Statins are increasingly being re-purposed as immune modulatory agents for non-cardiac systemic inflammatory diseases, including autoimmune disorders, inflammatory bowel disease, cognitive function/dementia, asthma and inflammatory lung disease, including COVID 191. Currently 1 in 10 pregnancies result in term preeclampsia, which is the most common form of preeclampsia, is currently neither predictable or preventable, increases morbidity and mortality risk to both the mother and baby, and elevates future premature cardiovascular disease (CVD) risk in women2. Endothelial dysfunction and inflammation have been hypothesized to be involved in preeclampsia, suggesting they may be treatment targets, however relatively little investigation has been conducted.
While statins are traditionally used for lowering low density lipoprotein (LDL)-cholesterol to reduce CVD risk, the relation between statin-related LDL-cholesterol lowering and systemic inflammatory diseases is not well characterized. In pregnancy, cholesterol is not routinely measured, in part due to the known rise in cholesterol levels due to elevated placental steroid hormones, lack of normal pregnancy reference values and limited treatment options in pregnancy. While elevated maternal hypercholesterolemia has been linked to the development of preeclampsia, LDL-cholesterol appears to be less associated compared to total cholesterol, non-HDL-cholesterol and triglyceride levels3. In addition, women with familial hypercholesterolemia have not been demonstrated to have a higher risk of eclampsia, preeclampsia, or pregnancy-induced hypertension4.
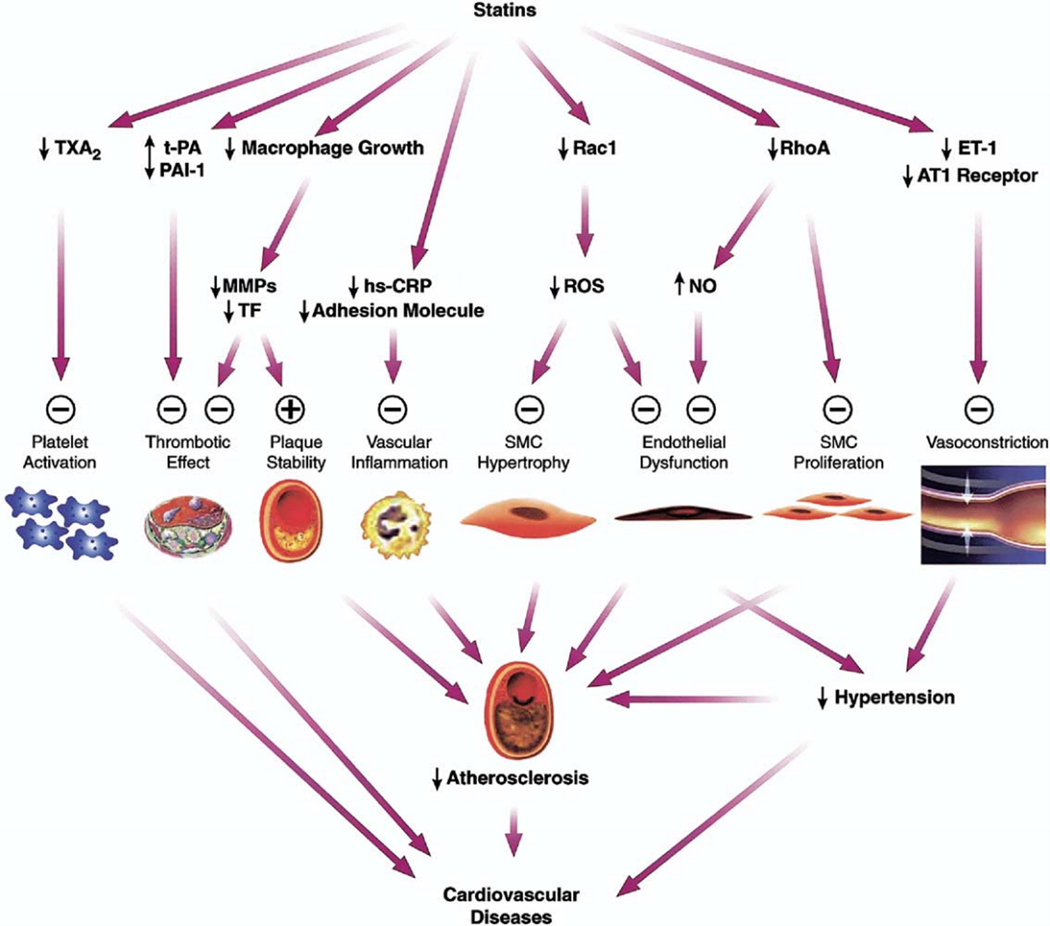
Rather, statin benefit in preeclampsia is hypothesized to rely on its non-cholesterol “pleiotropic” effects. Statins are thought to improve endothelial function and reduce inflammatory cytokines by reducing C-reactive protein (CRP) concentrations, inhibiting pro-inflammatory transcription factors, and blunting the T helper cell immune response1. Many of the proposed pleiotropic vascular effects of statins appear to involve restoring or improving endothelial function through increasing the bioavailability of nitric oxide, promoting re-endothelialization, reducing oxidative stress, and inhibiting inflammatory responses5. Yet more recent results from non-statin therapy CVD trials, including PCSK9 inhibitors, ezetimibe, and bempedoic acid, challenge the concept of statin pleiotropy, dominantly failing to show differential benefit above that of LDL-lowering1.
Given the emerging stance of healthy moms = healthy babies rationale stimulating the testing of medication for health conditions that adversely impact the health of mothers and therefore babies during pregnancy, in this issue the work of Döbert et al6 is a welcome addition. Preliminary data from animal studies provided a strong rational for the use of pravastatin in preeclampsia prevention, but human trials have been lacking. In their double-blind, placebo-controlled trial of 1,120 women with singleton pregnancies at high-risk of term preeclampsia, the authors evaluated the effects of pravastatin 20 mg daily vs placebo from 35–37 weeks of gestation until delivery. They found no benefit of pravastatin for reducing the incidence of preeclampsia, with no evidence of interaction between the effect of pravastatin, estimated risk of preeclampsia, prior history of preeclampsia, adherence, and aspirin intake in prespecified subgroup analyses. There was also no difference in the treatment effects on soluble fms-like tyrosine kinase-1 concentrations (sFlt-1) and serum placental growth factor (PlFG), biomarkers that tend to higher and lower in preeclamptic vs normal pregnancies, respectively.
What are some potential explanations for these negative trial results? Serum sFlt-1/PlGF levels may start to elevate as early as 24 weeks gestation in some women who later develop term preeclampsia7, thus starting pravastatin 20 mg at 35 weeks of pregnancy to reduce the later term pre-eclampsia outcome within 4–6 weeks may be too little statin given too late in the disease process. Indeed, a prior small clinical trial of pravastatin 10 mg starting at 12–17 weeks gestation had demonstrated promising trends toward higher PlGF and lower sFlt-1 levels with the statin7, contrary to this study. While cholesterol lowering was not measured in this trial, clinical CVD trials usually demonstrate outcome benefits following more than 4 months of statin therapy8, while the shorter-term benefits are typically observed with more potent statins at higher doses9. Thus, the lower potency and dose of the pravastatin may have contributed to the lack of benefit, as the more potent and higher dose atorvastatin has a documented shorter onset of benefit compared to other lower intensity statins8. Interestingly, despite comparable LDL lowering effects between simvastatin 40 mg and simvastatin 10 mg/ezetimibe 10 mg, simvastatin 40 mg produced greater flow-mediated dilation (improved endothelial function) than simvastatin 10 mg/ezetimibe 10 mg10. The findings of this study support the notion of statin pleiotropy that is dose-dependent and is in addition to the benefits of LDL lowering. Finally, the use of a hydrophilic statin, pravastatin, which has less vascular wall permeability compared to that of lipophilic statins, may be far less effective in preventing the detrimental effects of pre-eclampsia pro-inflammatory cytokines. It is also possible that the cytokine-mediated sFlt-1 and PlGF levels measured in the study, which did not differ by group in the current study, are not affected in the short-term by the pleiotropic effects of a low dose statin (Figure5). The low use of aspirin in the current study compared to the common use with statins in CVD trials may have also been a factor.
Figure : Pleiotropic effects of statins.
Plus sign = enhanced/activated; minus sign = inhibited; AT11 = angiotensin 1; ET-1 = endothelin 1; hs-CRP = high-sensitivity C-reactive protein; MMPs = matrix metalloproteinases; NO = nitric oxide; PAI-1 = plasminogen activator inhibitor-1; ROS = reactive oxygen species; SMC = smooth muscle cell; TF = tissue factor; t-PA = tissue-type plasminogen activator; TXA2 = thromboxane A2. (Reprinted with permission5)
Over the last decade, emerging data regarding statin safety in pregnancy has questioned the original classification of all statins as category X medication. Statins have not been shown to be independently associated with increased risk of congenital malformations when taken in the first-trimester11, with no adverse perinatal effects observed when taken in the second and third trimesters in two small double-blinded placebo-controlled randomized clinical trials7, 12. In these two trials, both daily pravastatin 10 mg and 40 mg initiated between 12–17 weeks or 24–32 weeks (respectively) resulted in drug concentrations in umbilical cord and maternal blood near or below the lowest level of quantification of the assay, supporting the limited transplacental transfer of the hydrophilic pravastatin. Although pravastatin reduced maternal cholesterol levels, umbilical cord blood cholesterol levels and infant birthweight did not differ7. As expected with an intermediate pravastatin dose with short duration at the end of the third trimester, Döbert and colleagues’ study provides some additional reassurance for lack of signal for adverse fetal, neonatal or maternal adverse outcomes. Consideration of a second-trimester potent statin might be considered in future trials, although sample size may be prohibitive unless a preeclampsia risk predictive model can be developed for second trimester.
Attempts to improve the prediction of preeclampsia have increased in the past decade, with the inclusion of angiogenic markers sFlt-1 and PlGF and blood pressure thresholds. An important contribution of the current investigation6 is the validation and demonstrated effectiveness of the Bayesian model that detects term preeclampsia 75% with a clinically relevant 10% screen positive. This Bayesian model includes maternal demographic characteristics and medical history, as well as late third trimester mean arterial pressure and maternal serum sFlt-1 and PlGF levels. Thus, new trials testing additional interventions can now be planned with appropriate power/sample size for this important and common condition in pregnant women.
In summary, while knowledge gaps remain regarding the pleiotropic effect of statins in CVD and non-cardiac systemic inflammatory diseases, the current study adds to the evidence that statins likely confer the majority of benefit through LDL lowering. Additionally, substantially more knowledge gaps remain in our understanding of the pathophysiology of preeclampsia, as well as the mechanistic links between preeclampsia and future CVD in women, in part due to the prior stance of excluding pregnant women in research studies. We and others have documented mechanistic links between adverse pregnancy outcomes including pre-eclampsia with subsequent premature hypertension13, ischemic heart disease with coronary microvascular dysfunction14, and left ventricular hypertrophy and myocardial fibrotic scar15. The NHLBI-sponsored NuMoM2b-HHS14 is exploring these gaps, and future investigation, including well-designed and rigorous randomized controlled trials such as Döbert and colleagues should be conducted in women during pregnancy.
Acknowledgements
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U01 64829, U01 HL649141, U01 HL649241, 1R01HL146158, 1U54AG065141, K23HL125941, 1R03 AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064, and the Edythe L. Broad Women’s Heart Research Fellowship, the Constance Austin Women’s Heart Health Fellowship, both at Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, the Linda Joy Pollin Women’s Heart Health Program, and the Erika Glazer Women’s Heart Health Project.
Footnotes
Disclosure Statement
Dr. C. Noel Bairey Merz, serves as Board of Director for iRhythm, fees paid through CSMC from Abbott Diagnostics and Sanofi. Dr. Janet Wei served on an advisory board for Abbott Vascular.
References
- 1.Yu D and Liao JK. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc Res. 2021. doi: 10.1093/cvr/cvab032. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, Rexrode KM, Mukamal KJ and Rich-Edwards JW. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol. 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spracklen CN, Smith CJ, Saftlas AF, Robinson JG and Ryckman KK. Maternal hyperlipidemia and the risk of preeclampsia: a meta-analysis. Am J Epidemiol. 2014;180:346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toleikyte I, Retterstol K, Leren TP and Iversen PO. Pregnancy outcomes in familial hypercholesterolemia: a registry-based study. Circulation. 2011;124:1606–14. [DOI] [PubMed] [Google Scholar]
- 5.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobert M, Varouxaki AN, Mu AC, Syngelaki A, Ciobanu A, Akolekar R, De Paco Matallana C, Cicero S, Greco E, Singh M, et al. Pravastatin versus Placebo in Pregnancies at High Risk of Term Preeclampsia. Circulation. 2021. [DOI] [PubMed] [Google Scholar]
- 7.Costantine MM, Cleary K, Hebert MF, Ahmed MS, Brown LM, Ren Z, Easterling TR, Haas DM, Haneline LS, Caritis SN, et al. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214:720 e1–720 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter PJ and Waters DD. Variations in time to benefit among clinical trials of cholesterol-lowering drugs. J Clin Lipidol. 2018;12:857–862. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, de Lemos JA, Cannon CP, Blazing M, Murphy SA, McCabe CH, Califf R and Braunwald E. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406–14. [DOI] [PubMed] [Google Scholar]
- 10.Liu PY, Liu YW, Lin LJ, Chen JH and Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman BT, Hernandez-Diaz S, Fischer MA, Seely EW, Ecker JL, Franklin JM, Desai RJ, Allen-Coleman C, Mogun H, Avorn J, et al. Statins and congenital malformations: cohort study. BMJ. 2015;350:h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Williams DJ, Cheed V, Middleton LJ, Ahmad S, Wang K, Vince AT, Hewett P, Spencer K, Khan KS, et al. Pravastatin for early-onset pre-eclampsia: a randomised, blinded, placebo-controlled trial. BJOG. 2020;127:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas DM, Parker CB, Marsh DJ, Grobman WA, Ehrenthal DB, Greenland P, Bairey Merz CN, Pemberton VL, Silver RM, Barnes S, et al. Association of Adverse Pregnancy Outcomes With Hypertension 2 to 7 Years Postpartum. J Am Heart Assoc. 2019;8:e013092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas DM, Ehrenthal DB, Koch MA, Catov JM, Barnes SE, Facco F, Parker CB, Mercer BM, Bairey-Merz CN, Silver RM, et al. Pregnancy as a Window to Future Cardiovascular Health: Design and Implementation of the nuMoM2b Heart Health Study. Am J Epidemiol. 2016;183:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quesada O, Park K, Wei J, Handberg E, Shufelt C, Minissian M, Cook-Wiens G, Zarrini P, Pacheco C, Tamarappoo B, et al. Left ventricular mass and myocardial scarring in women with hypertensive disorders of pregnancy. Open Heart. 2020;7:e001273. doi: 10.1136/openhrt-2020-001273 [DOI] [PMC free article] [PubMed] [Google Scholar]