. doi: 10.1111/j.1749-6632.1993.tb23033.x [DOI] [PubMed] [Google Scholar]
- 69.Volkow ND, Wang GJ, Fowler JS, et al. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0 [DOI] [PubMed] [Google Scholar]
- 70.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344 [DOI] [PubMed] [Google Scholar]
- 71.Cham R, Studenski SA, Perera S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res. 2008;185:391–398. doi: 10.1007/s00221-007-1161-3 [DOI] [PubMed] [Google Scholar]
- 72.Metti AL, Rosano C, Boudreau R, et al. Catechol-O-methyltransferase genotype and gait speed changes over 10 years in older adults. J Am Geriatr Soc. 2017;65:2016–2022. doi: 10.1111/jgs.14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31:523–531. doi: 10.1016/j.neurobiolaging.2008.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542 [DOI] [PubMed] [Google Scholar]
- 75.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u [DOI] [PubMed] [Google Scholar]
- 76.Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J. 2016;16:430–438. doi: 10.1038/tpj.2016.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Damoiseaux JS. Effects of aging on functional and structural brain connectivity. Neuroimage. 2017;160:32–40. doi: 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- 78.Kilgour AH, Todd OM, Starr JM. A systematic review of the evidence that brain structure is related to muscle structure and their relationship to brain and muscle function in humans over the lifecourse. BMC Geriatr. 2014;14:85. doi: 10.1186/1471-2318-14-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bogen B, Moe-Nilssen R, Aaslund MK, Ranhoff AH. Muscle strength as a predictor of gait variability after two years in community-living older adults. J Frailty Aging. 2020;9:23–29. doi: 10.14283/jfa.2019.24 [DOI] [PubMed] [Google Scholar]
- 81.Pinter D, Ritchie SJ, Gattringer T, et al. Predictors of gait speed and its change over three years in community-dwelling older people. Aging (Albany NY). 2018;10:144–153. doi: 10.18632/aging.101365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carson RG. Inter-hemispheric inhibition sculpts the output of neural circuits by co-opting the two cerebral hemispheres. J Physiol. 2020;598:4781–4802. doi: 10.1113/JP279793 [DOI] [PubMed] [Google Scholar]
- 83.Di Paola M, Caltagirone C, Spalletta G. What does the corpus callosum tell us about brain changes in the elderly? Expert Rev Neurother. 2011;11:1557–1560. doi: 10.1586/ern.11.130 [DOI] [PubMed] [Google Scholar]
- 84.Alexander DC, Barker GJ, Arridge SR. Detection and modeling of non-Gaussian apparent diffusion coefficient profiles in human brain data. Magn Reson Med. 2002;48:331–340. doi: 10.1002/mrm.10209 [DOI] [PubMed] [Google Scholar]
- 85.Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34:2747–2766. doi: 10.1002/hbm.22099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577–582. doi: 10.1002/mrm.10268 [DOI] [PubMed] [Google Scholar]
- 87.Ruddy KL, Leemans A, Carson RG. Transcallosal connectivity of the human cortical motor network. Brain Struct Funct. 2017;222:1243–1252. doi: 10.1007/s00429-016-1274-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985;44:578–591. doi: 10.1097/00005072-198511000-00004 [DOI] [PubMed] [Google Scholar]
- 89.Wang P, Meng C, Yuan R, et al. The organization of the human corpus callosum estimated by intrinsic functional connectivity with white-matter functional networks. Cereb Cortex. 2020;30:3313–3324. doi: 10.1093/cercor/bhz311 [DOI] [PubMed] [Google Scholar]
- 90.Liu X, Gao X, Zhang L, et al. Age-related changes in fiber tracts in healthy adult brains: a generalized q-sampling and connectometry study. J Magn Reson Imaging. 2018;48:369–381. doi: 10.1002/jmri.25949 [DOI] [PubMed] [Google Scholar]
- 91.Vien C, Boré A, Lungu O, et al. Age-related white-matter correlates of motor sequence learning and consolidation. Neurobiol Aging. 2016;48:13–22. doi: 10.1016/j.neurobiolaging.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 92.Wang D, Chen YJ, Li YH. Application of super-resolution track-density technique: earlier detection of aging-related subtle alterations than morphological changes in corpus callosum from normal population? J Magn Reson Imaging. 2019;49:164–175. doi: 10.1002/jmri.26051 [DOI] [PubMed] [Google Scholar]
- 93.Kuhn T, Jin Y, Huang C, et al. The joint effect of aging and HIV infection on microstructure of white matter bundles. Hum Brain Mapp. 2019;40:4370–4380. doi: 10.1002/hbm.24708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sexton CE, Walhovd KB, Storsve AB, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34:15425–15436. doi: 10.1523/JNEUROSCI.0203-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bender AR, Völkle MC, Raz N. Differential aging of cerebral white matter in middle-aged and older adults: a seven-year follow-up. Neuroimage. 2016;125:74–83. doi: 10.1016/j.neuroimage.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Annweiler C, Montero-Odasso M. Vascular burden as a substrate for higher-level gait disorders in older adults. A review of brain mapping literature. Panminerva Med. 2012;54:189–204. [PubMed] [Google Scholar]
- 97.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meyns P, Van Gestel L, Leunissen I, et al. Macrostructural and microstructural brain lesions relate to gait pathology in children with cerebral palsy. Neurorehabil Neural Repair. 2016;30:817–833. doi: 10.1177/1545968315624782 [DOI] [PubMed] [Google Scholar]
- 99.Tian Q, Chastan N, Bair WN, Resnick SM, Ferrucci L, Studenski SA. The brain map of gait variability in aging, cognitive impairment and dementia-A systematic review. Neurosci Biobehav Rev. 2017;74(Pt A):149–162. doi: 10.1016/j.neubiorev.2017.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou J, Poole V, Wooten T, et al. Multiscale dynamics of spontaneous brain activity is associated with walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2020;75:1566–1571. doi: 10.1093/gerona/glz231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian Q, Resnick SM, Davatzikos C, et al. A prospective study of focal brain atrophy, mobility and fitness. J Intern Med. 2019;286:88–100. doi: 10.1111/joim.12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryberg C, Rostrup E, Paulson OB, et al. Corpus callosum atrophy as a predictor of age-related cognitive and motor impairment: a 3-year follow-up of the LADIS study cohort. J Neurol Sci. 2011;307:100–105. doi: 10.1016/j.jns.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 103.Venkatraman VK, Steward CE, Cox KL, et al. Baseline white matter is associated with physical fitness change in preclinical Alzheimer’s disease. Front Aging Neurosci. 2020;12:115. doi: 10.3389/fnagi.2020.00115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van der Holst HM, Tuladhar AM, Zerbi V, et al. White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 2018;17:731–738. doi: 10.1016/j.nicl.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tian Q, Ferrucci L, Resnick SM, et al. The effect of age and microstructural white matter integrity on lap time variation and fast-paced walking speed. Brain Imaging Behav. 2016;10:697–706. doi: 10.1007/s11682-015-9449-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosario BL, Rosso AL, Aizenstein HJ, et al. ; Health ABC Study . Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J Gerontol A Biol Sci Med Sci. 2016;71:968–973. doi: 10.1093/gerona/glv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anstey KJ, Mack HA, Christensen H, et al. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 108.Cunningham EE, Noble JW, Krassioukov A, Boyd LA, Eng JJ. Decreased white matter fractional anisotropy is associated with poorer functional motor skills following spinal cord injury: a pilot study. Spinal Cord. 2019;57:206–213. doi: 10.1038/s41393-018-0191-y [DOI] [PubMed] [Google Scholar]
- 109.Wang LX, Guo L, Guo F, et al. Brain white matter fiber tracts involved in post-transjugular intrahepatic portosystemic shunt hepatic myelopathy. Neuroreport. 2017;28:1164–1169. doi: 10.1097/WNR.0000000000000898 [DOI] [PubMed] [Google Scholar]
- 110.Moscufo N, Wolfson L, Meier D, et al. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr). 2012;34:405–414. doi: 10.1007/s11357-011-9242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]