Abstract
The limited heritability of human life spans suggests an important role for gene–environment (G × E) interactions across the life span (T), from gametes to geronts. Multilevel G × E × T interactions of aging phenotypes are conceptualized in the Gero-Exposome as Exogenous and Endogenous domains. Stochastic variations in the Endogenous domain contribute to the diversity of aging phenotypes, shown for the diversity of inbred Caenorhabditis elegans life spans in the same culture environment, and for variegated gene expression of somatic cells in nematodes and mammals. These phenotypic complexities can be analyzed as 3-way interactions of gene, environment, and stochastic variations, the Tripartite Phenotype of Aging. Single-cell analyses provide tools to explore this broadening frontier of biogerontology.
Keywords: APOE, Exposome, Gerogens, Tripartite Phenotype
Background
The low heritability of life spans has long perplexed biogerontologists. For human twins and the inbred worm, the heritability of life spans is less than 35%, and may be below 10% (1–6). Despite their 1000-fold differences, the variance of human and Caenorhabditis elegans life spans is similar when scaled to life span as the coefficient of variation (COV) (3). We propose that the limited heritability of aging patterns and longevity in humans is an outcome of gene–environment (G × E) interactions for individual longevity haplotypes, together with stochastic (chance) somatic variations. For inbred nematodes in the same environment, the individual differences represent multilevel stochastic variations (2,7,8). For humans, the 20-year gap of longevity across socioeconomic status (9) involves modifiable environmental factors of diet, physical activity, and inhaled toxins from air pollution (AirPoll) and cigarette smoke (CigS). While many longevity genes are known, their G × E over time (T) has received less attention and the contribution of stochastic variations is undefined. Y chromosome genes also have undefined G × E × T interactions on longevity.
The Gero-Exposome
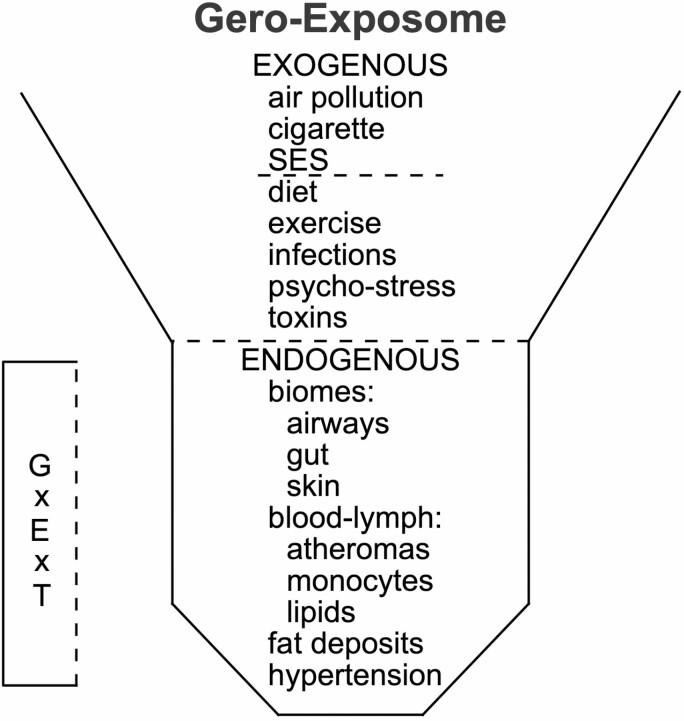
G × E × T is considered during the human life course in the framework of the Gero-Exposome (Figure 1). The exposome concept was developed by Wild in 2005 and 2012 for cancer epidemiology (10,11) and has been widely applied (12,13). The limitations of the “one-by-one” analysis of carcinogens demanded a more comprehensive analysis of environmental and lifestyle factors. Wild identified 3 domains in the exposome: the Exogenous Macrolevel Exposome (rural vs urban; social stratification; ambient toxins from CigS and AirPoll); the Exogenous Individual Exposome (diet, infections); and the Endogenous Exposome (biomes of gut and airways, fat depots, tissue injuries) (10,12). We applied Wild’s concept to Alzheimer’s disease (AD) in the “AD-Exposome” (12) to analyze multiple levels of pathogenesis for G × E × T (12), and in the Paleo-Exposome for G × E in the evolution of human longevity (13). We suggest explicit consideration of stochastic components that were often understood as implicit within E in the traditional binary formulation of G × E. The Endogenous Exposome should also include cell-to-cell variations of gene expression described as “variegated gene expression” (14). This innovative study identified human genes that controlled the variability of expression, measured as COV, for SIRT1, a longevity-related gene. Future studies of variegated gene expression should consider expression of APOE and its neighboring genes, whose haplotypes influence heart disease, obesity, hypertension, AD, and longevity (15). Age-related increase of somatic cell mutations and epigenomic modifications of DNA and histones will add noise to G × E × T.
Figure 1.
The Gero-Exposome with exogenous and endogenous components. G × E × T = interactions of gene by environment over age and time, from the prefertilization oocyte into later ages; SES = socioeconomic status. Finch and Kulminski (12) gives further details of the Exogenous and Endogenous Exposomes.
Fat depots are important to the Endogenous Gero-Exposome because of their contribution to systemic inflammation: IL-6, TNFα, and C-reactive protein are higher in venous blood from fat depots than in arterial blood (16–18). Inflammatory secretions emanate from the macrophages surrounding adipocytes that increase with obesity (19). Fat depots also secrete C-reactive protein (CRP), assessed in obese patients before and after bariatric surgery (20). The antibacterial CRP is also made by the liver during acute-phase inflammatory responses. APOE alleles influence obesity and neurodegeneration in mouse models, discussed below. Both exogenous and endogenous stressors can induce inflammatory damage with G × E interactions, as shown for the influence of APOE allele on vascular and neurodegenerative diseases (15,21). Obesity increases the risk of AD (22) and degeneration of brain myelin (white matter) (23), and is augmented by APOE4. Transgenic mice fed fat show G × E for the human APOE4 with accelerated brain amyloid deposits (24,25). The gut biome also varies by APOE allele (26).
Environmental toxicants that influence adult health were identified in the HELIX project (Human Early Life-Exposome). Childhood obesity was increased by maternal CigS (27), while children’s telomeres were shortened in white blood cells by both elevated urban AirPoll (28) and secondhand CigS (29). AirPoll and CigS promote obesity and elevated blood glucose and lipids (endogenous individual domain) that are risk factors for atherosclerosis, various cancers, hypertension, and AD and are preventable causes of premature aging (30).
Ambient Toxins of the Exogenous Macro Exposome: AirPoll and CigS
Inhaled toxins from AirPoll and CigS are associated with excess mortality and shortened longevity. In 2019, AirPoll and CigS were attributed to 16 million excess deaths worldwide, which is 2-fold more than the 8 million deaths attributed to, infections and road injuries, the next ranked causes of death (30). Mortality from AirPoll and CigS is likely to have increased in 2020 because coronavirus disease 2019 (COVID-19) mortality is increased by CigS (31) and elevated AirPoll (32).
For AD, CigS accounts for 11% U.S. cases, and 14% worldwide (population attributable risk) (33). Cognitively normal smokers older than 60 years incur accelerated brain aging from CigS, with greater accumulation of brain amyloid (34) and earlier atrophy of cerebral cortex (35). Secondhand smoke also increased risk of dementia and accelerated cognitive decline in large population studies from the United States (36) and China (37,38). AirPoll accelerates brain atrophy and cognitive decline (39,40), and increases the risk of AD (39,41,42).
AirPoll and CigS may be considered as gerogens as they promote pathogenesis of the major morbidities of aging: atherosclerosis, cancer, and dementia (43). For heart disease, lung cancer, and cognitive decline, AirPoll and CigS synergize with super-additivity (44–46). Women have greater neurological vulnerability to AirPoll by 3% higher risk than men of AD and Parkinson disease, shown for 63 million U.S. Medicare older people in the largest population-based study to date (42).
AirPoll Neurotoxicity of G × E in Mouse Models
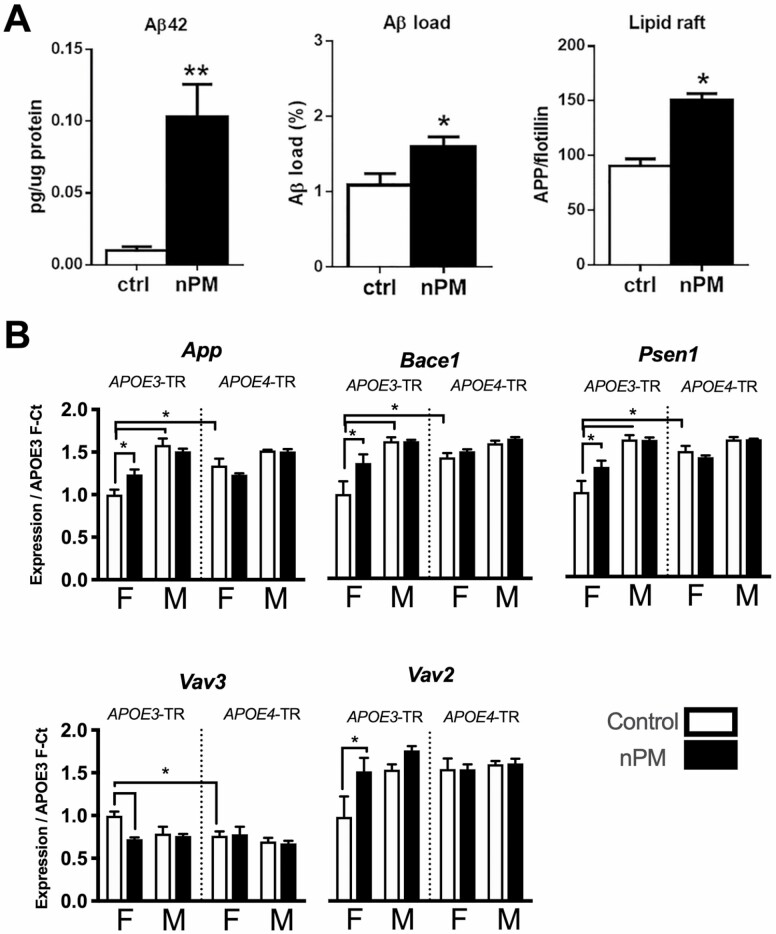
Several mouse models of AirPoll show G × E for APOE alleles and sex. For Rodent exposures to AirPoll, our lab has used a nanoscale subfraction of particular matter (nPM) collected from a Los Angeles freeway designated here as AirPoll-nPM; other labs use direct traffic exposure, or AirPoll components such as ozone (O3) or diesel exhaust particles (47). Mice transgenic for human familial AD-dominant mutations respond to chronic AirPoll-nPM with increased soluble Aβ42 peptide and increased plaque load (Figure 2A) (39,48). Subcellular mechanisms include increased pro-amyloidogenic processing of the amyloid precursor protein (APP) in lipid rafts (Figure 2B).
Figure 2.
AirPoll-nPM induces amyloidogenic responses in mouse cerebral cortex after exposure for 8–10 wk of high traffic levels. (A) Amyloidogenic responses of J20 male mice carrying APPswe (familial AD gene) showing increase of soluble Aβ42 and Aβ plaque load (39), and increased lipid raft levels of amyloid precursor protein (APP) (39). *p<.05, **p<0.01 (B) Amyloid metabolic gene response of APOE-TR mice carrying human APOE3 and APOE4 alleles by targeted replacement (no familial AD genes) (49). * p<.05 in ANOVA test after FDR multiple test correction. AirPoll = air pollution; nPM = nanoscale subfraction of particular matter.
Older women who carry APOE4 are at higher risk of AirPoll-associated dementia (39). We find corresponding sex differences for APOE alleles in mouse brain transcriptome responses to AirPoll-nPM (49). Only female APOE3-TR mice responded to AirPoll-nPM in genes for production of the amyloid Aβ peptide (APP, Bace11, Psen1) and for Aβ phagocytosis (Vav2, Vav3) (Figure 2B). This suggests that APOE3 carriers efficiently clear amyloid oligomers, which may counteract AirPoll, consistent with impaired Aβ clearance in APOE4 carriers. Male APOE-TR did not respond to AirPoll-nPM in amyloid metabolic gene pathways. The female excess risk for AD decline may be due to sex differences in brain genomic response to AirPoll, with 2-fold more gene responses and different pathways in young female mice than in male mice (49). Similarly, ozone exposure caused memory decline in young male APOE3-TR mice, but not in APOE4 mice (50). The higher baseline levels of enzymes for oxidative stress response in E4 males suggest a ceiling effect for their lack of response to ozone. These findings suggest epidemiological studies.
Two transcription factors, NRF2 and NFκB, showed the same pattern for APOE4-TR of baseline differences and lack of responsiveness to AirPoll. More than 80 genes downstream of NRF2 responded to AirPoll in mouse cerebral cortex. AirPoll-nPM induced nuclear translocation of NRF2 in liver and lung, as well as brain (51), suggesting bodywide systemic responses. While aging also increased NRF2 expression (51), middle-aged mice had minimal NRF2 response to AirPoll (52). This apparent ceiling in AirPoll response effect extends to neurite atrophy in hippocampal CA1 neurons (52,53). The selective neurotoxicity of CA1 neurites to AirPoll-nPM parallels the selective CA1 neurodegeneration during AD.
We anticipate complex interactions of sex and aging for APOE alleles and other AD risk genes with AirPoll that may differ by age. A causal chain of responses to AirPoll across the human life course may include the following: (i) high baseline oxidative stress in aging or APOE4 individuals; (ii) AirPoll-nPM excessive induction of NRF2 antioxidant responses that increase the nuclear NRF2 baseline; (iii) increase of NFκB baseline; and (iv) increasing neurological damage, with diminished response to exogenous and endogenous stressors. This pattern occurred in multiple organs of middle-aged mice chronically exposed to nPM (51). The developmental impact of AirPoll and CigS is discussed below.
Cigarette Smoke
G × E interactions with CigS neurotoxicity are shown in 2 studies. Cognitively normal APOE4 smoker aged 56–94 had greater brain amyloid and deficits in glucose utilization than E3 smokers; deficits in memory and auditory-verbal learning also differed by APOE (Alzheimer Disease Neuroimaging Initiative) (34). Middle-aged twins also showed G × E for CigS in cerebral cortex area (Veterans Twin Study of Aging) (54). Smoking history has been neglected by most genetic studies of dementia, and of other genes that influence longevity.
Rodents exposed to CigS confirm human findings. Mice transgenic for familial AD genes and exposed to chronic CigS accumulated 50% more brain amyloid and phosphorylated tau (55). Similarly, wild-type rats responded to CigS with increased AD biomarkers of sAPPa and phospho-tau (56). Reactive glia were increased in both models, as observed for AirPoll-nPM.
Sex is a neglected factor in human and rodent studies of CigS. In collaboration with demographer Eileen Crimmins, we showed that female heavy smokers had a higher risk of earlier age of death and stroke than males of the Health and Retirement Study (57). APOE alleles were not available.
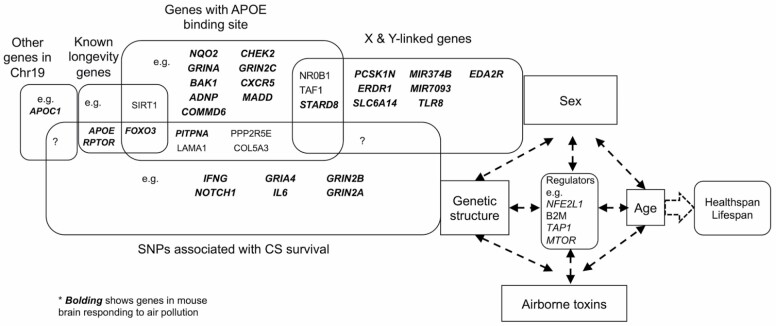
AirPoll and CigS independently shorten the life span with dose dependence and promote many of the same aging processes. We asked if these gerogens might share some of the same gene targets by comparing gene SNPs of long-lived smokers with genes activated in mouse brain responses to AirPoll. In 2016, Levine and Crimmins (58) identified 215 SNPs in long-lived smokers of the Health and Retirement Study, a U.S.-wide longitudinal study of health and aging. The groups compared were smokers who survived to age 80 versus smokers up to age 70, with Caucasian predominance. The long-lived smokers had mortality rates similar to same-age “never smokers.” We find considerable overlap of genes identified by SNPs in smoking-survivors with AirPoll-nPM-responding genes in mouse brain (Figure 3). Initial analysis showed that most (63%, 136/215) of the genes with SNPs in cigarette-survivor also respond to AirPoll-nPM in mouse (49). Functional analysis of genes associated with these 215 SNPs shows enrichment for shared pathways associated with immune response, oxidative stress, and development. SNPs are located near or within known human longevity genes (FOXO3, HLADRB1). Twenty shared genes have association with AD (eg, GAD2, GRIN2A, and GRIN2B). The 2016 gene database of the Health Retirement Survey did not include APOE alleles. Many other G × E interactions of airborne toxins with APOE alleles and sex may shape the healthspan and life span.
Figure 3.
Schema for interactions of genetic background, sex, airborne toxins, and age on individual health span and longevity. Bolding identifies genes that responded to AirPoll in the mouse brain (49,53,60) that are shared with older surviving smokers (58). AirPoll transcriptome- and CigS-associated SNPs show extensive overlap, suggesting shared mechanisms. AirPoll = air pollution; CigS = cigarette smoke.
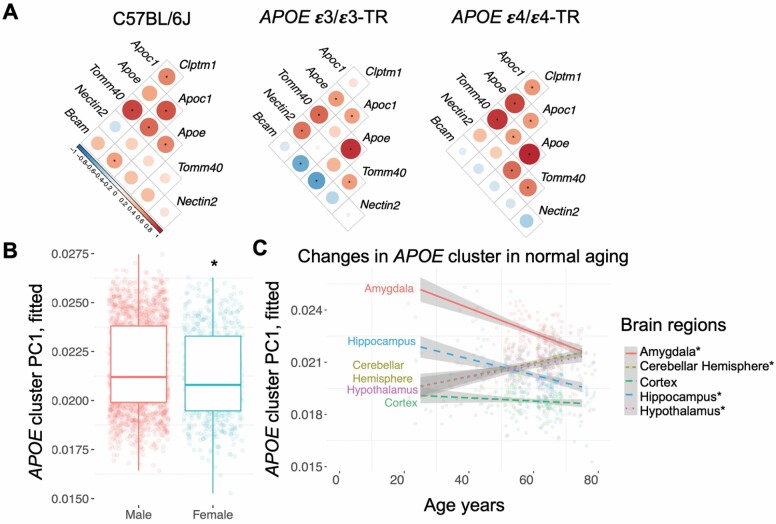
The APOE gene does not act alone as an AD risk factor on chromosome 19.3. Kulminski et al. has identified AD risk-haplotypes in more than 5 neighboring genes (59). The APOE gene clusters of mouse and human share many genes, but with inverted synteny (60). While its genetic variants have received the most attention, little is known of how the APOE cluster genes respond to environmental factors. Initial studies of mouse brain and archived data show that APOE cluster genes are highly responsive to components of AirPoll (60). Mouse cerebral cortex responded to AirPoll-nPM with increased Apoe mRNA levels together with various combinations of Tomm40, Apoc1, and other gene neighbors (Figure 4A). Human brain cDNA libraries also showed co-expression of APOE cluster genes, differing by sex (Figure 4B) and age with brain region specificity (Figure 4C). DNA methylation in the APOE cluster shows a complex epigenetic architecture that differs for AD and the cognitively normal participants (61).
Figure 4.
APOE gene cluster expression in mouse and human brain. (A) Heat maps showing transcriptional response of the mouse Apoe gene cluster to AirPoll-nPM in cerebral cortex, for C57BL/6J (B6) and transgenic for human APOE allele, APOE3-TR and APOE4-TR, both sexes. Principal component analysis of human APOE cluster expression for 5 brain regions (321 individuals, GTEx data) shows APOE PC1 differed by sex (B) and age (C) with brain region specificity. Figure adapted from Haghani et al. (60). AirPoll = air pollution; CigS = cigarette smoke.
The diverse APOE cluster responses to AirPoll components were explored with archived data from humans and rodents (60). Mouse lung responded differently from brain with other Apoe cluster subsets to coal tar and another AirPoll sample different from that used in our studies. Coal tar increased mRNAs of Apoc1, Apoe, and Nectin2, while AirPoll induced Apoc1, but not Tomm40. Antioxidant and inflammatory responses of other chromosomal genes include the expressions of Nqo1 and Il1b, which also responded to AirPoll-PM in Nrf2-regulated phase II gene expression in lung and brain (51). These findings give insights for the heterogeneity of AD risk from APOE4. Divergent patterns of co-expression of APOE cluster genes to the above AirPoll may arise from variations in local AirPoll chemistry for oxidative activity, despite the same density of AirPoll particles (62,63).
Developmental Impact of AirPoll and CigS
The Gero-Exposome (Figure 1) includes developmental exposures in the Exogenous domain. The analysis is necessarily multigenerational because human primary oocytes are fully formed before birth; thus, our prenatal oocyte was exposed to our grandmaternal environment (64). Multigenerational toxic influences are documented for CigS and lead (Pb) gestational exposure in rodent models (43,65). Robust effects in the first generation include elevated brain cytokines, neuronal deficits, and impaired glucose metabolism, together with increased depressive behaviors shown by us (66) and others (67,68). The male bias of many rodent gestational exposures parallels the male bias of autism, which is increased by early exposure to AirPoll (69). These sex differences anticipate other G × E × T interactions for the impact of childhood obesity on later-life cardiovascular and neurodegenerative diseases. APOE alleles have not been studied for the impact of CigS or AirPoll on pre- or postnatal development. Several examples illustrate the developmental harm of CigS and AirPoll. Fetal growth is consistently impaired by maternal smoking. Third-trimester exposure to maternal smoking shortened femur length and skull width (biparietal diameter; meta-analysis of 10 000 pregnancies in 16 studies) (70). In turn, maternal smoking is associated with higher adult body mass index (71), a risk factor in many age-related pathologies. There is a likely convergence of CigS and AirPoll in fetal growth retardation (72).
Epigenetic effects of maternal smoking include postnatal DNA methylation, robustly shown for GFI1, a transcriptional repressor which was hypomethylated at 8 CpG sites in adult children of maternal smokers (meta-analysis of 18 212 individuals from 17 populations) (73). Several hypomethylated GFI1-CpGs are associated with low birth weight, and adult adiposity and hypertension, which are AD risk factors. Whole-genome methylomes (bisulfite cleavage) show additional DNA methylation responses to maternal smoking in enhancers and other gene regulatory domains (74,75). Similarly, the gene promoter of SLC7A8, an amino acid transporter, had parallel changes of DNA-CpG sites in cord blood and adults of the Maternal and Child Health Study (75). Placental DNA methylation of CYP1A1 (detoxification gene) may also respond to maternal smoking (76,77).
The risk of childhood obesity is increased by maternal smoking. A meta-analysis of 236 687 children worldwide showed robust associations of maternal smoking during pregnancy with overweight (odds ratio [OR] 1.37) and obesity (OR 1.55) (78). Moreover, the combination of secondhand CigS and AirPoll promotes childhood obesity (44). In turn, childhood obesity increases risk for adult dyslipidemias that promote cardiovascular disease (79). As noted above, fat depots secrete inflammatory proteins, worsened by obesity. APOE alleles were not included in these studies.
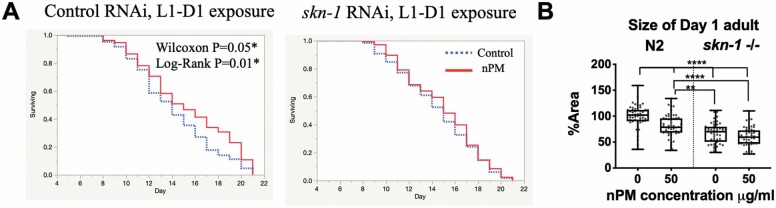
Resolving the complex G × E relationships of AirPoll would be facilitated with a high-throughput, short-duration screening model. We developed the nematode C elegans as a new model for developmental influences of AirPoll (80). Concentrations of AirPoll-nPM in culture media were identified that did not alter survival curves. Initial studies showed responses of C elegans to AirPoll-nPM in the culture media that corresponded to findings in rodents. For example, rapid responses to 1 hour of nPM exposure induced genes for oxidative stress responses (eg, gst-4), inflammation (eg, tol-1), and of the human AD pathway (eg, apl-1/APP homolog, and sel-12/PSEN1 homolog) (80). These rapid responses paralleled the rapid increase of Aβ42 in mouse brain from 3-hour exposure to nickel nanoparticles in FVBM mice (47,81). RNAi knockdown of the NRF2 homolog skn-1 eliminated the long-term developmental and life-span hormetic effects of early-life acute exposure to AirPoll-nPM (80). One-hour exposure of young adult worms to 50 µg/mL AirPoll-nPM in L1 and day 1 adulthood increased life span by 1.1 days, which was blocked by skn-1 RNAi (Figure 5A). Developmental exposure at L1 caused smaller-sized adults. Again, the developmental effect of nPM was blocked by skn-1 knockout (Figure 5B). The pleiotropic effect of SKN-1 activation by AirPoll-nPM is consistent with the metabolic reallocations of skn-1 gain-of-function mutants that increased longevity at the expense of decreased resistance to pathological bacteria (82). Because oxidative stress from paraquat decreased pathogen resistance, AirPoll may also impair immunity, as indicated by covariance of elevated AirPoll with risk of COVID-19 infections.
Figure 5.
Caenorhabditis elegans impact of SKN-1 perturbation on AirPoll-nPM exposure during development. (A) Survival curves showing increased longevity from nPM exposure (hormesis) and its abrogation by skn-1 knockdown. (B) Body size (area, arbitrary units) of adult day 1 wild-type (N2) and skn-1(zu135) mutant. Adapted from Haghani et al. (80). AirPoll = air pollution; CigS = cigarette smoke.
These findings merit extension to mouse models of Nrf2 manipulation by drugs and inducible gene knockdown to attenuate AirPoll toxicity. The hsp-16.2 and skg genes are interesting prospects for interactions with AirPoll, and for genes that alter the variability of stem cell number (83). The temperature dependence of life span in sgk-1(ok538) (84) would be interesting to study for interactions with AirPoll-nPM. Transgenerational effects of AirPoll may be anticipated from the growing list of environmental toxins and stressors with multigenerational effects that extend 3 or more generations, for example, for maternal exposure of mice to the flame retardant tetrabromobisphenol (85) or Pb (65,86). Mechanisms include altered methylation of DNA in mice (86) and of histones in C elegans (87). Other toxicants with experimentally reported transgenerational effects include vinclozolin (88) and other endocrine disruptors. Vinclozolin exposure can also cause “tertiary epimutations” by altering germline DNA methylation and somatic mutations that persist at least 3 generations (88). We anticipate other examples of epimutations from environment toxicants with transgenerational persistence and complex G × E interactions that will threaten future global health.
Stochastic Variation in the Total Phenotype
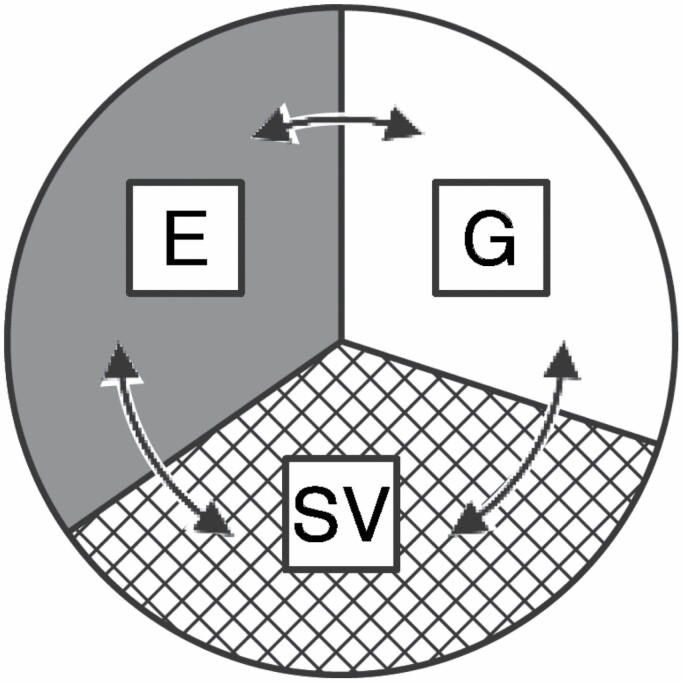
The unexplained heritability of life spans may also arise from interactions of environmental and stochastic factors with the genome, which we summarize as the Tripartite Phenotype of Aging (Figure 5). Bidirectional arrows indicate their interactions and plasticity of overlap, and extend earlier analyses (2,89,90).
Life span of C elegans varies as widely as humans when expressed as COV (3). Despite their isogenic status in the same culture dish, young worms swim at different rates, lay different numbers of eggs, and vary widely in their loss of these functions during aging. The loss of locomotion is attributed to sarcopenia from scattered myocyte cytopathology with loss of myofibrils, while neurons apparently remain intact (7). While these variations remain undefined for sarcopenia, stochastic variations of life spans are better understood for the heat shock gene promoter hsp-16.2 in benchmark studies by Tom Johnson, Alex Mendenhall, and colleagues (91). The life spans of individual worms scaled with hsp-16.2 gene expression over a 2-fold range. In contrast, 2 other stress inducible genes (myo-2 and mtl-2) lacked association of expression levels with longevity (92). Locomotor activity paralleled the longevity trends (93), confirming a prior study (7). Genetic manipulations that decreased insulin signaling also decreased life-span COV (94,95).
Subcellular analysis of stochasticity by Mendenhall’s group showed cell-to-cell differences in hsp-16.2 protein levels of intestine cells of young worms (8). The scale of differences in cell expression for this key chaperone greatly exceeded the intrinsic noise in gene expression, as determined by reporters for each gene. The “variegated gene expression” of mammalian genes in vitro described above was associated with SIRT1, a human longevity gene (14). There may be stochastic components in the sex-APOE differences of Sirt1 expression in brain, which was 50% lower in APOE3 females than for other sex-APOE genotypes (49). A physiology of stochasticity is suggested by the regulation of intestinal cell variations by neurosecretions from thermosensory neurons (94). Further variations may be found in neuronal contacts of C elegans. While worms are known for (almost) identical numbers of each cell type, nonetheless, adult individuals vary widely in subcellular location and type of motor neuron synapses, implying variations during development (96,97). Subcellular mechanisms of stochasticity include gene silencing through small RNAi that are transmitted at least 3 generations (98). Stochastic processes have been modeled for individual trajectories of aging (99) and G × E (100) that confirm the large scale of stochastic epigenetic variations during development with later consequences to adult health and aging (3). Lastly, we recall Gärtner’s pioneering studies from 3 decades ago, which compared of twins derived from separate ova of inbred mice with those from artificially cleaved single ova. More than 70% of postnatal growth variance preexisted in oocytes at or before fertilization (101).
Concluding Perspectives
The search for individual determinants of health and longevity can be expanded to include environmental factors across the life span, from gametes to geronts.
Environmental interactions with genes over the lifetime (G × E × T) should consider interactions of the Exogenous and Endogenous domains of the Gero-Exposome, for example, adiposity from gestational exposure to CigS and AirPoll.
The Tripartite Phenotype of Aging includes cell heterogeneity that arises from developmental variations and variegated gene expression. The next phase of Systems Gerontology could include stochastic features of aging organ and cell data for modeling.
The frontier of stochastics in biogerontology can be explored with single-cell transcriptomes and ChIPseq.
Acknowledgments
Excellent graphics were contributed by Troy Palmer (Figures 1 and 6). We thank Sean P. Curran (USC) for helpful comments.
Figure 6.
The Tripartite Phenotype: G, heritable genes with variable impacts from the environment (E), in both the Exogenous and Endogenous domains of the Gero-Exposome (Figure 1), and from stochastic variations (SVs) during individual development and stochastic anomalies resulting in postnatal molecular damage, both intracellular and extracellular.
Funding
Funding was provided by NIH grants to C.E.F. (R01-AG051521, P50-AG005142, and P01-AG055367) and training support was provided to A.H. (PI: Kelvin Davis, T32- AG052374).
Conflict of Interest
None declared.
References
- 1.Bazopoulou D, Knoefler D, Zheng Y, et al. . Developmental ROS individualizes organismal stress resistance and lifespan. Nature. 2019;576:301–305. doi: 10.1038/s41586-019-1814-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch CE, Kirkwood TB.. Chance, Development, and Aging. Oxford University Press; 2000. [Google Scholar]
- 3.Finch CE, Tanzi RE. Genetics of aging. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407 [DOI] [PubMed] [Google Scholar]
- 4.Govindaraju D, Atzmon G, Barzilai N. Genetics, lifestyle and longevity: lessons from centenarians. Appl Transl Genom. 2015;4:23–32. doi: 10.1016/j.atg.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruby JG, Wright KM, Rand KA, et al. . Estimates of the heritability of human longevity are substantially inflated due to assortative mating. Genetics. 2018;210:1109–1124. doi: 10.1534/genetics.118.301613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yashin AI, Arbeev KG, Wu D, et al. . Genetics of human longevity from incomplete data: new findings from the Long Life Family Study. J Gerontol A Biol Sci Med Sci. 2018;73:1472–1481. doi: 10.1093/gerona/gly057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. . Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135 [DOI] [PubMed] [Google Scholar]
- 8.Burnaevskiy N, Sands B, Yun S, et al. . Chaperone biomarkers of lifespan and penetrance track the dosages of many other proteins. Nat Commun. 2019;10:5725. doi: 10.1038/s41467-019-13664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crimmins EM, Kim JK, Seeman TE. Poverty and biological risk: the earlier “aging” of the poor. J Gerontol A Biol Sci Med Sci. 2009;64:286–292. doi: 10.1093/gerona/gln010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456 [DOI] [PubMed] [Google Scholar]
- 11.Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41:24–32. doi: 10.1093/ije/dyr236 [DOI] [PubMed] [Google Scholar]
- 12.Finch CE, Kulminski AM. The Alzheimer’s disease exposome. Alzheimers Dement. 2019;15:1123–1132. doi: 10.1016/j.jalz.2019.06.3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trumble BC, Finch CE. The exposome in human evolution: from dust to diesel. Q Rev Biol. 2019;94:333–394. doi: 10.1086/706768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Burnaevskiy N, Annis J, et al. . Cell-to-cell variation in gene expression for cultured human cells is controlled in trans by diverse genes: implications for the pathobiology of aging. J Gerontol A Biol Sci Med Sci. 2020;75:2295–2298. doi: 10.1093/gerona/glaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finch CE, Kulminski AM. The ApoE locus and COVID-19: are we going where we have been? J Gerontol A Biol Sci Med Sci. 2021;76:e1–e3. doi: 10.1093/gerona/glaa200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656 [DOI] [PubMed] [Google Scholar]
- 17.Madani R, Karastergiou K, Ogston NC, et al. . RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am J Physiol Endocrinol Metab. 2009;296:E1262–E1268. doi: 10.1152/ajpendo.90511.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips CL, Grayson BE. The immune remodel: weight loss-mediated inflammatory changes to obesity. Exp Biol Med (Maywood). 2020;245:109–121. doi: 10.1177/1535370219900185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larabee CM, Neely OC, Domingos AI. Obesity: a neuroimmunometabolic perspective. Nat Rev Endocrinol. 2020;16:30–43. doi: 10.1038/s41574-019-0283-6 [DOI] [PubMed] [Google Scholar]
- 20.Paepegaey AC, Genser L, Bouillot JL, Oppert JM, Clément K, Poitou C. High levels of CRP in morbid obesity: the central role of adipose tissue and lessons for clinical practice before and after bariatric surgery. Surg Obes Relat Dis. 2015;11:148–154. doi: 10.1016/j.soard.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 21.Yassine HN, Finch CE. APOE alleles and diet in brain aging and Alzheimer’s disease. Front Aging Neurosci. 2020;12:150. doi: 10.3389/fnagi.2020.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumsden AL, Mulugeta A, Zhou A, Hyppönen E. Apolipoprotein E (APOE) genotype-associated disease risks: a phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine. 2020;59:102954. doi: 10.1016/j.ebiom.2020.102954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mole JP, Fasano F, Evans J, et al. . Genetic risk of dementia modifies obesity effects on white matter myelin in cognitively healthy adults. Neurobiol Aging. 2020;94:298–310. doi: 10.1016/j.neurobiolaging.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 24.Jones NS, Watson KQ, Rebeck GW. Metabolic disturbances of a high-fat diet are dependent on APOE genotype and sex. eNeuro. 2019;6:ENEURO.0267-0219.2019. doi: 10.1523/ENEURO.0267-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen A, Pike CJ. APOE genotype affects metabolic and Alzheimer-related outcomes induced by Western diet in female EFAD mice. FASEB J. 2019;33:4054–4066. doi: 10.1096/fj.201801756R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran TTT, Corsini S, Kellingray L, et al. . APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J. 2019;33:8221–8231. doi: 10.1096/fj.201900071R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrijheid M, Fossati S, Maitre L, et al. . Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ Health Perspect. 2020;128:67009. doi: 10.1289/EHP5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clemente DBP, Vrijheid M, Martens DS, et al. . Prenatal and childhood traffic-related air pollution exposure and telomere length in European children: the HELIX project. Environ Health Perspect. 2019;127:87001. doi: 10.1289/EHP4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osorio-Yáñez C, Clemente DBP, Maitre L, et al. . Early life tobacco exposure and children’s telomere length: the HELIX project. Sci Total Environ. 2020;711:135028. doi: 10.1016/j.scitotenv.2019.135028 [DOI] [PubMed] [Google Scholar]
- 30.National Academies of Sciences E, Medicine. Using 21st Century Science to Improve Risk-Related Evaluations. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 31.Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J Med Virol. 2021;93:1045–1056. doi: 10.1002/jmv.26389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brandt EB, Beck AF, Mersha TB. Air pollution, racial disparities, and COVID-19 mortality. J Allergy Clin Immunol. 2020;146:61–63. doi: 10.1016/j.jaci.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durazzo TC, Mattsson N, Weiner MW; Alzheimer’s Disease Neuroimaging Initiative . Interaction of cigarette smoking history with APOE genotype and age on amyloid level, glucose metabolism, and neurocognition in cognitively normal elders. Nicotine Tob Res. 2016;18:204–211. doi: 10.1093/ntr/ntv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutherland MT, Riedel MC, Flannery JS, et al. . Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016;12:16. doi: 10.1186/s12993-016-0100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schubert CR, Fischer ME, Pinto AA, et al. . Brain aging in midlife: the Beaver Dam Offspring Study. J Am Geriatr Soc. 2019;67:1610–1616. doi: 10.1111/jgs.15886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, Kwong JC, Copes R, et al. . Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int. 2017;108:271–277. doi: 10.1016/j.envint.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 38.He F, Li T, Lin J, et al. . Passive smoking exposure in living environments reduces cognitive function: a prospective cohort study in older adults. Int J Environ Res Public Health. 2020;17. doi: 10.3390/ijerph17041402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cacciottolo M, Wang X, Driscoll I, et al. . Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7:e1022. doi: 10.1038/tp.2016.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Younan D, Petkus AJ, Widaman KF, et al. . Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer’s disease. Brain. 2020;143:289–302. doi: 10.1093/brain/awz348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livingston G, Huntley J, Sommerlad A, et al. . Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi L, Wu X, Danesh Yazdi M, et al. . Long-term effects of PM2.5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health. 2020. doi: 10.1016/S2542-5196(20)30227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finch CE, Morgan TE. Developmental exposure to air pollution, cigarettes, and lead: implications for brain aging. Ann Rev Dev Psychol. 2020;2:585–614. doi: 10.1146/annurev-devpsych-042320-044338 [DOI] [Google Scholar]
- 44.McConnell R, Shen E, Gilliland FD, et al. . A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ Health Perspect. 2015;123:360–366. doi: 10.1289/ehp.1307031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Forman HJ, Finch CE. A critical review of assays for hazardous components of air pollution. Free Radic Biol Med. 2018;117:202–217. doi: 10.1016/j.freeradbiomed.2018.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner MC, Cohen A, Burnett RT, et al. . Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ Res. 2017;154:304–310. doi: 10.1016/j.envres.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 47.Haghani A, Morgan TE, Forman HJ, Finch CE. Air pollution neurotoxicity in the adult brain: emerging concepts from experimental findings. J Alzheimers Dis. 2020;76:773–797. doi: 10.3233/JAD-200377 [DOI] [PubMed] [Google Scholar]
- 48.Cacciottolo M, Morgan TE, Saffari AA, et al. . Traffic-related air pollutants (TRAP-PM) promote neuronal amyloidogenesis through oxidative damage to lipid rafts. Free Radic Biol Med. 2020;147:242–251. doi: 10.1016/j.freeradbiomed.2019.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghani A, Cacciottolo M, Doty KR, et al. . Mouse brain transcriptome responses to inhaled nanoparticulate matter differed by sex and APOE in Nrf2-Nfkb interactions. Elife. 2020;9. doi: 10.7554/eLife.54822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang C, Stewart LT, Kuo HC, et al. . Cyclic O3 exposure synergizes with aging leading to memory impairment in male APOE ε3, but not APOE ε4, targeted replacement mice. Neurobiol Aging. 2019;81:9–21. doi: 10.1016/j.neurobiolaging.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Liu H, Davies KJ, et al. . Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radic Biol Med. 2012;52:2038–2046. doi: 10.1016/j.freeradbiomed.2012.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodward NC, Pakbin P, Saffari A, et al. . Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging. 2017;53:48–58. doi: 10.1016/j.neurobiolaging.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morgan TE, Davis DA, Iwata N, et al. . Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect. 2011;119:1003–1009. doi: 10.1289/ehp.1002973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prom-Wormley E, Maes HH, Schmitt JE, et al. . Genetic and environmental contributions to the relationships between brain structure and average lifetime cigarette use. Behav Genet. 2015;45:157–170. doi: 10.1007/s10519-014-9704-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno-Gonzalez I, Estrada LD, Sanchez-Mejias E, Soto C. Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nat Commun. 2013;4:1495. doi: 10.1038/ncomms2494 [DOI] [PubMed] [Google Scholar]
- 56.Ho YS, Yang X, Yeung SC, et al. . Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE. 2012;7:e36752. doi: 10.1371/journal.pone.0036752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haghani A, Arpawong TE, Kim JK, Lewinger JP, Finch CE, Crimmins E. Female vulnerability to the effects of smoking on health outcomes in older people. PLoS ONE. 2020;15:e0234015. doi: 10.1371/journal.pone.0234015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine ME, Crimmins EM. A genetic network associated with stress resistance, longevity, and cancer in humans. J Gerontol A Biol Sci Med Sci. 2016;71:703–712. doi: 10.1093/gerona/glv141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulminski AM, Shu L, Loika Y, et al. . Genetic and regulatory architecture of Alzheimer’s disease in the APOE region. Alzheimers Dement (Amst). 2020;12:e12008. doi: 10.1002/dad2.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haghani A, Thorwald M, Morgan TE, Finch CE. The APOE gene cluster responds to air pollution factors in mice with coordinated expression of genes that differs by age in humans. Alzheimers Dement. 2021;17:175–190. doi: 10.1002/alz.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nazarian A, Yashin AI, Kulminski AM. Summary-based methylome-wide association analyses suggest potential genetically driven epigenetic heterogeneity of Alzheimer’s disease. J Clin Med. 2020;9:1489. doi: 10.3390/jcm9051489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Haghani A, Mousavi AH, et al. . Cell-based assays that predict in vivo neurotoxicity of urban ambient nano-sized particulate matter. Free Radic Biol Med. 2019;145:33–41. doi: 10.1016/j.freeradbiomed.2019.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haghani A, Johnson R, Safi N, et al. . Toxicity of urban air pollution particulate matter in developing and adult mouse brain: comparison of total and filter-eluted nanoparticles. Environ Int. 2020;136:105510. doi: 10.1016/j.envint.2020.105510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finch CE, Loehlin JC. Environmental influences that may precede fertilization: a first examination of the prezygotic hypothesis from maternal age influences on twins. Behav Genet. 1998;28:101–106. doi: 10.1023/a:1021415823234 [DOI] [PubMed] [Google Scholar]
- 65.Sobolewski M, Abston K, Conrad K, et al. . Lineage- and sex-dependent behavioral and biochemical transgenerational consequences of developmental exposure to lead, prenatal stress, and combined lead and prenatal stress in mice. Environ Health Perspect. 2020;128:27001. doi: 10.1289/EHP4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haghani A, Johnson RG, Woodward NC, et al. . Adult mouse hippocampal transcriptome changes associated with long-term behavioral and metabolic effects of gestational air pollution toxicity. Transl Psychiatry. 2020;10:218. doi: 10.1038/s41398-020-00907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen JL, Liu X, Weston D, et al. . Developmental exposure to concentrated ambient ultrafine particulate matter air pollution in mice results in persistent and sex-dependent behavioral neurotoxicity and glial activation. Toxicol Sci. 2014;140:160–178. doi: 10.1093/toxsci/kfu059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun. 2014;37:30–44. doi: 10.1016/j.bbi.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 69.Volk HE, Perera F, Braun JM, et al. . Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environ Res. 2020:110320. doi: 10.1016/j.envres.2020.110320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp Neurol. 2018;299:241–251. doi: 10.1016/j.expneurol.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Power C, Atherton K, Thomas C. Maternal smoking in pregnancy, adult adiposity and other risk factors for cardiovascular disease. Atherosclerosis. 2010;211:643–648. doi: 10.1016/j.atherosclerosis.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 72.Burris HH, Baccarelli AA. Air pollution and in utero programming of poor fetal growth. Epigenomics. 2017;9:213–216. doi: 10.2217/epi-2017-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parmar P, Lowry E, Cugliari G, et al. ; BIOS Consortium; GLOBAL Meth QTL Consortium . Association of maternal prenatal smoking GFI1-locus and cardio-metabolic phenotypes in 18,212 adults. EBioMedicine. 2018;38:206–216. doi: 10.1016/j.ebiom.2018.10.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bauer T, Trump S, Ishaque N, et al. . Environment-induced epigenetic reprogramming in genomic regulatory elements in smoking mothers and their children. Mol Syst Biol. 2016;12:861. doi: 10.15252/msb.20156520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Howe CG, Zhou M, Wang X, et al. . Associations between maternal tobacco smoke exposure and the cord blood [Formula: see text] DNA methylome. Environ Health Perspect. 2019;127:47009. doi: 10.1289/EHP3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janssen BG, Gyselaers W, Byun HM, et al. . Placental mitochondrial DNA and CYP1A1 gene methylation as molecular signatures for tobacco smoke exposure in pregnant women and the relevance for birth weight. J Transl Med. 2017;15:5. doi: 10.1186/s12967-016-1113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luyten LJ, Saenen ND, Janssen BG, et al. . Air pollution and the fetal origin of disease: a systematic review of the molecular signatures of air pollution exposure in human placenta. Environ Res. 2018;166:310–323. doi: 10.1016/j.envres.2018.03.025 [DOI] [PubMed] [Google Scholar]
- 78.Rayfield S, Plugge E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J Epidemiol Community Health. 2017;71:162–173. doi: 10.1136/jech-2016-207376 [DOI] [PubMed] [Google Scholar]
- 79.Yan Y, Bazzano LA, Juonala M, et al. . Long-term burden of increased body mass index from childhood on adult dyslipidemia: the i3C Consortium Study. J Clin Med. 2019;8. doi: 10.3390/jcm8101725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haghani A, Dalton HM, Safi N, et al. . Air pollution alters Caenorhabditis elegans development and lifespan: responses to traffic-related nanoparticulate matter. J Gerontol A Biol Sci Med Sci. 2019;74:1189–1197. doi: 10.1093/gerona/glz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SH, Knight EM, Saunders EL, et al. . Rapid doubling of Alzheimer’s amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Res. 2012;1:70. doi: 10.12688/f1000research.1-70.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nhan JD, Turner CD, Anderson SM, et al. . Redirection of SKN-1 abates the negative metabolic outcomes of a perceived pathogen infection. Proc Natl Acad Sci USA. 2019;116:22322–22330. doi: 10.1073/pnas.1909666116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katsanos D, Koneru SL, Mestek Boukhibar L, et al. . Stochastic loss and gain of symmetric divisions in the C. elegans epidermis perturbs robustness of stem cell number. PLoS Biol. 2017;15:e2002429. doi: 10.1371/journal.pbio.2002429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newell Stamper BL, Cypser JR, Kechris K, Kitzenberg DA, Tedesco PM, Johnson TE. Movement decline across lifespan of Caenorhabditis elegans mutants in the insulin/insulin-like signaling pathway. Aging Cell. 2018;17. doi: 10.1111/acel.12704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu F, Luo Q, Zhang Y, et al. . Trans-generational effect of neurotoxicity and related stress response in Caenorhabditis elegans exposed to tetrabromobisphenol A. Sci Total Environ. 2020;703:134920. doi: 10.1016/j.scitotenv.2019.134920 [DOI] [PubMed] [Google Scholar]
- 86.Sen A, Heredia N, Senut MC, et al. . Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep. 2015;5:14466. doi: 10.1038/srep14466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woodhouse RM, Ashe A. How do histone modifications contribute to transgenerational epigenetic inheritance in C. elegans? Biochem Soc Trans. 2020;48:1019–1034. doi: 10.1042/BST20190944 [DOI] [PubMed] [Google Scholar]
- 88.McCarrey JR, Lehle JD, Raju SS, Wang Y, Nilsson EE, Skinner MK. Tertiary epimutations—a novel aspect of epigenetic transgenerational inheritance promoting genome instability. PLoS ONE. 2016;11:e0168038. doi: 10.1371/journal.pone.0168038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkwood TB, Finch CE. Ageing: the old worm turns more slowly. Nature. 2002;419:794–795. doi: 10.1038/419794a [DOI] [PubMed] [Google Scholar]
- 90.Kirkwood TB, Melov S. On the programmed/non-programmed nature of ageing within the life history. Curr Biol. 2011;21:R701–R707. doi: 10.1016/j.cub.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 91.Mendenhall AR, Tedesco PM, Taylor LD, Lowe A, Cypser JR, Johnson TE. Expression of a single-copy hsp-16.2 reporter predicts life span. J Gerontol A Biol Sci Med Sci. 2012;67:726–733. doi: 10.1093/gerona/glr225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37:894–898. doi: 10.1038/ng1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cypser JR, Wu D, Park SK, et al. . Predicting longevity in C. elegans: fertility, mobility and gene expression. Mech Ageing Dev. 2013;134:291–297. doi: 10.1016/j.mad.2013.02.003 [DOI] [PubMed] [Google Scholar]
- 94.Mendenhall A, Crane MM, Leiser S, et al. . Environmental canalization of life span and gene expression in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2017;72:1033–1037. doi: 10.1093/gerona/glx017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mendenhall A, Crane MM, Tedesco PM, Johnson TE, Brent R. Caenorhabditis elegans genes affecting interindividual variation in life-span biomarker gene expression. J Gerontol A Biol Sci Med Sci. 2017;72:1305–1310. doi: 10.1093/gerona/glw349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085 [DOI] [PubMed] [Google Scholar]
- 97.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086 [DOI] [PubMed] [Google Scholar]
- 98.Houri-Zeevi L, Korem Kohanim Y, Antonova O, Rechavi O. Three rules explain transgenerational small RNA inheritance in C. elegans. Cell. 2020;182:1186–1197.e12. doi: 10.1016/j.cell.2020.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He L, Zhbannikov I, Arbeev KG, Yashin AI, Kulminski AM. A genetic stochastic process model for genome-wide joint analysis of biomarker dynamics and disease susceptibility with longitudinal data. Genet Epidemiol. 2017;41:620–635. doi: 10.1002/gepi.22058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Farrell S, Stubbings G, Rockwood K, Mitnitski A, Rutenberg A. The potential for complex computational models of aging. Mech Ageing Dev. 2020;193:111403. doi: 10.1016/j.mad.2020.111403 [DOI] [PubMed] [Google Scholar]
- 101.Gärtner K. A third component causing random variability beside environment and genotype. A reason for the limited success of a 30 year long effort to standardize laboratory animals? Lab Anim. 1990;24:71–77. doi: 10.1258/002367790780890347 [DOI] [PubMed] [Google Scholar]