Abstract
Autophagy, a process catabolizing intracellular components to maintain energy homeostasis, impacts aging and metabolism. Spermidine, a natural polyamine and autophagy activator, extends life span across a variety of species, including mice. In addition to protecting cardiac and liver tissue, spermidine also affects adipose tissue through unexplored mechanisms. Here, we examined spermidine in the links between autophagy and systemic metabolism. Consistently, daily injection of spermidine delivered even at late life is sufficient to cause a trend in life-span extension in wild-type mice. We further found that spermidine has minimal metabolic effects in young and old mice under normal nutrition. However, spermidine counteracts high-fat diet (HFD)-induced obesity by increasing lipolysis in visceral fat. Mechanistically, spermidine increases the hepatokine fibroblast growth factor 21 (FGF21) expression in liver without reducing food intake. Spermidine also modulates FGF21 in adipose tissues, elevating FGF21 expression in subcutaneous fat, but reducing it in visceral fat. Despite this, FGF21 is not required for spermidine action, since Fgf21−/− mice were still protected from HFD. Furthermore, the enhanced lipolysis by spermidine was also independent of autophagy in adipose tissue, given that adipose-specific autophagy-deficient (Beclin-1flox/+Fabp4-cre) mice remained spermidine-responsive under HFD. Our results suggest that the metabolic effects of spermidine occur through systemic changes in metabolism, involving multiple mechanistic pathways.
Keywords: Aging, FGF21, High-fat diet, Metabolism, Mice
Aging is a complex process involving multiple organs and regulatory pathways. It is deemed as the driving force for numerous age-dependent diseases (1). As aging progresses, physiological functions decline, with decreased or dysregulated autophagy activity as one driving mechanisms in aging (2). Autophagy is a critical regulator of cellular homeostasis that promotes degradation of damaged proteins and intracellular organelles, recycling macromolecules to replenish energy reserves (3). This catabolic function allows cells to survive nutrient-limited conditions and is a central mechanism by which damaged components are removed. However, autophagic activity wanes with age and contributes to the accumulation of damaged macromolecules and organelles in late life (4).
Prolongevity interventions enhancing autophagy activity, either genetically or pharmaceutically, extend life span across a variety of taxa (3,5). Nutritional interventions, such as calorie restriction (CR) and rapamycin, extend healthy life span through the molecular mechanisms converging on suppression of the mechanistic target of rapamycin complex 1 (mTORC1) (5,6), which antagonizes autophagy induction (7). Directly targeting autophagy, through ubiquitous overexpression of Atg5 (autophagy-related 5, a key component of autophagy machinery), delays aging phenotypes, maintaining leanness, increasing insulin sensitivity, and, most importantly, extending life span (8). In contrast, cardiac-specific deletion of Atg5 leads to an age-dependent cardiomyopathy (9). In another example, a gain-of-function mutation in the Beclin-1 gene (Beclin1F121A/F121A), a critical gene in the initiating the formation of autophagosomes, results in decreased interaction between BECLIN-1 and its negative regulator BCL2, in turn promoting autophagy and extending mouse life span (10).
As the primordial system for energy production during nutrient deficiency at the cellular level, autophagic activity intimately connects this cellular metabolism to overall systemic metabolism (11) and longevity (12). Spermidine, an endogenous biological polyamine (ie, natural part of our body), exhibits broad longevity-extending activities, mainly via the induction of autophagy in multiple tissues (13), and in species including yeast, flies, worms (14), and mice (15,16). Spermidine improves cardiovascular and liver function during aging (15,16), but whether spermidine mediates age- and diet-related metabolic disorders, or affects adipose tissue, remains unexplored. Moreover, it has not been determined whether other pathways mediated by spermidine, such as metabolism, are interrelated with autophagy (in an additive or synergistic way) (13). Thus, it will be important to define actionable molecular targets that explain the beneficial effects of spermidine in diverse pathophysiological settings (13). Given the role of autophagy in systemic metabolism, we envisioned a possible role for altered metabolism in life extension by spermidine.
Method
Mouse Husbandry
Young and old mice (C57BL/6J genetic background) were obtained from Charles River Laboratory. Fgf21+/− mice (C57BL/6 genetic background; Lilly Research Laboratories) were crossed to generate Fgf21 knockout mice (Fgf21−/− mice) as described previously (17). Homozygous floxed Beclin-1 (Beclin-1f/ox/f/ox, The Jackson Laboratory, stock number 028794) mice were bred with the Fabp4(aP2)-Cre transgenic mice (The Jackson Laboratory, stock number 005069) to generate adipose-specific autophagy (Beclin-1) deficient mice (Beclin-1flox/+Fabp4-Cre mice, C57BL/6J genetic background). Fabp4-Cre transgenic mice express Cre recombinase protein in brown and white adipose tissues (WATs) under the control of adipose-specific Fabp4 promoter (18). The offspring were genotyped and screened for the recombinant allele using conventional polymerase chain reaction analysis of tail genomic DNA. All mice were housed under temperature- and light-controlled conditions (72 °F, 12-hour light/12-hour dark cycle) with ad libitum access to water and food (Harlan Teklad diet number TD.2918 with 6.2% fat, 44.2% carbohydrate, and 18.6% protein). For the longevity study, mice were allowed to die naturally and no mice were used for any other biochemical or metabolic tests. In the high-fat diet (HFD)-induced obesity study, mice at 2 months of age were randomly placed on either a standard laboratory rodent chow (Harlan Teklad diet number TD.2918) or a HFD (Research Diets number D12492 with 34.9% fat, 26.3% carbohydrate, and 26.2 % protein), and monitored for 6 months. All mice were maintained in a barrier animal facility and all procedures were approved by the Institutional Animal Care and Use Committee at the Buck Institute for Research on Aging.
Injection of Spermidine
Spermidine (S0266-5G, Sigma-Aldrich, St. Louis, MO) was delivered as previously reported (19). In brief, spermidine was dissolved in ddH2O to 1 M aqueous solution. This was titrated to pH 7.2–7.4, sterile filtered, aliquoted into 1 mL portions, and stored at −80 °C for long-term storage. Daily injections of 50 mg/kg spermidine were performed intraperitoneally (i.p.) using 29-gauge, 0.3 cc insulin syringes for a period of 6 months. Control mice were injected with phosphate-buffered saline (PBS).
Body Composition
Whole-body composition (fat mass, lean mass, and free water) analysis was conducted using quantitative nuclear magnetic resonance machine (EchoMRI-2018; Echo Medical Systems, Houston, TX) without anesthesia.
Glucose Tolerance Test and Insulin Tolerance Test
Glucose tolerance test (GTT) was performed on unanesthetized animals. Mice were fasted with ad libitum access to water for 16 hours (overnight) before being given a single injection i.p. with 20% glucose at a dose of 2 g/kg of body weight (BW). The tail prick was used for blood glucose measurement at time points 0, 30, 60, 90, 120, and 180 minutes with an ACCU-CHEK Aviva glucometer (Roche Diagnostics, Dallas, TX) and test strips. For the insulin tolerance test (ITT), the same procedures were made except receiving an i.p. injection of 0.75 U/kg insulin and blood glucose concentration were measured at time points 0, 15, 30, 60, 90, and 120 minutes. Insulin was Humulin R (U-100) from Lilly.
Indirect Calorimetry
Metabolism was measured in a Promethion metabolic cage system (Sable Systems International) equipped with GA-3 small mammal gas analyzers. Metabolic cages were used while the mice were awake to simultaneously measure BW, oxygen consumption (VO2), carbon dioxide output (VCO2), spontaneous activity, and food intake with ad libitum access to food and water. Mice were housed individually in metabolic cages and acclimatized for 24 hours before the beginning of the recording and the analysis. Data were subsequently analyzed using Sable System ExpeDATA software (v1.4.9).
Tissue Harvesting and Immunoblotting
Liver and 2 WATs (subcutaneous fat and visceral fat) were harvested from mice after 6 months of treatment and immediately frozen in liquid nitrogen for Western blotting analysis. Tissue sections were homogenized on ice using the Omni TH homogenizer (Omni International, Kennesaw, GA) in radioimmunoprecipitation assay buffer (300 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris [pH 8.0], protease inhibitor cocktail [Roche], phosphatase inhibitor 2, 3 [Sigma]) and then centrifuged at 13,200 rpm for 10 minutes at 4 °C. The supernatants were collected and protein concentrations were determined using the DC protein assay (Bio-Rad). Equal amounts of protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (4%–12% Bis-Tris gradient gel, Invitrogen), transferred to membranes, and analyzed by western blotting with protein-specific antibodies. The antibodies against the phosphorylated rsS6S240/244 (5364), S6 (2217), adipose triglyceride lipase (ATGL; 2439), the phosphorylated HSLS563 (4139), hormone-sensitive lipase (HSL; 4107), Beclin-1 (3495), p62 (5114), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 2118) were purchased from Cell Signaling Technology (Danvers, MA). Fibroblast growth factor 21 (FGF21; ab171941) and monoacylglycerol lipase (MGL; ab24701) were purchased from Abcam (Boston, MA). Protein bands were revealed using the Amersham ECL detection system (GE Healthcare) and quantified by densitometry using ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical Analysis
All statistical analyses were conducted using GraphPad Prism 8 (GraphPad). The survival curves were completed using a Kaplan–Meier curve. We used a log-rank (Mantel–Cox) test to perform the statistical analyses of the survival curves. All the other data are shown as mean ± SEM. Where measurements were taken longitudinally, such as BW and adiposity, a mixed-effects model (restricted maximum likelihood) or 2-way repeated measures analysis of variance was used. The statistical significance of differences between 2 groups (or the indicated groups) was determined using unpaired, 2-tailed Student’s t test.
Results
A prior study showed that spermidine improves survival of mice, whether initiated at young or old age, and provides cardioprotection (15). Spermidine has been touted as a mTOR-independent inducer of autophagy, which also plays a major role in life-span regulation and systemic metabolism. Given this, it is intriguing to note that spermidine extends life span in mice with minimal effects on the metabolism (15). Here, we revisited spermidine’s effects on metabolism in mice of 2 different ages (young and old), including a study of spermidine’s effects on young mice treated with a HFD (60% of calories from fat). Specifically, we examined the effects of spermidine on mice at young (started at 2 months), old (started at 19–21 months), and young mice under HFD. All groups included both male and female mice and were treated and monitored for up to 6 months.
Survival of Aged Mice Treated With Daily Injection of Spermidine
While the purpose of our study was not to study the effects of spermidine on life span, we did maintain a small cohort, with inception of treatment from 19 to 21 months, until death to assess whether our study would generate consistent results with previous reports. Instead of supplying spermidine in the drinking water, we delivered the spermidine by daily i.p. injection of 50 mg/kg (19). We found the trend toward life extension by i.p. injection of spermidine with 6% and 13% increase of mean life span in females and males (848.4 vs 898.8 days and 934.6 vs 1059.3 days), respectively, although the data did not reach the statistical significance, likely due to the small sample size (Supplementary Figure S1A and B). There was also a trend that did not reach significance when genders were combined (8.8% increase; 891.8 vs 970.1 days; Supplementary Figure S1C). Our results with a limited number of old mice are consistent with the life-span extension observed when spermidine was added to the drinking water (15,16), supporting the conclusion that late-life treatment with spermidine extends murine life span (15).
Spermidine on Metabolism in Mice
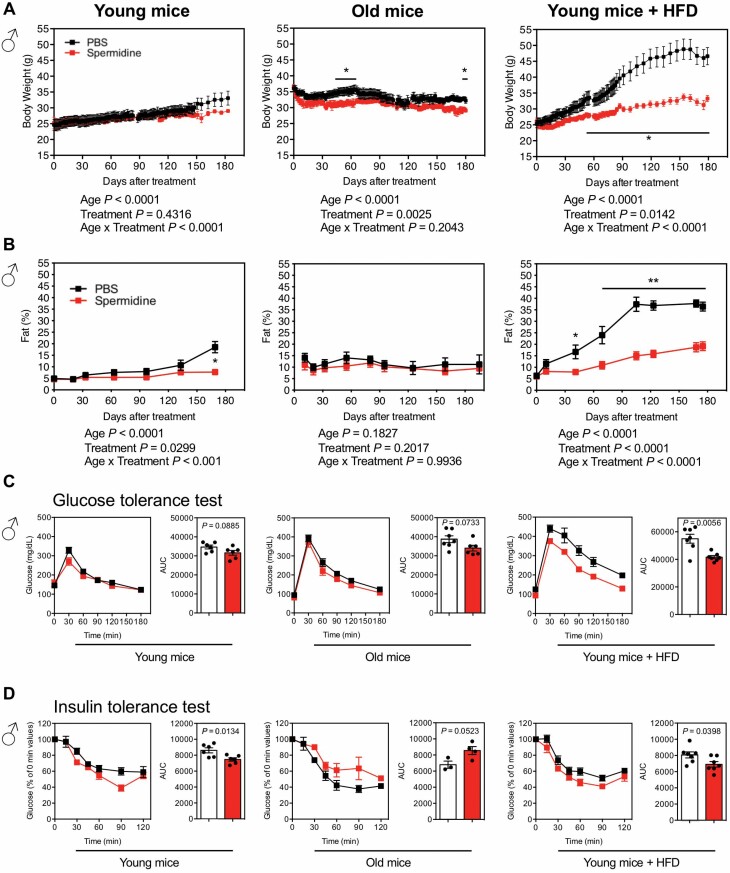
BW gradually increases as young male mice age, and this is not altered by spermidine (Figure 1A); BW almost completely overlapped between PBS- and spermidine-treated young mice, both males (Figure 1A) and females (Supplementary Figure S2A). This effect was not observed in old mice, where there is a peak weight gain with a drop off toward the end of life. Interestingly, we noticed a slightly reduction of BW by spermidine in both males (Figure 1A) and females (Supplementary Figure S2A). To determine whether weight gain corresponded to adiposity, mice were subjected to magnetic resonance imaging (MRI) analysis. In line with BW data, adiposity was similar in mice treated with spermidine, except after long-term spermidine treatment in young males (Figure 1B) and early stage of treatment in old females (Supplementary Figure S2B).
Figure 1.
Effects of spermidine on metabolism in male mice. (A) Serial BW of young mice (started at 2-month old; PBS, n = 6; spermidine, n = 6), old mice (started at 21-month old; PBS, started with n = 9; spermidine, started with n = 11), and young mice under HFD (started at 2-month old; PBS, n = 7; spermidine, n = 7). (B) Adiposity [(percent body fat = (fat mass/BW) × 100)] was measured in young mice (PBS, n = 6; spermidine, n = 6), old mice (PBS, started with n = 7; spermidine, started with n = 7), and young mice under HFD (PBS, n = 7; spermidine, n = 7). (A, B) Statistics for the overall effects of age, treatment, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood) or 2-way analysis of variance. (C) GTT in young mice (PBS, n = 6; spermidine, n = 6), old mice (PBS, n = 7; spermidine, n = 6), and young mice under HFD (PBS, n = 7; spermidine, n = 7). The AUC for GTT is shown on right panel. (D) ITT in young mice (PBS, n = 6; spermidine, n = 6), old mice (PBS, n = 3; spermidine, n = 4), and young mice under HFD (PBS, n = 7; spermidine, n = 7). The AUC for ITT is shown on right panel. Each symbol in the AUC panels represents a single mouse. PBS, black; spermidine, red. All bars represent mean ± SEM. Significant differences were taken when p <.05. *p < .05; **p < .01. AUC = area under the curve; BW = body weight; GTT = glucose tolerance test; HFD = high-fat diet; ITT = insulin tolerance test; PBS = phosphate-buffered saline.
Remarkably, a HFD exacerbated the difference of body composition between control and spermidine treatment at early stages in both males (Figure 1A and B) and females (Supplementary Figure S2A and B). The HFD accentuated weight gain as well as adiposity, while spermidine dramatically offset these changes. Collectively, our data are consistent with a previous report (15), showing no major difference by spermidine in terms of metabolism in normal chow-fed mice, either young or old mice. Interestingly, however, spermidine dramatically attenuates HFD-induced obesity.
Accumulation of adiposity and BW is usually concomitant with glucose intolerance and/or insulin resistance. We set out to evaluate the role of spermidine in peripheral glucose metabolism by systemic GTT and ITT. In line with the effect of spermidine on BW and adiposity, we did not observe significant improvement of glucose tolerance by spermidine in young and old male mice, although we detected modest trend toward improved glucose tolerance (Figure 1C). Interestingly, spermidine significantly improved glucose tolerance in young mice under HFD (Figure 1C). We observed the same results for females—no significant differences unless mice were on HFD (Supplementary Figure S2C). For ITT, spermidine improved the insulin sensitivity in both young males (Figure 1D) and females on a normal diet (Supplementary Figure S2D). Interestingly, spermidine increased the insulin resistance in both old males (Figure 1D; although not significant) and females (Supplementary Figure S2D). Under HFD, spermidine only improved insulin sensitivity in male mice (Figure 1D), but not female mice (Supplementary Figure S2D).
The reduced BW and adiposity by spermidine, especially in mice under HFD (Figure 1), led us to speculate that this potentially negative energy balance was due to reduced food intake. We then employed indirect calorimetry to characterize the metabolic profiles of mice treated with spermidine at different ages to decipher the cause(s) of the lean phenotype. Interestingly, no statistically significant reduction of food intake was observed by spermidine treatment across these 3 groups; young, old, and young mice under HFD (Supplementary Figure S3A), although old mice of both sexes had less food intake in general (Supplementary Figures S3A and 4A). These results suggest that spermidine did not directly affect the amount of food intake, seemingly eliminating appetite suppression as a mechanism underlying differential weight gain on a HFD.
In the same setting, we also employed indirect calorimetry to characterize the metabolic profiles of the young, old, and young mice under HFD treated with spermidine to decipher the cause(s) of the lean phenotype. Specifically, we measured energy expenditure (EE) and spontaneous activity. While spermidine did not affect EE in young mice, increased expenditure was detected both in old female mice and in mice of both sexes on a HFD (Supplementary Figures S3B and 4B). Spermidine also increased spontaneous activity (Supplementary Figure S3C) and running distance (Supplementary Figure S3D), as assessed by wheel meter running in young male mice under HFD (Supplementary Figure S3C). Furthermore, the running distance is correlated with wheel speed in old females (Supplementary Figure S4C and D). In summary, the leanness induced by spermidine, especially in HFD-treated mice, maybe due to the increased spontaneous activity-associated EE.
Spermidine on mTORC1 Signaling and Autophagy Activity
Suppressing mTORC1 signaling, either by nutritional or genetic manipulation, is a conserved mechanism of life-span extension of mice (5). We thus set out to determine whether spermidine mediates mTORC1 signaling, focusing on rpS6 phosphorylation and autophagic activity. Unexpectedly, spermidine significantly increases mTORC1 activity, as indicated by phosphorylation of rpS6 (p-S6 S240/244), in subcutaneous fat (WAT) of young mice (Supplementary Figure S5A), while mTORC1 is inhibited in old mice (Supplementary Figure S5B) and especially in mice under HFD (Supplementary Figure S5C). Intriguingly, similar patterns were observed with Beclin-1 protein expression (Supplementary Figure S5), a key protein in the regulation of the initiation of autophagosome formation. On the other hand, the reduction of p62/SQSTM1 (sequestosome 1; hereafter referred to as p62) did not reach the statistical significance unless mice were on a HFD (Supplementary Figure S5). The significant reduction of p62 protein levels likely indicates an increase in autophagic flux because p62, a protein that binds ubiquitinated proteins targeted for autophagic degradation, is itself degraded during the autophagic process (20).
In the liver, phosphorylation of rpS6 was indistinguishable between PBS- and spermidine-treated mice, across young, old, and young mice under HFD (Supplementary Figure S5D–F). Interestingly, spermidine increased autophagic Beclin-1 in both young and old mice, but not young mice under HFD (Supplementary Figure S5D–F). However, spermidine only reduced p62 protein in young mice under HFD (Supplementary Figure S5F). Collectively, these results suggest spermidine regulates mTORC1 and autophagy activity in tissue-, age-, and diet-dependent manner.
Spermidine Increases Lipolysis in WATs, Especially in Visceral Fat of HFD-Treated Mice
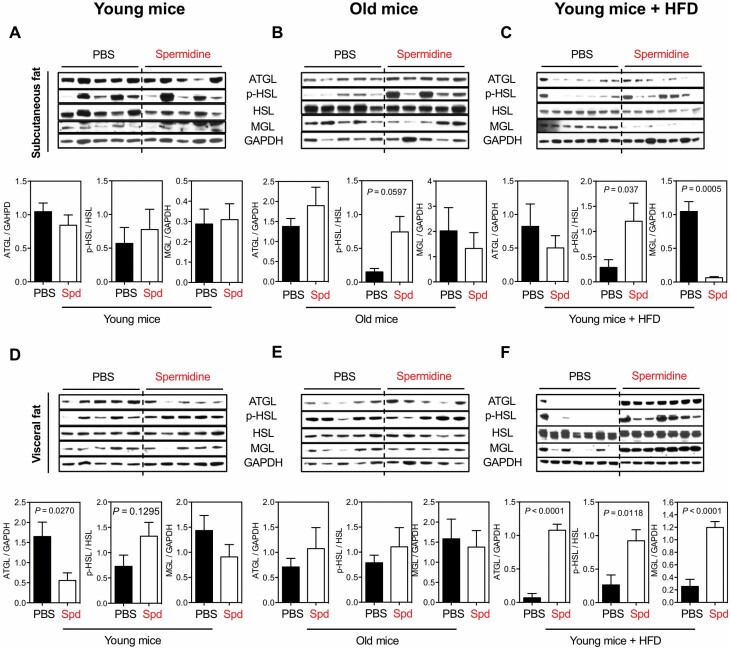
To follow the change in BW and adiposity by spermidine, especially in mice under HFD, lipolysis was analyzed in 2 main metabolic tissues: liver and adipose tissue (WAT). Three key proteins were assessed that act in sequence in lipolysis: ATGL, HSL, and MGL. In subcutaneous fat, spermidine did not mediate lipolysis, evaluated by ATGL, phosphorylation of HSL S563 (p-HSL), and MGL protein expressions, in young mice (Figure 2A), but there was a trend toward increased ATGL and p-HSL in old mice (Figure 2B). However, the lipolysis activity is mixed in young mice under HFD, given that p-HSL is significantly increased by spermidine but MGL is dramatically suppressed (Figure 2C). Thus, in subcutaneous fat, spermidine has no consistent effect on expression of enzymes involved in lipolysis.
Figure 2.
Spd on lipolysis of white adipose tissues. Western blots for the activity of lipolysis, indicated by ATGL, phosphorylation of HSL S563 (p-HSL), and MGL, in subcutaneous fat of (A) young mice, (B) old mice, and (C) young mice under HFD after 6 mo of Spd treatment. Relative ATGL levels (normalized to GAPDH), p-HSL levels (normalized to HSL), and MGL levels (normalized to GAPDH) were quantified underneath. Activity of lipolysis in the other white adipose tissue, visceral fat, of (D) young mice, (E) old mice, and (F) young mice under HFD. All bars represent mean ± SEM. Significant differences were taken when p <.05. ATGL = adipose triglyceride lipase; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HFD = high-fat diet; HSL = hormone-sensitive lipase; MGL = monoacylglycerol lipase; PBS = phosphate-buffered saline; Spd = spermidine.
In visceral fat, spermidine did not alter level of lipolysis enzymes in young and old mice (Figure 2D and E, respectively), except in young mice where ATGL expression was reduced (Figure 2D). Unlike in subcutaneous fat, spermidine drastically and significantly increased ATGL, p-HSL, and MGL expressions in visceral fat of HFD-fed mice (Figure 2F). Taken together, these data suggest that while spermidine minimally affects lipolysis under normal nutrition, it is dramatically increased under HFD, especially in visceral fat. This increased lipolysis in visceral fat by spermidine may contribute to the reduced BW and adiposity in HFD-fed mice (Figure 1A and B).
Spermidine Manifests Tissue-Specific Expression of FGF21
Given that the starvation hormone FGF21 regulates glucose and insulin sensitivity and lipid metabolism, we set out to examine the FGF21 protein expression by Western blot in major tissues where it is known to be secreted, namely liver and WAT. In liver, FGF21 protein expression was significantly elevated by spermidine in young mice (Figure 3A) and young mice under HFD (Figure 3C), and a similar (although not significant) trend was observed in old mice (Figure 3B). We also observed a similar pattern of FGF21 protein expression in subcutaneous fat across these 3 groups of mice (Figure 3). Intriguingly, this expression pattern was opposite in visceral fat, where FGF21 protein expression was significantly reduced in young mice and young mice under HFD (Figure 3). Given that we did not observe any perceivable reduction of food intake by spermidine treatment (Supplementary Figures S3A and S4A), these results suggest FGF21 modulation by spermidine is not due to reduced food intake, but rather a more direct metabolic effect of spermidine.
Figure 3.
Spd modulates FGF21 protein expression in liver and white adipose tissues. FGF21 protein levels were determined by Western blots from liver, sub. fat, and vis. fat of (A) young mice, (B) old mice, and (C) young mice under HFD after 6 mo of Spd treatment. Relative FGF21 levels (normalized to GAPDH) were quantified underneath. All bars represent mean ± SEM. Significant differences were taken when p <.05. FGF21 = fibroblast growth factor 21; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; HFD = high-fat diet; PBS = phosphate-buffered saline; Spd = spermidine; sub. fat = subcutaneous fat; vis. fat = visceral fat.
Genetic Fgf21 Knockdown Did Not Ablate the Metabolic Improvements by Spermidine
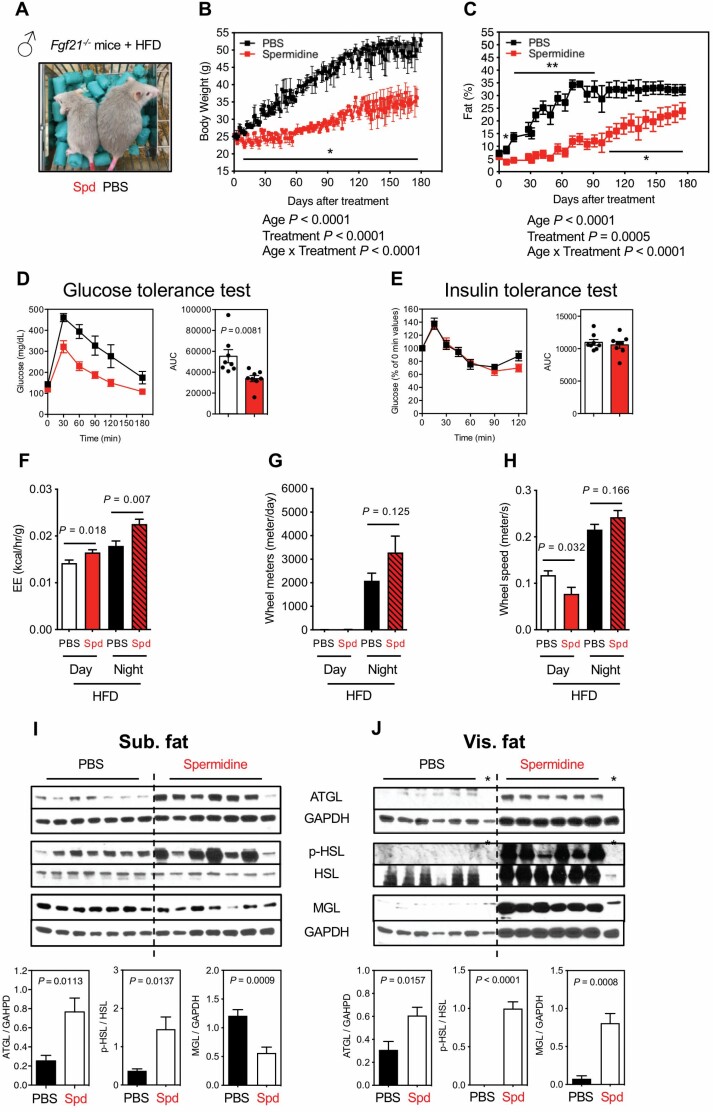
Since FGF21 protein expression is enhanced, especially in HFD mice (Figure 3), we determined whether it was required for the protective effects of spermidine. Two-month-old Fgf21−/− mice (kindly supplied by Lilly) were fed a HFD and treated with spermidine by i.p. injection for 6 months (Figure 4A) with BW and body composition monitored (Figure 4B and C). Contrary to our hypothesis, we observed reduced BW and adiposity by spermidine in Fgf21−/− mice under HFD (Figure 4B and C). Moreover, spermidine still improved glucose sensitivity, while insulin sensitivity was unaltered in Fgf21−/− mice (Figure 4D and E). Consistently, spermidine enhanced the EE (Figure 4F) and led to a trend toward increased wheel meter distance and speed at night, although the data in this case did not reach significance (Figure 4G and H). We generally observed the same results from female Fgf21−/− mice (Supplementary Figure S6A, B, and D), although spermidine’s effect on glucose sensitivity (Supplementary Figure S6C) and EE (Supplementary Figure S6E) did not reach significance, while behavioral activity (wheel meter distance at night and wheel speed at day) was significantly increased (Supplementary Figure S6F and G). At the molecular level, lipolysis enzymes showed variable changes in subcutaneous fat of Fgf21−/− mice, with a significant elevation by spermidine both in ATGL and p-HSL but a suppression in MGL protein (Figure 4I). In visceral fat (Figure 4J), the pattern of lipolysis mediated by spermidine in Fgf21−/− mice under a HFD mirrors that of wild-type mice (Figure 2F), showing that reducing BW by spermidine is likely linked to enhanced lipolysis in visceral fat and does not require FGF21. In summary, ablation of FGF21 does not abolish spermidine-mediated protection in the context of overnutrition, suggesting that other mechanisms underlie the phenotype, or at least that there are other redundant pathways that are also regulated.
Figure 4.
Effects of Spd on Fgf21-deficient male mice under HFD. (A) Image of Fgf21-deficient male mice (Fgf21−/−) under HFD after 2.5 mo of Spd or PBS treatment (started at 2-month old). (B) Body weight (PBS, black, n = 8; Spd, red, n = 8). (C) Adiposity (PBS, n = 6; Spd, n = 7). (B, C) Statistics for the overall effects of age, treatment, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood) or 2-way analysis of variance. (D) GTT (PBS, n = 8; Spd, n = 8). (E) ITT (PBS, n = 8; Spd, n = 8). The AUC for GTT or ITT is shown on right panel and each symbol represents a single mouse. (F) EE (kcal/h/g). (G) Wheel meters (m/d). (H) Wheel speed (m/s). n = 4 for PBS and Spd groups from indirect calorimetry (F, G, H). Western blots for the activity of lipolysis, indicated by ATGL, p-HSL, and MGL, from (I) sub. fat and (J) vis. fat of Fgf21−/− mice under HFD after 6 mo of Spd treatment. Relative ATGL levels (normalized to GAPDH), p-HSL levels (normalized to HSL), and MGL levels (normalized to GAPDH) were quantified underneath. *Samples were censored for quantifications in ATGL and p-HSL levels (J). All bars represent mean ± SEM. Significant differences were taken when p <.05. *p < .05; **p < .01. ATGL = adipose triglyceride lipase; AUC = area under the curve; EE = energy expenditure; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; GTT = glucose tolerance test; HFD = high-fat diet; HSL = hormone-sensitive lipase; ITT = insulin tolerance test; MGL = monoacylglycerol lipase; PBS = phosphate-buffered saline; Spd = spermidine; sub. fat = subcutaneous fat; vis. fat = visceral fat.
Genetic Ablation of Autophagy Specifically in Adipose Tissue Does Not Abrogate the Beneficial Effects of Spermidine on Metabolism
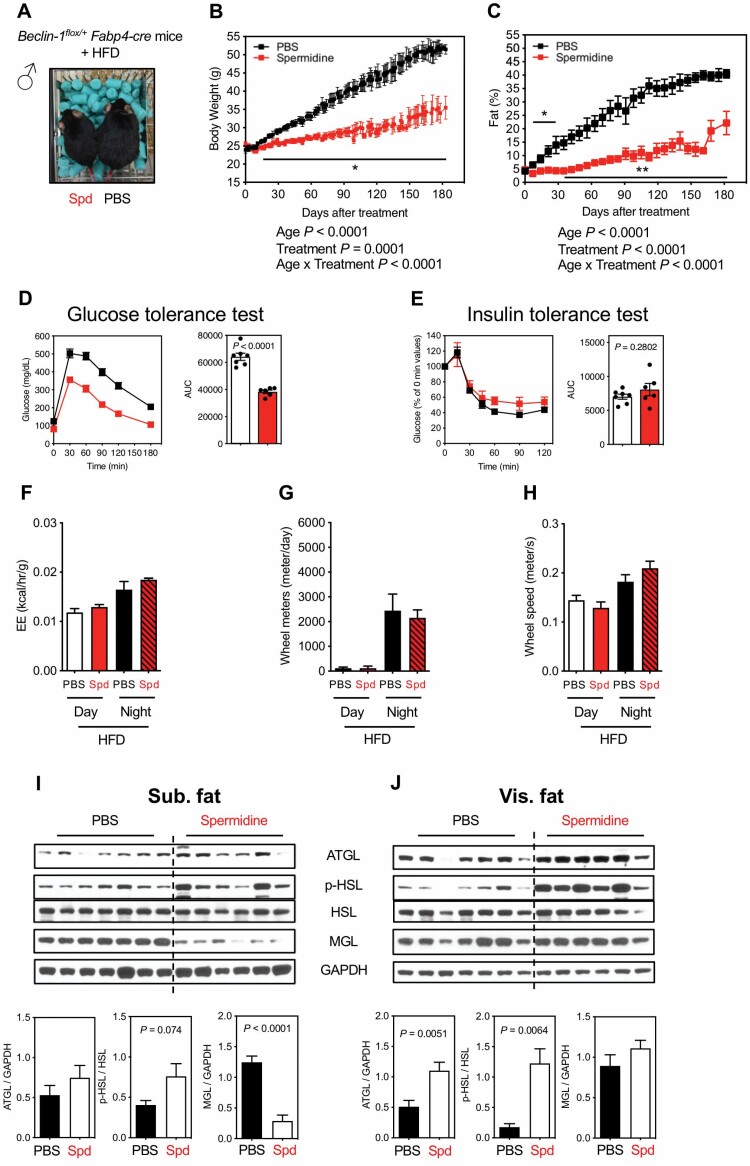
Given that autophagy is modulated by spermidine (Supplementary Figure S5) and that adipose is one of the main target tissues of spermidine (Figure 2), we further tested the hypothesis that autophagy in adipose tissue is required for spermidine’s action in mice under HFD. To test this, we employed an adipose-specific Beclin-1-deficient mouse model by crossing Beclin-1flox/flox mice with the Fabp4-cre mice, in which CRE (causes recombination) expression is under the control of an adipose tissue-specific aP2 (fatty acid-binding protein 4 [FABP4]) promoter. Two-month-old adipose-specific autophagy-deficient mice (therein referred to Beclin-1flox/+Fabp4-cre) were subjected to HFD and treated with either PBS or spermidine by i.p. injection for 6 months (Figure 5A). Again and contrary to our hypothesis, Beclin-1flox/+Fabp4-cre mice under a HFD remain sensitive to spermidine treatment, with reduced BW and adiposity (Figure 5B and C) and improved glucose sensitivity (Figure 5D), but not insulin sensitivity (Figure 5E), while the control PBS-treated Beclin-1flox/+Fabp4-cre mice recapitulated many of the characteristics of HFD-treated WT mice (Figure 1). However, spermidine did not affect the EE, wheel meters, and wheel speed in Beclin-1flox/+Fabp4-cre mice under HFD (Figure 5F–H). We observed the same results from female Beclin-1flox/+Fabp4-cre mice (Supplementary Figure S7A–E), although spermidine did enhance EE and behavioral activity (Supplementary Figure S7F–H).
Figure 5.
Effects of Spd on adipose-specific autophagy deficiency male mice under HFD. (A) Image of adipose-specific autophagy-deficient male mice (Beclin-1flox/+Fabp4-cre) under HFD after 3 mo of Spd or PBS treatment (started at 2-month old). (B) Body weight (PBS, black, n = 7; Spd, red, n = 6). (C) Adiposity (PBS, n = 7; Spd, n = 7). (B, C) Statistics for the overall effects of age, treatment, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood) or 2-way analysis of variance. (D) GTT (PBS, n = 7; Spd, n = 6). (E) ITT (PBS, n = 7; Spd, n = 6). The AUC for GTT or ITT is shown on right panel and each symbol represents a single mouse. (F) EE (kcal/h/g). (G) Wheel meters (m/d). (H) Wheel speed (m/s). n = 4 for PBS and Spd groups from indirect calorimetry (F, G, H). Western blots for the activity of lipolysis, indicated by ATGL, p-HSL, and MGL, from (I) sub. fat and (J) vis. fat of Beclin-1flox/+Fabp4-cre mice under HFD after 6 mo of Spd treatment. Relative ATGL levels (normalized to GAPDH), p-HSL levels (normalized to HSL), and MGL levels (normalized to GAPDH) were quantified underneath. All bars represent mean ± SEM. Significant differences were taken when p < .05. *p <.05; **p <.01. ATGL = adipose triglyceride lipase; AUC = area under the curve; EE = energy expenditure; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; GTT = glucose tolerance test; HFD = high-fat diet; HSL = hormone-sensitive lipase; ITT = insulin tolerance test; MGL = monoacylglycerol lipase; PBS = phosphate-buffered saline; Spd = spermidine; sub. fat = subcutaneous fat; vis. fat = visceral fat.
At the molecular level, lipolysis activity is mixed in subcutaneous fat, with a trend toward increased p-HSL, but a significant suppression in MGL (Figure 5I). While in visceral fat, ATGL and p-HSL, but not MGL, were statistically elevated by spermidine (Figure 5J). These patterns of lipolysis in WAT mediated by spermidine in Beclin-1flox/+Fabp4-cre mice under HFD mirror that from wild-type mice (Figure 2C and F). Furthermore, FGF21 protein expression patterns in Beclin-1flox/+Fabp4-cre mice recapitulated the same patterns in wild-type mice (Figure 3C), with higher FGF21 protein expression in liver and subcutaneous fat, but lower levels in visceral fat (Supplementary Figure S7I). Taken together, results derived from Beclin-1flox/+Fabp4-cre mice indicate that spermidine also mediates general metabolism in a manner that does not depend on autophagic activity in fat.
Discussion
The objective of this study was to explore the potential role of spermidine in systemic metabolism during aging and in an elevated nutritional environment. We hypothesized that spermidine extends life span in mice by rescuing age-related metabolic dysregulation. Our foremost results showing spermidine protects against diet-induced obesity echoed that of a previous study showing spermine, another polyamine synthesized from spermidine by spermine synthase, reduces BW and improves glucose challenge in HFD-induced obese mice (21). Consistently, Fernandez et al. reported that autophagy induction by spermidine protects from the HFD-induced metabolic deficit in wild-type (C57BL/6J) mice (22). Our results further demonstrated that spermidine prevents this early-onset HFD-induced obesity and modulates autophagy, FGF21 protein expression, and lipolysis in a tissue-specific manner. Interestingly, spermidine continued to protect against a HFD and stimulated lipolysis in FGF21- and adipose-specific autophagy-deficient mice. Therefore, we can conclude that while enhanced FGF21 and adipose-specific autophagy may contribute to the beneficial effects of spermidine in this context, that neither alone are required.
In our experimental setting, spermidine has minimal metabolic effects on young mice (and to some extent on old mice), with no changes in BW, glucose sensitivity, EE, and behavioral activity, despite the induction of autophagy in multiple tissues, as indicated by Beclin-1 protein expression. These metabolic responses to spermidine treatment (administered 50 mg/kg spermidine daily injection) are consistent with the original life-span study showing spermidine (given orally in drinking water, 0.3 and 3 mM) extends life span in mice with no major effect on the metabolism (15). Nonetheless, spermidine administrated late in life still extends life span (late-life treatment) (15) (and a similar trend in this study), and produces a marginal reduction in BW in old mice in this study. Spermidine also ameliorates the HFD-induced obesity and glucose intolerance, presumably by enhancing EE and behavioral activity. These results suggest that spermidine mediates age- and diet-dependent metabolism. It will be of interest to determine whether spermidine can more robustly extend the life span of genetic- or diet-induced obese mice.
Spermidine bears similarity to CR and rapamycin, which extend life span, delay cardiovascular aging, and regulate autophagy (15,23). Life extension by CR and rapamycin in mice may also be due to the activation of autophagy in the other metabolic tissues, such as liver (6). Unlike CR or rapamycin, however, spermidine has been shown to induce autophagy by means of a mTORC1-independent pathway, as spermidine did not alter the phosphorylation status of mTOR or its downstream substrate, ribosomal protein S6 kinase (19). Interestingly, our results showed spermidine modulates mTORC1 activity, a conserved regulator of autophagy, and autophagy activity in age-, diet-, and tissue-dependent fashion. For instance, spermidine increased mTORC1 activity, indicated by phosphorylation of rpS6 (p-S6 S240/244), as well as Beclin-1 protein levels in subcutaneous fat of young mice, whereas these effects were reversed in mice fed a HFD. Since a HFD is reported to induce adipose-specific autophagy, it is possible that spermidine directly counteracts this response (24). In liver, HFD suppresses autophagy (24), which was not reversed by spermidine. This age- and tissue-dependent autophagic response was also observed (which was not limited to spermidine) in cardiac tissue where CR suppressed autophagy, indicated by Beclin-1 protein, in young mice, while it accelerated autophagy in middle-aged and old mice (25). As a catabolic process, it is worth noting that autophagy levels have to be tightly regulated and dynamic in response to aging and energetic demands (26). Furthermore, although mTORC1 activity is generally associated with antagonizing the induction of autophagy, it is interesting to note that induction of autophagy can occur through both mTORC1-dependent and -independent pathways (27).
We noticed an age-dependent response in insulin intolerance, where spermidine increases systemic insulin sensitivity in young mice while decreases it in old mice (both males and females) (Figure 1 and Supplementary Figure S2). This is intriguing given that insulin resistance is generally associated with obesity-related diseases and shorter life span in organisms (28). The reason why spermidine promotes insulin resistance in old mice is as of yet unclear. However, it has been suggested that insulin resistance can be beneficial in late age by protecting the animals or particular organ systems from the detrimental actions of hyperinsulinemia (28), or eliciting a cellular antioxidant defense mechanisms (29). Indeed, spermidine elicits antioxidant defense mechanisms in some lower organisms (13), and this should be further evaluated in mammals. Along with some rodent models that show a lack of association between insulin resistance and longevity (30), spermidine-induced insulin resistance in old mice further fuels the debate regarding the role of insulin resistance in life span. Furthermore, sex may also complicate the interpretation of the role of spermidine in response to insulin challenge, since spermidine increases systemic insulin sensitivity in male mice but not female mice in the context of a HFD (Figure 1 and Supplementary Figure S2). The intrinsic differences between males and females, including endocrine systems and deposition of adipose tissue (more discussion below) (31), should be further evaluated, given that spermidine extends mouse life span in both sexes (15,16).
A puzzling result from our study is that spermidine divergently affects lipolytic protein expression in 2 types of WAT: subcutaneous and visceral fat. This differential expression in 2 kinds of fat depots likely speaks to the fundamental differences between subcutaneous and visceral fat in metabolism (32). For instance, an age-related accumulation of visceral fat, such as epididymal fat, is accompanied by a decrease in insulin sensitivity (33), while surgical removal of visceral fat in rats improved insulin sensitivity (34) and, more importantly, increased life span (35). On the other hand, simple transplantation of subcutaneous fat into the visceral cavity significantly improved glucose tolerance in mice (36). In humans, increased amount of subcutaneous fat is considered to be protective against the development of insulin resistance (37). Our results demonstrate that increased lipolysis, indicated by elevated ATGL and p-HSL, by spermidine is more robust in visceral than in subcutaneous fat of mice fed HFD. Given that the current literature suggests that in lean rodents, ATGL and HSL are the major lipases for triglycerides and diglyceride, respectively, and account for ~95% of lipase activity in murine WAT (38), we postulate that visceral fat contributes to the majority of lipolysis induced by spermidine in wild-type, Fgf21−/− and Beclin-1flox/+Fabp4-Cre mice fed HFD (Figures 2F, 4J, and 5J). Although our measurement technique (MRI) did not distinguish fat depots, the enhanced lipolysis in visceral fat is associated with improving glucose sensitivity in our setting. Therefore, our data suggest that visceral fat is the major target of spermidine-mediated lipolysis since visceral fat is more lipotoxic than subcutaneous fat.
Another notable finding is the divergent FGF21 response to spermidine in 2 kinds of WATs, with higher FGF21 protein levels in subcutaneous fat and reduced levels in visceral fat of young mice and HFD-fed mice. This expression pattern is also apparent in Beclin-1flox/+Fabp4-Cre mice suggesting autophagy activity is dispensable in spermidine-mediated FGF21 expression in adipose tissues. Although the reason why subcutaneous fat is more prone to express FGF21 upon spermidine than visceral fat is as of yet unclear, one possible explanation is the reduced βklotho levels in visceral fat of HFD-induced obese mice. Klotho is an auxiliary protein that interacts with FGFRs (FGF receptors) to confer the FGF21 binding and subsequent signaling activation (39).
That the starvation hormone FGF21 was induced by spermidine in liver may come as a surprise, since food intake was not different across age and diet. Initially identified as a hepatokine, endogenous FGF21 regulates lipid and glucose metabolism upon fasting, starvation, or overnutrition (such as HFD) (39). No difference in food intake in our setting not only suggests FGF21 secretion by spermidine is not due to starvation or a CR effect, but instead suggests that the favorable effects of spermidine are independent of calorie intake. Nonetheless, this increased FGF21 protein secreted from liver could result in the increase of lipolysis in WAT, given adipose tissue is one of the primary sites of FGF21 action (40), and may further play a pleiotropic role in systemic metabolism and behavior (41). Mouse genetic models indicate that FGF21 stimulates lipolysis in the WAT: the expression of Atgl and Hsl, the 2 predominant lipase genes of lipolysis in the WAT, was significantly increased in the WAT of Fgf21 transgenic mice (42), while the expression was decreased in the subcutaneous WAT of Fgf21−/− mice (43). Intriguingly, FGF21 protein is dispensable in the context of spermidine, since Fgf21−/− mice maintain spermidine responsiveness. Consistently, a study employing the same Fgf21−/− mice demonstrated endogenous FGF21 is not required for the in vivo efficacy of antiobesity actions of the thiazoladinediones (44). However, we cannot rule out the potential role of spermidine-induced FGF21 in (i) increasing insulin sensitivity by promoting the healthy expansion of adipose tissue, especially subcutaneous fat (45), and (ii) regulating systemic metabolism through adipose-independent mechanisms (46).
Prior to our findings, the prevailing concept for the prolongevity effects of spermidine was due to improved cardiovascular (15) and hepatic function (16) through activation of autophagy. Our study suggests that spermidine also mediates systemic metabolism for life-span extension possibly by circumventing the induction of autophagy to mediate FGF21 protein expression in metabolic tissues and enhance lipolysis in adiposity. Given that spermidine is a natural metabolite, it is of interest to know whether its levels are correlated with health status in humans. Indeed, the polyamines spermidine and spermine are enriched in whole blood of nonagenarians/centenarians (47). Furthermore, the nutritional diets rich in spermidine are also linked to improved cognition with age (48) and lower overall, cardiovascular and cancer-related mortality in humans (49). Whether endogenous or supplemented spermidine induces autophagy in humans remains unanswered. Interestingly, the serum levels of the autophagy biomarker Beclin-1 is increased in centenarians (50). Despite these promising results in humans, deciphering the molecular mechanism underlying spermidine’s actions still require further research. Our findings suggest that, in addition to normal aging, spermidine represents a potential treatment strategy to counteract obesity-associated disorders and may have clinical translatability in this context as a largely safe supplement.
Supplementary Material
Acknowledgments
We would like to thank the staff at the Buck Institute for Research on Aging for their expert monitoring of the mice. B.K.K. is an Ellison Medical Foundation Senior Scholar in Aging. We apologize to our colleagues whose contributions could not be cited due to space constraint.
Funding
This work was funded by the National Institute on Aging R01 AG050441. C.-Y.L. was supported by a Glenn/AFAR postdoctoral fellowship.
Conflict of Interest
None declared.
Author Contributions
C.-Y.L. and B.K.K. participated in the design of all the experiments. C.-Y.L., O.M.P.K., A.M.B., N.A., J.A., D.M.M., I.B., A.M.S., Y.-M.H., B.D.W. II, S.M.B.M., A.N.M., G.T., A.R.T., A.M.D., R.B.O., H.D.P., J.W., and J.K.S. performed the experiments. C.-Y.L. analyzed the data. C.-Y.L. and B.K.K. wrote the manuscript with input from the coauthors.
References
- 1.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–593. doi: 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030 [DOI] [PubMed] [Google Scholar]
- 5.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. doi: 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fok WC, Zhang Y, Salmon AB, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci. 2013;68(2):108–116. doi: 10.1093/gerona/gls127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. doi: 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pyo JO, Yoo SM, Ahn HH, et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taneike M, Yamaguchi O, Nakai A, et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6(5):600–606. doi: 10.4161/auto.6.5.11947 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez AF, Sebti S, Wei Y, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Pietrocola F, Levine B, Kroemer G. Metabolic control of autophagy. Cell. 2014;159(6):1263–1276. doi: 10.1016/j.cell.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López-Otín C, Galluzzi L, Freije JMP, Madeo F, Kroemer G. Metabolic control of longevity. Cell. 2016;166(4):802–821. doi: 10.1016/j.cell.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 13.Madeo F, Eisenberg T, Pietrocola F, Kroemer G. Spermidine in health and disease. Science. 2018;359(6374):eaan2788. doi: 10.1126/science.aan2788 [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi: 10.1038/ncb1975 [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–1438. doi: 10.1038/nm.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yue F, Li W, Zou J, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res. 2017;77(11):2938–2951. doi: 10.1158/0008-5472.CAN-16-3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology. 2009;150(11):4931–4940. doi: 10.1210/en.2009-0532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morselli E, Mariño G, Bennetzen MV, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192(4):615–629. doi: 10.1083/jcb.201008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myeku N, Figueiredo-Pereira ME. Dynamics of the degradation of ubiquitinated proteins by proteasomes and autophagy: association with sequestosome 1/p62. J Biol Chem. 2011;286(25):22426–22440. doi: 10.1074/jbc.M110.149252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadasivan SK, Vasamsetti B, Singh J, et al. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur J Pharmacol. 2014;729(8):94–99. doi: 10.1016/j.ejphar.2014.01.073 [DOI] [PubMed] [Google Scholar]
- 22.Fernandez AF, Bárcena C, Martínez-García GG, et al. Autophagy counteracts weight gain, lipotoxicity and pancreatic beta-cell death upon hypercaloric pro-diabetic regimens. Cell Death Dis. 2017;8(8):e2970. doi: 10.1038/cddis.2017.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082 [DOI] [PubMed] [Google Scholar]
- 24.Maixner N, Kovsan J, Harman-Boehm I, Blüher M, Bashan N, Rudich A. Autophagy in adipose tissue. Obes Facts. 2012;5(5):710–721. doi: 10.1159/000343983 [DOI] [PubMed] [Google Scholar]
- 25.Sheng Y, Lv S, Huang M, et al. Opposing effects on cardiac function by calorie restriction in different-aged mice. Aging Cell. 2017;16:1155–1167. doi: 10.1111/acel.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmeisser K, Parker JA. Pleiotropic effects of mTOR and autophagy during development and aging. Front Cell Dev Biol. 2019;7:192. doi: 10.3389/fcell.2019.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans. 2013;41(5):1103–1130. doi: 10.1042/BST20130134 [DOI] [PubMed] [Google Scholar]
- 28.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci USA. 2009;106(42):17787–17792. doi: 10.1073/pnas.0902380106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barzilai N, Ferrucci L. Insulin resistance and aging: a cause or a protective response? J Gerontol A Biol Sci Med Sci. 2012;67(12):1329–1331. doi: 10.1093/gerona/gls145 [DOI] [PubMed] [Google Scholar]
- 31.Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63(3):453–461. doi: 10.1007/s00125-019-05040-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415 [DOI] [PubMed] [Google Scholar]
- 33.Barzilai N, Banerjee S, Hawkins M, Chang CJ, Chen W, Rossetti L. The effect of age-dependent increase in fat mass on peripheral insulin action is saturable. J Gerontol A Biol Sci Med Sci. 1998;53(2):B141–B146. doi: 10.1093/gerona/53a.2.b141 [DOI] [PubMed] [Google Scholar]
- 34.Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51(10):2951–2958. doi: 10.2337/diabetes.51.10.2951 [DOI] [PubMed] [Google Scholar]
- 35.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7(3):438–440. doi: 10.1111/j.1474-9726.2008.00391.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–E1760. doi: 10.1210/jc.2011-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweiger M, Schreiber R, Haemmerle G, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281(52):40236–40241. doi: 10.1074/jbc.M608048200 [DOI] [PubMed] [Google Scholar]
- 39.Salminen A, Kaarniranta K, Kauppinen A. Regulation of longevity by FGF21: interaction between energy metabolism and stress responses. Ageing Res Rev. 2017;37:79–93. doi: 10.1016/j.arr.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 40.Whittle AJ. FGF21 conducts a metabolic orchestra and fat is a key player. Endocrinology. 2016;157(5):1722–1724. doi: 10.1210/en.2016-1193 [DOI] [PubMed] [Google Scholar]
- 41.Lewis JE, Ebling FJP, Samms RJ, Tsintzas K. Going back to the biology of FGF21: new insights. Trends Endocrinol Metab. 2019;30(8):491–504. doi: 10.1016/j.tem.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 42.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5(6):415–425. doi: 10.1016/j.cmet.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 43.Hotta Y, Nakamura H, Konishi M, et al. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150(10):4625–4633. doi: 10.1210/en.2009-0119 [DOI] [PubMed] [Google Scholar]
- 44.Adams AC, Coskun T, Cheng CC, O Farrell LS, Dubois SL, Kharitonenkov A. Fibroblast growth factor 21 is not required for the antidiabetic actions of the thiazoladinediones. Mol Metab. 2013;2(3):205–214. doi: 10.1016/j.molmet.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Wu G, Fang Q, et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat Commun. 2018;9(1):272. doi: 10.1038/s41467-017-02677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.BonDurant LD, Ameka M, Naber MC, et al. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017;25(4):935–944.e4. doi: 10.1016/j.cmet.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pucciarelli S, Moreschini B, Micozzi D, et al. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15(6):590–595. doi: 10.1089/rej.2012.1349 [DOI] [PubMed] [Google Scholar]
- 48.Wirth M, Benson G, Schwarz C, et al. The effect of spermidine on memory performance in older adults at risk for dementia: a randomized controlled trial. Cortex. 2018;109:181–188. doi: 10.1016/j.cortex.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 49.Kiechl S, Pechlaner R, Willeit P, et al. Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr. 2018;108(2):371–380. doi: 10.1093/ajcn/nqy102 [DOI] [PubMed] [Google Scholar]
- 50.Emanuele E, Minoretti P, Sanchis-Gomar F, et al. Can enhanced autophagy be associated with human longevity? Serum levels of the autophagy biomarker beclin-1 are increased in healthy centenarians. Rejuvenation Res. 2014;17(6):518–524. doi: 10.1089/rej.2014.1607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.