Abstract
Human adult laughter is characterized by vocal bursts produced predominantly during exhalation, yet apes laugh while exhaling and inhaling. The current study investigated our hypothesis that laughter of human infants changes from laughter similar to that of apes to increasingly resemble that of human adults over early development. We further hypothesized that the more laughter is produced on the exhale, the more positively it is perceived. To test these predictions, novice (n = 102) and expert (phonetician, n = 15) listeners judged the extent to which human infant laughter (n = 44) was produced during inhalation or exhalation, and the extent to which they found the laughs pleasant and contagious. Support was found for both hypotheses, which were further confirmed in two pre-registered replication studies. Likely through social learning and the anatomical development of the vocal production system, infants' initial ape-like laughter transforms into laughter similar to that of adult humans over the course of ontogeny.
Keywords: laughter, vocalization, positive emotion, affect, ontogeny, primates
1. Introduction
In social mammalian species, joint laughter contributes to the establishment and enhancement of social bonds (e.g. great apes: [1,2]; rodents: [3]). Laughter evolved from the laboured breathing of physical play and in humans ritualized into a signal that is primarily produced during exhalation (‘ha-ha’; [4,5]). Compared to human adults, infants have little control over their vocal production apparatus and have had limited opportunities for social learning. Based on these observations, we sought to test two hypotheses: (i) the extent to which human laughter is perceived to be produced during exhalation increases over the course of early ontogeny, and (ii) whether this change maps onto a shift in listeners' perception, such that laughter produced more during exhalation is perceived as more positive.
Similar to many other expressions of emotion, human laughter has its origins in ancestral non-human primate displays [6,7]. Despite considerable similarities in laughter patterns across great apes and humans, some notable differences have also been established. In a study examining tickle-induced vocalizations from infant and juvenile great apes, including humans, Davila Ross et al. [1] found that all non-human ape species produced laughter during exhalation, as well as during mixed exhalation–inhalation (egressive–ingressive) phases. By contrast, humans exclusively produced egressive laughter. The authors proposed that over the course of human evolution, egressive laughter may have been exaggerated after the divergence of hominins from a common ancestor with chimpanzees and bonobos. Davila Ross and colleagues included laughter from human infants between 11 and 19 months. However, laughter emerges in human infants as young as three-months old [8,9]. It may be that the production of laughter vocalizations changes over the course of development, since the vocal tract of a newborn infant is similar to that of a great ape [10], and vocal production undergoes dramatic changes within the first 2 years of life [11,12]. Compared to human adults, infant vocalizations are generally more likely to include ingressive sound production [13]. We therefore hypothesized that infant laughter would be characterized by more ingressive vocalizations compared to adults, and that the degree of laughter occurring on exhalation would increase over ontogeny.
Laughter is intrinsically social [14–17]. In fact, laughter is 30 times more likely to occur in social, as compared to solitary, situations [18]. When people laugh, it functions as a social glue: contagious laughter is associated with longer social interactions in humans [19], as well as in other species (e.g. chimpanzees: [20]; geladas: [21]). Shared laughter is particularly important early in ontogeny in order to strengthen the essential bond between the infant and the caregiver [22], and indeed young infants laugh a great deal: the frequency of laughter between mothers and infants over a period of 20 min is within the same range as that occurring in a 24 h period for adults [15]. Social learning may shape laughter production, given that infants are strongly biased to learn communication skills that result in the caregiver satisfying the infant's drives [23]. Through processes of mimicry, imitation and social learning, infants may learn that voiced, songlike laughs, which are typically produced during exhalation, yield the most preferable outcomes in interaction partners [24]. Infants may thus come to produce more egressive laughter in order to elicit positive affect from listeners. We therefore hypothesized that the extent to which laughter was produced on the exhalation would be positively correlated with adult listeners' judgements of the laughs’ contagiousness and pleasantness.
In the present study, we thus sought to empirically test two predictions on breathing patterns in laughter vocalizations over the course of early ontogeny. First, we predicted that the proportion of laughter produced on the exhalation would be lower in infants than in adults, and that the proportion of egressive laughter would increase over the course of infant development (3–18 months). Second, we sought to test whether egressive laughter would be positively associated with perceived positive affect, potentially making the shift in vocal production of laughter functionally adaptive in terms of social relationships.
2. Method
The study consisted of three experiments, with Experiment 2 being a pre-registered replication of Experiment 1 with new participants and completely novel stimulus materials (https://osf.io/j2d5w) and Experiment 3 a control experiment which is explained at the end of the result section. As it is not possible to identify whether vocalizations are produced on an ingressive or egressive airstream based on a measure derived from the acoustic signal alone [25,26], we chose to take a ratings-based approach in which novice and expert listeners made perceptual judgements of inhalation and exhalation. In Experiment 1, 102 novices (89 female, mean age 23.5 years, range 18–58 years) and 15 phoneticians (14 female, mean age 35.3 years, range 26–58 years) participated online. The judgements made by the novices closely matched those made by the experts (see electronic supplementary material, table S1), and consequently only novices were included in Experiment 2 (102 novices, 94 female, mean age 19.1 years, range 18–23 years). All participants gave informed consent, and the studies were approved by the local ethics committee of Leiden University (CEP16-1206/365 and CEP19-1015/503).
Sound clips of infant laughter were collected from video-sharing websites (e.g. YouTube) and the authors' personal networks. The laughs used in Experiment 1 and Experiment 2 were produced by different infants. The lower age limit was set to three months, in order to include the youngest age at which infants have been found to produce laughter [9], and the upper limit to 18 months. In general, we determined the infant's age based on information provided by caregivers. For the videos collected from video-sharing platforms, this meant that we used explicit information mentioned in the title or description of the video. For example: ‘Austin giggling while I'm tickling his feet! 7 months 3 days' or ‘Laughing Baby Vee @ 5 months’. Only videos with such explicit information were included in the study. The number of clips was 44 in Experiment 1 and 64 in Experiment 2. No selection criteria other than the age of the infant and audio quality (no interruptions, dominant background noise or vocalizations produced by others) were employed. For each clip, the duration, the infant's age and sex, and the cause of the laughter was noted (see electronic supplementary material, table S2 and S3; clip duration was not correlated with age1). In addition, we included adult laughter sounds (five clips for the novices in Experiment 1, and eight clips for the experts in Experiment 1 and the novices in Experiment 2) in order to test whether, compared to adults, infants would laugh more on the inhalation. All clips had a duration between 4 and 7 s.
Before the start of the main survey, participants were familiarized with ingressive (produced during inhalation) and egressive (produced during exhalation) vocalizations by listening to ingressive and egressive non-laughter vocalizations produced by human adults (one clip of each). Then, the laughter clips were played in a random order, and participants were asked, for each clip, to state their agreement with the following four statements: (i) the laugh is produced during inhalation; (ii) the laugh is produced during exhalation; (iii) the laugh is pleasant to listen to; (iv) the laugh is contagious (when listening to this laugh, I feel like laughing too). The response format for all judgements was a continuous slider with a five-point scale below which had one-decimal accuracy. The scales ranged from never (0) to always (4) for the first two statements and from strongly disagree to strongly agree for the last two statements. By using the slider, participants could not only opt for 0, 1, 2, 3, 4, but also for a rating in between two round numbers (e.g. 2.3; for an overview of reliability scores for all ratings see electronic supplementary material, table S4).
A proportion score for egressive laughter was calculated by dividing the perceived exhalation score by the sum of the perceived inhalation and exhalation scores. A combined positive affect score was calculated by taking the average value of the pleasantness score and the contagiousness score2. Data were analysed using R v. 4.0.5 (2021-03-31) [27].
3. Results
A paired samples t-test comparing perceptions of laughter produced by infants to that of adults confirmed that the proportion of laughter produced during exhalation was significantly lower in infants than in adults (Experiment 1: Minfants = 0.62 (s.d. = 0.09), Madults = 0.74 (s.d. = 0.16), t114 = −9.09, p < 0.001; Experiment 2: Minfants = 0.59 (s.d. = 0.09), Madults = 0.62 (s.d. = 0.12), t101 = −3.26, p < 0.01).
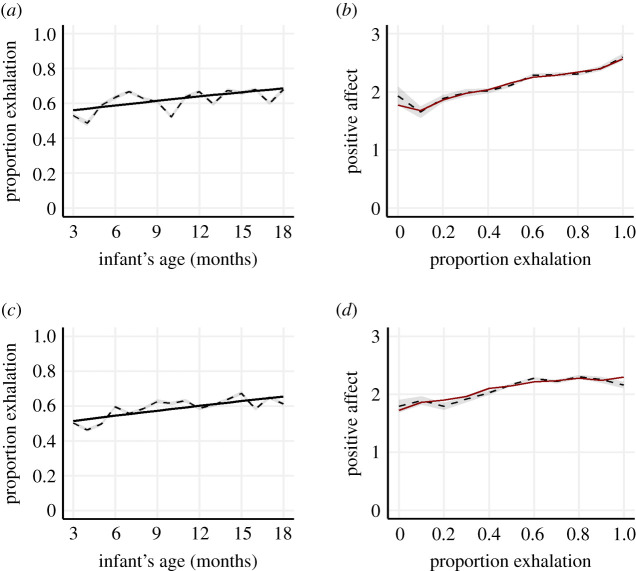
To test our hypothesis that laughter would be produced increasingly on the exhalation over the course of infancy, two identical multilevel beta regression models were generated, one for each experiment. Infant age was used as a predictor variable, and the proportion of laughter produced during exhalation as an outcome variable. The models included a random intercept per participant and a random slope as a function of infant age. As hypothesized, egressive laughter was found to increase with age (Exp 1: β = 0.04, odds ratio (OR) = 1.04, s.e. = 0.00, z = 10.54, p < 0.001; electronic supplementary material, table S5 and figure 1a; Exp 2: β = 0.04, OR = 1.04, s.e. = 0.00, z = 9.99, p < 0.001; electronic supplementary material, table S6; figure 1c). For exploratory purposes, we investigated whether this effect was modulated by the gender of the infant and the gender of the listener. No reliable effects were observed (electronic supplementary material, tables S11–S14).
Figure 1.
The proportion of laughter produced during exhalation (a,c) and positive affect scores (b,d) in Experiment 1 (a,b) and Experiment 2 (c,d). The solid line shows predicted data based on the described multilevel models. The dashed line shows observed data, and the shaded error band indicates 1 s.e.
Using linear multilevel models including a random intercept per participant, we investigated whether the degree to which laughter was produced on the exhalation would predict the amount of positive affect evoked in adult perceivers. The proportion of exhalation was used as a predictor variable and listener positive effect as an outcome variable. As hypothesized, the perceived proportion of laughter produced on the exhalation positively predicted positive affect scores (Exp 1: F1,5111.5 = 135.09, p < 0.001, electronic supplementary material, table S15; figure 1b; Exp 2: F1,6490.9 = 127.24, p < 0.001; electronic supplementary material, table S16; figure 1d)3. Thus, the more the laughter was produced on the exhalation, the more positively it was perceived.
In order to rule out that the positivity judgements were influenced by the judgements of whether the laughs were egressive, we conducted a third, pre-registered study (https://aspredicted.org/blind.php?x=8e5tx3) in which 102 novices (69 male, mean age 29.8 years, range 18–74 years) were asked to judge the pleasantness and contagiousness of a combination of all audio clips used in Experiment 1 and Experiment 2 (108 infant clips and 13 adult control clips) without being asked to answer questions about airflow direction. The average score on the proportion of laughter produced during exhalation assigned to each clip in Experiment 1 and Experiment 2 was used as a predictor for the positive affect scores. The pleasantness and contagiousness scores were combined, as they were highly positively correlated with each other (r = 0.77, p < 0.001). In line with the results from Experiments 1 and 2, the proportion of laughter produced on the exhalation positively predicted positive affect scores (F1,11014 = 524.26, p < 0.001, electronic supplementary material, table S17). This finding rules out a potential confound and provides additional support for our hypothesis that more egressive laughter evokes more positive responses in adult listeners.
4. Discussion
The present study examined changes in the production of human laughter in early ontogeny. In two experiments, we found that the proportion of laughter perceived to be produced during exhalation was lower in infants than in adults, and that the older the infants, the more their laughter was egressive. Over the course of early development, human laughter thus deviates increasingly from the laughter vocalizations of non-human primates [1]. Our findings also point to a likely role of social feedback in developmental changes of laughter, with laughs produced more on the exhalation eliciting more positive affect in adult listeners. This result was confirmed in a third independent control experiment where listeners only had to evaluate the audio clips on positive affect and not on the extent to which the laughter was produced during exhalation or inhalation.
Two pathways may explain the shift towards egressive laughter over ontogeny and the enhanced interpersonal effects of egressive laughter. First, developmental changes in the acoustic features of laughter are likely to relate to human anatomical development: the vocal tract of human infants initially resembles that of non-human primates [10], but undergoes major developmental changes during the first years [28]. Functionally, infants greatly improve in terms of vocal control [29] as they start to produce proto-speech vocalizations like babbling around seven to eight months [30,31]. Early human infant laughter may thus resemble the laughter of non-human primates in part due to similarities in terms of vocal production systems and associated (lack of) vocal control.
Second, developmental changes in laughter production may also reflect social learning processes. Infants as young as six months have been found to mimic sounds produced by their caregivers [32], and infants are highly receptive to caregivers' responses to their pre-linguistic vocalizations [33]. In particular, infants adapt subsequent vocalizations based on social feedback [29] and human adults have a preference for voiced, songlike laughs which are produced during exhalation [24]. Processes of imitation and social learning may thus support the development of gradually more adult-like laughter [34]. Since laughter induces positive affect in others [24], infants may over time come to produce laughter with a higher proportion of exhalation in order to elicit maximally positive responses from their social environment.
The present study establishes developmental changes in breathing during laughter production. Further work will be needed to examine whether these findings map onto changes in other important acoustic features of laughter, such as duration, the spectral centre of gravity and harmonics-to-noise ratio (e.g. [35,36]). Moreover, future work might examine whether other types of non-verbal vocalizations (e.g. crying) have similar or different trajectories in terms of the development of egressive vocal production whether laughter production changes further beyond the age range examined in the present study. Moreover, it would be interesting to more thoroughly study the developmental trajectory by including a greater number of audio clips per age category (in months). Finally, another potential avenue for future research would be to determine whether similar developmental changes occur in the laughter vocalizations of non-human primates.
In conclusion, this study provides novel insights into the ontogeny of human laughter. Our findings demonstrate that infants appear to increasingly produce egressive laughter over the course ontogeny, with more egressive laughter also being perceived more positively by adults. Thus, human laughter changes over ontogeny from vocalizations similar to those of other great apes to laughter resembling that of human adults.
Acknowledgements
We thank Tom Wilderjans for his advice on the statistical analyses.
Endnotes
There was no confounding correlation between age and the length of the clip (Experiment 1: r = −0.033, p = 0.834; Experiment 2: r = 0.165, p = 0.192).
A proportion score for exhalation was calculated because the continuous inhalation and exhalation scores were highly negatively correlated (Experiment 1: r = −0.91, p < 0.001; Experiment 2: r = −0.85, p < 0.001). The pleasantness and contagiousness scores were combined because they were highly positively correlated with each other (Experiment 1: r = 0.76, p < 0.001; Experiment 2: r = 0.72, p < 0.001). For the interested reader, we include separate results in the supplementary material (electronic supplementary material, tables S7, S8, S9, and S10).
In two control analyses we ruled out that this effect was the result of a positive relationship between age and positive affect. Specifically, after we added age to the models of both experiments, the relationship between the proportion of laughter produced on the exhalation and the perceived pleasantness remained highly significant (Exp 1: F1,5109.7) = 94.07, p < 0.001; Exp 2: F1,6490.2 = 53.27, p < 0.001). Variance inflation factors (VIF) were below 4 in both experiments (Experiment 1 VIF = 1.02; Experiment 2 VIF = 1.05) indicating no issues of multicollinearity between age and the exhalation score.
Ethics
All participants gave informed consent, and the studies were approved by the local ethics committee of Leiden University (CEP16-1206/365 and CEP19-1015/503).
Data accessibility
All data and study materials are available in the electronic supplementary material and will become available after publication on Dataverse.nl
Authors' contributions
M.E.K. developed the study concept. M.E.K., D.V. and D.A.S. designed the study. D.V. programmed the tasks and collected the data. B.G.E. reached out to phoneticians to rate the stimuli. D.V. and D.A.S. pre-registered Experiment 1 and 2. Data were analysed by I.S. and D.V. under the supervision of M.E.K. D.V. and M.E.K. drafted the manuscript, and D.A.S. and B.G.E. provided several critical revisions. All authors approved the final version of the manuscript and agree to be held accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Netherlands Science Foundation 016.VIDI.185.036, the Templeton World Charity Organization TWCF0267 (to M.E.K.) and the European Research Council (ERC) under the European Union's Horizon 2020 Programme for Research and Innovation grant no. 714977 (to D.A.S.).
References
- 1.Davila Ross M, Owren MJ, Zimmermann E. 2009Reconstructing the evolution of laughter in great apes and humans. Curr. Biol. 19, 1106-1111. ( 10.1016/j.cub.2009.05.028) [DOI] [PubMed] [Google Scholar]
- 2.Van Hooff JARAM. 1972A comparative approach to the phylogeny of laughter and smiling. In Non-verbal communication (ed. Hinde RA), pp. 209-241. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Panksepp J, Burgdorf J. 2003‘Laughing’ rats and the evolutionary antecedents of human joy? Physiol. Behav. 79, 533-547. [DOI] [PubMed] [Google Scholar]
- 4.Provine RR, Yong YL. 1991Laughter: a stereotyped human vocalization. Ethology 89, 115-124. ( 10.1111/j.1439-0310.1991.tb00298.x) [DOI] [Google Scholar]
- 5.Todt D, Vettin J. 2005Human laughter, social play, and play vocalizations of non-human primates: an evolutionary approach. Behaviour 142, 217-240. ( 10.1163/1568539053627640) [DOI] [Google Scholar]
- 6.Darwin C, Ekman P, Prodger P. 1998The expression of the emotions in man and animals, 3rd edn. London, UK: Harper Collins. [Google Scholar]
- 7.Gervais M, Wilson DS. 2005The evolution and functions of laughter and humor: a synthetic approach. Q. Rev. Biol. 80, 395-430. ( 10.1086/498281) [DOI] [PubMed] [Google Scholar]
- 8.Washburn RW. 1929A study of the smiling and laughing of infants in the first year of life. Genet. Psychol. Monogr. 6, 403-537. [Google Scholar]
- 9.Addyman C, Addyman I. 2013The science of baby laughter. Comedy Studies 4, 143-153. ( 10.1386/cost.4.2.143_1) [DOI] [Google Scholar]
- 10.Karmiloff-Smith A. 2013Developmental change. In Encyclopedia of autism spectrum disorders (ed. Volkmar FR). New York, NY: Springer. ( 10.1007/978-1-4419-1698-3_1426) [DOI] [Google Scholar]
- 11.Negus VE. 1949The comparative anatomy and physiology of the larynx. New York, NY: Hafner Publishing Company. [Google Scholar]
- 12.Stark RE, Bernstein LE, Demorest ME. 1993Vocal communication in the first 18 months of life. J. Speech Lang. Hear Res. 36, 548-558. ( 10.1044/jshr.3603.548) [DOI] [PubMed] [Google Scholar]
- 13.Grau SM, Robb MP, Cacace AT. 1995Acoustic correlates of inspiratory phonation during infant cry. J. Speech Lang. Hear Res. 38, 373-381. ( 10.1044/jshr.3802.373) [DOI] [PubMed] [Google Scholar]
- 14.LaFrance M. 1983Felt versus feigned funniness: issues in coding smiling and laughing. In Handbook of humor research (eds McGhee PE, Goldstein JH), pp. 1-12. New York, NY: Springer. [Google Scholar]
- 15.Young PT. 1937Laughing and weeping, cheerfulness and depression: a study of moods among college students. J. Soc. Psychol. 8, 311-334. ( 10.1080/00224545.1937.9920012) [DOI] [Google Scholar]
- 16.Scott SK, Lavan N, Chen S, McGettigan C. 2014The social life of laughter. Trends Cogn. Sci. 18, 618-620. ( 10.1016/j.tics.2014.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant GA, Fessler DM, Fusaroli R, Clint E, Aarøe L, Apicella CL, De Smet D. 2016Detecting affiliation in colaughter across 24 societies. Proc. Natl Acad. Sci. USA 113, 4682-4687. ( 10.1073/pnas.1524993113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provine RR, Fischer KR. 1989Laughing, smiling and talking: relation to sleeping and social context in humans. Ethology 83, 295-305. ( 10.1111/j.1439-0310.1989.tb00536.x) [DOI] [Google Scholar]
- 19.Provine RR. 1992Contagious laughter: laughter is a sufficient stimulus for laughs and smiles. Bullet. Psychonomic Soc. 30, 1-4. ( 10.3758/BF03330380) [DOI] [Google Scholar]
- 20.Davila-Ross M, Allcock B, Thomas C, Bard KA. 2011. Aping expressions? Chimpanzees produce distinct laugh types when responding to laughter of others. Emotion 11, 1013-1020. ( 10.1037/a0022594) [DOI] [PubMed] [Google Scholar]
- 21.Mancini G, Ferrari PF, Palagi E. 2013In play we trust: rapid facial mimicry predicts the duration of playful interactions in geladas. PLoS ONE 8, 6. ( 10.1371/journal.pone.0066481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowlby J. 1969. Attachment and loss: attachment. New York: Basic Books. 1994. In The handbook of humor: clinical applications in psychotherapy, vol. I (ed. Buckman ES), Malabar, FL: Krieger. [Google Scholar]
- 23.Halliday M. 1975Learning how to mean: explorations in the development of language. New York, NY: Elsevier. [Google Scholar]
- 24.Bachorowski JA, Owren MJ. 2001Not all laughs are alike: voiced but not unvoiced laughter readily elicits positive affect. Psychol. Sci. 12, 252-257. ( 10.1111/1467-9280.00346) [DOI] [PubMed] [Google Scholar]
- 25.Eklund R. 2008Pulmonic ingressive phonation: diachronic and synchronic characteristics, distribution and function in animal and human sound production and in human speech. J. Int. Phonetic Assoc. 38, 235-325. ( 10.1017/S0025100308003563) [DOI] [Google Scholar]
- 26.Sundqvist P. 2012Pulmonic ingressive speech in Shetland English. World Englishes 31, 434-448. ( 10.1111/j.1467-971X.2012.01772.x) [DOI] [Google Scholar]
- 27.R Core Team. 2021R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 28.Mugitani R, Hiroya S. 2012Development of vocal tract and acoustic features in children. Acoust. Sci. Technol. 33, 215-220. ( 10.1250/ast.33.215) [DOI] [Google Scholar]
- 29.Gaultier C, Gallego J. 2005Development of respiratory control: evolving concepts and perspectives. Respir. Physiol. Neurobiol. 149, 3-15. ( 10.1016/j.resp.2005.04.018) [DOI] [PubMed] [Google Scholar]
- 30.Oller DK. 1980The emergence of the sounds of speech in infancy. In Child phonology, vol. 1: production (eds Yeni-Komshian GH, Kavanagh JF, Ferguson CA), pp. 93-112. New York, NY: Academic Press. [Google Scholar]
- 31.Stark RE. 1980Stage of speech development in the first year of life. In N, child phonology: production, vol. 1 (eds Yeni-Komshian GH, Kavanagh JF, Ferguson CA), pp. 73-92. New York, NY: Academic Press. [Google Scholar]
- 32.Imafuku M, Kanakogi Y, Butler D, Myowa M. 2019Demystifying infant vocal imitation: the roles of mouth looking and speaker's gaze. Dev. Sci. 22, 1-12. ( 10.1111/desc.12825) [DOI] [PubMed] [Google Scholar]
- 33.Albert RR, Schwade JA, Goldstein MH. 2018The social functions of babbling: acoustic and contextual characteristics that facilitate maternal responsiveness. Dev. Sci. 21, 1-11. ( 10.1111/desc.12641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snowdon CT, Hausberger M. 1997Social influences on vocal development. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Szameitat DP, Alter K, Szameitat AJ, Wildgruber D, Sterr A, Darwin CJ. 2009Acoustic profiles of distinct emotional expressions in laughter. J. Acoust. Soc. Am. 126, 354-366. ( 10.1121/1.3139899) [DOI] [PubMed] [Google Scholar]
- 36.Lavan N, Scott SK, McGettigan C. 2016Laugh like you mean it: authenticity modulates acoustic, physiological and perceptual properties of laughter. J. Nonverbal Behav. 40, 133-149. ( 10.1007/s10919-015-0222-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and study materials are available in the electronic supplementary material and will become available after publication on Dataverse.nl