Abstract
Background
Cholangiocarcinoma (cancer in the bile duct) is an aggressive tumour for which surgical resection is a mainstay of treatment. Despite complete resection, recurrences of the cancer are common and lead to poor prognosis in patients. Postoperative adjuvant chemotherapy given after surgical resection may reduce the risk of cancer recurrence by eradicating residual cancer and micrometastatic lesions. The benefits and harms of postoperative adjuvant chemotherapy versus placebo, no intervention, or other adjuvant chemotherapies are unclear.
Objectives
To assess the benefits and harms of postoperative adjuvant chemotherapy versus placebo, no intervention, or other adjuvant chemotherapies for people with cholangiocarcinoma after curative‐intent resection.
Search methods
We performed electronic searches in the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials, MEDLINE, Embase, LILACS, Science Citation Index Expanded, and Conference Proceedings Citation Index ‐ Science for trials that met the inclusion criteria up to 28 April 2021.
Selection criteria
Randomised clinical trials irrespective of blinding, publication status, or language comparing postoperative adjuvant chemotherapy versus placebo, no intervention, or a different postoperative adjuvant chemotherapy regimen for participants with curative‐intent resection for cholangiocarcinoma.
Data collection and analysis
We used standard Cochrane methods to develop and conduct the review. We conducted meta‐analyses and presented results, where feasible, using a random‐effects model and risk ratios (RR) with 95% confidence intervals (CI). We assessed risk of bias according to predefined domains suggested by Cochrane. We rated the certainty of evidence using the GRADE approach and presented outcome results in a summary of findings table.
Main results
We included five published randomised clinical trials. The trials included 931 adults (18 to 83 years old) who underwent curative‐intent resection for cholangiocarcinoma. Four trials compared postoperative adjuvant chemotherapy (mitomycin‐C and 5‐fluorouracil (5‐FU); gemcitabine; gemcitabine plus oxaliplatin; or capecitabine) versus no postoperative adjuvant chemotherapy (surgery alone) in 867 participants with cholangiocarcinoma only. A fifth trial compared postoperative adjuvant S‐1 (a novel oral fluoropyrimidine derivative) chemotherapy versus gemcitabine in 70 participants with intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma (64 participants), and gallbladder carcinoma (6 participants). We assessed all of the included trials at overall high risk of bias. One trial was conducted in France, three in Japan, and one in the United Kingdom. We could not perform all planned comparison analyses due to lack of data. Three trials used intention‐to‐treat analyses. Another trial used per‐protocol analysis. In the remaining trial one participant in the intervention group and one in the control group were lost to follow‐up. However, the outcomes of these two participants were not described.
Postoperative adjuvant chemotherapy versus no postoperative adjuvant chemotherapy
We are very uncertain as to whether postoperative adjuvant chemotherapy has little to no effect on all‐cause mortality versus no postoperative adjuvant chemotherapy (RR 0.92, 95% CI 0.84 to 1.01; 4 trials, 867 participants, very low‐certainty evidence). We are very uncertain of the effect of postoperative adjuvant chemotherapy on serious adverse events (RR 17.82, 95% CI 2.43 to 130.82; 1 trial, 219 participants, very low‐certainty evidence). The trial indicated that postoperative adjuvant chemotherapy could increase serious adverse events, as 19/113 (20.5%) of participants developed an adverse event, compared to 1/106 (1.1%) of participants in the no‐postoperative adjuvant chemotherapy group. None of the included trials reported data on health‐related quality of life, cancer‐related mortality, time to recurrence of the tumour, and non‐serious adverse events in participants with only cholangiocarcinoma.
Adjuvant S‐1 chemotherapy (fluoropyrimidine derivative) versus adjuvant gemcitabine‐based chemotherapy
The only available trial analysed all participants with intrahepatic, perihilar cholangiocarcinoma and gallbladder carcinoma together, with data on participants with cholangiocarcinoma not provided separately. The authors reported that one‐year overall mortality after adjuvant S‐1 therapy was lower than with adjuvant gemcitabine‐based therapy following major hepatectomy for biliary tract cancer. There were no differences in two‐year overall mortality.
Funding: two trials received support from drug companies; one trial received funding from the Japan Society of Clinical Oncology; one trial received support from "Programme Hospitalier de Recherche Clinique (PHRC2009) and Ligue Nationale Contre le Cancer"; and one trial did not provide information on support or sponsorship.
We identified six ongoing randomised clinical trials.
Authors' conclusions
Based on the very low‐certainty evidence found in four trials in people with curative‐intent resection for cholangiocarcinoma, we are very uncertain of the effects of postoperative adjuvant chemotherapy (mitomycin‐C and 5‐FU; gemcitabine; gemcitabine plus oxaliplatin; or capecitabine) versus no postoperative adjuvant chemotherapy on mortality. The effects of postoperative adjuvant chemotherapy compared with no postoperative adjuvant chemotherapy on serious adverse events are also very uncertain, but the result of the single trial showed 20% higher occurrences of haematologic adverse events. We assessed the certainty of the evidence as very low due to overall high risk of bias, and imprecision. Due to insufficient power of the only identified trial, the best postoperative adjuvant chemotherapy regimen in people with only cholangiocarcinoma could not be established. We also lack randomised clinical trials with outcome data on adjuvant S‐1 chemotherapy versus adjuvant gemcitabine‐based chemotherapy in people with cholangiocarcinoma alone. There is a need for further randomised clinical trials designed to be at low risk of bias and with adequate sample size exploring the best adjuvant chemotherapy treatment after surgery in people with cholangiocarcinoma.
Plain language summary
Postoperative chemotherapy for cholangiocarcinoma that can be surgically removed
Review question
What are the benefits and harms of postoperative adjuvant chemotherapy (drugs administered after surgery, aiming to kill any cancer left after the operation) versus no postoperative adjuvant chemotherapy or another type or form of chemotherapy in people who underwent resection for cholangiocarcinoma (cancer in the bile ducts) with intent to cure.
Background
Cholangiocarcinoma is an aggressive tumour for which surgical resection is a mainstay of treatment. Bile ducts are the drainage 'pipes' that carry bile from the liver to the gallbladder, and from the gallbladder to the small intestine. Despite complete surgical removal (resection) of the cholangiocarcinoma, recurrences of tumour are common, leading to a poor prognosis for patients. It is thought that postoperative adjuvant chemotherapy (supplementary treatment after initial treatment) given after surgical resection may reduce the risk of cancer recurrence by eliminating residual cancer and micrometastatic lesions (microtumours that have spread from a cancer to distant areas of the body). The overall benefits and harms of this type of treatment are unclear.
Study characteristics
We searched for published articles describing randomised clinical trials (a type of study where participants are randomly assigned to one of two or more treatment groups) to identify the role of postoperative adjuvant chemotherapy, and found five studies with a total of 931 participants. Four studies (867 participants) compared surgery and postoperative adjuvant chemotherapy (mitomycin‐C and 5‐fluorouracil (5‐FU); gemcitabine; gemcitabine plus oxaliplatin; or capecitabine) with surgery alone (no postoperative adjuvant chemotherapy). One study (70 participants; 64 with cholangiocarcinoma and 6 with gallbladder carcinoma) compared surgery and a new adjuvant oral chemotherapy (S‐1) (fluoropyrimidine derivative) versus surgery and adjuvant gemcitabine‐based chemotherapy.
Funding: two trials received support from drug companies; one trial received funding from the Japan Society of Clinical Oncology; one trial received support from "Programme Hospitalier de Recherche Clinique (PHRC 2009) and Ligue Nationale Contre le Cancer"; and one trial did not provide information on support or sponsorship.
We also identified six ongoing randomised clinical trials.
Key results
We are very unsure as to whether postoperative adjuvant chemotherapy has any effect on death from any cause. Only one trial reported on adverse events. Whilst this trial indicated that postoperative adjuvant chemotherapy could increase serious adverse events, this result is very uncertain due to the lack of studies (i.e. only one found) and the low number of participants. No information was available on quality of life, death from cancer, time to tumour recurrence, and non‐serious adverse events.
Conclusions
Due to poor study quality, an insufficient number of studies, and low number of participants, the effects of postoperative adjuvant chemotherapy versus no postoperative adjuvant chemotherapy on mortality and serious adverse events are very uncertain. More randomised clinical trials designed with better study methods and larger participant numbers are needed.
Summary of findings
Background
Description of the condition
Cholangiocarcinoma is a malignant epithelial tumour arising in the bile duct (intrahepatic and extrahepatic bile ducts). Several conditions have been established as risk factors associated with cholangiocarcinoma including cirrhosis, hepatitis B, hepatitis C, chronic inflammation with liver injury secondary to the primary sclerosing cholangitis, and infection with Opisthorchis viverrini (South‐East Asian liver fluke) and Clonorchis sinensis (Chinese liver fluke) (Bergquist 2015; Luvira 2018). The age‐standardised incidence rates of cholangiocarcinoma vary widely between the different geographical regions, largely due to variations in regional environmental risk factors, diets, and lifestyles (Bergquist 2015). Cholangiocarcinoma is relatively rare in Western countries, with age‐standardised incidence rates between 0.3 and 2.0 per 100,000 person‐years (Bridgewater 2014). The age‐standardised incidence rate of cholangiocarcinoma in Asian countries ranges from 1 to 85 per 100,000 person‐years (Bridgewater 2014). The highest age‐standardised incidence rate of cholangiocarcinoma is found in the northeast provinces of Thailand, where the liver fluke O viverrini is endemic (up to 113 per 100,000 person‐years in men and 50 per 100,000 person‐years in women) (Parkin 2002; Sripa 2008; Bergquist 2015; Khuntikeo 2016).
Cholangiocarcinoma is commonly classified based on anatomical location. The intrahepatic type is defined as a cholangiocarcinoma that arises in a bile duct proximal to a second‐degree bile duct. The perihilar type is located in the bile duct between a second‐degree bile duct and a junction of cystic duct and a common bile duct. The distal type is localised in the area between the insertion of a cystic duct and the ampulla of Vater (Nakeeb 1996). The American Joint Committee staging for Cancer (AJCC) tumour‐nodes metastasis (TNM) staging system for cholangiocarcinoma is displayed in Appendix 1 (NCCN 2019).
Complete surgical resection remains the gold standard of treatment for cholangiocarcinoma (Nagino 2013; Luvira 2017a). Malignancy‐positive surgical margins and regional lymph node metastasis negatively impact on the survival of people with cholangiocarcinoma who undergo curative‐intent resection (Isa 2001; Weber 2001; Pattanathien 2013; Titapun 2015; Luvira 2017a). Median survival of people who have malignancy‐negative resection margins range from 25 to 61 months, compared to 12 to 20 months in people with malignancy‐positive resection margins (Isa 2001; Pattanathien 2013; Titapun 2015). Median survivals were 10 to 12 months in people with a lymph node metastasis and 27 to 63 months for people without a lymph node metastasis (Isa 2001; Pattanathien 2013; Titapun 2015).
Description of the intervention
Chemotherapy is a drug therapy used to treat cancer, principally by inhibiting the growth and division of cancer cells and promoting cell death. Adjuvant (supplementary treatment after initial treatment) chemotherapy can be defined as an additional chemotherapy given after primary surgery or primary radiotherapy in an attempt to lessen the risk of the cancer returning by eradicating residual diseases, particularly micrometastatic lesions that are supposed to be outside the field of the primary treatment covered (Carter 1986; Powell 1987). Chemotherapy agents can be administered locally (e.g. topical application or intracavitary injection) or systemically (e.g. oral, intramuscular injection, or intravenous injection) administered. The choice of chemotherapy agents for each patient mainly depends on the type and stage of cancer, patient's performance status, and details of previous treatment received (Carter 1986; Powell 1987). Although the best chemotherapy for cholangiocarcinoma remains to be determined, 5‐fluorouracil (5‐FU) and gemcitabine, as a single chemotherapeutic agent or in combination with other drugs (e.g. cisplatin or oxaliplatin), have been proposed as active and well‐tolerated regimens (Thongprasert 2005).
How the intervention might work
The mainstay of treatment of cholangiocarcinoma is surgical resection (Razumilava 2014; Luvira 2016). The operation for intrahepatic cholangiocarcinoma is resection of the involved hemi‐liver. For perihilar cholangiocarcinoma, additional bile duct resection, complete caudate lobe resection, and hepato‐duodenal lymph node dissection are required. Pancreaticoduodenectomy is the operation of choice for people with distal cholangiocarcinoma (Razumilava 2014). Although complete resection offers the best chance of cure for people with cholangiocarcinoma, recurrence can be high, ranging from 46% to 61% (Weber 2001; Yamamoto 2001; Hyder 2013). Cholangiocarcinoma can recur locally in the remaining liver or systemically in the distant organs. It is generally accepted that the recurrence of cancers arises not only because of inadequacy of surgical resection, but it is also secondary to pre‐existing microscopic cancer that spread systematically (Al Ustwani 2012). This concept motivates the attempt of postoperative adjuvant chemotherapy to supplement surgical resection in order to eradicate microscopic disease following resection of clinically detectable lesions, and in order to achieve long‐term prevention of cancer growth of cholangiocarcinoma.
A number of Cochrane Reviews have addressed the benefits of adjuvant chemotherapy given after primary surgery in lengthening survival in a variety of cancers (Figueredo 2008; Petersen 2012; Burdett 2015; Lawrie 2015). In people with completely resected stage II colon cancer, adjuvant chemotherapy reduced the risk of cancer recurrence by approximately 17% compared with the control group who underwent surgery alone (risk ratio (RR) for disease‐free survival 0.83, 95% confidence interval (CI) 0.75 to 0.92; certainty of evidence: not available) (Figueredo 2008). In people with rectal cancer undergoing curative‐intent surgery, adjuvant chemotherapy reduced the risk of death by 17% (hazard ratio (HR) 0.83, 95% CI 0.76 to 0.91; certainty of evidence: not available) and disease recurrence by 25% (HR 0.75, 95% CI 0.68 to 0.83; certainty of evidence: not available) amongst people undergoing postoperative adjuvant chemotherapy compared with people undergoing observation (Petersen 2012). When compared with the control group, postoperative adjuvant chemotherapy given to women with early‐stage ovarian cancer reduced the 10‐year risk of death (RR 0.76, 95% CI 0.62 to 0.94; certainty of evidence: high) and the risk of disease progression (RR 0.72, 95% CI 0.60 to 0.87; certainty of evidence: high) (Lawrie 2015). Additionally, compared with surgery alone, adjuvant chemotherapy in people with resected early‐stage non‐small cell lung cancer reduced the risk of death by approximately 14% (HR 0.86, 95% CI 0.81 to 0.92; certainty of evidence: not available) (Burdett 2015). There is evidence suggesting benefit of chemotherapy in advanced biliary tract cancer (Glimelius 1996; Marin 2020). Various retrospective studies investigated the efficacy of numerous chemotherapeutic agents in the adjuvant setting that previously had demonstrated activity in locally advanced and metastatic biliary tract cancer, and found that adjuvant chemotherapy was associated with longer survival (Horgan 2012; Belkouz 2020).
Why it is important to do this review
Although various novel surgical techniques for treating cholangiocarcinoma have been developed, prognosis of patients after resection remains poor. Interventions for improving treatment outcomes amongst people with cholangiocarcinoma operated for cure are therefore needed. The high tendency for cholangiocarcinomas to recur provides a rationale for adjuvant therapy after definitive surgery (Yang 2014). Postoperative adjuvant chemotherapy appears to be a promising adjuvant treatment for people with cholangiocarcinoma. However, to our knowledge, there has been no systematic review or meta‐analysis evaluating the effectiveness of postoperative adjuvant chemotherapy following surgical resection of cholangiocarcinoma. We therefore conducted this Cochrane Review to evaluate the effectiveness and safety of postoperative adjuvant chemotherapy for people with cholangiocarcinoma undergoing curative‐intent surgery.
Objectives
To assess the benefits and harms of postoperative adjuvant chemotherapy versus placebo, no intervention, or other adjuvant chemotherapies for people with cholangiocarcinoma after curative‐intent resection.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials irrespective of blinding, publication status, or language. We planned to extract and present narrative data on harm from quasi‐randomised and observational studies if these were retrieved with the search results, as adverse events are rarely reported in randomised clinical trials (Storebø 2018). We planned to include unpublished trials only if trial data and methodological descriptions were provided in written form or obtained through direct contact with study authors. We did not expect to find trials with cross‐over or cohort designs because of the nature of the interventions.
Types of participants
Adults (aged 18 years or older) of any sex who underwent curative‐intent resection for cholangiocarcinoma and received any type of postoperative adjuvant chemotherapy compared with people with the same condition, but receiving placebo, no postoperative adjuvant chemotherapy, or other adjuvant chemotherapies.
We only included trials with participants whose diagnosis of cholangiocarcinoma was established by pathological examination of surgical specimens. If studies included many types of biliary tract cancer (cholangiocarcinoma, gallbladder and ampulla of Vater carcinoma), we contacted authors to obtain disaggregated information specific for cholangiocarcinoma participants (See Characteristics of included studies for details). We intended to include people receiving any type of neoadjuvant treatment prior to operation, who were subsequently randomised to postoperative adjuvant chemotherapy, if the co‐interventions were equally applied in the trial groups.
Types of interventions
Experimental interventions
All regimens of postoperative adjuvant chemotherapy (intravenous infusion, intravenous bolus, intraportal infusion, and oral administration of a single regimen or a combination of chemotherapy regimens).
Control group
No postoperative adjuvant chemotherapy (surgery alone), placebo, or a different regimen or form of chemotherapy.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Serious adverse events. We used the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice's definition of a serious adverse event (ICH‐GCP 1997), i.e. any untoward medical occurrence that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect. We considered all other adverse events as non‐serious (see below).
Health‐related quality of life (as reported by the participants and as assessed by standard grading systems measured on a valid scale (e.g. Functional Assessment of Cancer Therapy‐Hepatobiliary cancers) (FACT‐Hep 2015).
Secondary outcomes
Cancer‐related mortality (death from cancer).
Time to recurrence of the tumour.
Non‐serious adverse events.
We performed our primary analyses using the outcome data at the last follow‐up.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (maintained and searched internally by the Cochrane Hepato‐Biliary Group Information Specialist via the Cochrane Register of Studies Web; 28 April 2021), the Cochrane Central Register of Controlled Trials (2021, Issue 4; date of search 28 April 2021) in the Cochrane Library, MEDLINE Ovid (1946 to 28 April 2021), Embase Ovid (1974 to 28 April 2021), LILACS (Latin American and Caribbean Health Science Information database) (BIREME; 1982 to 28 April 2021), Science Citation Index Expanded (Web of Science; 1900 to 28 April 2021), and Conference Proceedings Citation Index ‐ Science (Web of Science; 1990 to 28 April 2021). The search strategies for identification of relevant studies for the review are provided in Appendix 2.
There was no language restriction for this systematic review.
Searching other resources
We handsearched reference lists of articles retrieved by the search and contacted trial authors to obtain additional data where necessary.
We searched the online trial registries ClinicalTrials.gov (www.clinicaltrials.gov/), European Medicines Agency (EMA) (www.ema.europa.eu/ema/), World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp), Google Scholar (scholar.google.com), US Food and Drug Administration (FDA) (www.fda.gov), and pharmaceutical company sources for ongoing or unpublished trials (date of search 11 May 2021).
We contacted the Cholangiocarcinoma Foundation of Thailand (cloud.cascap.in.th) on 3 May 2021, and obtained one ongoing trial.
We also contacted the Cholangiocarcinoma Foundation (cholangiocarcinoma.org/) and the Alan Morement Memorial Fund (ammf.org.uk/) on 21 May 2021 and 3 June 2021, but obtained no additional trials.
Data collection and analysis
We performed the review following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We performed the analyses using Review Manager 5 (Review Manager 2020), and performed Trial Sequential Analysis (TSA) to evaluate imprecision for our primary outcomes (Thorlund 2017; TSA 2017; Wetterslev 2017). We contacted the authors of two trials for information specific to participants with cholangiocarcinoma alone. The authors of one trial provided the requested information (Edeline 2019), whilst the authors of the other trial did not reply to our requests (Kobayashi 2019).
Selection of studies
We downloaded all titles and abstracts retrieved by the electronic searches to a reference management database (EndNote). We removed duplicate publications, and then transferred the remaining results to Covidence. After excluding studies that clearly did not match the review criteria, we obtained the full texts of potentially relevant references for detailed reviewing. Two review authors (VL and ES) independently assessed the eligibility of the retrieved publications. Any disagreements were resolved through discussion or by consulting a third review author (AP or CK) when necessary. We identified no multiple publications of the included trials nor publications with follow‐up trial data. We used the details of the selection process in Covidence to create a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Two review authors (VL and ES) independently extracted study characteristics and outcome data from the included trials using Covidence. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. Any disagreements were resolved by consensus or by involving a third review author (AP or CK). One review author (PP) transferred data into Review Manager 5 (Review Manager 2020). Another review author (PL) checked the study characteristics for accuracy against the trial report.
We extracted the following data from the included trials: author, year of publication, and journal citation (including language); country; setting; inclusion and exclusion criteria; study methodology; study population and characteristics; total number enrolled, participant characteristics, age, comorbidities, other baseline characteristics, stage of cholangiocarcinoma, tumour size in largest diameter, histopathological type, status of surgical margin and lymph nodes, volume of residual lesion; intervention details, regimens of adjuvant chemotherapy, dose of adjuvant chemotherapy; comparison details; risk of bias in study (see below); duration of follow‐up; outcomes; results; trial funding/sponsorship, notable conflicts of interest of trial authors.
We planned the following.
For time‐to‐event data (e.g. time to recurrence of the tumour), we would extract the log of the hazard ratio (log(HR)) and its standard error from the trial reports. If these were not reported, we would attempt to estimate the log (HR) and its standard error using the methods of Parmar 1998.
For dichotomous outcomes (e.g. all‐cause mortality and adverse events), we extracted the number of people in each intervention group who experienced the outcome of interest and the number of people assessed at the end of follow‐up, to estimate a risk ratio (RR).
For continuous outcomes (e.g. health‐related quality of life), we planned to extract the final value and standard deviation of the outcome of interest and the number of people assessed at the end of follow‐up in each intervention group, to estimate the mean difference between the treatment arms. There were no continuous data to be extracted.
We calculated 95% confidence intervals (CI) for each estimate.
Where possible, all data extracted were to be those relevant to the conductance of intention‐to‐treat analysis, in which participants were analysed in the groups to which they had been assigned.
Assessment of risk of bias in included studies
Two review authors (VL and ES) independently assessed the risk of bias of each included trial according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018). We used the following definitions in our risk of bias assessment.
Allocation sequence generation
Low risk of bias: the study performed sequence generation using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent person not otherwise involved in the study.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation, so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit a judgement of 'low risk' or 'high risk', or the trial did not address this outcome.
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but it is likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; or blinding of outcome assessment ensured, and it is unlikely that the blinding could have been broken.
Unclear risk of bias: any of the following: insufficient information to permit a judgement of 'low risk' or 'high risk', or the trial did not address this outcome.
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; or blinding of outcome assessment, but it is likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, serious adverse events, and time to recurrence of the tumour. If the original trial protocol is available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. ClinicalTrials.gov), the outcomes sought should be those enumerated in the original protocol if the trial protocol was registered before or at the time the trial was begun. If the trial protocol was registered after the trial was begun, we will not consider those outcomes to be reliable.
Unclear risk of bias: the study authors did not report all predefined outcomes fully, or it is unclear whether the study authors recorded data on these outcomes or not.
High risk of bias: the study authors did not report one or more predefined outcomes.
Other bias
Low risk of bias: the trial appeared to be free of other factors that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other factors that could have put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
We judged a trial to be at an overall low risk of bias if all of the above domains were assessed at low risk of bias. We judged a trial to be at an overall high risk of bias if one or more of the above domains were assessed at unclear or high risk of bias.
Any differences in opinion were resolved through discussion; in the case of unsettled disagreements, a third review author adjudicated.
Measures of treatment effect
We used the following measures of the effect of treatment.
For dichotomous outcomes, we analysed data based on the number of events and the number of people assessed in the experimental and comparison groups. We used these to calculate the RR and 95% CI and Trial Sequential Analysis‐adjusted CI.
For continuous outcomes, we planned to analyse data based on the mean, standard deviation, and number of people assessed for both the experimental and comparison groups to calculate the mean difference between treatment groups with a 95% CI and Trial Sequential Analysis‐adjusted CI. We planned that if the mean difference was reported without individual group data, we would use this mean difference to report the study results. If more than one study measured the same outcome using different tools, we would calculate the standardised mean difference and 95% CI using the inverse‐variance method.
Unit of analysis issues
The unit of analysis was the trial and trial participants randomised to each intervention group. If a trial had two or more experimental intervention groups that shared a common control group, we would divide the control group into the respective number of experimental intervention groups of interest to us if they were part of the same comparison. We did not identify any trials with multiple intervention arms. We did not expect to find trials with a cross‐over design or cluster‐randomised trials.
Dealing with missing data
We attempted to contact study authors to obtain missing data. We did not impute missing outcome data and only analysed the data available in the trial reports.
Assessment of heterogeneity
We planned to assess heterogeneity amongst the included trials using visual inspection of the forest plots to consider both the direction and magnitude of effects and the degree of overlap between confidence interval. We also assessed statistical heterogeneity in each meta‐analysis using the I2 statistic and Chi2 test (Higgins 2003). We regarded heterogeneity as substantial if the I2 statistic value was greater than 50%, or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity (Deeks 2001; Higgins 2011). If there was substantial statistical heterogeneity, we would carry out subgroup analyses to assess differences between the included trials in terms of clinical and methodological characteristics as described in Subgroup analysis and investigation of heterogeneity.
Assessment of reporting biases
Given the small number of included trials, we did not examine funnel plots corresponding to meta‐analysis of the primary outcome all‐cause mortality to assess the potential for small‐study effects such as publication bias. In future updates, if 10 or more trials are identified, we will assess funnel plot asymmetry visually, and if asymmetry of funnel plots is identified, we will perform exploratory analyses to investigate it (see Differences between protocol and review) (Sterne 2011).
Data synthesis
Meta‐analysis
See Differences between protocol and review.
We performed the analysis using Review Manager 5 (Review Manager 2020). Where feasible, the results were meta‐analysed. We planned to perform meta‐analysis using random‐effects and fixed‐effect models in all meta‐analyses, and decided post hoc to explore differences between the two models using the fixed‐effect model in sensitivity analysis (if more than one trial provided data for meta‐analysis).
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias (as defined previously).
Surgical margin status (histologically cancer negative compared to histologically cancer positive).
Status of regional lymph node (negative compared to positive).
Location of tumour (intrahepatic compared to extrahepatic).
Type of adjuvant chemotherapy regimen (gemcitabine‐based compared to 5‐FU based).
We assessed subgroup differences by interaction tests and reported the results of subgroup analyses quoting the Chi2 test and P value, the interaction test, and the I2 statistic value whenever data were available.
Sensitivity analysis
We planned to apply 'best‐worst‐case' and 'worst‐best‐case' scenarios based on the outcomes all‐cause mortality, quality of life, and serious adverse events.
We also used the fixed‐effect model to conduct meta‐analyses. We planned to report both the fixed‐effect and random‐effects models if results differed.
We used Trial Sequential Analysis to assess imprecision (Jakobsen 2014), and compared the result with our GRADE assessment of imprecision.
Trial Sequential Analyses
We applied Trial Sequential Analysis (TSA) (Thorlund 2017; TSA 2017) to control random errors in our meta‐analysis (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Wetterslev 2017). We calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) which should also consider the diversity observed in the meta‐analysis (Wetterslev 2008; Wetterslev 2009; Wetterslev 2017).
We calculated the required information size for dichotomous outcomes based on the event proportion in the control group of the included trials; assumption of an a priori risk ratio of 10%; a risk of type I error of 2.5% for both primary and secondary outcomes as we have three outcomes of both (Jakobsen 2014); a risk of type II error of 10% (power 90%); and the observed diversity of the meta‐analysis (Wetterslev 2017). We planned also to conduct a TSA using the risk ratio in trials at low risk of bias, but as we found no such trials, this analysis will have to wait for their appearance. In future updates, for continuous outcomes, we will estimate the required information size based on the standard deviation observed in the control group of the meta‐analysis and a minimal relevant difference of 50% of this standard deviation, and the observed diversity in the trials in the meta‐analysis. For hazard ratio (HR), we will conduct robustness analyses by changing them into risk ratio as described above, or we will use software that can handle HRs (Miladinovic 2013).
We added the trials according to year of publication. Based on the required information size, we constructed trial sequential monitoring boundaries (Wetterslev 2008; Thorlund 2017). These boundaries determine the statistical inference that one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed before the required information size is reached, firm evidence may perhaps be established, and further trials may be superfluous. In contrast, if the boundary is not surpassed, it is most likely necessary to continue conducting trials to detect or reject a certain intervention effect. This can be determined by assessing whether or not the cumulative Z‐curve crosses the trial sequential monitoring boundary for futility (Wetterslev 2008). We conducted TSA using software from the Copenhagen Trial Unit (Thorlund 2017; TSA 2017).
We reported and compared the results with TSA as sensitivity analysis to imprecision assessed by GRADE. We downgrade imprecision in TSA by two levels if the accrued number of participants is below 50% of the diversity‐adjusted required information size (DARIS), and one level if it is between 50% and 100% of DARIS. We do not downgrade if the cumulative Z‐curve crosses the monitoring boundaries for benefit, harm, or futility, or if DARIS is reached.
Summary of findings and assessment of the certainty of the evidence
We created two summary of findings tables with all review outcomes, with data collected at the longest follow‐up (all‐cause mortality (median follow‐up range 46.5 to 79.4 months), serious adverse events (median follow‐up 79.4 months), health‐related quality of life, cancer‐related mortality (death from cancer), time to recurrence of the tumour, and non‐serious adverse events) obtained from the included trials using GRADEpro GDT software (GRADEpro GDT). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed (GRADEpro GDT). The certainty of a body of evidence considers within‐study risk of bias, indirectness of the evidence, heterogeneity of the data, imprecision of effect estimates, and risk of publication bias (Balshem 2011; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2011a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Guyatt 2013a; Mustafa 2013; Guyatt 2017).
Results
Description of studies
Results of the search
Our searches identified 472 records (Figure 1). We found and removed 114 duplicate records. After examining the titles and abstracts of the remaining 358 records, we selected 26 records for full‐text review. We found five randomised clinical trials that fulfilled our inclusion criteria (see Included studies; Characteristics of included studies).
1.

Study flow diagram. Date of search 28 April 2021.
We excluded a total of 15 studies (see Excluded studies; Characteristics of excluded studies). We did not find any quasi‐randomised or observational studies describing harmful effects of the intervention.
We identified six ongoing studies (see Ongoing studies; Characteristics of ongoing studies). We did not identify any unpublished studies.
Included studies
We included five randomised clinical trials (see Characteristics of included studies) (Takada 2002; Ebata 2018; Edeline 2019; Kobayashi 2019; Primrose 2019). One trial recruited only participants with cholangiocarcinoma (Ebata 2018). The other four trials recruited participants with cholangiocarcinoma and participants with gallbladder cancer (Takada 2002; Edeline 2019; Kobayashi 2019; Primrose 2019). Two of these four trials provided separate information on participants with cholangiocarcinoma in their reports (Takada 2002; Primrose 2019). The other two trials did not provide information specific to participants with cholangiocarcinoma in their reports (Edeline 2019; Kobayashi 2019). We contacted the authors of Edeline 2019 and Kobayashi 2019 to obtain specific information on participants with cholangiocarcinoma alone. The authors of Edeline 2019 provided the requested information, whilst the authors of Kobayashi 2019 did not reply to our requests. As a result, we combined data on participants with cholangiocarcinoma and gallbladder cancer from Kobayashi and colleagues in our analyses.
The five included trials involved a total of 931 participants with cholangiocarcinoma and were conducted in France, Japan, and the United Kingdom; all trials had a parallel‐group design. Four trials (Takada 2002; Ebata 2018; Edeline 2019; Primrose 2019), with 867 participants (who underwent surgery), compared the efficacy of postoperative adjuvant chemotherapy (mitomycin‐C and 5‐fluorouracil (5‐FU); gemcitabine; gemcitabine plus oxaliplatin; or capecitabine) versus no postoperative adjuvant chemotherapy. The remaining trial was a phase II trial, with 64 participants, comparing two different regimens of postoperative adjuvant chemotherapy (S‐1 versus gemcitabine) (Kobayashi 2019). All participants underwent surgery before the postoperative treatment.
Takada 2002 randomised 508 trial participants with resected pancreatic, bile duct, gallbladder, or ampulla of Vater carcinomas to either the mitomycin‐C and 5‐FU experimental intervention group or the control intervention group, who received no postoperative adjuvant chemotherapy (surgery alone). There were 118 trial participants with bile duct carcinoma, that is cholangiocarcinoma. The trial randomised participants from April 1986 to June 1992 in 31 centres in Japan. The trial followed up all participants for five years.
Ebata 2018 randomised 225 participants with resected extrahepatic cholangiocarcinoma (recruited from 1 September 2007 to 31 January 2011) at 48 hospitals in Japan. The trial participants were randomised in a 1:1 ratio to postoperative adjuvant gemcitabine (experimental intervention group) versus no postoperative adjuvant chemotherapy (surgery alone) (control intervention group). The trial followed up all but two participants for a median of 79.4 months.
Edeline 2019 randomised 196 participants with macroscopically resected biliary tract cancer, which included cholangiocarcinoma and gallbladder carcinoma (enrolled from July 2009 and February 2014) at 33 centres across France. There were 156 participants with cholangiocarcinoma. The trial participants (with macroscopically resected cholangiocarcinoma) were randomly assigned (1:1) within three months after resection to receive either postoperative adjuvant chemotherapy GEMOX (gemcitabine plus oxaliplatin) regimen (experimental intervention group) or surveillance with no postoperative adjuvant chemotherapy (control intervention group). The trial followed up all participants for a median of 46.5 months (95% CI 42.6 to 49.3 months).
Primrose 2019 randomised 447 participants who had undergone macroscopically resection for cholangiocarcinoma and muscle‐invasion gallbladder cancer (from 15 March 2006 to 4 December 2014) across 44 specialist hepato‐pancreato‐biliary centres in the United Kingdom. The trial participants were randomly assigned (1:1) to receive postoperative adjuvant oral capecitabine (experimental intervention group) versus observation (no postoperative adjuvant chemotherapy) (control intervention group) commencing within 16 weeks after surgery. There were 368 participants with cholangiocarcinoma (284 extrahepatic and 84 intrahepatic cholangiocarcinoma). The trial followed up all participants for a median of 60 months (interquartile range (IQR) 37 to 60).
Kobayashi 2019 randomised 70 participants who had undergone major hepatectomy for biliary tract cancer, which included intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma, and carcinoma of gallbladder (from February 2013 to June 2016) at centres across Japan. There were 64 participants with cholangiocarcinoma. The participants were randomly assigned (1:1) to receive postoperative adjuvant chemotherapy S‐1 (experimental intervention group) versus gemcitabine (control intervention group). The authors reported one‐ and two‐year survival rates based on all participants, but did not report the follow‐up time. There were no losses to follow‐up at the end of the study.
There were no losses to follow‐up in four trials (Takada 2002; Edeline 2019; Kobayashi 2019; Primrose 2019). Three included trials used intention‐to‐treat analyses (Edeline 2019; Kobayashi 2019; Primrose 2019). One trial used a per‐protocol analysis, although there were no loss to follow‐up (Takada 2002). The fifth trial had one loss to follow‐up in each group (Ebata 2018); however, the outcomes of these two participants were not described, therefore it was unclear whether or not this trial used intention‐to‐treat analysis.
Excluded studies
We excluded 15 studies that did not fulfil our inclusion criteria (see Characteristics of excluded studies) (Lim 2009; Di Girolamo 2011; Ghidini 2015; Hoehn 2015; Toyoki 2015; Kim 2017; Mizuno 2017; Okabayashi 2017; Schweitzer 2017; Woo 2017; Krasnick 2018; Nassour 2018; Siebenhuner 2018; NCT02798510; NCT03081039). Eleven studies were retrospective (Lim 2009; Di Girolamo 2011; Ghidini 2015; Hoehn 2015; Toyoki 2015; Kim 2017; Mizuno 2017; Okabayashi 2017; Schweitzer 2017; Krasnick 2018; Nassour 2018); one study was designed to determine whether adjuvant concurrent chemoradiotherapy improved overall survival (NCT02798510); two studies were phase II studies in which all participants received postoperative adjuvant chemotherapy (Woo 2017; Siebenhuner 2018); and one study was withdrawn (NCT03081039).
Amongst these, we did not find any relevant quasi‐randomised or other non‐randomised studies reporting on adverse events.
Ongoing studies
We identified six ongoing studies that appear to meet our inclusion criteria (see Characteristics of ongoing studies) (Stein 2015; Nakachi 2018; NCT02548195; NCT03079427; NCT03609489; TCTR20161101003). As of 9 July 2021, no further information than that described below was available.
Stein 2015 has planned a multicentre, two‐stage randomised controlled phase III trial, comparing postoperative adjuvant chemotherapy with gemcitabine plus cisplatin versus capecitabine after curative‐intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA‐1 Trial). The primary outcome is disease‐free survival. The estimated enrolment is 781 participants. The trial is still recruiting. Last follow‐up date: 23 September 2020.
Nakachi 2018 has planned a randomised phase III trial comparing postoperative adjuvant chemotherapy with S‐1 versus surgery alone (no postoperative adjuvant chemotherapy) in individuals with resectable biliary tract cancer (JCOG1202: aSCOT; Japan Clinical Oncology Group Study: Adjuvant S‐1 for Cholangiocarcinoma Trial). The primary outcome is overall survival. The planned total sample size is 440. The trial is still recruiting.
NCT02548195 is a randomised, open‐label, phase III trial comparing oxaliplatin plus gemcitabine versus capecitabine alone as postoperative adjuvant treatment in the prevention of recurrence of intrahepatic cholangiocarcinoma. The primary outcome is recurrence‐free survival. The estimated enrolment is 286 participants. The trial is still recruiting. Last follow‐up date: 14 September 2015.
NCT03079427 is a randomised, open‐label, phase II trial of postoperative adjuvant capecitabine versus gemcitabine plus cisplatin in individuals with resected extrahepatic cholangiocarcinoma with regional lymph node metastasis. The primary outcome is two‐year disease‐free survival. The estimated enrolment is 100 participants. The trial is still recruiting. Last follow‐up date: 16 June 2020.
NCT03609489 is a randomised, open‐label, phase II trial of postoperative apatinib (a tyrosine kinase inhibitor that selectively inhibits the vascular endothelial growth factor receptor‐2) plus capecitabine versus capecitabine as adjuvant treatment for individuals with surgical resection for histologically confirmed biliary tract cancer. The primary outcome is progression‐free survival. The estimated enrolment is 40 participants. This trial is not yet recruiting. Last follow‐up date: 9 August 2018.
TCTR20161101003 is a randomised, single‐blinded, phase III trial of gemcitabine alone versus gemcitabine plus cisplatin as adjuvant chemotherapy after curative‐intent resection of cholangiocarcinoma. The primary outcome is overall survival. The estimated enrolment was not available. The trial is still recruiting. Last follow‐up date: 10 August 2020.
Risk of bias in included studies
We assessed the risk of bias of the included trials. For graphical presentation, see Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both allocation sequence generation and allocation concealment were clearly reported in two trials (Ebata 2018; Primrose 2019), whereas it was not described in the other three trials (Takada 2002; Edeline 2019; Kobayashi 2019).
Blinding
We judged all trials to be at high risk of performance and detection bias, since four trials were described as "open label" trials (Ebata 2018; Edeline 2019; Kobayashi 2019; Primrose 2019), and the remaining trial did not provide information regarding blinding (Takada 2002), but it was likely that the blinding was broken because participants in the control group received neither treatment nor placebo.
Incomplete outcome data
There was a low risk of attrition bias in all of the included trials.
Selective reporting
We judged four trials to be at low risk of reporting bias (Ebata 2018; Edeline 2019; Kobayashi 2019; Primrose 2019), as all predefined outcomes in their respective protocols were reported in the results (UMIN000000820; NCT01313377; NCT01815307; NCT00363584). We judged the remaining trial as at unclear risk of reporting bias as the protocol of the trial was not available (Takada 2002).
Other potential sources of bias
We found no evidence of any other biases in the included trials.
Effects of interventions
Summary of findings 1. Adjuvant chemotherapy compared with no chemotherapy for resectable cholangiocarcinoma.
| Adjuvant chemotherapy compared with no chemotherapy for resectable cholangiocarcinoma | ||||||
| Patient or population: people with resectable cholangiocarcinoma Setting: secondary care Intervention: adjuvant chemotherapy Comparison: no chemotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no chemotherapy | Risk with adjuvant chemotherapy | |||||
| All‐cause mortality at 5 years | Study population | RR 0.92 (0.84 to 1.01) | 867 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 | We used Trial Sequential Analysis to calculate diversity‐adjusted required information size (DARIS). DARIS was calculated based on event proportion in the control group of 59.3% (255/430), risk ratio of 10%, type I error of 2.5%, type II error of 10% (90% power), and heterogeneity correction based on model variance (trial diversity was 0%). The required information size was 3473 participants. | |
| 593 per 1000 | 546 per 1000 (498 to 599) | |||||
| Serious adverse events Follow‐up: median: 79.4 months |
Study population | RR 17.82 (2.43 to 130.82) | 219 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 2 | Serious adverse events in the trial report were described as any serious adverse event, and not a specific toxicity. | |
| 9 per 1000 | 168 per 1000 (23 to 1000) | |||||
| Health‐related quality of life | There was no difference in time to definitive deterioration of global health‐related quality of life between those who received postoperative adjuvant GEMOX (gemcitabine and oxaliplatin) and those who did not. Participants receiving postoperative adjuvant capecitabine had worse social functioning compared with those who did not receive adjuvant chemotherapy. |
‐ | Both trials reported overall outcomes for all participants (cholangiocarcinoma and gallbladder carcinoma). | |||
| Cancer‐related mortality | Not reported | ‐ | None of the included trials reported this outcome for participants with cholangiocarcinoma alone. | |||
| TIme to recurrence of the tumour | Not reported | ‐ | None of the included trials reported this outcome for participants with cholangiocarcinoma alone. | |||
| Non‐serious adverse events | Not reported | ‐ | None of the included trials reported this outcome for participants with cholangiocarcinoma alone. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded due to methodological limitations (two levels): most trials had a high risk of performance and detection bias; and imprecision (one level); the optimal information size (OIS) of 3473 participants was not reached (see comment). 2Downgraded due to methodological limitations (two levels): the trial had a high risk of performance and detection bias; and imprecision (one level); the optimal information size (OIS) was not met (based on OIS using a conventional sample size calculation, the control group risk of 0.009, relative risk reduction (RRR) of 20%, α of 0.05, β of 0.20, the required sample size was 38,926 participants in each trial group).
Summary of findings 2. Adjuvant S‐1 compared to gemcitabine for resectable cholangiocarcinoma.
| Adjuvant S‐1 compared to gemcitabine for resectable cholangiocarcinoma | ||||||
| Patient or population: people with resectable cholangiocarcinoma Setting: secondary care Intervention: adjuvant S‐1 Comparison: adjuvant gemcitabine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with adjuvant gemcitabine | Risk with adjuvant S‐1 | |||||
| All‐cause mortality: 1‐year overall survival | Study population | RR 0.38 (0.15 to 0.96) | 70 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | 64 of 70 participants in this trial had cholangiocarcinoma; 6 participants had gallbladder carcinoma. | |
| 371 per 1000 | 141 per 1000 (56 to 357) | |||||
| All‐cause mortality: 2‐year overall survival | Study population | RR 0.81 (0.58 to 1.13) | 70 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | 64 of 70 participants in this trial had cholangiocarcinoma; 6 participants had gallbladder carcinoma. | |
| 743 per 1000 | 602 per 1000 (431 to 839) | |||||
| Serious adverse events | Study population | Not estimable | 70 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 | 64 of 70 participants in this trial had cholangiocarcinoma; 6 participants had gallbladder carcinoma. | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded due to methodological limitations (one level): the trial had a high risk of performance and detection bias; indirectness (one level): participants included people with other types of cancer; and imprecision (two levels): the sample size was not met, and there was a low number of events and a wide confidence interval.
Four included trials compared postoperative adjuvant chemotherapy following curative‐intent resection versus no postoperative adjuvant chemotherapy (surgical resection alone). See Included studies; Characteristics of included studies; and Table 1.
Adjuvant chemotherapy versus no adjuvant chemotherapy
Primary outcomes
All‐cause mortality
All four trials reported five‐year survival with the number of people with cholangiocarcinoma who were alive. We converted these data into number of people who died to obtain all‐cause mortality at five years. The median follow‐up time was 46.5 months for Edeline 2019, 60 months for Primrose 2019, and 79.4 months for Ebata 2018, and at least five years for all trial participants in Takada 2002. There seemed to be little to no difference in all‐cause mortality at five years between postoperative adjuvant chemotherapy and no postoperative adjuvant chemotherapy (RR 0.92, 95% CI 0.84 to 1.01; 4 trials, 867 participants, I2 = 0%, Analysis 1.1, very low‐certainty evidence).
1.1. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 1: All‐cause mortality
Sensitivity analysis
We observed similar findings with the TSA: the TSA‐adjusted RR was 0.92 (0.74 to 1.16). The required information size was not met (3473 participants; Figure 4). As only 25% of the required information size was accrued, we downgraded imprecision by two levels with TSA unlike our assessment of imprecsion by one level with GRADE.
4.
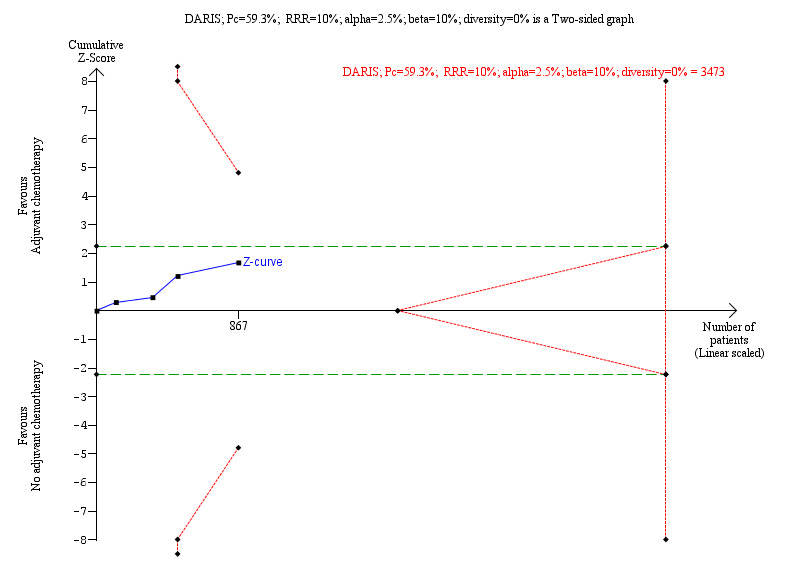
Trial Sequential Analysis (TSA) on all‐cause mortality
Four trials provided data on all‐cause mortality. The diversity‐adjusted required information size (DARIS) was calculated based on event proportion in the control group of 59.3% (255/430), relative risk reduction (RRR) of 10%, type I error of 2.5%, type II error of 10% (90% power), and heterogeneity correction based on model variance (trial diversity was 0%). The required information size was 3473 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm (red inward‐sloping lines), or futility (red outward lines). The accrued number of randomised participants is only 25% of the DARIS. The green dashed lines show conventional boundaries (2.5%). The TSA‐adjusted risk ratio was 0.92 (TSA‐adjusted confidence interval 0.74 to 1.16); two‐sided hypothesis testing, O’Brien‐Fleming α‐spending function.
Serious adverse events
Only one trial focused on adverse events in participants with cholangiocarcinoma who received postoperative adjuvant chemotherapy (Ebata 2018), finding that postoperative adjuvant chemotherapy increased serious adverse events (RR 17.82, 95% CI 2.43 to 130.82; 1 trial, 219 participants, I2 = 0%, very low‐certainty evidence).
Ebata 2018 found that the postoperative adjuvant chemotherapy (gemcitabine) group had more grade 3/4 haematologic toxicities compared with the no postoperative adjuvant chemotherapy (observation) group (Table 3). The risk ratio was 15.5 (95% CI 3.8 to 62.9) for abnormal leucocyte level and 15.5 (95% CI 5.8 to 40.9) for abnormal neutrophil level. Non‐haematological adverse events were rare and comparable between the two groups.
1. Grade 3/4 haematologic adverse events.
| Event | Intervention | Study | Number of participants with an event | Total number of participants | Proportion (%) |
| Leucocytes | Adjuvant gemcitabine | Ebata 2018 | 33 | 113 | 29.2 |
| Neutrophils | Adjuvant gemcitabine | Ebata 2018 | 66 | 113 | 58.4 |
The trials by Takada 2002, Edeline 2019, and Primrose 2019 did not report data on adverse events for participants with cholangiocarcinoma.
Health‐related quality of life
Two trials reported some data on health‐related quality of life (Edeline 2019; Primrose 2019); however, the reported information did not focus specifically on participants with cholangiocarcinoma alone, but on participants with cholangiocarcinoma and carcinoma of the gallbladder together.
Edeline 2019 reported that there was no difference in time to definitive deterioration of global health‐related quality of life, defined as the time between randomisation and worsening of global, physical functioning, or fatigue using European Organization for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 scores of at least 5 points (log‐rank P value = 0.39), and physical functioning (log‐rank P value = 0.15) between those who received postoperative adjuvant GEMOX (gemcitabine and oxaliplatin) and those who did not.
Primrose 2019 assessed each quality of life domain by comparison of standardised area under the curve via a Mann‐Whitney test, reporting that trial participants who received postoperative adjuvant capecitabine had worse social functioning compared with those who did not, with a median standardised area under the curve of 76.2 (IQR 56.9 to 91.7) in the capecitabine group and 83.3 (IQR 64.6 to 95.8) in the observation (no postoperative adjuvant chemotherapy) group (P = 0.006).
Secondary outcomes
Cancer‐related mortality (death from cancer)
None of the included trials planned to assess this outcome.
Time to recurrence of the tumour
None of the included trials planned to assess this outcome.
Non‐serious adverse events
None of the included trials planned to assess this outcome.
Subgroup analysis and investigation of heterogeneity
Whilst there was no significant heterogeneity in the main analysis, we followed our plan to perform subgroup analyses to estimate treatment effects for specific subgroups of participants (i.e. surgical margin status, status of regional lymph node, location of the tumour, and type of chemotherapy regimen).
We could perform all but one of the five prespecified subgroup analyses, that is trials at low risk of bias compared to trials at high risk of bias. There was no statistical difference between postoperative adjuvant chemotherapy and no postoperative adjuvant chemotherapy in any of the subgroups, that is surgical margin status, status of regional lymph node, location of the tumour, and type of chemotherapy regimen, regarding all‐cause mortality (Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.5). These findings suggest that surgical margin status, status of regional lymph node, location of the tumour, and types of chemotherapy regimen did not modify effects of postoperative adjuvant chemotherapy in comparison to no postoperative adjuvant chemotherapy.
1.2. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 2: All‐cause mortality (margin status). Subgroup analysis
1.3. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 3: All‐cause mortality (lymph node status). Subgroup analysis
1.4. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 4: All‐cause mortality (location of tumour). Subgroup analysis
1.5. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 5: All‐cause mortality (chemotherapy regimen). Subgroup analysis
Sensitivity analysis
We performed sensitivity analyses using worst‐case and best‐case scenarios, as described in the protocol. Three trials had no losses to follow‐up (Takada 2002; Edeline 2019; Primrose 2019). The fourth trial in this comparison had only one loss to follow‐up in each study arm (Ebata 2018). The results did not change, mainly because the number of loss to follow‐up was very small. We do not present these two analyses.
We also performed the sensitivity analyses using the fixed‐effect model. The results were not different, and we, therefore, reported only the results of the random‐effects model.
We performed TSA for one dichotomous outcome, all‐cause mortality at five years (Figure 4). As only 25% of the required information size was accrued, we downgraded imprecision by two levels with TSA unlike our assessment of imprecsion by one level with GRADE. We did not perform TSA for continuous outcomes as no trials provided continuous data.
Certainty of the evidence
Our assessment of the certainty of the evidence for the comparison 'Adjuvant chemotherapy versus no adjuvant chemotherapy' is presented in Table 1. We judged the overall certainty of the evidence for all‐cause mortality as very low (downgraded three levels) because of methodological limitations (two levels): most trials had a high risk of performance and detection bias; and imprecision (one level): the optimal information size (OIS) was not reached. We judged the overall certainty of the evidence for serious adverse events as very low (downgraded three levels) because of methodological limitations (two levels): the single trial was at high risk of performance bias and detection bias; and imprecision (one level): the OIS size was not reached (based on OIS using a conventional sample size calculation for a single trial).
We did not downgrade for indirectness, inconsistency of evidence, or publication bias because we only included participants with cholangiocarcinoma; there was no substantial heterogeneity; and there were fewer than 10 included trials, respectively.
Adjuvant S‐1 versus gemcitabine
One trial, conducted in Japan, compared postoperative adjuvant S‐1 versus gemcitabine (Kobayashi 2019). However, this trial included participants with intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma, and gallbladder carcinoma, and analysed the results together. We contacted the author on 22 May 2020 and 5 June 2020 to obtain specific data for participants with cholangiocarcinoma, but did not receive a response. Nevertheless, since 64 out of 70 participants in this trial had cholangiocarcinoma, we decided to analyse all trial data as provided, keeping in mind the indirectness of the evidence.
Primary outcomes
All‐cause mortality
The trial by Kobayashi and colleagues reported one‐ and two‐year survival rates, with the number of participants alive, without specifying details on the participant disease initially (Kobayashi 2019). We converted these data into numbers of people who died to obtain all‐cause mortality at five years. At one year, adjuvant S‐1 showed a reduction in all‐cause mortality compared with adjuvant gemcitabine, but the evidence is very uncertain (RR 0.38, 95% CI 0.15 to 0.96; 1 trial, 70 participants, Analysis 2.1, very low‐certainty evidence). At two years, adjuvant S‐1 may have little to no effect on all‐cause mortality compared with adjuvant gemcitabine, but the evidence is very uncertain (RR 0.81, 95% CI 0.58 to 1.13; 1 trial, 70 participants, Analysis 2.2, very low‐certainty evidence).
2.1. Analysis.

Comparison 2: Adjuvant S‐1 versus gemcitabine, Outcome 1: All‐cause mortality: 1 year
2.2. Analysis.

Comparison 2: Adjuvant S‐1 versus gemcitabine, Outcome 2: All‐cause mortality: 2 years
Serious adverse events
The Kobayashi 2019 trial reported that no participants suffered serious adverse events (very low‐certainty evidence).
Health‐related quality of life
Kobayashi 2019 did not plan to assess this outcome.
Secondary outcomes
Cancer‐related mortality (death from cancer)
Kobayashi 2019 did not plan to assess this outcome.
Time to recurrence of the tumour
Kobayashi 2019 did not plan to assess this outcome.
Non‐serious adverse events
Kobayashi 2019 reported that the S‐1 group was associated with higher rates of biliary infection (18.1% versus 8.5%).
Certainty of the evidence
Our assessment of the certainty of the evidence for the comparison 'Adjuvant S‐1 versus gemcitabine' is presented in Table 2. We judged the overall certainty of the evidence for all‐cause mortality and serious adverse events as very low (downgraded three levels) because of methodological limitations (one level): the trial had a high risk of performance and detection bias; indirectness (one level): participants included people with other types of cancer; and imprecision (two levels): low number of events and a wide confidence interval. In addition, the sample size was not met.
Discussion
Summary of main results
Four randomised clinical trials involving a total of 867 participants with cholangiocarcinoma compared postoperative adjuvant chemotherapy versus no postoperative adjuvant chemotherapy. Only one trial focused on participants with cholangiocarcinoma, whereas the other three trials also included participants with other biliary tract cancers. However, it was possible to extract data on the participants with cholangiocarcinoma from three of the trials, and we obtained the required data for the remaining trial through contact with the study authors. There was little to no difference in all‐cause mortality between postoperative adjuvant chemotherapy versus no postoperative adjuvant chemotherapy; however, we are very uncertain on the result due to the very low certainty of the evidence. We are also very uncertain as to whether postoperative adjuvant chemotherapy is associated with more serious adverse events compared with no postoperative adjuvant chemotherapy, also due to very low‐certainty evidence. The increased occurrence of adverse events in the chemotherapy group makes pharmacological sense and seems likely. Moreover, the adverse event is more likely to be worse than observed. We identified no trials assessing postoperative chemotherapy versus placebo.
Subgroup analyses of location of the tumour, surgical margin status, status of regional lymph node, and types of chemotherapy regimen did not seem to modify the effects of postoperative adjuvant chemotherapy in comparison to no postoperative adjuvant chemotherapy. However, due to the limited data available in the included studies, the subgroup analyses do not permit any meaningful interpretation.
In the comparison between different chemotherapy regimens, one randomised clinical trial assessed the efficacy and feasibility of postoperative adjuvant S‐1 chemotherapy versus gemcitabine after major hepatectomy for bile tract cancer, including 64 cholangiocarcinoma and 6 gallbladder carcinoma participants. Unfortunately, this trial did not report data specifically for cholangiocarcinoma participants only. At one year, adjuvant S‐1 may reduce all‐cause mortality compared with adjuvant gemcitabine. At two years, adjuvant S‐1 may have little to no effect on all‐cause mortality compared with adjuvant gemcitabine, but the evidence for this outcome is very uncertain.
We await the results of six identified ongoing randomised clinical trials to provide more data for these comparisons.
Overall completeness and applicability of evidence
Given that we included five trials with a relatively small number of participants with cholangiocarcinoma, at overall high risk of bias, our confidence in the effect estimation of the intervention is very low. Only one trial included participants with cholangiocarcinoma alone (Ebata 2018). The other four included trials involved participants with cholangiocarcinoma and gallbladder carcinoma; however, we obtained information for participants with cholangiocarcinoma alone from three of these trials (Edeline 2019; Primrose 2019; Takada 2002).
The included trials evaluated the effectiveness and adverse events of the two most important chemotherapy regimens for cholangiocarcinoma. One trial evaluated S‐1 chemotherapy, which is quite unique for Japan. Unfortunately, data on outcomes for participants with cholangiocarcinoma were not available. Moreover, the distribution of the countries in which the trials were conducted was limited: three trials were conducted in Japan (Takada 2002; Ebata 2018; Kobayashi 2019), and two trials in Europe (Edeline 2019; Primrose 2019), which may raise the suspicion of publication bias and applicability of the evidence in general.
Quality of the evidence
We judged the overall risk of bias of the included trials as high due to lack of blinding in all five included trials. We assessed the overall certainty of the evidence using the GRADE approach (Balshem 2011; GRADEpro GDT). For assessment of the outcome of interest, we judged the overall certainty of the evidence for all‐cause mortality as very low (downgraded three levels) due to methodological limitations (two levels): most trials had a high risk of performance and detection bias; and imprecision (one level): the OIS was not reached (the OIS determined using TSA). We also judged the overall certainty of the evidence for serious adverse events as very low (downgraded three levels) due to methodological limitations (two levels): the single trial had a high risk of performance and detection bias; and imprecision (one level): the OIS was not reached (the OIS determined using a conventional sample size calculation; the control group risk of 0.009, relative risk reduction (RRR) of 20%, α of 0.05, β of 0.20 (80% power), a minimum sample size was 38,926 participants in each arm).
Potential biases in the review process
We performed an extensive search of the databases. We searched for randomised clinical trials including participants with curative‐intent resection of cholangiocarcinoma who had received postoperative adjuvant chemotherapy versus no postoperative adjuvant chemotherapy. The search strategies were very broad. However, amongst the 334 references retrieved, only five publications were of interest, describing five randomised clinical trials. The paucity of trials of interest to our review may be due to the rarity of cholangiocarcinoma. Amongst our included trials, only Ebata 2018 focused on participants with cholangiocarcinoma. The remaining trials included participants with other kinds of bile duct cancers, resulting in heterogeneity amongst the trials. The lack of reporting of adverse events of the intervention in the included trials is another potential bias in the review process. We did not find any relevant observational studies reporting on harms in our search for randomised trials. By focusing mainly on randomised trials, we are aware that the review result regarding the potential harms of the intervention may be biased towards assessments of benefits. We were unable to construct funnel plots due to the limited number of trials, which means that we may unwittingly have perpetuated a publication bias.
None of the review authors has any links to drug companies or a financial interest in the prescription of the chemotherapeutic agents assessed in this review, nor were they involved in the conduct of any included trial, thus there were no issues associated to bias secondary to conflicts of interests in this review.
Agreements and disagreements with other studies or reviews
Six systematic reviews evaluating the benefits of postoperative adjuvant therapy for biliary tract cancer have been published (Horgan 2012; Zhu 2014; Ghidini 2017; Ma 2019; Wang 2019; Rangarajan 2020). None of these reviews assessed the adverse effects of adjuvant chemotherapy. Horgan 2012 included 20 studies involving 6712 participants, but the majority of the studies were observational studies, and the studies included all types of bile duct cancer. The adjuvant therapy of interest in this meta‐analysis included adjuvant chemotherapy and adjuvant chemo‐radiation. Horgan 2012 recommended adjuvant therapy for bile duct cancer, especially for those with lymph node‐positive disease and R1 (microscopically not free surgical margin) disease. Zhu 2014 included 12 studies involving 1361 participants with all types of bile duct cancer and assessed the benefits of various adjuvant modalities. This review claimed that adjuvant chemotherapy with gemcitabine increased survival. Ghidini 2017 included 30 studies involving a total of 22,499 participants with cholangiocarcinoma and gallbladder carcinoma. The intervention of interest was adjuvant chemotherapy, but the majority of the included studies were observational studies. The authors of this review claimed that adjuvant chemotherapy administration led to an overall survival benefit in resected bile duct tumour. Ma 2019 included 15 retrospective cohort studies involving 5060 participants with resectable intrahepatic cholangiocarcinoma. The intervention of interest was adjuvant chemotherapy, but all of the studies included in this review were retrospective cohort studies. Ma 2019 concluded that adjuvant chemotherapy is associated with improved overall survival and should be considered in patients with intrahepatic cholangiocarcinoma following curative resection, and in particular in those with advanced disease. Wang 2019 included 19 studies involving 11,458 participants with cholangiocarcinoma. The intervention of interest was adjuvant chemotherapy. One included study was a randomised clinical trial, whilst the other 18 included studies were retrospective observational studies. Wang 2019 concluded that postoperative adjuvant chemotherapy improves overall survival in intrahepatic and hilar cholangiocarcinoma patients, but that distal cholangiocarcinoma gained no benefit from adjuvant chemotherapy. Rangarajan 2020 included 35 studies involving 42,917 participants with cholangiocarcinoma and gallbladder carcinoma. The intervention of interest was adjuvant chemotherapy. The review included five randomised trials and 30 retrospective cohort studies and advocated the use of adjuvant therapy in bile duct cancer after curative‐intent resection.
All of the aforementioned reviews included both randomised clinical trials and observational studies. Four reviews recruited participants with all types of bile duct cancer (cholangiocarcinoma and gallbladder carcinoma) (Horgan 2012; Zhu 2014; Ghidini 2017; Rangarajan 2020). Three reviews included both adjuvant chemotherapy and adjuvant chemo‐radiation (Horgan 2012; Zhu 2014; Ghidini 2017).
Our review includes only randomised clinical trials aimed to assess the benefits and harms of adjuvant chemotherapy for participants with cholangiocarcinoma after curative‐intent resection. We found insufficient evidence to support or refute the use of adjuvant chemotherapy for this condition.
Authors' conclusions
Implications for practice.
Based on the very low‐certainty evidence found in four trials in people with curative‐intent resection for cholangiocarcinoma, we are very uncertain about the effects of postoperative adjuvant chemotherapy (mitomycin‐C and 5‐fluorouracil (5‐FU); gemcitabine; gemcitabine plus oxaliplatin; or capecitabine) versus no postoperative adjuvant chemotherapy on mortality. The effects of postoperative adjuvant chemotherapy on serious adverse events compared with no postoperative adjuvant chemotherapy are also very uncertain, but the result of the single included trial showed 20% higher occurrences of haematologic adverse events. We rated the certainty of the evidence as very low due to the overall trial risk of bias and imprecision, therefore these findings should be interpreted with caution, as a rating of very low certainty means that the true effect is likely to be substantially different from the estimate of effect. Due to insufficient power of the only identified trial, the best postoperative adjuvant chemotherapy regimen in people with only cholangiocarcinoma could not be established.
Implications for research.
There is a need for high‐quality randomised clinical trials assessing the benefit of postoperative adjuvant treatment, including chemotherapy and chemo‐radiotherapy, for people with cholangiocarcinoma. It is very important that future trials focus on only people with cholangiocarcinoma. Moreover, there are at least two main subtypes of cholangiocarcinoma, that is papillary and tubular cholangiocarcinoma, which may need separate assessments. Future randomised clinical trials should plan to conduct subgroup analysis between these two subtypes. Moreover, future trials should include placebo as well as nocebo controls. Outcomes should include mortality, health‐related quality of life, and serious adverse events. The trials must be designed following the SPIRIT statement (Standard Protocol Items: Recommendations for Interventional Trials; www.spiritstatement.org), and their reporting should be according to the CONSORT statement (www.consort-statement.org).
History
Protocol first published: Issue 9, 2017
Acknowledgements
We thank Dimitrinka Nikolova for contribution to the editorial process, and Sarah Louise Klingenberg for help with the search strategies and the electronic searches.
Protocol:
Peer reviewers: Dhananjaya Sharma, India; Frederik Keus, the Netherlands Contact editor: Saboor A Khan, the UK Sign‐off editor: Christian Gluud, Denmark
Review:
Peer reviewers: Ib Christian Rasmussen, Sweden; Shailesh Advani, the USA Contact editors: Omar Abdel‐Rahman, Egypt and Canada; Luit Penninga, Denmark Sign‐off editor: Christian Gluud, Denmark Abdomen and Endocrine Network Editor: Rachel Richardson, UK
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, the Capital Region, Rigshospitalet, Copenhagen, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
We would like to thank Julien Edeline and team (Centre Eugène Marquis, Rennes, France), Reham Abdel‐Wahab (Cholangiocarcinoma Foundation), and Helen Morement (The Alan Morement Memorial Fund) for their kind reply and generous sharing of study data.
Appendices
Appendix 1. American Joint Committee staging for Cancer (AJCC) tumour‐node metastasis (TNM) staging system for cholangiocarcinoma
| Intrahepatic bile duct tumours | |
| Stage | Findings |
| 0 | Carcinoma in situ (intraductal tumour) |
| IA | Solitary tumour < 5 cm without vascular invasion |
| IB | Solitary tumour > 5 cm without vascular invasion |
| II | Solitary tumour with vascular invasion |
| Multiple tumours, with or without vascular invasion | |
| IIIa | Tumour perforating the visceral peritoneum |
| IIIb | Tumour involving local extrahepatic structures by direct invasion |
| Regional lymph node metastasis present | |
| IV | Distant metastasis |
| Perihilar bile duct tumours | |
| Stage | Findings |
| 0 | Carcinoma in situ/high‐grade dysplasia |
| I | Tumour confined to the bile duct, with extension up to the muscle layer or fibrous tissue |
| II | Tumour invaded beyond the wall of the bile duct to surrounding adipose tissue |
| Tumour invaded adjacent hepatic parenchyma | |
| IIIA | Tumour invaded unilateral branches of the portal vein or hepatic artery |
| IIIB | Tumour invades main portal vein or its branches bilaterally; or the common hepatic artery; or the second‐order biliary radicals with contralateral portal vein or hepatic artery involvement |
| IIIC | One to three positive lymph nodes typically involving the hilar, cystic duct, common bile duct, hepatic artery, posterior pancreatoduodenal, and portal vein lymph nodes |
| IVA | Four or more positive lymph nodes from the sites described for N1 |
| IVB | Distant metastasis |
| Distal bile duct tumours | |
| Stage | Findings |
| 0 | Carcinoma in situ/high‐grade dysplasia |
| I | Tumour invades the bile duct wall with a depth less than 5 mm |
| IIA | Tumour invades the bile duct wall with a depth less than 5 mm with metastasis to one to three regional lymph nodes |
| Tumour invades the bile duct wall with a depth 5 to 12 mm | |
| IIB | Tumour invades the bile duct wall with a depth 5 to 12 mm with metastasis to one to three regional lymph nodes |
| Tumour invades the bile duct wall with a depth greater than 5 mm, with or without metastasis to one to three regional lymph nodes | |
| IIIA | Metastsis to four or more regional lymph nodes |
| IIIB | Tumour involves the celiac axis, superior mesenteric artery, and/or common hepatic artery |
| IV | Distant metastasis |
Appendix 2. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register (via the Cochrane Register of Studies Web) | April 2021 | ((adjuvant or adjunct*) and (chemotherap* or drug* or therap*)) and (cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumour*))) |
| Cochrane Central Register of Controlled Trials in the Cochrane Library | 2021, Issue 4 | #1 MeSH descriptor: [Chemotherapy, Adjuvant] explode all trees #2 (adjuvant or adjunct*) and (chemotherap* or drug* or therap*) #3 #1 or #2 #4 MeSH descriptor: [Cholangiocarcinoma] explode all trees #5 MeSH descriptor: [Bile Duct Neoplasms] explode all trees #6 cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumour*)) #7 #4 or #5 or #6 #8 #3 and #7 |
| MEDLINE Ovid | 1946 to April 2021 | 1. exp Chemotherapy, Adjuvant/ 2. ((adjuvant or adjunct*) and (chemotherap* or drug* or therap*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 3. 1 or 2 4. exp Cholangiocarcinoma/ 5. exp Bile Duct Neoplasms/ 6. (cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumor*))).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 7. 4 or 5 or 6 8. 3 and 7 9. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier] 10. 8 and 9 |
| Embase Ovid | 1974 to April 2021 | 1. exp adjuvant chemotherapy/ 2. ((adjuvant or adjunct*) and (chemotherap* or drug* or therap*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp bile duct tumor/ 5. (cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumor*))).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. 3 and 6 8. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 and 8 |
| LILACS (Bireme) | 1982 to April 2021 | ((adjuvant or adjunct$) and (chemotherap$ or drug$ or therap$)) [Words] and (cholangiocarcinom$ or (bile duct and (cancer$ or carcinom$ or malignan$ or neoplasm$ or tumor$))) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to April 2021 | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumor*))) #1 TS=((adjuvant or adjunct*) and (chemotherap* or drug* or therap*)) |
| Conference Proceedings Citation Index ‐ Science (Web of Science) | 1990 to April 2021 | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analys*) #3 #2 AND #1 #2 TS=(cholangiocarcinom* or (bile duct and (cancer* or carcinom* or malignan* or neoplasm* or tumor*))) #1 TS=((adjuvant or adjunct*) and (chemotherap* or drug* or therap*)) |
Data and analyses
Comparison 1. Adjuvant chemotherapy versus no chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1.1 Overall | 4 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.2 All‐cause mortality (margin status). Subgroup analysis | 2 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.16] |
| 1.2.1 Surgical margin positive | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 1.2.2 Surgical margin negative | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.73, 1.15] |
| 1.3 All‐cause mortality (lymph node status). Subgroup analysis | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.51] |
| 1.3.1 Lymph node positive | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.89, 1.56] |
| 1.3.2 Lymph node negative | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.51, 1.23] |
| 1.4 All‐cause mortality (location of tumour). Subgroup analysis | 3 | 749 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.83, 1.02] |
| 1.4.1 Intrahepatic | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.04] |
| 1.4.2 Extrahepatic | 3 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.08] |
| 1.5 All‐cause mortality (chemotherapy regimen). Subgroup analysis | 4 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.84, 1.01] |
| 1.5.1 Gemcitabine‐based chemotherapy | 2 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.84, 1.05] |
| 1.5.2 5‐FU‐based chemotherapy | 2 | 486 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.05] |
| 1.6 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.6. Analysis.

Comparison 1: Adjuvant chemotherapy versus no chemotherapy, Outcome 6: Serious adverse events
Comparison 2. Adjuvant S‐1 versus gemcitabine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality: 1 year | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 All‐cause mortality: 2 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ebata 2018.
| Study characteristics | ||
| Methods | Open‐label, randomised, multicentre phase III trial, with a parallel‐group design Location: Japan Intention‐to‐treat used. |
|
| Participants |
Inclusion criteria Participants with histologically proven extrahepatic bile duct cancer (perihilar and distal cholangiocarcinomas) who underwent macroscopically curative resection Exclusion criteria Confirmed recurrence before registration; clinically significant pleural effusion or ascites; pulmonary fibrosis or interstitial pneumonia; uncontrollable diarrhoea; severe heart failure or myocardial infarction within 6 months before registration; active infectious disease; blood transfusion within 2 weeks before trial registration; severe surgical complications; other concomitant malignant disease; pregnant or lactating women; childbearing potential; or other serious disorders incompatible with the trial, based on the investigator’s judgement |
|
| Interventions |
Group I: postoperative adjuvant gemcitabine (117 participants):
intravenous gemcitabine at dose 1000 mg/m2 over 30 minutes on day 1, 8, and 15 followed by a rest period of 1 week Group II: no postoperative adjuvant chemotherapy; surgery alone (108 participants) |
|
| Outcomes |
Primary outcome: overall survival Secondary outcome: relapse‐free survival and toxicity |
|
| Notes | Multicentre trial from Japan Trial authors contacted for additional data: not required |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned randomly to either the gemcitabine or observation (surgery alone) group in a 1: 1 ratio by a modified minimization method.... Treatment allocation was performed centrally through a web‐based randomization system managed by the data centre." |
| Allocation concealment (selection bias) | Low risk | "Patients were assigned randomly to either the gemcitabine or observation (surgery alone) group in a 1: 1 ratio by a modified minimization method.... Treatment allocation was performed centrally through a web‐based randomization system managed by the data centre." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The Bile Duct Cancer Adjuvant Trial (BCAT) was a randomised, open‐label, multicentre phase III trial conducted at 48 Japanese hospitals" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "The Bile Duct Cancer Adjuvant Trial (BCAT) was a randomised, open‐label, multicentre phase III trial conducted at 48 Japanese hospitals" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient in each group was lost to follow‐up within 5 years after randomisation." |
| Selective reporting (reporting bias) | Low risk | The trial reported all predefined protocol outcomes; trial protocol was available. |
| Other bias | Low risk | No other obvious biases |
Edeline 2019.
| Study characteristics | ||
| Methods | Randomised clinical trial with a parallel‐group design Location: France Intention‐to‐treat used. |
|
| Participants |
Inclusion criteria Participants deemed eligible were at least 18 years old and had undergone a curative intent, macroscopically complete (R0 or R1) resection of a localised biliary tract cancer (intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, or gallbladder carcinoma; ampullary carcinomas excluded). Exclusion criteria None |
|
| Interventions |
Group I: postoperative adjuvant chemotherapy (arm A: 95 participants): gemcitabine (1000 mg/m2 intravenously) over 100 minutes (fixed‐dose infusion rate, 10 mg/m2/min) on day 1 Oxaliplatin (85 mg/m2 intravenously) over 2 hours on day 2 every 2 weeks for 12 cycles Group II: no postoperative adjuvant chemotherapy; surveillance only (arm B: 99 participants) |
|
| Outcomes |
Primary outcomes: relapse‐free survival and time to definitive deterioration of health‐related quality of life Secondary outcomes: overall survival, toxicity, and exploratory translational outcomes (including study of potential, predictive, and prognostic factors) |
|
| Notes | Multicentre study (phase III) from France Trial authors contacted for additional data: yes. Date: 6 November 2019. Reply received: 12 November 2019 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description regarding method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description regarding concealment of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "PRODIGE 12‐ACCORD 18 (UNICANCER GI Group) was a multicentre, open‐label, randomised phase III trial conducted in 33 centres in France within the PRODIGE French Group of Digestive Oncology (FFCD), UNICANCER GI Group (UCGI), Multidisciplinary Cooperative Group in Oncology (GERCOR) intergroup" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "PRODIGE 12‐ACCORD 18 (UNICANCER GI Group) was a multicentre, open‐label, randomized phase III trial conducted in 33 centres in France within the PRODIGE French Group of Digestive Oncology (FFCD), UNICANCER GI Group (UCGI), Multidisciplinary Cooperative Group in Oncology (GERCOR) intergroup" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in both arms were followed up completely. |
| Selective reporting (reporting bias) | Low risk | The trial reported all predefined outcomes in the protocol, which was available. |
| Other bias | Low risk | No other obvious biases |
Kobayashi 2019.
| Study characteristics | ||
| Methods | Multicentre phase II, open‐label, randomised clinical trial with a parallel‐group design Location: Japan Intention‐to‐treat used. |
|
| Participants |
Inclusion criteria Patients more than 20 years old who had undergone major hepatectomy (hemihepatectomy, trisectionectomy, or more) due to biliary tract cancer, who had received no other therapy than surgery Exclusion criteria Presence of pulmonary fibrosis or intestinal pneumonia, severe heart disease, uncontrolled diabetes mellitus, active infection, pregnancy or lactation, women of childbearing age unless using effective contraception, severe drug hypersensitivity, mental disorder, concurrent double cancer, or other serious medical conditions |
|
| Interventions |
Group I: postoperative adjuvant chemotherapy gemcitabine (35 participants): gemcitabine (1000 mg/m2; intravenously) every 2 weeks for 24 weeks (6 months) Group II: postoperative adjuvant chemotherapy S‐1 (35 participants): S‐1 (80 mg/m2; orally) for 28 days every 6 weeks for 24 weeks (6 months) |
|
| Outcomes |
Primary outcomes: 1‐year recurrence‐free survival rate Secondary outcomes: 2‐year recurrence‐free survival, 1‐year overall survival, and 2‐year overall survival rates, recurrence‐free survival and overall survival curves, adverse events, completion rate, and relative dose intensity |
|
| Notes | Multicentre study (phase II) from Japan Trial authors contacted for additional data: 22 May 2020 and 5 June 2020. Reply received: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description regarding method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No description regarding concealment of the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "We designed this open‐label, multicenter, randomised phase II study to explore and compare the efficacy and feasibility of adjuvant gemcitabine or S‐1 therapy for BTC." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "We designed this open‐label, multicenter, randomised phase II study to explore and compare the efficacy and feasibility of adjuvant gemcitabine or S‐1 therapy for BTC." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in both arms were followed up completely. |
| Selective reporting (reporting bias) | Low risk | The trial reported all predefined outcomes in the protocol, which was available. |
| Other bias | Low risk | No other obvious biases |
Primrose 2019.
| Study characteristics | ||
| Methods | Randomised clinical trial with a parallel‐group design Location: the United Kingdom Intention‐to‐treat used. |
|
| Participants |
Inclusion criteria: Patients at least 18 years old with histologically confirmed cholangiocarcinoma or muscle‐invasive gallbladder cancer who had a macroscopically complete resection with curative intent Exclusion criteria: Patients who had not completely recovered from previous surgery, or who had previous chemotherapy or radiotherapy for biliary tract cancer |
|
| Interventions |
Group I: postoperative adjuvant chemotherapy capecitabine (223 participants): oral capecitabine (1250 mg/m2) was given postoperatively twice a day on days 1 to 14 of a 3‐weekly cycle for 24 weeks (8 cycles) Group II: no postoperative adjuvant chemotherapy; observation (224 participants) |
|
| Outcomes |
Primary outcomes: overall survival Secondary outcomes:
|
|
| Notes | Multicentre study (phase III) from the United Kingdom Trial authors contacted for additional data: not required. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned 1:1 to the capecitabine group or the observation group" |
| Allocation concealment (selection bias) | Low risk | "allocation concealment was achieved using a computerised minimisation algorithm that stratified patients by surgical centre, site of disease, resection status, and performance status" Allocation concealment was done by a central unit. "Concealment remained until the interventions were assigned by a central telephone‐based randomisation service hosted by the Cancer Research UK Clinical Trials Unit "(Birmingham)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Treatment was not masked" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Treatment was not masked" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in both arms were followed up completely. |
| Selective reporting (reporting bias) | Low risk | The trial reported all predefined outcomes in the protocol, which was available. |
| Other bias | Low risk | No other obvious biases |
Takada 2002.
| Study characteristics | ||
| Methods | Randomised clinical trial with a parallel‐group design Location: Japan Intention‐to‐treat used. |
|
| Participants |
Inclusion criteria
Exclusion criteria None |
|
| Interventions |
Group I: postoperative adjuvant mitomycin‐C (232 participants): mitomycin‐C (6 mg/m2; intravenously) rapid infusion on the day of surgery 5‐FU (310 mg/m2) in 2 courses of treatment for 5 consecutive days (each during postoperative weeks 1 and 3) 5‐FU orally; daily until disease recurrence Group II: no postoperative adjuvant chemotherapy; surgery alone (204 participants) |
|
| Outcomes |
Primary outcomes: overall survival Secondary outcomes: disease‐free survival, ECOG PS, improvement in body weight, and adverse effects |
|
| Notes | The 139 participants with bile duct carcinoma included in this trial received adjuvant mitomycin (67 participants) and surgery alone (72 participants). Multicentre trial (phase III) from Japan Trial authors contacted for additional data: not required |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description provided. |
| Allocation concealment (selection bias) | Unclear risk | No description provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "A randomised design, stratified according to institution and disease, was used.... The control group was not treated with any drugs including placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A randomised design, stratified according to institution and disease, was used.... The control group was not treated with any drugs including placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in both arms were followed up completely. |
| Selective reporting (reporting bias) | Unclear risk | Protocol of the trial was not available. |
| Other bias | Low risk | No other obvious biases |
5‐FU: 5‐fluorouracil
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Di Girolamo 2011 | Retrospective analysis of clinical data through reviewing medical records |
| Ghidini 2015 | Retrospective analysis of clinical data through reviewing medical records. No description regarding toxicity of chemotherapy in available published data |
| Hoehn 2015 | Retrospective analysis of national database that evaluated the efficacy of adjuvant chemotherapy and adjuvant radiotherapy. No description regarding toxicity of chemotherapy in available published data |
| Kim 2017 | Retrospective analysis of clinical data through reviewing medical records. No description regarding toxicity of chemotherapy in available published data |
| Krasnick 2018 | Retrospective multi‐institutional analysis involving various types of adjuvant treatment. No description regarding toxicity of chemotherapy in available published data |
| Lim 2009 | Retrospective analysis. Participants received adjuvant concurrent chemo‐radiation before chemotherapy. No description regarding toxicity of chemotherapy in available published data |
| Mizuno 2017 | Retrospective analysis of clinical data through reviewing medical records. No description regarding toxicity of chemotherapy in available published data |
| Nassour 2018 | Retrospective analysis of national database. No description regarding toxicity of chemotherapy in available published data |
| NCT02798510 | Study was designed to determine whether adjuvant concurrent chemoradiotherapy improves overall survival. |
| NCT03081039 | Study was withdrawn by the researcher because of a competing study. A phase III, multicentre, open‐label, randomised study of cisplatin or carboplatin with gemcitabine versus gemcitabine alone as adjuvant therapy in individuals with resected or ablated intra‐hepatic cholangiocarcinoma |
| Okabayashi 2017 | Retrospective analysis of clinical data through reviewing medical records. No description regarding toxicity of chemotherapy in available published data |
| Schweitzer 2017 | Retrospective analysis of clinical data through reviewing medical records. No description regarding toxicity of chemotherapy in available published data |
| Siebenhuner 2018 | Phase II study that evaluated the feasibility and efficacy of cisplatin and gemcitabine. All participants received adjuvant chemotherapy, no observation arm. No description regarding toxicity of chemotherapy in cholangiocarcinoma participants in available published data |
| Toyoki 2015 | Retrospective analysis of clinical data through reviewing medical records, for evaluation of efficacy of adjuvant S‐1 chemotherapy for resected advanced extra‐hepatic bile duct cancer. No description regarding toxicity of chemotherapy in available published data |
| Woo 2017 | Phase II study that evaluated the feasibility and efficacy of gemcitabine. All participants received adjuvant chemotherapy, no observation arm. No description regarding toxicity of chemotherapy in available published data |
Characteristics of ongoing studies [ordered by study ID]
Nakachi 2018.
| Study name | A randomized phase III trial comparing adjuvant chemotherapy with S‐1 vs. surgery alone in patients with resectable biliary tract cancer (JCOG1202: aSCOT) |
| Methods | Randomised, multicentre, open‐label, phase III study |
| Participants | People with the following:
|
| Interventions | Group I: surgery alone Group II: S‐1 |
| Outcomes | Primary endpoint:
Secondary endpoint:
|
| Starting date | September 2013 |
| Contact information | Kohei Nakachi, Otaru General Hospital, 1‐1‐1, Wakamatsu, Otaru, Hokkaido 047‐8550, Japan E‐mail: knakachi‐gi@umin.org |
| Notes | The planned total sample size is 440. |
NCT02548195.
| Study name | Oxaliplatin plus gemcitabine versus capecitabine alone as adjuvant treatment in the prevention of recurrence of intrahepatic cholangiocarcinoma |
| Methods | Randomised, open‐label, phase III study |
| Participants | People with:
|
| Interventions | Group I: oxaliplatin and gemcitabine Group II: capecitabine |
| Outcomes | Primary outcome:
Secondary outcome:
Other outcome:
|
| Starting date | July 2015 |
| Contact information | Hui‐Chuan Sun, MD, PhD +86 21 04041990 ext 610559; sun.huichuan@zs‐hospital.sh.cn |
| Notes | Estimated enrolment: 286 participants |
NCT03079427.
| Study name | Randomized phase 2 study of capecitabine versus gemcitabine plus cisplatin in patients with resected extrahepatic cholangiocarcinoma with regional lymph node metastasis |
| Methods | Randomised, open‐label, phase II study |
| Participants | People with the following:
|
| Interventions | Group I: gemcitabine plus cisplatin Group II: capecitabine |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 15 May 2017 |
| Contact information | Chanhoon Yoo, MD +82‐2‐3010‐1727; yooc@amc.seoul.kr |
| Notes | Estimated enrolment: 100 participants |
NCT03609489.
| Study name | A study to investigate apatinib combined capecitabine on adjuvant therapy of biliary carcinoma |
| Methods | Phase II, open‐label, randomised study |
| Participants | People with the following:
|
| Interventions | Group I: apatinib with capecitabine Group II: capecitabine |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 10 September 2018 |
| Contact information | Jie Ma, Deputy Director, First Affiliated Hospital of Guangxi Medical University |
| Notes | This study has not yet started recruiting participants. |
Stein 2015.
| Study name | Adjuvant chemotherapy with gemcitabine and cisplatin compared to standard of care after curative intent resection of cholangiocarcinoma and muscle invasive gall bladder carcinoma (ACTICCA‐1 Trial) |
| Methods | Multicentre, 2‐stage, prospective, randomised controlled phase III trial |
| Participants | People with the following:
|
| Interventions | Group I: gemcitabine plus visplatin Group II: vapecitabine |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | April 2014 |
| Contact information | Alexander Stein 004940741056882; a.stein@uke.de Jun Li 004940741058572; j.li@uke.de |
| Notes | Estimated enrolment: 781 participants: April 2022 |
TCTR20161101003.
| Study name | A randomised controlled trial of gemcitabine alone versus gemcitabine plus cisplatin as an adjuvant chemotherapy after curative intent resection of cholangiocarcinoma (GeCiCCA) |
| Methods | Multicentre, prospective, randomised controlled phase III trial |
| Participants | People with the following:
|
| Interventions | Group I: gemcitabine plus cisplatin Group II: gemcitabine |
| Outcomes | Primary outcome:
Secondary outcome:
|
| Starting date | 16 November 2016 |
| Contact information | Wilaiphorn Thinkhamrop; w.think@gmail.com |
| Notes | Expected completion date: 16 November 2022 |
ALP: alkaline phosphate ALT: alanine aminotransferase AST: aspartate aminotransferase CT: computed tomography CTCAE: Common Terminology Criteria for Adverse Events ECOG: Eastern Cooperative Oncology Group UICC: Union for International Cancer Control ULN: upper limits of normal
Differences between protocol and review
Unit of analysis
We have expanded this section to read: "The unit of analysis was the trial and trial participants randomised in each intervention group. If a trial had two or more experimental intervention groups that shared a common control group, we planned to divide the control group into the respective number of experimental intervention groups of interest to us if they were part of the same comparison. However, we did not identify any trials with multiple intervention arms. We did not expect to have, neither found, trials with cross‐over design or cluster randomised trials."
Data synthesis: meta‐analysis
In the review protocol, we planned to perform meta‐analysis using random‐effects and fixed‐effect models, and wrote that "we will report the most conservative finding, using a P value of 0.025 or less, two‐tailed, as statistically significant due to the three primary and three secondary outcomes." In the review, we applied the random‐effects model for all primary meta‐analyses, and the fixed‐effect model as sensitivity analysis.
Data synthesis: Trial Sequential Analysis
We conducted Trial Sequential Analysis by setting a risk of type II error of 10% instead of 90% (typographical error in the protocol). In addition, we did not perform Trial Sequential Analysis for continuous outcomes because the available data were not suitable for analysis. In future updates of this review, for continuous outcomes we plan to estimate the required information size based on the standard deviation observed in the control group and a minimal relevant difference of 50% of this standard deviation, and the observed diversity in the trials in the meta‐analysis.
Contributions of authors
VL: developed and drafted the protocol; selected trials for inclusion; extracted trial data; interpreted data; assessed certainty of the evidence; drafted the final review. ES: selected trials for inclusion; extracted trial data; commented on the final review. AP: selected trials for inclusion; extracted trial data; commented on the final review. CK: developed and drafted the protocol; interpreted data; commented on the final review. PL: developed and commented on the protocol; interpreted data; commented on the final review. PP: commented on the protocol; performed Trial Sequential Analysis; interpreted data; assessed the certainty of the evidence; commented on the final review.
All authors have approved the publication of the review.
Sources of support
Internal sources
Department of Surgery, Faculty of Medicine, Khon Kaen University, Thailand
Department of Obstetrics and Gynaecology, Faculty of Medicine, Khon Kaen University, Thailand
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand
Cochrane Thailand, Thailand
External sources
Thailand Research Fund (Distinguished Professor Award), Thailand
Declarations of interest
VL: none known ES: none known AP: none known CK: none known PL: none known PP: none known
New
References
References to studies included in this review
Ebata 2018 {published data only}
- Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. British Journal of Surgery 2018;105(3):192-202. [PMID: ] [DOI] [PubMed] [Google Scholar]
Edeline 2019 {published data only}
- Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. Journal of Clinical Oncology 2019;37(8):658-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kobayashi 2019 {published data only}
- Kobayashi S, Nagano H, Tomokuni A, Gotoh K, Sakai D, Hatano E, et al. A prospective, randomized phase ii study of adjuvant gemcitabine versus s-1 after major hepatectomy for biliary tract cancer (KHBO 1208): Kansai Hepato-Biliary Oncology Group. Annals of Surgery 2019;270(2):230-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Primrose 2019 {published data only}
- Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncology 2019;20(5):663-73. [DOI] [PubMed] [Google Scholar]
Takada 2002 {published data only}
- Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002;95(8):1685-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Di Girolamo 2011 {published data only}
- Di Girolamo S, Nobili E, Derenzini E, Rosa F, Agostini V, Ercolani G, et al. Impact of adjuvant chemotherapy on time to relapse in cholangiocarcinoma. Journal of Clinical Oncology 2011;29(4 Suppl):299. [Google Scholar]
Ghidini 2015 {published data only}
- Ghidini M, Lampis A, Braconi C, Trevisani F, Cheong IH, Hahne JC, et al. Retrospective analysis of the role of adjuvant chemotherapy and microRNAs expression in resected cholangiocarcinomas (CCAs). Annals of Oncology 2015;26(Suppl 6):L22. [Google Scholar]
Hoehn 2015 {published data only}
- Hoehn RS, Wima K, Ertel AE, Meier A, Ahmad SA, Shah SA, et al. Adjuvant chemotherapy and radiation therapy is associated with improved survival for patients with extrahepatic cholangiocarcinoma. Annals of Surgical Oncology 2015;22(Suppl 3):S1133-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim YS, Oh SY, Go SI, Kang JH, Park I, Song HN, et al. The role of adjuvant therapy after R0 resection for patients with intrahepatic and perihilar cholangiocarcinomas. Cancer Chemotherapy and Pharmacology 2017;79(1):99-106. [PMID: ] [DOI] [PubMed] [Google Scholar]
Krasnick 2018 {published data only}
- Krasnick BA, Jin LX, Davidson JT 4th, Sanford DE, Ethun CG, Pawlik TM, et al. Adjuvant therapy is associated with improved survival after curative resection for hilar cholangiocarcinoma: a multi-institution analysis from the U.S. Extrahepatic Biliary Malignancy Consortium. Journal of Surgical Oncology 2018;117(3):363-71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2009 {published data only}
- Lim KH, Oh DY, Chie EK, Jang JY, Im SA, Kim TY, et al. Adjuvant concurrent chemoradiation therapy (CCRT) alone versus CCRT followed by adjuvant chemotherapy: which is better in patients with radically resected extrahepatic biliary tract cancer?: a non-randomized, single center study. BMC Cancer 2009;9:345. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizuno 2017 {published data only}
- Mizuno T, Ebata T, Yokoyama Y, Igami T, Sugawara G, Yamaguchi J, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surgery Today 2017;47(2):182-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nassour 2018 {published data only}
- Nassour I, Mokdad AA, Porembka MR, Choti MA, Polanco PM, Mansour JC, et al. Adjuvant therapy is associated with improved survival in resected perihilar cholangiocarcinoma: a propensity matched study. Annals of Surgical Oncology 2018;25(5):1193-201. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT02798510 {published data only}
- NCT02798510. Clinical trial of adjuvant chemotherapy followed by concurrent chemoradiotherapy compared with adjuvant chemotherapy alone in patients with gallbladder carcinoma and extrahepatic cholangiocarcinoma. clinicaltrials.gov/ct2/show/NCT02798510 (first received 14 June 2016).
NCT03081039 {published data only}
- NCT03081039. A study of cisplatin or carboplatin with gemcitabine versus gemcitabine alone as adjuvant therapy in patients with resected or ablation intra-hepatic cholangiocarcinoma. clinicaltrials.gov/ct2/show/NCT03081039 (first received 15 March 2017).
Okabayashi 2017 {published data only}
- Okabayashi T, Shima Y, Iwata J, Morita S, Sumiyoshi T, Sui K, et al. Characterization of prognostic factors and the efficacy of adjuvant s-1 chemotherapy in patients with post-surgery extrahepatic bile duct cancer. Anticancer Research 2017;37(12):7049-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schweitzer 2017 {published data only}
- Schweitzer N, Weber T, Kirstein MM, Fischer M, Kratzel AM, Reineke-Plaass T, et al. The effect of adjuvant chemotherapy in patients with intrahepatic cholangiocarcinoma: a matched pair analysis. Journal of Cancer Research and Clinical Oncology 2017;143(7):1347-55. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siebenhuner 2018 {published data only}
- Siebenhuner AR, Seifert H, Bachmann H, Seifert B, Winder T, Feilchenfeldt J, et al. Adjuvant treatment of resectable biliary tract cancer with cisplatin plus gemcitabine: a prospective single center phase II study. BMC Cancer 2018;18(1):72. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toyoki 2015 {published data only}
- Toyoki Y, Ishido K, Kudo D, Kimura N, Hakamada K. Adjuvant chemotherapy using S-1 improves survival in patients with resected advanced extra-hepatic bile duct cancer: a propensity score matching analysis. Journal of Clinical Oncology 2015;33(15 Suppl):e15170. [Google Scholar]
Woo 2017 {published data only}
- Woo SM, Yoon K-A, Hong EK, Park WS, Han S-S, Park SJ, et al. A phase II study of gemcitabine as adjuvant treatment for biliary tract cancer after surgical resection. Journal of Clinical Oncology 2017;35(4 Suppl):330. [Google Scholar]
References to ongoing studies
Nakachi 2018 {published data only}
- Nakachi K, Konishi M, Ikeda M, Mizusawa J, Eba J, Okusaka T, et al. A randomized phase III trial of adjuvant S-1 therapy vs. observation alone in resected biliary tract cancer: Japan Clinical Oncology Group Study (JCOG1202, ASCOT). Japanese Journal of Clinical Oncology 2018;48(4):392-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT02548195 {published data only}
- NCT02548195. Oxaliplatin+gemcitabine vs capecitabine as adjuvant therapy for intrahepatic cholangiocarcinoma. clinicaltrials.gov/ct2/show/NCT02548195 (first received 14 September 2015).
NCT03079427 {published data only}
- NCT03079427. Adjuvant capecitabine vs gemcitabine plus cisplatin in resected extrahepatic cholangiocarcinoma. clinicaltrials.gov/ct2/show/NCT03079427 (first received 14 March 2017).
NCT03609489 {published data only}
- NCT03609489. A study to investigate apatinib combined capecitabine on adjuvant therapy of biliary carcinoma. clinicaltrials.gov/ct2/show/NCT03609489 (first received 1 August 2018).
Stein 2015 {published data only}
- Stein A, Arnold D, Bridgewater J, Goldstein D, Jensen LH, Klumpen HJ, et al. Adjuvant chemotherapy with gemcitabine and cisplatin compared to observation after curative intent resection of cholangiocarcinoma and muscle invasive gallbladder carcinoma (ACTICCA-1 trial) - a randomized, multidisciplinary, multinational phase III trial. BMC Cancer 2015;15:564. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20161101003 {published data only}
- TCTR20161101003. A randomized controlled trial of gemcitabine alone versus gemcitabine plus cisplatin as an adjuvant chemotherapy after curative intent resection of cholangiocarcinoma. www.thaiclinicaltrials.org/show/TCTR20161101003 (first received 31 October 2016).
Additional references
Al Ustwani 2012
- Al Ustwani O, Iancu D, Yacoub R, Iyer R. Detection of circulating tumor cells in cancers of biliary origin. Journal of Gastrointestinal Oncology 2012;3(2):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Belkouz 2020
- Belkouz A, Wilmink JW, Haj Mohammad N, Hagendoorn J, Vos-Geelen J, Dejong CH, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Critical Reviews in Oncology/Hematology 2020;151:102975. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bergquist 2015
- Bergquist A, Seth E. Epidemiology of cholangiocarcinoma. Best Practice & Research. Clinical Gastroenterology 2015;29(2):221-32. [DOI] [PubMed] [Google Scholar]
Bridgewater 2014
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. Journal of Hepatology 2014;60(6):1268-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. Journal of Clinical Epidemiology 2008;61:763-9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analyses may be inconclusive - Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI] [PubMed] [Google Scholar]
Burdett 2015
- Burdett S, Pignon JP, Tierney J, Tribodet H, Stewart L, Le Pechoux C, et al, the Non-Small Cell Lung Cancer Collaborative Group. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD011430. [DOI: 10.1002/14651858.CD011430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 1986
- Carter SK. Adjuvant chemotherapy of cancer. A review of its current status. Drugs 1986;31(4):337-67. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Deeks 2001
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London (UK): BMJ Publishing Group, 2001:285-312. [Google Scholar]
EndNote [Computer program]
- EndNote Version X7. Thomas Reuters. New York (NY): Thomas Reuters, 2015.
FACT‐Hep 2015
- FACIT Group. Functional assessment of cancer therapy - Hepatobiliary cancers (FACT-Hep). www.facit.org/measures/FACT-Hep (accessed 8 September 2021).
Figueredo 2008
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No: CD005390. [DOI: 10.1002/14651858.CD005390.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghidini 2017
- Ghidini M, Tomasello G, Botticelli A, Barni S, Zabbialini G, Seghezzi S, et al. Adjuvant chemotherapy for resected biliary tract cancers: a systematic review and meta-analysis. HPB: the Official Journal of the International Hepato-Pancreato-Biliary Association 2017;19(9):741-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Glimelius 1996
- Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Annals of Oncology 1996;7(6):593-600. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed March 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence - study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence - publication bias. Journal of Clinical Epidemiology 2011;64(12):1277-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence - imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence - inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence - indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables - binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles - continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2013d
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719-25. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines: 17. Assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Horgan 2012
- Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. Journal of Clinical Oncology 2012;30(16):1934-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hyder 2013
- Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153(6):811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1. Philadelphia: Barnett International/PAREXEL, 1997. [Google Scholar]
Isa 2001
- Isa T, Kusano T, Shimoji H, Takeshima Y, Muto Y, Furukawa M. Predictive factors for long-term survival in patients with intrahepatic cholangiocarcinoma. American Journal of Surgery 2001;181(6):507-11. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khuntikeo 2016
- Khuntikeo N, Loilome W, Thinkhamrop B, Chamadol N, Yongvanit P. A comprehensive public health conceptual framework and strategy to effectively combat cholangiocarcinoma in Thailand. PLOS Neglected Tropical Diseases 2016;10(1):e0004293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [DOI] [PubMed] [Google Scholar]
Lawrie 2015
- Lawrie TA, Winter-Roach BA, Heus P, Kitchener HC. Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD004706. [DOI: 10.1002/14651858.CD004706.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gotzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. BMJ (Clinical Research Ed.) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luvira 2016
- Luvira V, Nilprapha K, Bhudhisawasdi V, Pugkhem A, Chamadol N, Kamsa-Ard S. Cholangiocarcinoma patient outcome in northeastern Thailand: single-center prospective study. Asian Pacific Journal of Cancer Prevention 2016;17(1):401-6. [DOI] [PubMed] [Google Scholar]
Luvira 2017a
- Luvira V, Pugkhem A, Bhudhisawasdi V, Pairojkul C, Sathitkarnmanee E, Luvira V, et al. Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct. Journal of Gastroenterology and Hepatology 2017;32(2):527-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luvira 2018
- Luvira V, Kamsa-Ard S, Kamsa-Ard S, Luvira V, Srisuk T, Pugkhem A, et al. Association between repeated praziquantel treatment and papillary, and intrahepatic cholangiocarcinoma. Annals of Hepatology 2018;17(5):802-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ma 2019
- Ma KW, Cheung TT, Leung B, She BW, Chok KS, Chan AC, et al. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: a meta-analysis. Medicine 2019;98(5):e14013. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin 2020
- Marin JJ, Prete MG, Lamarca A, Tavolari S, Landa-Magdalena A, Brandi G, et al. Current and novel therapeutic opportunities for systemic therapy in biliary cancer. British Journal of Cancer 2020;123(7):1047-59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miladinovic 2013
- Miladinovic J, Hoz I, Djulbegovic D. Trial sequential boundaries for cumulative meta-analysis. Stata Journal 2013;13(11):77-91. [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352(9128):609-13. [DOI] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nagino 2013
- Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Annals of Surgery 2013;258(1):129-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nakeeb 1996
- Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Annals of Surgery 1996;224(4):463-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2019
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: hepatobiliary cancers version 4, 2019. www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 27 December 2019).
NCT00363584
- NCT00363584. Capecitabine or observation after surgery in treating patients with biliary tract cancer. clinicaltrials.gov/ct2/show/NCT00363584 (first received 15 August 2006).
NCT01313377
- NCT01313377. Gemcitabine hydrochloride and oxaliplatin or observation in treating patients with biliary tract cancer that has been removed by surgery. clinicaltrials.gov/ct2/show/NCT01313377 (first received 11 March 2011).
NCT01815307
- NCT01815307. Phase II study of gemcitabine versus S-1 adjuvant therapy after hemihepatectomy for biliary tract cancer. clinicaltrials.gov/ct2/show/NCT01815307 (first received 21 March 2013).
Parkin 2002
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer incidence in five continents, vol. VIII, 2002. publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Cancer-Incidence-In-Five-Continents-Volume-VIII-2002 (accessed 30 March 2021).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pattanathien 2013
- Pattanathien P, Khuntikeo N, Promthet S, Kamsa-Ard S. Survival rate of extrahepatic cholangiocarcinoma patients after surgical treatment in Thailand. Asian Pacific Journal of Cancer Prevention 2013;14(1):321-4. [DOI] [PubMed] [Google Scholar]
Petersen 2012
- Petersen SH, Harling H, Kirkeby LT, Wille-Jørgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database of Systematic Reviews 2012, Issue 3. Art. No: CD004078. [DOI: 10.1002/14651858.CD004078.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 1987
- Powell BL, Craig JB. Adjuvant chemotherapy of solid tumors. American Journal of the Medical Sciences 1987;294(1):33-41. [DOI] [PubMed] [Google Scholar]
Rangarajan 2020
- Rangarajan K, Simmons G, Manas D, Malik H, Hamady ZZ. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: a systematic review and meta-analysis. European Journal of Surgical Oncology 2020;46(4 Pt A):684-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Razumilava 2014
- Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383(9935):2168-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1-82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane Reviews: The ROBES Meta-Epidemiologic Study. American Journal of Epidemiology 2018;187(5):1113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Sripa 2008
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Current Opinion in Gastroenterology 2008;24(3):349-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomized controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non-randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD012069. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thongprasert 2005
- Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Annals of Oncology 2005;16(Suppl 2):ii93-6. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses. International Journal of Epidemiology 2009;38(1):276-86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clinical Epidemiology 2010;2:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2017
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA); 2nd edition. Copenhagen Trial Unit, 2017. Available from ctu.dk/tsa/learn-more.
Titapun 2015
- Titapun A, Pugkhem A, Luvira V, Srisuk T, Somintara O, Saeseow OT, et al. Outcome of curative resection for perihilar cholangiocarcinoma in Northeast Thailand. World Journal of Gastrointestinal Oncology 2015;7(12):503-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2017 [Computer program]
- Copenhagen Trial Unit TSA - Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2017. Available at ctu.dk/tsa/downloads/.
UMIN000000820
- UMIN000000820. Randomised phase III trial of adjuvant chemotherapy with gemcitabine vs. observation in patients with resectable bile duct cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000000983 (first received 20 July 2007).
Wang 2019
- Wang ML, Ke ZY, Yin S, Liu CH, Huang Q. The effect of adjuvant chemotherapy in resectable cholangiocarcinoma: a meta-analysis and systematic review. Hepatobiliary & Pancreatic Diseases International 2019;18(2):110-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Weber 2001
- Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. Journal of the American College of Surgeons 2001;193(4):384-91. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. Journal of Clinical Epidemiology 2008;61(1):64-75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random-effects meta-analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 2001
- Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. Journal of Hepato-Biliary-Pancreatic Surgery 2001;8(2):154–7. [DOI] [PubMed] [Google Scholar]
Yang 2014
- Yang H, Zhou J, Wei X, Wang F, Zhao H, Li E. Survival outcomes and prognostic factors of extrahepatic cholangiocarcinoma patients following surgical resection: adjuvant therapy is a favorable prognostic factor. Molecular and Clinical Oncology 2014;2(6):1069-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2014
- Zhu GQ, Shi KQ, You J, Zou H, Lin YQ, Wang LR, et al. Systematic review with network meta-analysis: adjuvant therapy for resected biliary tract cancer. Alimentary Pharmacology & Therapeutics 2014;40(7):759-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Luvira 2017b
- Luvira V, Satitkarnmanee E, Pugkhem A, Kietpeerakool C, Lumbiganon P, Kamsa-Ard S, et al. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012814. [DOI: 10.1002/14651858.CD012814] [DOI] [PMC free article] [PubMed] [Google Scholar]


