Abstract
Transcriptional activators function in vivo via binding sites that may be packaged into chromatin. Here we show that whereas the transcriptional activator GAL4 is strongly able to perturb chromatin structure via a nucleosomal binding site in yeast, GCN4 does so poorly. Correspondingly, GCN4 requires assistance from an accessory protein, RAP1, for activation of the HIS4 promoter, whereas GAL4 does not. The requirement for RAP1 for GCN4-mediated HIS4 activation is dictated by the DNA-binding domain of GCN4 and not the activation domain, suggesting that RAP1 assists GCN4 in gaining access to its binding site. Consistent with this, overexpression of GCN4 partially alleviates the requirement for RAP1, whereas HIS4 activation via a weak GAL4 binding site requires RAP1. RAP1 is extremely effective at interfering with positioning of a nucleosome containing its binding site, consistent with a role in opening chromatin at the HIS4 promoter. Furthermore, increasing the spacing between binding sites for RAP1 and GCN4 by 5 or 10 bp does not impair HIS4 activation, indicating that cooperative protein-protein interactions are not involved in transcriptional facilitation by RAP1. We conclude that an important role of RAP1 is to assist activator binding by opening chromatin.
Eukaryotic transcriptional activators function in part by overcoming repressive effects of chromatin (14, 40). First, however, the activators must bind to sites in chromatin. In vitro, nucleosomes can impede access of transcriptional activators such as heat shock factor and GAL4 to DNA (56, 63). Activation domains can contribute to activator binding to chromatin in vivo, either by cooperative interactions with general transcription factors or by recruiting chromatin remodeling activities which alter chromatin structure to enhance binding (6, 29, 34, 51, 54, 55, 59). However, these interactions do not completely alleviate the repressive effects of chromatin on activator binding, as diminished activator binding is seen in vivo at positions near the center of a positioned nucleosome relative to outside or near the edge of a positioned nucleosome (62, 69). Activator binding to nucleosomal sites in vitro can be aided by cooperative effects in which nucleosome perturbation by one activator facilitates binding of a second (1, 42), and this may also occur in vivo (60, 62). In spite of these advances, however, the rules and mechanisms governing access of transcriptional activators to chromatin in vivo remain to be established.
In this work, we compare the abilities of and the requirements for two transcriptional activators from the yeast Saccharomyces cerevisiae, GAL4 and GCN4, to interact with chromatin in vivo. GCN4, the proximal positive regulator in general amino acid control, coordinately activates at least 40 different genes upon amino acid starvation (53). These genes encode the enzymes needed for a variety of amino acid biosynthetic pathways. One of these, the HIS4 gene, is regulated by two independent systems, general control and basal control. Basal control is regulated by the BAS1 and BAS2 transcription factors under conditions of phosphate or adenine limitation. General control is regulated by GCN4 upon amino acid starvation. At the HIS4 promoter, a RAP1 binding site which overlaps a high-affinity GCN4 binding site is required for both BAS1/BAS2 and GCN4-dependent transcription of the HIS4 gene, although RAP1 alone cannot activate transcription of the HIS4 gene (11). Consequently, it has been suggested that RAP1 functions to increase accessibility of GCN4 and BAS1/BAS2 binding sites in HIS4 chromatin. Consistent with this idea, RAP1 competes with GCN4 in vitro for binding to a DNA fragment containing the RAP1 site and the partially overlapping GCN4 site from the HIS4 promoter, and increased amounts of GCN4 can displace RAP1 from the same DNA (3). Furthermore, mutation of the RAP1 binding site in the HIS4 promoter causes reduced micrococcal nuclease sensitivity of the HIS4 promoter region containing both the GCN4 binding site and BAS1/BAS2 binding sites in chromatin made from yeast cells (11).
Interestingly, GCN4 can activate transcription from promoters of other target genes independently of RAP1. A poly(dA-dT) tract is required for GCN4-dependent transcription of HIS3. Because of the rigid structure of poly(dA-dT), it was suggested that its function is to prevent nucleosomes from occluding the GCN4 binding site (26). Thus, it is possible that GCN4-mediated transactivation of target genes may require either intrinsic DNA structure or other trans-acting factors to overcome repression by chromatin. It is not clear at present whether GCN4 is unusual in this regard, since direct comparison with other activators, such as GAL4, has not been made. In this work, we have performed direct comparisons between different activators, principally GCN4 and GAL4, to examine their abilities to perturb a nucleosome containing their cognate binding sites and also to compare their abilities to activate HIS4 transcription in the presence and absence of a RAP1 binding site. Our results indicate that different activators do indeed vary in their abilities to perturb chromatin and that this ability correlates with the ability to activate HIS4 independently of RAP1. Furthermore, these differences are attributable to differences in binding affinity and not to properties of the activation domain.
MATERIALS AND METHODS
Plasmids.
To create the yeast plasmid TAGCN1Δ80, the consensus GCN4 binding site 5′-ATG-ACT-CAT-3′ was inserted into pRS104-17Δ80 (34) to replace the GAL4 binding site by two-step PCR (22) with primers A and B (Table 1) and verified by DNA sequencing. Yeast DNA sequence was excised by SacI and HindIII and ligated with the complementary SacI-HindIII fragment of pRS110 (35) and then transformed into yeast (23). Transformants were verified by Southern analysis. The yeast plasmid TAR/GCN1Δ80, which contains a wild-type RAP1 binding site adjacent to the GCN4 binding site, was created in the same way with primers C and D (Table 1). This RAP1 site is the same as that in the wild-type HIS4 promoter. Similarly, TARmut/GCN1Δ80, created with primers E and F (Table 1), contains a mutated RAP1 binding site adjacent to the GCN4 binding site. A HindIII site was created in TARmut/GCN1Δ80, so the SacI-HindIII fragment used for further ligation of the yeast plasmid was generated by partial digestion. The yeast plasmid TA17Δ80 was created as previously described (34) and introduced into yeast along with pRS426GAL4, a multicopy plasmid bearing the GAL4 gene (45).
TABLE 1.
Primers used in this study
| Purpose | Primersa | Restriction site created |
|---|---|---|
| Introduce GCN4 binding site in TAGCN1Δ80 | (A) 5′-ATGACTCATAAAACATAAAATCTG-3′ | |
| (B) 5′-ATGAGTCATCGATCTTTTATGC-3′ | ClaI | |
| Introduce wild-type RAP1 binding site in TAR/GCN1Δ80 | (C) 5′-GCTAAACCCATGCACAATGACTCATAAAACATAAAATCTGTTGAC-3′ | |
| (D) 5′-GTGCATGGGTTTAGCGATCTTTATGCTTGCTTTTCAAAAGGCCTGC-3′ | ||
| Introduce mutated RAP1 binding site in TARmut/GCN1Δ80 | (E) 5′-GATCGCTAAAGCTTTGCACAATGACTC-3′ | HindIII |
| (F) 5′-GCAAAGCTTTAGCGATCTTTATGCTTGC-3′ | HindIII | |
| Amplify the GCN4 coding sequence | (G) 5′-AAGAATTCTAAAATGTCCGAATATCAGCCAAGTTTATTTGC-3′ | EcoRI |
| (H) 5′-GGTAACTCGAGTCAGCGTTCGCCAACTAATTTCTTTAATCTGGC-3′ | XhoI | |
| Introduce a strong GAL4 site into HIS4 promoter with the wild-type RAP1 site | (R) 5′-CGGAAGACTCTCCTCCGGTTTTTATCAGTCATTCG-3′ | |
| (S) 5′-CGGAGGAGAGTCTTCCGTGTGCATGGGTTTAGCAA-3′ | ||
| Introduce a strong GAL4 site into HIS4 promoter with the mutated RAP1 site | (T) 5′-CGGAGGAGAGTCTTCCGGTGTGCAAAGCTTTAGC-3′ | HindIII |
| (S) 5′-CGGAAGACTCTCCTCCGGTTTTTATCAGTCATTCG-3′ | ||
| Introduce a weak GAL4 site into HIS4 promoter with the wild-type RAP1 site | (U) 5′-AGGAAGACTCTCCTCCGGTTTTTATCAGTCATTCG-3′ | |
| (V) 5′-CGGAGGAGAGTCTTCCTTGTGCATGGGTTTAGC-3′ | ||
| Introduce a weak GAL4 site into HIS4 promoter with the mutated RAP1 site | (U) 5′-AGGAAGACTCTCCTCCGGTTTTTATCAGTCATTCG-3′ | |
| (W) 5′-CGGAGGAGAGTCTTCCTGTGTGCAAAGCTTTAGC-3′ | HindIII | |
| Introduce an XhoI site into HIS4 promoter with the wild-type RAP1 site | (O) 5′-ACATCGCGACTCGAGGTTTTTTTATCAGTCATTCG-3′ | XhoI |
| (P) 5′-CTCGAGTCGCGATGTTGTGCATGGGTTTAG-3′ | NruI | |
| Introduce an XhoI site into HIS4 promoter with the mutated RAP1 site | (Q) 5′-CTCGAGTCGCGATGTTGTGCAAAGCTTTAGC-3′ | XhoI, NruI |
| (O) 5′-ACATCGCGACTCGAGGTTTTTTTATCAGTCATTCG-3′ | HindIII | |
| Introduce 4 Bicoid sites | (Top strand) 5′-TCGAATCTAATCCCTATCTAATCCCTATCTAATCCCTATCTAATCCCT-3′ | |
| (Bottom strand) 5′-TCGAAGGGATTAGATAGGGATTAGATAGGGATTAGATAGGGATTAGAT-3′ | ||
| Introduce 5 bp between the wild-type RAP1 site and the strong GCN4 site in the HIS4 promoter | (I) 5′-CATTTCAGTGACTCACGTTATCAGTCATTCGATATAG-3′ | |
| (J) 5′-CGTGAGTCACTGAAATGTGCATGGGTTTAGCAATTA-3′ | ||
| Introduce 10 bp between the wild-type RAP1 site and the strong GCN4 site in the HIS4 promoter | (K) 5′-TTTTATCACAGTGACTCACGTGTCATTCGATATAGAAGGTAAG-3′ | |
| (L) 5′-CACGTGAGTCACTGTGATAAAATGTGCATGGGTTTAGCAATTA-3′ | ||
| Introduce 5 bp between the mutated RAP1 site and the strong GCN4 site in the HIS4 promoter | (I) 5′-CATTTCAGTGACTCACGTTATCAGTCATTCGATATAG-3′ | |
| (M) 5′-CGTGAGTCACTGAAATGTGCAAAGCTTTAGCAATTA-3′ | HindIII | |
| Introduce 10 bp between the mutated RAP1 site and the strong GCN4 site in the HIS4 promoter | (K) 5′-TTTTATCACAGTGACTCACGTGTCATTCGATATAGAAGGTAAG-3′ | |
| (N) 5′-CACGTGAGTCACTGTGATAAAATGTGCAAAGCTTTAGCAATTA-3′ | HindIII |
Restriction sites (column 3) are underlined.
Plasmid pAB71 (5) (a gift of Alex Bortvin), which expresses the GCN4 gene from the DED1 promoter, was constructed by subcloning the SmaI-EcoRI fragment containing the GCN4 gene driven by the DED1 promoter from YCp88-GCN4 (24) into the CEN-containing, LEU2-marked plasmid YCplac111 (18). GAL4 was expressed either from the endogenous GAL4 gene (see Fig. 4) or from pCL1 (15), which expresses GAL4 from the ADH1 promoter (see Fig. 2). Bicoid protein was expressed from a GAL-inducible promoter with plasmid pDB1.2 (7) (a gift of David Burz). GAL4-GCN4 (the first 147 amino acids of GAL4 fused to all of GCN4 except for the amino-terminal 53 amino acids) was expressed from the DED1 promoter with plasmid pLY236, a CEN-containing plasmid with a LEU2 marker. This plasmid was created in three steps. First, the HpaI-XbaI fragment of pMA235 (2) was cloned into p416/GAL4, which contains the GAL4 gene fused to the ADH1 promoter in vector pRS416 (9, 52). The XbaI-PstI fragment of this new clone was then subcloned into pAB71 to construct pLY235. Plasmid pLY235 is a CEN-containing plasmid with a LEU2 marker and expresses the GAL4-GCN4 fusion protein from the ADH1 promoter. A PstI-HindIII fragment from pLY235 was cloned into pAB71 to construct pLY236.
FIG. 4.
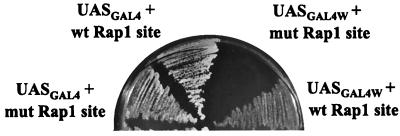
HIS4 expression mediated by GAL4 through a weak binding site depends on the RAP1 binding site. Cells containing the strong GAL4 binding site (UASGAL4) or the weak GAL4 binding site (UASGAL4W) with a wild-type (wt) or mutated (mut) RAP1 binding site were streaked from rich medium onto SC-His/galactose. GAL4 was expressed from the endogenous GAL4 promoter.
FIG. 2.
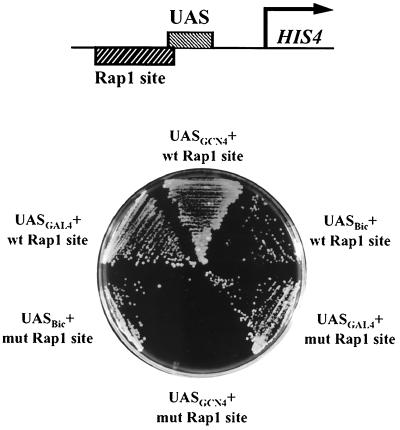
The RAP1 binding site is required for HIS4 expression mediated by GCN4 but not by GAL4 or Bicoid. Yeast strains containing integrated HIS4 promoters (diagrammed at the top), differing in the presence of a wild-type (wt) or mutated (mut) RAP1 site and in the activator binding site, were streaked from raffinose medium containing histidine onto galactose medium lacking histidine. GCN4 was expressed from the DED1 promoter, GAL4 was expressed from the ADH1 promoter, and Bicoid was expressed from a modified GAL1 promoter.
The GAL1pr-GCN4 plasmid, which expresses GCN4 from the GAL1 promoter, was constructed as a multicopy plasmid containing the LEU2 gene. The GCN4 coding sequence was amplified from genomic DNA with primers G and H (Table 1). Restriction sites for EcoRI and XhoI were introduced for further cloning. The PCR product was digested with EcoRI and XhoI, and the fragment was then introduced into pLY5C1. pLY5C1 was created by cloning the BamHI-KpnI fragment of pBC103 (10) containing the LEU2 gene into the multicopy plasmid phRF4-40 (16), which contains a GAL1 promoter and an ADH1 terminator.
Plasmids that contain the modified HIS4 promoter with a wild-type RAP1 binding site combined with either a GAL4 or Bicoid binding site were derivatives of pCB576 (11) (kindly provided by Kim Arndt). Plasmids that contain the HIS4 promoter with a mutated RAP1 binding site combined with either a GAL4 or Bicoid binding site were derivatives of pCB599 (11). The primers used to introduce a 17-bp weak or strong GAL4 binding site are shown in Table 1 (primers R to W). The EcoRI-PstI fragments of the PCR products were inserted into either pCB576 or pCB599 to replace the wild-type HIS4 promoter fragment. For introduction of four Bicoid sites, an XhoI restriction site was introduced into the HIS4 promoter fragment by PCR with primers O and P (in conjunction with the wild-type RAP1 binding site) or O and Q (in conjunction with the mutated RAP1 binding site) (Table 1), and the two phosphokinase-treated oligonucleotides containing four strong Bicoid sites (Table 1) were then inserted into the fragment. This fragment was cut with EcoRI and PstI and then cloned into pCB576 or pCB599. Introduction of the 5- and 10-bp insertions between the GCN4 and RAP1 sites was accomplished by PCR with primers shown in Table 1 (primers I to N) and either pCB599 or pCB576 as a template. The PCR products were cloned into pCB576 and verified by sequencing.
Strains and media.
The S. cerevisiae strains used in this study are derivatives of either FY24 or AY883 and are listed in Table 2. Yeast cells were grown at 30°C in complete synthetic dropout medium (Bio 101) containing 2% glucose, 1.5% raffinose, or 2% galactose. Cell transformations were performed by a standard lithium acetate method (23). To induce endogenous GCN4, 3-aminotriazole (3-AT) was added to a 10 mM final concentration from a freshly made 1 M solution to early log-phase cells and cells grown for 2.5 h.
TABLE 2.
Yeast strains used in this study
| Strain | Genotype or description | Reference or source |
|---|---|---|
| FY24 | MATα ura3-52 trp1Δ63 leu2Δ1 | 66 |
| LYY50 | Same as FY24, but with gcn4Δ | This study |
| AY883 | MATα gcn4-2 bas1-2 bas2-2 ura3-52 leu2-3,112; URA3 at position −123 of HIS4 | 11 |
| LYY596 | Same as AY883 but with wild-type HIS4 | This study |
| LYY599 | Same as AY883 but with mutated RAP1 site at HIS4 | This study |
| LYY11 | Same as LYY596 but with a GAL4 site replacing the strong GCN4 site at HIS4 | This study |
| LYY13 | Same as LYY599 but with a GAL4 site replacing the strong GCN4 site at HIS4 | This study |
| LYY12 | Same as LYY596 but with 4 Bicoid sites replacing the strong GCN4 site at HIS4 | This study |
| LYY14 | Same as LYY599 but with 4 Bicoid sites replacing the strong GCN4 site at HIS4 | This study |
| LYY15 | Same as LYY596 but with a weak GAL4 site replacing the strong GCN4 site at HIS4 | This study |
| LYY16 | Same as LYY599 but with a weak GAL4 site replacing the strong GCN4 site at HIS4 | This study |
| LYY596+5 | Same as LYY596 but with 5-bp insertion between the RAP1 site and the strong GCN4 site at HIS4 | This study |
| LYY596+10 | Same as LYY596 but with 10-bp insertion between the RAP1 site and the strong GCN4 site at HIS4 | This study |
| LYY599+5 | Same as LYY599 but with 5-bp insertion between the mutated RAP1 site and the strong GCN4 site at HIS4 | This study |
| LYY599+10 | Same as LYY599 but with 10-bp insertion between the mutated RAP1 site and the strong GCN4 site at HIS4 | This study |
The gcn4Δ strain LYY50 was constructed from FY24 by two-step gene disruption with the insertion plasmid YIp56-SC3674 (26) (generously provided by Kevin Struhl). GCN4 gene disruption was confirmed by Southern analysis.
For construction of strains containing modified genomic HIS4 promoters, plasmids containing either the wild-type HIS4 promoter or a modified HIS4 promoter were constructed from pCB576 and pCB599 as described above and verified by DNA sequencing. The XhoI-SpeI fragments of the corresponding plasmids were transformed into AY883 cells, in which the URA3 gene has been placed upstream in the HIS4 promoter. Transformed cells were divided into separate culture tubes (to ensure eventual isolation of independent clones), grown in liquid yeast extract-peptone-dextrose (YEPD) medium overnight at 30°C, and plated on 5-fluoroorotic acid (5-FOA) plates. 5-FOA-resistant cells were patched onto YEPD plates. PCR products from yeast genomic DNA were amplified with HIS4 promoter-specific primers, used to identify the desired HIS4 substitution by size, and confirmed by sequencing. The above procedure produced an isogeneic set of yeast strains that differ only at the chromosomal HIS4 locus. LEU2-marked expression vectors for Bicoid, GCN4, GAL4, or GAL4-GCN4 were introduced into the corresponding strains.
Analysis of chromatin structure.
Chromatin was prepared from yeast nuclei (47) or spheroplast lysates (28) and analyzed by the indirect end label technique (37, 68), as described previously (51).
RESULTS
Nucleosome perturbation elicited by GCN4 via a nucleosomal binding site is weaker than that elicited by GAL4 at a similar site.
Previous work has suggested that binding of the transcriptional activator GCN4 to promoter sites in yeast is sometimes assisted by accessory proteins or DNA structural elements that open chromatin structure (11, 26). In contrast, GAL4 can bind to nucleosomal sites in yeast, with concomitant perturbation of nucleosome positioning, without apparent assistance from other DNA-binding proteins (34, 45, 51, 69). These findings suggest that different transactivators might differ in their abilities to bind to sites in chromatin in vivo.
To compare more directly the abilities of GAL4 and GCN4 to bind to sites in chromatin, we constructed two yeast episomes differing only in the activator binding site (Fig. 1A). TA17Δ80 is a TRP1 ARS1-derived yeast episome containing a strong 17-bp GAL4 binding site which is situated near the middle of a positioned nucleosome in the absence of GAL4 (34). TAGCN1Δ80 is identical except that the GAL4 binding site has been replaced by a 9-bp consensus GCN4 binding site. These two episomes were introduced into yeast, and nucleosome positioning was examined by the indirect end label technique (37, 68). In this assay, micrococcal nuclease (MNase) cleavage sites are compared in naked DNA and chromatin, and regions of 140 to 160 bp that are protected in chromatin, but not in naked DNA, are diagnostic of positioned nucleosomes (50, 57).
FIG. 1.
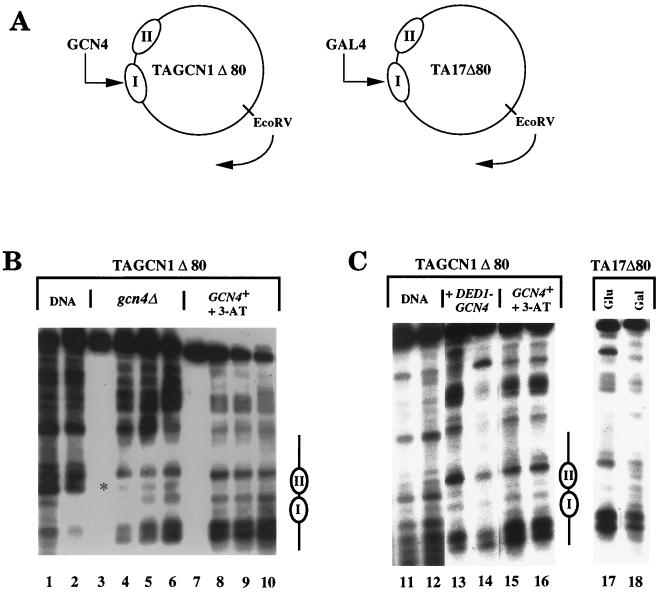
Perturbation of nucleosome positioning elicited by GCN4 via a nucleosomal binding site is poorer than that elicited by GAL4. (A) Schematic diagram of plasmids TAGCN1Δ80 and TA17Δ80. Positioned nucleosomes I and II are shown as ellipses. (B) Induction of GCN4 by 3-AT results in minimal perturbation of nucleosome positioning in TAGCN1Δ80. MNase cleavage sites were mapped clockwise from the EcoRV site, as indicated, in naked DNA (lanes 1 and 2) or in chromatin from cells lacking GCN4 or from GCN4+ cells induced with 3-AT (lanes 3 to 10). Note that the cleavage seen in the region of nucleosome II (especially lanes 4 to 6, denoted by an asterisk) corresponds to a site cleaved very strongly in naked DNA; we observed some variability in this cleavage in different experiments (see Fig. 6, lanes 4 and 5). (C) Comparison of nucleosome perturbation in TAGCN1Δ80 by GCN4 expressed from the DED1 promoter (lanes 13 and 14) or endogenous GCN4 induced with 3-AT (lanes 15 and 16) with perturbation in TA17Δ80 by GAL4 expressed from a multicopy plasmid bearing the GAL4 gene (lane 18). Lane 17 contains chromatin from cells grown in glucose medium and containing only the endogenous GAL4 gene. Lanes 11 to 18 were run on the same gel. Samples were digested with MNase at 0 U/ml (lanes 3 and 7), 0.5 U/ml (lane 4), 1 U/ml (lanes 1, 5, 8, and 11), 2 U/ml (lanes 6 and 9), 4 U/ml (lanes 2 and 12), 5 U/ml (lanes 10, 13, and 15), or 20 U/ml (lanes 14 and 16 to 18). The locations of nucleosomes I and II are indicated by ellipses.
Nucleosomes I and II were positioned equivalently in TA17Δ80 in cells grown in glucose (Fig. 1C, lane 17) and in TAGCN1Δ80 in gcn4Δ cells (Fig. 1B, lanes 4 to 6), as expected. Growth of cells containing TA17Δ80 and a 2μm GAL4-containing plasmid in galactose results in GAL4 synthesis and disruption of nucleosome positioning, as observed previously (Fig. 1C, lane 18) (34). In contrast, both constitutive GCN4 synthesis from the DED1 promoter and induction from the endogenous GCN4 gene result in only slight perturbation of nucleosome positioning in the reporter containing a nucleosomal GCN4 binding site (Fig. 1; compare lanes 4 to 6 with lanes 8 to 10 and 13 to 16). High-level expression of GCN4 from a GAL4-driven promoter (see below) resulted in only a marginal increase in nucleosome perturbation of TAGCN1Δ80 (data not shown). Thus, GCN4 perturbs nucleosome positioning via a nucleosomal binding site in yeast more weakly than does GAL4, suggesting that it binds to sites in chromatin less well.
In contrast to GCN4, neither GAL4 nor Bicoid require a RAP1 binding site to activate HIS4 transcription.
GCN4-dependent transcription of HIS4 depends strongly on the RAP1 binding site, and it has been suggested that RAP1 perturbs chromatin structure at the HIS4 promoter to allow GCN4 to bind (11). Since nucleosome perturbation elicited by GAL4 appears to be stronger than that by GCN4 in vivo (Fig. 1), we wanted to test whether GAL4-mediated transcription of HIS4 would require the RAP1 binding site.
Isogenic bas1 bas2 yeast strains having a GAL4 or GCN4 binding site and a wild-type or mutant RAP1 binding site were constructed in the genomic HIS4 promoter (Table 2). To monitor HIS4 expression, cells were plated onto synthetic complete medium without histidine (SC-His) and incubated for 2 to 3 days at 30°C. Cells containing a GCN4 binding site in the HIS4 promoter and constitutively expressing GCN4 from the DED1 promoter required a RAP1 site for growth on SC-His/galactose, consistent with previous work (11) (Fig. 2). In contrast, GAL4 expressed from a multicopy plasmid (Fig. 2) or endogenous GAL4 (see Fig. 4) supported growth on SC-His/galactose plates with or without an intact RAP1 binding site. HIS4 mRNA expression levels varied in accordance with the ability of cells to grow on media lacking histidine (70), consistent with earlier work (11). When glucose was used as the carbon source to repress GAL4 synthesis, cells containing the mutated RAP1 binding site in combination with a GAL4 binding site at HIS4 did not grow on SC-His but cells having a wild-type RAP1 site in combination with a GAL4 binding site at the HIS4 promoter showed slight growth (70). Similarly, weak histidine prototrophy was recently reported in yeast having the RAP1 binding site in the HIS4 promoter replaced by two GAL4 binding sites, independent of GAL4, in a BAS1+ BAS2+ background (29a). This slight growth may result from weak binding by another activator, such as PUT3, in conjunction with RAP1. (GAL4 binds to the sequence CGGN11CCG, and PUT3 binds the sequence CGGN10CCG [49].) Taken together, these results indicate that, in contrast to GCN4, GAL4 can activate HIS4 expression sufficiently well to allow histidine prototrophy without assistance from RAP1.
We also examined activation of HIS4 by another transcriptional activator, Bicoid, from Drosophila melanogaster. Bicoid contains a DNA-binding domain from the homeodomain class and has an activation domain distinct from the acidic activation domains of GAL4 and GCN4 (13, 24, 32). Inclusion of four consensus Bicoid binding sites (two Bicoid dimer sites) in a nucleosomal site in a yeast episome analogous to TA17Δ80 (Fig. 1) results in strong perturbation of nucleosome positioning upon expression of Bicoid in yeast cells, similar to the effect of GAL4 on TA17Δ80 (4). (We chose to use four Bicoid binding sites to create a high-affinity binding site, as two sites bind Bicoid weakly in vivo and in vitro [7]). Yeast strains having the same four Bicoid binding sites in the HIS4 locus, along with either the wild-type or a mutant RAP1 site, were constructed (Table 2). Expression of Bicoid protein from a GAL4-driven promoter allowed growth of cells on SC-His/galactose with or without an intact RAP1 binding site (Fig. 2). Thus Bicoid, like GAL4, has a strong ability to perturb nucleosome positioning via a high-affinity nucleosomal binding site and does not require RAP1 for efficient HIS4 activation at such a site.
The GCN4 activation domain can activate HIS4 efficiently in the absence of a RAP1 binding site.
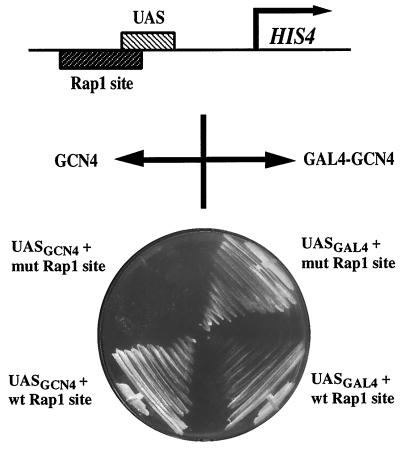
GAL4 and GCN4 each have distinct DNA-binding and activation domains (24, 32). The requirement for RAP1 for efficient activation of HIS4 by GCN4 but not by GAL4 could be due to differences in either or both domains. To address this issue, we asked whether a GAL4-GCN4 fusion protein acting via a GAL4 site at the HIS4 promoter could confer histidine prototrophy independently of a RAP1 site. A low-copy-number CEN-containing plasmid expressing a GAL4-GCN4 fusion protein (see Materials and Methods) from the DED1 promoter was introduced into yeast strains containing the GAL4 site at the HIS4 promoter. The DED1 promoter is expected to generate levels of GCN4 mRNA comparable to the native GCN4 promoter (24) (Fig. 2). Figure 3 shows that the resulting yeast cells are His+ in the presence or absence of a RAP1 binding site. Thus, the GCN4 activation domain is capable of efficiently activating HIS4 transcription in the absence of a RAP1 binding site, suggesting that the function of RAP1 binding at the HIS4 promoter is to help the GCN4 DNA-binding domain bind to chromatin.
FIG. 3.
HIS4 expression mediated by the GCN4 activation domain through a GAL4 binding site does not require the RAP1 binding site. Cells containing the GAL4 binding site (UASGAL4) with a wild-type (wt) or mutated (mut) RAP1 site in the HIS4 promoter, and expressing GAL4-GCN4 from the DED1 promoter, were streaked from SC-Leu/glucose onto SC-His-Leu/glucose, as were cells containing the GCN4 binding site (UASGCN4) with a wild-type or mutated RAP1 binding site.
Transactivator binding affinity affects the requirement for a RAP1 binding site for efficient HIS4 activation.
The binding affinities of GAL4 [Kd, 2 × 10−9 M−1 for GAL4(1–100) (43)] and Bicoid (apparent Kd, about 2 × 10−10 M−1 for four sites [7]) for the sites used at the HIS4 promoter in this work are considerably stronger than that of GCN4 (apparent Kd, 2 × 10−8 M−1 [65]). This suggested that binding site affinity could be an important determinant as to whether RAP1 was needed for a given transcriptional activator to efficiently activate HIS4. Alternatively, it could be that binding affinity is less important than the type of DNA-binding domain used; some modes of DNA binding could be more compatible with the chromatin structure at the HIS4 promoter than others. To address this question, we replaced the GCN4 binding site in the HIS4 promoter with a weak GAL4 binding site from the GAL1-10 promoter. In vitro binding of the GAL4 DNA-binding domain (amino acids 1 to 140) to this site is eightfold weaker than to the consensus GAL4 site (61), so the binding affinity should be comparable to that of GCN4.
When cells containing the weak GAL4 binding site were grown on glucose plates, they exhibited a His− phenotype. On galactose media, cells having the weak GAL4 binding site combined with the wild-type RAP1 binding site showed some growth but grew much more slowly than cells containing a strong GAL4 binding site at the HIS4 promoter (Fig. 4). Cells containing the weak GAL4 binding site and the mutated RAP1 binding site at the HIS4 promoter exhibited a His− phenotype on galactose plates. These findings indicate that when HIS4 transcription is mediated from a weak GAL4 binding site, RAP1 is needed for efficient transactivation.
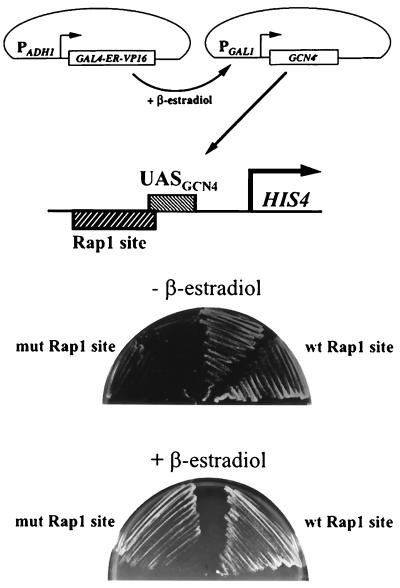
If the dependence on the RAP1 binding site for GCN4-mediated activation of HIS4 is due to the relatively weak binding of GCN4, then high levels of GCN4 might allow efficient HIS4 expression independently of the RAP1 binding site. We tested this idea by overexpressing GCN4. We fused the GCN4 coding sequence to the GAL1 promoter in a multicopy plasmid and induced expression with the hormone-dependent activator GAL4-ER-VP16 (30). Cell growth was then examined on SC-His/glucose in the presence or absence of 100 nM β-estradiol. In the absence of β-estradiol, cells containing the wild-type RAP1 binding site exhibited some growth, indicating that the low levels of GCN4 produced from the expression vector in the absence of hormone are sufficient to activate the wild-type HIS4 promoter (Fig. 5). However, these low levels were not sufficient to allow growth of cells lacking the RAP1 binding site (Fig. 5). In the presence of 100 nM β-estradiol, cells containing the mutated RAP1 binding site exhibited some growth on SC-His, although growth was weaker than that of cells having the wild-type HIS4 promoter (Fig. 5). These results were corroborated by monitoring cell growth in liquid SC-His in the absence or presence of β-estradiol (70). Thus, overexpression of GCN4 can partially complement the histidine auxotrophy seen in the absence of the RAP1 binding site.
FIG. 5.
Overexpression of GCN4 partially overcomes the requirement for a RAP1 binding site for GCN4-mediated HIS4 expression. GCN4 was overexpressed by using the hormone-dependent activator GAL4-ER-VP16 to activate the GAL1pr-GCN4 promoter (top). Cells containing the GCN4 binding site (UASGCN4) and a wild-type (wt) or mutated (mut) RAP1 binding site, and harboring the GAL1pr-GCN4 plasmid and an expression vector for GAL4-ER-VP16, were streaked onto SC-His-Ura-Leu/glucose plates containing no β-estradiol or containing 100 nM β-estradiol, as indicated.
A RAP1 binding site strongly interferes with nucleosome positioning in vivo.
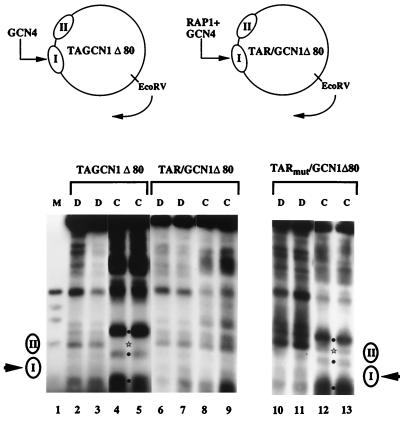
Based on the apparent ability of RAP1 to open chromatin structure in the HIS4 promoter to allow activation by GCN4 (Fig. 2) (11) and on its high affinity for its binding site (Kd, 10−11 M−1 [64]), we expected that RAP1 might show a strong ability to perturb chromatin structure in vivo. To test this hypothesis, we constructed the yeast episome TAR/GCN1Δ80. This plasmid is identical to TAGCN1Δ80, except that a RAP1 binding site has been introduced adjacent to the GCN4 binding site in nucleosome I (Fig. 6). Since RAP1 is an essential gene and therefore cannot be deleted (48), we introduced a mutated RAP1 site into nucleosome I as a control. The mutation is the same one that abolished GCN4-dependent transcription of HIS4 in vivo. Chromatin structure of TAR/GCN1Δ80 and TARmut/GCN1Δ80 was examined by MNase cleavage, followed by indirect end labeling in gcn4Δ yeast cells, so that any effects on chromatin structure should be attributable to RAP1. Nucleosomes I and II were positioned in TARmut/GCN1Δ80 as in TAGCN1Δ80, although somewhat less strongly (Fig. 6, lanes 2 to 5 and 10 to 13). In contrast, the chromatin structure of TAR/GCN1Δ80 was dramatically changed, with the positioning of nucleosomes I and II essentially abolished (Fig. 6, lanes 6 to 9). These results demonstrate that RAP1 is extremely effective in creating a localized region of open chromatin.
FIG. 6.
Perturbation of nucleosome positioning by RAP1 via a nucleosomal binding site. MNase cleavage sites in plasmids TAGCN1Δ80 and TAR/GCN1Δ80, schematized at the top, as well as TARmut/GCN1Δ80, were mapped clockwise from the EcoRV site, as indicated. Cleavage sites were mapped in naked DNA (D) or in chromatin (C) from cells grown in glucose media. Lane 1 contains ΦX/HaeIII marker DNA. Locations of positioned nucleosomes I and II are indicated by ellipses. The closed circles between lanes 4 and 5 and lanes 12 and 13 indicate cleavages enhanced in chromatin relative to DNA, and the star indicates a site protected in chromatin. Each pair of lanes, beginning with lanes 2 and 3, differs only in the concentration of MNase used. Lanes 10 to 13 were derived from a gel separate from lanes 1 to 9.
Altering the spacing between the RAP1 and GCN4 binding sites does not impair HIS4 activation.
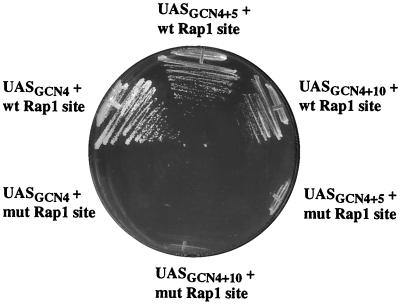
RAP1 assists activator binding at some promoters via protein-protein interactions (12). One piece of evidence supporting such interactions was a demonstration that altering the distance between binding sites for RAP1 and GCR1 at the PYK1 promoter results in a loss of GCR1 binding and upstream activating sequence (UAS) activity in vivo (12). To test whether RAP1 helps GCN4 bind to the HIS4 promoter via direct cooperative interactions between RAP1 and GCN4, 5 or 10 nucleotides were inserted between the RAP1 and GCN4 binding sites at the HIS4 promoter locus. Such alterations in spacing would be expected to disrupt protein-protein interactions important for cooperative binding, as was found for the PYK1 promoter (12). This is particularly true of the 5-bp increase, which would place the RAP1 binding site on the opposite face of the DNA double helix relative to its position in the wild-type promoter. In contrast, if the function of RAP1 at the HIS4 promoter is principally to open chromatin, the precise spacing should not be critical.
We then tested the ability of yeast harboring these variant HIS4 promoters to grow on media lacking histidine. The two isogenic strains containing the wild-type RAP1 site and either the 5- or 10-bp insertion between the RAP1 and GCN4 sites grew on media lacking histidine (Fig. 7). To rule out the possibility that the additional DNA sequences introduced between the RAP1 and GCN4 sites at the HIS4 promoter create a binding site for another protein and/or change the binding affinity of the GCN4 site, the same 5 or 10 nucleotides were introduced between GCN4 and the mutated RAP1 site in LYY599 to create LYY599+5 and LYY599+10 (Table 2). These strains failed to grow on SC-His (Fig. 7). These results indicate that direct cooperative interactions between RAP1 and GCN4 at the HIS4 promoter are very unlikely and support the idea that RAP1 binding to the HIS4 promoter facilitates GCN4 binding by overcoming the repressive effect of chromatin.
FIG. 7.
Altering the spacing between the RAP1 and GCN4 sites does not impair HIS4 transactivation. Yeast strains (Table 2) contain integrated HIS4 promoters with either a wild-type (wt) or mutated (mut) RAP1 site and have either wild-type spacing between the RAP1 and GCN4 sites or 5 or 10 bp inserted in the UAS (UASGCN4+5 and UASGCN4+10). Cells were streaked from SC-Leu/glucose onto SC-His-Leu/glucose. GCN4 was expressed from the DED1 promoter.
DISCUSSION
A prerequisite for transcriptional activation in eukaryotes is the binding of activator proteins to DNA. Eukaryotic DNA is packaged into chromatin, which poses a potential impediment to activator binding. In vitro studies have shown that activator binding to nucleosomal sites is hindered to various degrees, depending on variables such as the type of factor, the location of binding sites, the acetylation status of the histone amino termini, and the presence of chromatin remodeling activities (39, 67). Much less has been done to examine activator binding to nucleosomal sites in vivo, and consequently little is known regarding issues such as the relative abilities of distinct activators to perturb chromatin structure via nucleosomal binding sites. We report here that whereas GAL4 is able to substantially perturb nucleosome positioning via a nucleosomal binding site in a yeast episome, GCN4 does so very poorly. Consistent with this difference, a RAP1 binding site is required for GCN4-dependent transcription of HIS4, in agreement with previous work (11), but is not needed for efficient activation of HIS4 by GAL4. RAP1 is needed by the GCN4 DNA-binding domain and not the activation domain for HIS4 activation, as shown by the ability of GAL4-GCN4 to activate HIS4 via a GAL4 site in the presence of a mutated RAP1 site. Overexpression of GCN4 can partially bypass the requirement for RAP1 at the HIS4 promoter, whereas weakening the GAL4 binding site in the modified HIS4 promoter leads to a requirement for RAP1 for efficient activation by GAL4. The ability of RAP1 to assist activation by two entirely distinct proteins (GCN4 and, at a weak binding site, GAL4) suggests that direct protein-protein interactions are unlikely to be involved in RAP1-facilitated activation at the HIS4 promoter, in contrast to its role in assisting binding of GCR1 to promoters for genes encoding enzymes in the glycolytic pathway (12). A lack of direct cooperative interactions is further supported by the finding that altering the spacing between the RAP1 and GCN4 binding sites by 5 or 10 bp does not significantly affect HIS4 activation. These results, in sum, point to a role for RAP1 in opening chromatin to allow activator access to weak binding sites.
Based on MNase mapping of chromatin structure, the HIS4 promoter does not appear to be packaged into highly positioned nucleosomes, although differences between MNase cleavages of naked DNA and HIS4 promoter chromatin indicate nonrandom packaging (27, 70). Therefore, although the ability of different transcription factors to perturb chromatin structure via nucleosomal binding sites in TRP1 ARS1-based plasmids, such as TA17Δ80 and TAGCN1Δ80, provides a useful indicator of the ability of these factors to overcome histone-mediated repression, we do not necessarily expect this correlation to be perfect at a given promoter. For example, GAL4-GCN4, which can activate the HIS4 promoter without help from RAP1, does not perturb nucleosome positioning in TA17Δ80 (52). This most likely reflects the requirement for a strong activation domain to bind to nucleosomal sites in vivo (51, 52). We have also found that in specific mutant backgrounds that alleviate the requirement for a RAP1 site to allow activation of the HIS4 promoter by GCN4, GCN4 is nevertheless unable to perturb TAGCN1Δ80 chromatin structure (70). Mutation of the RAP1 site in the HIS4 promoter decreases the intensity of MNase cleavage sites near the GCN4 binding site, consistent with a more repressive chromatin structure (11), but further work will be required to understand in detail how that chromatin structure prevents activation by GCN4 and how RAP1 affects chromatin structure to facilitate GCN4-mediated activation.
Binding affinities affect the abilities of activators to access sites in chromatin.
Our results indicate that the Kd of binding and the abundance of the activator are important in determining its ability to access sites in chromatin. This simple chemical basis for a differential ability to bind to sites in chromatin can have physiological consequences, as shown by the requirement for RAP1 in conjunction with weak but not strong activator binding sites in the HIS4 promoter. This finding is consistent with previous work showing that yeast heat shock factor can activate transcription from the HSP82 promoter from a high-affinity site but does not activate from low-affinity sites unless overexpressed (21). In this example, the high-affinity site plays the role of the RAP1 binding site at the HIS4 promoter, opening chromatin structure to allow binding of heat shock factor to nearby low-affinity sites. Factor abundance has also been shown to affect binding of activators to sites in chromatin in vivo: the yeast activators GAL4 and PHO4 are both inhibited from binding to nucleosomal sites at endogenous levels but can be induced to bind such sites by overexpression (62, 69).
The dependence on Kd for activator binding to sites in chromatin in vivo and the findings that overexpression of an activator can partially compensate for a low-affinity binding site and/or a repressive chromatin structure (references 21, 62 and 69 and this work) indicate that nucleosomes do not provide an absolute kinetic blockade to activator binding in vivo. Rather, binding appears to be governed at least in part by standard equilibrium chemistry. This picture is consistent with a model in which binding of factors to chromatin is governed by equilibria including both activator-binding site interactions and histone-DNA interactions (41). However, this model cannot provide a complete explanation, as activation domains have also been shown to contribute to in vivo binding (6, 34, 51, 54, 55). Whether activation domains enhance factor binding by interactions with the basal transcriptional machinery, by recruitment of chromatin remodeling complexes, or by another mechanism is not yet known. However, it has been demonstrated that nucleosome perturbation by both GAL4 and Bicoid via nucleosomal binding sites can occur in the absence of functional SWI/SNF complex and in nonreplicating cells (4, 45).
A role for RAP1 in opening chromatin.
RAP1 has roles in transcriptional activation, silencing, and telomere maintenance (19, 48). The ability of RAP1 to bind to and perturb chromatin demonstrated here is likely to contribute to its ability to perform these various roles. RAP1 binding sites are found at numerous yeast promoters, generally in combination with other transcription factor binding sites (12, 19, 48). Mutation of the RAP1 binding sites in such promoters often severely reduces the transcription level of target genes, although the RAP1 sites alone function either weakly or not at all as UAS elements (reference 12 and references therein). It thus seems likely that the principal role of RAP1 at such promoters is to open chromatin to facilitate binding of other transcription factors. This has been suggested explicitly, as we have noted, for the HIS4 promoter (11). A similar proposal has been made for a role of RAP1 in facilitating GCR1 access to glycolytic gene promoters (12). The latter proposal was based on studies of the TPI1 promoter; in this instance it is likely that direct cooperative effects between RAP1 and GCR1 also contribute to RAP1 facilitating GCR1 binding (12, 58). Our results strongly support a role for RAP1 in opening chromatin to facilitate access of transcriptional activators by demonstrating that RAP1 has a potent ability to interfere with nucleosome positioning and that RAP1 can facilitate efficient HIS4 activation by disparate activators.
One possible mechanism for such chromatin-mediated cooperativity was suggested on the basis of in vitro studies. In this scenario, one protein may bind to a nucleosomal site, by virtue of high affinity or its location in the nucleosome, and allow binding of a second protein to a less favorable site (1, 38, 41, 42). A recent study showing that GAL4 and LexA derivatives could cooperate in transcriptional activation in yeast suggests that chromatin-mediated cooperativity may pertain in vivo as well (60). Further work will be required to determine whether the results observed in vivo in that instance or in the present study can be explained by the proposed mechanism.
RAP1 is not likely to be the only protein to function in opening chromatin to allow transactivator access. Other proteins, such as ABF1 and GRF2 (REB1) in yeast and the Drosophila protein GAGA factor, appear to play similar roles at some promoters (8, 20, 31, 33, 44, 46), and ABF1 is able to remodel chromatin in vivo (25). It will be interesting to determine whether such proteins can function interchangeably, as is typically the case for transcriptional activators, and to determine whether domains apart from the DNA-binding domain contribute to chromatin opening.
The reorganization of chromatin structure by RAP1 in the episome TAR/GCN1Δ80 is remarkable (Fig. 6). The MNase cleavage pattern in the vicinity of the RAP1 binding site in this episome is essentially identical in naked DNA and chromatin. In contrast, protections and cleavages characteristic of positioned nucleosomes are seen in TARmut/GCN1Δ80, bearing the mutant RAP1 site, and in the related plasmids TAGCN1Δ80 and TA17Δ80, bearing GCN4 and GAL4 binding sites, respectively, in the absence of the activators. Furthermore, although GAL4 elicits strong perturbation of nucleosome positioning in TA17Δ80, the resulting MNase cleavage pattern retains features seen in the absence of GAL4, appearing intermediate between the patterns seen with naked DNA and with chromatin in the absence of GAL4 (34) (Fig. 1C). This difference between the abilities of GAL4 and RAP1 to reorganize chromatin could reflect more extensive interactions of RAP1 with chromatin; for example, RAP1 binding to DNA induces bending via its amino-terminal region (36). However, the difference could also indicate more complete occupancy by RAP1 than by GAL4, as suggested by inhibition of GAL4 binding to the center of a positioned nucleosome compared to positions nearer the edge observed in another yeast study (69). Perhaps this potent ability of RAP1 to reorganize chromatin contributes to the lack of dependence on GCN5 for HIS4 activation by GCN4, in contrast to the dependence seen at other promoters activated by GCN4 (17).
ACKNOWLEDGMENTS
We thank Kim Arndt, Alex Bortvin, Dave Burz, Steve Hanes, Alan Hinnebusch, C. J. Ingles, Kevin Struhl, and Fred Winston for providing plasmids and yeast strains; Karen Arndt, Bhuvana Balasubramanian, Susan Gasser, Grace Stafford, and Fred Winston for helpful discussions; Steve Hanes, Joan Curcio, and Mike Ryan for critically reading the manuscript; and Tim Moran and Matt Schudt of the Wadsworth Center Molecular Genetics Core Facility for DNA sequencing and oligonucleotide synthesis.
This work was supported by NIH grant GM51993 to R.H.M.
REFERENCES
- 1.Adams C C, Workman J L. Binding of disparate transcriptional activators to nucleosomal DNA is inherently cooperative. Mol Cell Biol. 1995;15:1405–1421. doi: 10.1128/mcb.15.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison L A, Ingles C J. Mutations in RNA polymerase II enhance or suppress mutations in GAL4. Proc Natl Acad Sci USA. 1989;86:2794–2798. doi: 10.1073/pnas.86.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arndt K, Fink G R. GCN4 protein, a positive transcription factor in yeast, binds general control promoters at all 5′ TGACTC 3′ sequences. Proc Natl Acad Sci USA. 1986;83:8516–8520. doi: 10.1073/pnas.83.22.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian B, Morse R H. Binding of Gal4p and Bicoid to nucleosomal sites in yeast in the absence of replication. Mol Cell Biol. 1999;19:2977–2985. doi: 10.1128/mcb.19.4.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bortvin, A. Unpublished data.
- 6.Bunker C A, Kingston R E. Activation domain-mediated enhancement of activator binding to chromatin in mammalian cells. Proc Natl Acad Sci USA. 1996;93:10820–10825. doi: 10.1073/pnas.93.20.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burz D S, Rivera-Pomar R, Jäckle H, Hanes S D. Cooperative DNA-binding by Bicoid provides a mechanism for threshold-dependent gene activation in the Drosophila embryo. EMBO J. 1998;18:5998–6009. doi: 10.1093/emboj/17.20.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasman D I, Lue N F, Buchman A R, LaPointe J W, Lorch Y, Kornberg R D. A yeast protein that influences the chromatin structure of UASG and functions as a powerful auxiliary activator. Genes Dev. 1990;4:503–514. doi: 10.1101/gad.4.4.503. [DOI] [PubMed] [Google Scholar]
- 9.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 10.Cohen, B., and R. Brent. Unpublished data.
- 11.Devlin C, Tice-Baldwin K, Shore D, Arndt K T. RAP1 is required for BAS1/BAS2- and GCN4-dependent transcription of the yeast HIS4 gene. Mol Cell Biol. 1991;11:3642–3651. doi: 10.1128/mcb.11.7.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drazinic C M, Smerage J B, Lopez M C, Baker H V. Activation mechanism of the multifunctional transcription factor repressor-activator protein 1 (Rap1p) Mol Cell Biol. 1996;16:3187–3196. doi: 10.1128/mcb.16.6.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Driever W, Ma J, Nüsslein-Volhard C, Ptashne M. Rescue of bicoid mutant Drosophila embryos by Bicoid fusion proteins containing heterologous activating sequences. Nature. 1989;342:149–153. doi: 10.1038/342149a0. [DOI] [PubMed] [Google Scholar]
- 14.Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992;355:219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- 15.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:145–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 16.Finley, R., and R. Brent. Unpublished data.
- 17.Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992;11:4145–4152. doi: 10.1002/j.1460-2075.1992.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 19.Gilson E, Gasser S M. Repressor activator protein 1 and its ligands: organising chromatin domains. Nucleic Acids Mol Biol. 1995;9:308–327. [Google Scholar]
- 20.Gonçalves P M, Griffioen G, Minnee R, Bosma M, Kraakman L S, Mager W H, Planta R J. Transcription activation of yeast ribosomal genes requires additional elements apart from binding sites for Abf1p and Rap1p. Nucleic Acids Res. 1995;23:1475–1480. doi: 10.1093/nar/23.9.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross D S, Adams C C, Lee S, Stentz B. A critical role for heat shock transcription factor in establishing a nucleosome-free region over the TATA-initiation site of the yeast HSP82 heat shock gene. EMBO J. 1993;12:3931–3945. doi: 10.1002/j.1460-2075.1993.tb06071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill J, Ian K A, Donald G, Griffiths D E. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hope I A, Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986;46:885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y-F, Hao Z L, Li R. Chromatin remodeling and activation of chromosomal DNA replication by an acidic transcriptional activation domain from BRCA1. Genes Dev. 1999;13:637–642. doi: 10.1101/gad.13.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iyer V, Struhl K. Poly(dA:dT), a ubiquitous promoter element that stimulates transcription via its intrinsic DNA structure. EMBO J. 1995;14:2570–2579. doi: 10.1002/j.1460-2075.1995.tb07255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang W Y, Stillman D J. Regulation of HIS4 expression by the Saccharomyces cerevisiae SIN4 transcriptional regulator. Genetics. 1995;140:103–114. doi: 10.1093/genetics/140.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent N A, Bird L E, Mellor J. Chromatin analysis in yeast using NP-40 permeabilized spheroplasts. Nucleic Acids Res. 1993;21:4653–4654. doi: 10.1093/nar/21.19.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingston R E, Bunker C A, Imbalzano A N. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 29a.Kirkpatrick D T, Fan Q, Petes T D. Maximal stimulation of meiotic recombination by a yeast transcription factor requires the transcription activation domain and a DNA-binding domain. Genetics. 1999;152:101–115. doi: 10.1093/genetics/152.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louvion J-F, Havaux-Copf B, Picard D. Fusion of GAL4-VP16 to a steroid binding domain provides a tool for gratuitous induction of galactose-responsive genes in yeast. Gene. 1993;131:129–134. doi: 10.1016/0378-1119(93)90681-r. [DOI] [PubMed] [Google Scholar]
- 31.Lu Q, Wallrath L L, Elgin S C R. The role of a positioned nucleosome at the Drosophila melanogaster hsp26 promoter. EMBO J. 1995;14:4738–4746. doi: 10.1002/j.1460-2075.1995.tb00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma J, Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987;48:847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- 33.Martens J A, Brandl C J. GCN4p activation of the yeast TRP3 gene is enhanced by ABF1p and uses a suboptimal TATA element. J Biol Chem. 1994;269:15661–15667. [PubMed] [Google Scholar]
- 34.Morse R H. Nucleosome disruption by transcription factor binding in yeast. Science. 1993;262:1563–1566. doi: 10.1126/science.8248805. [DOI] [PubMed] [Google Scholar]
- 35.Morse R H, Roth S Y, Simpson R T. A transcriptionally active tRNA gene interferes with nucleosome positioning in vivo. Mol Cell Biol. 1992;12:4015–4025. doi: 10.1128/mcb.12.9.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Müller T, Gilson E, Schmidt R, Giraldo R, Sogo J, Gross H, Gasser S M. Imaging the asymmetrical DNA bend induced by repressor activator protein 1 with scanning tunneling microscopy. J Struct Biol. 1994;113:1–12. doi: 10.1006/jsbi.1994.1027. [DOI] [PubMed] [Google Scholar]
- 37.Nedospasov S A, Georgiev G P. Non-random cleavage of SV40 DNA in the compact minichromosome and free in solution by micrococcal nuclease. Biochem Biophys Res Commun. 1980;92:532–539. doi: 10.1016/0006-291x(80)90366-6. [DOI] [PubMed] [Google Scholar]
- 38.Ng K W, Ridgway P, Cohen D R, Tremethick D J. The binding of a Fos/Jun heterodimer can completely disrupt the structure of a nucleosome. EMBO J. 1997;16:2072–2085. doi: 10.1093/emboj/16.8.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen-Hughes T, Workman J L. Experimental analysis of chromatin function in transcriptional control. Crit Rev Eukaryot Gene Expr. 1994;4:403–441. [PubMed] [Google Scholar]
- 40.Paranjape S M, Kamakaka R T, Kadonaga J T. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–297. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- 41.Polach K J, Widom J. Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J Mol Biol. 1995;254:130–149. doi: 10.1006/jmbi.1995.0606. [DOI] [PubMed] [Google Scholar]
- 42.Polach K J, Widom J. A model for the cooperative binding of eukaryotic regulatory proteins to nucleosomal target sites. J Mol Biol. 1996;258:800–812. doi: 10.1006/jmbi.1996.0288. [DOI] [PubMed] [Google Scholar]
- 43.Reece R J, Ptashne M. Determinants of binding-site specificity among yeast C6 zinc cluster proteins. Science. 1993;261:909–911. doi: 10.1126/science.8346441. [DOI] [PubMed] [Google Scholar]
- 44.Rolfes R J, Zhang F, Hinnebusch A G. The transcriptional activators BAS1, BAS2, and ABF1 bind positive regulatory sites as the critical elements for adenine regulation of ADE5,7. J Biol Chem. 1997;272:13343–13354. doi: 10.1074/jbc.272.20.13343. [DOI] [PubMed] [Google Scholar]
- 45.Ryan M P, Jones R, Morse R H. SWI-SNF complex participation in transcriptional activation at a step subsequent to activator binding. Mol Cell Biol. 1998;18:1774–1782. doi: 10.1128/mcb.18.4.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder S C, Weil P A. Genetic tests of the role of Abf1p in driving transcription of the yeast TATA box binding protein-encoding gene, SPT15. J Biol Chem. 1998;273:19884–19891. doi: 10.1074/jbc.273.31.19884. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu M, Roth S Y, Szent-Gyorgyi C, Simpson R T. Nucleosomes are positioned with base pair precision adjacent to the α2 operator in Saccharomyces cerevisiae. EMBO J. 1991;10:3033–3041. doi: 10.1002/j.1460-2075.1991.tb07854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shore D. RAP1: a protean regulator in yeast. Trends Genet. 1994;10:408–412. doi: 10.1016/0168-9525(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui A H, Brandriss M C. A regulatory region responsible for proline-specific induction of the yeast PUT2 gene is adjacent to its TATA box. Mol Cell Biol. 1988;8:4634–4641. doi: 10.1128/mcb.8.11.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson R T. Nucleosome positioning: occurrence, mechanisms, and functional consequences. Prog Nucleic Acids Res Mol Biol. 1991;40:143–184. doi: 10.1016/s0079-6603(08)60841-7. [DOI] [PubMed] [Google Scholar]
- 51.Stafford G A, Morse R H. Chromatin remodeling by transcriptional activation domains in a yeast episome. J Biol Chem. 1997;272:11526–11534. doi: 10.1074/jbc.272.17.11526. [DOI] [PubMed] [Google Scholar]
- 52.Stafford, G. A., M. P. Ryan, and R. H. Morse. Unpublished data.
- 53.Struhl K. Yeast GCN4 regulatory factor. In: McKnight S A, Yamamoto K R, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 833–859. [Google Scholar]
- 54.Svaren J, Schmitz J, Horz W. The transactivation domain of Pho4 is required for nucleosome disruption at the PHO5 promoter. EMBO J. 1994;13:4856–4862. doi: 10.1002/j.1460-2075.1994.tb06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka M. Modulation of promoter occupancy by cooperative DNA binding and activation-domain function is a major determinant of transcriptional regulation by activators in vivo. Proc Natl Acad Sci USA. 1996;93:4311–4315. doi: 10.1073/pnas.93.9.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor I C A, Workman J L, Schuetz T J, Kingston R E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991;5:1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- 57.Thoma F. Nucleosome positioning. Biochim Biophys Acta. 1992;1130:1–19. doi: 10.1016/0167-4781(92)90455-9. [DOI] [PubMed] [Google Scholar]
- 58.Tornow J, Zeng X, Gao W, Santangelo G M. GCR1, a transcriptional activator in Saccharomyces cerevisiae, complexes with RAP1 and can function without its DNA binding domain. EMBO J. 1993;12:2431–2437. doi: 10.1002/j.1460-2075.1993.tb05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vashee S, Kodadek T. The activation domain of GAL4 protein mediates cooperative promoter binding with general transcription factors in vivo. Proc Natl Acad Sci USA. 1995;92:10683–10687. doi: 10.1073/pnas.92.23.10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vashee S, Melcher K, Ding W V, Johnston S A, Kodadek T. Evidence for two modes of cooperative DNA binding in vivo that do not involve direct protein-protein interactions. Curr Biol. 1998;8:452–458. doi: 10.1016/s0960-9822(98)70179-4. [DOI] [PubMed] [Google Scholar]
- 61.Vashee S, Xu H, Johnston S A, Kodadek T. How do “Zn2Cys6” proteins distinguish between similar upstream activation sites? J Biol Chem. 1993;268:24699–24706. [PubMed] [Google Scholar]
- 62.Venter U, Svaren J, Schmitz J, Schmid A, Hörz W. A nucleosome precludes binding of the transcription factor Pho4 in vivo to a critical target site in the PHO5 promoter. EMBO J. 1994;13:4848–4855. doi: 10.1002/j.1460-2075.1994.tb06811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vettese-Dadey M, Walter P, Chen H, Juan L J, Workman J L. Role of the histone amino termini in facilitated binding of a transcription factor, GAL4-AH, to nucleosome cores. Mol Cell Biol. 1994;14:970–981. doi: 10.1128/mcb.14.2.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vignais M L, Huet J, Buhler J M, Sentenac A. Contacts between the factor TUF and RPG sequences. J Biol Chem. 1990;265:14669–14674. [PubMed] [Google Scholar]
- 65.Weiss M A, Ellenberger T, Wobbe C R, Lee J P, Harrison S C, Struhl K. Folding transition in the DNA-binding domain of GCN4 on specific binding to DNA. Nature. 1990;347:575–578. doi: 10.1038/347575a0. [DOI] [PubMed] [Google Scholar]
- 66.Winston F, Dollard C, Ricupero-Hovasse S L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- 67.Workman J L, Kingston R E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 68.Wu C. The 5′ ends of Drosophila heat-shock genes in chromatin are sensitive to DNase I. Nature. 1980;286:854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- 69.Xu M, Simpson R T, Kladde M P. Gal4p-mediated chromatin remodeling depends on binding site position in nucleosomes but does not require DNA replication. Mol Cell Biol. 1998;18:1201–1212. doi: 10.1128/mcb.18.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu, L. Unpublished data.