Abstract
Objective
to investigate whether the combination of dynapenia and abdominal obesity is worse than these two conditions separately regarding gait speed decline over time.
Methods
a longitudinal study was conducted involving 2,294 individuals aged 60 years or older free of mobility limitation at baseline (gait speed >0.8 m/s) who participated in the English Longitudinal Study of Ageing. Dynapenia was determined as a grip strength <26 kg for men and <16 kg for women. Abdominal obesity was determined as a waist circumference >102 cm for men and >88 cm for women. The participants were divided into four groups: non-dynapenic/non-abdominal obese (ND/NAO); only abdominal obese (AO); only dynapenic (D) and dynapenic/abdominal obese (D/AO). Generalised linear mixed models were used to analyse gait speed decline (m/s) as a function of dynapenia and abdominal obesity status over an 8-year follow-up period.
Results
over time, only the D/AO individuals had a greater gait speed decline (−0.013 m/s per year, 95% CI: −0.024 to −0.002; P < 0.05) compared to ND/NAO individuals. Neither dynapenia nor abdominal obesity only was associated with gait speed decline.
Conclusion
dynapenic abdominal obesity is associated with accelerated gait speed decline and is, therefore, an important modifiable condition that should be addressed in clinical practice through aerobic and strength training for the prevention of physical disability in older adults.
Keywords: waist circumference, grip strength, gait speed, mobility limitation, trajectories, older adults, ELSA study
Key Points
Dynapenic abdominal obesity is associated with accelerated gait speed decline.
Dynapenic obesity, measured by BMI, was not associated with gait speed decline.
Neither dynapenia nor abdominal obesity only was associated with gait speed decline.
Introduction
Mobility measured by gait speed is considered the sixth vital sign in the assessment of older adults [1, 2]. Mobility limitation compromises independence and increases the risk of falls, functional loss, hospitalization and death [3]. The decline in gait speed is a complex process associated with increasing age, low schooling and income, physical inactivity, smoking, joint diseases, diabetes, hypertension, heart disease, stroke, depression, cognitive decline [4, 5], age-related decline in muscle strength (dynapenia) and body fat accumulation [6].
Dynapenia is characterised by deficiencies in neural activation and motor recruitment patterns, the loss of alpha motor neurons, the replacement of type II fibres with type I fibres, as well as changes in muscle mass and architecture [7]. Moreover, with increasing age, there is an accumulation of intramuscular and abdominal fat, along with a reduction in subcutaneous fat [8–11]. Independently of intramuscular fat, an increase in abdominal fat stimulates pro-inflammatory activity [8–10, 12] and protein catabolism and blocks the effect of insulin on both muscle anabolism and the repair process of motor neurons [9, 10] compromising muscle strength [8, 13], which has a negative impact on mobility.
The co-existence of obesity and dynapenia, which is denominated dynapenic obesity, has been considered as a risk factor associated with gait speed decline. In a cross-sectional study, Yang and collaborators [14] found an association between dynapenic obesity (defined as a body mass index [BMI] ≥ 25 kg/m2 and the lowest tertile of grip strength) and slower gait speed. In another cross-sectional study, Bouchard and Janssen [15] found a similar association, defining dynapenic obesity as the highest tertile of total fat mass (determined by dual-energy x-ray absorptiometry) and the lowest tertile of leg extensor strength. On the other hand, the few longitudinal studies addressing this issue report conflicting results. Stenholm and collaborators [16] found that dynapenic obesity (BMI ≥ 30 kg/m2 and the lowest tertile of knee extensor strength) was associated with gait speed decline, whereas Batsis and collaborators [17], using the same definition of dynapenic obesity as Stenholm and collaborators [16], did not find such an association.
At present, there is a lack of consensus about the concept of dynapenic obesity. In addition, to the best of our knowledge, the association between dynapenic abdominal obesity (defined by a waist circumference >88 cm for women and >102 cm for men and a grip strength <16 kg for women and <26 kg for men) and mobility decline has not been analysed, despite being related to worse trajectories of basic and instrumental activities of daily living (BADLs and IADLs) disability [18, 19], the occurrence of falls [20] and mortality [21]. Therefore, the present study aimed to test the following hypothesis: among individuals free of mobility limitations at baseline, the trajectory of gait speed decline over an 8-year follow-up period is greater in individuals with dynapenic abdominal obesity than those with only dynapenia or only abdominal obesity.
Methods
Study population
Data were extracted from the English Longitudinal Study of Ageing (ELSA), an ongoing panel study involving community-dwelling individuals in England aged 50 years or older. ELSA began in 2002 with a sample composed of participants of the Health Survey for England (HSE), a nationally representative survey using a random probability sample stratified in different stages [22]. ELSA follow-up interviews occur every 2 years and health examinations (i.e. nurse visits), carried out for the first time in 2004, every 4 years. A detailed description of the study can be found in a previous publication [23]. The present sample was composed of 2,294 individuals aged 60 years or older free of mobility limitation at baseline (gait speed >0.8 m/s) [3, 24] that participated in ELSA in 2004–2005, reassessed in 2008–2009 and 2012–2013 totalizing 8-year follow-up period.
Gait speed
Usual gait speed was determined by the best time between two consecutive trials for 2.4 m on a flat surface without the use of a gait-assistance device [25–27]. The total course in meters was divided by the time in seconds for conversion into meters/second (m/s). In the trajectory analysis, gait speed in m/s was considered a continuous variable [28]. To ensure the inclusion of individuals free of mobility limitation at baseline, only participants with a gait speed greater than 0.8 m/s were selected. This cut-off point was chosen because it is more sensitive and the most frequently used to identify adverse health outcomes in older adults, such as the incidence of mobility limitation, BADLs disability and mortality, than cut-offs of 1.0 or 1.2 m/s [3, 24].
Anthropometric measures and classification of groups
Muscle strength was determined using a hand dynamometer (Smedley; range: 0–100 kg). Grip strength is widely used as a measure of muscle strength and is considered a reliable predictor of negative outcomes [29–31], such as mobility limitation and BADLs disability [29, 32–35]. Moreover, previous studies show similarities between grip strength and knee extensor strength [36, 37] in the association with gait speed decline [32–34]. The test was performed with the participant standing, arms alongside the trunk and elbow flexed at 90° [38]. Three maximum trials were performed with the dominant hand, respecting a 1-min interval between trials. The highest value was considered in the analysis. Dynapenia was defined as grip strength <26 kg for men and <16 kg for women [18, 19, 24].
Waist circumference was measured using a metric tape at the midpoint between the lowest rib and upper margin of the iliac crest. The participant remained standing and two measurements were made at the end of the expiratory phase of the respiratory cycle [38]. If the difference between readings exceeded 3 cm, a third measurement was taken. Abdominal obesity was defined as waist circumference >102 cm for men and >88 cm for women [39, 40].
The participants were divided into four-category time-varying groups based on their dynapenia and abdominal obesity status: non-dynapenic/non-abdominal obese (ND/NAO); only abdominal obese (AO); only dynapenic (D) and dynapenic/abdominal obese (D/AO) [19].
Co-variates
The co-variates included in the present analysis constitute a broad spectrum of factors associated with gait speed decline [4]. All co-variates were treated as having fixed and a random effect over time.
The sociodemographic variables were sex, age, marital status (with or without conjugal life), educational level (0–11 years; 12–13 years; >13 years) and total household wealth, including financial, housing and physical wealth, such as jewellery and artwork (divided into quintiles).
Health-related behaviours included the classification of the participants as non-smoker, former smoker (individuals who quit smoking at least 1 year earlier) or current smoker. Frequency of alcohol consumption was classified as non-drinkers or drinking 1 day a week, drinking on 2–6 days a week or drinking daily [18]. Sedentary lifestyle (vigorous or moderate physical activity once per week, one to three times per month, hardly ever or never; any mild physical activity) or active lifestyle (vigorous or moderate physical activity more than once a week) [19] was defined based on the level of physical activity determined by the instrument validated by the Health Survey for England [41].
Health status was determined by self-reported medical diagnosis of cancer, stroke, systemic arterial hypertension, heart disease, lung disease, joint disease, osteoporosis and falls in the previous 12 months. Diabetes was recorded in the occurrence of glycated haemoglobin ≥6.5% [42].
Memory was assessed using word-list learning test with higher scores indicating a better memory (range: 0–20 words) [43]. Depressive symptoms were determined using the Center for Epidemiologic Studies Depression Scale (CES-D), considering a cut-off of ≥4 points [44].
Weight (kg) was measured using a Tanita electronic scale without shoes and wearing light clothing. Height (m) was measured using a standardised Leicester portable stadiometer. BMI was calculated using the standard formula [weight (kg)/height (m) squared].
Statistical analysis
Differences in the baseline characteristics among the four analytical groups according to dynapenia and abdominal obesity status were evaluated using the chi-square test, analysis of variance (ANOVA), and post hoc Tukey’s test. A P value <0.05 was considered indicative of statistical significance.
To estimate the trajectories of gait speed over time, we used generalised linear mixed models using the XTMIXED procedure in Stata 14 SE (Stata Corp, College Station, TX, USA). This model deals better with unbalanced data in studies with repeated measures, enabling the statistical modelling of time-dependent changes in the outcome and the magnitude of associations between variables [45, 46]. The rates of gait speed decline were compared using ß coefficients and 95% confidence intervals (CI). In the final models, the intercept represents the estimated mean difference in gait speed at baseline among individuals according to dynapenia and abdominal obesity status, taking the ND/NAO as the reference category. On the slope, time (in years) indicates the magnitude of the trajectory of gait speed decline independently of the co-variates (as if time per se was determinant of the decline). The interaction between time and each dynapenia and abdominal obesity status represents the estimated difference in the annual rate of gait speed decline (slope) between each of the three groups (AO, D and D/AO) and the reference group (ND/NAO), evaluating the annual rate of the change in gait speed in each group.
Three sensitivity analyses were also performed. First, to investigate whether dynapenia alone (yes/no) and abdominal obesity alone (yes/no), i.e. as independent conditions, would be capable of modifying the associations found in the original models; second, to investigate whether dynapenic obesity using BMI ≥ 30 kg/m2 instead of abdominal obesity is associated with gait speed decline; and third, to investigate whether the results would be consistent with the original model when considering the 1,452 individuals with complete data in the 8 years of follow-up.
Results
Among the 2,294 participants free of mobility limitation at baseline, 1,749 and 1,452 were re-evaluated after 4 and 8 years, respectively. Little more than 63% of the initial analytical sample participated in the three waves of the study and 76% participated in two waves. The baseline characteristics according to dynapenia and abdominal obesity status are displayed in Table 1.
Table 1 .
Baseline characteristics of 2,294 older adults of ELSA study (2004) according to dynapenia and abdominal obesity status
| ND/NAO | AO | D | D/AO | |
|---|---|---|---|---|
| n = 1,180 | n = 991 | n = 74 | n = 49 | |
| Age, (mean ± SD) | 68.3 ± 6.5 | 68.2 ± 6.0 | 74.0 ± 8.4a,b | 71.2 ± 9.0a,b |
| Sex (female), (%) | 46.4 | 55.1a | 48.6 | 63.3 |
| Marital status (without conjugal life), (%) | 28.5 | 26.3 | 39.2 | 40.8 |
| Household wealth, (%) | ||||
| 1st quintile (highest) | 31.9 | 25.1a | 21.6 | 14.3a |
| 2nd quintile | 25.1 | 22.6 | 29.7 | 22.4 |
| 3rd quintile | 19.4 | 23.0 | 21.6 | 32.7 |
| 4th quintile | 14.4 | 16.8 | 10.8 | 12.2 |
| 5th quintile (lowest) | 8.0 | 11.0 | 16.3 | 18.4a |
| Not reported (%) | 1.2 | 1.5 | - | - |
| Educational level (%) | ||||
| >13 years | 30.8 | 26.0 | 24.3 | 8.2a,b |
| 12–13 years | 25.5 | 22.7 | 20.3 | 30.6 |
| 0–11 years | 43.7 | 51.3 | 55.4 | 61.2 |
| Smoking status (%) | ||||
| Non-smoker | 42.2 | 37.0 | 24.3a | 30.6 |
| Ex-smoker | 46.9 | 53.3a | 66.2a | 59.2 |
| Smoker | 10.9 | 9.7 | 9.5 | 10.2 |
| Alcohol intake (%) | ||||
| Non-drinker or rare drinker | 12.4 | 15.4 | 16.2 | 20.4 |
| Frequent drinker | 44.2 | 44.0 | 44.6 | 42.9 |
| Daily drinker | 38.1 | 34.3 | 31.1 | 20.4a |
| Did not answer | 5.3 | 6.3 | 8.1 | 16.3a,b |
| Physical activity status (sedentary) (%) | 20.3 | 28.0a | 27.0 | 42.9a |
| Hypertension (%) | 33.7 | 49.9a | 35.1b | 53.1 |
| Diabetes (%) | 4.8 | 8.9a | 1.4 | 10.2 |
| Cancer (%) | 8.1 | 9.2 | 13.5 | 2.0 |
| Lung disease (%) | 14.2 | 16.4 | 10.8 | 22.4 |
| Heart disease (%) | 19.7 | 19.3 | 18.9 | 36.7a,b |
| Stroke (%) | 3.1 | 2.4 | 2.7 | 6.1 |
| Joint disease (%) | 26.8 | 36.9a | 63.5a,b | 63.3a,b |
| Osteoporosis (%) | 6.2 | 5.0 | 10.8 | 12.2 |
| Falls (%) | 23.7 | 26.1 | 31.1 | 32.7 |
| Depressive symptoms (%) | 6.7 | 9.1 | 13.5 | 12.2 |
| Memory score, points (mean ± SD) | 10.3 ± 3.1 | 10.3 ± 3.1 | 9.1 ± 3.9a,b | 9.4 ± 3.2 |
| Body mass index, kg/m2 (mean ± SD) | 24.8 ± 2.5 | 30.4 ± 3.6a | 24.1 ± 2.8a,b | 29.5 ± 4.5a,c |
| Grip strength, kg (mean ± SD) | ||||
| Men | 40.0 ± 7.3 | 41.1 ± 7.5a | 18.5 ± 7.8a,b | 20.9 ± 5.1a,b |
| Women | 24.8 ± 4.9 | 25.3 ± 4.7 | 11.9 ± 3.0a,b | 12.4 ± 3.1a,b |
| Waist circumference, cm (mean ± SD) | ||||
| Men | 93.7 ± 6.2 | 109.8 ± 6.5a | 92.1 ± 7.9b | 111.3 ± 10.1a,c |
| Women | 80.3 ± 5.4 | 98.1 ± 8.2a | 80.2 ± 6.2b | 97.4 ± 7.3a,c |
| Gait speed, m/s (mean ± SD) | 1.10 ± 0.2 | 1.05 ± 0.2a | 1.02 ± 0.2a | 1.00 ± 0.2a |
Data expressed as percentage, mean and standard deviation (SD). Hyphen (−) indicates absence of observations. ND/NAO: non-dynapenic/non-abdominal obese; AO: only abdominal obese; D: only dynapenic; D/AO: dynapenic/abdominal obese. Statistical significance P < 0.05
aSignificantly different from ND/NAO
bSignificantly different from AO
cSignificantly different from D
Table 2 displays the estimated parameters of the generalised linear mixed model for changes in gait speed as a function of dynapenia and abdominal obesity status in 8-year follow-up period. Time per se was not an independent predictor of gait speed decline. Among the groups analysed, the AO group had worse gait speed on the intercept (−0.024 m/s 95% CI: −0.046 to −0.001; P < 0.05) than ND/NAO. Over an 8-year follow-up, only the D/AO group had greater gait speed decline compared to the ND/NAO group. The estimated parameter for the difference in the slope was −0.013 m/s per year (95% CI: −0.024 to −0.002; P < 0.05), which corresponds to −0.15 m/s at the end of the 8-year follow-up (Figure 1 and Table 5) after adjusting for age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score, height and weight. Table 5 and Figure 1 show the predicted mean values of gait speed for the ND/NAO, AO, D and D/AO groups in each year during the 8-year follow-up period in 2,294 English older adults.
Table 2 .
Generalised linear mixed model estimated for gait speed decline as a function of dynapenia and abdominal obesity status in 8-year follow-up (2004–2012) of 2,294 English older adults—main analysis
| Dynapenia and abdominal obesity status | Estimated parameters (lower to upper 95%CI) | |
|---|---|---|
| Time, years | 0.022 | (−0.045 to 0.090) |
| Intercept | ||
| Non-dynapenic/non-abdominal obese | Reference | |
| Only abdominal obese | −0.024 | (−0.046 to −0.001)* |
| Only dynapenic | −0.032 | (−0.076 to 0.013) |
| Dynapenic/abdominal obese | −0.021 | (−0.075 to 0.033) |
| Slope | ||
| Time × Non-dynapenic/non-abdominal obese | Reference | |
| Time × Only abdominal obese | −0.004 | (−0.010 to 0.001) |
| Time × Only dynapenic | −0.002 | (−0.011 to 0.008) |
| Time × Dynapenic/abdominal obese | −0.013 | (−0.024 to −0.002)* |
Dynapenia was defined by grip strength <26 kg for men and <16 kg for women. Abdominal obesity was defined by waist circumference >102 cm for men and >88 cm for women. Estimated parameters represent difference in slope (estimated changes in gait speed per unit of time) between groups in question and reference. Model adjusted by age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score, height and weight
*P < 0.05
Figure 1 .
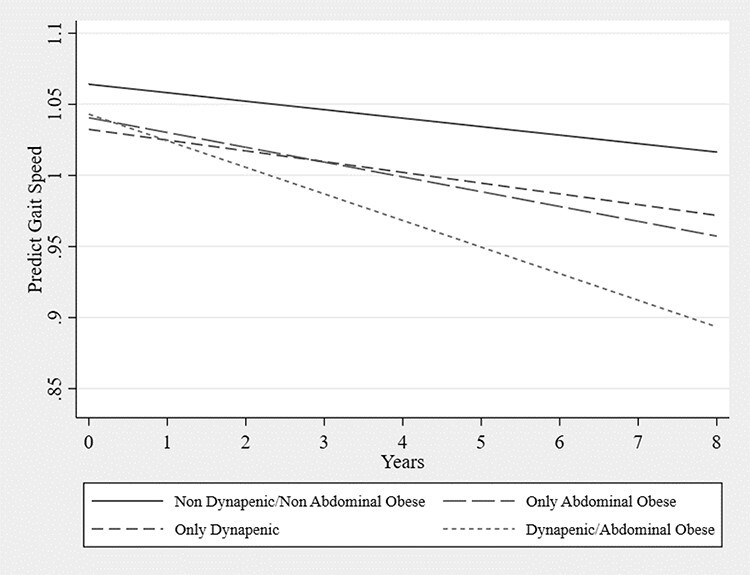
Gait speed trajectory according to dynapenia and abdominal obesity status adjusted for age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score, height and weight—main analysis ELSA Study 2004–2012.
Table 5 .
Prediction of average annual gait speed as a function of dynapenia and abdominal obesity status in 8-year follow-up (2004–2012) of 2,294 English older adults—main analysis
| ND/NAO | AO | D | D/AO | |||||
|---|---|---|---|---|---|---|---|---|
| Predicted | 95% CI | Predicted | 95% CI | Predicted | 95% CI | Predicted | 95% CI | |
| values | values | values | values | |||||
| Baseline | 1.064 | 1.051–1.078 | 1.041 | 1.026–1.055 | 1.032 | 0.989–1.076 | 1.043 | 0.991–1.095 |
| Year 1 | 1.058 | 1.047–1.070 | 1.030 | 1.018–1.043 | 1.025 | 0.987–1.063 | 1.024 | 0.980–1.069 |
| Year 2 | 1.052 | 1.042–1.063 | 1.020 | 1.008–1.031 | 1.017 | 0.984–1.051 | 1.006 | 0.967–1.044 |
| Year 3 | 1.046 | 1.035–1.057 | 1.009 | 0.998–1.021 | 1.010 | 0.978–1.041 | 0.987 | 0.952–1.022 |
| Year 4 | 1.040 | 1.028–1.052 | 0.999 | 0.986–1.011 | 1.002 | 0.970–1.034 | 0.968 | 0.934–1.003 |
| Year 5 | 1.034 | 1.020–1.048 | 0.989 | 0.974–1.003 | 0.995 | 0.960–1.030 | 0.950 | 0.913–0.986 |
| Year 6 | 1.028 | 1.012–1.045 | 0.978 | 0.961–0.995 | 0.987 | 0.947–1.027 | 0.931 | 0.890–0.972 |
| Year 7 | 1.022 | 1.003–1.042 | 0.968 | 0.948–0.988 | 0.979 | 0.933–1.026 | 0.912 | 0.865–0.960 |
| Year 8 | 1.016 | 0.994–1.039 | 0.957 | 0.934–0.981 | 0.972 | 0.918–1.029 | 0.893 | 0.838–0.949 |
Dynapenia was defined by grip strength <26 kg for men and <16 kg for women. Abdominal obesity was defined by waist circumference >102 cm for men and >88 cm for women. ND/NAO: non-dynapenic/non-abdominal obese; AO: only abdominal obese; D: only dynapenic; D/AO: dynapenic/abdominal obese. Adjusted by age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score, height and weight.
Compared to our main analysis, the results of the first sensitivity analysis confirmed that abdominal obesity alone and dynapenia alone (as independent conditions) were not associated with gait speed decline over time (Table 3). The second sensitivity analysis confirmed that dynapenic obesity (BMI ≥ 30 kg/m2 and grip strength <26 kg for men and <16 kg for women) was not associated with gait speed decline over time (Table 4). The third sensitivity analysis, including 1,452 individuals with complete data during the 8-year follow-up period (Supplementary Figure S1, Supplementary Tables S1 and S2 available in Age and Ageing online), did not alter the results found in the main analysis (Figure 1 and Table 5).
Table 3 .
Generalised linear mixed model estimated for gait speed decline as a function of dynapenia alone and abdominal obesity alone, as independent conditions, in 8-year follow-up (2004–2012) of 2,294 English older adults—sensitivity analysis
| Dynapenia alone and abdominal obesity alone (as independent conditions) | Estimated parameters (lower to upper 95% CI) | |
|---|---|---|
| Time, years | 0.021 | (−0.046 to 0.088) |
| Intercept | ||
| Abdominal obesity alone | -0.021 | (−0.043 to 0.001) |
| Dynapenia alone | -0.018 | (−0.052 to 0.017) |
| Slope | ||
| Time × Abdominal obesity alone | -0.005 | (−0.010 to 0.001) |
| Time × Dynapenia alone | -0.004 | (−0.012 to 0.003) |
Sensitivity analysis using dynapenia alone (yes/no) and abdominal obesity alone (yes/no) as independent conditions, instead of combining both conditions. Dynapenia was defined by grip strength <26 kg for men and <16 kg for women. Abdominal obesity was defined by waist circumference >102 cm for men and >88 cm for women. Estimated parameters represent difference in slope (estimated changes in gait speed per unit of time) between groups in question and reference. Model adjusted by age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score, height and weight
*P < 0.05
Table 4 .
Generalised linear mixed model estimated for gait speed decline as a function of dynapenia and obesity (BMI) status in 8-year follow-up (2004–2012) of 2,294 English older adults—sensitivity analysis
| Dynapenia and obesity (BMI) status | Estimated parameters (lower to upper 95% CI) | |
|---|---|---|
| Time, years | −0.002 | (−0.031 to 0.026) |
| Intercept | ||
| Non-dynapenic/non-obese | Reference | |
| Only obese | −0.031 | (−0.054 to −0.009)* |
| Only dynapenic | −0.022 | (−0.059 to 0.016) |
| Dynapenic/obese | −0.050 | (−0.137 to 0.036) |
| Slope | ||
| Time × Non-dynapenic/non-obese | Reference | |
| Time × Only obese | −0.002 | (−0.004 to 0.008) |
| Time × Only dynapenic | −0.004 | (−0.012 to 0.004) |
| Time × Dynapenic/obese | −0.003 | (−0.020 to 0.013) |
Sensitivity analysis using dynapenic obesity (BMI ≥ 30 kg/m2 and grip strength <26 kg for men and <16 kg for women) instead of dynapenic abdominal obesity (waist circumference >102 cm for men and >88 cm for women and grip strength <26 kg for men and <16 kg for women). Estimated parameters represent difference in slope (estimated changes in gait speed per unit of time) between groups in question and reference. Model adjusted by age, sex, household wealth, smoking status, physical activity status, depressive symptoms, diabetes, hypertension, heart disease, falls, osteoporosis, joint disease, mean memory score and waist circumference
*P < 0.05
Discussion
Our main findings in individuals free of mobility limitation at baseline showed that dynapenic abdominal obesity is worse than only dynapenia and only obesity with regard to gait speed decline over time in older adults, underscoring the relevance of this condition as a clinical entity. In contrast, dynapenic obesity was not associated with gait speed decline.
Cross-sectional studies using different methodologies have investigated the relationship between dynapenic obesity and slower gait speed. Evaluating 616 Chinese individuals aged 60 years or older considering BMI ≥ 25 kg/m2 and lowest tertile of grip strength, Yang and collaborators [14] found that dynapenic obesity was associated with slower gait speed in older adults. Bouchard and Janssen [15] reported the same association in an evaluation of 2,039 men and women aged 55 years or older considering the highest tertile of total fat mass (determined by dual-energy x-ray absorptiometry) and lowest tertile of leg extensor strength.
The only two longitudinal studies found addressing this issue showed contradictory results. Analysing 930 males and females aged 65 years or older free of self-reported mobility limitation at baseline, Stenholm et al. [16] found that dynapenic obesity (BMI ≥ 30 kg/m2 and lowest tertile of knee extensor strength) increases the rate of the gait speed decline over a 6-year follow-up. However, using the same operational definition, Batsis and colleagues [17] did not find this association in an analysis of 2,025 individuals aged 60 years or older with knee osteoarthritis over a 4-year follow-up period.
Although the sample of the present study had a similar mean age (68.5 years) as that in the Batsis et al. study [17] (68.2 years) and both had a lower mean age than that in the study conducted by Stenholm et al. [16] (74.1 years), the present results using dynapenic abdominal obesity were similar to those in the latter study [16]. The lack of association between dynapenic obesity and gait speed decline in the study conducted by Batsis et al. [17] may have occurred because the mean strength was greater in their sample and, consequently, the distribution of tertiles resulted in higher cut-off points for the definition of dynapenia compared to those from Stenholm et al. study (37.3 vs. 21.5 kg for men and 23.9 vs. 14.3 kg for women). However, these results cannot be compared to the present findings due to the fact that we have used cut-off points for grip strength previously used in the literature to define dynapenia [18–21]. Moreover, the individuals that composed the sample at baseline in Batsis et al. study [17] had higher gait speed in the four groups studied (ND/NAO, AO, D and D/AO) than those in the Stenholm et al. study [16] and in the present investigation. Finally, the follow-up time of Batsis et al.’s study [17] was shorter. Therefore, as the sample had higher gait speed with greater strength at baseline, the decline might only be evident in a longer follow-up period.
We found that dynapenic abdominal obesity was associated with gait speed decline, which did not occur with dynapenic obesity (measured by BMI). The explanation for this finding may lie in the fact that waist circumference is better than BMI for measuring fat redistribution over time in a metabolically more active region [8–10] that exerts a greater negative impact on muscle strength [8, 13] and, consequently, gait speed [6]. Besides the reduction in grip strength that occurs with increasing age [47, 48], there is cross-sectional [49–51] and longitudinal [13] evidence showing that abdominal obesity measured by waist circumference may accelerate this process, whereas the contrary occurs with BMI. For instance, Keevil et al. [49] analysed 8,441 participants from the European Prospective Investigation into Cancer-Norfolk aged 48–92 years and found a reduction in grip strength of 3.56 kg in men and 1.00 kg in women for every 10 cm increase in waist circumference, whereas an increase in grip strength of 4.28 kg in men and 1.26 kg in women was found for every 4.0 kg/m2 increase in BMI. This highlights the importance of using waist circumference rather than BMI and the definition of dynapenic abdominal obesity rather than dynapenic obesity on the assessment of older adults.
Ageing is marked by numerous physical and neurophysiological changes that can lead to dynapenia. The pathways involved in this process include deficiencies in neural activation and motor recruitment patterns, a reduction in muscle quantity and contractile quality, and fat infiltration in muscle fibres [2]. Moreover, a significant accumulation of age-related central fat can exacerbate the process of dynapenia [8, 13] mediated by the increase in the expression of circulating inflammatory cytokines, such as tumor necrosis factor (TNF-α and TNF-β) and interleukin (IL-6) [8–10, 12]. The oxidative stress found with this low-grade inflammation can promote the degradation of muscle fibres as well as attenuate anabolic action in muscle tissue and regenerative action in neural tissue due to insulin growth factor (IGF-1) [8–10, 12]. Therefore, individuals with dynapenic abdominal obesity may be more predisposed to gait speed decline.
Time per se was not an independent predictor of gait speed decline. Similar results have been described in previous studies on the trajectory of BADLs and IADLs disability [18, 19]. Such findings indicate that mobility decline is more dependent on adverse socioeconomic, behavioural and clinical factors than time per se. Noteworthy, the difference in gait speed between the ND/NAO and D/AO groups was 0.02 m/s at baseline and increased to nearly 0.12 m/s at the end of the 8-year follow-up, which is relevant as the literature indicates that 0.10 m/s is the minimal clinically important difference capable of affecting gait and exerting an impact on the daily living of older adults [3]. Thus, as dynapenic abdominal obesity has been demonstrated to be a risk factor for mortality, falls and worse trajectories of BADLs and IADLs disability [18, 19], the present study by linking this condition to gait speed decline over time presents further evidence that dynapenic abdominal obesity should be evaluated as a clinical entity.
The present study has several strong points. The first is the use of a large representative national English sample of community-dwelling older adults. Analyses involving three waves of the study with a long follow-up time make important contributions regarding the incidence of gait speed decline in older adults. The use of an objective physical performance measure and the adjustment of the models by a broad spectrum of important variables associated with the exposure and outcome are further strengths of this study. Moreover, to the best of our knowledge, this is one of the few studies to consider the influence of the regional distribution of adipose tissue in ageing.
This study also has some limitations that should be recognised. First, losses to follow-up could be a source of bias. Such losses are unavoidable in longitudinal studies involving community-dwelling older adults; however, they did not interfere with our final results. Second, the sample had a small number of individuals with dynapenic abdominal obesity. However, this fact did not impede us from finding an association with gait speed decline over the 8 years of follow-up. Third, the cut-off points could have led to the non-classification of individuals in pre-clinical states in the groups. While this occurs in all studies that use cut-off points, generalised linear mixed models offer the advantage of analysing variations in the same individual over time, enabling the identification and incorporation of those that cross over such thresholds in the subsequent analyses. Fourth, the lack of information on nutrition and the history of obesity (onset and duration) may also be considered a limitation. Finally, waist circumference does not provide a direct estimate of visceral adiposity, for which more sophisticated exams are required. However, waist circumference is an especially useful screening tool in clinical practice.
Conclusions
Unlike only dynapenia or only abdominal obesity, dynapenic abdominal obesity is associated with accelerated gait speed decline in older adults free of mobility limitation at baseline and is, therefore, an important condition to be addressed in clinical practice through aerobic and strength training for the prevention of physical disability in this population.
Supplementary Material
Contributor Information
Roberta de Oliveira Máximo, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil.
Dayane Capra de Oliveira, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil.
Paula Camila Ramírez, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil; Escuela de Fisioterapia, Universidad Industrial de Santander, Colômbia.
Mariane Marques Luiz, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil.
Aline Fernanda de Souza, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil.
Maicon Luís Bicigo Delinocente, Gerontology Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil.
Andrew Steptoe, Department of Epidemiology and Public Health, University College London, London, UK.
Cesar de Oliveira, Department of Epidemiology and Public Health, University College London, London, UK.
Tiago da Silva Alexandre, Physical Therapy Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil; Gerontology Postgraduate Program, Federal University of Sao Carlos, Sao Carlos, Brazil; Department of Epidemiology and Public Health, University College London, London, UK; Department of Gerontology, Federal University of Sao Carlos, Sao Carlos, Brazil.
Acknowledgments
The authors are grateful to the team and participants of the ELSA study. The authors’ responsibilities were as follows—study design: R.O.M. and T.S.A.; provision of data: A.S., C.O. and T.S.A.; consistency of database: R.O.M., P.C.R. and T.S.A.; analysis and interpretation of data: R.O.M. and T.S.A.; writing of manuscript: R.O.M. and T.S.A.; revision of manuscript: R.O.M., D.C.O., P.C.R., M.M.L., A.F.S.C., M.L.B.D., A.S., C.O. and T.S.A. R.O.M. assumes responsibility for the integrity of the data analysis.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was supported by the Sao Paulo Research Foundation—FAPESP [grants numbers: 17/26377-4 to Roberta de Oliveira Máximo and 18/13917-3 to Tiago da Silva Alexandre]—the Coordination for the Improvement of Higher Education Personnel—CAPES [Finance code 001]—the National Council of Scientific and Technological Development—CNPq [grant number: 303981/2017-2 and 303577/2020-7]. The funding for the English Longitudinal Study of Ageing is provided by the USA National Institute on Aging Grant R01AG017644 and a consortium of UK government departments co-ordinated by the Economic and Social Research Council (ESRC). The funders had no involvement in the manuscript.
Ethical Approval and Informed Consent
The English Longitudinal Study of Ageing received approval from the National Research Ethics Service (London Multicentre Research Ethics Committee [MREC/01/2/91]) and all participants signed a statement of informed consent. The authors confirm that all research and methods were performed in accordance with approved guidelines and regulations.
References
- 1.Fritz S, Lusardi M. White paper: ‘walking speed: the sixth vital sign. J Geriatr Phys Ther 2009; 32: 2–5. [PubMed] [Google Scholar]
- 2.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act 2015; 23: 314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abellan Van Kan G, Rolland Y, Andrieu Set al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people. Cah l’Annee Gerontol 2009; 13: 13–23. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci 2016; 71: 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeom HA, Fleury J, Keller C. Risk factors for mobility limitation in community-dwelling older adults: a social ecological perspective. Geriatr Nurs 2008; 29: 133–40. [DOI] [PubMed] [Google Scholar]
- 6.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev 2013; 35: 51–65. [DOI] [PubMed] [Google Scholar]
- 7.Clark BC, Manini TM. Sarcopenia # dynapenia. J Gerontol A Biol Sci Med Sci 2008; 63: 829–34. [DOI] [PubMed] [Google Scholar]
- 8.Schrager MA, Metter EJ, Simonsick Eet al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2007; 102: 919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser M, Pahor M, Taaffe DRet al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci 2002; 57: M326–32. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis 2005; 183: 308–15. [DOI] [PubMed] [Google Scholar]
- 11.Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009; 8: 339–48. [DOI] [PubMed] [Google Scholar]
- 12.Weinbrenner T, Schröder H, Escurriol Vet al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr 2006; 83: 30–5. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho DHT, Scholes S, Santos JLF, Oliveira C, Alexandre T d S. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English longitudinal study of ageing. J Gerontol A Biol Sci Med Sci 2019; 74: 1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Jiang J, Hao Q, Luo L, Dong B. Dynapenic obesity and lower extremity function in elderly adults. J Am Med Dir Assoc 2015; 16: 31–6. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci 2010; 65A: 71–7. [DOI] [PubMed] [Google Scholar]
- 16.Stenholm S, Alley D, Bandinelli Set al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes 2010; 33: 635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batsis JA, Zbehlik AJ, Pidgeon D, Bartels SJ. Dynapenic obesity and the effect on long-term physical function and quality of life: data from the osteoarthritis initiative physical functioning, physical health and activity. BMC Geriatr 2015; 15: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandre T da S, Scholes S, Ferreira Santos JL, Duarte YA de O, Oliveira C. The combination of dynapenia and abdominal obesity as a risk factor for worse trajectories of IADL disability among older adults. Clin Nutr 2018; 37: 2045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexandre T d S, Scholes S, Santos JLF, Oliveira C. Dynapenic abdominal obesity as a risk factor for worse trajectories of ADL disability among older adults: the ELSA cohort study. J Gerontol Ser A 2019; 74: 1112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Máximo R d O, Santos JLF, Perracini MR, Oliveira C d, Duarte YA d O, Alexandre T d S. Abdominal obesity, dynapenia and dynapenic-abdominal obesity as factors associated with falls. Brazilian J Phys Ther 2019; 23: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva Alexandre T, Scholes S, Ferreira Santos JL, Oliveira Duarte YA, Oliveira C. Dynapenic abdominal obesity increases mortality risk among English and Brazilian older adults: a 10-year follow-up of the ELSA and SABE studies. J Nutr Health Aging 2018; 22: 138–44. [DOI] [PubMed] [Google Scholar]
- 22.Mindell J, Biddulph JP, Hirani Vet al. Cohort profile: the health survey for England. Int J Epidemiol 2012; 41: 1585–93. [DOI] [PubMed] [Google Scholar]
- 23.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 2013; 42: 1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Studenski SA, Peters KW, Alley DEet al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69 A: 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci Let al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995; 332: 556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Pieper CFet al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000; 55: M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perracini MR, Mello M, Oliveira Máximo Ret al. Diagnostic accuracy of the short physical performance battery for detecting frailty in older people. Phys Ther 2019; 100: 90–98. [DOI] [PubMed] [Google Scholar]
- 29.Rijk JM, Roos PRRKM, Deckx L, Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int 2015; 16: 5–20. [DOI] [PubMed] [Google Scholar]
- 30.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 2008; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 31.McGrath R, Johnson N, Klawitter Let al. What are the association patterns between handgrip strength and adverse health conditions? A topical review. SAGE Open Med 2020; 8: 205031212091035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks GE, Shardell M, Alley DEet al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2012; 67 A: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauretani F, Russo CR, Bandinelli Set al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003; 95: 1851–60. [DOI] [PubMed] [Google Scholar]
- 34.Fragala MS, Alley DE, Shardell MDet al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc 2016; 64: 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alley DE, Shardell MD, Peters KWet al. Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci 2014; 69 A: 559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martien S, Delecluse C, Boen Fet al. Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch Gerontol Geriatr 2015; 60: 252–8. [DOI] [PubMed] [Google Scholar]
- 37.Alonso AC, Ribeiro SM, Silva Luna NMet al. Association between handgrip strength, balance, and knee flexion/extension strength in older adults. PLoS One 2018; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banks J, Breeze E, Lessof C, Nazroo J. Retirement, health and relationships of the older population in England: the 2004 English Longitudinal Study of Ageing. London: Institute for Fiscal Studies; 2006.
- 39.World Health Organization . Waist Circumference and Waist-Hip Ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. World Health, 2011. [Google Scholar]
- 40.National Institutes of Health . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. 1998; 6 Suppl 2: 51S–209S. [PubMed]
- 41.Rivilis I, Hay J, Cairney J, Klentrou P, Liu J, Faught BE. Joint health surveys unit, National Centre for social research and University College London research Department of Epidemiology and Public Health. The health survey for England 2008. Res Dev Disabil 2011; 32: 894–910. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res Clin Pract 2011; 93: 299–309. [DOI] [PubMed] [Google Scholar]
- 43.Steel N, Huppert FA, McWilliams B, Melzer D.. Physical and cognitive function. In: Marmot M, Banks J, Blundell R, Lessof C, Nazroo J, editors. Health, Wealth and Lifestyles of the Older Population in England: The 2002 English Longitudinal Study of Ageing. 2003: 249–300.
- 44.Radloff LS. The CES-D scale. Appl Psychol Meas 1977; 1: 385–401. [Google Scholar]
- 45.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986; 73: 13–22. [Google Scholar]
- 46.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42: 121. [PubMed] [Google Scholar]
- 47.Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci 1993; 84: 331–7. [DOI] [PubMed] [Google Scholar]
- 48.Frederiksen H, Hjelmborg J, Mortensen J, Mcgue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol 2006; 16: 554–62. [DOI] [PubMed] [Google Scholar]
- 49.Keevil VL, Luben R, Dalzell Net al. Cross-sectional associations between different measures of obesity and muscle strength in men and women in a British cohort study. J Nutr Health Aging 2014; 19: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stenholm S, Sallinen J, Koster Aet al. Association between obesity history and hand grip strength in older adults—exploring the roles of inflammation and insulin resistance as mediating factors. J Gerontol A Biol Sci Med Sci 2011; 66 A: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayer AA, Syddall HE, Dennison EMet al. Grip strength and the metabolic syndrome: findings from the Hertfordshire cohort study. QJM 2007; 100: 707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


