To the Editor:
The health impact of coronavirus disease (COVID-19) appears disproportionate across populations. Biological mechanisms of prognosis of COVID-19 (1) suggest that long-term exposure to ambient air pollution may contribute to health disparities. Mortality impacts including those from pneumonia and cardiovascular events due to long-term exposure to air pollution are well established (2–5). Early studies covering multiple neighborhoods (e.g., U.S. counties) suggest that long-term exposure to particulate matter ⩽2.5 μm in aerodynamic diameter (PM2.5) and ozone (O3) may be linked with higher risk of COVID-19 mortality (6–8).
However, many questions remain regarding linkages between COVID-19 and air pollution. Particularly, pollution levels vary by community, and evidence based on high spatially resolved data such as for neighborhoods is lacking (9). Potential determinants of transmission and prognosis of COVID-19 (e.g., urbanicity, socioeconomic conditions, race/ethnicity, health behaviors, access to health care) are disproportionately distributed across neighborhoods. These factors may have impacted findings of early studies because of possible residual confounding.
We conducted cross-sectional analysis to investigate association between long-term exposure to PM2.5 and O3 and COVID-19 confirmed mortality in 177 neighborhoods (i.e., five-digit modified ZIP code tabulation areas [MZCTAs]) in New York City, New York. COVID-19 data from February 29, 2020, to January 5, 2021, were obtained from the New York City (NYC) Health Department’s COVID-19 Data Website. The date of the first confirmed COVID-19 case and death is February 29 and March 11, 2020, respectively. Annual average PM2.5 levels from December 2008 to December 2018 and summer (June to August) average O3 levels from 2009 to 2018 in 300 m raster were obtained from NYC Open Data. MZCTA-level associations between PM2.5 and O3 and COVID-19 confirmed mortality rate were estimated using Poisson regression. We considered nonlinear terms (e.g., natural cubic spline) for associations between pollutants and COVID-19 mortality. A random MZCTA-level intercept and coordinates were included to consider overdispersion and adjust for spatial autocorrelation. We applied generalized propensity score (GPS) weighting to adjust for transmission (i.e., COVID-19 confirmed case rate), age, sex, race/ethnicity, population density, socioeconomic position, smoking, obesity, preexisting diseases (stroke, lung cancer, asthma, and poorly controlled diabetes), and healthcare availability (model 1). A list of variables is shown in Table 1 and its footnote. GPS was estimated by linear regression of the logarithm of PM2.5 or O3 on covariates. To test mediation through COVID-19 transmission and/or preexisting diseases, we omitted these variables (models 2–4). We further adjusted for covariates whose GPS-weighted absolute correlations with air pollutants exceeded 0.10. MZCTA-level associations between variables other than air pollution and COVID-19 mortality were also evaluated. Data were obtained from NYC Neighborhood Health Atlas, Community Health Profiles, American Community Survey 2014–2018 five-year estimate, hospital beds of Definitive Healthcare of Esri COVID-19 GIS Hub, and the New York State Health Department. We used area-weighting to align different spatial tabulations.
Table 1.
Relative Rate and 95% Confidence Interval of COVID-19 Mortality Rate for One Standard Deviation Increase in Non–Air Pollution Neighborhood-Level Variables
| Covariates* (SD) | Model A |
Model B |
Model C |
|||
|---|---|---|---|---|---|---|
| RR (95% CI) | VIF | RR (95% CI) | VIF | RR (95% CI) | VIF | |
| COVID-19 | ||||||
| Number of COVID-19 tests performed from February 29, 2020, to January 5, 2021 (11,729.64 tests) | 1.08 (1.03–1.14) | 1.3 | 1.05 (1.00–1.10) | 1.5 | 1.05 (1.00–1.1) | 1.6 |
| Age-adjusted COVID-19 confirmed case rate from February 29, 2020, to January 5, 2021 (1,421.50 cases/100,000 persons) | 1.22 (1.14–1.31) | 2.4 | 1.24 (1.16–1.33) | 3.0 | 1.24 (1.15–1.33) | 3.2 |
| Demographics | ||||||
| % of population age 45–65 yr (3.61%) | 0.97 (0.91–1.04) | 1.8 | 1.00 (0.93–1.06) | 2.6 | 1.00 (0.94–1.07) | 2.7 |
| % of population age ≥65 yr (5.10%) | 1.25 (1.17–1.35) | 2.4 | 1.27 (1.18–1.36) | 2.9 | 1.27 (1.18–1.36) | 3.0 |
| Female per 100 males (11.15 persons) | 1.01 (0.94–1.07) | 2.1 | 1.01 (0.95–1.07) | 2.3 | 1.00 (0.95–1.07) | 2.4 |
| % of population that is Black/African American (24.80%) | 1.19 (1.12–1.27) | 2.1 | 1.15 (1.09–1.22) | 2.5 | 1.13 (1.06–1.21) | 3.4 |
| % of population that is Hispanic/Latino (19.48%) | 1.23 (1.14–1.33) | 3.3 | 1.08 (0.99–1.17) | 5.0 | 1.07 (0.98–1.17) | 5.6 |
| Population density (12,370.08 persons/km2) | 0.97 (0.91–1.04) | 2.2 | 0.98 (0.92–1.04) | 2.5 | 0.98 (0.92–1.04) | 2.6 |
| Socioeconomic conditions | ||||||
| % of population of all ages living below the federal poverty level in the past 12 mo (9.67%) | — | — | 1.10 (1.03–1.18) | 3.0 | 1.11 (1.03–1.20) | 4.4 |
| % of civilian noninstitutionalized population with health insurance (3.83%) | — | — | 0.88 (0.82–0.93) | 2.6 | 0.87 (0.82–0.93) | 2.7 |
| % of occupied housing units with >1.00 occupant per room (4.89%) | — | — | 1.00 (0.93–1.07) | 3.8 | 0.99 (0.92–1.07) | 4.6 |
| Average household size (0.35 person) | — | — | 0.94 (0.87–1.01) | 3.5 | 0.93 (0.87–1.01) | 3.6 |
| Availability of adult ICU beds† (1.00 beds/100,000 persons) | — | — | 0.95 (0.88–1.04) | 4.8 | 0.95 (0.87–1.04) | 4.9 |
| % of adult current smokers‡ (3.00%) | — | — | — | — | 0.99 (0.94–1.04) | 1.8 |
| % of obese adults§ (8.71%) | — | — | — | — | 1.02 (0.94–1.11) | 4.5 |
| Standardized incidence ratio of lung cancer (0.20) | — | — | — | — | 0.98 (0.93–1.03) | 1.7 |
Definition of abbreviations: CI = confidence interval; COVID-19 = coronavirus disease; MZCTA = modified ZIP code tabulation area; RR = relative rate; VIF = variance inflation factor.
All variables were adjusted for one another.
The covariates in this table were selected based on their potential role as confounders and correlation analysis. The following covariates were not included because they were highly correlated with the covariates in this table: percentage of population age 15–44 years; percentage of population that is Asian/Pacific islanders; percentage of population age ⩾25 years whose highest level of education is less than a high school diploma or General Education Development; median household income in the past 12 months; stroke hospitalization; preventable asthma hospitalization; poorly controlled diabetes; staffed beds; licensed beds.
. AMZCTA is spatial availability of adult ICU beds at each MZCTA, N is the number of population unit (100 ft2) in that MZCTA, di,j is the travel time from the centroid of population unit i to the hospital j, Rj is the supply ratio of the hospital j, Wj is the weight by the theory of distance decay (10), and Pi is the number of people at population unit i. The supply ratio, where Sj is the number of adult ICU beds and Dr is a set of travel time rings from the hospital j up to r minutes, where r is 5, 10, 15, 20, 25, 30, 35, 40, 45, or 60 minutes. The value of 1 mile/6 min was based on calculation of Vehicle Trip Distance and Duration reported in the Annual Report of Citywide Mobility Survey 2019 by the NYC Department of Transportation.
Percentage of people aged ⩾18 years who report being current smokers (smoking ⩾100 cigarettes and now report smoking every day or some days) from NYC Community Health Profiles.
Percentage of people aged ⩾18 years with body mass index >30 from NYC Community Health Profiles.
From February 29, 2020, to January 5, 2021, the average crude COVID-19 mortality rate was 226.8 deaths per 100,000 persons. Crude COVID-19 mortality rates by neighborhood ranged from 0 (financial district in Manhattan) to 744.9 cases per 100,000 persons (East New York neighborhood of Brooklyn). MZCTA-level long-term PM2.5 and O3 ranged from 7.21 to 13.45 μg/m3 and 22.22 to 38.67 ppb, respectively. Correlation between PM2.5 and O3 was −0.90.
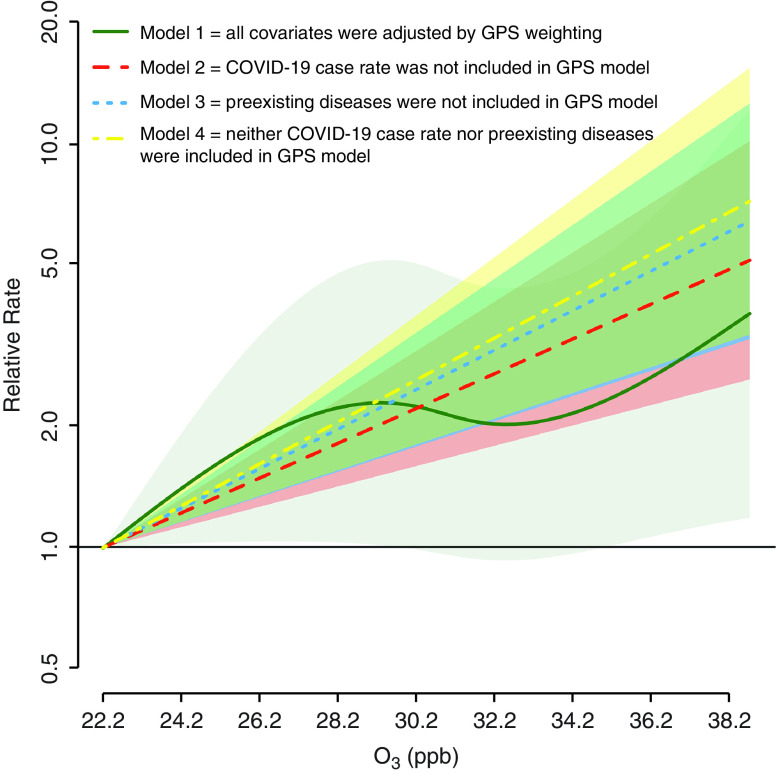
For O3, covariates, including PM2.5, were generally well balanced. A few variables needed to be additionally adjusted using disease models. Figure 1 presents MZCTA-level associations between long-term O3 exposure and COVID-19 mortality risk from models 1–4. Four models showed consistent associations. We should note that preexisting diseases and COVID-19 case rate were well balanced by GPS-weighting even for models 2–4. These well-balanced covariates suggest inability to isolate O3–COVID-19 infection and O3–preexisting disease condition mediation pathways. In model 2, a 1-ppb increase in O3 concentration was associated with a 10.43% (95% confidence interval, 5.97–15.08%) increase in COVID-19 mortality. For PM2.5, covariates were not well balanced, so covariate adjustment was needed. A 1-μg/m3 increase of PM2.5 was associated with a −5.00% (95% confidence interval, −13.62% to 4.47%) increase in COVID-19 mortality.
Figure 1.
Association between long-term exposure to O3 and coronavirus disease (COVID-19) mortality risk in New York City. All models were adjusted by GPS weighting. Covariates in Table 1 and its footnote were generally well balanced (absolute correlation <0.10). In addition to the weighting, some covariates were additionally adjusted by covariate adjustment in disease models because their absolute correlations with O3 after GPS weighting were >0.10 (all the correlations were <0.30). These covariates were number of COVID-19 tests performed, percentage of Hispanic population, poverty rate, and asthma (models 1–2); number of COVID-19 tests performed, percentage of population that is white, percentage of population that is Hispanic, poverty rate, crowded housing, average household size, and obesity (model 3); and number of COVID-19 tests performed, percentage of population that is white, percentage of population that is Hispanic, and poverty rate (model 4). The relative rate compares COVID-19 mortality risk at a given level of long-term O3 exposure to the risk at the lowest observed O3 level (22.2 ppb). Shaded areas represent 95% confidence intervals. For model 1, nonlinear terms were better fit than a linear term based on Akaike Information Criteria (1,138.99 vs. 1,140.37). GPS = generalized propensity score; O3 = ozone.
Table 1 presents MZCTA-level associations between neighborhood characteristics and COVID-19 mortality. We excluded preexisting disease variables because of their high correlation with obesity (>0.70) except for lung cancer. Results suggest that neighborhoods with a higher percentage of Black individuals, Hispanic individuals, individuals aged ⩾65 years, individuals living below the poverty level, and individuals without health insurance are linked with higher COVID-19 mortality risk.
Our results indicate that populations living in neighborhoods with higher O3 levels may have a poorer prognosis of COVID-19. This association was present at pollution levels below Environmental Protection Agency regulatory standards.
We speculate some reasons why we did not find associations for PM2.5. First, PM2.5 was highly correlated with O3, suggesting possible residual confounding by O3. O3 was poorly balanced by GPS-weighting for PM2.5–COVID-19 mortality association, unlike PM2.5, which was well balanced by GPS-weighting for O3–COVID-19 mortality association. There may exist residual confounding by factors other than O3, although we used GPS-weighting and covariate adjustments. Finally, residual variability of PM2.5 and COVID-19 mortality after adjustment may be inadequate for detecting the association between them.
There are limitations. Our analysis is cross-sectional, so the temporality of links between air pollution and COVID-19 mortality rate is not established. The ecological fallacy may not be fully eliminated, although our spatial unit is the most highly resolved to date for this area of research in the United States. Undiagnosed COVID-19 deaths could not be considered. Future research should consider microclimate in exposures for within-neighborhood variability, the complex pollution mixture, and COVID-19 infection as well as mortality.
To the best of our knowledge, this is the first study to investigate associations between long-term PM2.5 and O3 exposure with COVID-19 mortality using neighborhood-level data in the United States, for which race/ethnicity, socioeconomic conditions, population density, availability of healthcare, and PM2.5 and O3 concentrations are heterogeneously distributed. Our findings also support the theory of disproportionate health burden of COVID-19 by socioeconomic conditions and race/ethnicity. Complex social, economic, cultural, and historical factors may contribute to these health disparities. Our results for multiple stressors may be generalizable to other areas and provide important scientific evidence to aid global efforts to tackle disproportionate impacts of COVID-19. Individual-level studies such as prospective cohort studies should be conducted to confirm links between air pollution and prognosis of COVID-19.
Footnotes
Supported by an Assistance Agreement awarded by the U.S. Environmental Protection Agency to Yale University (RD835871). It has not been formally reviewed by the U.S. Environmental Protection Agency. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. The U.S. Environmental Protection Agency does not endorse any products or commercial services mentioned in this publication. Research reported in this publication was also supported by the National Institute on Minority Health and Health Disparities of the NIH under grant R01MD012769. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: M.L.B. was the principal investigator. H.K. and M.L.B. designed the study. H.K. collected data and conducted analysis. H.K. and M.L.B. interpreted data and analysis. H.K. wrote the draft. H.K. and M.L.B. edited the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202010-3844LE on May 3, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 2. Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. American Heart Association Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease; Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3. Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med. 2010;181:47–53. doi: 10.1164/rccm.200901-0160OC. [DOI] [PubMed] [Google Scholar]
- 4. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 5. Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. doi: 10.1016/j.envint.2019.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandt EB, Beck AF, Mersha TB. Air pollution, racial disparities, and COVID-19 mortality. J Allergy Clin Immunol. 2020;146:61–63. doi: 10.1016/j.jaci.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci Adv. 2020;6:eabd4049. doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang D, Shi L, Zhao J, Liu P, Sarnat JA, Gao S, et al. Urban air pollution may enhance COVID-19 case-fatality and mortality rates in the United States. Innovation (N Y) 2020;1:100047. doi: 10.1016/j.xinn.2020.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konstantinoudis G, Padellini T, Bennett J, Davies B, Ezzati M, Blangiardo M. Long-term exposure to air-pollution and COVID-19 mortality in England: A hierarchical spatial analysis. Environ Int. 2021;146:106316. doi: 10.1016/j.envint.2020.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delamater PL, Messina JP, Grady SC., WinklerPrins V, Shortridge AM. Do more hospital beds lead to higher hospitalization rates? A spatial examination of Roemer’s Law. PLoS One. 2013;8:e54900. doi: 10.1371/journal.pone.0054900. [DOI] [PMC free article] [PubMed] [Google Scholar]