Abstract
Background
Initial orthostatic hypotension (OH) is a clinical syndrome of exaggerated transient orthostasis associated with higher risks of falls, frailty and syncope in older adults.
Objective
To provide a prevalence estimate of initial OH in adults aged 65 years or older.
Methods
Literature search of MEDLINE (from 1946), Embase (from 1947) and Cochrane Central Register of Controlled Trials was performed until 6 December 2019, using the terms ‘initial orthostatic hypotension’, ‘postural hypotension’ and ‘older adults’. Articles were included if published in English and participants were 65 years or older. Random effects models were used for pooled analysis.
Results
Of 5,136 articles screened, 13 articles (10 cross-sectional; 3 longitudinal) reporting data of 5,465 individuals (54.5% female) from the general (n = 4,157), geriatric outpatient (n = 1,136), institutionalised (n = 55) and mixed (n = 117) population were included. Blood pressure was measured continuously and intermittently in 11 and 2 studies, respectively. Pooled prevalence of continuously measured initial OH was 29.0% (95% CI: 22.1–36.9%, I2 = 94.6%); 27.8% in the general population (95% CI: 17.9–40.5%, I2 = 96.1%), 35.2% in geriatric outpatients (95% CI: 24.2–48.1%, I2 = 95.3%), 10.0% in institutionalised individuals (95% CI: 2.4–33.1%, I2 = 0%) and 21.4% in the mixed population (95% CI: 7.0–49.6, I2 = 0%). Pooled prevalence of intermittently measured initial OH was 5.6% (95% CI: 1.5–18.9%, I2 = 81.1%); 1.0% in the general population (95% CI: 0.0–23.9%, I2 = 0%) and 7.7% in geriatric outpatients (95% CI: 1.8–27.0%, I2 = 86.7%).
Conclusion
The prevalence of initial OH is high in older adults, especially in geriatric outpatients. Proper assessment of initial OH requires continuous blood pressure measurements.
Keywords: aged, blood pressure, frail older people, hypotension, orthostatic intolerance, posture
Key Points
Initial orthostatic hypotension (OH) is associated with higher risks of falls, frailty and syncope in older adults.
Initial OH affects 27.8% (95% CI:17.9–40.5%) of older general populations.
Initial OH affects 35.2% (95% CI: 24.2–48.1%) of geriatric outpatients.
Continuous blood pressure monitoring is recommended to diagnose initial OH.
Introduction
Initial orthostatic hypotension (OH) is a clinical syndrome of exaggerated transient orthostasis and is defined as a decrease of ≥40 mmHg in systolic blood pressure (SBP) and/or ≥20 mmHg in diastolic blood pressure (DBP) within 15 seconds of active standing [1,2]. It differs distinctly from classical OH, defined as a decrease of ≥20 mmHg in SBP and ≥10 mmHg in DBP within 3 minutes of standing or to at least 60° upright tilting [1]. Initial OH may be accompanied by orthostatic intolerance symptoms of light-headedness, dizziness, unsteadiness or visual disturbance [1,2], which has been attributed to impaired cerebral perfusion [3]. Older adults with initial OH have higher risks of falls, frailty and syncope [2,4–7], regardless of the presence of symptoms [5,8]. Age-related changes such as low skeletal muscle strength and cardiorespiratory fitness may further impair restoration of systemic blood pressure and aggravate negative outcomes of initial OH [4,9,10].
The negative implications associated with initial OH highlight the need to identify this variant of OH, particularly in older adults who may already be susceptible to pre-syncopal episodes and falls [10]. Unlike classical OH which can be detected intermittently, the rapid blood pressure changes that occur in initial OH are less likely be detected with a sphygmomanometer [10,11]. Therefore, continuous blood pressure monitoring is recommended to capture the transient changes in blood pressure upon immediate active standing or passive tilting [1,12]. Limited availability of beat-to-beat blood pressure monitoring devices in clinical settings may cause initial OH to be underdiagnosed.
The aim of this systematic review and meta-analysis is to provide pooled estimates of the prevalence of initial OH in adults aged 65 years or older by both intermittent and continuous blood pressure monitoring.
Methods
Data search and sources
The review protocol was registered with the PROSPERO International prospective register of systematic reviews (CRD42020170696) and developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13]. MEDLINE (from 1946), EMBASE (from 1974) and Cochrane Central Register of Controlled Trials were systematically searched for articles published until 6 December 2019, in assistance of research librarian Lindy Cochrane of the University of Melbourne. The search strategy included the following keywords: ‘initial orthostatic hypotension’, ‘postural hypotension’ and ‘older adults’. The complete search strategy is presented in Supplementary Table S1, Supplementary data are available in Age and Ageing online.
Article selection
All identified articles were managed with EndNote (Version: X9 Clarivate Analytics, Philadelphia, USA). After the removal of duplicates, articles were exported into Covidence and assessed by screening titles and abstracts for potential eligibility by two independent reviewers (J.T. and S.L.H.). Subsequent full texts of eligible articles were screened by the same reviewers. Disagreements between the reviewers were resolved by a third reviewer (R.K.I.). Articles were eligible if they met the following inclusion criteria: cohorts with a mean or median age of 65 years or older, diagnosis of initial OH that encompassed a decrease of ≥40 mmHg in SBP and/or ≥20 mmHg in DBP up to 1 minute after postural change and written in English. Studies that experimentally induced initial OH with medication were excluded. Conference abstracts, case reports (less than five participants), reviews, editorials and letters to the editor were excluded. Reference lists from the included full-text articles were searched to identify potential additional articles.
Data extraction
The following variables were extracted independently by two reviewers (J.T., S.L.H.): first author, year of publication, population, study design, study setting, number of participants, % of valid blood pressure measurements, % female, mean or median age, initial OH definition, resting period (min), standing period (min), type of postural change (active stand, passive stand, active supine-to-sit or passive supine-to-sit), blood pressure measurement (continuous or intermittent) and types of devices used to measure initial OH. Active stand was defined as activation of lower limb muscles upon standing up, whereas passive stand or supine-to-sit was defined as immobility or inactivation of lower limb muscles during the transition from supine to standing or sitting. The prevalence of initial OH was extracted for the total population and, if given, for subpopulations. If initial OH prevalence was reported at more than one time point, the first point in time with the highest reported number of participants was included.
Study quality
The quality and risk of bias of individual articles were assessed by two independent reviewers (J.T., S.L.H.) using the nine-point Newcastle–Ottawa Scale (NOS) [14] adapted for cross-sectional studies. Articles with an NOS score between 0–3, 4–6 and 7–9 points were defined as low, moderate and high quality, respectively [14]. The specified NOS is provided in Supplementary Table S2, Supplementary data are available in Age and Ageing online.
Meta-analysis
Analyses were stratified by method of blood pressure measurement (continuously and intermittently), initial OH definition (with symptoms and without symptoms) and study population (categorised as general population, geriatric outpatients, institutionalised or mixed population). Additional analyses were performed excluding studies using passive postural changes to test for initial OH. The pooled prevalence of initial OH was presented as a percentage and 95% confidence interval (CI). A random effects model was used to account for heterogeneity. Heterogeneity was assessed using the I2-test (<25% low; 25–75% moderate and >75% high) [15]. P-values less than 0.05 were considered statistically significant. All analyses were performed using Comprehensive Meta-Analysis (version3.3; Biostat Inc., EnglewoodNK).
Results
Article selection
Figure 1 shows the PRISMA flow diagram. The search yielded 8,311 articles. After duplicate removal, 5,136 titles and abstracts were screened of which 210 were selected for full text screening. Thirteen articles were included in the systematic review and meta-analysis.
Figure 1 .
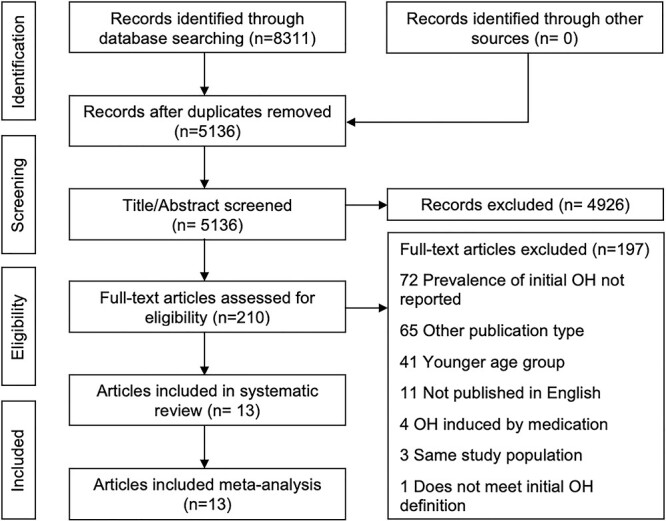
PRISMA Flowchart—article screening and selection.
Study and participant characteristics
Table 1 and Supplementary Table S3, Supplementary data are available in Age and Ageing online, list the study characteristics. A total of 5,465 participants (54.5% females ranging between 43.0 and 71.7%) with a mean age ranging between 67.8 ± 6.1 and 84.2 ± 0.9 years, representing the general population (n = 4,157) [16–18], geriatric outpatients (n = 1,136) [4,10,19–24], institutionalised population (n = 55) [7] and mixed population (n = 117) [5], were included. Ten studies were cross-sectional (n = 4,914) [4,5,10,16,18–22,24] and three studies were longitudinal (n = 551) [7,17,23].
Table 1 .
Study characteristics, stratified by population
| First author, year (reference) | Study design | Population | N | Valid measures, N (%) | Female, Na (%) | Age, years mean (SD) | Period R/S (min) | Postural change | Devices used |
|---|---|---|---|---|---|---|---|---|---|
| Geriatric outpatients | |||||||||
| Bengtsson-Lindberg, 2015 [19] | C | AD, Advasc, DLB | 154 | 154 (100) | 95 (61.7) | 76 (6.3) | 10/10 | AS | Digital sphyg. |
| Control | 50 | 48 (96) | 33 (66) | 76 (7) | 10/10 | AS | Digital sphyg. | ||
| Breeuwsma, 2017 [20] | C | CVD, DM, HTN | 127 | 104 (81.9) | 45 (43) | 68.8 (8.5) | 5/4 | AS,ASS | Finometer Pro |
| De Bruine, 2017 [21] | C | Mobility, cogn, morbid | 24 | 24 (100) | 14 (58.3) | 79.3 (7.7) | 5/3 | AS | Nexfin |
| De Bruine, 2019 [22] | C | Mobility | 62 | 58 (93.5) | 33 (56.9) | 80.6 (7.0) | 5/3 | PS | Finometer Pro |
| Hayakawa, 2015 [23] | L | MCI | 141 | 130 (93) | 76 (54) | 74 (6.8) | 10/3 | AS | Finometer |
| Control | 75 | 69 (92) | 32 (43) | 69 (7.1) | 10/3 | AS | Finometer | ||
| McJunkin, 2015 [10] | C | General | 115 | 115 (100) | 57 (49.5) | 71.1 | 5–10/3 | AS | Manual sphyg. |
| Mol, 2018 [24] | C | Mobility, cogn, mixed | 109 | 109 (100) | 60 (55) | 81.7 (7.0) | 5/3 | AS | Nexfin |
| Romero-Ortuno, 2011 [4] | C | General | 608 | 442 (72.7) | 317 (71.7) | 72.1 (7.1) | 10/3 | AS | Finometer Pro |
| General population | |||||||||
| Finucane, 2014 [16] | C | Community | 2,647 | 2,409 (91) | 1,266 (52.6) | 67.8 (6.1) | 10/2 | AS | Finometer |
| McDonald, 2017 [17] | L | Community | 353 | 297 (84.1) | 40 (50.6) | 73 (6.8) | 10/3 | AS | Portapres; Finapres |
| Saedon, 2019 [18] | C | Community | 1,245 | 1,245 (100) | 693 (55.7) | 67.9 (6.9) | 5/3 | AS | Taskforce; CNSys |
| Institutionalised | |||||||||
| Shaw, 2019 [7] | L | Long-term care | 55 | 55 (100) | 65 (56)* | 84.2 (0.9)* | 15/15 | PSS | Finometer Pro |
| Mixed population | |||||||||
| Saedon, 2016 [5] | C | Fallers AED, primary care, geriatrics, hospital | 155 | 117 (75.5) | 107 (69.0) | 75 (7) | 10/3 | AS | Portapres; Finapres |
| Control | 112 | 89 (79.5) | 77 (68.7) | 72 (6) | 10/3 | AS | Portapres; Finapres | ||
C, Cross-sectional; L, Longitudinal; AD, Alzheimer’s disease; ADVASC, AD and vascular components; DLB, Dementia with Lewy bodies; MCI, Mild Cognitive Impairment; Cogn, Cognition; Morbid, Morbidity; CVD, Cardiovascular Disease; DM, Diabetes Mellitus; HTN, Hypertension; OH, Orthostatic Hypotension; AED, Accident and Emergency Department; R, Resting; S, Standing/Sitting; AS, Active Stand; ASS, Active Supine-to-Sit; PS, Passive Stand; PSS, Passive Supine-to-Sit; Sphyg., Sphygmomanometer; CNSys, CNSystems.
*Sample size for these characteristics, n = 116.
Diagnosis
Initial OH was defined as a decrease of ≥40 mmHg in SBP and/or ≥20 mmHg in DBP within 15 seconds of standing [4,5,10,16–18,20–24], within 30 seconds of sitting [7] and within the first minute of standing [19]. The diagnosis of initial OH included the presence of symptoms in three articles [4,16,20] and the recovery of blood pressure within 30–60 seconds in one article [10]. Blood pressure was measured continuously using Finometer Pro [4,5,7,16,17,20,22,23], Nexfin [21,24] and Task Force CNSystems [18] or intermittently using either a digital sphygmomanometer [19] or manual sphygmomanometer [10]. The resting period prior to postural change varied between 5 [10,18,20–22,24], 10 [4,5,16,17,19,23] and 15 minutes [7]. Standing or sitting period varied between 2 [16], 3 [4,5,10,17,18,21–24], 4 [20], 10 [19] and 15 minutes [7]. The postural change included active stand [4,5,10,16–21,23,24], passive-transition-to-stand [22], active supine-to-sit [20] or passive supine-to-sit [7]. In one article using a passive-transition-to-stand, participants were asked to stand unsupported after reaching a standing position [22]. In one article, both active stand and active supine-to-sit were tested in all participants [20], in which active stand showed a significant larger SBP drop between 0 and 40 seconds after postural change [20] and was included in the meta-analysis. Valid blood pressure measurements were reported to be 100% in five articles [7,10,18,21,24]; the lowest reported percentage of valid measurements was 77.2% [4].
Quality assessment
Ten articles, including general populations [16–18], geriatric outpatients [4,20–24] and a mixed population [5], were of high quality (NOS score ≥ 7). The remaining three articles were of moderate quality (NOS score 4–6) [7,10,19] (Table 3).
Table 3 .
Risk of bias quality assessment using the NOS
| First author, year | Selection | Comparability | Outcome | Score | Quality | ||||
|---|---|---|---|---|---|---|---|---|---|
| Represent-ativeness of exposed cohort | Sample size | Selection of non-exposed cohort | Ascertainment of exposure: continuous BP | Adjustment for potential confounders | Assessment of outcome | Statistical test | |||
| Bengtsson-Lindberg, 2015 [19] | * | * | * | – | * | – | * | 5 | Moderate |
| Breeuwsma, 2017 [20] | * | * | * | * | – | ** | * | 7 | High |
| De Bruine, 2017 [21] | * | * | * | * | – | ** | * | 7 | High |
| De Bruine, 2019 [22] | * | * | * | * | * | ** | * | 8 | High |
| Finucane, 2014 [16] | * | * | * | * | – | ** | * | 7 | High |
| Hayakawa, 2015 [23] | * | * | * | * | ** | ** | * | 9 | High |
| McDonald, 2017 [17] | * | * | * | * | * | ** | * | 8 | High |
| McJunkin, 2015 [10] | * | * | * | – | – | ** | * | 6 | Moderate |
| Mol, 2018 [24] | * | * | * | * | * | ** | * | 8 | High |
| Romero-Ortuno, 2011 [4] | * | * | * | * | – | ** | * | 7 | High |
| Saedon, 2016 [5] | * | * | * | * | ** | ** | * | 9 | High |
| Saedon, 2019 [18] | * | * | * | * | ** | ** | * | 9 | High |
| Shaw, 2019 [7] | * | * | * | * | – | * | * | 6 | Moderate |
Maximum total score of 9 points. 0–3, low quality; 4–6, moderate quality; 7–9, high quality.
Meta-analysis: prevalence of initial OH
Initial OH prevalence ranged between 0.0 and 72.5% (Table 2). Pooled prevalence of continuously measured initial OH was 29.0% (95% CI: 22.1–36.9%, I2 = 94.6%); 27.8% (95% CI: 17.9–40.5%, I2 = 96.1%) in the general population; 35.2% (95% CI: 24.2–48.1%, I2 = 95.3%) in geriatric outpatients; 10.0% (95% CI: 2.4–33.1%, I2 = 0%) in institutionalised individuals and 21.4% (95% CI: 7.0–49.6, I2 = 0%) in mixed populations (Figure 2a). Excluding studies using passive postural change resulted in a slightly higher pooled prevalence of continuously measured initial OH of 29.8% (95% CI: 22.3–38.6%, I2 = 95.2%) (Supplemental Figure S1a, Supplementary data are available in Age and Ageing online). When studies were stratified by initial OH definition, pooled prevalence of continuously measured initial OH was 31.4% (95% CI: 21.0–44.0%, I2 = 95.2%) without symptoms and 29.4% (95% CI: 27.8–31.1%, I2 = 94.2%) with symptoms in the definition. Prevalence of continuously measured initial OH without symptoms was 26.7% (95% CI: 13.5–45.9%, I2 = 96.3%) in the general population, 47.2% (95% CI: 27.5–67.9%, I2 = 90.3%) in geriatric outpatients, 10.0% (95% CI: 1.7–41.6%, I2 = 0%) in institutionalised individuals and 21.4% (95% CI: 4.8–59.6%, I2 = 0%) in mixed populations (Figure 2b). Excluding studies using passive postural change resulted in a slightly higher pooled prevalence of continuously measured initial OH without symptoms in the definition of 33.5% (95% CI: 21.1–48.7%, I2 = 96.0%) (Supplemental Figure S1b, Supplementary data are available in Age and Ageing online). Prevalence of continuously measured initial OH with symptoms was 31.4% (95% CI: 29.6–33.3%, I2 = 0%) in the general population and 18.7% (95% CI: 15.7–22.2%, I2 = 0%) in geriatric outpatients (Figure 2c). Pooled prevalence of intermittently measured initial OH was 5.6% (95% CI: 1.5–18.9%, I2 = 81.1%). Prevalence of intermittently measured initial OH was 1.0% (95% CI: 0.0–23.9%, I2 = 0%) in the general population and 7.7% in geriatric outpatients (95% CI: 1.8–27.0%, I2 = 86.7%) (Figure 2d).
Table 2 .
Definition and prevalence of initial OH, stratified by populations
| First author, year (reference) | BP measurement | Initial OH definition | Initial OH prevalence (%) | Initial OH prevalence in subgroups (%) |
|---|---|---|---|---|
| Geriatric outpatients | ||||
| Bengtsson-Lindberg, 2015a [19] | Int. | IOH-60 | 14.3 | AD (4) |
| ADvasc (6) | ||||
| DLB (34) | ||||
| Breeuwsma, 2017 [20] | Cont. | IOH-15 + S | 16.3 | AS (16.3) |
| 5.8 | ASS (5.8) | |||
| De Bruine, 2017 [21] | Cont. | IOH-15 | 54.2 | |
| De Bruine, 2019 [22] | Cont. | IOH-15 | 41.1 | |
| Hayakawa, 2015a [23] | Cont. | IOH-15 | 65.4 | MCI (65.4) |
| McJunkin, 2015 [10] | Int. | IOH-15 + R30 | 3.5 | |
| Mol, 2018 [24] | Cont. | IOH-15 | 29.4 | |
| Romero-Ortuno, 2011 [4] | Cont. | IOH-15 + S | 19.2 | |
| General population | ||||
| Bengtsson-Lindberg, 2015b [19] | Int. | IOH-60 | 0.0 | Control (0) |
| Finucane, 2014 [16] | Cont. | IOH-15 + S | 31.4 | |
| Hayakawa, 2015b [23] | Cont. | IOH-15 | 72.5 | Control (72.5) |
| McDonald, 2017 [17] | Cont. | IOH-15 | 16.2 | |
| Saedon, 2016b [5] | Cont. | IOH-15 | 9.0 | Non-fallers (9.0) |
| Saedon, 2019 [18] | Cont. | IOH-15 | 24.9 | |
| Institutionalised | ||||
| Shaw, 2019 [7] | Cont. | IOH-30 | 10 | Frail (15) |
| Non-frail (4) | ||||
| Mixed population | ||||
| Saedon, 2016a [5] | Cont. | IOH-15 | 21.4 | Fallers (21.4) |
BP, Blood Pressure; Int., Intermittent; Cont., Continuous beat-to-beat analyses; IOH, Initial Orthostatic Hypotension; IOH-15, SBP or DBP drop within 15 seconds; IOH-15 + S, plus, symptoms; IOH-15 + R30, correcting within 30–60 seconds; IOH-30, SBP or DBP drop within 30 seconds; IOH-60, SBP or DBP within 60 seconds; AD, Alzheimer’s disease; ADVASC, AD and vascular components; DLB, Dementia with Lewy bodies; MCI, Mild Cognitive Impairment; AS, Active Stand; ASS, Active Supine-to-Sit.
Figure 2 .
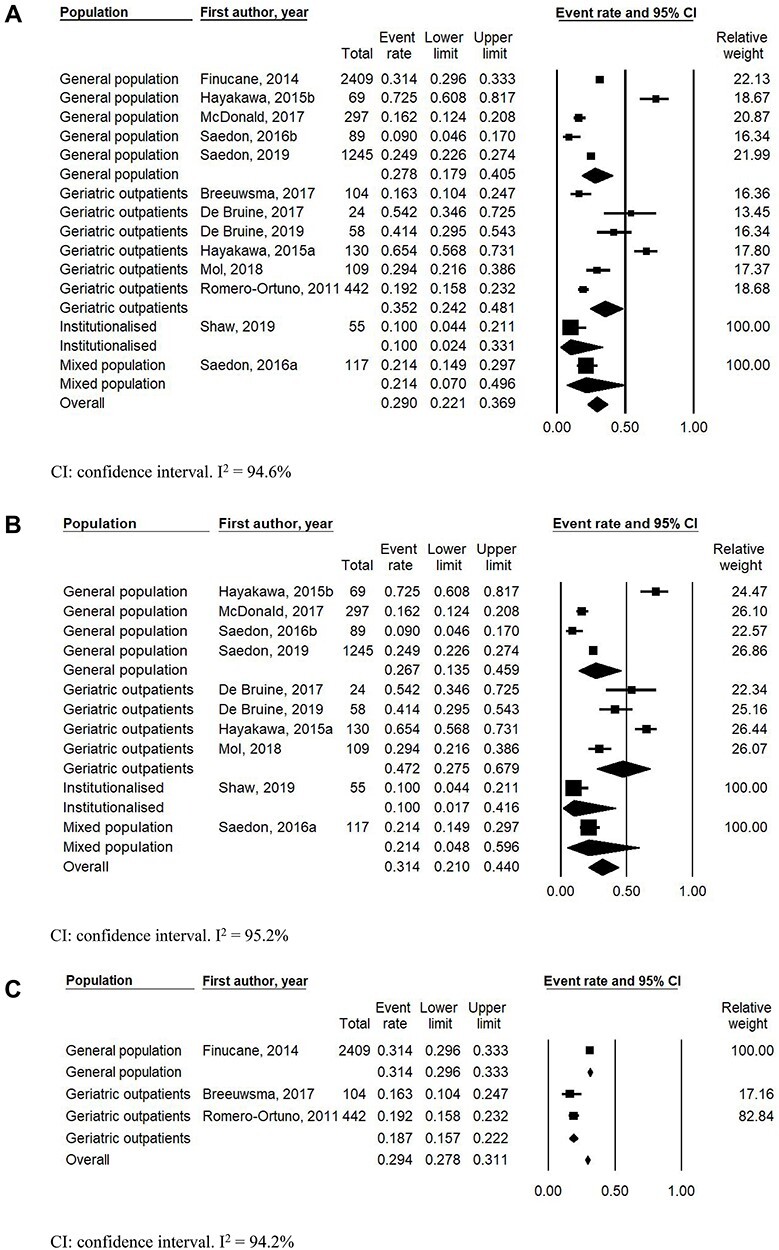
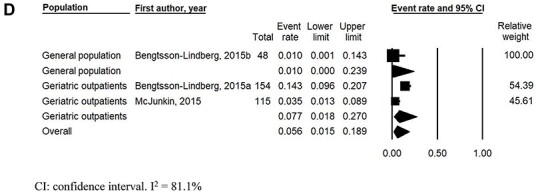
(a) Pooled initial orthostatic hypotension prevalence using continuous blood pressure monitoring, stratified by population. (b) Pooled initial orthostatic hypotension prevalence using continuous blood pressure monitoring and initial orthostatic hypotension definition without inclusion of symptoms, stratified by population. (c) Pooled initial orthostatic hypotension prevalence using continuous blood pressure monitoring and initial orthostatic hypotension definition with inclusion of symptoms, stratified by population. (d) Pooled initial orthostatic hypotension prevalence using intermittent blood pressure monitoring.
Discussion
This systematic review and meta-analysis demonstrate a high prevalence of initial OH in older adults aged 65 years or older. Over a quarter of older adults from the general population and over a third of geriatric outpatients are affected by initial OH. The pooled prevalence of initial OH in older adults is higher when blood pressure is measured continuously compared with intermittently.
There were considerable variations in the diagnosis of initial OH. Currently, the consensus statement on the definition of initial OH [1] does not clearly specify whether the presence of symptoms, in addition to the hemodynamic criteria, is required to diagnose initial OH. Inclusion of symptoms in the initial OH definition excludes asymptomatic older adults who exhibit a large initial blood pressure drop. However, asymptomatic older adults with a substantial initial blood pressure drop may still be at risk of negative consequences of initial OH such as falls or syncope [5,8]. Thus, the inclusion of symptoms in the definition of initial OH seems implausible and results in a lower prevalence as demonstrated in our findings. Participants with dementia with Lewy bodies [19] and diabetes mellitus [25] are at higher risk of initial OH due to autonomic dysfunction and therewith delayed compensatory response to postural change. Damaged elastin fibres and increased arterial stiffness caused by diabetes mellitus can lead to decreased vascular resistance [25] and impaired baroreceptor function [26]. Given that initial OH is associated with increased risks of falls [18], frailty [7] and syncope [2] in older adults, it is clinically important to assess initial OH to target interventions to minimise these poor clinical outcomes.
Active stand is commonly used because it allows clinicians to assess the cardiovascular responses to standing [1,12]. However, frail older adults may find it difficult to complete an active stand test [27], so passive sitting is a safe alternative [7]. Given that institutionalised older adults are more likely to be frail, with increased morbidity and mortality compared with the general population [28], they may require a longer transition time during postural change. The slow transition time during the passive seated orthostatic stress may explain the low prevalence in the institutionalised population [7]. Slow transition times allow for adaptation and counter manoeuvring such as activation of muscles for effective use of the skeletal muscle pump, thereby counteracting the relative blood pressure drop in the first 15 seconds [21]. Another effective intervention to counteract the effects of initial OH include lower body muscle tensing immediately after standing [29] as it decreases venous pooling in the lower limbs and helps to sustain cardiac output [11]. Salt supplementation [30] and medication reviews may also be considered.
Orthostatic blood pressure should be measured continuously to capture the immediate blood pressure changes that occur upon standing [12]. The rapid blood pressure changes that occur in initial OH cannot be detected using a conventional sphygmomanometer because there is insufficient time to inflate the cuff upon standing [12]. This results in an underestimation of the initial OH prevalence, which is in line with the presented results. Additionally, continuous blood pressure monitoring facilitates the analysis of blood pressure recovery [31]. Currently, the consensus statement on the definition of initial OH does not specify the duration of the blood pressure drop in initial OH [1]. The recent availability of a structured protocol using continuous blood pressure devices for the active stand test will help clinicians to improve the standardisation of beat-to-beat blood pressure measurements in clinical practice and research studies [31], thereby optimising the detection of initial OH.
Strengths and limitations
This is the first systematic review to estimate the prevalence of initial OH in older adults aged 65 years or older. Selection bias was avoided by including all populations and definitions of initial OH that met the hemodynamic criteria of a decrease of ≥40 mmHg in SBP and ≥20 mmHg in DBP up to 1 minute after postural change. Articles that were not published in English were excluded and hence may be open to reporting bias. The high heterogeneity in the pooled analyses can be attributed to variations in study populations, measurement protocols and initial OH definitions.
Conclusion
Initial OH is highly prevalent in older adults aged 65 years or older, particularly when measured continuously, highlighting the need to screen and diagnose initial OH. Future research should investigate whether the inclusion of symptoms in the definition of initial OH is clinically relevant. Furthermore, there is a need to establish a consensus on the diagnosis of initial OH using continuous blood pressure devices to consistently identify participants with initial OH.
Supplementary Material
Acknowledgements
We kindly acknowledge research librarian Lindy Cochrane of the University of Melbourne for assisting with the search of literature.
Contributor Information
Jennifer Tran, Department of Medicine and Aged Care, @AgeMelbourne, The University of Melbourne, The Royal Melbourne Hospital, Parkville, Victoria 3050, Australia.
Sarah L Hillebrand, Department of Human Movement Sciences, @AgeAmsterdam, Amsterdam Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Carel G M Meskers, Department of Rehabilitation Medicine, Amsterdam UMC, Vrije Universiteit, Amsterdam Movement Sciences, Amsterdam, The Netherlands.
Rebecca K Iseli, Department of Medicine and Aged Care, @AgeMelbourne, The University of Melbourne, The Royal Melbourne Hospital, Parkville, Victoria 3050, Australia.
Andrea B Maier, Department of Medicine and Aged Care, @AgeMelbourne, The University of Melbourne, The Royal Melbourne Hospital, Parkville, Victoria 3050, Australia; Department of Human Movement Sciences, @AgeAmsterdam, Amsterdam Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Healthy Longevity Program, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; Centre for Healthy Longevity, @AgeSingapore, National University Health System, Singapore.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This research was funded by an unrestricted grant of the University of Melbourne received by Professor Andrea B. Maier and the Medical Research Future Fund (MRFF) provided by the Melbourne Academic Centre for Health (MACH). The funders had no role in the development of the protocol.
References
- 1.Freeman R, Wieling W, Axelrod FBet al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69–72. [DOI] [PubMed] [Google Scholar]
- 2.Twist DJL, Dinh T, Bouwmans EME, Kroon AA. Initial orthostatic hypotension among patients with unexplained syncope: an overlooked diagnosis? Int J Cardiol 2018; 271: 269–73. [DOI] [PubMed] [Google Scholar]
- 3.Mehagnoul-Schipper DJ, Vloet LC, Colier WN, Hoefnagels WH, Jansen RW. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke 2000; 31: 1615–20. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 2011; 59: 655–65. [DOI] [PubMed] [Google Scholar]
- 5.Saedon NI, Zainal-Abidin I, Chee KHet al. Postural blood pressure electrocardiographic changes are associated with falls in older people. Clin Auton Res 2016; 26: 41–8. [DOI] [PubMed] [Google Scholar]
- 6.Wijnen VK, Harms MP, Go-Schon IKet al. Initial orthostatic hypotension in teenagers and young adults. Clin Auton Res 2016; 26: 441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw BH, Borrel D, Sabbaghan Ket al. Relationships between orthostatic hypotension, frailty, falling and mortality in elderly care home residents. BMC Geriatr 2019; 19: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman R, Illigens BMW, Lapusca Ret al. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension 2020; 75: 1325–32. [DOI] [PubMed] [Google Scholar]
- 9.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med 2007; 120: 841–7. [DOI] [PubMed] [Google Scholar]
- 10.McJunkin B, Rose B, Amin Oet al. Detecting initial orthostatic hypotension: a novel approach. J Am Soc Hypertens 2015; 9: 365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wieling W, Krediet CT, Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci (Lond) 2007; 112: 157–65. [DOI] [PubMed] [Google Scholar]
- 12.Wijnen VK, Finucane C, Harms MPMet al. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 2017; 282: 468–83. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Shamseer L, Clarke Met al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margulis AV, Pladevall M, Riera-Guardia Net al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa scale and the RTI item bank. Clin Epidemiol 2014; 6: 359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 16.Finucane C, O'Connell MD, Fan CWet al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish longitudinal study on ageing (TILDA). Circulation 2014; 130: 1780–9. [DOI] [PubMed] [Google Scholar]
- 17.McDonald C, Pearce M, Kerr SR, Newton J. A prospective study of the association between orthostatic hypotension and falls: definition matters. Age Ageing 2017; 46: 439–45. [DOI] [PubMed] [Google Scholar]
- 18.Saedon NI, Frith J, Goh CHet al. Orthostatic blood pressure changes and physical, functional and cognitive performance: the MELoR study. Clin Auton Res 2020; 30: 129–137. [DOI] [PubMed] [Google Scholar]
- 19.Bengtsson-Lindberg M, Larsson V, Minthon L, Wattmo C, Londos E. Lack of orthostatic symptoms in dementia patients with orthostatic hypotension. Clin Auton Res 2015; 25: 87–94. [DOI] [PubMed] [Google Scholar]
- 20.Breeuwsma AC, Hartog LC, Kamper AMet al. Standing orthostatic blood pressure measurements cannot be replaced by sitting measurements. Hypertens Res 2017; 40: 765–70. [DOI] [PubMed] [Google Scholar]
- 21.Bruine ES, Reijnierse EM, Trappenburg MCet al. Standing up slowly antagonises initial blood pressure decrease in older adults with orthostatic hypotension. Gerontology 2017; 63: 137–43. [DOI] [PubMed] [Google Scholar]
- 22.Bruine ES, Reijnierse EM, Trappenburg MCet al. Diminished dynamic physical performance is associated with orthostatic hypotension in geriatric outpatients. J Geriatr Phys Ther 2019; 42: E28–34. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa T, McGarrigle CA, Coen RFet al. Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J Am Geriatr Soc 2015; 63: 1868–73. [DOI] [PubMed] [Google Scholar]
- 24.Mol A, Reijnierse EM, Trappenburg MC, Wezel RJA, Maier AB, Meskers CGM. Rapid systolic blood pressure changes after standing up associate with impaired physical performance in geriatric outpatients. J Am Heart Assoc 2018; 7: e010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozakova M, Palombo C. Diabetes mellitus, arterial wall, and cardiovascular risk assessment. Int J Environ Res Public Health 2016; 13: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi M, Miyai N, Nagano Set al. Orthostatic blood pressure changes and subclinical markers of atherosclerosis. Am J Hypertens 2015; 28: 1134–40. [DOI] [PubMed] [Google Scholar]
- 27.Bohannon RW. Five-repetition sit-to-stand test: usefulness for older patients in a home-care setting. Percept Mot Skills 2011; 112: 803–6. [DOI] [PubMed] [Google Scholar]
- 28.Hartog LC, Hendriks SH, Cimzar-Sweelssen Met al. Orthostatic changes in blood pressure and mortality in a nursing home population. J Hypertens 2016; 34: 1068–74. [DOI] [PubMed] [Google Scholar]
- 29.Wieling W, Dijk N, Thijs RD, Lange FJ, Krediet CTP, Halliwill JR. Physical countermeasures to increase orthostatic tolerance. J Intern Med 2015; 277: 69–82. [DOI] [PubMed] [Google Scholar]
- 30.Claydon VE, Hainsworth R. Salt supplementation improves orthostatic cerebral and peripheral vascular control in patients with syncope. Hypertension (Dallas, Tex : 1979)2004; 43: 809–13. [DOI] [PubMed] [Google Scholar]
- 31.Finucane C, Wijnen VK, Fan CWet al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res 2019; 29: 427–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


