Abstract
Objectives
frail older adults may be more vulnerable to stressors, resulting in steeper declines in cognitive function. Whether the frailty–cognition link differs by cognitive domain remains unclear; however, it could lend insight into underlying mechanisms.
Methods
we tested whether domain-specific cognitive trajectories (clock-drawing test, (CDT), immediate and delayed recall, orientation to date, time, president and vice-president naming) measured annually (2011–2016) differ by baseline frailty (physical frailty phenotype) in the National Health and Aging Trends Study (n = 7,439), a nationally representative sample of older adult U.S. Medicare beneficiaries, using mixed effects models to describe repeated measures of each cognitive outcome. To determine if the association between frailty and subsequent cognitive change differed by education, we tested for interaction using the Wald test.
Results
we observed steeper declines for frail compared to non-frail participants in each domain-specific outcome, except for immediate recall. Largest differences in slope were observed for CDT (difference = −0.12 (standard deviations) SD/year, 95%CI: −0.15, −0.08). By 2016, mean CDT scores for frail participants were 1.8 SD below the mean (95%CI: −1.99, −1.67); for non-frail participants, scores were 0.8 SD below the mean (95%CI: −0.89, −0.69). Associations differed by education for global cognitive function (Pinteraction < 0.001) and for each domain-specific outcome: CDT (Pinteraction < 0.001), orientation (Pinteraction < 0.001), immediate (Pinteraction < 0.001) and delayed (Pinteraction < 0.001) word recalls.
Conclusion
frailty is associated with lower levels and steeper declines in cognitive function, with strongest associations for executive function. These findings suggest that aetiologies are multifactorial, though primarily vascular related; further research into its association with dementia sub-types and related pathologies is critical.
Keywords: cognitive ageing, cognition, frailty, epidemiology, dementia, older people
Key Points
Frailty is associated with lower levels and steeper declines in cognitive performance in a nationally representative cohort.
Greatest differences were observed for executive function.
Aetiologies are multifactorial, though likely vascular in nature.
Introduction
Cognitive impairment is common in the United States, affecting 16–20% of older adults [1]. Mild cognitive impairment (MCI) is increasingly recognised as a prodromal phase of many types of dementias [1–5], which has become a leading contributor to disability and dependence among older adults [6], as well as mounting economic and social burden [1]. However, its complex pathophysiology, slow progression and heterogeneous clinical manifestations [2, 7] complicate efforts to pinpoint underlying causes and diagnose early. Cognitive decline, the hallmark of dementia, is a critical endpoint for studies of cognitive impairment and dementia [1–5]. Therefore, it has become increasingly important to investigate potential modifiable risk factors of cognitive decline to identify prevention strategies for later life cognitive impairment and dementia.
Physical frailty, a syndrome [8–10] occurring in approximately 10–15% of community-living older adults [10, 11], is described as a deterioration in physiologic reserve manifested as a vulnerability to stressors [8–10]. While there are at least 67 identified frailty instruments [12], including the commonly investigated deficit accumulation index [13, 14], the physical frailty phenotype (PFP) [8] is the most widely used measure [12], especially in etiologic research given its distinction from co-morbidity and disability [12, 15]. Frailty is a dynamic process that is potentially preventable and modifiable as suggested by prior studies [16–18]. Together with evidence of a frailty–cognition link, it is important to investigate the role of frailty in subsequent cognitive decline to provide a potential hopeful avenue for intervention.
Studies that have demonstrated a frailty–cognition link have collectively suggested that frailty is associated with lower levels and steeper declines in cognitive function [19–23]. However, due to common research challenges including use of small samples, lack of generalizability and lack of sufficient follow-up to observe changes, results have been inconsistent. Among the few that have demonstrated a significant relationship between frailty and cognition by specific cognitive domain, stronger associations for executive function compared to memory were observed [21–24]. It is likely that in the face of acute/everyday stressors, frail older adults have lower cognitive function and steeper cognitive decline than non-frail counterparts, with strongest associations for executive function—a critical domain that deteriorates with onset of vascular dementia [25, 26]. It is also likely that education—a strong predictor of cognitive reserve, which protects against damaging effects of neuropathology—modifies this association [27].
To better understand the association between frailty and subsequent cognitive decline, we leveraged the National Health and Aging Trends Study (NHATS), a prospective, nationally representative sample of U.S. Medicare beneficiaries aged 65+ years. Our goals were to: (i) assess global and domain-specific cognitive trajectories overall; (ii) test whether global and domain-specific cognitive levels and trajectories differ by baseline frailty and (iii) investigate whether associations are modified by educational attainment. Examining the frailty–cognition relationship among a large, heterogeneous population with repeated measures of domain-specific cognitive function would provide critical insight into underlying mechanisms and aetiologies.
Methods
Study design
We used NHATS, a prospective cohort study of a nationally representative sample of U.S. Medicare beneficiaries aged 65+ years [28]. Our analytic sample included community-dwelling older adults with measures of at least three of five frailty criteria and longitudinal cognitive measures (n = 7,439), as described below (Supplementary Figure S1); as in prior studies [11, 19, 26], participants who had three or more frailty assessments missing were excluded from the study. All participants were followed annually for a maximum of 5 years (2011–2016), and data were collected via 2-hour, in-person interviews.
Demographic factors including age, sex, race/ethnicity, education and annual income were considered, as well as self-reported medical conditions. Additionally, participants were classified as having possible/probable dementia based on cognitive testing and self-/proxy reports using NHATS criteria [29] and based on prior studies [26, 30] (Supplementary Table S1 available in Age and Ageing online). Depressive symptoms (PHQ-2), subjective well-being, lower extremity function (short physical performance battery [SPPB]) and dependence in activities of daily living (ADLs) and instrumental activities of daily living (IADLs) were also considered.
Frailty
Baseline frailty was measured using the PFP [9] criteria (exhaustion, low activity, shrinking, slowness and weakness) previously operationalised in NHATS [8, 9, 19, 26] using validated interview and performance measures of functioning [31] (Supplementary Table S1 available in Age and Ageing online).
Each criterion was scored as 0 or 1 representing the absence or presence of the component. Criteria were then summed to create a total score (range: 0–5), and participants were defined as frail with a score of ≥3, as has been validated in clinical settings for purposes of risk stratification [20, 32, 33].
Cognitive function
Trajectories for four domain-specific cognitive measures were assessed in NHATS during in-person interviews. A word-list memory test [34] required participants to recall 10 words immediately after the list of words was presented (immediate word recall) and again after a five-minute delay (delayed word recall). The number of words recalled at a given timepoint was used as either an immediate or a delayed word recall score, ranging from 0 to 10, where higher scores represent more words recalled. A clock-drawing test (CDT) for executive function was also administered to participants. Each participant was given a two-minute time limit to draw a clock face telling the time ‘10 after 11.’ Clocks were rated according to standard criteria, where higher scores represent more complete/accurate drawings [35]. Orientation to date and time was measured by asking participants for the current day, month, year and day of the week and for the name of the current president and vice-president.
We standardised scores of all four component cognitive tests using their baseline means and standard deviations (SD) and then averaged them into a global cognitive composite score, as described in prior studies [36].
Statistical analyses
We tested differences in participant characteristics by frailty using t-tests from logistic regressions incorporating survey weights from the sample design to compare means for all continuous variables and frequency distributions for all categorical variables by frailty. We then described cognitive trajectories for each cognitive outcome using multiple linear regression with fixed and random effects for people and time, applying analytic weights to generate nationally representative estimates. We generated autocorrelation functions to assess the variance–co-variance structure of repeated measures of each cognitive outcome and selected a first-order autoregressive model with robust variance accordingly. Models were controlled for demographic and health characteristics including centred age, sex, race, highest level of education, income quartile and number of co-morbidities. To determine if the association between frailty and subsequent cognitive change was modified by education, we tested for interaction of the association using the Wald test.
All analyses were performed using Stata 15.0, and we used a statistical significance cut-off of α < 0.05.
Sensitivity analyses
We conducted a series of sensitivity analyses to evaluate whether findings remained robust after restricting to those without possible/probable dementia, addressing for ceiling effects (random-effects Tobit models) and addressing potential differential rates of attrition (two complementary approaches: multiple imputations by chained equations in conjunction with generalised estimating equations (GEE) and inverse probability weighting in conjunction with GEE).
Results
Study population
Of 7,439 community-dwelling older adults (mean age = 75.23 years) in NHATS followed for a mean of 3.21 years (SE = 0.03), 56.44% were women, 81.53% self-reported as White, and 20.30% had possible/probable dementia at baseline (Table 1).
Table 1 .
Baseline participant characteristics by frailty status of community-dwelling older adults in the National Health and Aging Trends Study, 2011–2016 (n = 7,439)
| Characteristic | Overall (n = 7,439) |
Non-frail (n = 6,126) |
Frail (n = 1,313) |
|---|---|---|---|
| Age (years)** | 75.23 (0.10) | 74.62 (0.10) | 78.94 (0.24) |
| Female (%)** | 4,320 (56.44) | 3,479 (55.25) | 841 (63.72) |
| Race (%)** | |||
| White | 5,129 (81.53) | 4,346 (82.72) | 783 (74.27) |
| Black | 1,641 (8.20) | 1,263 (7.59) | 378 (11.93) |
| Hispanic | 440 (6.68) | 322 (6.00) | 118 (10.81) |
| Other | 229 (3.59) | 195 (3.69) | 34 (2.99) |
| Income (%)** | |||
| 1st quartile | 2,302 (25.37) | 1,683 (22.68) | 619 (41.74) |
| 4th quartile | 1,339 (22.92) | 1,245 (25.27) | 94 (8.60) |
| Education (%)** | |||
| 8th Grade or less | 953 (10.14) | 643 (8.50) | 310 (20.13) |
| 9th–12th Grade (no diploma) | 1,042 (11.36) | 790 (10.33) | 252 (17.64) |
| High school diploma or equivalent | 2,041 (27.58) | 1,694 (27.41) | 347 (28.59) |
| Some college but no degree | 1,483 (21.54) | 1,285 (22.18) | 198 (17.60) |
| Associates or bachelor’s degree | 1,182 (17.94) | 1,034 (18.89) | 148 (12.18) |
| Graduate degree | 714 (11.44) | 665 (12.68) | 49 (3.86) |
| Subjective well-being score** | 17.30 (0.05) | 17.69 (0.05) | 14.44 (0.18) |
| Depressive symptoms** | 1,179 (14.39) | 690 (10.36) | 489 (38.93) |
| Co-morbidity (%)a** | |||
| 0 Co-morbidities | 1,256 (18.37) | 1,188 (20.56) | 68 (5.04) |
| 1 Co-morbidities | 2,184 (30.19) | 1,952 (32.47) | 232 (16.27) |
| 2 Co-morbidities | 2,048 (26.65) | 1,681 (26.48) | 367(27.68) |
| 3 Co-morbidities | 1,163 (14.86) | 843(13.30) | 320 (24.35) |
| 4+ Co-morbidities | 788 (9.93) | 462(7.18) | 326 (26.66) |
| SPPB, mean (SD) | 6.73 (0.06) | 2.37 (0.09) | 7.41 (0.05) |
| ADL (%)** | |||
| Moderate | 1,684 (21.15) | 1,343 (20.12) | 341 (27.44) |
| Severe | 1,284 (13.66) | 544 (7.11) | 740 (53.53) |
| IADL (%)** | |||
| Moderate | 1,625 (21.06) | 1,276 (19.55) | 349 (30.28) |
| Severe | 1,327 (13.23) | 586 (7.16) | 741 (50.17) |
| Global cognition composite score** | 0.20 (0.01) | 0.26 (0.01) | -0.26 (0.03) |
| Clock-drawing test score** | 0.16 (0.02) | 0.25 (0.02) | -0.39 (0.04) |
| Immediate word recall** | 0.18 (0.21) | 0.25 (0.02) | −0.33 (0.04) |
| Delayed word recall** | 0.20 (0.02) | 0.25 (0.02) | −0.22 (0.04) |
| Orientation to date & time** | 0.15 (0.02) | 0.24 (0.02) | −0.41 (0.04) |
| Dementia (possible or probable) (%)** | 1,941 (20.30) | 1,270 (16.27) | 671 (44.84) |
Note: Raw numbers and weighted percentages (%) for categorical characteristics, as well as weighted means and standard deviations for continuous characteristics are presented.
aCo-morbidities were self-reported and included history of cancer, hip fracture, heart disease, high blood pressure, arthritis, osteoporosis, diabetes, lung disease or stroke. Per characteristic, comparisons were statistically significant at **P < 0.05
Frailty at baseline
At baseline, 1,313 (14.1%) were frail. Frail participants tended to be older (mean = 78.94 versus74.62 years) and were more likely than non-frail participants to be female (63.72% versus 55.25%), Black (11.93% versus 7.59%) and Hispanic (10.81% versus 6.00%). Frail older adults were also more likely to have a highest education attainment of ≤8th grade (20.13% versus 8.50%) and be within the lowest income quartile (41.74% versus 22.68%). Additionally, frail older adults were more likely to have ≥4 co-morbidities (26.66% versus 7.18%), severe depressive symptoms (38.93% versus 10.36%), dependence (IADL: 50.17% versus 7.16%; ADL: 53.53% versus 7.11%) and lower mean SPPB scores (2.37 versus7.41) (Table 1).
Cognitive levels and trajectories among all community-dwelling older adults
After adjustment, reference participants (a 75-year-old, White male with 0 co-morbidities, educational attainment of ≤8th grade and lowest income quartile) scored significantly below the mean for all cognitive tests at baseline (Figure 1). On average, reference participant scores ranged from 0.40 SD below the mean level for the delayed word recall (−0.40 SD, 95% confidence interval (CI): −0.48, −0.32) to 0.60 SD below the mean level for immediate word recall (−0.60 SD, 95%CI: −0.78, −0.32).
Figure 1 .
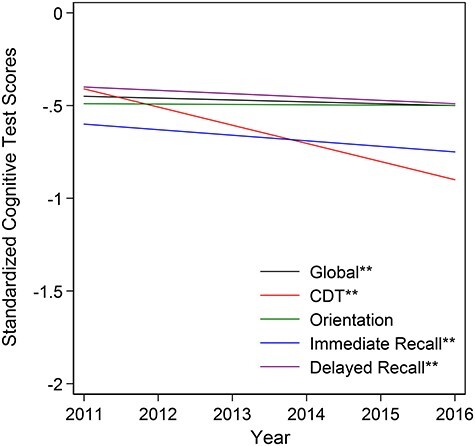
Adjusted cognitive trajectories by specific cognitive domain among community-dwelling older adult participants in the National Health and Aging Trends Study, 2011–2016 (n = 7,439). The global cognition composite score was created by standardising each of the four cognitive tests to a mean of 0 and a standard deviation of 1 based on the baseline visit, and taking the average of those standardised scores. Adjusted estimates were controlled for follow-up time, age (centred at 75 years), sex, race, education, income quartile and co-morbidity index (out of 7), including arthritis, diabetes, heart disease, high blood pressure, lung disease, osteoporosis and stroke. A reference participant represents a 75-year-old, White male with 0 co-morbidities, educational attainment of 8th grade or less and in the lowest income quartile. Cognitive domains with two stars (**) represent statistical significance slopes at a cut-off of P = 0.05.
Over the 5-year follow-up, community-dwelling older adults demonstrated decline in global cognitive function (−0.010 SD/year, 95%CI: −0.014, −0.006) and in all domain-specific cognitive performance tests with the exception of orientation (Figure 1). Steepest declines occurred for CDT (−0.098 SD/year, 95%CI: −0.107, −0.089), immediate word recall (−0.030 SD/year, 95%CI: −0.036, −0.025) and delayed word recall (−0.018 SD/year, 95%CI: −0.024, −0.013), respectively (Table 2).
Table 2 .
Adjusted linear mixed models quantifying global and domain-specific cognitive level and slope estimates among all community-dwelling older adults in the National Health and Aging Trends Study, 2011–2016 (n = 7,439). The global cognition composite score was created by standardising each of the four cognitive tests to a mean of 0 and a standard deviation of 1 based on the baseline visit, and taking the average of those standardised scores. Adjusted estimates were controlled for follow-up time, age (centred at 75 years), sex, race, education, income quartile and co-morbidity index (out of 7), including arthritis, diabetes, heart disease, high blood pressure, lung disease, osteoporosis and stroke. Cognitive level represents the average level for a reference participant (75-year-old White male with 0 co-morbidities, educational attainment of 8th grade or less and in the lowest income quartile)
| Cognitive outcome | Adjusted estimates Cohen’s d (95% confidence interval) |
|
|---|---|---|
| Level | Slope | |
| Global cognition composite | −0.45 (−0.51, −0.39)** | −0.010 (−0.014, −0.006)** |
| Clock-drawing test | −0.41 (−0.50, −0.33)** | −0.098 (−0.107, −0.089)** |
| Orientation | −0.49 (−0.58, −0.41)** | −0.002 (−0.007, 0.004) |
| Immediate recall | −0.60 (−0.78, −0.52)** | −0.030 (−0.036, −0.025)** |
| Delayed recall | −0.40 (−0.48, −0.32)** | −0.018 (−0.024, −0.013)** |
**Statistical significance at a cut-off of P = 0.05
Baseline cognitive levels by frailty
After adjustment, frail community-dwelling older adults had lower global cognitive scores (−0.64 SD, 95%CI: −0.72, −0.57) compared to non-frail community-dwelling older adults (−0.42 SD, 95%CI: −0.48, −0.35) at baseline, and this difference was statistically significant (Cohen’s d = −0.23 SD, 95%CI: −0.27, −0.19). Additionally, across all domain-specific cognitive tests, frail older adults had significantly lower scores than non-frail older adults at baseline, with the greatest differences in observed for CDT (Cohen’s d = −0.47 SD, 95%CI: −0.54, −0.39) and orientation (Cohen’s d = −0.39 SD, 95%CI: −0.45, −0.33) (Table 3). At baseline, frail older adults had CDT scores that were more than two times lower (−0.82 SD, 95%CI: −0.92, −0.71) than non-frail older adults (−0.35 SD, 95%CI: −0.44, −0.26) (Figure 2).
Table 3 .
Adjusted linear mixed models quantifying global and domain-specific cognitive level and slope estimates comparing frail (n = 1,313) versus non-frail (n = 6,126) among community-dwelling older adults in the National Health and Aging Trends Study, 2011–2016. The global cognition composite score was created by standardising each of the four cognitive tests to a mean of 0 and a standard deviation of 1 based on the baseline visit and taking the average of those standardised scores. Adjusted estimates were controlled for follow-up time, age (centred at 75 years), sex, race, education, income quartile, and comorbidity index (out of 7), including arthritis, diabetes, heart disease, high blood pressure, lung disease, osteoporosis and stroke
| Cognitive outcome | Baseline cognitive levels Standard deviations |
Slopes Standard deviations per year |
||||
|---|---|---|---|---|---|---|
| Frail | Non-frail | Difference Cohen’s d (95% CI) |
Frail | Non-frail | Difference Cohen’s d (95% CI) |
|
| Global cognition composite | −0.64** | −0.42** | −0.23 (−0.27, −0.19)** | −0.03** | −0.01** | −0.02 (−0.03, −0.01)** |
| Clock-drawing test | −0.82** | −0.35** | −0.47 (−0.54, −0.39)** | −0.20** | −0.09** | −0.12 (−0.15, −0.08)** |
| Orientation | −0.83** | −0.44** | −0.39 (−0.45, −0.33)** | −0.02** | −0.0001 | −0.02 (−0.04, −0.001)** |
| Immediate recall | −0.79** | −0.56** | −0.23 (−0.28, −0.17)** | −0.04** | −0.03** | −0.01 (−0.03, 0.01) |
| Delayed recall | −0.53** | −0.38** | −0.15 (−0.21, −0.10)** | −0.04** | −0.02** | −0.02 (−0.04, −0.01)** |
**Statistical significance at a cut-off of P = 0.05
Figure 2 .
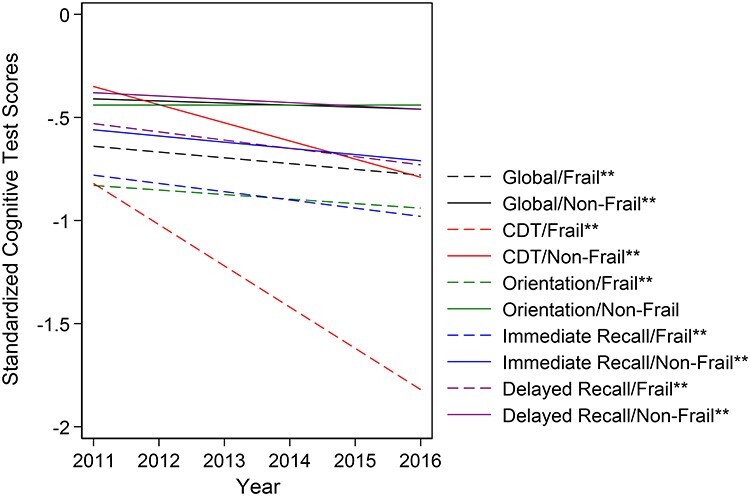
Adjusted cognitive trajectories by specific cognitive domain and by frailty status among community-dwelling older adult participants in the National Health and Aging Trends Study, 2011–2016 (n = 7,439). The global cognition composite score was created by standardising each of the four cognitive tests to a mean of 0 and a standard deviation of 1 based on the baseline visit, and taking the average of those standardised scores. Adjusted estimates were controlled for follow-up time, age (centred at 75 years), sex, race, education, income quartile and co-morbidity index (out of 7), including arthritis, diabetes, heart disease, high blood pressure, lung disease, osteoporosis and stroke. A reference participant represents a 75-year-old, White male with 0 co-morbidities, educational attainment of 8th grade or less and in the lowest income quartile. Dotted lines represent frail participants; solid lines represent non-frail participants. Trajectories with two stars (**) represent statistical significance slopes at a cut-off of P = 0.05.
Cognitive trajectories by frailty
Over the 5-year follow-up, frail older adults had steeper declines in global cognitive function (−0.03 SD/year, 95%CI: −0.04, −0.01) than non-frail older adults (−0.01 SD/year, 95%CI: −0.012, −0.005), and the slopes were significantly different (Cohen’s d = −0.02 SD/year, 95%CI: −0.03, −0.01). Frail older adults had significantly steeper declines in cognitive function than non-frail older adults across all domain-specific cognitive tests except for the immediate word recall (Cohen’s d = −0.01 SD/year, 95%CI: −0.03, 0.01) (Figure 2). However, greatest difference in slopes comparing frail to non-frail older adults occurred for CDT (Cohen’s d = −0.12 SD/year, 95%CI: −0.15, −0.08), followed by orientation (Cohen’s d = −0.02 SD/year, 95%CI: −0.04, −0.001) and delayed word recall (Cohen’s d = −0.02 SD/year, 95%CI: −0.04, −0.01) (Table 3). By the end of the 5-year follow-up, CDT scores for frail older adults were about 1 SD lower than non-frail older adults (Cohen’s d = −1.04, 95%CI: −1.18, −0.89), where non-frail older adults were 0.79 SD below the mean (95%CI: −0.89, −0.69), while frail older adults were 1.83 SD below the mean (95%CI: −1.99, −1.67).
Education significantly modified the association between baseline frailty and subsequent trajectories in global cognitive function (βinteraction = −0.015 SD/year, P < 0.001), as well as in all domain-specific cognitive performance, including CDT (βinteraction = −0.018 SD/year, P < 0.001), orientation (βinteraction = −0.019 SD/year, P < 0.001), immediate word recall (βinteraction = −0.015 SD/year, P < 0.001) and delayed word recall (βinteraction = −0.017 SD/year, P < 0.001).
Sensitivity analyses
Across all sensitivity analyses, inferences remained consistent for differences in cognitive levels at baseline, with greatest observed differences by frailty for CDT and orientation. For cognitive trajectories, however, CDT was the only cognitive outcome that consistently demonstrated significant differences in slopes by frailty (Supplementary Tables S2–S5 available in Age and Ageing online).
Discussion
To our knowledge, this is the first nationally representative study of older adult U.S. Medicare beneficiaries examining the association between frailty and repeated measures of domain-specific cognitive function. Our findings are consistent with prior studies suggesting that frail older adults have lower global cognitive function (Cohen’s d = −0.23 SD, 95%CI: −0.27, −0.19) and experience steeper declines in global cognitive function (Cohen’s d = −0.02 SD/year, 95%CI: −0.03, −0.01) compared to their non-frail counterparts [21–24]. However, while some studies have found that greater frailty severity was predictive of cognitive decline [37–39], other studies have found that baseline cognitive performance was associated with incident frailty [40, 41]. The causal mechanisms underlying this association remain unclear, with evidence of a potential bidirectional relationship. Nevertheless, collectively, evidence suggests that many of the ageing processes catalysing frailty may also be responsible for brain ageing and cognitive decline [22, 23]. Our study extends those findings to a large, nationally representative, diverse sample with domain-specific measures, demonstrating that frail older adults had significantly lower scores in memory, executive function and orientation. Our findings suggest that with low physical reserve and the body’s inability to bounce back from stressors, frail older adults are more vulnerable to lower cognitive functioning than non-frail older adults. With lower cognitive function, and steeper declines in cognitive function as they age, frail older adults may be at greater risk for cognitive impairment and dementia.
Though we observed differences by frailty in memory and orientation, we found the strongest evidence for executive function. Specifically, frail older adults had CDT scores that were more than two times lower (−0.82 SD, 95%CI: −0.92, −0.71) than non-frail older adults (−0.35 SD, 95% CI: −0.44, −0.26) at baseline (Cohen’s d = −0.47, 95%CI: −0.54, −0.39). Furthermore, by the end of the 5-year follow-up, CDT scores for frail older adults were over 1.5 SD below the mean (Cohen’s d = −1.83, 95%CI: −1.99, −1.67), while scores for non-frail older adults were less than 1 SD below the mean (Cohen’s d = −0.79 SD, 95%CI: −0.89, −0.69). These results are consistent with hypotheses that frailty has a stronger association with markers of executive function compared to memory [19, 21–24]. Two of the most common types of dementias, Alzheimer’s disease (AD) and vascular dementia, are often distinguished by disproportionate impairments in episodic memory and executive function, respectively [25]. We therefore hypothesise that frailty’s link to cognitive function likely has multifactorial aetiologies that are primarily vascular in nature. Further research into its association with dementia sub-types and related pathologies is critical.
This study has several limitations. First, NHATS lacks any biomarker data and heavily relies on participants self-reporting for their health history. However, self-report of chronic conditions has been shown to be reasonably accurate against medical records and claims [42]. Second, using different cognitive performance measures with varying sensitivities is especially challenging. For executive function, the CDT is known to have less education bias and language barrier and is better able to identify executive dysfunction among people with normal MMSE [43]. Additionally, testing participants’ ability for delayed verbal memory (word recall) is a strong predictor of AD pathologies [44]. Therefore, in NHATS, these tests were appropriately selected for a variety of evaluation settings where speed and ease of assessment are important in this large, diverse sample compared to more comprehensive neuropsychological batteries, which can be time-consuming and burdensome for participants. A third limitation relates to that of attrition, as is often the case with any longitudinal study; however, after accounting for potential biases due to attrition using two complementary approaches, results remained robust across all cognitive domains for differences in cognitive performance at baseline by frailty status; differences in cognitive change by frailty status were less consistent across cognitive domains, though remained robust by frailty status for global cognitive function and executive function. Finally, despite being the most widely used measure of frailty, particularly for etiologic research [12], frailty as measured by the PFP can sometimes be challenging to measure in clinical practice given time constraints, imprecision in self-reported items and inability to complete a task [45]; investigation of other rapid tools used in clinical practice, such as the FRAIL scale [46] or the Clinical Frailty Scale [47], is warranted.
Despite these limitations, this large, nationally representative study of U.S. Medicare beneficiaries aged 65 years and older has increased the generalizability of previous findings that frailty is associated with lower and steeper declines in global and domain-specific cognitive function. With widely used, repeated measures of domain-specific cognitive tests, this study has supported hypotheses that frail older adults are more vulnerable to cognitive decline, particularly in executive function. Further investigation into the direction of this relationship would lend insight into mechanisms that underlie this association, which is crucial to the development of effective, targeted interventions that may potentially prevent cognitive impairment and dementia among frail older adults.
Supplementary Material
Contributor Information
Nadia M Chu, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Qian-Li Xue, Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Division of Geriatric Medicine and Gerontology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Mara A McAdams-DeMarco, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Michelle C Carlson, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA; Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Mental Health, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Karen Bandeen-Roche, Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Biostatistics, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Alden L Gross, Department of Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, USA; Center on Aging and Health, Johns Hopkins Medical Institutions, Baltimore, MD, USA; Department of Mental Health, Johns Hopkins School of Public Health, Baltimore, MD, USA.
Declaration of Sources of Funding
This work was supported by the National Institute on Aging at the National Institutes of Health to N.M.C. (T32AG000247 and K01AG064040), Q.X. (R03AG053743), M.M.D. (R01AG055781), K.B.R. (P30AG021334 and P50AG005146) and A.L.G. (K01AG050699).
Declaration of Conflicts of Interest
None.
References
- 1.Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med 2013; 29: 753–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LAet al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts RO, Knopman DS, Mielke MMet al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014; 82: 317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183–94. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is mild cognitive impairment prodromal for vascular dementia like Alzheimer’s disease? Stroke 2002; 33: 1981–5. [DOI] [PubMed] [Google Scholar]
- 6.Prince M, Wilmo A, Guerchet Met al. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer's Disease International (ADI), 2015. [Google Scholar]
- 7.Jack CR, Knopman DS, Jagust WJet al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12: 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston Jet al. Frailty in older adults evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–57. [DOI] [PubMed] [Google Scholar]
- 9.Bandeen-Roche K, Xue QL, Ferrucci Let al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 2006; 61: 262–6. [DOI] [PubMed] [Google Scholar]
- 10.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandeen-Roche K, Seplaki CL, Huang Jet al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015; 70: 1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buta BJ, Walston JD, Godino JGet al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016; 26: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007; 62: 722–7. [DOI] [PubMed] [Google Scholar]
- 14.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59: M255–63. [DOI] [PubMed] [Google Scholar]
- 16.Puts MT, Toubasi S, Andrew MKet al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017; 46: 383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Isaacs K, Darko Let al. Changes in frailty after kidney transplantation. J Am Geriatr Soc 2015; 63: 2152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apóstolo J, Cooke R, Bobrowicz-Campos Eet al. Effectiveness of interventions to prevent pre-frailty and frailty progression in older adults: a systematic review. JBI Database System Rev Implement Rep 2018; 16: 140–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu NM, Bandeen-Roche K, Xue Q-L, Carlson MC, Sharrett AR, Gross AL. Physical frailty phenotype criteria and their synergistic association on cognitive functioning. J Gerontol A 2020. 10.1093/gerona/glaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu NM, Gross AL, Shaffer AAet al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol, 2019; 30: 336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brigola AG, Rossetti ES, Santos BRet al. Relationship between cognition and frailty in elderly: a systematic review. Dement Neuropsychol 2015; 9: 110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev 2013; 12: 840–51. [DOI] [PubMed] [Google Scholar]
- 23.Canevelli M, Cesari M, Kan GA. Frailty and cognitive decline: how do they relate? Curr Opin Clin Nutr Metab Care 2015 Jan; 18: 43–50. [DOI] [PubMed] [Google Scholar]
- 24.Gross AL, Xue QL, Bandeen-Roche Ket al. Declines and impairment in executive function predict onset of physical frailty. J Gerontol A Biol Sci Med Sci 2016; 71: 1624–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci 2004; 226: 3–7. [DOI] [PubMed] [Google Scholar]
- 26.Chu NM, Bandeen-Roche K, Tian Jet al. Hierarchical development of frailty and cognitive impairment: clues into etiological pathways. J Gerontol A 2019; 74: 1761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002; 8: 448–60. [PubMed] [Google Scholar]
- 28.Kasper JD, Freedman VA. National Health and Aging Trends Study User Guide: Rounds 1, 2, 3 & 4 Final Release. Baltimore: Johns Hopkins University School of Public Health. Available at www.NHATS.org.
- 29.Kasper JD, Freedman VA, Spillman BC. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. Technical Paper #5, 2013. Available at www.NHATS.org.
- 30.Ge ML, Carlson MC, Bandeen-Roche Ket al. US national profile of older adults with cognitive impairment alone, physical frailty alone, and both. J Am Geriatr Soc 2020. 10.1111/jgs.16769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman VA, Kasper JD, Cornman JCet al. Validation of new measures of disability and functioning in the National Health and Aging Trends Study. J Gerontol A Biol Sci Med Sci 2011; 66: 1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandeen-Roche K, Gross AL, Varadhan Ret al. Principles and issues for physical frailty measurement and its clinical application. J Gerontol A 2020; 75: 1107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan DB, Maxwell CJ, Afilalo Jet al. A scoping review of frailty and acute care in middle-aged and older individuals with recommendations for future research. Can Geriatr J 2017; 20: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JC, Heyman A, Mohs RCet al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39: 1159–65. [DOI] [PubMed] [Google Scholar]
- 35.Shulman KI, Pushkar GD, Cohen CA, Zucchero CA. Clock-drawing and dementia in the community: a longitudinal study. Int J Geriatr Psychiatry 1993; 8: 487–96. [Google Scholar]
- 36.Wilson RS, Mendes De Leon CF, Barnes LLet al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002; 287: 742–8. [DOI] [PubMed] [Google Scholar]
- 37.Auyeung TW, Lee JS, Kwok T, Woo J. Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging 2011; 15: 690–4. [DOI] [PubMed] [Google Scholar]
- 38.Samper-Ternent R, Snih SA, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc 2008; 56: 1845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitnitski A, Fallah N, Rockwood MR, Rockwood K. Transitions in cognitive status in relation to frailty in older adults: a comparison of three frailty measures. J Nutr Health Aging 2011; 15: 863–7. [DOI] [PubMed] [Google Scholar]
- 40.Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci 2010 Nov; 65: 1228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doba N, Tokuda Y, Goldstein NE, Kushiro T, Hinohara S. A pilot trial to predict frailty syndrome: the Japanese Health Research Volunteer Study. Exp Gerontol 2012; 47: 638–43. [DOI] [PubMed] [Google Scholar]
- 42.Miller DR, Rogers WH, Kazis LE, Spiro A 3rd, Ren XS, Haffer SC. Patients' self-report of diseases in the Medicare Health Outcomes Survey based on comparisons with linked survey and medical data from the Veterans Health Administration. J Ambul Care Manage 2008; 31: 161–77. [DOI] [PubMed] [Google Scholar]
- 43.Nair AK, Gavett BE, Damman Met al. Clock drawing test ratings by dementia specialists: interrater reliability and diagnostic accuracy. J Neuropsychiatry Clin Neurosci 2010Winter; 22: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyness SA, Lee AY, Zarow C, Teng EL, Chui HC. 10-Minute delayed recall from the modified mini-mental state test predicts Alzheimer’s disease pathology. JAD 2014; 39: 575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walston J, Buta B, XQ-L. Frailty screening and interventions: considerations for clinical practice. Clin Geriatr Med 2018; 34: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc 2008; 9: 71–2. [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Song X, Mac Knight Cet al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


