Abstract
Background:
Cisplatin and paclitaxel are active in triple-negative breast cancer (TNBC). Despite different mechanisms of action, effective predictive biomarkers to preferentially inform drug selection have not been identified. The homologous recombination deficiency (HRD) assay (Myriad Genetics, Inc.) detects impaired double-strand DNA break repair and may identify patients with BRCA1/2-proficient tumors that are sensitive to DNA-targeting therapy. The primary objective of TBCRC 030 was to detect an association of HRD with pathologic response (RCB-0/1) to single-agent cisplatin or paclitaxel.
Patients and Methods:
This prospective phase II study randomized patients with germline BRCA1/2 wild-type/unknown stage I-III TNBC to 12 weeks of preoperative cisplatin or paclitaxel. The HRD assay was performed on baseline tissue; positive HRD was defined as a score ≥33. Crossover to an alternative chemotherapy was offered if there was inadequate response.
Results:
139 patients were evaluable for response, including 88 (63.3%) who had surgery at 12 weeks and 51 (36.7%) who crossed over due to inadequate clinical response. HRD results were available for 104 tumors (74.8%); 74 (71.1%) were HRD positive. RCB-0/1 rate was 26.4% with cisplatin and 22.3% with paclitaxel. No significant association was observed between HRD score and RCB response to either cisplatin (odds ratio [OR] for RCB 0/1 if HRD positive 2.22 [95% CI: 0.39–23.68]), or paclitaxel (OR for RCB 0/1 if HRD positive 0.90 [95% CI: 0.19–4.95]). There was no evidence of an interaction between HRD and pathologic response to chemotherapy.
Conclusions:
In this prospective preoperative trial in TNBC, HRD was not predictive of pathologic response. Tumors were similarly responsive to preoperative paclitaxel or cisplatin chemotherapy.
Keywords: Triple-negative breast cancer (TNBC), cisplatin, paclitaxel, neoadjuvant, preoperative, HRD
Introduction
Triple-negative breast cancer (TNBC), lacking expression of estrogen receptor (ER) and progesterone receptor (PR), and overexpression/amplification of HER2, accounts for approximately 15% of invasive breast cancer. TNBC is highly proliferative, and may have higher rates of recurrent disease compared to other breast cancer subtypes. There are currently no targeted therapies for early TNBC, so chemotherapy remains the standard systemic treatment[1].
Platinum chemotherapy has demonstrated activity against TNBC in the preoperative and metastatic settings, as a single agent[2–5] or in combination with an anthracycline/taxane backbone[6–8]. The addition of platinum to a multi-agent preoperative chemotherapy regimen increases response at surgery, but also increases chemotherapy-related toxicity. Moreover, TNBC is a heterogeneous disease comprised of multiple subtypes, with potential differential sensitivity to different chemotherapies[9]. Markers that can predict the benefit of specific chemotherapy agents in TNBC are lacking, which creates challenges when trying to select an optimal chemotherapy regimen for individual patients.
Cancers with deficiencies in homologous recombination (HR) have impaired double-strand DNA break repair, and thus may have preferential sensitivity to DNA-damaging agents like platinum chemotherapy[10]. Approximately 15–20% of patients with TNBC harbor germline or somatic mutations in BRCA1/2[11], which are crucial components of the HR pathway. Rates of pathologic complete response (pCR) in BRCA1/2-deficient breast cancer populations treated with preoperative cisplatin monotherapy have ranged from 20–60%[5, 12]. In addition to patients with BRCA1/2 mutations, HR deficiency can also occur in BRCA-proficient tumors through a variety of mechanisms including methylation of the BRCA1 promoter and mutations in other genes involved in HR; this may be referred to as a “BRCA-like” phenotype[13].
Since defects in HR could inform chemotherapy choice, it may be beneficial to assess HR status in TNBC patients. To this end, the homologous recombination deficiency (HRD) assay was developed to detect HR deficiency regardless of etiology or mechanism[14]. The HRD assay uses next-generation sequencing of formalin-fixed paraffin-embedded (FFPE) tumor tissue to measure genomic instability[15–17], with scores of ≥ 33 determined to be “positive” for HRD[18]. Retrospective analyses of TNBC cohorts treated with platinum-containing multiagent preoperative regimens have suggested that high-HRD tumors are more likely to have a pathologic response at surgery[18–20]. Taxane chemotherapy, while a backbone of breast cancer treatment, does not exploit defects in HR as a mechanism of cytotoxicity. Thus, it is possible that the HRD assay may identify tumors that are preferentially responsive to platinum chemotherapy.
Given the heterogeneity of TNBC, appropriate selection and tailoring of therapy would maximize therapeutic benefit and minimize risks of unnecessary toxicity. Thus, the TBCRC030 study was designed to prospectively evaluate the predictive capacity of the HRD biomarker for pathologic response to single-agent preoperative cisplatin or taxane chemotherapy in TNBC.
Methods
Study Population
This study was an investigator-initiated, prospective, open-label, randomized phase II trial designed to evaluate the ability of the HRD assay to predict pathologic response to preoperative chemotherapy. Eligible patients had invasive breast cancer that was ER ≤5%, PR ≤5%, and HER2 IHC 0/1+ or FISH ratio <2.0, and clinical stage I (T1 ≥1.5 cm) or stage II-III. Patients with a known germline BRCA1/2 mutation were ineligible; however, baseline genetic testing was not mandated. BRCA1/2 mutation status was subsequently ascertained through both commercial testing (germline) and single-gene results within HRD testing (germline and somatic). Clinical axillary lymph node status had to be known; indication of lymph node positivity necessitated further confirmation with biopsy.
Study Procedures
Patients were randomized to either cisplatin 75 mg/m2 every 3 weeks for 4 cycles, or weekly paclitaxel 80 mg/m2 for 12 weeks. Following completion of preoperative chemotherapy, patients underwent surgery, and then could receive further provider-choice adjuvant chemotherapy to ensure comprehensive systemic therapy. However, protocol therapy ended at surgery, and data on adjuvant therapy was not collected. Patients with inadequate clinical response after 12 weeks (as judged either clinically or radiologically by a provider) were able to “crossover” to an alternative provider-selected preoperative chemotherapy regimen. Adverse events were graded using CTCAE v4.0. Research FFPE and frozen biopsies were collected at baseline for all patients. Tissue was also collected at surgery and, whenever possible, when crossover occurred before surgery. Baseline clinical diagnostic tissue was collected for HRD analysis, performed by Myriad Genetics, Inc. At least 100 mm2 of tumor tissue was required for testing. The positive threshold for HRD of ≥33 was used in the primary analysis[18].
Statistical Analysis
The primary objective of the study was to compare pathologic response in TNBC tumors that were HR-proficient versus HR-deficient (defined as an HRD score ≥33) after preoperative chemotherapy (platinum or taxane-based). The primary endpoint was pathologic response, assessed using residual cancer burden (RCB) score[21]. Patients with RCB-0/1 were considered responders, whereas patients with RCB-2/3 were considered non-responders. Patients who crossed over after the 12 weeks of preoperative chemotherapy were considered non-responders and categorized as RCB-2/3.
Target study accrual was 160 patients. Patients were randomized in a 1:1 ratio stratified by initial lymph node assessment (positive vs. negative) as well as by tumor size (pretreatment T1–2 vs T3–4). Estimating that up to 12.5% of tissue would be inevaluable based on prior retrospective experiences with HRD testing, 140 evaluable patients were planned for the analysis. The consideration of sample size and power was based on co-primary objectives: to determine if HR-deficiency was predictive of pathologic response to cisplatin, and, symmetrically and separately, to determine if there was a negative association between HR-deficiency and pathologic response to taxane.
Each analysis for the relationship between HR status and pathologic response to specific chemotherapy was conducted using a univariate logistic regression model (to link HR-deficiency and response) and a likelihood ratio test with a one-sided type I error of alpha = 0.05. Under the assumption that the prevalence of HR deficiency would be 60%[22], 70 evaluable patients per arm (140 total) would provide >80% power to detect a response rate of 52% in HR-deficient patients versus a response rate of 16% in HR-non deficient patients. At time of trial design, pathologic response estimates were available for preoperative platinum but not for taxane monotherapy[19].
To test whether the association of HRD score and pathologic response to preoperative chemotherapy is agent-specific, an interaction between HRD score and treatment arm was evaluated in a logistic regression model using a likelihood ratio test and one-sided alpha = 0.05. With 140 patients, there was 82% power to detect an interaction corresponding to the following rates: platinum in high HR-deficient (52%), platinum in low HR-non deficient (16%), taxane in high HR-deficient (27%), taxane in low HR-non deficient (37%), which corresponds to odds ratios (HR-deficient vs. HR-non deficient) of 5.7 and 0.62 for response to cisplatin and taxane, respectively. Power for the test of interaction was calculated by simulation of binary data under the logistic regression model and represents the proportion of p-values from the 10,000 simulated datasets that were less than 0.05[23].
As a secondary analysis of HRD score and RCB-0/1 within each treatment arm, a multivariate logistic regression model was used to estimate the odds of pathologic response after adjusting for stratification factors and other patient and tumor characteristics with a known or observed association with response to preoperative therapy.
Compliance with Ethical Standards
This trial was approved by the institutional review boards at participating cancer centers and conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent before any study-related procedures.
Results
Patient and Tumor Characteristics
From April 2014 through January 2018, 147 participants were randomized, 75 to cisplatin and 72 to paclitaxel. Accrual to the study was terminated prematurely prior to goal accrual due to withdrawal of sponsor support. Seven patients were unable to proceed with protocol therapy (4 withdrew before starting, 1 was ineligible before starting, and 2 stopped within the first cycle due to hypersensitivity), therefore 140 received protocol therapy. One patient was not evaluable after completion of protocol therapy (lost to follow-up before surgery), leaving 139 response-evaluable patients (Figure 1). Patient characteristics for the entire study cohort are shown in Table 1. Notably, most of the enrolled patients had grade III tumors, T1–2, clinically node-negative, and 94% were ER/PR<1%. Although eligibility criteria were designed to enroll a germline BRCA wild-type population, post-enrollment genetic testing revealed 4 patients with germline mutations (2 in BRCA1, 2 in BRCA2) and 3 with somatic BRCA1 mutations.
Figure 1.
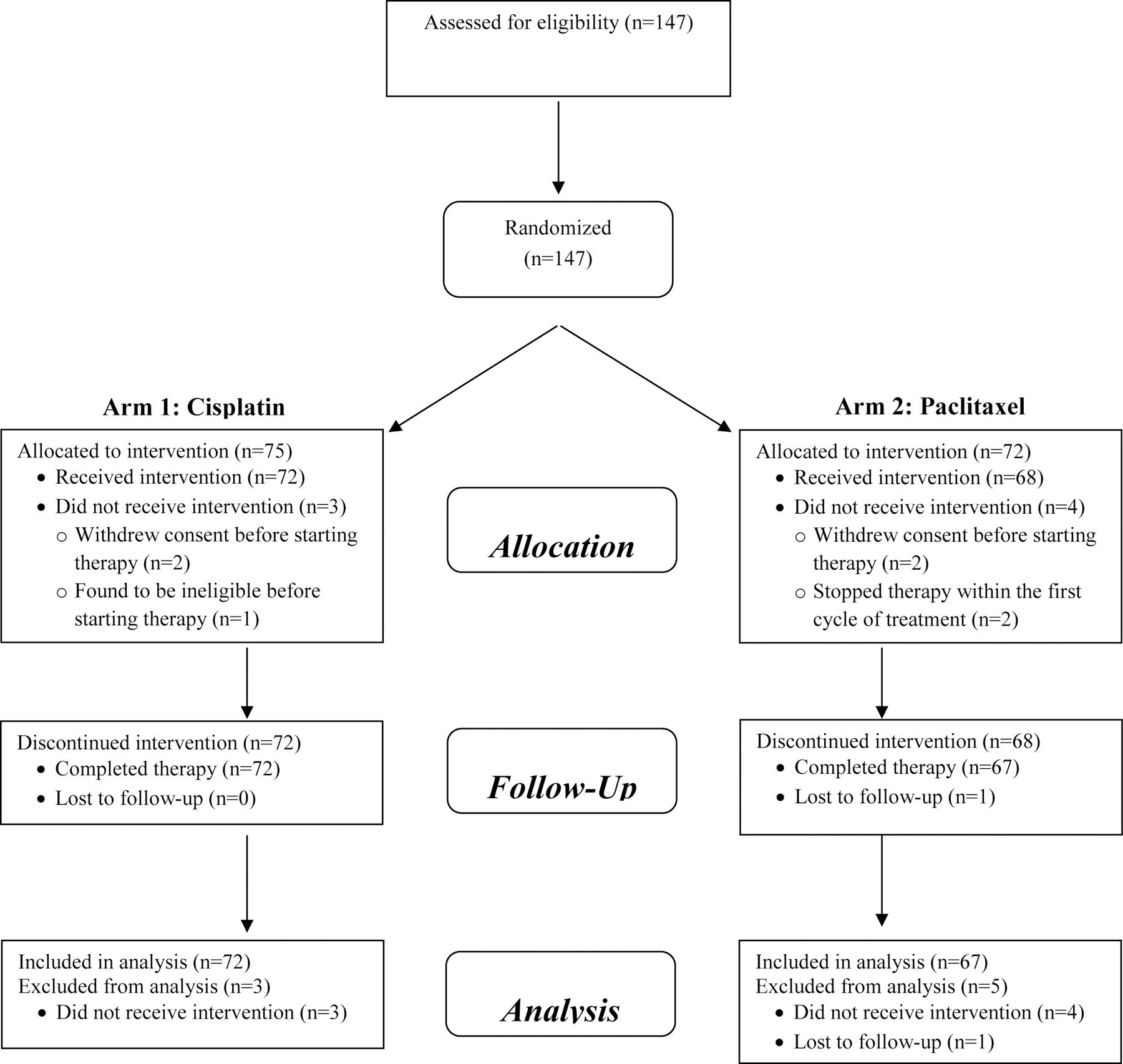
Consort diagram.
Table 1.
Patient characteristics
| Patient Characteristics (n = 147) | Cisplatin (n = 75) | Paclitaxel (n = 72) |
|---|---|---|
|
| ||
| Patient Factors | ||
|
| ||
| Age, median (range), years | 53 (28–82) | |
|
| ||
| Race/Ethnicity | ||
| White/Caucasian | 60 (80%) | 51 (71%) |
| Black/African American | 8 (11%) | 11 (15%) |
| Hispanic/Latino | 7 (9%) | 4 (6%) |
| Asian | 0 (0%) | 2 (3%) |
| Other | 0 (0%) | 4 (6%) |
|
| ||
| BRCA1/2 Status | ||
| Germline/somatic intact | 69 (92%) | 71 (99%) |
| Germline and/or somatic mutation* | 6 (8%) | 1 (1%) |
|
| ||
| Tumor Factors | ||
|
| ||
| Histologic Grade | ||
| II | 5 (7%) | 9 (13%) |
| III | 70 (93%) | 62 (86%) |
| Unknown | 0 (0%) | 1 (1%) |
|
| ||
| Clinical lymph node status | ||
| Positive | 29 (39%) | 26 (36%) |
| Negative | 46 (61%) | 46 (64%) |
|
| ||
| Tumor Size | ||
| T1–2 | 59 (79%) | 61 (85%) |
| T3–4 | 15 (20%) | 11 (15%) |
| Unknown | 1 (1%) | 0 (0%) |
Cisplatin arm: BRCA1/2 germline 4, BRCA1/2 somatic 2. Paclitaxel arm: BRCA1/2 somatic 1.
Preoperative Treatment and Clinical Results
Of the 139 response evaluable patients, 88 (63.3%) had surgery at 12 weeks and 51 (36.7%) crossed over to an alternative preoperative chemotherapy and were considered non-responders. For those who crossed over, the subsequent chemotherapy regimens included doxorubicin/cyclophosphamide (n = 33), doxorubicin/cyclophosphamide and paclitaxel (n = 8), cisplatin (n = 5), and paclitaxel (n = 5).
Pathologic outcomes after preoperative chemotherapy are shown in Table 2. The rate of pathologic response after 12 weeks of chemotherapy, with response defined as RCB-0/1 and non-response as RCB-2/3 or crossover, was 26.4% with cisplatin and 22.3% with paclitaxel. The rate of pathologic complete response (pCR) after 12 weeks of chemotherapy was 15.3% with cisplatin and 11.9% with paclitaxel. The rate of pathologic response (RCB-0/1) for all patients at time of surgery, including those who received additional chemotherapy before surgery, was 37.5% with cisplatin, and 41.8% with paclitaxel. No significant associations were seen between likelihood of RCB-0/1/pCR and clinicopathologic features, including age, grade, tumor size, or nodal status in either the cisplatin or paclitaxel arms.
Table 2.
Pathologic outcomes after preoperative chemotherapy
| Response | Cisplatin (n = 72) | Paclitaxel (n = 67) | Total (n = 139) |
|---|---|---|---|
| Responder (RCB-0/1) | 19 (26.4%) | 15 (22.3%) | 34 (24.5%) |
| Non-Responder (RCB-2/3 or crossover) | 53 (73.6%) | 52 (77.6%) | 105 (75.5%) |
| pCR | 11 (15.3%) | 8 (11.9%) | 19 (13.7%) |
| Non-pCR | 61 (84.7%) | 59 (88.1%) | 120 (86.3%) |
Abbreviations: pCR, pathologic complete response; RCB, residual cancer burden.
Safety
All adverse events with protocol therapy are described in Table 3. Overall, therapy was well-tolerated with expected toxicities for these agents. Greater rates of tinnitus and thrombocytopenia were seen with cisplatin, whereas greater rates of sensory neuropathy and liver function abnormality were seen with paclitaxel.
Table 3.
Adverse events occurring in ≥ 20% of patients
| Toxicity | Cisplatin (n = 72) | Paclitaxel (n = 68) | ||
|---|---|---|---|---|
| Any Grade | Grade 3/4 | Any Grade | Grade 3/4 | |
| Nausea | 57 (76%) | 1 (1%) | 31 (43%) | 0 (0%) |
| Fatigue | 55 (73%) | 0 (0%) | 52 (72%) | 0 (0%) |
| Neutrophil count decreased | 40 (53%) | 1 (1%) | 26 (36%) | 0 (0%) |
| Anemia | 34 (45%) | 1 (1%) | 38 (53%) | 0 (0%) |
| Constipation | 31 (41%) | 0 (0%) | 19 (26%) | 0 (0%) |
| White blood cell count decreased | 29 (39%) | 0 (0%) | 25 (35%) | 0 (0%) |
| Tinnitus | 26 (35%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Hypomagnesemia | 23 (31%) | 0 (0%) | 5 (7%) | 0 (0%) |
| Headache | 19 (25%) | 0 (0%) | 25 (35%) | 0 (0%) |
| Hypertension | 19 (25%) | 2 (3%) | 22 (31%) | 0 (0%) |
| Anxiety | 17 (23%) | 0 (0%) | 24 (33%) | 0 (0%) |
| Diarrhea | 16 (21%) | 1 (1%) | 23 (32%) | 0 (0%) |
| Platelet count decreased | 16 (21%) | 0 (0%) | 3 (4%) | 0 (0%) |
| Insomnia | 15 (20%) | 0 (0%) | 24 (33%) | 0 (0%) |
| Dysgeusia | 11 (15%) | 0 (0%) | 15 (21%) | 0 (0%) |
| Peripheral sensory neuropathy | 10 (13%) | 0 (0%) | 41 (57%) | 0 (0%) |
| Alanine aminotransferase increased | 6 (8%) | 0 (0%) | 16 (22%) | 0 (0%) |
HRD Results
HRD testing was attempted on baseline FFPE tumor samples for all evaluable patients. HRD testing was successfully completed for 104 patients (74.8%) and was unavailable for 35 patients (25.2%; n = 16 cisplatin, n = 19 paclitaxel) due to inadequate tissue or inconclusive results. Of the patients with known HRD results, 74 (71.1%) had HRD-high tumors and 30 (28.8%) had HRD-low tumors. The median HRD score in the cisplatin arm was 51, with 39 tumors HRD-high (69.6%) and 17 low (30.4%). In the paclitaxel arm, the median HRD score was 47, with 35 tumors HRD-high (72.9%) and 13 low (27.0%). Figure 2 shows the range of HRD scores within the entire study population. Of note, 6 of the 7 patients with BRCA1/2-deficient tumors had an HRD result; all of these tumors were HRD-high, as expected based on testing methodology.
Figure 2.
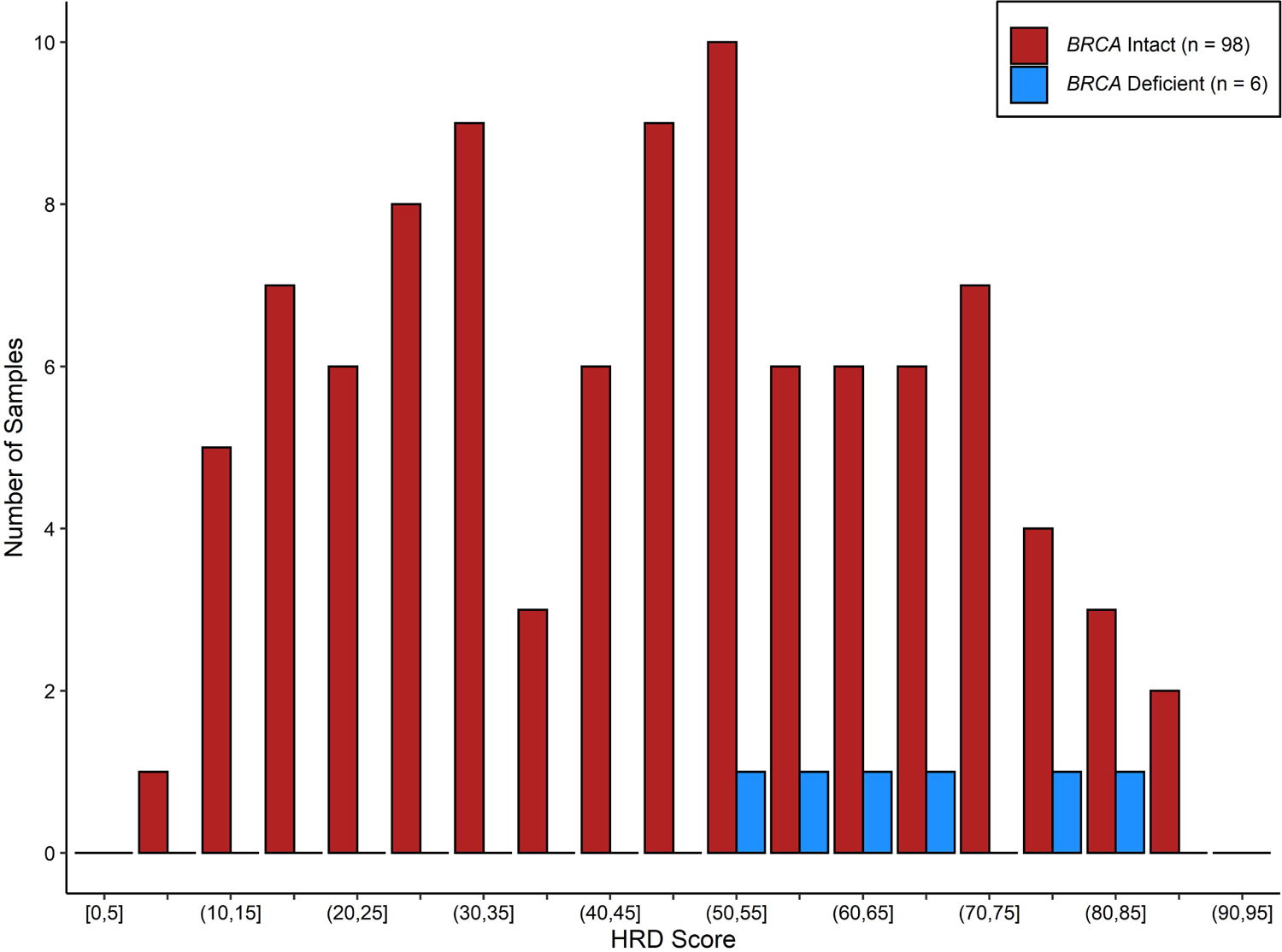
HRD score distribution for the response-evaluable population. BRCA-deficient tumors include those with germline or somatic BRCA1/2 gene mutations.
Abbreviations: HRD, homologous recombination deficiency.
For the primary analysis of association between HRD score and response after preoperative chemotherapy, no significant association was seen between HRD score and RCB 0/1 response to either preoperative cisplatin or paclitaxel. As shown in Table 4, the OR for RCB 0/1 response with cisplatin if HRD-high was 2.22 (95% CI: 0.39–23.68), and the OR for RCB 0/1 response with paclitaxel if HRD-high was 0.90 (95% CI: 0.19–4.95). Similarly, the OR for pCR response with cisplatin if HRD-high was 2.32 (95% CI: 0.23–118.07), and the OR for pCR response with paclitaxel if HRD-high was 0.55 (95% CI: 0.09–4.14). There was no evidence of odds ratios significantly different than 1, with p-values ranging from 0.3–0.9. These data are shown graphically in Figure 3. Analyses were repeated using the prior HRD threshold for positivity, ≥42, and results were not significantly changed (Supplemental Table 1).
Table 4.
Associations between clinical outcomes and HRD scores for the response-evaluable cohort with available HRD scores.
| Cisplatin (n = 56) | Paclitaxel (n = 48) | |||||
|---|---|---|---|---|---|---|
| HRD+ | HRD− | OR (95% CI) | HRD+ | HRD− | OR (95% CI)_ | |
| RCB-0/1 RCB-2/3/Crossover |
9 (23%) 30 (77%) |
2 (12%) 15 (88%) |
2.22 (0.39–23.68) | 10 (29%) 25 (71%) |
4 (31%) 9 (69%) |
0.90 (0.19–4.95) |
| pCR No pCR |
5 (13%) 34 (87%) |
1 (6%) 16 (94%) |
2.32 (0.23–118.07) | 5 (14%) 30 (86%) |
3 (23%) 10 (77%) |
0.55 (0.09–4.14) |
Abbreviations: CI, confidence interval; HRD, homologous recombination deficiency; OR, odds ratio; pCR, pathologic complete response; RCB, residual cancer burden.
Figure 3.
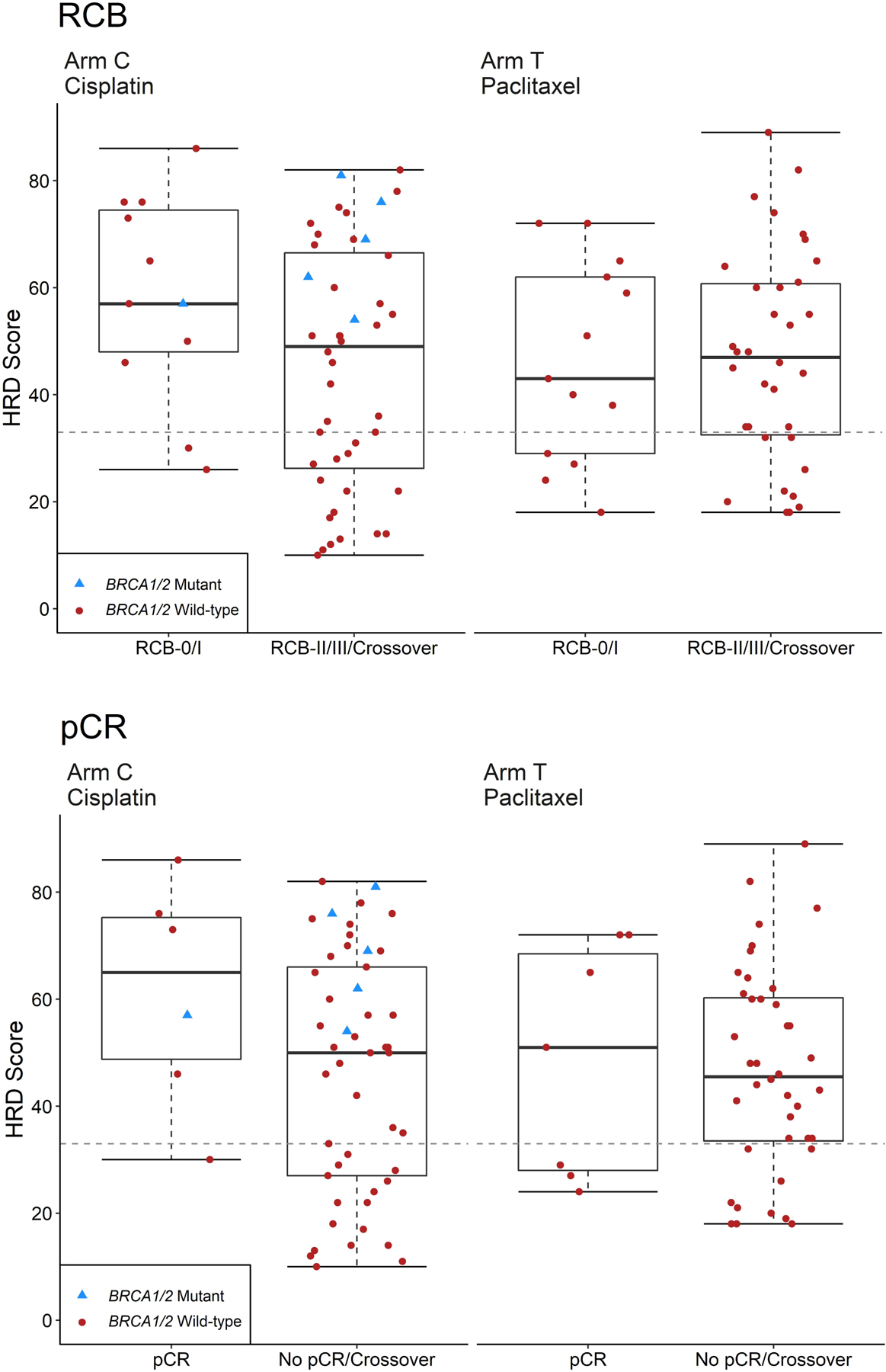
Box plots of HRD score versus pathologic response for all response-evaluable patients.
A. Response classified by RCB score, where RCB-0/1 = response, and RCB-2/3/crossover = non-response
B. Response classified by pCR, where pCR = response and no pCR/crossover = no response. Box outlines the 25th and 75th percentiles; solid line is the median; whiskers extend to the most extreme data point that is no more than 1.5 times the interquartile range.
Abbreviations: HRD, homologous recombination deficiency; pCR, pathologic complete response; RCB, residual cancer burden.
Of note, 6 of the 7 patients with BRCA1/2 altered tumors had known HRD results and were randomized to cisplatin; only one of these patients (1/6) had an RCB-0/1 response to cisplatin. The 7th patient without known HRD was randomized to paclitaxel and had a RCB-0/1 response. No significant association between HRD score and pathologic response was observed after removal of patients with BRCA1/2 altered tumors.
Discussion
This prospective randomized phase II study was designed to evaluate the ability of the HRD biomarker to predict response to either platinum or taxane chemotherapy for TNBC. Patients treated with 12 weeks of preoperative cisplatin or paclitaxel had an RCB-0/1 response rate of approximately 24%, with similar response results regardless of chemotherapy arm. HRD testing on baseline FFPE clinical samples was successful in 75% of cases. No predictive relationship was observed between binary HRD score and pathologic response to either cisplatin or paclitaxel.
There has been great interest in the role of platinum chemotherapy for TNBC, particularly as part of preoperative management. Single agent studies have yielded pCR rates of 20–30% in unselected patients[2, 3], and RCB-0/1 rates greater than 50% have been observed in BRCA1/2-deficient patients[12] and in patients with HR-deficient tumors[19]. When added to multiagent chemotherapy, platinum chemotherapy can increase the response rate at surgery, as seen in CALGB 40603 and GeparSixto[6–8, 24]. It is well established that achievement of pCR at surgery predicts favorable survival outcomes for TNBC patients[25]. However, preoperative trials are typically not powered for survival endpoints, and interpretation of long-term follow-up data from these large randomized trials has been mixed regarding whether the addition of platinum to multi-agent preoperative chemotherapy improves the chance of remaining disease-free[6–8, 24]. Adjuvant trials evaluating the use of platinum chemotherapy, either as part of an adjuvant regimen (NRG BR003; NCT02488967) or in the post-preoperative setting (EA1131; NCT02445391), are ongoing. As the addition of an extra chemotherapy agent increases the risk of toxicity, identification of which TNBC are platinum-sensitive would be desirable. Determining differential sensitivity to various chemotherapy agents among TNBC might also allow de-escalation of multidrug regimens in an effort to maximize efficacy and reduce toxicity.
The HRD assay was developed as a biomarker to help identify tumors with preferential sensitivity to DNA-targeting strategies, including platinum chemotherapy, and potentially direct use of these strategies in BRCA-proficient TNBC[14]. Early retrospective studies suggested the potential predictive capacity of the HRD biomarker for pathologic response to platinum-based chemotherapy[19], and the HRD assay may help select candidates with platinum-sensitive ovarian cancer for maintenance PARP inhibitor therapy[26]. However, more recent analyses derived from larger randomized parent trials have not confirmed the ability of specific HRD score to predict a higher response to platinum chemotherapy. In GeparSixto, the addition of carboplatin increased rates of pCR in HR-deficient tumors (high HRD score and BRCA1/2 mutation), but no interaction between HRD score and carboplatin benefit was detected[8]. In BrighTNess, breast cancers with high HRD scores had higher responses to chemotherapy. However, binary HRD score did not significantly predict benefit with platinum versus other chemotherapy[18]. In the TNT study[4], which randomized patients with metastatic TNBC to platinum versus taxane chemotherapy, HRD score did not predict response to a specific agent, despite greater response to carboplatin in BRCA1/2-deficient tumors. Consistent with these previous analyses, in the current study, HRD score was not prospectively predictive of response to platinum chemotherapy in patients with TNBC. This relationship was consistent whether using the modern threshold of 33 or the prior less sensitive threshold of 42. Despite strong preclinical rationale, it is unclear why the HRD assay has not demonstrated consistent capacity as a biomarker for specific chemotherapy response. There are potential explanations for these observations, for example, possibly reflecting a disconnect between measurement of prior DNA damage and re-emergence of functional capacity for homologous recombination[27]. However, based on the current data, the HRD assay cannot be used as a biomarker for platinum response, nor used to select a specific preoperative chemotherapy regimen for patients with TNBC.
Alternative biomarkers predictive of response or resistance of TNBC to specific chemotherapy agents remain under development. Immune infiltrating cells, immune gene signatures, or other genomic signatures in development may prove to be more consistent predictors of general therapeutic response[28, 29]. Furthermore, results from KEYNOTE-522 have suggested benefit from the addition of a PD-1 inhibitor to a preoperative chemotherapy backbone for TNBC[30], and future biomarker investigation may need to reflect potential routine use of immunotherapy in this setting. It is not known if immune signatures may have the ability to predict activity for specific chemotherapy agents in TNBC. Ongoing correlative analyses of research samples from TBCRC030 will contribute to this exploration, as well as a comprehensive evaluation of other predictors of benefit from specific chemotherapy.
This study has several potential limitations. First, HRD analysis was successful in only 75% of potentially evaluable cases. Assay failure was related primarily to obtaining inadequate tissue from a standard clinical diagnostic biopsy. Although the assay failure rate was estimated to be 12.5% from prior studies evaluating HRD retrospectively, in this prospective study within a network of academic centers, the ability to obtain adequate tissue for HRD analysis from a diagnostic core biopsy was lower than predicted. The study was designed to analyze a larger number of evaluable samples, and the assay failure rate led to decreased power to evaluate a relationship between HRD score and response to chemotherapy. Ability to accrue to the planned original sample size and/or a lower assay failure rate would have provided greater power to detect a significant association between HRD score and response to specific chemotherapy. Additionally, despite efforts to enroll BRCA-proficient patients, a small number of patients with BRCA-altered tumors were enrolled, which had the potential to skew results. However, the INFORM study (TBCRC 031), which prospectively evaluated preoperative cisplatin in BRCA1/2 mutation carriers, showed a RCB-0/1 rate of 33%, suggesting the inclusion of a small number of these patients was unlikely to skew overall results[5]. Finally, this study was not designed to address recurrence or survival, and information about these endpoints is not available from this dataset.
There are few modern studies that have prospectively evaluated preoperative platinum monotherapy specifically in BRCA1/2 proficient patients with TNBC. Although not designed to precisely compare arms, results from TBCRC030 suggest no substantial clinical benefit of single-agent cisplatin over paclitaxel in the preoperative setting for TNBC, regardless of HRD score. Although the addition of a fourth chemotherapy to a three-drug preoperative regimen may improve responses at surgery, TBCRC030 does not suggest preferential activity of platinum over paclitaxel monotherapy for BRCA1/2 proficient tumors. Inclusion of preoperative platinum chemotherapy into regimens for patients with TNBC should be considered carefully for select candidates. It is hoped that further research will allow optimization of preoperative chemotherapy selection, including escalation and de-escalation, for patients with TNBC.
Supplementary Material
Highlights.
This trial prospectively evaluated the predictive capacity of the HRD biomarker for pathologic response in early TNBC.
Pathologic responses to preoperative cisplatin or taxane monotherapy in germline BRCA1/2 wild-type TNBC were similar.
HRD score was not predictive of pathologic response to either cisplatin or paclitaxel chemotherapy.
HRD testing cannot be recommended as a tool to select chemotherapy agents in the management of early-stage TNBC.
Acknowledgements:
Writing: Editorial support was provided by Timothy Erick, PhD and Kaitlyn Bifolck, BA.
Funding:
This work was supported by an unrestricted research grant from Myriad Genetics, Inc (no grant number is applicable).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations: Oral presentation at the 2019 Annual Meeting of the American Society of Clinical Oncology, May 31 – June 4, 2019, Chicago, IL, USA
Clinical Trial Registration: NCT01982448
Disclosures: The authors have declared no conflicts of interest.
References
- 1.Lin NU, Vanderplas A, Hughes ME et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012;118:5463–5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver DP, Richardson AL, Eklund AC et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isakoff SJ, Mayer EL, He L et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2015;33:1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tutt A, Tovey H, Cheang MCU et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med 2018;24:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tung N, Arun B, Hacker MR et al. TBCRC 031: Randomized Phase II Study of Neoadjuvant Cisplatin Versus Doxorubicin-Cyclophosphamide in Germline BRCA Carriers With HER2-Negative Breast Cancer (the INFORM trial). J Clin Oncol 2020;38:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sikov WM, Berry DA, Perou CM et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015;33:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Schneeweiss A, Loibl S et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014;15:747–756. [DOI] [PubMed] [Google Scholar]
- 8.Loibl S, Weber KE, Timms KM et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol 2018;29:2341–2347. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann BD, Jovanovic B, Chen X et al. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS One 2016;11:e0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy RD, Quinn JE, Mullan PB et al. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst 2004;96:1659–1668. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Angulo AM, Timms KM, Liu S et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res 2011;17:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byrski T, Huzarski T, Dent R et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 2014;147:401–405. [DOI] [PubMed] [Google Scholar]
- 13.Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016;16:110–120. [DOI] [PubMed] [Google Scholar]
- 14.Timms KM, Abkevich V, Hughes E et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res 2014;16:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birkbak NJ, Wang ZC, Kim JY et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov 2012;2:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popova T, Manie E, Rieunier G et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res 2012;72:5454–5462. [DOI] [PubMed] [Google Scholar]
- 17.Abkevich V, Timms KM, Hennessy BT et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer 2012;107:1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telli ML, Metzger O, Timms K et al. Evaluation of homologous recombination deficiency (HRD) status with pathological response to carboplatin +/− veliparib in BrighTNess, a randomized phase 3 study in early stage TNBC. J Clin Oncol 2018;36:519–519. [Google Scholar]
- 19.Telli ML, Timms KM, Reid J et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin Cancer Res 2016;22:3764–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telli ML, Hellyer J, Audeh W et al. Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer. Breast Cancer Res Treat 2018;168:625–630. [DOI] [PubMed] [Google Scholar]
- 21.Symmans WF, Peintinger F, Hatzis C et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414–4422. [DOI] [PubMed] [Google Scholar]
- 22.Richardson AL, Silver DP, Szallasi Z, et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Cisplatin Neoadjuvant Chemotherapy in Patients with Triple Negative Breast Cancer. San Antonio Breast Cancer Symposium. San Antonio, TX: 2014. [Google Scholar]
- 23.Landau S, Stahl D. Sample size and power calculations for medical studies by simulation when closed form expressions are not available. Stat Methods Med Res 2013;22:324–345. [DOI] [PubMed] [Google Scholar]
- 24.Sikov WM, Polley M-Y, Twohy E et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/− carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol 2019;37:591–591. [Google Scholar]
- 25.Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008;26:1275–1281. [DOI] [PubMed] [Google Scholar]
- 26.Mirza MR, Monk BJ, Herrstedt J et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375:2154–2164. [DOI] [PubMed] [Google Scholar]
- 27.Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res 2014;16:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filho OM, Stover DG, Asad S et al. Immunophenotype and proliferation to predict for response to neoadjuvant chemotherapy in TNBC: Results from BrighTNess phase III study. J Clin Oncol 2019;37:510–510. [Google Scholar]
- 29.Loibl S, Sinn BV, Karn T et al. Exome analysis of oncogenic pathways and tumor mutational burden (TMB) in triple-negative breast cancer (TNBC): Results of the translational biomarker program of the neoadjuvant double-blind placebo controlled GeparNuevo trial. J Clin Oncol 2019;37:509–509. [Google Scholar]
- 30.Schmid P, Park YH, Ferreira M, et al. KEYNOTE-522: phase 3 study of neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy, followed by adjuvant pembrolizumab versus placebo for early triple-negative breast cancer: pathologic complete response in key subgroups and by treatment exposure and residual cancer burden. San Antonio Breast Cancer Symposium. San Antonio, TX: 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


