Abstract
Background
Bacteria colonizing the upper respiratory tract (URT) of young children play a key role in the pathogenesis of lower respiratory tract infection (LRTI).
Objectives
To systematically review the literature on the association between bacteria colonizing the URT and LRTI among young children.
Data sources
MEDLINE, Academic Search Premier, Africa-Wide Information and CINAHL, Scopus and Web of Science.
Study eligibility criteria
Studies published between 1923 and 2020, investigating URT bacteria from LRTI cases and controls.
Participants
Children under 5 years with and without acute LRTI.
Methods
Three reviewers independently screened titles, abstracts and full texts. Meta-analysis was done using Mantel–Haenszel fixed- or random-effects models.
Results
Most eligible studies (41/50) tested nasopharyngeal specimens when investigating URT bacteria. Most studies were of cross-sectional design (44/50). Twenty-four studies were performed in children in lower- or lower-middle-income countries (LMICs). There was higher prevalence of Haemophilus influenzae (pooled OR 1.60; 95% CI 1.23–2.07) and Klebsiella spp. (pooled OR 2.04; 95% CI 1.17–3.55) from URT specimens of cases versus controls. We observed a positive association between the detection of Streptococcus pneumoniae from URT specimens and LRTI after excluding studies where there was more antibiotic treatment prior to sampling in cases vs. controls (pooled OR 1.41; 95% CI 1.04–1.90). High density colonization with S. pneumoniae (>6.9 log10 copies/mL) was associated with an increased risk for LRTI. The associations between both Streptococcus and Haemophilus URT detection and LRTI were supported, at genus level, by 16S rRNA sequencing. Evidence for the role of Moraxella catarrhalis and Staphylococcus aureus was inconclusive.
Conclusions
Detection of H. influenzae or Klebsiella spp. in the URT was associated with LRTI, while evidence for association with S. pneumoniae was less conclusive. Longitudinal studies assessing URT microbial communities, together with environmental and host factors are needed to better understand pathogenesis of childhood LRTI.
Keywords: Aetiology, Bacteria, Children, Lower respiratory tract infection, Microbiome, Upper respiratory tract colonization
Introduction
Over the past two decades, widespread introduction of conjugate vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b (Hib) has contributed to a global reduction in the incidence and severity of lower respiratory tract infection (LRTI) [1,2]. Yet LRTI remains the leading cause of morbidity and mortality among children under 5 years, causing 808 920 deaths in 2017 [3]. The Global Burden of Disease Study 2017 reported pneumococcal pneumonia as the leading cause of LRTI deaths [2,4].
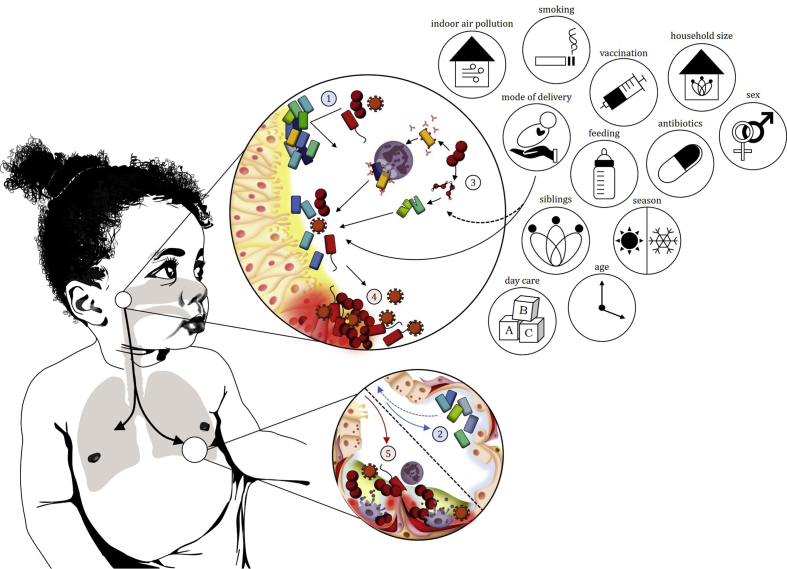
The conventional model of LRTI pathogenesis has been challenged with the advent of sequence-based methods for characterizing microbial communities. The concept that a pathogen from the upper respiratory tract (URT) invades and infects sterile lungs has been replaced by the notion that the lungs harbour transient or persistent bacterial communities derived from the URT. Shifts in URT bacterial communities may shape bacterial communities in the lungs associated with health or disease (Fig. 1) [[5], [6], [7]].
Fig. 1.
Imbalances in upper respiratory tract (URT) bacterial communities may result in translocation of dysbiotic bacterial communities to the lower respiratory tract (LRT), causing infection. (1) Commensal bacteria with low pathogenic potential confer colonization resistance against potential pathogens. (2) Bacterial communities from the URT translocate to the lungs where they are detected as stable resident or transient LRT communities. (3) Bacterial–bacterial interactions in the URT may be competitive or synergistic allowing potential pathogens to colonize. Environmental exposures may influence bacterial–bacterial and bacterial–host interactions, or directly impact on select bacteria within the community. (4) Perturbations of the bacterial community (dysbiosis), resulting from pathogen exposure, viral-bacterial, bacterial-bacterial interactions and environmental risk factors, may result in inflammation and damage to the URT epithelium. (5) Dysbiotic bacterial communities translocate to the LRT and cause inflammation and damage to the respiratory epithelium.
However, studies of the association between URT bacterial communities and LRTI in children have shown varying results. We therefore systematically reviewed data from studies investigating the association between URT bacterial prevalence and LRTI in children under the age of five years.
Materials and methods
A comprehensive description of the methodology is provided in Appendix 1. The review protocol (CRD42020200544) is available from the International Prospective Register of Systematic Reviews (PROSPERO) [8].
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria for reporting of systematic reviews [9] (Appendix 1). We searched six databases for articles reporting on the detection of URT bacteria in children with LRTI and controls (Appendix 1; Table 1). Definitions used are outlined in Appendix 1.
Table 1.
Eligibility criteria
| PICOS strategy | Inclusion criteria (all needed) | Exclusion criteria (any) |
|---|---|---|
| Population |
|
|
| Intervention |
|
|
| Comparator |
|
|
| Outcome |
|
|
| Study design |
|
|
PICOS, patients, intervention, comparator, outcomes, study design; (L)/(U)RTI, (lower)/(upper) respiratory tract infection.
We extracted data from eligible studies using a predefined data extraction template (Appendix 1) and evaluated eligible studies using the Newcastle–Ottawa Quality Assessment Scale (NOS) for case–control studies [10].
We determined the association between the prevalence of URT bacterial species and the risk of LRTI using pooled odds ratios (ORs) and corresponding confidence intervals (CIs). Pooled ORs were calculated in RevMan [11] using Mantel Haenszel fixed- or random-effects models based on between-study heterogeneity. We excluded studies with potentially overlapping participants from all quantitative analyses to avoid “double counting” aetiological data from a single set of participants.
Results
Study selection and characteristics of the included studies
We identified 6432 studies for title and abstract screening (Appendix 2). A total of 6309 studies were excluded based on titles and abstracts screened. Of the 6309 studies, 15 full texts could not be assessed for eligibility due to translational issues (Appendix 2). Of the 123 studies identified for full-text review, 73 were identified as ineligible (Appendix 2). In total, 50 studies were included in the systematic review (The reference list of eligible studies included in the review is provided in Appendix 2).
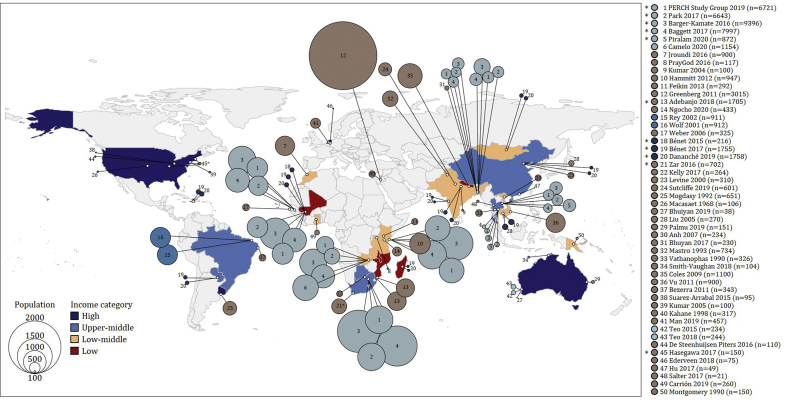
The characteristics of the 50 eligible studies, published between June 1968 and June 2020, are summarised in Appendix 2. Forty single-centre and ten multicentre studies were identified across 29 countries (high-income (five countries), upper-middle-income (six countries), lower-middle-income (12 countries) and low-income (six countries)) (Fig. 2; Appendix 2). Most participants were from low-income (n = 12 954/55 495, 23%) or lower-middle-income (n = 21 764/55 495, 39%) countries (Appendix 2).
Fig. 2.
Eligible studies included in this review (N = 50) were performed across 29 countries. The 50 eligible studies are listed on the right of the figure. The reference list of eligible studies included in the review is provided in Appendix 2. Multicentre studies are denoted with an asterisk. Studies shown in the same shade of blue represent potential overlap in participants across these studies.
Five studies followed children longitudinally, whilst 44 used a cross-sectional case–control design (Appendix 2). One [study 34] included cross-sectional and longitudinal components.
URT specimens tested included nasopharyngeal swabs (25 studies), nasopharyngeal aspirates (five studies), nasal swabs (five studies), throat swabs (two studies) or oropharyngeal swabs (one study) (Appendix 2). Seven studies by the Pneumonia Etiology Research for Child Health (PERCH) Group [studies 1-6] and Feikin and colleagues [study 11] used a combined nasopharyngeal and oropharyngeal swab, while a further five studies tested two different specimens. Overall, 82% of studies included nasopharyngeal specimens (swabs, aspirates, or combined specimens).
Most studies (70%) screened for more than one bacterium using culture (n = 10), PCR (n = 13), culture and PCR (n = 3) or short fragment sequencing of 16S rRNA gene amplicons (n = 9) (Appendix 2). The nine 16S rRNA profiling studies were carried out in high-income (n = 6) and upper-middle-income (n = 3) countries.
The 50 eligible studies primarily enrolled cases from hospital (n = 28), ambulatory (n = 8) and community (n = 7) settings, while controls were primarily enrolled from community (n = 17), ambulatory (n = 16) and hospital (n = 6) settings (Appendix 2).
Reporting of antibiotic treatment prior to sampling was variable (Appendix 2). Half of eligible studies did not provide vaccination details (n = 25) (Appendix 2). Nineteen studies reported on pneumococcal conjugate vaccine (PCV). Other vaccinations included Hib (n = 9); diphtheria, tetanus, and pertussis (DTP/DTaP) (n = 4); measles (n = 3) and influenza (n = 2).
Studies primarily included participants up to 5 years (68%), 2 years (14%) or 1 year of age (12%) (Appendix 2). More males were included as cases compared with controls in 40% of studies, while more females were included as cases compared with controls in 18% of studies. Fifteen studies did not report on sex. Studies adjusted for age (n = 27), season (n = 15), sex (n = 4), site (n = 4), HIV status (n = 1) and nutritional status (n = 1), while 21 studies did not provide adjustments for potential confounders.
Overall, 72% (36/50) studies were considered to have low risk of bias (NOS ≥7) (Appendix 2).
Associations between URT bacteria and LRTI
Seven studies including potentially overlapping participants were excluded to avoid “double counting” (Fig. 2; Appendix 2), and one further study was excluded as no prevalence data were provided. Data from 42 studies were available for meta-analysis (Appendix 2).
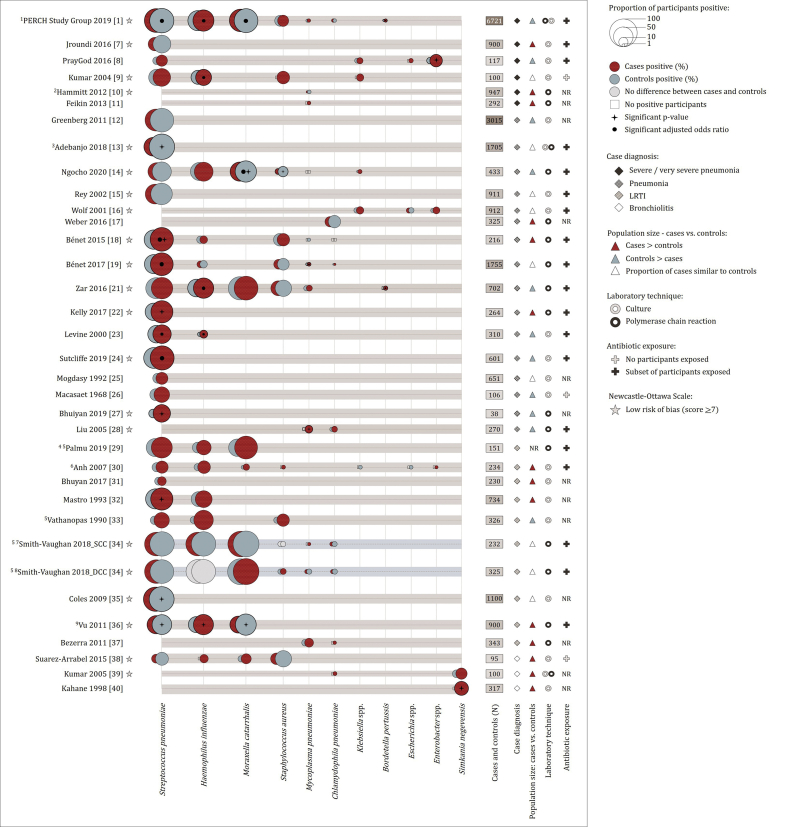
Fig. 3 represents URT bacterial prevalence from culture or PCR, for bacteria which were reported in at least two studies (34 studies). Among these, participants were most frequently screened for S. pneumoniae (n = 26), H. influenzae (n = 14), M. catarrhalis (n = 8), S. aureus (n = 10), Mycoplasma pneumoniae (n = 10) and Chlamydophila pneumoniae (n = 8).
Fig. 3.
Proportion of lower respiratory tract infection (LRTI) cases and controls from which bacteria were detected in the upper respiratory tract (URT) using culture or PCR. Only bacteria for which two or more eligible studies provided prevalence data are represented. Proportions of cases/controls from which each of the respective bacteria were detected are represented by bubbles of different sizes. Proportions were calculated using the number of participants positive divided by the number of participants tested. The group (cases or controls) with a higher proportion of participants positive is plotted in front of the group with a lower proportion of participants positive (Red bubbles: cases; Blue bubbles: controls). Grey bubbles: no differences between case control groups. Unfilled squares: zero prevalence. The reference list of eligible studies included in the review is provided in Appendix 2. 1Data from chest radiography positive cases. 2Data excludes controls with respiratory tract infection. 3Results from culture not PCR. PCR was only performed for culture negative cases (not controls). Antibiotic exposure among cases was higher compared to controls (97% vs. 27%). 4Control specimens include specimens collected asymptomatically or during URTI/acute otitis media episodes. 5Data from specimens screened, not participants. 6Data from cases represent both pneumonia and acute bronchiolitis groups. 7SCC, Same Child Control cohort (LRTI specimen matched to non-LRTI specimen from the same child). 8DCC, Different Child Control cohort (LRTI specimen matched to non-LRTI specimen from a different child). 9Data from cases represent both pneumonia and other LRTI groups.
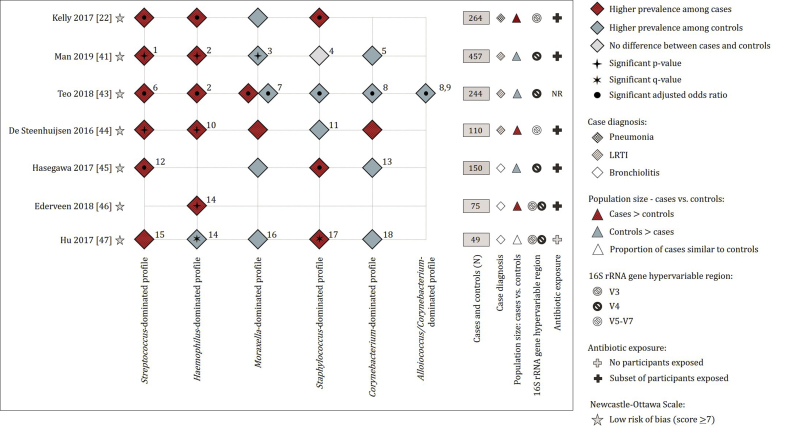
Five of eight studies using 16S rRNA sequencing designated specimens into “microbiota profile groups” based on the genus detected at the highest relative abundance from each of the specimens. Case–control prevalence data were primarily provided for Streptococcus-, Haemophilus-, Moraxella-, Staphylococcus-, Corynebacterium- and Alloiococcus/Corynebacterium-dominated profiles (microbiota profile groups) (Fig. 4). Two [studies 46 and 47] reported relative abundances of bacterial genera from cases and controls (Fig. 4). One study, excluded from Fig. 4, longitudinally investigated microbiota profiling groups between healthy and LRTI states [study 48]. No distinct microbiota profiles were reported for healthy and LRTI states within this cohort, however, the study reported that respiratory illness coincided with a perturbation in bacterial community profiles which were unique for each child and each illness episode [study 48].
Fig. 4.
Bacterial profiles from 16S rRNA gene amplicon sequencing of upper respiratory tract samples from LRTI cases and controls. Red diamonds: microbiota profile group/genus was more prevalent among cases compared to controls. Blue diamonds: microbiota profile group/genus was more prevalent among controls compared to cases. Grey diamonds: no differences in the prevalence of microbiota profile group/genus among cases and controls. The reference list of eligible studies included in the review is provided in Appendix 2. (1) Streptococcus pneumoniae-dominated profile. (2) Haemophilus influenzae/haemolyticus-dominated profile. (3) Moraxella catarrhalis/nonliquefaciens-dominated profile. (4) Staphylococcus aureus/epidermidis-dominated profile. (5) Corynebacterium propinquum/Dolosigranulum pigrum-dominated profile. (6) Streptococcus pneumoniae/pseudopneumoniae-dominated profile. (7) Moraxella catarrhalis/lincolnii-dominated profile. (8) Corynebacterium propinquum/pseudodiphtheriticum-dominated profile. (9) Alloiococcus otidis (Dolosigranulum pigrum)-dominated profile. (10) Haemophilus influenzae-dominated profile. (11) Staphylococcus aureus-dominated profile. (12) Mixed profile (Streptococcus/Tumebacillus-dominated profile). (13) Corynebacterium/Dolosigranulum-dominant profile. (14) Haemophilus genus. (15) Streptococcus genus. (16) Moraxella genus. (17) Staphylococcus genus. (18) Corynebacterium genus.
Streptococcus pneumoniae
The majority (16/26, 62%) of studies using culture or PCR showed a higher prevalence of S. pneumoniae among cases compared to controls (Fig. 3); however, the pooled OR did not show a significant association (pooled OR 1.16; 95% CI 0.86–1.57) (Appendix 3: Fig. S1). A sensitivity analysis, including data from 13 studies with NOS scores ≥7 and > 200 participants, showed a higher prevalence among controls than cases (pooled OR 0.93; 95% CI 0.60–1.43) (Appendix 3: Fig. S2). Subgroup analysis of eight studies where antibiotic treatment prior to sampling was more prevalent among cases than controls showed no significant difference in prevalence of S. pneumoniae among these groups (pooled OR 0.75; 95% CI 0.43–1.31) (Appendix 3: Table S1). Following the exclusion of these eight studies, a significant association was observed between S. pneumoniae and LRTI (n = 18) (pooled OR 1.41; 95% CI 1.04–1.90).
The PERCH Study [study 1], which investigated aetiology in settings with high PCV coverage, reported a significantly higher prevalence of S. pneumoniae among controls compared to hospitalised LRTI cases (Fig. 3; Appendix 3: Fig. S1,S2). After applying a pathogen-specific PCR density threshold (>6.9 log10 copies/mL), PERCH reported that S. pneumoniae was associated with an increased risk for LRTI. Another study reported a significantly higher median density of S. pneumoniae from participants with radiologically confirmed pneumonia compared to healthy controls (7.8 × 106 copies/mL vs. 7.9 × 105 copies/mL; p < 0.0001) [study 36].
Eight of twelve studies reporting on pneumococcal serotypes showed higher prevalence of vaccine type pneumococci from cases compared to controls (Appendix 4). Of these, two studies reported higher PCV vaccination from controls [studies 7 and 13], while two studies reported no PCV vaccination of either cases and controls [studies 12 and 18]. Five of the eight studies provided no details on PCV status of individual participants [studies 15,32,36 and 50], two of which reported a higher prevalence of non-vaccine pneumococcal serotypes from controls compared with cases [studies 32 and 36] (Appendix 4). Longitudinal analysis showed that pneumococcal serotypes 1, 5, 7, 9, 10, 14, 18, 45 and 46, classified as invasive serotypes [study 50], were more frequently detected during LRTI episodes compared to controls [study 50].
All six studies performing 16S rRNA gene amplicon sequencing reported higher prevalence of Streptococcus-dominated profiles or higher relative abundance of Streptococcus genus among cases than controls (Fig. 4), with statistically significant findings in five studies.
Haemophilus influenzae
Haemophilus influenzae was more prevalent among cases than among controls in 86% (12/14) of studies using culture or PCR (Fig. 3) (pooled OR 1.60; 95% CI 1.23–2.07)] (Appendix 3: Fig. S3). Sensitivity analysis including studies with NOS scores ≥7 and > 200 participants (seven studies) showed similar findings (pooled OR 1.24; 95% CI 0.96–1.62)] (Appendix 3: Fig. S4), as did subgroup analysis (Appendix 3: Table S2).
Only five of the 14 studies performed H. influenzae serotyping [studies 1,14,21,23 and 32], three of which provided non-typeable H. influenzae (NTHI), non-type b H. influenzae (non-type b) and/or Hib prevalence data. NTHI prevalence was reported at 91.3% from cases and 96.4% from controls [study 32]. Non-type b H. influenzae prevalence was higher among cases than in controls (55.4% vs. 49.6%) (OR 1.22; 95% CI 1.07–1.40) [study 1]. Hib prevalence was low overall, but higher among cases than controls (2.4% vs. 1.9%) (OR 1.74; 95% CI 1.14–2.66) [study 1]): and (7.3% vs. 1.9%) (OR 4.12; 95% CI 1.01–16.80) [study 23]. Hib vaccine coverage was reported by 7/14 studies [studies 1,14,18,19,21,23,30], of which two reported no Hib vaccination [studies 23 and 30] while three reported higher coverage of Hib vaccination in controls [studies 1,14 and 19].
Five of six 16S rRNA gene amplicon sequencing studies reported significantly higher prevalence of Haemophilus-dominated profiles or higher relative abundance of Haemophilus genus from cases compared to controls (Fig. 4).
Moraxella catarrhalis
Four culture or PCR studies reported higher prevalence of M. catarrhalis from cases, whilst four studies reported higher prevalence among controls (Fig. 3) (pooled OR 1.09; 95% CI 0.55–2.15; Appendix 3: Fig. S5). Moraxella catarrhalis prevalence was borderline lower among cases when including studies with NOS scores ≥7 and > 200 participants (five studies) (pooled OR 0.71; 95% CI 0.51–1.00) (Appendix 3: Fig. S6). Studies including participants under 5 years, and those where more cases had antibiotic treatment prior to sampling than controls, showed significantly higher prevalence of M. catarrhalis among controls than among cases (Appendix 3: Table S3).
One study showed that M. catarrhalis URT density was significantly higher among LRTI cases when compared to healthy controls (p < 0.0001) [study 36], while another study reported higher bacterial densities among controls [study 21].
One 16S rRNA gene amplicon sequencing study reported higher prevalence of Moraxella-dominated profiles from cases, whilst four reported higher prevalence of Moraxella-dominated profiles or higher relative abundance of Moraxella genus from cases among controls (Fig. 4). One study [study 43] investigated profiles longitudinally and reported that specimens collected 1–2 weeks prior to an LRTI were significantly enriched with Moraxella-dominated profiles compared to all other healthy specimens.
Staphylococcus aureus
There was no overall association between S. aureus on culture or PCR and LRTI (10 studies) (pooled OR 0.89; 95% CI 0.62–1.26) (Fig. 3; Appendix 3: Fig. S7), or when including only studies with NOS scores ≥7 and > 200 participants (six studies) (Appendix 3: Fig. S8). Subgroup analyses suggested lower prevalence of S. aureus in cases versus controls in participants diagnosed with pneumonia versus controls (four studies) (pooled OR 0.69; 95% CI 0.57–0.84) and in younger participants (<24 months) (three studies) (pooled OR 0.76; 95% CI 0.56–1.02) (Appendix 3: Table S4).
There were inconsistent findings for difference between cases and controls at the Staphylococcus genus level reported by studies using 16S rRNA gene amplicon sequencing (Fig. 4).
Bacteria detected at low prevalence among LRTI cases and controls
Mycoplasma pneumoniae (nine studies) and C. pneumoniae (seven studies) were detected at low prevalence (Fig. 3) and were similar among cases and controls: M. pneumoniae (pooled OR 1.35; 95% CI 0.96–1.90) (Appendix 3: Figs S9, S10; Appendix 3: Table S5); C. pneumoniae (pooled OR 0.83; 95% CI 0.59–1.18) (Appendix 3: Figs S11, S12; Appendix 3: Table S6).
Four of the five studies using culture or PCR reported higher prevalence of Klebsiella spp. from cases compared to controls (Fig. 3) (pooled OR 2.04; 95% CI 1.17–3.55) (Appendix 3: Figure S13). Limited subgroup analyses could be performed, all of which had ORs >1 (Appendix 3: Table S7).
Bordetella pertussis, Enterobacter spp. and Simkania negevensis data were only available from ≤3 studies (Fig. 3), and therefore meta-analysis was not done.
Bacterial-bacterial co-detection in cases and controls
Ten studies provided bacterial–bacterial co-detection data using culture or PCR from both cases and controls (Appendix 5). Of these, six provided co-detection data at species-level [studies 14,21,32-34 and 36]. Two studies reported statistically significant differences: higher prevalence of S. pneumoniae/H. influenzae co-detection in cases versus controls [studies 14 and 36]; higher prevalence of H. influenzae/M. catarrhalis co-detection in cases versus controls [study 36]; higher prevalence of S. pneumoniae/M. catarrhalis co-detection in controls versus cases [study 36] and higher prevalence of S. pneumoniae/H. influenzae/M. catarrhalis co-detection in controls versus cases [studies 14 and 36] (Appendix 5).
Discussion
Although S. pneumoniae is recognized as the leading bacterial cause of LRTI in children [2,4], several large recent studies have not found evidence of an association between URT carriage and LRTI [12,13]. Our meta-analysis did not identify a significant association, however, after excluding studies reporting more antibiotic treatment prior to sampling in cases than controls, we found a positive association between S. pneumoniae URT colonization and LRTI. We further highlight the importance of bacterial density thresholds when investigating the aetiological role of URT bacteria in LRTI, identified by two studies [studies 1 and 36]. These data are supported by all six studies performing 16S rRNA gene amplicon sequencing, which reported higher prevalence of Streptococcus-dominated profiles or higher relative abundance of Streptococcus genus among cases vs. controls. However, genus level analysis may mask the contribution of streptococcal species other than Streptococcus pneumoniae to LRTI. We observed higher prevalence of vaccine type pneumococci from cases compared to controls in eight of 12 studies) (Appendix 4) but could not assess the role of PCV immunisation as five of the eight studies reporting on pneumococci serotypes did not provide details on PCV status of individual participants.
Similar to pneumococcus, the number of Hib associated LRTI deaths among young children has significantly decreased with implementation of Hib-conjugate vaccines [2]. However, the widespread use of Hib-conjugate vaccines has resulted in NTHI strains emerging as a common cause of paediatric LRTI [14]. Our meta-analysis confirmed the association between H. influenzae and LRTI, but, since most studies did not serotype strains, we could not assess the role of Hib and NTHI specifically.
While M. catarrhalis colonization was variably associated with LRTI, one study reported significant enrichment of Moraxella-dominated profiles from specimens collected 1–2 weeks prior to an LRTI [study 43]. Furthermore, co-detection with H. influenzae was associated with LRTI [study 36]. Interactions between species may be key to understand the role of M. catarrhalis in LRTI. M. catarrhalis enhances the development of stable polymicrobial biofilms by promoting the survival of non-typeable H. influenzae in the presence of S. pneumoniae [15,16]. Interactions have also been demonstrated between other species, for example, an inverse correlation between S. pneumoniae and S. aureus has been ascribed to hydrogen peroxide production by S. pneumoniae [17], inflammation triggered by the pneumococcal pilus [[18], [19], [20]] or cross-reactive antibodies [21]. No overall association between S. aureus and LRTI was found.
Other URT bacteria commonly associated with LRTI, particularly in LMICs, include Mycobacterium tuberculosis, B. pertussis, E. coli and K. pneumoniae [22]. M. tuberculosis may present with acute LRTI and can be detected from nasopharyngeal samples [23]; yet, no studies included in this review screened for M. tuberculosis using URT specimens [24,25]. Furthermore, only two studies included B. pertussis despite its high burden in LMICs [26].
Five studies screened for Klebsiella spp. and meta-analysis showed a higher prevalence among cases. Klebsiella spp. have been identified as an important cause of neonatal infections [27] and death [28], particularly in LMICs [29]. PCR was commonly done for M. pneumoniae and C. pneumoniae [30], yet, their prevalence was low and showed no clear association with LRTI. M. pneumoniae and C. pneumoniae are more commonly detected from school-aged children, compared with younger children [31]. Sputum, as opposed to NP specimens, has been reported as preferred specimen type to screen for M. pneumoniae and C. pneumoniae [32].
We identified heterogeneity between studies in the association of the four major bacterial species with LRTI, which may be accounted for by several factors. Firstly, definitions of cases and controls differed between studies. The WHO case definition for pneumonia was used by most studies [33], but lacks specificity [34,35]. Other case definitions included LRTI and bronchiolitis. Cases with bronchiolitis were generally younger than two years of age. Difference in age is likely to be an important source of heterogeneity, given age-related differences in aetiology of LRTI [31]. Controls were primarily classified as asymptomatic. Children with URTI may harbour a different URT microbiota than asymptomatic children [36], hence an ideal population-based control group for LRTI should be balanced between healthy children and those with URTI where dysbiotic URT bacterial communities could control for inflated ORs [37]. Secondly, study populations may contribute to heterogeneity. Hospitalised cases with more severe illness or prior medical treatment may differ from cases diagnosed at community clinics. Hospitalised controls may similarly not reflect the general population. Thirdly, sampling niches with differences in bacterial biomass and composition could also contribute to heterogeneity [38]. The nasopharynx was most frequently sampled [39,40], and shows high overlap with bacterial colonizers of topographically proximate niches such as the anterior nares and the oropharynx [41], yet, the combined study of nasopharyngeal and oropharyngeal niches may be of value [42,43]. Fourthly, age, vaccination coverage, antibiotic treatment, mode of delivery, feeding practices, indoor air pollution, tobacco smoke exposure, crowding, HIV or seasonal changes have been associated with changes in nasopharyngeal microbial communities [[44], [45], [46]]. Only 29 studies (58%) adjusted for confounders, primarily age and season.
A limitation of many of the studies is the targeted detection of only a few bacterial species. A more comprehensive approach towards assessment of the bacterial component is through 16S rRNA gene amplicon sequencing. However, 16S rRNA sequencing may introduce amplification bias and short-read 16S rRNA sequencing used by studies included in this review has limited ability to discriminate at the species level. Metagenomic sequencing may be preferable to allow for de novo identification of pathogens at species-level [47].
This review focused on investigation of URT bacteria, which may not accurately represent the site of infection. LRT specimens, such as induced sputum or bronchoalveolar lavage are likely to provide more direct evidence for aetiology [48,49]. Using transthoracic lung aspirates (TLA) and pleural fluid (PF), the PERCH study identified S. pneumoniae and S. aureus as predominant pathogens from a select group of children with severe pneumonia [50]. However, sampling of LRT specimens is relatively invasive and requires expertise [51]. Furthermore, LRT specimens are frequently contaminated by URT microbiota [51]. URT specimens are therefore most commonly sampled to investigate LRTI aetiology in children [42,[52], [53], [54]]. It is, however, important to note that although URT specimens are useful to “rule out” causes of LRTI, these specimens are less useful to “rule in” aetiological agents [55].
Studies included in this review identified associations between URT bacteria and LRTI but causality cannot be directly inferred. Furthermore, molecular techniques used by several studies included in this review may improve bacterial detection rates, but do not distinguish between live pathogenic organisms and residual nucleic acid from non-viable organisms, nor do they distinguish between commensal and pathogenic organisms.
This systematic review was limited to bacterial pathogens and did not include studies investigating associations between non-bacterial pathogens and LRTI. The aetiological role of respiratory viruses in LRTI among children under the age of five has recently been reviewed [56]. Furthermore, although viruses have been described as the most common aetiologic agent of LRTI, the severity of disease associated with bacterial infection and increasing antibiotic resistance makes bacterial pneumonia a major public health concern. A previous systematic review examining temporal associations between respiratory viruses and bacteria concluded that viruses both alter bacterial communities in the URT and contribute to bacterial colonization of the LRT [57]. However, the latter review could not quantify the contribution of bacteria to LRTI in the context of viral infection.
Data included in this review were derived from epidemiological studies of association. Such studies have limited ability to address pathogenetic mechanisms. However, they raise important questions around pathogenesis. Does bacterial colonization of the URT always precede LRTI, and how does bacterial density relate to the risk of subsequent LRTI? How does the balance shift between colonization and invasive disease? How does signalling within URT bacterial communities influence progression to LRTI? Further studies aimed at elucidating these pathogenetic process are needed.
Transparency declaration
The authors declare no conflict of interest. Research reported in this publication was supported by the National Institutes of Health Common Fund, through the Office of Strategic Coordination/Office of the NIH Director, National Institute of Environmental Health Sciences and National Human Genome Institute of Health of the National Institutes of Health (H3Africa awards grant numbers U54HG009824 and 1U01AI110466); Bill & Melinda Gates Foundation, Seattle, WA (grant number OPP1017641, OPP1017579). M.P.N. is supported by an Australian National Health and Medical Research Council Investigator Grant (APP1174455). S.C. received funding from the National Research Foundation South Africa and the L’Oréal-UNESCO For Women in Science South African Young Talents Award. H.J.Z. received funding from the South African Medical Research Council. The funding bodies had no role in the study design, the collection, analysis or interpretation of the data or the writing of the manuscript.
Contributors
S.C. was involved in study conception and design, conducted the study searches, screening, data extraction, analysis and interpretation and drafted the final report. K.Y.L.L. and C.M. were involved in screening, data extraction checks and contributed to the final report. H.J.Z. was involved in study conception and design and contributed to the final report. M.P.N. was involved in study conception and design and drafting of the final report. All authors approved the submitted version.
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.05.034.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Le Roux D.M., Zar H.J. Community-acquired pneumonia in children—a changing spectrum of disease. Pediatr Radiol. 2017;47:1392–1398. doi: 10.1007/s00247-017-3827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troeger C.E., Khalil I.A., Blacker B.F., Biehl M.H., Albertson S.B., Zimsen S.R.M. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2020;20:60–79. doi: 10.1016/S1473-3099(19)30410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth G.A., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeedi P., Salimian J., Ahmadi A., Imani Fooladi A.A. The transient but not resident (TBNR) microbiome: a Yin Yang model for lung immune system. Inhal Toxicol. 2015;27:451–461. doi: 10.3109/08958378.2015.1070220. [DOI] [PubMed] [Google Scholar]
- 6.Dickson R.P., Erb-Downward J.R., Freeman C.M., McCloskey L., Beck J.M., Huffnagle G.B. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson R.P., Erb-Downward J.R., Martinez F.J., Huffnagle G.B. The microbiome and the respiratory tract. Annu Rev Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claassen-Weitz S., Lim K.Y., Mullally C., Zar H.J., Nicol M.P. PROSPERO; 2020. The association between detection of bacteria in the upper respiratory tract with lower respiratory tract infection in young children: protocol for a systematic review.https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200544 CRD42020200544. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 10.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available from:
- 11.Review manager (RevMan) [computer program]. Version 5.4. Copenhagen: the nordic cochrane centre. The Cochrane Collaboration; 2020. [Google Scholar]
- 12.The Pneumonia Etiology Research for Child Health (PERCH) Study Group Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394:757–779. doi: 10.1016/S0140-6736(19)30721-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar H.J., Barnett W., Stadler A., Gardner-Lubbe S., Myer L., Nicol M.P. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463–472. doi: 10.1016/S2213-2600(16)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slack M.P.E. The evidence for non-typeable Haemophilus influenzae as a causative agent of childhood pneumonia. Pneumonia. 2017;9:2–5. doi: 10.1186/s41479-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bair K.L., Campagnari A.A. Moraxella catarrhalis promotes stable polymicrobial biofilms with the major otopathogens. Front Microbiol. 2020;10:1–9. doi: 10.3389/fmicb.2019.03006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettigrew M.M., Gent J.F., Revai K., Patel J.a., Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis. 2008;14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiss-Mandel A., Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: myth or reality? Hum Vaccines Immunother. 2016;12:351–357. doi: 10.1080/21645515.2015.1081321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regev-Yochay G., Lipsitch M., Basset A., Rubinstein E., Dagan R., Raz M. The pneumococcal pilus predicts the absence of Staphylococcus aureus Co-colonization in pneumococcal carriers. Clin Infect Dis. 2009;48:760–763. doi: 10.1086/597040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barocchi M.A., Ries J., Zogaj X., Hemsley C., Albiger B., Kanth A. A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci USA. 2006;103:2857–2862. doi: 10.1073/pnas.0511017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson A.L., Ries J., Bagnoli F., Dahlberg S., Fälker S., Rounioja S. RrgA is a pilus-associated adhesin in Streptococcus pneumoniae. Mol Microbiol. 2007;66:329–340. doi: 10.1111/j.1365-2958.2007.05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lijek R.S., Luque S.L., Liu Q., Parker D., Bae T., Weiser J.N. Protection from the acquisition of Staphylococcus aureus nasal carriage by cross-reactive antibody to a pneumococcal dehydrogenase. Proc Natl Acad Sci USA. 2012;109:13823–13828. doi: 10.1073/pnas.1208075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marangu D., Zar H.J. Childhood pneumonia in low-and-middle-income countries: an update. Paediatr Respir Rev. 2019;32:3–9. doi: 10.1016/j.prrv.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togun T.O., MacLean E., Kampmann B., Pai M. Biomarkers for diagnosis of childhood tuberculosis: a systematic review. PLoS One. 2018;13:1–19. doi: 10.1371/journal.pone.0204029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliwa J.N., Karumbi J.M., Marais B.J., Madhi S.A., Graham S.M. Tuberculosis as a cause or comorbidity of childhood pneumonia in tuberculosis-endemic areas: a systematic review. Lancet Respir Med. 2015;3:235–243. doi: 10.1016/S2213-2600(15)00028-4. [DOI] [PubMed] [Google Scholar]
- 25.Martinez L., Roux D.M., Barnett W., Stadler A., Nicol M.P., Zar H.J. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town , South Africa. Lancet Child Adolesc Heal. 2018;2:46–55. doi: 10.1016/S2352-4642(17)30149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeung K.H.T., Duclos P., Nelson E.A.S., Hutubessy R.C.W. An update of the global burden of pertussis in children younger than 5 years : a modelling study. Lancet Infect Dis. 2017;17:974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 27.Abdel-Wahab F., Ghoneim M., Khashaba M., El-Gilany A.-H., Abdel-Hady D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J Hosp Infect. 2013;83:196–199. doi: 10.1016/j.jhin.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Chawana R., Baillie V., Izu A., Solomon F., Bassat Q., Blau D.M. Potential of minimally invasive tissue sampling for attributing specific causes of childhood deaths in South Africa: a pilot , epidemiological study. Clin Infect Dis. 2019;69:361–373. doi: 10.1093/cid/ciz550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chisti M.J., Tebruegge M., Vincente S La, Graham S.M., Duke T. Pneumonia in severely malnourished children in developing countries – mortality risk, aetiology and validity of WHO clinical signs: a systematic review. Trop Med Int Heal. 2009;14:1173–1189. doi: 10.1111/j.1365-3156.2009.02364.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y., Wang Q., Li D., Yao K., Wang T. Abundance of the nasopharyngeal microbiome effects pertussis diagnosis and explains the sensitivity difference between bacterial culture and real-time PCR. Eur J Clin Microbiol Infect Dis. 2020;39:501–507. doi: 10.1007/s10096-019-03750-5. [DOI] [PubMed] [Google Scholar]
- 31.Popovsky E.Y., Florin T.A. Community-acquired pneumonia in childhood. Ref Modul Biomed Sci. 2020 doi: 10.1016/B978-0-08-102723-3.00013-5. [DOI] [Google Scholar]
- 32.Loens K., Van Heirstraeten L., Malhotra-Kumar S., Goossens H., Ieven M. Optimal sampling sites and methods for detection of pathogen possibly causing community-acquired lower respiratory tract infections. J Clin Microbiol. 2009;47:21–31. doi: 10.1128/JCM.02037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott J., Brooks W., Peiris J., Holtzman D., Mulholland E. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardoso M.R.A., Nascimento-Carvalho C.M., Ferrero F., Alves F.M., Cousens S.N. Adding fever to WHO criteria for diagnosing pneumonia enhances the ability to identify pneumonia cases among wheezing children. Arch Dis Child. 2011;96:58–61. doi: 10.1136/adc.2010.189894. [DOI] [PubMed] [Google Scholar]
- 35.Saha D., Ronan A., Khan W.A., Salam M.A. Diagnosis of pneumonia in children with dehydrating diarrhoea. J Heal Popul Nutr. 2014;32:14–18. [PMC free article] [PubMed] [Google Scholar]
- 36.Chonmaitree T., Jennings K., Golovko G., Khanipov K., Pimenova M., Patel J.A. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellussi L.M., Passali F.M., Ralli M., Vincentiis M.D.E., Greco A., Passali D. An overview on upper respiratory tract infections and bacteriotherapy as innovative therapeutic strategy. Eur Rev Med Pharmacol Sci. 2019;23:27–38. doi: 10.26355/eurrev_201903_17345. [DOI] [PubMed] [Google Scholar]
- 38.Hammitt L.L., Kazungu S., Welch S., Bett A., Onyango C.O., Gunson R.N. Added value of an oropharyngeal swab in detection of viruses in children hospitalized with lower respiratory tract infection. J Clin Microbiol. 2011;49:2318–2320. doi: 10.1128/JCM.02605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chochua S., D’Acremont V., Hanke C., Alfa D., Shak J., Kilowoko M. Increased nasopharyngeal density and concurrent carriage of streptococcus pneumoniae, haemophilus influenzae, and Moraxella catarrhalis are associated with pneumonia in febrile children. PLoS One. 2016;11:1–13. doi: 10.1371/journal.pone.0167725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zar H.J., Workman L., Isaacs W., Munro J., Black F., Eley B. Rapid molecular diagnosis of pulmonary tuberculosis in children using nasopharyngeal specimens. Clin Infect Dis. 2012;55:1088–1095. doi: 10.1093/cid/cis598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Steenhuijsen Piters W.A.A., Sanders E.A.M., Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philos Trans R Soc B Biol Sci. 2015;370:20140294. doi: 10.1098/rstb.2014.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh R.L., Kaestli M., Chang A.B., Binks M.J., Pope C.E., Hoffman L.R. The microbiota in bronchoalveolar lavage from young children with chronic lung disease includes taxa present in both the oropharynx and nasopharynx. Microbiome. 2016;4:1–18. doi: 10.1186/s40168-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odutola A., Antonio M., Owolabi O., Bojang A., Foster-Nyarko E., Donkor S. Comparison of the prevalence of common bacterial pathogens in the oropharynx and nasopharynx of Gambian infants. PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0075558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch A.A.T.M., De Steenhuijsen Piters W.A.A., Van Houten M.A., Chu M.L.J.N., Biesbroek G., Kool J. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. Am J Respir Crit Care Med. 2017;196:1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- 45.Vanker A., Nduru P.M., Barnett W., Dube F.S., Sly P.D., Gie R.P. Indoor air pollution and tobacco smoke exposure: impact on nasopharyngeal bacterial carriage in mothers and infants in an African birth cohort study. ERJ Open Res. 2019;5 doi: 10.1183/23120541.00052-2018. 00052–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogaert D., Keijser B., Huse S., Rossen J., Veenhoven R., van Gils E. Variability and diversity of nasopharyngeal microbiota in children: a metagenomic analysis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson J.S., Spakowicz D.J., Hong B., Petersen L.M., Demkowicz P., Chen L. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10:5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettigrew M.M., Gent J.F., Kong Y., Wade M., Gansebom S., Bramley A.M. Association of sputum microbiota profiles with severity of community-acquired pneumonia in children. BMC Infect Dis. 2016;16:1–12. doi: 10.1186/s12879-016-1670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai W., Wang H., Zhou Q., Feng X., Lu Z., Li D. The concordance between upper and lower respiratory microbiota in children with Mycoplasma pneumoniae pneumonia article. Emerg Microbe. Infect. 2018;7:92. doi: 10.1038/s41426-018-0097-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebruke B.E., Knoll M.D., Haddix M., Zaman S.M., Prosperi C., Feikin D.R. The Aetiology of pneumonia from analysis of Lung aspirate and Pleural fluid samples: findings from the PERCH study. Clin Infect Dis. 2020;ciaa1032 doi: 10.1093/cid/ciaa1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zar H.J., Andronikou S., Nicol M.P. Advances in the diagnosis of pneumonia in children. BMJ. 2017;358:1–13. doi: 10.1136/bmj.j2739. [DOI] [PubMed] [Google Scholar]
- 52.Huxley E.J., Viroslav J., Gray W.R., Pierce A.K. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64:564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 53.Bassis C.M., Erb-Downward J.R., Dickson R.P., Freeman C.M., Schmidt T.M., Young V.B. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6:1–10. doi: 10.1128/mBio.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segal L.N., Clemente J.C., Tsay J.C.J., Koralov S.B., Keller B.C., Wu B.G. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016;1:1–11. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu A.Z., Shi P., Wang L.B., Qian L.L., Zhang X.B. Diagnostic value of nasopharyngeal aspirates in children with lower respiratory tract infections. Chin Med J (Engl) 2017;130:647–651. doi: 10.4103/0366-6999.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi T., McLean K., Campbell H., Nair H. Aetiological role of common respiratory viruses in acute lower respiratory infections in children under five years: a systematic review and meta–analysis. J Glob Health. 2015;5:1–10. doi: 10.7189/jogh.05.010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K.H., Gordon A., Foxman B. The role of respiratory viruses in the etiology of bacterial pneumonia. Evol Med Public Heal. 2016;2016:95–109. doi: 10.1093/emph/eow007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.