Abstract
Phototrophic microorganisms (microbial phototrophs) use light as an energy source to carry out various metabolic processes producing biomaterials and bioenergy and supporting their own growth. Among them, microalgae and cyanobacteria have been utilized extensively for bioenergy, biomaterials, and environmental applications. Their superior photosynthetic efficiency, lipid content, and shorter cultivation time compared to terrestrial biomass make them more suitable for efficient production of bioenergy and biomaterials. Other phototrophic microorganisms, especially anoxygenic phototrophs, demonstrated the ability to survive and flourish while producing renewable energy and high-value products under harsh environmental conditions. This review presents a comprehensive overview of microbial phototrophs on their (i) production of bioenergy and biomaterials, (ii) emerging and innovative applications for environmental conservation, mitigation, and remediation, and (iii) physical, genetic, and metabolic pathways to improve light harvesting and biomass/biofuel/biomaterial production. Both physical (e.g., incremental irradiation) and genetic approaches (e.g., truncated antenna) are implemented to increase the light-harvesting efficiency. Increases in biomass yield and metabolic products are possible through the manipulation of metabolic pathways and selection of a proper strain under optimal cultivation conditions and downstream processing, including harvesting, extraction, and purification. Finally, the current barriers in harnessing solar energy using phototrophic microorganisms are presented, and future research perspectives are discussed, such as integrating phototrophic microorganisms with emerging technologies.
Keywords: Phototrophs, photosynthesis, bio-energy, biomaterials, high-value products, environmental applications
Graphical Abstract
1. Introduction
A sustainable and renewable water-energy-food nexus system is highly desirable for modern societies and harnessing the solar energy can be an integral factor to that system. It is estimated that the total incident solar power at the Earth’s surface is 124,000 terrawatt, and a small fraction (~0.07%) of it is utilized by all photosynthetic organisms [1]. Phototrophic microorganisms (microbial phototrophs) can be a potential tool for efficient conversion of the virtually unlimited supply of solar energy into bioenergy and renewable materials [[2], [3], [4]] (Fig. 1). These microorganisms have a photosynthetic efficiency (~12%) that is much higher than terrestrial biomass (1.8–2.2%) [5,6]. Moreover, microalgae and cyanobacteria have a 10-fold higher lipid content while requiring one-fourth cultivation time compared to conventional terrestrial plants [7]. They are highly adaptive to dynamic environmental conditions and do not compete for food-producing land [8]. Therefore, unlike the current practices of producing biofuels from crops and edible vegetable oils, they generally do not pose any threat to food security [2]. Also, it is possible to offset production costs (i.e., cost of nutrients) through the use of wastewater during cultivation [9]. The high yield of lipid and biomass makes them economically more suitable for bioenergy and biomaterial production.
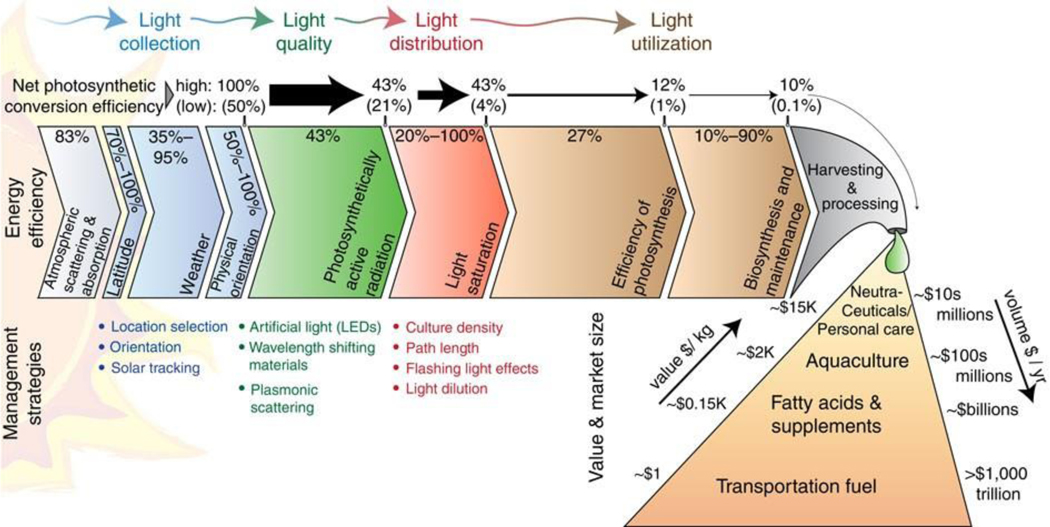
Fig. 1.
Energy conversion efficiencies from sunlight to biomass and associated geological and biological factors (lateral flow diagram). The management strategies for efficient conversion of solar energy into biomass and the value of different bioproducts are presented in bulleted points and triangle shape, respectively. Reprinted with permission from Ref. [5]. Copyright © Springer Nature.
Not only is the growth potential for microbial phototrophs impressive, but different forms of energy and materials can be derived from the same biomass [8,10,11]. The biomass of these microorganisms is used for extracting numerous high-value compounds such as pigments, nutraceuticals, pharmaceuticals, and antioxidants [12,13]. They have been proven useful for environmental applications such as environmental monitoring and early warning, wastewater treatment, heavy metal removal, greenhouse gas sequestration, and emerging contaminant removal [[14], [15], [16], [17]].
The unique photosynthetic activities of microbial phototrophs allow them to convert sunlight to bioenergy and biomaterials. The presence of the photosensitive pigment known as chlorophyll allows them to capture light for their growth. Unlike plants that mostly use visible sunlight, some phototrophic microorganisms have developed the ability to convert a much broader spectrum of light energy (visible and/or infrared) into sugars [18]. One of the most attractive features of aquatic phototrophic microorganisms is their inducible CO2-concentrating mechanisms that allow them to optimize carbon acquisition [19]. Both cyanobacteria and eukaryotic microalgae can accumulate bicarbonate to use as a source of CO2. They can increase internal CO2 concentration through the dehydration of accumulated bicarbonate [19]. During photosynthesis, the production of oxygen depends on the type of microorganisms. Microbial phototrophs capable of producing oxygen during the photosynthesis process are known as oxygenic phototrophs (e.g., cyanobacteria), otherwise known as anoxygenic phototrophs (e.g., green and purple sulfur bacteria) [20].
1.1. Anoxygenic phototrophs
Two and half billion years ago, with little atmospheric oxygen, the photosynthetic niche was mostly populated by anoxygenic phototrophs [21,22]. Anoxygenic phototrophs contain bacteriochlorophyll(s) instead of chlorophyll as the oxygenic phototrophs and require only a single type of photochemical reaction center (RCI or RCII) for conducting photosynthesis [23]. Phototrophs of seven phyla (Proteobacteria, Acidobacteria, Chloroflexi, Cyanobacteria, Chlorobi, Firmicutes, and Gemmatimonadetes) have the ability to perform anoxygenic photosynthesis [20]. They are capable of growing at low light conditions using specialized pigments that can absorb sunlight from spectral regions usually unavailable to oxygenic phototrophs [24]. Almost all the species of phyla Chlorobi, Chloroflexi, and Acidobacteria contain a unique light-harvesting pigment known as chlorosome to absorb sunlight at 740–750 nm, while the purple phototrophic bacteria (PPB) harvest sunlight from the infrared region (800–1020 nm) of the light spectrum [18]. Thus, different anoxygenic entities may co-exist in the same environment without competing for the same light source. Unlike oxygenic phototrophs, anoxygenic phototrophs can harness energy from both the visible and infrared regions (Table 1).
Table 1.
A comparison between the anoxygenic and oxygenic microbial phototrophs.
| Parameters | Anoxygenic phototrophs | Oxygenic phototrophs |
|---|---|---|
|
| ||
| Electron donors | Reduced sulfur species, elemental sulfur, dihydrogen, and ferrous ion [20, 24] | Water |
| Electron acceptors | Ferredoxin, flavodoxin, N2, H+, quinone, nicotinamide adenine dinucleotide phosphate (NADP) [20, 25] | Quinone, NADP, H+ [25, 26] |
| Typical spectral range | Visible and/or infrared spectra [27] | Visible spectra [27] |
| Maximum specific growth rate (μmax) | 0.20–5.86 d−1 [28, 29] | 0.48–3.60 d−1 [30] |
| Photosynthetic oxygen production | No | Yes [20] |
| Oxidative damage to photosystems | Yes, sensitive to O2 [20] | Yes [31] |
The growth and metabolisms in anoxygenic phototrophs are dependent on their light-harvesting capability and tolerance to oxygen [20]. Both green and purple sulfur bacteria are anaerobic and mostly live in sunlit and sulfidic conditions. A group of anoxygenic phototrophs, known as aerobic anoxygenic phototrophs (AAP), is unique in their ability to grow in the presence of oxygen owing to their photoheterotrophic nature [28]. While oxygenic phototrophs convert inorganic carbon, in the form of carbon dioxide, into biomass using electrons from water, anoxygenic phototrophs get electrons from a wide range of external electron donors like elemental sulfur, sulfide, thiosulphate, ferrous iron, and hydrogen [24,32]. According to one study, the purple sulfur bacterium Thiocapsa roseopersicina has a maximum specific growth rate of 0.07 h−1 (1.63 d−1) on dimethyl sulfide culture [33]. AAP can grow even faster, with a reported maximum specific growth rate of 5.86 d−1 [29].
The unique growth features of anoxygenic phototrophs compared to oxygenic phototrophs (Table 1) make them a suitable candidate for cultivating in a wide range of inclement environmental conditions. Depending on their level of tolerance to extreme conditions, anoxygenic phototrophs can be thermophilic, acidophilic, alkaliphilic, halophilic, and so on. Chloroflexus, Heliothrix, and Roseiflexus are the most renowned thermophilic anoxygenic phototrophs. Notably, Chloroflexus aurantiacus is abundant in Yellowstone hot springs (USA), grows best at 55 °C, and is capable of surviving at temperatures up to 70 °C [34]. They adapt to high temperatures either through structural adaptation of their cell components or by accumulating compatible solutes to protect cellular structures [34]. By contrast, Rhodoferax antarcticus found in Antarctic lakes and fjords grow best at 15–18 °C and cannot tolerate temperatures above 25 °C. Acidiphilium can grow aerobically at a pH as low as 1.5 [34]. Anoxygenic phototrophs are generally rich in lipids, proteins, and carbohydrates, especially, purple phototrophic bacteria, which can be utilized to produce biofuels (e.g., biodiesel, hydrogen) and valuable biomaterials (e.g., pharmaceuticals, nutraceuticals) via internal electron recycling.
1.2. Oxygenic phototrophs
Oxygenic phototrophs such as cyanobacteria, green algae, and diatoms are capable of producing oxygen during photosynthesis using water as the electron donor [1,20]. While most oxygenic microbial phototrophs are championed for their metabolites, some other species, such as diatoms, have also been proven to be very useful owing to their diverse cellular patterns and structures. More than 120,000 diatom species have been identified, with each species having unique structural features such as patterns, shape, spikes, and nano-scale pore size [35]. Most oxygenic phototrophs absorb solar energy from the visible light region of the solar spectrum (400–700 nm), while anoxygenic phototrophs can perform photosynthesis with light from both visible and infrared regions [27]. Oxygenic phototrophs require more input energy for photosynthesis (shorter wavelength light) compared to anoxygenic phototrophs (longer wavelength light) owing to the inverse relationship between photon energy and wavelength. The purple bacteria Blastochloris viridis, for example, can use wavelengths beyond 1000 nm for photosynthesis [27]. The maximum wavelength (corresponding to its threshold energy) for the oxygenic photosynthesis process has been identified in the cyanobacterium Acaryochloris marina, which extends to 740 nm [27].
The key elements of oxygenic photosynthesis are photosystem I (PSI), photosystem II (PSII), adenosine triphosphate (ATP) synthase, and cytochrome b6f complex [36]. Both the photosystems (PSI and PSII) play essential roles in photosynthesis, involving an electron transport system in the thylakoid membranes of the microorganisms [31]. During oxygenic photosynthesis, the light-harvesting pigments (chlorophyll) photo-oxidize the reaction centers (P700 and P680). Electrons are transferred from PSII to oxidized P700 in PSI via plastoquinone in this process and involve generating a proton gradient across the membranes, which allows the production of ATP via ATP synthase. In addition, NADP+ is reduced to NADPH in PSI using the electrons from P700 via ferredoxin and ferredoxin-NADP+ reductase. Oxygen is produced during the oxidation of water (as electron donor) through oxidized P680 in PSII. These photosynthetic electron transport reactions collectively provide energy in the form of ATP and NADPH for carbon dioxide assimilation in the Calvin-Benson-Bassham cycle [31]. However, an excess supply of ATP and NADPH can facilitate the production of reactive oxygen species (ROS), which can cause oxidative damage to PSI and PSII [31]. Excess light or high proton irradiance also contributes to the oxidative damage of PSI and PSII. More photosynthesis-limiting factors will be reviewed in the later sections of this article.
2. Using phototrophic microorganisms for bioenergy and electricity production
Phototrophic microorganisms are capable of harnessing solar energy in the form of biodiesel [2], bio-oil [37], bio-alcohol [38], hydrogen [39], biogas [40], as well as electricity [10]. Some species can be utilized for energy storage as well due to their unique structural properties [41]. This section discusses significant findings on the production of biofuels (liquid and gaseous), electricity, as well as energy storage systems using microbial phototrophs.
2.1. Liquid biofuels
Owing to their high lipid and carbohydrate contents, phototrophic microorganisms are considered a promising source for producing biodiesel, bio-oil, and bioethanol. While high lipid content in algal cells is desirable for bio-oil and biodiesel production, high carbohydrate content is sought for bio-alcohol production. Transesterification, pyrolysis, and fermentation techniques are most commonly used to produce biodiesel, bio-oil, and bio-alcohol, respectively [37,42,43].
2.1.1. Biodiesel
Phototrophic microorganisms such as microalgae are a promising source of biodiesel as some of the species have the lipid content of up to 60% of their dry weight [44]. Biodiesel has very similar physical and chemical properties (carbon chain length, energy density, and viscosity) to that of crude oil-derived diesel and can be potentially used as a “drop-in” fuel in the existing internal combustion engines [45].
Algal strain selection is crucial to achieve desired biodiesel production. High lipid content, environmental tolerance, high growth rate, and ease of harvesting and extraction are the decisive factors when selecting a proper microalgal strain [2]. Algae from the genera Botryococcus, Chlorella, Scenedesmus, Chlamydomonas, Dunuliella, and Nannochloropsis are preferable for biodiesel production due to their high internal lipid content [46]. For example, Nannochloropsis salina cultivated in a batch photobioreactor (PBR) can achieve a lipid content of 50% by dry weight and an average annual growth rate of 25 g/(m2·day). The net energy ratio (NER) for algal biodiesel is 0.93 MJ of energy consumed per MJ of energy produced. Microalgae biodiesel has 30% less energy input per unit of product than conventional soybean-based biodiesel, which has the NER of 1.64 MJ/MJ [47].
Biomass pretreatments such as dewatering and cell disruption are frequently used before lipid extraction to enhance lipid production [48]. Lipid extraction from dry biomass is typically done using the organic solvent extraction method. In one study, lipids were extracted from the dry biomass of Chlorococcum sp. Using a hexane/isopropanol mixture (3:2 v/v) under vigorous mixing (800 rpm) for 450 min at 25 °C [37]. The extracted lipids were then converted into biodiesel and glycerol through transesterification in methanol. The lipid mixed with potassium hydroxide (catalyst) was stirred for 3 h at 60 °C during this transesterification process. Biodiesel was finally separated from the biodiesel/glycerol mixture through phase separation [49]. An exhaustive list of different microalgal species and their corresponding lipid, protein, and carbohydrate contents, as well as their potential bioenergy yields, are presented in Table 2.
Table 2.
The chemical composition (lipid, protein, and carbohydrate) and potential biodiesel, ethanol, and methane yield from the dry algae as a substrate. Adapted from [8, 11, 40].
| Microalgal species | Lipid (%) | Protein (%) | Carbohydrate (%) | Biodiesel (g/kg-S) | Ethanol (g/kg-S) | Methane (L/g-S) |
|---|---|---|---|---|---|---|
|
| ||||||
| Anabaena cylindrica | 4–7 | 43–56 | 25–30 | 12–21 | 125–150 | 0.22–0.28 |
| Aphanizomenon flos-aquae | 3 | 62 | 23 | 9 | 115 | 0.31 |
| Arthrospira maxima | 6–7 | 60–71 | 13–16 | 18–21 | 65–80 | 0.30–0.36 |
| Botryococcus braunii | 25–75 | - | - | - | - | - |
| Chaetoceros calcitrans | 39 | 58 | 10 | - | - | - |
| Chaetoceros muelleri | 22–33 | 46–64 | 12–19 | - | - | - |
| Chlamydomonas rheinhardii | 21 | 48 | 17 | 63 | 85 | 0.24 |
| Chlorella emersonii | 29 | - | - | - | - | - |
| Chlorella minutissima | 31 | - | - | - | - | - |
| Chlorella zofingiensis | 26–46 | 11–20 | 25–28 | 78–138 | 125–140 | 0.06–0.10 |
| Chlorella protothecoides | 55 | 10–52 | 10–15 | - | - | - |
| Chlorella pyrenoidosa | 2 | 57 | 26 | 6 | 130 | 0.29 |
| Chlorella sorokiniana | 22 | - | - | |||
| Chlorella vulgaris | 14–25 | 51–58 | 9–17 | 42–66 | 60–85 | 0.26–0.29 |
| Chlorogloeopsis fritschii | 50 | 44 | 7 | - | - | - |
| Crypthecodinium cohnii | 20 | - | - | - | - | - |
| Dunaliella bioculata | 8 | 49 | 4 | 24 | 20 | 0.25 |
| Dunaliella primolecta | 23 | - | - | - | - | - |
| Dunaliella salina | 6 | 57 | 32 | 18 | 160 | 0.29 |
| Dunaliella tertiolecta | 28 | - | - | - | - | - |
| Ettlia oleoabundans | 35–54 | - | - | - | - | - |
| Euglena gracilis | 14–18 | 39–61 | 14–20 | 42–60 | 70–90 | 0.20–0.31 |
| Haematococcus pluvialis | 25 | - | - | - | - | - |
| Isochrysis galbana | 23–30 | 7–25 | 30–45 | - | - | - |
| Monoraphidium minutum | ~52 | - | - | - | - | - |
| Nannochloropsis gaditana | 23.3 | 48.3 | 9.31 | - | - | - |
| Navicula saprophila | ~51 | - | - | - | - | - |
| Nitzschia closterium | 27 | - | - | - | - | - |
| Phaeodactylum tricornutum | 20–30 | - | - | - | - | - |
| Porphyridium cruentum | 9–14 | 28–39 | 40–57 | 27–42 | 200–285 | 0.20–0.29 |
| Prymnesium parvum | 22–38 | 28–45 | 25–33 | 66–114 | 125–165 | 0.14–0.23 |
| Scenedesmus dimorphus | 16–43 | 8–18 | 18–52 | 48–120 | 105–260 | 0.04–0.09 |
| Scenedesmus obliquus | 35–55 | 50–56 | 10–17 | 36–42 | 50–85 | 0.25–0.28 |
| Scenedesmus quadricauda | 1.9 | 47 | - | 5.7 | - | 0.24 |
| Skeletonema costatum | 21 | - | - | - | - | - |
| Spirogyra sp. | 11–21 | 6–20 | 33–64 | 33–63 | 165–320 | 0.03–0.10 |
| Spirulina maxima | 6–7 | 60–71 | 13–16 | 18–21 | ||
| Spirulina platensis | 4–9 | 46–65 | 8–20 | 12–27 | 40–70 | 0.23–0.32 |
| Synechococcus sp. | 11 | 63 | 15 | 33 | 75 | 0.32 |
| Tetraselmis maculata | 3 | 52 | 15 | 9 | 75 | 0.26 |
| Tetraselmis suecica | 15–23 | - | - | - | - | - |
| Thalassiosira pseudonana | 20 | - | - | - | - | - |
Many studies have described metabolic and genetic improvements of different algal species to enhance biodiesel production. The green alga Chlamydomonas reinhardtii has been studied extensively due to its known genome [50]. Metabolic engineering strategies such as manipulating acylation of diacylglycerol and recycling of membrane lipids during triacylglycerol derivation have been attempted to increase the metabolic flux of triacylglycerol production. It has been reported that inactivation of ADP-glucose pyrophosphorylase can increase triacylglycerols production tenfold [51]. Both acetyl CoA carboxylase and type-II fatty acid synthase have been identified as the rate-limiting enzymes during fatty acid synthesis [52]. Lipid production can be enhanced by genetically modifying those enzymes. It was also reported that the targeted knockdown of a multifunctional lipase/phospholipase/acyltransferase of diatom Thalassiosira pseudonana could result in a lipid yield that is three-fold higher than wildtype diatoms [53]. Moreover, nitrogen deficiency and higher initial iron concentration in the medium, along with metabolic engineering, lead to higher lipid content [54]. Nevertheless, algae-based biodiesel production is currently not economically viable. The cost of biodiesel production is 4.4-times higher, and the process is 2.5-times as energy-intensive compared to petroleum-based diesel [2, 55].
2.1.2. Bio-alcohol
Algae-based alcohols, namely ethanol and butanol, have been attracting much attention due to their impressive fuel quality and heating values (bioethanol 19.6 MJ/L, biobutanol 29.2 MJ/L) [43]. With their similar ranges of energy density, biobutanol can be used as a “drop-in” product replacing diesel and gasoline [43].
In bioethanol production, algal biomass is less resistant to conversion into simple sugars compared to the plant biomass with plant cell wall containing lignin that is difficult for industrial processing [38]. Also, the algal cellulose has a unique crystalline structure (triclinic crystalline form) compared to plant cell structure (monoclinic crystalline form) and, therefore, is easy to transform as it contains a weaker hydrogen bond [38]. For bioethanol production, it is very critical to select algae with a high content of carbohydrates (e.g., 40–57% w/w from Porphyridium cruentum) [45]. Chlorella vulgaris has been reported to yield 40% w/w ethanol owing to its high carbohydrate content [56]. The most commonly used microalgal species for bioethanol production are Chlamydomonas reinhardtii UTEX 90, Porphyridium cruentum, Scenedesmus obliquus, Chlorella vulgaris, Chlorococcum sp. owing to their high starch accumulation capability [57].
The bioethanol production process generally requires hydrolysis as the first step to breakdown carbohydrate biomass into monosaccharides [42]. One study utilized separate hydrolysis and fermentation processes to produce bioethanol from Chlorococcum infusionum biomass [42]. After hydrolyzing the biomass with NaOH, fermentation was carried out using Saccharomyces cerevisiae under vigorous mixing (200 rpm) at 30 °C for 72 hours. This whole process resulted in a bioethanol yield of 26.1 wt% (g ethanol/g biomass) when pre-treated with 0.75% (w/v) NaOH at 120°C for 30 min [42]. The same hydrolysis process can be used for biobutanol production. After hydrolysis, Acetone-Butanol-Ethanol fermentation can be carried out using saccharolytic butyric acid-producing microorganisms such as Clostridium acetobutylicum [58].
Genetic engineering and manipulation approaches have been applied to enhance bioethanol production. Researchers have cloned the code of bacterium Zymomonas mobiliz into a vector and then used it to transform the cyanobacterium Synechococcus sp. so that it can create a metabolic pathway for carbon utilization and directly synthesize ethanol without fermentation [59]. A similar study created a Synechocystis sp. PCC 6803 strain that is capable of self-production of bioethanol [60]. This self-production can increase overall energy efficiency and make the process more cost-effective. However, such manipulations can inhibit the growth of many algal species.
Other methods, such as using acid pre-treatment of the algal biomass, are proven to enhance ethanol production by chain depolymerization [61]. One major drawback of bioethanol production is that it produces CO2 as a byproduct, which can accelerate the greenhouse effect if it is not correctly contained. Besides, acid hydrolysis can produce byproducts, including 5-hydroxy-methyl-furfural and levulinic acid, which can significantly reduce ethanol production efficiency [62]. If enzymatic hydrolysis is utilized, recovering the enzyme from the product can also be challenging [63].
2.1.3. Bio-oil
Phototrophic microorganisms can be used to produce bio-oil with proper thermochemical conversion techniques (e.g., pyrolysis). While it has some similarities to biodiesel, bio-oil is a dark, renewable fuel derived from biomass through the thermal cracking of molecules. Bio-oil can be used directly as a “drop-in” fuel or as a blend [8]. Pyrolysis and liquefaction are widely used for bio-oil production from biomass [64, 65]. Both processes are performed at a high temperature in the absence of oxygen [8]. While liquefaction is carried out at 200–400 °C, pyrolysis usually requires a temperature above 400 °C and under pressure [43]. Algal species such as Cladophora fracta and Chlorella protothecoides can produce bio-oil up to 48.2 and 55.3 wt%, respectively, at a temperature of 750–775 °C [65]. An exhaustive list of hydrocarbon (mostly bio-oil and bio-crude) yields from corresponding microalgal species and associated conversion techniques can be found elsewhere [65, 66].
2.2. Gaseous biofuels
Both anoxygenic and oxygenic phototrophs can be utilized to produce hydrogen gas [67, 68]. For other biogas production, either fresh or lipid-extracted biomass can be used as the raw materials [8].
2.2.1. Hydrogen
Photosynthetic production of molecular hydrogen is considered one of the most promising approaches to producing renewable energy. Hydrogen gas is highly portable and contains a heating value of 142 MJ/kg, equivalent to 10.1 MJ/L (liquefied). When combusted, it emits only water as the end product [39]. Both anoxygenic and oxygenic phototrophic microorganisms can produce hydrogen using solar energy through either photofermentation or co-metabolism of N2-fixation [67, 68]. Oxygen produced during oxygenic photosynthesis inhibits hydrogen production [58], making anoxygenic phototrophs (e.g., Rhodobacter sphaeroides CIP 60.6 with a maximum rate of H2 production at 40 mL/L∙h) preferable for hydrogen production [69]. Two enzymes, hydrogenase, and nitrogenase are mainly responsible for hydrogen production through biophotolysis during the co-metabolism of N2-fixing. Many cyanobacterial species convert atmospheric nitrogen into ammonia (NH3) and discharge H2 as a by-product as part of the nitrogen-fixing process. Cyanobacteria use carbohydrates for H2 production if atmospheric nitrogen is not available [70]. One study used the purple photosynthetic bacteria Rhodobacter capsulatus to produce hydrogen through single-stage photofermentation of glucose with a maximum hydrogen yield of 5.5±0.15 mol H2/mol glucose under optimal cultivation conditions with a light intensity of 175 W/m2, 35 mM glucose, and 4.5 mM glutamate [71]. More information on direct and indirect biophotolysis, photofermentation, and dark fermentation is available elsewhere [72].
The hydrogen production potential in phototrophic microorganisms is dependent on strain-specific enzymatic activities and environmental conditions [39]. The most commonly used microalgae and cyanobacteria for photobiological hydrogen production are from the genera Botryococcus, Chlamydomonas, Chlorococcum, Chlorella, Scenedesmus, Synechocystis, Tetraspora, Anabaena, and Nostoc due to significant presences of hydrogenase enzyme in their cells [73]. Among these diverse species, Chlamydomonas reinhardtii, and Anabaena spp. are reported to have the highest hydrogen production [50, 65]. Generally, nitrogen-fixing species (Anabaena and Nostoc) can produce more hydrogen than the non-nitrogen fixing microorganisms (e.g., Synechocystis) due to the higher presence of enzymes responsible for hydrogen production [74].
Metabolic and genetic engineering approaches have been utilized to increase hydrogen production. For example, to enhance hydrogen production, the hydrogen-uptake hydrogenase (Hup-hydrogenase) is inactivated since the Hup-hydrogenase reduces the net hydrogen production in nitrogen-fixing cyanobacteria [39]. Higher hydrogen production can also be achieved in hydrogen-uptake deficient mutants of Anabaena and Nostoc (e.g., Anabaena variabilis AVM13 (ΔhupSL), Nostoc punctiforme NHM5 (ΔhupL)) compared to the wild types [39]. Similarly, another study has reported that hydrogen production could be increased five-fold by redirecting the electrons to the hydrogenase enzyme using targeted ferredoxin and ferredoxin-NADP+-oxidoreductase variants [75]. Hydrogen production can also be enhanced by eliminating pathways competing for electrons (viz; carbon fixation, nitrate assimilation, and respiratory electron transport system) [39]. Since biological hydrogen production is often inhibited due to the extreme oxygen sensitivity of hydrogen synthesizing enzymes [50], a sulfur-deprived condition can increase the respiration rate in order to reduce oxygen sensitivity [11, 76].
Other techniques such as immobilization of phototrophic microorganisms are reported to increase hydrogen production. For instance, alginate films can immobilize the hydrogen-producing microalgae for higher output [77]. This enhanced hydrogen output is mainly attributed to higher cell density, favorable kinetics, and the capability of the alginate polymer to effectively restrict oxygen diffusion into the matrix. Furthermore, manipulating the light conditions of algal cultures can affect hydrogen production [76]. A shift from continuous light to a train of light pulses interrupted by longer dark phases is reported to have higher hydrogen production [78]. Also, it has been reported that Chlamydomonas reinhardtii tla1 strain, CC-4169, with truncated light-harvesting antenna produced 4–6 times more hydrogen than the parental CC-425 strain [79]. CC-4169 exhibited 4-times higher maximum specific growth rate at 285 μEm−2 s−1 and an 8.5-times higher rate at 350 μEm−2 s−1 when immobilized at the same cell density as compared with CC-425 strain [79]. Despite numerous attempts, biological hydrogen production is still not commercially attractive. Even under currently optimized conditions, biologically derived hydrogen gas may cost 3–6 dollars/kg while conventional steam reforming of methane process costs about 1 dollar/kg (1 kg H2 ≈ 1-gallon gasoline in terms of energy content) [79, 80].
2.2.2. Biogas
Green energy in the form of biogas can be produced from phototrophic microorganisms with established technologies such as anaerobic digestion. Both the virgin biomass and the lipid-extracted biomass can be used for biogas production [8]. Biogas typically consists of 45–70% methane, 20–55% CO2, as well as a small amount of H2S, H2O, and hydrocarbons [40].
Potent biogas producing microbial phototrophs should have a high growth rate, thin cell wall with low holocellulose content, higher environmental adaptability, and more cytoplasmic components for improved digestion efficiency and gas content [81]. Anabaena cylindrica, Chlorella sorokiniana, Scenedesmus obliquus, Spirulina platensis, and Synechococcus sp. have been identified as the highest biogas yielding species based on the criteria mentioned above [82]. Not surprisingly, the culture conditions affect their growth and biogas production to a great extent. A comprehensive review of biogas from microalgae is available elsewhere [40].
2.3. Electricity
Phototrophic microorganisms possess an excellent ability to facilitate direct/indirect transfer of electrons outside of the cell, known as exoelectrogenic activity [83]. Accordingly, bioelectrochemical systems (BESs) such as microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) are used to generate electricity that can power remote electronic devices [84, 85]. A critical review of BESs integrated with membrane technologies for harvesting energy and water treatment is available elsewhere [86]. Phototrophic microorganisms used in MFCs are versatile. They can be introduced to the anodic chamber to serve as a substrate (source of carbon), but mostly, they are present in the cathodic chamber with roles that include CO2 capture from the anode and simultaneous wastewater treatment with electricity generation [10, 87]. The power generation of an MFC can be as high as 1.78 W/m2 when consisting of a dry algae biomass of 5 g/L (Scenedesmus obliquus) and activated sludge used as the substrate [88]. Often, microalgae-bacteria consortia are also used in MFCs as well. For example, an MFC was constructed using microalgae Chlamydomonas reinhardtii (catholyte) and sulfur-reducing bacteria Geobacter sulferreducens (anolyte) [10]. This MFC exhibited a power output of 630 mW/m2 when acetate was used as the substrate. The microalgae-bacteria consortia-based MFCs may have lower electricity generation than the pure microalgal cultures because of the adverse effects posed by the oxygen generated in the anode as well as the low concentration of electron donors [89].
Phototrophic microorganisms are reported to be used to develop biophotovoltaic systems (BPVs) [83]. Unlike typical photovoltaic systems, the photosensitive components are living organisms capable of reproduction, damage repair, as well as storing energy for power generation in the absence of light [83]. Furthermore, photosynthetic proteins are emerging photovoltaic materials due to their inherent delicate architecture and internal circuitry [90]. While the conventional p–n junction solar cells have a conversion efficiency of 34%, the photosynthetic proteins demonstrated almost 100% quantum efficiency [91–93]. However, the power generation efficiency of these systems is still too low due to the weak exoelectrogenic activity of photosynthetic microorganisms. Recently, researchers used an energy carrier (D-lactate) to enhance the extracellular electron transport efficiency of the system resulting in a power density (over 150 mW/m2) of at least one order of magnitude higher than the conventional energy carrier-less BPVs [94]. The power density also could be significantly enhanced by decoupling storage and power delivery in a BPV as well [95]. In this system, two chambers spatially separated the charging and power delivery operations so that they can be optimized independently for enhanced power density. Despite clean energy production along with synergistic activities, BESs come with limitations of low power density, power intensity, and power generation efficiency, especially in scale-up of such systems. The power intensity (W per m3 of reactor volume) from reactors with volumes larger than 1 L is often much less than 1 kW/m3, a threshold for industrial application for energy recovery from organic matter [96].
2.4. Energy storage
Supercapacitors with high power densities and fast charging rates have been developed using diatoms and transitional metal oxides [97, 98]. One study reported achieving a capacity of 202.6 Faraday per gram using hierarchical and porous MnO2-modified diatoms [97]. The same material resulted in an even higher power capacity of 297.8 Fg−1 after etching the diatomite [97]. The highest capacitance of 425 Fg−1 was achieved when TiO2 nanospheres, diatom silica, and MnO2 mesoporous nanosheets were combined [98]. Since diatom silica is an inexpensive natural biomaterial, the potential of diatom as economical and high-performance electrode material for supercapacitors is enormous. Diatomite with small size, high sorption capacity, large surface area, and thermal stability is a perfect fit for hydrogen storage [41]. Pristine diatomite has demonstrated a hydrogen adsorption capacity of 0.46 wt% at 2.63 MPa and 298 K. The hydrogen adsorption capacity can be further increased to 0.83 wt% through acid-thermal activation of diatomite [41]. Diatoms are utilized as thermal energy storage as well due to their unique porous nanostructure. The integration of diatomite with conventional phase change materials can improve the thermal storage capacity and stability as well [99]. A summary of diatom-based thermal storage composites and the corresponding latent heats are reported elsewhere [41].
3. Using phototrophic microorganisms for value-added products and biomaterials
Being rich in proteins and carbohydrates, phototrophic microorganisms have the potential for food and feed [57], bioactive compounds [12], bioplastics [100], fertilizers [101, 102], stable isotopes [103], and many more. This section of the review focuses on the production of value-added products and high-value biomaterials using phototrophic microorganisms.
3.1. Pigments and bioactive compounds
One major high-value compound of nutritional and pharmaceutical importance is microalgal pigments (mostly carotenoids) [13]. In phototrophic microorganisms, they mainly function as accessory pigments during photosynthesis, CO2 fixation, culture coloration, and protection of photosystems from oxidative damage [104]. Carotenoids are hydrophobic pigments having two major groups: carotenes (non-oxygenated molecules) and xanthophylls (oxygenated molecules) [12]. Astaxanthin, β-carotene, and lutein are the three carotenoids abundantly found in microalgae [13]. Many algal species can accumulate these carotenoids. Dunaliella salina, Haematococcus pluvialis, Chlorella zofingiensis, and Chlorella vulgaris are extensively studied due to their large-scale production capability [12]. The algal species used for producing lutein and astaxanthin are summarized elsewhere [13].
Phycobiliproteins are another major group of pigments mainly found in cyanobacteria, Rhodophyta, Glaucophyta, and cryptomonads [104, 105]. Four types of phycobiliproteins (phycoerythrin, phycocyanin, phycoerythrocyanin, and allophycocyanin) are identified so far [105]. Phycobiliproteins demonstrate significant efficacy in anti-oxidant, anti-cancer, anti-inflammatory, anti-microbial, anti-tumor, anti-diabetes, and other essential bioactivities [105].
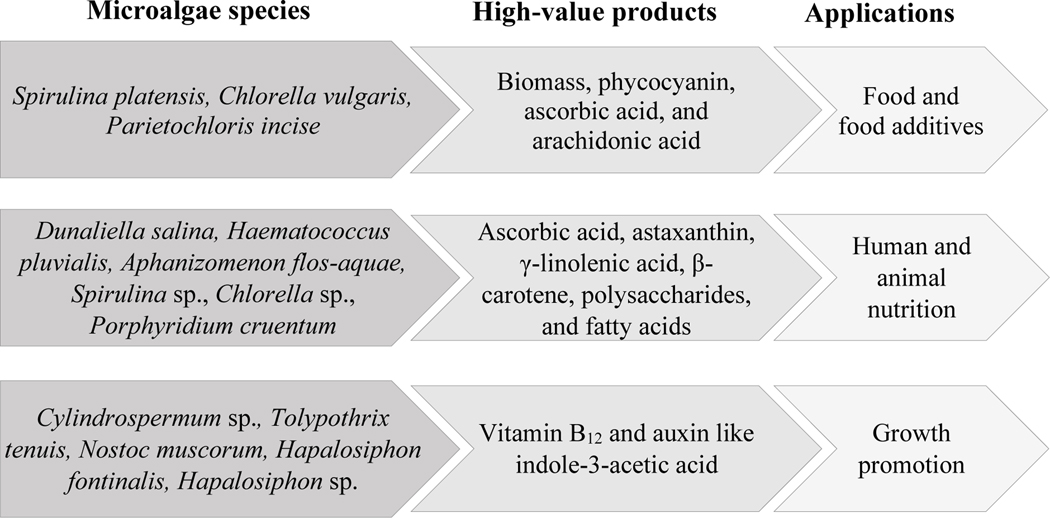
Phototrophic microorganisms can also be used as the source of other high-value bioactive compounds such as fatty acids, glycerol, vitamins, and polysaccharides [12, 100, 106]. Microalgal species such as Dunaliella tertiolecta, Nannochloropsis oculate, Spirulina platensis, Tetraselmis suecica, and Euglena gracilis are capable of producing vitamins [12]. Genetically modified Synechocystis sp., PCC6803, can produce a higher content of fatty acids (197 ± 14 mg/L) compared to its parental wild type (1.8 ± 0.06 mg/L) [106]. A shortlist of high-value products produced from microalgae species and their applications are presented in Fig. 2.
Fig. 2.
High-value products produced from microalgae species and their applications [13, 110–112].
Many studies have been conducted to increase the concentration of those compounds in microalgae and cyanobacteria, especially under stress conditions [12]. One study reported an increase in fatty acid yield when cultivating Chlorella vulgaris NIES-227 under nitrogen starved conditions [107]. Higher glycerol content can be achieved in green algae Dunaliella with increased salinity in the culture medium [108]. Alteration of the culture medium’s chemical composition can affect biomass, pigments, and fatty acid production. For instance, a 2000-fold increase in the astaxanthin yield was observed when 5.6% (v/v) of ethanol was used in the culture medium of Schizochytrium limacinum B4D1 [109].
3.2. Pharmaceutical and personal care products
Microalgae and cyanobacteria are promising sources of antibiotic, antimicrobial, neurotoxic, hepatotoxic, anti-oxidant, anti-cancer, and anti-inflammatory compounds [104, 110]. They can be a reliable source of many carotenoids (e.g., astaxanthin, lutein, canthaxanthin, and zeaxanthin), which are medicinally beneficial as well. The most common species of microalgae used for personal care products include Chlorella vulgaris, Spirulina platensis, Dunaliella salina, and Nannochloropsis oculata [110]. Caution should be taken, however, as some cyanobacteria contain various inherent toxins (dermatotoxins, hepatotoxins, cytotoxins, neurotoxins) [113]. For instance, cyanobacterial species such as Anabaena flos-aquae, Anabaena circinalis, Aphanizomenon sp., Cylindrospermum sp., Planktothrix sp., and Microcystis aeruginosa can produce anatoxin-a, which can potentially impact the nervous system [113].
Physical, genetic, and metabolic approaches have been employed to increase the production of various pharmaceuticals compounds from microalgae. The use of organic dye (Rhodamine 8G) can increase the chlorophyll and carotenoid concentrations in Chlamydomonas reinhardtii by 45 and 36 wt%, respectively [114]. Genetic and metabolic approaches mainly deal with the rate-limiting steps of the high-value compound production process to accelerate the process. For instance, a genetically modified Chlorella zofingiensis can produce 32.1% more total carotenoids and 54.1% more astaxanthin than its wild type [111]. In another study, down-regulation was used to enhance the lutein and zeaxanthin production in Chlamydomonas reinhardtii. By knocking out the gene for zeaxanthin epoxidase, the genetically modified Chlamydomonas reinhardtii had a much higher zeaxanthin content (56-fold) without interrupting lutein production [115]. Metabolic pathway modification by introducing a bkt gene from Haematococcus pluvialis encoding β-carotene ketolase increases ketocarotenoids production with maximum astaxanthin and canthaxanthin contents of 3.5 and 1.9 μg/g dry weight, respectively [112]. However, sometimes even successful genetic and metabolic transformations do not guarantee long-term stability. Also, modulation of carotenogenesis and its networking with other metabolic processes need further study for successful and stable modification [116].
3.3. Bioplastics and biopolymers
Bioplastics can be produced from lipids, protein, and carbohydrates, which makes phototrophic microorganisms the best fit because they are rich in those compounds [100]. The main bioplastic products with commercial-scale production are polyhydroxyalkanoates (PHAs), polyhydroxybutyrates (PHBs), polylactic acid (PLA), protein plastics, starch plastics, and cellulose plastics [100]. Spirulina sp., Nostoc sp., Oscillatoria sp., Synechocystis sp., and Calothrix sp. are recommended for PHA production, while phototrophic microorganisms with high carbohydrate content (e.g., Chlorella sp., Scenedesmus sp., Synechococcus sp.) are good for PLA production [117].
Accumulation of PHAs in different cyanobacterial and microalgal species under different growth conditions is summarized elsewhere [118]. Enhanced PHA and PHB production often occurs under nutrient-limiting conditions [119]. For instance, Nostoc muscorum Agardh was reported to yield 69% PHAs under phosphate-limited conditions [120]. However, the nutrient limitation can potentially slow down algal growth since cellular growth is directly related to the nutrient availability [119]. As such, genetic modification can be an alternative to inducing nutrient-limiting conditions to enhance PHA and PHB production without compromising the growth. In one study, overexpression of native biosynthetic genes in strain Synechocystis sp. PCC 6803 resulted in an observed 2.6-fold increase in PHB (26% of dry cell weight) compared to the wild type (10% of dry cell weight) [121]. In another study, Synechocystis sp. PCC6714 strain had a higher PHB yield (37 ± 4% dry cell weight) compared to that of the wild-type (14 ± 0.5% dry cell weight) when point mutations in the phosphate-specific transport system integral membrane protein-A and ABC-transport complex were introduced [122].
3.4. Other high-value products through phototrophic synthesis
Phototrophic microorganisms, including cyanobacteria and algae, are capable of synthesizing noble metal nanoparticles such as silver, gold, platinum, palladium, zirconium, titanium, and some of their oxides as well [123]. While conventional synthesis involves energy-intensive processes and toxic solvents, phototrophic synthesis of these nanomaterials, either extra- or intracellularly, can be a viable alternative [124, 125]. For instance, green microalga Coccomyxa actinabiotis can uptake and survive at the silver concentration range from 10−7 to 10−2 M Ag+, resulting in the accumulation of AgS and/or Ag nanoparticles that are dependent on Ag+ concentrations [126]. Even the lipid-extracted biomass has been reported being useful in the synthesis of silver nanoparticles [127]. The intracellular silver contents are 0.057 and 0.011 g/g biomass in Spirulina platensis and Nostoc linckia, respectively, when exposed to AgNO3 suspension [124]. Similarly, the rapid biosynthesis of gold nanoparticles with particle sizes ranging from 8 to 12 nm has been reported [128]. For example, Chlorella vulgaris can accumulate up to 0.014 g gold/g biomass per hour until it reaches a total intracellular gold concentration of 0.042 g gold/g biomass [124]. Some microalgal species demonstrated simultaneous accumulation of metallic nanomaterials, including rhodium (Rh), palladium (Pd), and platinum (Pt) [129].
Phototrophic microorganisms are also used to produce stable isotopes of biogenic elements (H, C, N, O, S, Mg, Se) [103]. These stable isotopes can later be extracted either chemically or by hydrolysis of biomass [103]. While they provide a model for studying the effect of isotopes on metabolic processes, deuterated algae can serve as a commercial source of fully deuterated compounds, such as glucose, amino acids, chlorophylls, and carotenoids [103].
Microalgae-derived biofertilizer could be another high-value product for maintaining soil fertility and sustainable crop production[130]. The application of N-fixing cyanobacteria and microalgae enhances crop yields while reducing the use of chemical fertilizers [102].
4. Emerging and innovative applications of phototrophic microorganisms for environmental conservation, mitigation, and remediation
Phototrophic microorganisms have a broad range of applications in the environment. Such applications include, but are not limited to, CO2 sequestration [15], environmental sensing and monitoring [131, 132], and wastewater treatment [17, 133]. This section of the review discusses the emerging and innovative use of phototrophic microorganisms for environmental conservation, mitigation, remediation, and other applications.
4.1. Environmental conservation
Phototrophic microorganisms have been used to develop tools for environmental conservation such as biosensors (for contaminant detection and water quality monitoring) and early warning of algal blooms [14, 131]. These emerging tools can further advance the monitoring and management of natural environments and ecological communities.
4.1.1. Biosensors for environmental monitoring
Biosensors are analytical devices with a bioreceptor for biological sensing and a transducer to transform the biochemical signal to optical-electrical interpretation [134]. A recent comprehensive review of biosensors is available elsewhere [135]. The bioreceptor can be either based on the whole cell or proteins, peptides, enzymes, nucleic acids, and antibodies [136]. Phototrophic microorganisms-based biosensors have been developed to detect toxic heavy metals, biocides, dioxin, volatile organic compounds (VOCs), organophosphorus compounds, endocrine disruptors, and monitor water quality [137–139].
Studies have reported the use of whole-cell microalgae Chlorella vulgaris to detect Cd2+, Dictyosphaerium chlorelloides to detect Cu2+, and Chlamydomonas reinhardtii to detect Ni2+ [138]. A biosensor developed using red alga Porphyridium cruentum can detect arsenic (III) at a concentration as low as 2.5 parts per billion [140]. More recently, a microalgae-living sensor has been developed for the detection of metal ions with a breakthrough detection limit of 50 nM using nanocavity-enhanced photoelectrochemical techniques [131]. Even BESs (more discussion in section 2.3) can be potential environmental biosensors where phototrophic microbial metabolism acts to sense the signals from the environment, and the decrease in the light-dependent electrical response via these microorganisms is proportional to the concentration of toxicants, including heavy metals [141]. Overall, efforts in developing biosensors using phototrophic microorganisms continue along with the use of other emerging technologies, including bioluminescent [142], photoelectrochemical, and bioelectrochemical processes. Details about the development of nucleic acid-based and enzyme-based biosensorsare summarized elsewhere [136, 143].
Microalgal biosensors are also used for selective detection of pollutants such as pesticides and herbicides [132, 144]. Specific oxygen production rate measurement is a powerful tool for determining the toxicity to algae and cyanobacteria [145]. Similarly, a lab-on-chip electrochemical biosensor has been developed based on oxygen production using a wild type Chlamydomonas reinhardtii to measure the concentration of herbicides with a detection limit of approximately 0.1 μM [137]. The marine green alga Ostreococcus tauri was also used to develop a recombinant biosensor to detect two antifouling biocides (Diuron and Irgarol 1051) through luminescence monitoring [146]. While most biosensors are designed to detect target compounds in an aqueous solution, some biosensors can also detect contaminants in aerosol form. One study used Chlorella vulgaris cells immobilized on the membrane of an oxygen electrode to detect perchloroethylene, a volatile organic compound. The concentration of the perchloroethylene aerosol was estimated based on the modified oxygen generation in the presence of perchloroethylene [134].
4.1.2. Early warning of harmful algal blooms
Harmful algal blooms (HABs) are the result of eutrophication and are deemed as one of the most serious environmental hazards [147, 148]. Early warning of the magnitude, timing, and location of HABs is important to the environment and human health. However, the complex microbial growth and dynamics of algal biomass in continuously changing environments make it challenging to determine and predict HABs using traditional statistical models (e.g., multiple linear regression, autoregressive moving average models) [149]. Nevertheless, while conventional models use multiple input parameters, one study used algal biomass as the sole input parameter to simplify the model and reduce cost. The developers claimed to predict algal blooms 1–3 days in advance by combining wavelet analysis with artificial neural networks [149]. Researchers have also developed phytoplankton models as well as phyco-pigment remote sensing methods for HAB prediction [14, 150, 151]. However, the phytoplankton models and remote sensing methods are unable to identify species composition, specific microcystin producers, and toxin production.
Understanding the possible cyanobacterial community succession and their relationship with cyanotoxin production remains a major challenge. Researchers have recently developed molecular techniques using quantitative polymerase chain reaction (qPCR) and quantitative reverse transcription PCR (RT-qPCR) to determine and predict toxic cyanobacteria, including the producers of microcystin, anatoxin, saxitoxin, and cylindrospermopsin in eutrophic waters. Based on the acquired data, an early warning method has been developed with predictive powers of up to 50–60%, while a one-week early warning of microcystin exceedance above the US Environmental Protection Agency allowable concentration can be attained [14]. This molecular approach to determine cyanobacteria and their genes for cyanotoxins is an attractive alternative method for the protection of source water as well as an early warning tool to drinking water treatment plants [14].
4.2. Environmental mitigation and remediation
Other than atmospheric CO2 sequestration (see Table 3 for details) [15], phototrophic microorganisms are showing promise in mitigation and remediation activities such as ammonia gas mitigation from swine and dairy farms [152], NOx and SOx sequestration from flue gas [153], antibiotic resistance abatement [17], and heavy metal removal [16]. Scenedesmus dimorphus can remove more than 95% of the ammonia gas from the inlet stream of animal production operations at the ammonia mass loading rate of 42.4 mg/(L∙d) in a photobioreactor operated at the hydraulic retention time of 10 d [152]. Microalgae are also used for removing NOx from flue gas and subsequently assimilating it as a nitrogen source [153]. Dunaliella tertiolecta exhibited 96% (v/v) removal of NOx from flue gas containing 100 parts per million NOx in the inlet gas using a counter-flow tubular algae column [154].
Table 3.
Carbon dioxide fixation by different phototrophic microorganisms from flue gas [155–160]. Partially adapted with permission from [153].
| Phototrophic microorganisms | Reactor type and size | Light intensity (μmol/m2/s) | Operation strategy | CO2 fixation rate (mg/L∙d) |
|---|---|---|---|---|
|
| ||||
| Acutodesmus sp. | Photobioreactor, 2 L | 30 | Continuous feeding; Batch | 190 |
| Botryococcus braunii | Flat plate, 30 L | 800 | Continuous feeding; Batch | 830 |
| Chlamydomonas reinhardtii UTEX 90 | Airlift photobioreactor, 2L | 100 | Continuous feeding; batch | 512 |
| Chlamydomonas reinhardtii CC 2656 | Airlift photobioreactor, 2L | 100 | Continuous feeding; batch | 266 |
| Chlorella emersonii | Air-lift column, 5.5 L | 200 | On-off feeding; semi-batch | 113 |
| Chlorella sp. MTF-7 | Bubble column, 50 L | Variable (outdoor) | On-off feeding; batch | 677 |
| Chlorella sp. MTF-15 | Bubble column, 1 L | 300 | Continuous feeding; batch | 877 |
| Chlorella sp. MTF-15 | Bubble column, 1200 L | Variable (outdoor) | On-off feeding; batch | 370 |
| Chlorella sp. Cv | Bubble column, 0.33 L | 100 | Continuous feeding; batch | 1200 |
| Chlorella vulgaris | Raceway pond, 60 L | Variable (outdoor) | Semi-continuous | 103 |
| Chlorella vulgaris | Bubble column, 0.3 L | 1150 | Continuous feeding; batch | 4400 |
| Chlorococcum infusionum | Photobioreactor, 0.5 L | 107 | Continuous feeding; batch | 95 |
| Isochrysis sp. | Photobioreactor, 10 L | Variable (outdoor) | Continuous feeding; batch | 350 |
| Nannochloropsis oceanic KA2 | Raceway pond, 8000 L | Variable (outdoor) | On-off feeding; batch | 25 |
| Nannochloropsis salina | Raceway pond, 600 L | Variable (outdoor) | Continuous feeding; batch | 60 |
| Scenedesmus dimorphus | Bubble column, 0.1 L | 100 | On-off feeding; batch | 889 |
| Scenedesmus vacuolatus | Bubble column, 2 L | 3000 | Continuous feeding | 1150 |
Microalgae are effective in removing organic pollutants, radionuclides, heavy metals, and emerging contaminants from water as well [16, 161, 162]. They can remove antibiotics from wastewater treatment plant effluent through assimilation, adsorption, and/or degradation [17]. One study reported that the freshwater alga, Scenedesmus dimorphus, can remove up to 93% of ciprofloxacin from impaired water. Scenedesmus was capable of removing more than 93% of 17 β-estradiol in both laboratory and pilot-plant photobioreactor studies, although the bioremediation mechanisms such as bio-adsorption, bio-uptake, and biodegradation and their relative importance are yet to be determined [161, 163].
Species of the genera Anabaena, Chlorella, Cladophora, Oscillatoria, and Scenedesmus are known for their hyper-accumulation and hyper-adsorbing capabilities of heavy metals [161]. For example, a Chlorella strain can accumulate cadmium (Cd) up to 65% of its organic mass from a Cd-rich suspension [164]. Owing to their high absorption capacity, algae can be used to treat acid-mine drainage. For instance, copper (Cu) was mainly removed (approximately 90%) through microalgal metabolism (e.g., metal ions transferred across the membrane via particular organelles such as vacuoles and retained intracellularly) while the remaining Cu (10.70 ± 1.92%) was removed by adsorption in a batch study of treating acid mine water using the marine microalga Nannochloropsis oculata [165]. Similarly, algae have shown promising results in arsenic (As) removal with high arsenic uptake capacities (bioaccumulation factor of approximately 390 L/kg in 2 hours) [166]. Arsenic accumulation in Chlorella pyrenoidosa is dependent on the species (e.g., As(III), As (V)) and concentration of As with As (V) having higher uptake rate constants than As (III) [166]. Extracellular polymeric substances produced by the algae enhances adsorption, reduces intracellular As, and increases inorganic As tolerance of the algae [166].
New applications of phototrophic microorganisms for remediation and degradation of pollutants continue to grow. For instance, phototrophic microorganisms are deployed to treat reverse osmosis concentrate [167, 168]. The brackish water diatom Pseudostaurosira trainorii PEWL001 can remove 95% of reactive silica and 64% of calcium from the reverse osmosis concentrate [167]. Chlorella sp. ZTY4 and Scenedesmus sp. LX1 have been proven to have higher adaptability to simultaneously remove nitrogen, phosphorous, and hardness from reverse osmosis concentrate [168].
4.3. Other environmental applications
Several critical reviews on phototrophic microorganisms such as algae and cyanobacteria and their environmental applications are available [169–171]. More and more applications of phototrophic microorganisms are blooming for environmentally friendly solutions and sustainable development. For example, holistic approaches are now being adopted to improve indoor air quality and energy efficiency. Bio-reactive façade integrated into buildings can facilitate the photosynthesis process by acting as solar-thermal cells, absorb indoor air pollutants (including CO2), and produce renewable energy in the form of biomass [172]. They also provide shade, insulation, and natural ventilation, along with energy production. The BIQ building in Hamburg, Germany, is the first algae-powered building in the world. Since it’s inception, it has been closely monitored for performance and energy efficiency. After the first year of operation, the façade system of this building exhibited a conversion efficiency of 58% (10% biogas and 48% heat), and approximately 4500 kWh of electricity was generated by the 200 m2 algae façade [172]. It can reduce carbon directly by photosynthetic uptake and indirectly by reducing electrical demand. The reduction of CO2 by the 200 m2 façade of the BIQ building is estimated to be up to 6 tons/year [172]. Another building (Marina City Towers, Chicago, Illinois) was also renovated to integrate an on-site algal bioreactor that captures CO2 from the surrounding air using humidity swings [172]. However, one major challenge in adopting the microalgal façades and/or algae-urban system is the on-site provision of bioreactors and biorefinery infrastructures. Supply of light, CO2, nutrients, and water, as well as harvesting and extraction facilities, must be integrated as on-site infrastructure to avoid energy loss during transportation. Also, the structural viability and load of the microalgal façades needs to be considered during the architectural and engineering design of the building.
5. Challenges and research perspectives for applying phototrophic microorganisms and technologies
The challenges of using phototrophic microorganisms to harness solar energy for bioenergy, biomaterials, and environmental applications are substantial. The reported photosynthetic energy conversion efficiencies in current operations (~1%) are much lower than the theoretical maximum (~12%) [5]. In addition, low biomass yield and poor environmental adaptability of many algal and cyanobacterial strains remain a concern [40, 110]. It is also challenging to maintain a monospecific culture in the outdoor environment where it is prone to contamination with other indigenous species [173]. Moreover, the growth and metabolism of the phototrophic microorganisms are further affected by seasonal shifts [10, 174].
Perhaps the most critical barrier is the high cultivation, harvesting, and extraction costs [64]. While large-scale open ponds offer low-cost, simple, and easy to maintain cultivation of microalgae,they have limited utilization of CO2 and light, poor mixing, and prone to contamination [64]. By contrast, photobioreactors offer better control over key microalgal cultivation parameters but at high capital and operational costs. Current harvesting practices such as filtration, flotation, centrifugation, flocculation, and magnetic separation are expensive and have a high energy requirement as well, which in turn adds up to the production cost [175]. Hybrid refinery strategies such as combining biodiesel production with bio-products may improve the marketability of microalgae as a viable alternative and sustainable source [2, 110]. Also, the cultivation process integrated with wastewater treatment can improve the economic feasibility and environmental sustainability by reducing/eliminating the external nutrient requirements for cell growth and lowering overall waste discharge costs [176, 177]. However, using wastewater as the nutrient source can potentially be difficult due to high turbidity, fluctuating nutrient concentrations, the presence of contaminants such as toxic metals, pesticides, antibiotics, and undesired microorganisms [178].
Developing a biomaterial extraction process without dewatering requirements can reduce the production cost further but, there is still an ongoing debate about the potential adverse environmental effects of biofuels (biodiesel) and their production process and consumption [59, 179]. For instance, genetically modified strains can invade the natural habitat and may produce harmful toxins harming other organisms in the ecosystem [180]. More research is thus needed to overcome these challenges while minimizing the adverse effects of employing phototrophic microorganisms for bioenergy, biomaterial production, and environmental applications. Research perspectives and some promising research fields are discussed and described below.
5.1. New discoveries and applications of microbial phototrophs, particularly anoxygenic phototrophs, for harnessing solar energy
While oxygenic phototrophs such as algae and cyanobacteria are studied extensively, much less work has been done on anoxygenic phototrophs. For instance, AAP bacteria (see discussion in Section 1.1) are photoheterotrophic species that exist in a variety of aquatic environments [34, 181] and are critical to the cycling of both organic and inorganic carbon in the ocean [182]. Anoxygenic phototrophs have the unique ability to live in extreme conditions of temperature, pressure, pH, and salinity, can co-exist with oxygenic phototrophs in the same environment, and survive under both light-anaerobic or dark-aerobic conditions [183]. The light-derived energy facilitates their faster growth than heterotrophs [181], and yet their ecological role and significant attributes remain to be fully understood.
Purple phototrophic bacteria (see information in Section 1.1) are capable of growing photoheterotrophically with a high biomass yield (1 g COD biomass/g COD removed) [184]. Their biomass is highly preferable as the protein-rich feed for animal (e.g., livestock) consumption. Like oxygenic phototrophs, anoxygenic phototrophs have also been used for wastewater treatment to remove heavy metals, macro pollutants, dyes, as well as for producing electricity, bioplastics, and other value-added products [133, 183, 185, 186]. Some anoxygenic phototrophs have azo-reductase activities to remove azo-dyes (textile chemical) from wastewater. For example, Rhodopseudomonas palustris (AS1.2352) can remove the azo-dye under anaerobic conditions at a pH of 8. Anoxygenic microorganisms Rhodobacter blasticus, R. adriaticus, R. palustris, Rhodopseudomonas capsulatus, and Rhodovulum strictrum can also remove 96% of the dye within 2 days under anaerobic light conditions [183].
One possible way to improve the biomass yield is to co-culture both the oxygenic and anoxygenic phototrophs as they do not compete for the same light spectrum. Researchers have proposed a dichroic beam sharing technique for better light management [187]. They used this method to illuminate both Rhodobacter sphaeroides (anoxygenic) and Arthrospira platensis (oxygenic) at the same time and achieved a 71% increase in the overall photosynthetic activities. As the oxygen generated by the oxygenic metabolism may inhibit the growth of anoxygenic phototrophs, a process that separates oxygen-producing photosynthesis from the oxygen-sensitive proton reduction reaction both spatially and chronologically should be considered [188].
Algae-bacteria consortia, especially for biological hydrogen production [189], promote (i) direct biophotolysis (to split water molecules to hydrogen ion and oxygen via photosynthesis followed by converting H+ hydrogen gas by hydrogenase enzyme in microalgae such as Chlamydomonas reinhardtii), (ii) photofermentation (by purple non-sulfur photosynthetic bacteria that directly capture solar energy to drive H2 production from organic compounds), (iii) indirect biophotolysis (photosynthetic carbon fixation followed by the conversion of the stored carbohydrate to hydrogen by cyanobacteria) [190], and (iv) dark fermentation (by anaerobic fermentative bacteria) [190]. Recent research on bioelectrochemical systems that separate fast-growing AAP bacteria (in the anodic chamber) from algae (in algae-assisted MFCs) has shown great promise in improving energy and electricity production [191].
5.2. Genetic, metabolic, and process engineering to maximize the yield of phototrophic biomass along with functional structures and metabolites
Enhancing phototrophic biomass yield and metabolite concentration is key to the economical production of bioenergy and biomaterials [192]. From a research perspective, developing new strains via genetic and metabolic engineering approaches that can be grown easily and offer better environmental adaptability would be the first step toward enhancing biomass yield and metabolite concentration [7, 193]. Novel genome editing tools such as TALENs and CRISPR/Cas9 are used because of their rapid and precise mutagenesis capability for the desired products [194, 195]. Multiple efforts have been made to increase photosynthetic activity, light-harvesting efficiency, biomass accumulation, and concentration of metabolites [50, 196, 197], along with omics approaches to help increase biorefinery capabilities [194]. The truncated antenna size (see more discussion in Section 2.2.1) resulted in 44.5% greater biomass productivity under high light conditions [197]. Truncated chlorophyll antenna is capable of reducing the oxygen sensitivity of certain enzymes, such as hydrogenase, for greater hydrogen production [50].
Enhancing lipid metabolism is the goal when producing biofuels [106, 107]. Genetic transformation, including multi-gene manipulation and enzyme modification, is necessary for improving lipid production [198]. Comprehensive reviews on genome engineering tools for exploring and exploiting the metabolism of these organisms are available elsewhere [194, 195, 199, 200]. It is expected that more cutting-edge genome-editing technologies will be developed towards improved product-based genetic modification. Caution should be made, however, to avoid introducing genetically modified phototrophs into the natural ecosystem through strict regulation, sterilization, and monitoring, as they can potentially emerge as invasive species to that ecosystem.
Aside from cultivation, downstream processes such as harvesting, extraction, and purification are the most expensive steps in producing high-value compounds. The harvesting process is responsible for 20–30% of the total cost to produce microalgae [201]. Auto-flocculation can be a viable method for efficient harvesting, removing approximately 90% of suspended algal biomass [202]. It is mediated by the interaction of algal organic matter and the residual salts present in the algal culture [203]. Co-culturing algae (Chlorella vulgaris) and bioflocculant-producing bacteria (Rhizobium radiobacter) may be considered to facilitate harvesting [204]. The conventional solvent extraction method based on the “like dissolves like” concept is cheap and easy but requires a longer time and additional treatments. Super-/sub-critical solvent extraction can be a quick and efficient alternative. For instance, sub-critical extraction is a convenient way to extract high-value compounds from algal cells. Unlike supercritical extraction, it does not require high pressure to enhance the diffusivity of the solvent. One major problem with sub-critical extraction is that the high temperature may destroy the functional activity of the extracted compounds [205].
5.3. Integration of phototrophic microorganisms with emerging technologies
Emerging technologies will continue to drive future innovations for the growing microbial/algae industry, with several important research topics listed below.
Prediction and control of HABs with machine learning techniques, geographic information system (GIS), 5G wireless network technology, and molecular technologies. The application of machine learning techniques, along with the use of a historical record of HAB events obtained from GIS and physical monitoring, can help developing state-of-the-art detection systems for spatial and temporal characteristics of HAB events and the accurate prediction of HABs [206]. Along with the use of other emerging technologies such as 5G and artificial intelligence (AI), maximum detection and prediction accuracy can be achieved with this new HAB detection and prediction system for implementing HAB control and prevention measures.
Phototrophic microbial growth with membrane technology. Raceway ponds are the most commonly used outdoor facilities for the cultivation of microalgae and other phototrophic microorganisms. With the development of membrane technology and decreasing the cost of membrane materials, membrane bioreactors can decouple solids retention time (SRT) from hydraulic retention time (HRT) for high-density algal cultivation with benefits including improved biomass productivity, photosynthetic efficiency, and nutrient (phosphorus) recovery [207–210].
Improving microalgal cultivation and harvesting using nanotechnology. Recent efforts include supplementing nanoparticles as micronutrients, lipid inducer, and protector of cells against hostile environments [211]. The use of nanoparticles reportedly enhanced cell growth, biomass content, and biomethane potential as well [212]. A 47.2% increased production of carotenoids was achieved by internalizing gold nanoparticles into Chlorella zofingiensis cells [213]. High-efficiency (more than 95%) and low-cost magnetic harvesting were achieved by in-situ formation of Fe nanoparticles on the microalgal surface [214]. Integrating nanoparticles with membrane technology (i.e., cellulose-based scaffolds modified with TiO2 nanoparticles) can significantly increase microalgal growth by improving scaffold properties [215]. However, nanoparticle stability, cost-effective recycling procedures, and their environmental impacts are yet to be fully understood [211].
The exploitation of diatom nanotechnology. As single-celled photosynthetic microalgae, diatoms have species-specific cell walls (frustules) with distinct microporous to nanoporous siliceous structures. Diatoms are thus powerful tools in nanotechnology to manufacture nanostructured smart biomaterials such as DNA–silica hybrid materials with complex geometric structures [216]. Diatoms and silica nanotechnology along with other technologies (e.g., microfluidic technologies) are utilized for biosensing, drug and gene delivery, biopharmaceutical, and other value-added products [217, 218]. Also, titanium incorporated hierarchical structures of diatoms have shown promising results for selective removal of pharmaceuticals and personal care products from water [219].
Novel uses of phototrophic microorganisms in wastewater treatment. Examples of such innovative uses include, but are not limited to: (a) wastewater treatment with PPB in a continuous photo anaerobic membrane bioreactor [220], (b) forward osmosis with an algal draw solution to concentrate municipal wastewater and recover resources [221], (c) protein production from wastewater with phototrophic microorganisms by assimilating and up-cycling nutrients in wastewater [222], and (d) a new generation of energy-neutral [223] or even energy-positive wastewater treatment [224].
6. Conclusions
Phototrophic microorganisms have the potential to be a clean and sustainable means for harnessing solar energy and converting it to bioenergy, biomaterials, and high-value products. These microorganisms have broad environmental applications as well. However, large-scale production of these products is still limited because of the low biomass yield and high production costs of phototrophic microorganisms. Therefore, more research and economically feasible strategies are needed for commercialization. Future research perspectives, such as integrating phototrophic microorganisms with emerging technologies, are proposed. Emphasis should be given to integrating phototrophic microorganisms with nanotechnology to increase cell growth and metabolic contents and make downstream processes (i.e., harvesting, extraction, purification) more cost-effective. The exploitation of diatom nanotechnology is especially promising for not only manufacturing nanostructured smart, cheap biomaterials, but also opening new directions for sustainability and environmental solutions. Nonetheless, efforts to be made towards minimizing the adverse environmental impacts when adapting to these new technologies.
Highlights.
Both oxygenic and anoxygenic phototrophs convert solar energy to renewable energy.
Microbial phototrophs are a source of biofuels, biomaterials, and high-value products.
Microbial phototrophs provide solutions for environmental conservation and remediation.
Barriers in harnessing solar energy using phototrophic microorganisms are presented.
Research to integrate microbial phototrophs with emerging technologies is discussed.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Permission is attained for all copyrighted graphics, images, tables, and figures. The contribution by Jingrang Lu is through the USEPA Office of Research and Development’s (ORD’s) research programs: Safe and Sustainable Water Resources (SSWR: SSWR 4.2.2 and 4.3.1) in the research described here. The views expressed in this manuscript are those of authors and do not necessarily reflect on USEPA ORD. It has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
List of abbreviations, units, and nomenclature
- AAP
Aerobic Anoxygenic Phototrophs
- ADP
Adenosine Diphosphate
- ATP
Adenosine Triphosphate
- BES
Bioelectrochemical System
- BPV
Biophotovoltaic
- COD
Chemical Oxygen Demand
- DNA
Deoxyribonucleic Acid
- GIS
Geographic Information System
- HAB
Harmful Algal Bloom
- MEC
Microbial Electrolysis Cell
- MFC
Microbial Fuel Cell
- MJ
Megajoule
- NADP
Nicotinamide Adenine Dinucleotide Phosphate
- NER
Net Energy Ratio
- PBR
Photobioreactor
- PHAs
Polyhydroxyalkanoates
- PHBs
Polyhydroxybutyrates
- PLA
Polylactic Acid
- PPB
Purple Phototrophic Bacteria
- PS
Photosystem
- qPCR
Quantitative Polymerase Chain Reaction
- RC
Reaction Center
- ROS
Reactive Oxygen Species
- RT-qPCR
Quantitative Reverse Transcription Polymerase Chain Reaction
- v/v
Volume/Volume
- VOCs
Volatile Organic Compounds
- w/w
Weight/Weight
References
- [1].Junge W. Oxygenic photosynthesis: history, status and perspective. Q Rev Biophys. 2019;52:1–17. [DOI] [PubMed] [Google Scholar]
- [2].Goh BHH, Ong HC, Cheah MY, Chen W-H, Yu KL, Mahlia TMI. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew Sustain Energy Rev. 2019;107:59–74. [Google Scholar]
- [3].Abu-Ghosh S, Dubinsky Z, Verdelho V, Iluz D. Unconventional high-value products from microalgae: A review. Bioresource Technology. 2021;329:124895. [DOI] [PubMed] [Google Scholar]
- [4].Hussain F, Shah SZ, Ahmad H, Abubshait SA, Abubshait HA, Laref A, et al. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renewable and Sustainable Energy Reviews. 2021;137:110603. [Google Scholar]
- [5].Ooms MD, Dinh CT, Sargent EH, Sinton D. Photon management for augmented photosynthesis. Nat Commun. 2016;7:12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anyanwu RC, Rodriguez C, Durrant A, Olabi AG. Micro-macroalgae properties and applications. Reference Module in Materials Science and Materials Engineering: Elsevier Inc.; 2018. p. 1–28. [Google Scholar]
- [7].Ghosh A, Khanra S, Mondal M, Halder G, Tiwari ON, Saini S, et al. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Convers Manag. 2016;113:104–18. [Google Scholar]
- [8].Zhu LD, Hiltunen E, Antila E, Zhong JJ, Yuan ZH, Wang ZM. Microalgal biofuels: Flexible bioenergies for sustainable development. Renew Sustain Energy Rev. 2014;30:1035–46. [Google Scholar]
- [9].Nwoba EG, Mickan BS, Moheimani NR. Chlorella sp. growth under batch and fed-batch conditions with effluent recycling when treating the effluent of food waste anaerobic digestate. Journal of Applied Phycology. 2019;31:3545–56. [Google Scholar]
- [10].Saba B, Christy AD, Yu Z, Co AC. Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): An overview. Renew Sustain Energy Rev. 2017;73:75–84. [Google Scholar]
- [11].Shuba Eyasu S, Kifle D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew Sustain Energy Rev. 2018;81:743–55. [Google Scholar]
- [12].Markou G, Nerantzis E. Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv. 2013;31:1532–42. [DOI] [PubMed] [Google Scholar]
- [13].Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol Adv. 2018;36:54–67. [DOI] [PubMed] [Google Scholar]
- [14].Lu J, Struewing I, Wymer L, Tettenhorst DR, Shoemaker J, Allen J. Use of qPCR and RT-qPCR for monitoring variations of microcystin producers and as an early warning system to predict toxin production in an Ohio inland lake. Water Res. 2020;170:115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Venkata Mohan S, Modestra JA, Amulya K, Butti SK, Velvizhi G. A circular bioeconomy with biobased products from CO2 sequestration. Trends Biotechnol. 2016;34:506–19. [DOI] [PubMed] [Google Scholar]
- [16].Suresh Kumar K, Dahms HU, Won EJ, Lee JS, Shin KH. Microalgae - A promising tool for heavy metal remediation. Ecotoxicol Environ Saf. 2015;113:329–52. [DOI] [PubMed] [Google Scholar]
- [17].Leng L, Wei L, Xiong Q, Xu S, Li W, Lv S, et al. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere. 2020;238:124680. [DOI] [PubMed] [Google Scholar]
- [18].Hanada S. Anoxygenic photosynthesis -A photochemical reaction that does not contribute to oxygen reproduction. Microbes Environ. 2016;31:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spalding MH. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot. 2008;59:1463–73. [DOI] [PubMed] [Google Scholar]
- [20].Hamilton TL. The trouble with oxygen: The ecophysiology of extant phototrophs and implications for the evolution of oxygenic photosynthesis. Free Radic Biol Med. 2019;140:233–49. [DOI] [PubMed] [Google Scholar]
- [21].Johnston DT, Wolfe-Simon F, Pearson A, Knoll AH. Anoxygenic photosynthesis modulated Proterozoic oxygen and sustained Earth’s middle age. Proc Natl Acad Sci U S A. 2009;106:16925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Planavsky NJ, Crowe SA, Fakhraee M, Beaty B, Reinhard CT, Mills BJW, et al. Evolution of the structure and impact of Earth’s biosphere. Nature Reviews Earth & Environment. 2021;2:123–39. [Google Scholar]
- [23].Zeng Y, Selyanin V, Lukeš M, Dean J, Kaftan D, Feng F, et al. Characterization of the microaerophilic, bacteriochlorophyll a-containing bacterium Gemmatimonas phototrophica sp. nov., and emended descriptions of the genus Gemmatimonas and Gemmatimonas aurantiaca. Int J Syst Evol Microbiol. 2015;65:2410–9. [DOI] [PubMed] [Google Scholar]
- [24].Ozaki K, Thompson KJ, Simister RL, Crowe SA, Reinhard CT. Anoxygenic photosynthesis and the delayed oxygenation of Earth’s atmosphere. Nat Commun. 2019;10:3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Adessi A, De Philippis R. Purple bacteria: Electron acceptors and donors. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry (Second Edition). Waltham: Academic Press; 2013. p. 693–9. [Google Scholar]
- [26].Nelson N. Photosystems and global effects of oxygenic photosynthesis. Biochim Biophys Acta-Bioenergetics 2011;1807:856–63. [DOI] [PubMed] [Google Scholar]
- [27].Chen M, Blankenship RE. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci. 2011;16:427–31. [DOI] [PubMed] [Google Scholar]
- [28].Ferrera I, Gasol JM, Sebastián M, Hojerová E, Koblížek M. Comparison of growth rates of aerobic anoxygenic phototrophic bacteria and other bacterioplankton groups in coastal mediterranean waters. Appl Environ Microbiol. 2011;77:7451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferrera I, Sánchez O, Kolářová E, Koblížek M, Gasol JM. Light enhances the growth rates of natural populations of aerobic anoxygenic phototrophic bacteria. The ISME Journal. 2017;11:2391–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schediwy K, Trautmann A, Steinweg C, Posten C. Microalgal kinetics — a guideline for photobioreactor design and process development. Eng Life Sci. 2019;19:830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shimakawa G, Matsuda Y, Nakajima K, Tamoi M, Shigeoka S, Miyake C. Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci Rep. 2017;7:41022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hohmann-Marriott MF, Blankenship RE. Evolution of photosynthesis. Annu Rev Plant Biol. 2011;62:515–48. [DOI] [PubMed] [Google Scholar]
- [33].Visscher PT, van Gemerden H. Photo-autotrophic growth of Thiocapsa roseopersicina on dimethyl sulfide. FEMS Microbiol Lett. 1991;81:247–50. [Google Scholar]
- [34].Imhoff JF. Anoxygenic phototrophic bacteria from extreme environments. In: Hallenbeck PC, editor. Modern topics in the phototrophic prokaryotes. Switzerland: Springer International Publishing; 2017. p. 427–80. [Google Scholar]
- [35].Losic D, Mitchell JG, Voelcker NH. Diatomaceous lessons in nanotechnology and advanced materials. Adv Mater. 2009;21:2947–58. [Google Scholar]
- [36].Junge W, Nelson N. ATP synthase. Annu Rev Biochem. 2015;84:631–57. [DOI] [PubMed] [Google Scholar]
- [37].Halim R, Gladman B, Danquah MK, Webley PA. Oil extraction from microalgae for biodiesel production. Bioresour Technol. 2011;102:178–85. [DOI] [PubMed] [Google Scholar]
- [38].Daroch M, Geng S, Wang G. Recent advances in liquid biofuel production from algal feedstocks. Appl Energy. 2013;102:1371–81. [Google Scholar]
- [39].Khetkorn W, Rastogi RP, Incharoensakdi A, Lindblad P, Madamwar D, Pandey A, et al. Microalgal hydrogen production - A review. Bioresour Technol. 2017;243:1194–206. [DOI] [PubMed] [Google Scholar]
- [40].Zabed HM, Akter S, Yun J, Zhang G, Zhang Y, Qi X. Biogas from microalgae: Technologies, challenges and opportunities. Renew Sustain Energy Rev. 2020;117:109503. [Google Scholar]
- [41].Sun XW, Zhang YX, Losic D. Diatom silica, an emerging biomaterial for energy conversion and storage. J Mater Chem A. 2017;5:8847–59. [Google Scholar]
- [42].Lam MK, Lee KT. Chapter 12 - Bioethanol production from microalgae. In: Kim S-K, editor. Handbook of Marine Microalgae. Boston: Academic Press; 2015. p. 197–208. [Google Scholar]
- [43].Chen H, Zhou D, Luo G, Zhang S, Chen J. Macroalgae for biofuels production: Progress and perspectives. Renew Sustain Energy Rev. 2015;47:427–37. [Google Scholar]
- [44].Voloshin RA, Rodionova MV, Zharmukhamedov SK, Nejat Veziroglu T, Allakhverdiev SI. Review: Biofuel production from plant and algal biomass. Int J Hydrogen Energy. 2016;41:17257–73. [Google Scholar]
- [45].Rodionova MV, Poudyal RS, Tiwari I, Voloshin RA, Zharmukhamedov SK, Nam HG, et al. Biofuel production: Challenges and opportunities. Int J Hydrogen Energy. 2017;42:8450–61. [Google Scholar]
- [46].Ho SH, Ye X, Hasunuma T, Chang JS, Kondo A. Perspectives on engineering strategies for improving biofuel production from microalgae--a critical review. Biotechnol Adv. 2014;32:1448–59. [DOI] [PubMed] [Google Scholar]
- [47].Batan L, Quinn J, Willson B, Bradley T. Net energy and greenhouse gas emission evaluation of biodiesel derived from microalgae. Environ Sci Technol. 2010;44:7975–80. [DOI] [PubMed] [Google Scholar]
- [48].Halim R, Danquah MK, Webley PA. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol Adv. 2012;30:709–32. [DOI] [PubMed] [Google Scholar]
- [49].Faried M, Samer M, Abdelsalam E, Yousef RS, Attia YA, Ali AS. Biodiesel production from microalgae: Processes, technologies and recent advancements. Renew Sustain Energy Rev. 2017;79:893–913. [Google Scholar]
- [50].Majidian P, Tabatabaei M, Zeinolabedini M, Naghshbandi MP, Chisti Y. Metabolic engineering of microorganisms for biofuel production. Renew Sustain Energy Rev. 2018;82:3863–85. [Google Scholar]
- [51].Miller R, Wu G, Deshpande RR, Vieler A, Gärtner K, Li X, et al. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol. 2010;154:1737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Banerjee C, Dubey KK, Shukla P. Metabolic engineering of microalgal based biofuel production: Prospects and challenges. Front Microbiol. 2016;7:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Trentacoste EM, Shrestha RP, Smith SR, Glé C, Hartmann AC, Hildebrand M, et al. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci U S A 2013;110:19748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lü J, Sheahan C, Fu P. Metabolic engineering of algae for fourth generation biofuels production. Energy Environ Sci. 2011;4:2451–66. [Google Scholar]
- [55].Shirvani T, Yan X, Inderwildi OR, Edwards PP, King DA. Life cycle energy and greenhouse gas analysis for algae-derived biodiesel. Energy Environ Sci. 2011;4:3773–8. [Google Scholar]
- [56].Lee S, Oh Y, Kim D, Kwon D, Lee C, Lee J. Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl Biochem Biotechnol. 2011;164:878–88. [DOI] [PubMed] [Google Scholar]
- [57].Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact. 2018;17:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Abomohra AE-F, Elshobary M. Biodiesel, bioethanol, and biobutanol production from microalgae. In: Alam MA, Wang Z, editors. Microalgae biotechnology for development of biofuel and wastewater treatment. Singapore: Springer Singapore; 2019. p. 293–321. [Google Scholar]
- [59].Menetrez MY. An overview of algae biofuel production and potential environmental impact. Environ Sci Technol. 2012;46:7073–85. [DOI] [PubMed] [Google Scholar]
- [60].Dexter J, Fu P. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ Sci. 2009;2:857–64. [Google Scholar]
- [61].Nguyen MT, Choi SP, Lee J, Lee JH, Sim SJ. Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol. 2009;19:161–6. [DOI] [PubMed] [Google Scholar]
- [62].Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26. [DOI] [PubMed] [Google Scholar]
- [63].He Y, Bagley DM, Leung KT, Liss SN, Liao B-Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol Adv. 2012;30:817–58. [DOI] [PubMed] [Google Scholar]
- [64].Peng L, Fu D, Chu H, Wang Z, Qi H. Biofuel production from microalgae: a review. Environ Chem Lett. 2019;18:285–97. [Google Scholar]
- [65].Bahadar A, Bilal Khan M. Progress in energy from microalgae: A review. Renew Sustain Energy Rev. 2013;27:128–48. [Google Scholar]
- [66].Tan CH, Show PL, Chang JS, Ling TC, Lan JC. Novel approaches of producing bioenergies from microalgae: A recent review. Biotechnol Adv. 2015;33:1219–27. [DOI] [PubMed] [Google Scholar]
- [67].Vasiliadou IA, Berná A, Manchon C, Melero JA, Martinez F, Esteve-Nuñez A, et al. Biological and bioelectrochemical systems for hydrogen production and carbon fixation using purple phototrophic bacteria. Front Energy Res. 2018;6:107. [Google Scholar]
- [68].Bothe H, Schmitz O, Yates MG, Newton WE. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol Mol Biol Rev. 2010;74:529–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Akroum-Amrouche D, Abdi N, Lounici H, Mameri N. Effect of physico-chemical parameters on biohydrogen production and growth characteristics by batch culture of Rhodobacter sphaeroides CIP 60.6. Appl Energy. 2011;88:2130–5. [Google Scholar]
- [70].Alam MA, Wang Z. Microalgae biotechnology for development of biofuel and wastewater treatment. Singapore: Springer Nature Switzerland AG; 2019. [Google Scholar]
- [71].Ghosh D, Sobro IF, Hallenbeck PC. Optimization of the hydrogen yield from single-stage photofermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Bioresour Technol. 2012;123:199–206. [DOI] [PubMed] [Google Scholar]
- [72].Bolatkhan K, Kossalbayev BD, Zayadan BK, Tomo T, Veziroglu TN, Allakhverdiev SI. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int J Hydrogen Energy. 2019;44:5799–811. [Google Scholar]
- [73].Eroglu E, Melis A. Photobiological hydrogen production: Recent advances and state of the art. Bioresour Technol. 2011;102:8403–13. [DOI] [PubMed] [Google Scholar]
- [74].Khanna N, Raleiras P, Lindblad P. Fundamentals and recent advances in hydrogen production and nitrogen fixation in cyanobacteria. In: Borowitzka M, Beardall J, J R, editors. The physiology of microalgae. Cham: Springer International Publishing; 2016. p. 101–27. [Google Scholar]
- [75].Rumpel S, Siebel JF, Farès C, Duan J, Reijerse E, Happe T, et al. Enhancing hydrogen production of microalgae by redirecting electrons from photosystem I to hydrogenase. Energy Environ Sci. 2014;7:3296–301. [Google Scholar]
- [76].Kosourov S, Patrusheva E, Ghirardi ML, Seibert M, Tsygankov A. A comparison of hydrogen photoproduction by sulfur-deprived Chlamydomonas reinhardtii under different growth conditions. J Biotechnol. 2007;128:776–87. [DOI] [PubMed] [Google Scholar]
- [77].Kosourov SN, Seibert M. Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng. 2009;102:50–8. [DOI] [PubMed] [Google Scholar]
- [78].Kosourov S, Jokel M, Aro E-M, Allahverdiyeva Y. A new approach for sustained and efficient H2 photoproduction by Chlamydomonas reinhardtii. Energy Environ Sci. 2018;11:1431–6. [Google Scholar]
- [79].Kosourov SN, Ghirardi ML, Seibert M. A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int J Hydrogen Energy. 2011;36:2044–8. [Google Scholar]
- [80].Fino D. Hydrogen production in conventional, bio-based and nuclear power plants. In: Basile A, Iulianelli A, editors. Advances in hydrogen production, storage and distribution. Cambridge: Woodhead Publishing; 2014. p. 85–122. [Google Scholar]
- [81].Tijani H, Abdullah N, Yuzir A. Integration of microalgae biomass in biomethanation systems. Renew Sustain Energy Rev. 2015;52:1610–22. [Google Scholar]
- [82].Bose A, Lin R, Rajendran K, O’Shea R, Xia A, Murphy JD. How to optimise photosynthetic biogas upgrading: a perspective on system design and microalgae selection. Biotechnol Adv. 2019;37:107444. [DOI] [PubMed] [Google Scholar]
- [83].McCormick AJ, Bombelli P, Bradley RW, Thorne R, Wenzel T, Howe CJ. Biophotovoltaics: oxygenic photosynthetic organisms in the world of bioelectrochemical systems. Energy Environ Sci. 2015;8:1092–109. [Google Scholar]
- [84].Pant D, Singh A, Van Bogaert G, Irving Olsen S, Singh Nigam P, Diels L, et al. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012;2:1248–63. [Google Scholar]
- [85].Yamashita T, Hayashi T, Iwasaki H, Awatsu M, Yokoyama H. Ultra-low-power energy harvester for microbial fuel cells and its application to environmental sensing and long-range wireless data transmission. J Power Sources. 2019;430:1–11. [Google Scholar]
- [86].Yang E, Chae K-J, Choi M-J, He Z, Kim IS. Critical review of bioelectrochemical systems integrated with membrane-based technologies for desalination, energy self-sufficiency, and high-efficiency water and wastewater treatment. Desalination. 2019;452:40–67. [Google Scholar]
- [87].Hu X, Zhou J, Liu B. Effect of algal species and light intensity on the performance of an air-lift-type microbial carbon capture cell with an algae-assisted cathode. RSC Adv. 2016;6:25094–100. [Google Scholar]
- [88].Rashid N, Cui Y-F, Saif Ur Rehman M, Han J-I. Enhanced electricity generation by using algae biomass and activated sludge in microbial fuel cell. Sci Total Environ. 2013;456–457:91–4. [DOI] [PubMed] [Google Scholar]
- [89].Xiao L, He Z. Applications and perspectives of phototrophic microorganisms for electricity generation from organic compounds in microbial fuel cells. Renew Sustain Energy Rev. 2014;37:550–9. [Google Scholar]
- [90].Ravi SK, Tan SC. Progress and perspectives in exploiting photosynthetic biomolecules for solar energy harnessing. Energy Environ Sci. 2015;8:2551–73. [Google Scholar]
- [91].Blankenship RE, Tiede DM, Barber J, Brudvig GW, Fleming G, Ghirardi M, et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–9. [DOI] [PubMed] [Google Scholar]
- [92].Kamran M, Delgado JD, Friebe V, Aartsma TJ, Frese RN. Photosynthetic protein complexes as bio-photovoltaic building blocks retaining a high internal quantum efficiency. Biomacromolecules. 2014;15:2833–8. [DOI] [PubMed] [Google Scholar]
- [93].Polman A, Knight M, Garnett EC, Ehrler B, Sinke WC. Photovoltaic materials: Present efficiencies and future challenges. Science. 2016;352:4424. [DOI] [PubMed] [Google Scholar]
- [94].Zhu H, Meng H, Zhang W, Gao H, Zhou J, Zhang Y, et al. Development of a longevous two-species biophotovoltaics with constrained electron flow. Nat Commun. 2019;10:4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Saar KL, Bombelli P, Lea-Smith DJ, Call T, Aro E-M, Müller T, et al. Enhancing power density of biophotovoltaics by decoupling storage and power delivery. Nat Energy. 2018;3:75–81. [Google Scholar]
- [96].Yasri N, Roberts EPL, Gunasekaran S. The electrochemical perspective of bioelectrocatalytic activities in microbial electrolysis and microbial fuel cells. Energy Rep. 2019;5:1116–36. [Google Scholar]
- [97].Zhang YX, Huang M, Li F, Wang XL, Wen ZQ. One-pot synthesis of hierarchical MnO2-modified diatomites for electrochemical capacitor electrodes. J Power Sources. 2014;246:449–56. [Google Scholar]
- [98].Guo XL, Kuang M, Li F, Liu XY, Zhang YX, Dong F, et al. Engineering of three dimensional (3-D) diatom@TiO2@MnO2 composites with enhanced supercapacitor performance. Electrochim Acta. 2016;190:159–67. [Google Scholar]
- [99].Karaman S, Karaipekli A, Sarı A, Biçer A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Solar Energy Mater Sol Cells. 2011;95:1647–53. [Google Scholar]
- [100].Laurens LML, Markham J, Templeton DW, Christensen ED, Van Wychen S, Vadelius EW, et al. Development of algae biorefinery concepts for biofuels and bioproducts; a perspective on process-compatible products and their impact on cost-reduction. Energy Environ Sci. 2017;10:1716–38. [Google Scholar]
- [101].Zarezadeh S, Moheimani NR, Jenkins SN, Hülsen T, Riahi H, Mickan BS. Microalgae and Phototrophic Purple Bacteria for Nutrient Recovery From Agri-Industrial Effluents: Influences on Plant Growth, Rhizosphere Bacteria, and Putative Carbon- and Nitrogen-Cycling Genes. Frontiers in Plant Science. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Renuka N, Guldhe A, Prasanna R, Singh P, Bux F. Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol Adv. 2018;36:1255–73. [DOI] [PubMed] [Google Scholar]
- [103].Zachleder V, Vitova M, Hlavova M, Moudrikova S, Mojzes P, Heumann H, et al. Stable isotope compounds - production, detection, and application. Biotechnol Adv. 2018;36:784–97. [DOI] [PubMed] [Google Scholar]
- [104].Koller M, Muhr A, Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014;6:52–63. [Google Scholar]
- [105].Pagels F, Guedes AC, Amaro HM, Kijjoa A, Vasconcelos V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol Adv. 2019;37:422–43. [DOI] [PubMed] [Google Scholar]
- [106].Liu X, Sheng J, Curtiss R 3rd. Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci U S A. 2011;108:6899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shen XF, Chu FF, Lam PK, Zeng RJ. Biosynthesis of high yield fatty acids from Chlorella vulgaris NIES-227 under nitrogen starvation stress during heterotrophic cultivation. Water Res. 2015;81:294–300. [DOI] [PubMed] [Google Scholar]
- [108].Hadi MR, Shariati M, Afsharzadeh S. Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. Biotechnol Bioproc E. 2008;13:540. [Google Scholar]
- [109].Du H, Liao X, Gao Z, Li Y, Lei Y, Chen W, et al. Effects of methanol on carotenoids as well as biomass and fatty acid biosynthesis in Schizochytrium limacinum B4D1. Appl Environ Microbiol. 2019;85:01243–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rizwan M, Mujtaba G, Memon SA, Lee K, Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew Sustain Energy Rev. 2018;92:394–404. [Google Scholar]
- [111].Liu J, Sun Z, Gerken H, Huang J, Jiang Y, Chen F. Genetic engineering of the green alga Chlorella zofingiensis: a modified norflurazon-resistant phytoene desaturase gene as a dominant selectable marker. Appl Microbiol Biotechnol. 2014;98:5069–79. [DOI] [PubMed] [Google Scholar]
- [112].Anila N, Simon DP, Chandrashekar A, Ravishankar GA, Sarada R. Metabolic engineering of Dunaliella salina for production of ketocarotenoids. Photosynth Res. 2016;127:321–33. [DOI] [PubMed] [Google Scholar]
- [113].Rajneesh Singh SP, Pathak J Sinha RP. Cyanobacterial factories for the production of green energy and value-added products: An integrated approach for economic viability. Renew Sustain Energy Rev. 2017;69:578–95. [Google Scholar]
- [114].Ramanna L, Rawat I, Zerrouki D, Bux F. A novel organic dye-based approach to increase photon flux density for enhanced microalgal pigment production. J Clean Prod. 2018;198:187–94. [Google Scholar]
- [115].Baek K, Yu J, Jeong J, Sim SJ, Bae S, Jin E. Photoautotrophic production of macular pigment in a Chlamydomonas reinhardtii strain generated by using DNA-free CRISPR-Cas9 RNP-mediated mutagenesis. Biotechnol Bioeng. 2018;115:719–28. [DOI] [PubMed] [Google Scholar]
- [116].Saini DK, Chakdar H, Pabbi S, Shukla P. Enhancing production of microalgal biopigments through metabolic and genetic engineering. Crit Rev Food Sci Nutr. 2020;60:391–405. [DOI] [PubMed] [Google Scholar]
- [117].Zhang C, Show PL, Ho SH. Progress and perspective on algal plastics - A critical review. Bioresour Technol. 2019;289:121700. [DOI] [PubMed] [Google Scholar]
- [118].Costa SS, Miranda AL, de Morais MG, Costa JAV, Druzian JI. Microalgae as source of polyhydroxyalkanoates (PHAs) - A review. Int J Biol Macromol. 2019;131:536–47. [DOI] [PubMed] [Google Scholar]
- [119].Carpine R, Raganati F, Olivieri G, Hellingwerf KJ, Pollio A, Salatino P, et al. Poly-β-hydroxybutyrate (PHB) production by Synechocystis PCC6803 from CO2: Model development. Algal Res. 2018;29:49–60. [Google Scholar]
- [120].Bhati R, Mallick N. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 2015;7:78–85. [Google Scholar]
- [121].Khetkorn W, Incharoensakdi A, Lindblad P, Jantaro S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour Technol. 2016;214:761–8. [DOI] [PubMed] [Google Scholar]
- [122].Kamravamanesh D, Kovacs T, Pflügl S, Druzhinina I, Kroll P, Lackner M, et al. Increased poly-β-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour Technol. 2018;266:34–44. [DOI] [PubMed] [Google Scholar]
- [123].Hulkoti NI, Taranath TC. Biosynthesis of nanoparticles using microbes- a review. Colloids Surf B Biointerfaces. 2014;121:474–83. [DOI] [PubMed] [Google Scholar]
- [124].Dahoumane SA, Mechouet M, Wijesekera K, Filipe CDM, Sicard C, Bazylinski DA, et al. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology – a review. Green Chem. 2017;19:552–87. [Google Scholar]
- [125].Asmathunisha N, Kathiresan K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf B Biointerfaces. 2013;103:283–7. [DOI] [PubMed] [Google Scholar]
- [126].Leonardo T, Farhi E, Pouget S, Motellier S, Boisson AM, Banerjee D, et al. Silver accumulation in the green microalga Coccomyxa actinabiotis: Toxicity, in situ speciation, and localization investigated using Synchrotron XAS, XRD, and TEM. Environ Sci Technol. 2016;50:359–67. [DOI] [PubMed] [Google Scholar]
- [127].Chokshi K, Pancha I, Ghosh T, Paliwal C, Maurya R, Ghosh A, et al. Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Adv. 2016;6:72269–74. [Google Scholar]
- [128].Shah M, Fawcett D, Sharma S, Tripathy S, Poinern E. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8:7278–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Shams L, Turner A, Millward GE, Brown MT. Extra- and intra-cellular accumulation of platinum group elements by the marine microalga, Chlorella stigmatophora. Water Res. 2014;50:432–40. [DOI] [PubMed] [Google Scholar]
- [130].Li R, Tao R, Ling N, Chu G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil and Tillage Research. 2017;167:30–8. [Google Scholar]
- [131].Roxby D, Rivy H, Gong C, Gong X, Yuan Z, Chang G-E, et al. Microalgae living sensor for metal ion detection with nanocavity-enhanced photoelectrochemistry. Biosens Bioelectron. 2020;165:112420. [DOI] [PubMed] [Google Scholar]
- [132].Gall JL, Vasilijević S, Battaglini N, Mattana G, Noël V, Brayner R, et al. Algae-functionalized hydrogel-gated organic field-effect transistor. Application to the detection of herbicides. Electrochimica Acta. 2021;372:137881. [Google Scholar]
- [133].Cao K, Zhi R, Zhang G. Photosynthetic bacteria wastewater treatment with the production of value-added products: A review. Bioresour Technol. 2019:122648. [DOI] [PubMed] [Google Scholar]
- [134].Brayner R, Coute A, Livage J, Perrette C, Sicard C. Micro-algal biosensors. Anal Bioanal Chem. 2011;401:581–97. [DOI] [PubMed] [Google Scholar]
- [135].Hk Kordasht, Hassanpour S, Baradaran B, Nosrati R, Hashemzaei M, Mokhtarzadeh A, et al. Biosensing of microcystins in water samples; recent advances. Biosens Bioelectron. 2020;165:112403. [DOI] [PubMed] [Google Scholar]
- [136].Mehta J, Bhardwaj SK, Bhardwaj N, Paul AK, Kumar P, Kim KH, et al. Progress in the biosensing techniques for trace-level heavy metals. Biotechnol Adv. 2016;34:47–60. [DOI] [PubMed] [Google Scholar]
- [137].Tsopela A, Laborde A, Salvagnac L, Ventalon V, Bedel-Pereira E, Seguy I, et al. Development of a lab-on-chip electrochemical biosensor for water quality analysis based on microalgal photosynthesis. Biosens Bioelectron. 2016;79:568–73. [DOI] [PubMed] [Google Scholar]
- [138].Gutiérrez JC, Amaro F, Martín-González A. Heavy metal whole-cell biosensors using eukaryotic microorganisms: an updated critical review. Front Microbiol. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Verma ML, Rani V. Biosensors for toxic metals, polychlorinated biphenyls, biological oxygen demand, endocrine disruptors, hormones, dioxin, phenolic and organophosphorus compounds: a review. Environmental Chemistry Letters. 2020. [Google Scholar]
- [140].Zaib M, Saeed A, Hussain I, Athar MM, Iqbal M. Voltammetric detection of As(III) with Porphyridium cruentum based modified carbon paste electrode biosensor. Biosens Bioelectron. 2014;62:242–8. [DOI] [PubMed] [Google Scholar]
- [141].Labro J, Craig T, Wood SA, Packer MA. Demonstration of the use of a photosynthetic microbial fuel cell as an environmental biosensor. Int J Nanotechnol. 2017;14:213–25. [Google Scholar]
- [142].Wong LS, Judge SK, Voon BWN, Tee LJ, Tan KY, Murti M, et al. Bioluminescent microalgae-based biosensor for metal detection in water. IEEE Sens J. 2018;18:2091–6. [Google Scholar]
- [143].Li F, Yu Z, Han X, Lai RY. Electrochemical aptamer-based sensors for food and water analysis: A review. Anal Chim Acta. 2019;1051:1–23. [DOI] [PubMed] [Google Scholar]
- [144].Gosset A, Oestreicher V, Perullini M, Bilmes SA, Jobbágy M, Dulhoste S, et al. Optimization of sensors based on encapsulated algae for pesticide detection in water. Anal Methods. 2019;11:6193–203. [Google Scholar]
- [145].Tang T, Fadaei H, Hu Z. Rapid evaluation of algal and cyanobacterial activities through specific oxygen production rate measurement. Ecol Eng. 2014;73:439–45. [Google Scholar]
- [146].Sanchez-Ferandin S, Leroy F, Bouget FY, Joux F. A new, sensitive marine microalgal recombinant biosensor using luminescence monitoring for toxicity testing of antifouling biocides. Appl Environ Microbiol. 2013;79:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Lu J, Zhu B, Struewing I, Xu N, Duan S. Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci Rep. 2019;9:2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yindong T, Xiwen X, Miao Q, Jingjing S, Yiyan Z, Wei Z, et al. Lake warming intensifies the seasonal pattern of internal nutrient cycling in the eutrophic lake and potential impacts on algal blooms. Water Research. 2021;188:116570. [DOI] [PubMed] [Google Scholar]
- [149].Xiao X, He J, Huang H, Miller TR, Christakos G, Reichwaldt ES, et al. A novel single-parameter approach for forecasting algal blooms. Water Res. 2017;108:222–31. [DOI] [PubMed] [Google Scholar]
- [150].Randolph K, Wilson J, Tedesco L, Li L, Pascual DL, Soyeux E. Hyperspectral remote sensing of cyanobacteria in turbid productive water using optically active pigments, chlorophyll a and phycocyanin. Remote Sens Environ. 2008;112:4009–19. [Google Scholar]
- [151].Yim I, Shin J, Lee H, Park S, Nam G, Kang T, et al. Deep learning-based retrieval of cyanobacteria pigment in inland water for in-situ and airborne hyperspectral data. Ecol Indic. 2020;110:105879. [Google Scholar]
- [152].Kang J, Wen Z. Use of microalgae for mitigating ammonia and CO2 emissions from animal production operations — Evaluation of gas removal efficiency and algal biomass composition. Algal Res. 2015;11:204–10. [Google Scholar]
- [153].Yen HW, Ho SH, Chen CY, Chang JS. CO2, NOx and SOx removal from flue gas via microalgae cultivation: a critical review. Biotechnol J. 2015;10:829–39. [DOI] [PubMed] [Google Scholar]
- [154].Nagase H, Eguchi K, Yoshihara K-I, Hirata K, Miyamoto K. Improvement of microalgal NOx removal in bubble column and airlift reactors. J Ferment Bioeng. 1998;86:421–3. [Google Scholar]
- [155].Yadav G, Dubey BK, Sen R. A comparative life cycle assessment of microalgae production by CO2 sequestration from flue gas in outdoor raceway ponds under batch and semi-continuous regime. Journal of Cleaner Production. 2020;258:120703. [Google Scholar]
- [156].Yadav G, Dash SK, Sen R. A biorefinery for valorization of industrial waste-water and flue gas by microalgae for waste mitigation, carbon-dioxide sequestration and algal biomass production. Science of The Total Environment. 2019;688:129–35. [DOI] [PubMed] [Google Scholar]
- [157].Cheng D, Li X, Yuan Y, Yang C, Tang T, Zhao Q, et al. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas. Science of The Total Environment. 2019;650:2931–8. [DOI] [PubMed] [Google Scholar]
- [158].Banerjee S, Ray A, Das D. Optimization of Chlamydomonas reinhardtii cultivation with simultaneous CO2 sequestration and biofuels production in a biorefinery framework. Science of The Total Environment. 2021;762:143080. [DOI] [PubMed] [Google Scholar]
- [159].Lim YA, Chong MN, Foo SC, Ilankoon IMSK. Analysis of direct and indirect quantification methods of CO2 fixation via microalgae cultivation in photobioreactors: A critical review. Renewable and Sustainable Energy Reviews. 2021;137:110579. [Google Scholar]
- [160].Yahya L, Harun R, Abdullah LC. Screening of native microalgae species for carbon fixation at the vicinity of Malaysian coal-fired power plant. Scientific Reports. 2020;10:22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sutherland DL, Ralph PJ. Microalgal bioremediation of emerging contaminants - Opportunities and challenges. Water Res. 2019;164:114921. [DOI] [PubMed] [Google Scholar]
- [162].Mehta N, Benzerara K, Kocar BD, Chapon V. Sequestration of radionuclides Radium-226 and Strontium-90 by cyanobacteria forming intracellular calcium carbonates. Environ Sci Technol. 2019;53:12639–47. [DOI] [PubMed] [Google Scholar]
- [163].Parlade E, Hom-Diaz A, Blanquez P, Martinez-Alonso M, Vicent T, Gaju N. Effect of cultivation conditions on beta-estradiol removal in laboratory and pilot-plant photobioreactors by an algal-bacterial consortium treating urban wastewater. Water Res. 2018;137:86–96. [DOI] [PubMed] [Google Scholar]
- [164].de-Bashan LE, Bashan Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour Technol. 2010;101:1611–27. [DOI] [PubMed] [Google Scholar]
- [165].Martínez-Macías MdR, Correa-Murrieta MA, Villegas-Peralta Y, Dévora-Isiordia GE, Álvarez-Sánchez J, Saldivar-Cabrales J, et al. Uptake of copper from acid mine drainage by the microalgae Nannochloropsis oculata. Environ Sci Pollut Res. 2019;26:6311–8. [DOI] [PubMed] [Google Scholar]
- [166].Zhang J, Zhou F, Liu Y, Huang F, Zhang C. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa. Sci Total Environ. 2020;704:135368. [DOI] [PubMed] [Google Scholar]
- [167].Ikehata K, Zhao Y, Kulkarni HV, Li Y, Snyder SA, Ishida KP, et al. Water recovery from advanced water purification facility reverse osmosis concentrate by photobiological treatment followed by secondary reverse osmosis. Environ Sci Technol. 2018;52:8588–95. [DOI] [PubMed] [Google Scholar]
- [168].Wang X-X, Wu Y-H, Zhang T-Y, Xu X-Q, Dao G-H, Hu H-Y. Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Res. 2016;94:215–24. [DOI] [PubMed] [Google Scholar]
- [169].Vu HP, Nguyen LN, Zdarta J, Nga TTV, Nghiem LD. Blue-green glgae in surface water: Problems and opportunities. Curr Pollut Rep. 2020;6:105–22. [Google Scholar]
- [170].Nie J, Sun Y, Zhou Y, Kumar M, Usman M, Li J, et al. Bioremediation of water containing pesticides by microalgae: Mechanisms, methods, and prospects for future research. Sci Total Environ. 2020;707:136080. [DOI] [PubMed] [Google Scholar]
- [171].Antonacci A, Scognamiglio V. Biotechnological advances in the design of algae-based biosensors. Trends Biotechnol. 2020;38:334–47. [DOI] [PubMed] [Google Scholar]
- [172].Talaei M, Mahdavinejad M, Azari R. Thermal and energy performance of algae bioreactive façades: A review. J Building Eng. 2020;28. [Google Scholar]
- [173].Mandal S, Mallick N. Biodiesel production by the green microalga Scenedesmus obliquus in a recirculatory aquaculture system. Appl Environ Microbiol. 2012;78:5929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Snell MA, Barker PA, Surridge BWJ, Benskin CMH, Barber N, Reaney SM, et al. Strong and recurring seasonality revealed within stream diatom assemblages. Sci Rep. 2019;9:3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Okoro V, Azimov U, Munoz J, Hernandez HH, Phan AN. Microalgae cultivation and harvesting: Growth performance and use of flocculants - A review. Renewable and Sustainable Energy Reviews. 2019;115:109364. [Google Scholar]
- [176].Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W, et al. Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnology Advances. 2020;43:107567. [DOI] [PubMed] [Google Scholar]
- [177].Hu R, Chang H, Zou Y, Feng H, Wu H, Wu W, et al. Enhanced Reverse Osmosis Concentrate Disposal and Nutrients Conversion to Microalgae Bioenergy with a Gradient-Fed Strategy. ACS Sustainable Chemistry & Engineering. 2021;9:874–82. [Google Scholar]
- [178].Guldhe A, Kumari S, Ramanna L, Ramsundar P, Singh P, Rawat I, et al. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. Journal of Environmental Management. 2017;203:299–315. [DOI] [PubMed] [Google Scholar]
- [179].Abdullah B, Syed Muhammad SAFa, Shokravi Z, Ismail S, Kassim, Mahmood AN, et al. Fourth generation biofuel: A review on risks and mitigation strategies. Renew Sustain Energy Rev. 2019;107:37–50. [Google Scholar]
- [180].Flynn KJ, Mitra A, Greenwell HC, Sui J. Monster potential meets potential monster: pros and cons of deploying genetically modified microalgae for biofuels production. Interface Focus. 2013;3:20120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Koblížek M. Ecology of aerobic anoxygenic phototrophs in aquatic environments. FEMS Microbiol Rev. 2015;39:854–70. [DOI] [PubMed] [Google Scholar]
- [182].Kolber ZS, Plumley FG, Lang AS, Beatty JT, Blankenship RE, VanDover CL, et al. Contribution of aerobic photoheterotrophic bacteria to the carbon cycle in the ocean. Science. 2001;292:2492–5. [DOI] [PubMed] [Google Scholar]
- [183].Talaiekhozani A, Rezania S. Application of photosynthetic bacteria for removal of heavy metals, macro-pollutants and dye from wastewater: A review. J Water Proc Eng. 2017;19:312–21. [Google Scholar]
- [184].Capson-Tojo G, Batstone DJ, Grassino M, Vlaeminck SE, Puyol D, Verstraete W, et al. Purple phototrophic bacteria for resource recovery: Challenges and opportunities. Biotechnol adv. 2020;43:107567. [DOI] [PubMed] [Google Scholar]
- [185].Higuchi-Takeuchi M, Numata K. Marine purple photosynthetic bacteria as sustainable microbial production hosts. Front Bioeng Biotechnol. 2019;7:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Qi X, Ren Y, Liang P, Wang X. New insights in photosynthetic microbial fuel cell using anoxygenic phototrophic bacteria. Bioresour Technol. 2018;258:310–7. [DOI] [PubMed] [Google Scholar]
- [187].Redwood MD, Dhillon R, Orozco RL, Zhang X, Binks DJ, Dickinson M, et al. Enhanced photosynthetic output via dichroic beam-sharing. Biotechnol Lett. 2012;34:2229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Hallenbeck PC, Lazaro CZ, Sagir E. CHAPTER 1 Photosynthesis and hydrogen from photosynthetic microorganisms. In: Seibert Michael, Torzillo G, editors. Microalgal hydrogen production: Achievements and perspectives. London: The Royal Society of Chemistry; 2018. p. 1–30. [Google Scholar]
- [189].Fakhimi N, Gonzalez-Ballester D, Fernandez E, Galvan A, Dubini A. Algae-bacteria consortia as a strategy to enhance H2 production. Cells. 2020;9:1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Mona S, Kumar SS, Kumar V, Parveen K, Saini N, Deepak B, et al. Green technology for sustainable biohydrogen production (waste to energy): A review. Sci Total Environ. 2020;728:138481. [DOI] [PubMed] [Google Scholar]
- [191].Hou Q, Yang Z, Chen S, Pei H. Using an anaerobic digestion tank as the anodic chamber of an algae-assisted microbial fuel cell to improve energy production from food waste. Water Res. 2020;170:115305. [DOI] [PubMed] [Google Scholar]
- [192].Naduthodi MIS, Claassens NJ, D’Adamo S, van der Oost J, Barbosa MJ. Synthetic Biology Approaches To Enhance Microalgal Productivity. Trends in Biotechnology. 2021. [DOI] [PubMed] [Google Scholar]
- [193].Remmers IM, Wijffels RH, Barbosa MJ, Lamers PP. Can we approach theoretical lipid yields in microalgae? Trends Biotechnol. 2018;36:265–76. [DOI] [PubMed] [Google Scholar]
- [194].Fayyaz M, Chew KW, Show PL, Ling TC, Ng IS, Chang J-S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol adv. 2020;43:107554. [DOI] [PubMed] [Google Scholar]
- [195].Kroth PG, Bones AM, Daboussi F, Ferrante MI, Jaubert M, Kolot M, et al. Genome editing in diatoms: achievements and goals. Plant Cell Rep. 2018;37:1401–8. [DOI] [PubMed] [Google Scholar]
- [196].Jensen PE, Scharff LB. Engineering of plastids to optimize the production of high-value metabolites and proteins. Curr Opin Biotechnol. 2019;59:8–15. [DOI] [PubMed] [Google Scholar]
- [197].Shin W-S, Lee B, Jeong B-r, Chang YK, Kwon J-H. Truncated light-harvesting chlorophyll antenna size in Chlorella vulgaris improves biomass productivity. J Appl Phycol. 2016;28:3193–202. [Google Scholar]
- [198].Khan S, Fu P. Biotechnological perspectives on algae: a viable option for next generation biofuels. Curr Opin Biotechnol. 2020;62:146–52. [DOI] [PubMed] [Google Scholar]
- [199].Gomaa MA, Al-Haj L, Abed RM. Metabolic engineering of Cyanobacteria and microalgae for enhanced production of biofuels and high-value products. J Appl Microbiol. 2016;121:919–31. [DOI] [PubMed] [Google Scholar]
- [200].Huang W, Daboussi F. Genetic and metabolic engineering in diatoms. Philos Trans R Soc B. 2017;372:20160411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Georgianna DR, Mayfield SP. Exploiting diversity and synthetic biology for the production of algal biofuels. Nature. 2012;488:329–35. [DOI] [PubMed] [Google Scholar]
- [202].Rashid N, Nayak M, Suh WI, Lee B, Chang Y-K. Efficient microalgae removal from aqueous medium through auto-flocculation: investigating growth-dependent role of organic matter. Environ Sci Pollut Res. 2019;26:27396–406. [DOI] [PubMed] [Google Scholar]
- [203].Ding G, Li X, Lin W, Kimochi Y, Sudo R. Enhanced flocculation of two bioflocculation-producing bacteria by secretion of Philodina erythrophthalma. Water Res. 2017;112:208–16. [DOI] [PubMed] [Google Scholar]
- [204].Wang Y, Yang Y, Ma F, Xuan L, Xu Y, Huo H, et al. Optimization of Chlorella vulgaris and bioflocculant-producing bacteria co-culture: enhancing microalgae harvesting and lipid content. Lett Appl Microbiol. 2015;60:497–503. [DOI] [PubMed] [Google Scholar]
- [205].Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, et al. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr Rev Food Sci F. 2013;12:5–23. [Google Scholar]
- [206].Hill PR, Kumar A, Temimi M, Bull DR. HABNet: Machine learning, remote sensing-based detection of harmful algal blooms. IEEE J-STARS. 2020;13:3229–39. [Google Scholar]
- [207].Tang T, Hu Z. A comparison of algal productivity and nutrient removal capacity between algal CSTR and algal MBR at the same light level under practical and optimal conditions. Ecol Eng. 2016;93:66–72. [Google Scholar]
- [208].Tang T, Wan P, Hu Z. CO2 bubbling to improve algal growth, nutrient removal, and membrane performance in an algal membrane bioreactor. Water Environ Res. 2018;90:650–8. [DOI] [PubMed] [Google Scholar]
- [209].Xu M, Bernards M, Hu Z. Algae-facilitated chemical phosphorus removal during high-density Chlorella emersonii cultivation in a membrane bioreactor. Bioresour technol. 2014;153:383–7. [DOI] [PubMed] [Google Scholar]
- [210].Xu M, Li P, Tang T, Hu Z. Roles of SRT and HRT of an algal membrane bioreactor system with a tanks-in-series configuration for secondary wastewater effluent polishing. Ecol Eng. 2015;85:257–64. [Google Scholar]
- [211].Lee Y-C, Lee K, Oh Y-K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: A review. Bioresource Technology. 2015;184:63–72. [DOI] [PubMed] [Google Scholar]
- [212].Rana MS, Bhushan S, Prajapati SK. New insights on improved growth and biogas production potential of Chlorella pyrenoidosa through intermittent iron oxide nanoparticle supplementation. Scientific Reports. 2020;10:14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Li X, Sun H, Mao X, Lao Y, Chen F. Enhanced Photosynthesis of Carotenoids in Microalgae Driven by Light-Harvesting Gold Nanoparticles. ACS Sustainable Chemistry & Engineering. 2020;8:7600–8. [Google Scholar]
- [214].Li X, Liu B, Lao Y, Wan P, Mao X, Chen F. Efficient magnetic harvesting of microalgae enabled by surface-initiated formation of iron nanoparticles. Chemical Engineering Journal. 2021;408:127252. [Google Scholar]
- [215].Yap JX, Leo CP, Mohd Yasin NH, Derek CJC. Sustainable cultivation of Navicula incerta using cellulose-based scaffold incorporated with nanoparticles in air-liquid interface cultivation system. Chemosphere. 2021;273:129657. [DOI] [PubMed] [Google Scholar]
- [216].Liu X, Zhang F, Jing X, Pan M, Liu P, Li W, et al. Complex silica composite nanomaterials templated with DNA origami. Nature. 2018;559:593–8. [DOI] [PubMed] [Google Scholar]
- [217].Ozdalgic B, Ustun M, Dabbagh SR, Haznedaroglu BZ, Kiraz A, Tasoglu S. Microfluidics for microalgal biotechnology. Biotechnol Bioeng. 2021;118:1545–63. [DOI] [PubMed] [Google Scholar]
- [218].Dolatabadi JEN, de la Guardia M. Applications of diatoms and silica nanotechnology in biosensing, drug and gene delivery, and formation of complex metal nanostructures. TrAC Trends in Analytical Chemistry. 2011;30:1538–48. [Google Scholar]
- [219].Li Y, Zhang C, Hu Z. Selective removal of pharmaceuticals and personal care products from water by titanium incorporated hierarchical diatoms in the presence of natural organic matter. Water Research. 2021;189:116628. [DOI] [PubMed] [Google Scholar]
- [220].Hülsen T, Barry EM, Lu Y, Puyol D, Keller J, Batstone DJ. Domestic wastewater treatment with purple phototrophic bacteria using a novel continuous photo anaerobic membrane bioreactor. Water Res. 2016;100:486–95. [DOI] [PubMed] [Google Scholar]
- [221].Rood B, Zhang C, Inniss E, Hu Z. Forward osmosis with an algal draw solution to concentrate municipal wastewater and recover resources. Water Environ Res. 2020;92:689–97. [DOI] [PubMed] [Google Scholar]
- [222].Matassa S, Batstone DJ, Hülsen T, Schnoor J, Verstraete W. Can direct conversion of used nitrogen to new feed and protein help feed the world? Environ Sci Technol. 2015;49:5247–54. [DOI] [PubMed] [Google Scholar]
- [223].Passos F, Gutiérrez R, Uggetti E, Garfí M, García J, Ferrer I. Towards energy neutral microalgae-based wastewater treatment plants. Algal Res. 2017;28:235–43. [Google Scholar]
- [224].Shoener BD, Bradley IM, Cusick RD, Guest JS. Energy positive domestic wastewater treatment: the roles of anaerobic and phototrophic technologies. Environ Sci Process Impacts. 2014;16:1204–22. [DOI] [PubMed] [Google Scholar]